Abstract
Adipose tissue inflammation and reduced pancreatic β-cell function are key issues in the development of cardiovascular disease and progressive metabolic dysfunction in type 2 diabetes mellitus. The aim of this study was to determine the effect of the DPP IV inhibitor sitagliptin on adipose tissue and pancreatic islet inflammation in a diet-induced obesity model. C57Bl/6J mice were placed on a high-fat (60% kcal fat) diet for 12 wk, with or without sitagliptin (4 g/kg) as a food admix. Sitagliptin significantly reduced fasting blood glucose by 21% as well as insulin by ∼25%. Sitagliptin treatment reduced body weight without changes in overall body mass index or in the epididymal and retroperitoneal fat mass. However, sitagliptin treatment led to triple the number of small adipocytes despite reducing the number of the very large adipocytes. Sitagliptin significantly reduced inflammation in the adipose tissue and pancreatic islet. Macrophage infiltration in adipose tissue evaluated by immunostaining for Mac2 was reduced by sitagliptin (P < 0.01), as was the percentage of CD11b+/F4/80+ cells in the stromal vascular fraction (P < 0.02). Sitagliptin also reduced adipocyte mRNA expression of inflammatory genes, including IL-6, TNFα, IL-12(p35), and IL-12(p40), 2.5- to fivefold as well as 12-lipoxygenase protein expression. Pancreatic islets were isolated from animals after treatments. Sitagliptin significantly reduced mRNA expression of the following inflammatory cytokines: MCP-1 (3.3-fold), IL-6 (2-fold), IL-12(p40) (2.2-fold), IL-12(p35) (5-fold, P < 0.01), and IP-10 (2-fold). Collectively, the results indicate that sitagliptin has anti-inflammatory effects in adipose tissue and in pancreatic islets that accompany the insulinotropic effect.
Keywords: visceral fat, cytokines, macrophages, 12/15-lipoxygenase, insulin resistance
obesity is frequently associated with type 2 diabetes, and both conditions are characterized by chronic low-grade inflammation (47). Increased inflammation in adipose tissue and in the pancreatic islets is proposed as a key contributor to insulin resistance and type 2 diabetes, yet the mechanisms are not completely elucidated. Dipeptidyl peptidase IV (DPP IV) inhibitors have recently become widely used for treatment of type 2 diabetes. Sitagliptin is a commonly used DPP IV inhibitor in type 2 diabetic patients, and numerous randomized control trials have proved efficient in increasing insulin secretion in a glucose-dependent manner (20). In vivo DPP IV inhibition improves glucose homeostasis via multiple mechanisms. One of the mechanisms explaining the insulinotropic actions of sitagliptin is via an increase in the circulating incretin hormone glucagon-like peptide (GLP)-1, which is well known to suppress glucagon secretion and enhance insulin secretion (3, 15). In addition, in animal models of diabetes, sitagliptin showed favorable actions on islet and β-cell mass, morphology, and survival (21). Islet inflammation is an important contributor to islet and β-cell dysfunction in type 2 diabetes (17, 23). Several inflammatory pathways were reported to have relevance for impairment of islet and β-cell function and survival, including the lipoxygenase pathway (12, 36) and toll receptor activation (18, 24). A recent report indicates that GLP-2 receptor impacts on the susceptibility of islet damage in response to systemic inflammation (6). This raises the possibility that sitagliptin, in addition to the already known mechanisms, may act to improve islet and β-cell function via a reduction of systemic and/or local inflammation.
