Abstract
The deuterated water method is used extensively to measure gluconeogenesis in humans. This method assumes negligible exchange of the lower three carbons of fructose 6-phsophate via transaldolase exchange since this exchange will result in enrichment of carbon 5 of glucose in the absence of net gluconeogenesis. The present studies tested this assumption. 2H2O and acetaminophen were ingested and [1-13C]acetate infused in 11 nondiabetic subjects after a 16-h fast. Plasma and urinary glucuronide enrichments were measured using nuclear magnetic resonance spectroscopy before and during a 0.35 mU·kg FFM−1·min−1 insulin infusion. Rates of endogenous glucose production measured with [3-3H]- and [6,6-2H2]glucose did not differ either before (14.0 ± 0.7 vs. 13.8 ± 0.7 μmol·kg−1·min−1) or during the clamp (10.4 ± 0.9 vs. 10.9 ± 0.7 μmol·kg−1·min−1), consistent with equilibration and quantitative removal of tritium during triose isomerase exchange. Plasma [3-13C] glucose-to-[4-13C]glucose and urinary [3-13C] glucuronide-to-[4-13C]glucuronide ratios were <1.0 (P < 0.001) in all subjects both before (0.66 ± 0.04 and 0.60 ± 0.04) and during (059 ± 0.05 and 0.56 ± 0.06) the insulin infusion, respectively, indicating that ∼35–45% of the labeling of the 5th carbon of glucose by deuterium was due to transaldolase exchange rather than gluconeogenesis. When corrected for transaldolase exchange, rates of gluconeogenesis were lower (P < 0.001) and glycogenolysis higher (P < 0.001) than uncorrected rates both before and during the insulin infusion. In conclusion, assuming negligible dilution by glycerol and near-complete triose isomerase equilibration, these data provide strong experimental evidence that transaldolase exchange occurs in humans, resulting in an overestimate of gluconeogenesis and an underestimate of glycogenolysis when measured with the 2H2O method. Use of appropriate 13C tracers provides a means of correcting for transaldolase exchange.
Keywords: deuterated water method; transaldolase exchange reaction; triosephosphate isomerase; endogenous glucose production; [3-3H]glucose; [6,6-2H2]glucose
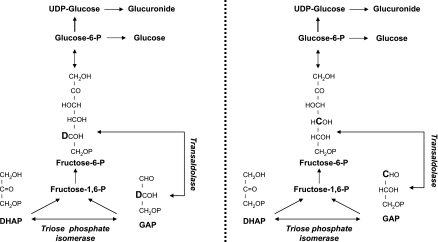
the deuterated water method is used extensively to measure gluconeogenesis in humans (1, 4, 10, 12, 16, 17, 32). This method assumes negligible exchange of the 4,5,6-triose fragment of fructose 6-phosphate and glyceraldehyde-3-phosphate via the transaldolase exchange reaction (27). If such exchange does occur (Fig. 1), then glucose can be labeled with deuterium on the 5th carbon (position 5) by simple exchange with a labeled three-carbon precursor without net hexose synthesis (24). We examined this question in nondiabetic subjects initially by infusing [3,5-2H2]glucose (9) and then subsequently by infusing [3,5-2H2]galactose (5). Whereas the ratio of deuterium on position 5 relative to position 3 of the infusate averaged 1.0, the ratio in either the plasma glucose or the uridyl diphosphate (UDP)-glucose pool was <1 in all subjects.
Fig. 1.
Schematic representation of the effect of transaldolase exchange on the incorporation of 13C (denoted as C) on the 4th carbon and 2H, i.e., deuterium (denoted as D), on the 5th carbon of fructose 6-phosphate during infusion of [1-13C]acetate, and following ingestion of 2H2O. UDP, uridyl diphosphate; DHAP, dihydroxyacetone phosphate; glucose-6-P, glucose 6-phosphate; GAP, glyceraldehyde 3-phosphate.
Although these experiments supported the presence of transaldolase exchange, they did not prove it since the ratio could have been decreased by loss of deuterium on the 5th carbon of glucose due to exchange with an unlabeled three-carbon precursor via the transaldolase exchange reaction, retention of deuterium on the 3rd carbon of glucose due to a kinetic isotope effect at the level of the triose phosphate isomerase reaction, or a combination of both (5, 9). Determination if transaldolase exchange occurs in humans is important since it would result in enrichment of glucose with deuterium on position 5 following administration of deuterated water, and therefore, it would cause an overestimation of the contribution of gluconeogenesis to endogenous glucose production (Fig. 1). Transaldolase exchange would similarly influence the glucose-labeling pattern observed with essentially all of the labeled precursor approaches that are used to measure gluconeogenesis (18–20).
Selective retention of tritium at the level of the triose phosphate isomerase could also influence measurement of glucose turnover using [3-3H]glucose, a widely used tracer in both animals and humans. If a kinetic isotope effect at level of the triose phosphate isomerase reaction caused retention of tritium (which presumably would be greater than retention of deuterium at the same position), this could result in subsequent release of [3-3H]glucose back into the systemic circulation via hepatic recycling of triose phosphate to glucose (15, 30). If this occurred, then glucose turnover measured with [3-3H]glucose would be lower than that measured with a tracer such as [6,6-2H2]glucose whose label is not influenced by the triose phosphate isomerase reaction.