Whereas DPP IV inhibition is well known to improve glycemic control in type 2 diabetes by mechanisms targeting mainly the pancreatic islet or liver function, considerably less is known about the effect of DPP IV inhibition on peripheral tissues that are key for the control of insulin sensitivity, such as the adipose tissue. Increasing evidence indicates that adipose tissue inflammation is an important determinant of insulin resistance, diabetes, and cardiovascular disease (31, 53). In particular, visceral adiposity in obesity is associated with increased inflammation and altered adipocyte metabolism in both mice and humans. DPP IV has been identified in human and mouse adipose tissue, and both the GLP-1 and gastric inhibitory polypeptide (GIP) receptors were reported for murine adipocytes (51, 59). Recently, a role for DPP IV was emphasized in potentiating the action of neuropeptide Y in isolated human adipocytes (32). Collectively, the data suggest a potentially important role for DPP IV (CD26) in the regulation of adipocyte metabolism by modulation of lipolysis and insulin sensitivity. Immune cell infiltration, notably of different subpopulations of macrophages and T cells, was recently shown to correlate with the development and maintenance of insulin resistance and diabetes in obesity (8, 26, 41, 56). Recently, sitagliptin treatment in nonobese diabetic mice was shown to decrease CD4+ T cell migration through incretin-dependent and -independent mechanisms (30). Also, DPP IV is increased in blood monocytes of obese human subjects (35). It is possible that inhibition of intrinsic DDP IV activity on adipocytes and/or immune cells may reduce immune cell activation and trafficking in the adipose tissue.
In this study, we determined the concurrent effect of sitagliptin treatment on inflammation in pancreatic islets and adipose tissue and effects on pancreatic function and adipose tissue remodeling in a model of diet-induced obesity. We measured inflammatory cytokine expression in pancreatic islets and adipocytes and determined macrophage infiltration in adipose tissue. The results indicate that sitagliptin improves glucose tolerance and ameliorates inflammation in both pancreatic islets and adipose tissue by reducing inflammatory cytokine expression and macrophage infiltration. Our study is the first to address the anti-inflammatory effects of sitagliptin treatment on both islets and adipose tissue in a mouse model of diet-induced obesity and insulin resistance.
EXPERIMENTAL PROCEDURES
Animals and treatments.
All procedures involving animals were approved by the Institutional Animal Care and Use Committee of Eastern Virginia Medical School. Male C57Bl/6J mice, 8 wk of age, were purchased from The Jackson Laboratory (Bar Harbor, ME) and randomly divided into two groups, those on a high-fat diet (60% kcal fat; Research Diets, New Brunswick, NJ) supplemented with 4 g/kg des-fluoro-sitagliptin (a sitagliptin analog provided by Merck Research Laboratories, Rahway, NJ) or on a high-fat diet only. Treatments (diet and sitagliptin) were continued for 12 wk for all mice. Mice on a low-fat diet, isocaloric with the high-fat diet (11% kcal fat; Research Diets), were treated with the same dose of sitagliptin or left untreated and were used as controls in some of the experiments. Mice were housed in a pathogen-free facility and caged individually, and food and water were provided ad libitum throughout the experiment. Body weight and food intake were measured weekly. Two separate trials were performed under identical experimental conditions, with each trial containing six to eight mice per experimental group. The body mass index (BMI) was calculated as described by others (42, 48).
Metabolic measurements.
Glucose and insulin tolerance tests were performed as described previously (9, 43). Two tests were performed in both of the mouse groups, the first after 1 wk on a high-fat diet and the second after 12 wk on a high-fat diet. For glucose tolerance tests (GTT), mice were fasted overnight and injected the following morning intraperitoneally (ip) with 2 g/kg glucose. Blood glucose was measured with an UltraTouch glucometer from cut tail tips at 0, 10, 20, 30, 60, 90, and 120 min following glucose injection. The insulin tolerance test (ITT) was performed in randomly fed mice by 1 U/kg ip insulin injection. Blood glucose was measured in tail vein samples collected at baseline and following insulin injection at 15, 30, 45, and 60 min. GTT and ITT were performed on the same set of mice 5 days apart. Plasma insulin was measured using a commercially available ELISA kit from Mercodia (Uppsala, Sweden) according to the manufacturer's instructions.
Pancreatic islet isolation and in vitro glucose-stimulated insulin secretion.
Pancreatic islets were isolated as described previously (43). Briefly, the pancreas was perfused through the common bile duct with 1.4 mg/ml collagenase P (Roche Applied Science) and then incubated briefly at room temperature and separated further from acinar tissue using a Histopaque 1077 gradient. All islets were incubated overnight in RPMI 1640 before the glucose-stimulated insulin secretion (GSIS) experiment. For GSIS, islets were incubated in Krebs-Ringer bicarbonate buffer (KRB) supplemented with 3 mM glucose for 1 h, followed by an additional 1-h incubation in KRB containing 28 mM glucose, as described previously (43). The supernatant was collected after each of the treatments and insulin was measured by an ELISA method (Mercodia).
Adipose tissue and pancreatic islet morphometry.
Epididymal tissue was collected in 10% buffered formalin, fixed overnight, and embedded in paraffin. Pancreatic tissue was fixed in 4% paraformaldehyde for 4 h, postfixed in 70% ethanol overnight, and then processed and embedded in paraffin. Hematoxylin and eosin staining was used for adipocyte and islet morphometry. Three representative images per section were obtained for a total of six tissue sections per mouse. For islet morphometric analysis the whole pancreas was embedded, and serial sections were performed on the entire tissue. Every seventh section (5 μm thick) was used to quantify islet area and size, as described previously (7). Four mice per experimental group were randomly selected and analyzed. Images were taken using a Zeiss Plan Apochromat ×20 objective, and adipocyte and islet area and number were obtained using the ImageJ software (National Institutes of Health, Bethesda, MD).
Adipocyte and stromal vascular fraction preparation.
Samples of epididymal adipose tissue (0.1–0.3 g) were digested with collagenase as described before, with minor modifications (13, 53). Briefly, finely minced adipose tissue was incubated for 1 h at 37°C in a shaking water bath in KRH buffer supplemented with 1% BSA, 20 nmol/l adenosine, and 1 mg/ml collagenase I. The resulting cell suspension was filtered through a 0.4-mm Nitex nylon mesh (Sefar Filtration, New York, NY). The floating adipocytes were collected and washed, and the infranatant was removed and centrifuged at 500 g for 5 min to pellet the stromal vascular fraction (SVF). Adipocytes were further used for real-time PCR cytokine expression, and SVF was used for flow cytometry to determine macrophage content.
Flow cytometry.
The SVF pellet obtained following adipose tissue digestion was suspended in fluorescence-activated cell sorting buffer following erythrocyte lysis. Counted SVF cells were incubated for 30 min at room temperature with the following fluorophore-conjugated primary antibodies: CD11b-FITC (BD Pharmigen, San Jose, CA), CD45-PerCP (BD Pharmigen), I-Ab-PE (BioLegend, San Diego, CA), and F4/80-Alexa 647 (BioLegend). Cells were analyzed on a BD upgraded fluorescence-activated cell sorting Caliber Flow Cytometer (8 colors) using FlowJo software (Tree Star, Ashland, OR). The macrophage content was defined by both F4/80- and CD11b-positive cells in a CD45-positive gate.
Real-time PCR.
Adipocytes isolated from epididymal samples (as described above) were lysed in Trizol (Invitrogen, Carlsbad, CA), and RNA was isolated using a RNeasy kit from Qiagen (Valencia, CA) according to the manufacturer's instructions. Isolated pancreatic islets were lysed in Trizol, and RNA was separated using the RiboPure kit (Ambion, Austin, TX). cDNA was prepared using a protocol, enzymes, and reagents provided by the Promega Reverse Transcription System (Promega, Madison, WI). Real-time PCR was performed using Taqman probes from Applied Biosystems (Carlsbad, CA). For assessing leukocyte 12-lipoxygenase expression, SYBR green with custom design primers was used. (forward: 5′-ctctcaaggcctgttcagga-3′; reverse: 5′-gtccattgtccccagaacct-3′). All thermal cycling was performed in the CFX96 Thermal Cycler (Bio-Rad, Hercules, CA). β-Actin was used to normalize the data. Results were expressed by the 2−ΔΔCT method, with the nontreated group used as a control.
Immunohistochemistry.