The present study was undertaken to determine whether transaldolase exchange occurs in humans, and if so, does it alter rates of gluconeogenesis measured using the deuterated water method? To address this question, 2H2O was given by mouth, and [1-13C]acetate was infused to assess transaldolase activity in nondiabetic humans. [1-13C]acetate is metabolized to yield [1-13C]glyceraldehyde-3-phosphate and [1-13C]dihydroxyacetone phosphate via Krebs cycle, gluconeogenic, and triose phosphate isomerase reactions. These precursors combine to form fructose 1,6-bisphosphate and then subsequently [4-13C]- or [3-13C]glucose (Fig. 1). We demonstrated recently that the ratio of [1,2,3-13C3]glucose to [4,5,6-13C3]glucuronide isotopomers derived from gluconeogenic metabolism of [U-13C]glycerol is altered by transaldolase exchange activity (23). On this basis, the ratio of “top” to “bottom” glucose triose isotopomers (e.g., [3-13C]- to [4-13C]glucose) allows the fraction of unlabeled glucose 6-phosphate that has participated in transaldolase exchange to be calculated (23). Therefore, in the present experiments, enrichment of glucose from [1-13C]acetate and 2H2O was measured simultaneously using 13C and 2H nuclear magnetic resonance (NMR) spectroscopy, allowing calculation of the contribution of transaldolase exchange to incorporation of deuterium on position 5 of glucose following 2H2O ingestion.
We report that the plasma [3-13C]glucose-to-[4-13C]glucose enrichment ratio observed during a [1-13C]acetate infusion was <1.0 in all subjects both before and during the insulin infusion. Assuming negligible dilution by glycerol and nearly complete triose isomerase equilibration, these data provide strong experimental evidence that transaldolase exchange occurs in humans. This resulted in an overestimation of gluconeogenesis and an underestimation of glycogenolysis that can be corrected by concurrent measurement of the 13C distribution in carbons 3 and 4 of plasma glucose.
RESEARCH DESIGN AND METHODS
After approval of the Mayo Institutional Review Board was given, 11 nondiabetic subjects (age 42 ± 4 yr, 2 females and 9 males, body mass index 30 ± 1 kg/m2) gave written, informed consent to participate in the study. Subjects were in good health and at stable weights and did not engage in regular vigorous exercise, and they did not have a history of diabetes in first-degree relatives. Subjects were admitted to the clinical research unit of the Mayo Clinic Center for Translational Science Activities at ∼1700 on the evening before the study and provided a standard supper (10 calories/kg; carbohydrate/fat/protein ratio 55:30:15). Subjects then ingested 1.67 g of 2H2O per lean body weight of water in three equally divided doses at 2200, 2400, and 0200. The subjects were permitted sips of water containing 2H2O if they so desired but otherwise remained fasted.
Subjects were awakened the following morning, and catheters were placed in forearm veins for tracer infusion and sampling of arterialized venous blood, as described previously (7). At 0600 (−180 min), infusions of [3-3H]glucose (12 μCi prime and 0.12 μCi/min continuous), [6,6-2H2]glucose (33 μmol/kg prime and 0.33 μmol·kg−1·min−1 continuous), and [1-13C]acetate (5.0 μmol·kg−1·min−1) were started and continued until the end of study at 1300. A urinary catheter was placed in the morning at 0500 for collection of urine. One gram of acetaminophen was given at ∼0630 and repeated at ∼0845 to enable measurement of urinary glucuronide. At time 0, somatostatin (60 ng·kg−1·min−1), insulin (0.35 mU·kg−1·min−1), glucagon (0.65 ng·kg−1·min−1), and human growth hormone (3 ng·kg−1·min−1) were started to ensure constant and equal portal concentrations of insulin and glucagon (7). Blood was sampled for glucose, and hormones were sampled at −30, 0, 60, 120, 180, 210, and 240 min. Samples for [3-3H]glucose-specific activity and [3-13C]-, [4-13C]-, [5-2H]-, [2-2H]-, and [6,6-2H2]glucose enrichment were obtained at −30, −20, −10, 0, 210, 220, 230, and 240 min.
An infusion of 50% dextrose containing [3-3H]- and [6,6-2H2]glucose was started at time 0 and given in amounts sufficient to clamp glucose at 110 mg/dl to match concentrations to those commonly present in people with prediabetes who were being studied as part of a separate experiment. In addition, the basal infusions of [3-3H]- and [6,6-2H2]glucose were tapered beginning at time 0 in a pattern that mimicked the anticipated changes in glucose production to minimize the changes in plasma glucose enrichment and specific activity, as described previously (2, 36).
Analytical methods.
Plasma samples were obtained at −30, −20, −10, and 0 min and at 210-, 220-, 230-, and 240-min urine samples at 0 and 240 min. All blood samples were immediately placed on ice, centrifuged at 4°C, separated, and stored at −80°C until analyses. Plasma glucose was analyzed using a GM9 Analox glucose analyzer (Analox Instruments, London, UK). Plasma insulin, C-peptide, and glucagon concentrations, [3-3H]glucose-specific activity, and [6,6-2H2]glucose enrichment were measured as described previously (2, 31).
For 13C enrichment analysis of glucose and glucuronide by NMR analysis of monoacetone glucose (MAG) and monoacetoneglucuronic lactone (MAGL) derivatives, we confirmed our previously published 13C NMR chemical shift assignments (21, 22) by 13C and 1H NMR spectroscopy of authentic compounds and derivatives formed from glucose enriched in various 13C positions. As reported previously (21), the 13C chemical shifts for the aliphatic MAGL carbons are arranged sequentially from carbon 1 downfield to carbon 5 upfield, with carbon 3 resonating at 81.5 ppm and carbon 4 resonating at 78.9 ppm, whereas for MAG the carbon 4 shift (80.6 ppm) is downfield of carbon 3 (74.8 ppm) (22). 13C longitudinal relaxation times (T1) for MAG and MAGL were quantified by the inversion recovery method, using natural abundance 13C signals from authentic standards. For quantifying plasma [3-13C]glucose-to-[4-13C]glucose excess enrichment ratios, MAG and MAGL samples were dissolved in 90/10 CD3CN/H2O and 100% CD3CN, respectively (21). 13C NMR spectra were obtained at 25°C with an 11.75 T Varian Unity spectrometer equipped with a 5-mm broadband probe. Based on T1 values of 2–4 s, quantitative 13C signals for MAG and MAGL aliphatic carbons were acquired with a 90° pulse angle, an acquisition time of 2.13 s during which 1H decoupling was applied, and a pulse delay of 21.77 s. 1H NMR spectra of MAG in 100% 2H2O were acquired with a 14.1 T Varian Unity spectrometer and indirect detection probe. Absolute 13C carbon 1 enrichment was quantified from the relative intensities of the 12C-1H central doublet and the 13C-1H satellites of the position 1 1H signal. Absolute enrichments of carbons 3 and 4 were estimated from the ratio of their 13C signals to that of carbon 1, multiplied by the absolute carbon 1-13C enrichment. Excess 13C enrichments of carbons 3 and 4 were calculated as the difference between absolute enrichment and background natural abundance value of 1.11%.
For all 13C signals measured, signal-to-noise ratios were >50:1 for MAG derived from plasma glucose and were typically 100:1 for MAGL derived from glucuronide. A signal-to-noise ratio of 50:1 represents a 2% uncertainty in the quantification of the observed natural 1.11% abundance 13C signal. This corresponds to an absolute uncertainty of 0.02% in determining the excess 13C enrichment level (i.e., the lower limit enrichment uncertainty would be 0.18 ± 0.02%).
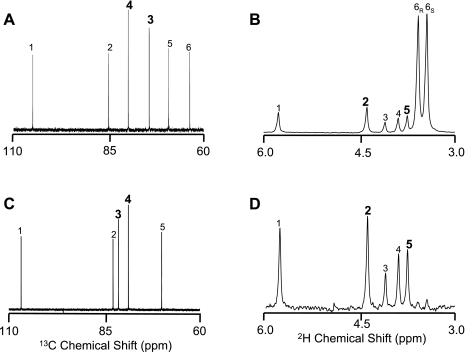
Because of the inability to extract sufficient quantities of glucuronide from small volumes of urine collected, urinary glucuronide enrichment could be measured in only nine of the subjects. Urinary acetaminophen glucuronide was derivatized to the MAGL for 13C NMR analysis, as described (22). This derivative was then reduced to MAG with lithium borohydride as described (21) for 2H NMR analysis of positional 2H enrichment. 2H NMR spectra were acquired at 50°C with a 14.1 T Varian VNMR system, as described previously (21, 22). 2H enrichment was calculated by comparing the hexose positional 2H signal intensities with those of the MAG methyl signals enriched to 2% 2H (21). NMR signals were quantified using the Nuts NMR spectral analysis program (Acorn NMR, Fremont, CA). Representative 13C and 2H spectra of MAG and MAGL derived from plasma and urine are shown in Fig. 2.
Fig. 2.
Examples of 13C (A) and 2H NMR spectra (B) of monoacetone glucose derivative from plasma glucose. Also shown is an example of a 13C NMR spectrum of the monoacetoneglucuronic lactone derivative from urinary glucuronide (C) and a 2H NMR spectrum of the same sample following its reduction to monoacetone glucose (D). All spectra were obtained from the same subject before (basal) the insulin infusion. The number above each signal represents its position in the hexose molecule.
Calculations.
All rates are expressed per lean body mass. Glucose turnover was calculated using the steady-state equations of Steele, as described previously (34, 36). The fraction of plasma glucose derived from transaldolase exchange was calculated as the plasma [3-13C]glucose-to-[4-13C]glucose and urinary [3-13C]glucuronide-to-[4-13C]glucuronide ratios. Endogenous glucose production (EGP) was calculated by subtracting the glucose infusion rate required to maintain euglycemia from total glucose appearance. Gluconeogenesis was calculated by multiplying the ratio of deuterium enrichment of positions 5 and 2 of glucose (2H5/2H2) times EGP. Since EGP rates did not differ with [3-3H]- and [6,6-2H2]glucose (see results), only those calculated with [3-3H]glucose are given in the text and figures. Glycogenolysis was calculated by subtracting gluconeogenesis from EGP, as described previously (7).
The observed enrichment of deuterium on position 5 relative to position 2 of glucose (2H5/2H2OBS) was adjusted for transaldolase exchange to give a corrected value (2H5/2H2CORR) as follows:
where 13C3/13C4 is the plasma [3-13C]glucose-to-[4-13C]glucose ratio.
The corrected gluconeogenic (GNGCORR) and glycogenolytic fluxes (GLYCORR) were then calculated as follows:
Statistical analysis.
Data in the text and figures are expressed as means ± SE. Values from −30, −20, −10, and 0 min were averaged as basal and at 210, 220, 230, and 240 min as clamp for statistical analysis and representation. Wilcoxon's signed-rank test was used to test the hypotheses that the plasma [3-13C]glucose-to-[4-13C]glucose and [3-13C]glucuronide-to-[4-13C]glucuronide ratios were less than one and no different baseline vs. clamp, whereas Student's one-tailed paired t-tests were used to test that insulin suppressed EGP, gluconeogenesis, and glycogenolysis. P < 0.05 was considered statistically significant.
RESULTS
Plasma glucose, insulin, C-peptide, and glucagon concentrations.