Epididymal adipose tissue was fixed in 10% buffered formalin overnight, then embedded in paraffin, and, following antigen retrieval, incubated with one of the following primary antibodies overnight at 4°C: rat anti-MAC2 antibody (1:100 dilution; Cedarlane Laboratories, Burlington, NC) or rabbit anti-12-lipoxygenase (1:100 dilution; Santa Cruz Biotechnology, Santa Cruz, CA). Detection was performed using the avidin-biotin peroxidase method, and slides were counterstained using Mayer's hematoxylin. Tissue sections incubated with goat IgG (Pierce) instead of the primary antibody were used as method controls. To quantify MAC2 immunostaining, labeled cells were counted in eight to 10 different fields for each sample and expressed as number of positive cells/area unit. All pictures were captured with an Olympus microscope using ×200 magnification. To determine 12/15-lipoxygenase staining intensity, MetaMorph software version 6.3 (Molecular Devices, Downingtown, PA) was used, and data were normalized/section area analyzed.
Statistical analysis.
Statistical analysis was performed using GraphPad Prism Software (GraphPad Software, La Jolla, CA). Student's t-test paired analysis was used for GSIS data and unpaired analysis for all other experiments. Data were expressed as means ± SE, and the null value was rejected for a P value <0.05.
RESULTS
Effects of sitagliptin on body weight, adiposity, and glucose homeostasis.
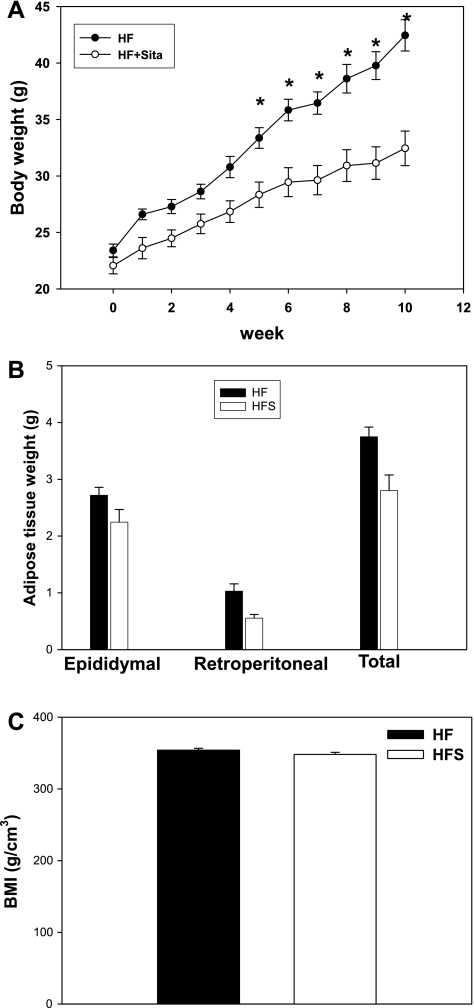
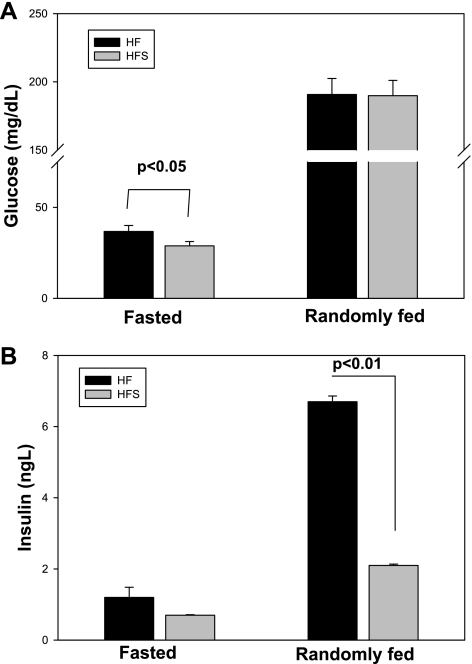
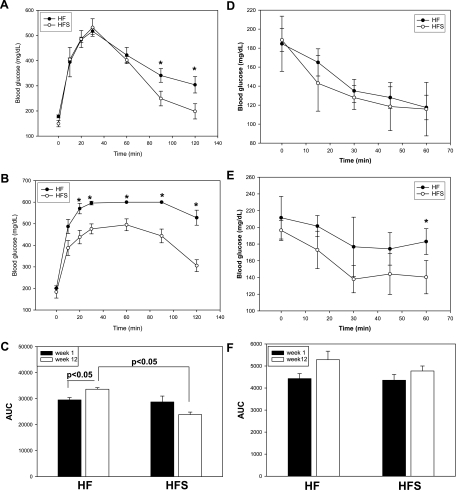
C57Bl/6J male mice were placed on a 60% kcal fat diet for 12 wk, and sitagliptin (0.4 g/kg) was simultaneously administered as a food admix to one of the groups. Body weight was measured weekly. As shown in Fig. 1A, starting with week 5 of diet, sitagliptin-treated mice had significantly lower body weights compared with the untreated high-fed group. There was no difference in the major visceral fat pad weights, as shown in Fig. 1B, although there was a strong trend for a lower total visceral adiposity in the sitagliptin-treated group (n = 0.067). The comparable adiposity was also reflected in the BMI, which shows no significant difference between the treated and control groups (Fig. 1C). In a group of lean mice on a low-fat diet, sitagliptin did not have a significant effect on body weight, and body weight curves were comparable with the high-fat sitagliptin-treated group (Supplemental Fig. S1A; Supplemental Material for this article can be found online at the AJP-Endocrinology and Metabolism web site). Also, food intake was not significantly different between the treated and nontreated groups (Supplemental Fig. S1B). To determine whether sitagliptin exerted insulinotropic effects