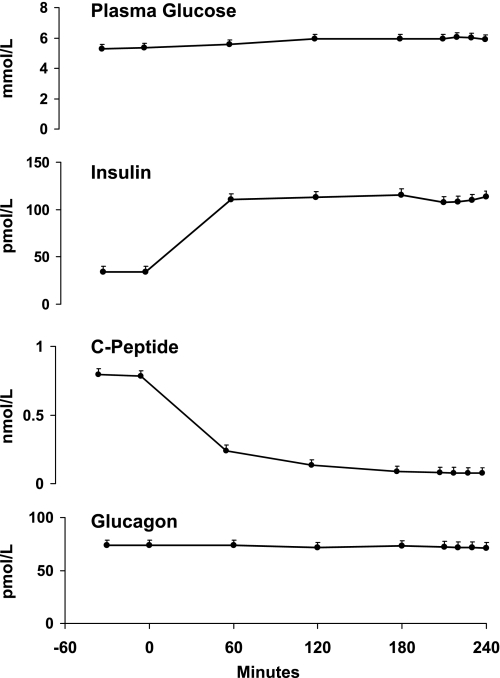
Glucose concentrations averaged 5.3 ± 0.0 mmol/l before the clamp and increased (P < 0.01) to 6.0 ± 0.1 mmol/l during the clamp (Fig. 3). Insulin concentrations averaged 34 ± 4 pmol/l before the clamp and increased (P < 0.001) to 110 ± 6 pmol/l during the clamp. C-peptide concentrations averaged 0.8 ± 0.05 nmol/l before the clamp and were suppressed (P < 0.001) to 0.07 ± 0.05 nmol/l during the clamp. Glucagon concentrations did not differ either before or during the clamp (73.6 ± 5.1 vs. 71.9 ± 3.4 pg/ml).
Fig. 3.
Plasma glucose, insulin, C-peptide, and glucagon concentrations observed before and during the infusion of insulin started at time 0 min.
EGP.
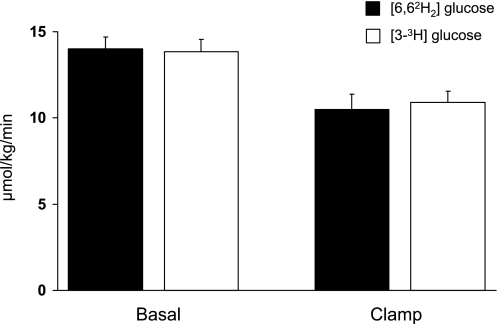
EGP did not differ when measured with [6,6-2H2]- or [3-3H]glucose either before (14.0 ± 0.7 vs. 13.8 ± 0.7 μmol·kg−1·min−1) or during (10.4 ± 0.9 vs. 10.9 ± 0.7 μmol·kg−1·min−1) the clamp (Fig. 4).
Fig. 4.
Rates of endogenous glucose production measured with [6,6,2H2]- (filled bars) and [3-3H]glucose (open bars) before (basal) and during the insulin infusion.
Plasma [3-13C]glucose-to-[4-13C]glucose and urinary [3-13C]glucuronide-to-[4-13C]glucuronide enrichments and ratios.
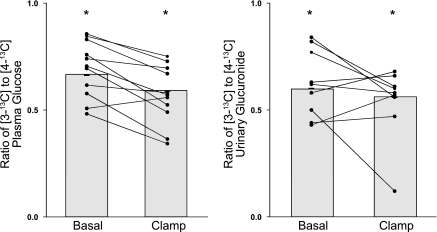
Excess 13C enrichment was observed in carbons 3 and 4 of plasma glucose and urinary glucuronide (Fig. 5). Plasma glucose excess 13C enrichment was 0.37 ± 0.05% for carbon 3 and 0.55 ± 0.06% for carbon 4 before the clamp and was 0.28 ± 0.06% for carbon 3 and 0.46 ± 0.08% for carbon 4 during the clamp. The glucose infused during the clamp was not enriched with 13C glucose, accounting for the decrease in plasma 13C glucose enrichment. Urinary glucuronide enrichment was 0.18 ± 0.03% for carbon 3 and 0.30 ± 0.05% for carbon 4 before the clamp and increased to 0.37 ± 0.06% for carbon 3 and 0.61 ± 0.07% for carbon 4 during the final 30 min of the clamp. This increase over time indicates progressive equilibration between the hepatic UDP glucose pool and urinary glucuronide and that, under these mild hyperinsulinemic hyperglycemic clamp conditions, the UDP-glucose pool was not substantially diluted by infused glucose.
Fig. 5.
The plasma [3-13C]glucose-to-[4-13C]glucose (left) and urinary [3-13C]glucuronide-to-[4-13C]glucuronide ratios (right) observed before (basal) and during the insulin infusion. *P < 0.0001 vs. 1.
The plasma [3-13C]glucose-to-[4-13C]glucose ratio was <1.0 (P < 0.0001) in all subjects both before (0.66 ± 0.04) and during (0.59 ± 0.05) the clamp, consistent with extensive transaldolase exchange (Fig. 5). The [3-13C]glucuronide-to-[4-13C]glucuronide ratio, measured in nine of the 11 subjects, was also <1.0 (P < 0.0001) in all subjects both before (0.60 ± 0.04) and during (0.56 ± 0.06) the clamp. The [3-13C]glucose-to-[4-13C]glucose ratio before the clamp did not differ from that observed during the clamp when measured in either plasma (P = 0.102) or urine (P = 0.82).
Deuterium enrichment on positions 5 and 2 of plasma glucose.
The corrected deuterium enrichment on position 5 of plasma glucose was lower (P < 0.0001) than the uncorrected enrichment both before (0.16 ± 0.02 vs. 0.23 ± 0.02) and during (0.06 ± 0.01 vs. 0.11 ± 0.02) the clamp (Table 1). This resulted in the corrected ratio of deuterium on position 5 to position 2 also being lower (P < 0.0001) both before (0.33 ± 0.03 vs. 0.49 ± 0.03) and during (0.29 ± 0.04 vs. 0.47 ± 0.04) the clamp. The corrected ratio of deuterium on position 5 to position 2 during the clamp did not differ from that present before the clamp.
Table 1.
Deuterium enrichment on positions 5 and 2 of plasma glucose
| Basal | Clamp | |
|---|---|---|
| Deuterium enrichment on position 2 | 0.47 ± 0.01 | 0.22 ± 0.03 |
| Deuterium enrichment on position 5 (uncorrected) | 0.23 ± 0.02 | 0.11 ± 0.02 |
| Ratio of deuterium enrichment on positions 5 and 2 (uncorrected) | 0.49 ± 0.03 | 0.47 ± 0.04 |
| Deuterium enrichment on position 5 (corrected) | 0.16 ± 0.02 | 0.06 ± 0.01 |
| Ratio of deuterium enrichment on positions 5 and 2 (corrected) | 0.33 ± 0.03 | 0.29 ± 0.04 |
Values are means ± SE.
Rates of gluconeogenesis and glycogenolysis uncorrected and corrected for transaldolase exchange reaction.
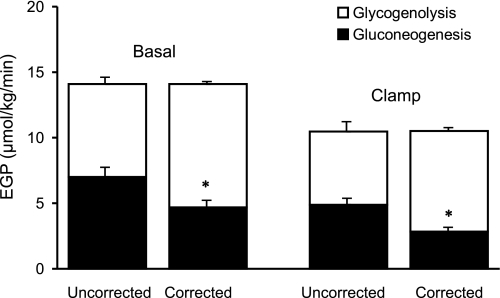
Corrected rates of gluconeogenesis were lower (P < 0.001) than uncorrected rates of gluconeogenesis both before (4.7 ± 0.6 vs. 6.9 ± 0.7 μmol·kg−1·min−1) and during (2.8 ± 0.3 vs. 4.9 ± 0.5 μmol·kg−1·min−1) the clamp (Fig. 6). Corrected rates were lower (P < 0.05) during the clamp than before the clamp, indicating suppression of gluconeogenesis.
Fig. 6.
Contribution of gluconeogenesis (filled bars) and glycogenolysis (open bars) to endogenous glucose production (EGP) observed before (basal) and during the insulin infusion, calculated using the uncorrected and corrected ratios of the deuterium enrichment on position 5 and position 2 of plasma glucose. *P < 0.001 vs. uncorrected.
Corrected rates of glycogenolysis were higher (P < 0.001) than uncorrected rates of gluconeogenesis both before (9.3 ± 0.6 vs. 7.1 ± 0.4 μmol·kg−1·min−1) and during (7.7 ± 0.9 vs. 5.6 ± 0.7 μmol·kg−1·min−1) the clamp. Corrected rates of glycogenolysis were lower (P < 0.05) during the clamp than before the clamp, indicating suppression of glycogenolysis.
DISCUSSION
The deuterated water method is used extensively to measure gluconeogenesis in humans (1, 4, 10, 12, 16, 17, 32). One of the key assumptions of this method is that there is negligible transaldolase exchange (25), since this would result in deuterium enrichment of position 5 in the absence of gluconeogenic hexose phosphate synthesis (Fig. 1). However, the present data indicate that there is substantial transaldolase exchange in humans. Therefore, measurements of gluconeogenesis based on deuterated water or other labeled gluconeogenic precursors need to account for this exchange.
The deuterated water method was pioneered by Landau et al. (27, 28). This method has tremendous appeal based on simplicity of calculation and by the fact that knowledge of the ratio of deuterium enrichment on positions 5 and 2 of glucose or UDP-glucose obviates the need for more complicated measurements of intracellular precursor enrichment (27, 28). However, as clearly articulated by Landau in his initial description of this method (27), the existence of transaldolase exchange following ingestion of deuterated water will contribute to the enrichment of glucose position 5 with deuterium and therefore will result in an overestimation of gluconeogenesis.
Since Basu et al. (5) and Bock et al. (9) recognized that this was a critical assumption, we undertook a series of experiments that sought to determine whether transaldolase exchange occurred in humans. We began by infusing [3,5-2H2]glucose that was enriched with deuterium on positions 3 and 5 of glucose at a ratio of 1.0 (9). We found that the position 5 to position 3 deuterium enrichment ratio of plasma glucose was less than one in all subjects. In subsequent experiments (5), we infused [3,5-2H2]galactose also labeled with deuterium on positions 3 and 5 at a ratio of 1.0 and measured enrichment of UDP-glucose positions 5 and 3 via the glucuronide method. Once again, the position 5 to position 3 deuterium enrichment ratio of glucuronide was less than one in all subjects. These experiments strongly suggested, but did not prove, that transaldolase exchange occurs in humans, since the position 5 to position 3 deuterium enrichment ratio also would be decreased if there was retention of position 3 deuterium due to a kinetic isotope effect at the level of the triosephosphate isomerase reaction (15, 30).
The current experiments used a 13C tracer to assess transaldolase exchange. Since metabolism of 13C relative to 12C is not subject to significant isotope effects, unequal enrichment on carbons 3 and 4 of glucose cannot be ascribed to isotopic fractionation. Theoretically, a glucose 13C3/13C4 ratio of <1.0 could occur if exchange of [1-13C]glyceraldehyde-3-phosphate and [1-13C]dihydroxyacetone phosphate via triose phosphate isomerase reaction was incomplete. We believe this to be unlikely in our experimental setting since previous human studies indicate that there is near-complete equilibration of gluconeogenic tracers into the triose halves of glucose or glucuronide via the triose phosphate isomerase reaction (15, 30). Furthermore, incomplete triose phosphate isomerase equilibration would result in retention of the [3-3H]glucose label relative to that of [6,6-2H2]glucose, causing reduced dilution of the former relative to the later and therefore lower estimates of EGP from [3-3H]glucose compared with [6,6-2H2]glucose. This did not happen. Rates of EGP rates measured with the two tracers were the same both before and during the clamp. However, some retention of tritium on the third carbon of glucose cannot be rigorously excluded since the intrahepatic specific activities of dihydroxyacetone phosphate and glyceraldehyde 3-phosphate were not measured directly.