or affected insulin sensitivity, we measured glucose and insulin tolerance in mice on a high-fat diet with or without sitagliptin treatment. Fasting blood glucose was significantly lower in sitagliptin-treated mice, with no difference in the blood glucose between groups in randomly fed mice (Fig. 2A). Also, plasma insulin concentrations were significantly lower in the randomly fed mice treated with sitagliptin, whereas the fasting insulin levels tended to be lower in the sitagliptin-treated group (Fig. 2B). The postprandial hyperinsulinemia and increased fasting plasma glucose in high-fat-fed obese mice indicates the presence of impaired glucose tolerance that is ameliorated by sitagliptin treatment. These findings were confirmed by the GTT. GTT showed a significant difference in the area under the curve (AUC) between the beginning and end of the high-fat diet in the control group of mice (Fig. 3, A and C). However, in the sitagliptin-treated mice there was no significant difference in glucose tolerance between weeks 1 and 12 (Fig. 3, A and C). Also, the AUC was significantly higher in the control vs. sitagliptin-treated group at the end of the study (Fig. 3, B and C). The result suggests that endogenous insulin secretion is impaired in response to a glucose challenge in the high-fat group, and this impairment is not seen in the sitagliptin-treated group. Insulin tolerance tests showed no significant difference between the treated and nontreated groups either at the beginning or the end of the dietary regimens (Fig. 3, D–F). The results indicate that sitagliptin lowered circulating insulin in randomly fed mice and reduced fasting hyperglycemia in the obese mice on the high-fat diet.
Fig. 1.
Effect of sitagliptin on body weight and adiposity. A: weekly measurements of body weight in mice on high-fat diets with or without sitagliptin treatment. B: weight of total visceral fat and of the 2 major visceral depots (epididymal and retroperitoneal). C: body mass index (BMI) calculated according to the following formula: [3√BW (g)/length (mm)] × 104. Data are from n = 10 mice/group and expressed as means ± SE; the null hypothesis was rejected for a P value <0.05. HF, control group on high fat; HFS, sitagliptin-treated group; HF + Sita, mice on HF diet with sitagliptin treatment. *Statistically significant compared with HFS.
Fig. 2.
Effect of sitagliptin treatment on plasma glucose and insulin. A: fasted and fed plasma glucose were determined using a glucometer as baseline values in the insulin and glucose tolerance tests and at the end of the study. B: plasma insulin concentration was determined at the end of the study by immunoassay. Results are from n = 12–14 mice/group and expressed as means ± SE.
Fig. 3.