In the present experiments, the plasma [3-13C]glucose-to-[4-13C]glucose ratio was used to correct estimates of gluconeogenesis obtained with the deuterated water method for transaldolase exchange. Corrected glycogenolysis was then calculated by subtracting corrected gluconeogenesis from endogenous glucose production. Of note, the pattern of change for uncorrected and corrected gluconeogenesis and glycogenolysis during the clamp was the same; the small increase in insulin and glucose used in the present experiments resulted in suppression of both gluconeogenesis and glycogenolysis. However, the percentage of endogenous glucose production derived from gluconeogenesis was lower and from glycogenolysis was higher both in the basal state and during the clamp when corrected than when uncorrected.
Although the present data provide strong experimental support that transaldolase exchange occurs in humans and provide a means to correct for this exchange, they do not determine the extent to which this approach reflects actual rates of gluconeogenesis. This is a difficult question to answer since there is no criteria standard for the measurement of gluconeogenesis in humans. In an effort to validate the deuterated water method, Chandramouli et al. (11) reasoned that if the deuterated water method provides an accurate estimate of gluconeogenesis, then the fraction of endogenous glucose production attributed to gluconeogenesis should increase with fasting and ultimately approach unity when hepatic glycogen is severely depleted by an extended fast. This pattern was indeed observed (11). However, unfortunately, this does not exclude transaldolase exchange since hepatic glucose 6-phosphate enrichment will approach that of body water and triose phosphate precursors when glycogen stores and therefore glycogenolysis approach zero.
Methods that do not rely upon assumptions regarding the pattern of precursor labeling have also been used to measure gluconeogenesis. The hepatic catheterization method enables determination of the rate of extraction of gluconeogenic precursors by the liver (13, 35). Although maximal rate of gluconeogenesis can be calculated by assuming that all precursors are converted to glucose 6-phosphate, the actual rate is not known. In vivo 13C magnetic resonance spectroscopy can be used to measure the rate of hepatic glycogenolysis; if rates of endogenous glucose production are also known, gluconeogenesis can be calculated by subtraction (33). However, this method assumes negligible hepatic recycling of the 13C glucose label and requires precise measurements of the magnitude of change in liver volume. In addition, since this method is only available in a few highly specialized research centers worldwide, it is less accessible to the research community compared with tracer methods.
Of note, although the present experiments used [1-13C]acetate to measure transaldolase exchange, rates of exchange can also be measured using other gluconeogenic tracers. At least in theory, unequal enrichment or specific activity of the triose moieties of glucose derived from tracers such as [U-13C]glycerol (23), [3-14C]lactate (29), or recycled isotopomers from [U-13C]glucose (18) can also provide an index of the rate of transaldolase exchange. Therefore, measurement of glucose triose isotopomer ratios (for example, [1,2,3-13C3]glucose to [4,5,6-13C3]glucose), could enable correction for transaldolase exchange without the need for an additional tracer.
The present study has several limitations. Plasma [3-13C]glucose-to-[4-13C]glucose and urinary [3-13C]glucuronide-to-[4-13C]glucuronide ratios were measured before and after a 4-h infusion of a relatively low dose of insulin. Additional studies at higher insulin concentrations will be required to more fully examine whether transaldolase exchange is modulated by insulin. In theory, high rates of hepatic lypolysis combined with incomplete equilibration at the level of the triose isomerase reaction could generate sufficient unlabeled dihydroxyacetone phosphate to disproportionately decrease labeling of C3 relative to C4 (26). However, we doubt whether this is the cause of the lower C3/C4 ratio in the current studies since equilibration of the triose isomerase reaction is nearly complete and lipolysis is not high following an overnight fast and is presumably further suppressed by infusion of insulin (3, 13, 33, 35).
We measured transaldolase exchange only in healthy nondiabetic subjects who had normal fasting glucose concentrations. Studies in individuals with impaired fasting glucose are ongoing to determine whether the alterations in the metabolic state that occur with prediabetes influence the extent of transaldolase exchange. If so, our previous conclusion that gluconeogenesis is increased in people with prediabetes may be incorrect (8). It is also not known whether other factors such as nutritional status, hepatic zonation (14), or disease states such as obesity or diabetes alter the rate of transaldolase exchange. On the other hand, if the rate of transaldolase exchange remains constant under a wide variety of experimental conditions, then, although the absolute rates of gluconeogenesis determined without correcting the deuterium enrichment of glucose position 5 may be incorrect, comparisons of relative differences among groups may remain valid.
In summary, the present data indicate that infusion of [1-13C]acetate results in asymmetric labeling of carbons 3 and 4 of glucose in the fasting state and during an insulin infusion. Assuming negligible dilution by glycerol and nearly complete equilibration of triose isomerase, these data provide strong experimental evidence that transaldolase exchange occurs in humans at a rate that is sufficient to increase the deuterium enrichment in position 5 following administration of 2H2O by ∼35–45%. [3-3H]- and [6,6-2H2]glucose rates provide equivalent estimates of endogenous glucose production, arguing against substantial hepatic recycling of the tritium label. Although the current experimental design provides a means for correcting position 5 deuterium enrichment for asymmetrical labeling of carbons 3 and 4, it is both complex and expensive. Future studies are needed to determine whether the extent of transaldolase exchange is influenced by differing experimental conditions and disease states. If not, the deuterated water method may still provide useful estimates of gluconeogenesis and glycogenolysis.
GRANTS
This study was supported by the US Public Health Service (DK-29953, 1-UL1-RR-024150) and the Mayo Clinic. The NMR spectrometers are part of the National NMR Network and were purchased in the framework of the Portuguese National Programme for Scientific Re-equipment, contract no. REDE/1517/RMN/2005, with funds from POCI 2010 (Fundo Europeu de Desenvolvimento Regional) and Fundação para a Ciência e a Tecnologia.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
We thank N. Uzhmanov (visiting medical student, Gazi Medical School, Ankara, Turkey), B. Dicke (Mayo Clinic), P. Reich (Mayo Clinic), and B. Norby (Mayo Clinic) for technical assistance, C. Nordyke (Mayo Clinic) for assistance with graphics, and the staff of the Mayo Clinical Research Unit and Center for Clinical and Translation Science Activities for assistance with the studies. Dr. Robert A. Rizza is the Earl and Annette R. McDonough Professor of Medicine. We are particularly grateful for the guidance of Dr. Bernie Landau. We miss his wisdom.
REFERENCES
- 1. Adkins A, Basu R, Persson M, Dicke B, Shah P, Vella A, Schwenk WF, Rizza R. Higher insulin concentrations are required to suppress gluconeogenesis than glycogenolysis in non-diabetic humans. Diabetes 52: 2213–2220, 2003 [DOI] [PubMed] [Google Scholar]
- 2. Basu A, Basu R, Shah P, Vella A, Johnson CM, Jensen M, Nair KS, Schwenk F, Rizza R. Type 2 diabetes impairs sphlanchnic uptake of glucose but does not alter intestinal glucose absorption during enteral glucose feeding: additional evidence for a defect in hepatic glucokinase activity. Diabetes 50: 1351–1362, 2001 [DOI] [PubMed] [Google Scholar]
- 3. Basu R, Basu A, Chandramouli V, Norby B, Dicke B, Shah P, Cohen O, Landau BR, Rizza RA. Effects of pioglitazone and metformin on NEFA-induced insulin resistance in type 2 diabetes. Diabetologia 51: 2031–2040, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Basu R, Chandramouli V, Dicke B, Landau B, Rizza R. Obesity and type 2 diabetes impair insulin-induced suppression of glycogenolysis as well as gluconeogenesis. Diabetes 54: 1942–1948, 2005 [DOI] [PubMed] [Google Scholar]
- 5. Basu R, Chandramouli V, Schumann W, Basu A, Landau BR, Rizza RA. Additional evidence that transaldolase exchange, isotope discrimination during the triose-isomerase reaction, or both occur in humans: effects of type 2 diabetes. Diabetes 58: 1539–1543, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Basu R, Di Camillo B, Toffolo G, Basu A, Shah P, Vella A, Rizza R, Cobelli C. Use of a novel triple-tracer approach to assess postprandial glucose metabolism. Am J Physiol Endocrinol Metab 284: E55–E69, 2003 [DOI] [PubMed] [Google Scholar]
- 7. Basu R, Schwenk F, Rizza RA. Both fasting glucose production and disappearance are abnormal in people with “mild” and “severe” type 2 diabetes. Am J Physiol Endocrinol Metab 287: E55–E62, 2004 [DOI] [PubMed] [Google Scholar]
- 8. Bock G, Chittilapilly E, Basu R, Toffolo G, Cobelli C, Chandramouli V, Landau BR, Rizza RA. Contribution of hepatic and extrahepatic insulin resistance to the pathogenesis of impaired fasting glucose: role of increased rates of gluconeogenesis. Diabetes 56: 1703–1711, 2007 [DOI] [PubMed] [Google Scholar]
- 9. Bock G, Schumann WC, Basu R, Burgess SC, Yan Z, Chandramouli V, Rizza RA, Landau BR. Evidence that processes other than gluconeogenesis may influence the ratio of deuterium on the fifth and third carbons of glucose: implications for the use of 2H2O to measure gluconeogenesis in humans. Diabetes 57: 50–55, 2008 [DOI] [PubMed] [Google Scholar]
- 10. Boden G, Chen X, Stein TP. Gluconeogenesis in moderately and severely hyperglycemic patients with type 2 diabetes mellitus. Am J Physiol Endocrinol Metab 280: E23–E30, 2001 [DOI] [PubMed] [Google Scholar]
- 11. Chandramouli V, Ekberg K, Schumann WC, Kalhan SC, Wahren J, Landau BR. Quantifying gluconeogenesis during fasting. Am J Physiol Endocrinol Metab 273: E1209–E1215, 1997 [DOI] [PubMed] [Google Scholar]
- 12. Chen X, Iqbal N, Boden G. The effects of free fatty acids on gluconeogenesis and glycogenolysis in normal subjects. J Clin Invest 103: 365–372, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Edgerton DS, Cardin S, Emshwiller M, Neal D, Chandramouli V, Schumann WC, Landau BR, Rossetti L, Cherrington AD. Small increases in insulin inhibit hepatic glucose production solely caused by an effect on glycogen metabolism. Diabetes 50: 1872–1882, 2001 [DOI] [PubMed] [Google Scholar]
- 14. Ekberg K, Chandramouli V, Kumaran K, Schumann WC, Wahren J, Landau BR. Gluconeogenesis and glucuronidation in liver in vivo and the heterogeneity of hepatocyte function. J Biol Chem 270: 21715–21717, 1995 [DOI] [PubMed] [Google Scholar]
- 15. Fletcher SJ, Herlihy JM, Albery WJ, Knowles JR. Energetics of triosephosphate isomerase: the appearance of solvent tritium in substrate glyceraldehyde 3-phosphate and in product. Biochemistry 15: 5612–5617, 1976 [DOI] [PubMed] [Google Scholar]