Glucose (GTT) and insulin tolerance tests (ITT) in control diet alone and with sitagliptin treatment groups. GTT was determined in mice with or without sitagliptin treatment after the 1st week of diet (A) and during the last week (week 12; B). Results are expressed as blood glucose concentration (mg/dl). C: areas under the curve (AUC) were calculated based on the GTT curves. ITT was measured in randomly fed mice at week 1 (D) and at week 12 (E) of the study. Results are expressed as blood glucose concentration (mg/dl). F: AUC was calculated based on the respective ITT curves. Results are from n = 12–14 mice/group and expressed as means ± SE. *Statistically significant (P < 0.05) compared with HFS group.
Sitagliptin improves GSIS in isolated islets.
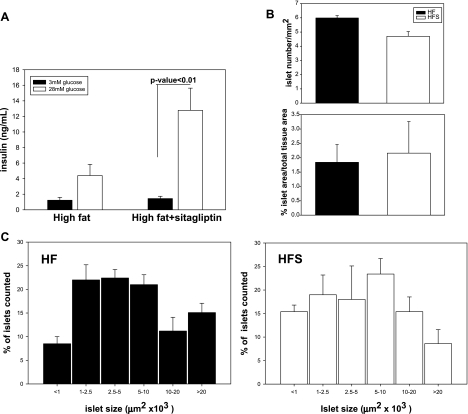
To directly assess whether sitagliptin treatment improves insulin secretion in response to an increase in glucose, isolated islets were incubated in 3 and 28 mmol/l glucose. The basal insulin secretion (3 mmol/l) was not different between sitagliptin-treated and control groups (Fig. 4A). The islets from control mice responded with an increase in insulin secreted in the medium when challenged with 28 mmol/l glucose; however, the increase was not significant (Fig. 4A). In contrast, islets from sitagliptin-treated mice showed a significantly greater increase in insulin release during a 28 mmol/l glucose exposure. To further determine whether sitagliptin had effects on islet morphology or number, paraffin-embedded sections were stained with hematoxylin and eosin and analyzed for islet number and area, neither of which showed differences between sitagliptin-treated and control groups (Fig. 4B). However, size distribution analysis showed a decrease in the percentage of large islets in the sitagliptin-treated group vs. nontreated high-fat control (area >0.02 mm2, P < 0.05) and a relative increase in the percentage of small islets (area <1,000 μm2, P < 0.05) (Fig. 4C).
Fig. 4.
Pancreatic islet function and morphometry. A: sitagliptin treatment improves in vitro glucose-stimulated insulin secretion in islets. Islets were treated with 3 mmol/l glucose for 1 h, followed by 28 mmol/l glucose for an additional hour. Insulin was measured in medium at both time points using an ELISA kit. Fifty islets were measured in each condition and assayed in triplicate. Data are expressed as means ± SE from 5 mice/group. Islet morphometry was determined in paraffin-embedded pancreatic sections stained with hematoxylin and eosin. B: islet size and number were not significantly changed by sitagliptin treatment. C: islet size distribution shows that sitagliptin treatment tends to reduce the number of hyperplasic islets (area >0.02 mm2), whereas it increases the number of small islets. Data represent means ± SE from 5 mice/group.
Sitagliptin reduces inflammatory cytokine expression in the pancreatic islets.