- 16. Gastaldelli A, Baldi S, Pettiti M, Toschi E, Camastra S, Natali A, Landau BR, Ferrannini E. Influence of obesity and type 2 diabetes on gluconeogenesis and glucose output in humans: a quantitative study. Diabetes 49: 1367–1373, 2000 [DOI] [PubMed] [Google Scholar]
- 17. Gastaldelli A, Toschi E, Pettiti M, Frascerra S, Quiñones-Galvan A, Sironi AM, Natali A, Ferrannini E. Effect of physiological hyperinsulinemia on gluconeogenesis in nondiabetic subjects and in type 2 diabetic patients. Diabetes 50: 1807–1812, 2001 [DOI] [PubMed] [Google Scholar]
- 18. Haymond MW, Sunehag AL. The reciprocal pool model for the measurement of gluconeogenesis by use of [U-13C]glucose. Am J Physiol Endocrinol Metab 278: E140–E145, 2000 [DOI] [PubMed] [Google Scholar]
- 19. Hellerstein MK, Christiansen M, Kaempfer S, Kletke C, Wu K, Reid JS, Mulligan K, Hellerstein NS, Shackleton CH. Measurement of de novo hepatic lipogenesis in humans using stable isotopes. J Clin Invest 87: 1841–1852, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hellerstein MK, Neese RA, Linfoot P, Christiansen M, Turner S, Letscher A. Hepatic gluconeogenic fluxes and glycogen turnover during fasting in humans. A stable isotope study. J Clin Invest 100: 1305–1319, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jones JG, Barosa C, Gomes F, Mendes AC, Delgado TC, Diogo L, Darcia P, Bastos M, Barros L, Fagulha A, Baptista C, Carvalheiro M, Caldeira MM. NMR derivatives for quantification of 2H and 13C-enrichment of human glucuronide from metabolic tracers. J Carb Chem 25: 203–217, 2006 [Google Scholar]
- 22. Jones JG, Fagulha A, Barosa C, Bastos M, Baptista C, Caldeira MM, Carvalheiro M. Noninasive analysis of hepatic glycogen kinetics before and after brakfast with deuterated water and acetaminophen. Diabetes 55: 2294–2300, 2006 [DOI] [PubMed] [Google Scholar]
- 23. Jones JG, Garcia P, Barosa C, Delgado TC, Caldeira MM, Diogo L. Quantification of hepatic transaldolase exchange activity and its effects on tracer measurements of indirect pathway flux in humans. Magn Reson Med 59: 423–429, 2008 [DOI] [PubMed] [Google Scholar]
- 24. Landau B, Bartsch GE. Estimations of pathway contributions to glucose metabolism and the transaldolase reactions. J Biol Chem 241: 741–749, 1966 [PubMed] [Google Scholar]
- 25. Landau BR, Fernandez CA, Previs SF, Ekberg K, Chandramouli V, Wahren J, Kalhan SC, Brunengraber H. A limitation in the use of mass isotopomer distributions to measure gluconeogensis in fasting humans. Am J Physiol Endocrinol Metab 269: E18–E26, 1995 [DOI] [PubMed] [Google Scholar]
- 26. Landau BR, Hastings AB, Nesbett FB. Origin of glucose and glycogen carbons formed from C14-labeled pyruvate by livers of normal and diabetic rats. J Biol Chem 214: 525–535, 1955 [PubMed] [Google Scholar]
- 27. Landau BR, Wahren J, Chandramouli V, Schumann WC, Ekberg K, Kalhan SC. Use of 2H2O for estimating rates of gluconeogenesis. Application to the fasted state. J Clin Invest 95: 172–178, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Landau BR, Wahren J, Chandramouli V, Schumann WC, Ekberg K, Kalhan SC. Contributions of gluconeogenesis to glucose production in the fasted state. J Clin Invest 98: 378–385, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Magnusson I, Schumann WC, Bartsch GE, Chandramouli V, Kumaran K, Wahren J, Landau BR. Noninvasive tracing of Krebs cycle metabolism in liver. J Biol Chem 266: 6975–6984, 1991 [PubMed] [Google Scholar]
- 30. Rieder SV, Rose IA. The mechanism of the triosephosphate isomerase reaction. J Biol Chem 234: 1007–1010, 1959 [PubMed] [Google Scholar]
- 31. Rizza RA, Mandarino LJ, Gerich JE. Dose-response characteristics for effects of insulin on production and utilization of glucose in man. Am J Physiol Endocrinol Metab 240: E630–E639, 1981 [DOI] [PubMed] [Google Scholar]
- 32. Roden M, Stingl H, Chandramouli V, Schumann WC, Hofer A, Landau BR, Nowotny P, Waldhäusl W, Shulman GI. Effects of free fatty acid elevation on postabsorptive endogenous glucose production and gluconeogenesis in humans. Diabetes 49: 701–707, 2000 [DOI] [PubMed] [Google Scholar]
- 33. Rothman DL, Magnusson I, Katz LD, Shulman RG, Shulman GI. Quantitation of hepatic glycogenolysis and gluconeogenesis in fasting humans with 13C NMR. Science 254: 573–576, 1991 [DOI] [PubMed] [Google Scholar]
- 34. Steele R, Wall J, DeBodo R, Altszuler N. Measurement of size and turnover rate of body glucose pool by the isotope dilution method. Am J Physiol 187: 15–24, 1956 [DOI] [PubMed] [Google Scholar]
- 35. Steiner KE, Mouton SM, Bowles CR, Williams PE, Cherrington AD. The relative importance of first- and second-phase isulin secretion in countering the action of glucagon on glucose turnover in the conscious dog. Diabetes 31: 964–972, 1982 [DOI] [PubMed] [Google Scholar]
- 36. Vella A, Shah P, Basu R, Basu A, Camilleri M, Schwenk WF, Rizza RA. Type I diabetes mellitus does not alter initial splanchnic glucose extraction or hepatic UDP-glucose flux during enteral glucose administration. Diabetologia 44: 729–737, 2001 [DOI] [PubMed] [Google Scholar]