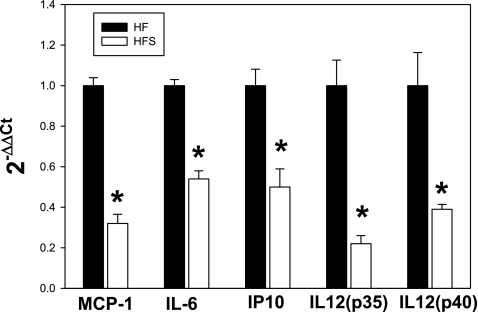
We next examined mRNA expression of several key proinflammatory cytokines in islets isolated from sitagliptin and control groups. This set of cytokines was chosen on the basis of previous data on C57Bl6/J mice showing an elevation of the proinflammatory cytokines in the IL-6/IL-12 pathways and a potential pathogenic role in insulin resistance and type 2 diabetes. The mRNA expression of the cytokines and chemokines measured in this study were all significantly increased in the high-fat diet-fed mice compared with lean, low-fat diet-fed counterparts (Supplemental Fig. S2A). For all of the cytokines tested, islets from sitagliptin-treated mice on the high-fat diet showed significantly reduced expression of the proinflammatory cytokines compared with the control group (Fig. 5). The most prominent decreases in mRNA expression were found for monocyte chemoattractant protein-1 (MCP-1; 3.3-fold), IL-12(p35) (5-fold), and IL-12(p40) (2.2-fold) (P < 0.01). Also, approximately twofold decreases in expression were detected for IL-6 and IP-10 (P < 0.05). In mice on the low-fat diet, sitagliptin treatment did not affect mRNA expression of the cytokines examined, suggesting a specific diet-related effect of the drug (Supplemental Fig. S2B).
Fig. 5.
Sitagliptin treatment reduces inflammatory cytokine expression in pancreatic islets. Data are normalized to β-actin, and the fold change in expression in the sitagliptin vs. control group was calculated using the 2−ΔΔCT method. Results are from n = 7–10 mice/group. MCP-1, monocyte chemoattractant protein-1. *Statistically significant (P < 0.05) compared with HF group.
Effect of sitagliptin on adipocyte inflammation.
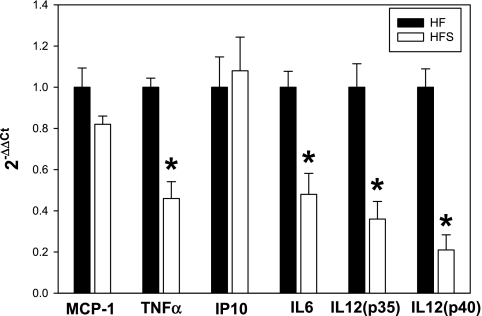
To investigate whether sitagliptin reduces inflammation in tissues other than pancreatic islets that are potential contributors to impaired glucose homeostasis, we examined proinflammatory cytokine expression in visceral adipocytes isolated from mice with or without sitagliptin treatment (Fig. 6). Also, leukocyte 12/15-lipoxygenase expression was measured (Fig. 7) since previous work from our group indicated a role of the enzyme in the adipose tissue inflammation and insulin resistance (43). Of the cytokines examined, there were significant ∼2.5-fold decreases in IL-6 and TNFα expression (P < 0.05) and a 4.5- to fivefold decrease in expression of IL-12(p35) and IL-12(p40) (P < 0.05) in the adipocytes isolated from the sitagliptin-treated group vs. controls (Fig. 6). There was no significant difference in mRNA expression of the chemokines MCP-1 and C-X-C motif chemokine 10 (IP-10) (Fig. 6). In mice on the low-fat diet, mRNA expression of TNFα, IL-6, IL-12(p35), and IL-12(p40) were significantly lower, by two- to 3.4-fold, compared with obese mice on the high-fat diet (Supplemental Fig. S3A). In addition, sitagliptin treatment of mice on the low-fat diet did not significantly change expression of any of the cytokines examined (Supplemental Fig. S3B). We also examined protein expression of 12/15-lipoxygenase enzyme in total adipose tissue by immunohistochemistry and mRNA expression by real-time PCR in isolated adipocytes (Fig. 7). The immunopositive cells were seen in both adipocytes (Fig. 7, dotted arrows) and immune cells localizing in crown-like structures (Fig. 7, solid arrows). Morphometric analysis revealed a significantly reduced level (P < 0.01) of 12/15-lipoxygenase protein in the adipose tissue from the sitagliptin-treated group (Fig. 7). Also, leukocyte 12/15-lipoxygenase levels were significantly reduced in adipocytes isolated from adipose tissue of sitagliptin-treated obese mice compared with untreated controls. Moreover, 12-lipoxygenase protein expression in adipose tissue of lean mice was not changed by sitagliptin treatment, showing a selective effect of the latter in adipose tissue of obese mice (Supplemental Fig. S4).
Fig. 6.
Sitagliptin reduces proinflammatory cytokine expression in adipocytes. Adipocytes were isolated from total epididymal tissue by collagenase digestion, and gene expression was analyzed by real-time PCR. Data are normalized to β-actin, and the fold change in expression in the sitagliptin vs. control group was calculated using the 2−ΔΔCT method. Results are from n = 7–10 mice/group. *Statistically significant (P < 0.05) compared to HF group.
Fig. 7.
Sitagliptin reduces 12-lipoxygenase (12-LO) mRNA expression in adipocytes (A) and reduces protein expression in adipose tissue (B). 12-LO expression was quantified by real-time PCR using specific primers and normalized to β-actin. Results are expressed as the mean of 1/ΔCT ± SE from n = 5 mice/group. Paraffin-embedded adipose tissue sections were immunostained using a 12-LO antibody. Representative immunostaining illustrates reduction of the immunostaining frequency in the sitagliptin-treated sample. Immunostaining is possibly associated with both the immune cells in the crown-like structures (solid arrows) and adipocytes (dotted arrows). Intensity of staining was quantified using the Metamorph software and expressed as relative units of intensity normalized to area analyzed/section. A number of 4–6 sections from 4 mice/group were analyzed. Data are expressed as means ± SE.
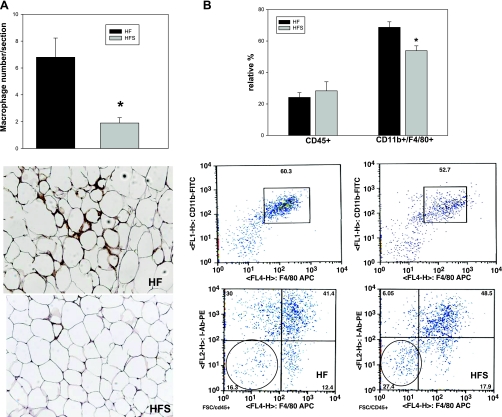
Sitagliptin reduces macrophage infiltration in adipose tissue.
A hallmark of adipose tissue inflammation is immune cell infiltration. Macrophages are among the most abundant immune cells infiltrating adipose tissue after chronic high-fat feeding. We determined macrophage relative abundance in adipose tissue from high-fat-fed mouse groups using both immunohistochemistry and flow cytometry (Fig. 8, A and B). Immunopositive cells for Mac2 were significantly more numerous in the control compared with the sitagliptin-treated group, suggesting reduced inflammation by sitagliptin treatment (Fig. 8A). Furthermore, we digested the adipose tissue and analyzed the stromal vascular fraction component for the presence of macrophages and lymphoid cells. Macrophages were identified as cells positive for CD45/F4/80/CD11b (Fig. 8B). The relative percentage of macrophages of the total CD45+ cells was 71.95 ± 2.96 in the high-fat group and was significantly reduced in the sitagliptin-treated group to 62.52 ± 5.85 compared with controls (P = 0.019). The number of CD45+ cells normalized to total adipose tissue was not significantly different between the two groups (data not shown). Also, the lymphoid cells characterized as CD45+/I-Ab−/F4/80− were comparable between the two groups (Fig. 8B). This indicates that, in addition to ameliorating inflammation in adipocytes, sitagliptin also reduced infiltration of macrophages in the adipose tissue in response to the high-fat feeding. In control lean mice, the number of macrophages infiltrating the adipose tissue was significantly lower compared with obese mice (1.22 ± 0.38 vs. 6.8 ± 1.1), and sitagliptin treatment did not significantly change the macrophage number (Supplemental Fig. S5A). This finding was confirmed by flow cytometry analysis of the CD11b+/F4/80+ double-positive cells that were not significantly changed by sitagliptin treatment in the lean mice (Supplemental Fig. S5B).
Fig. 8.
Sitagliptin reduces macrophage infiltration in adipose tissue after high-fat feeding. A: immunostaining using MAC2 antibody was performed in 5–6 mice/group and quantified as described in experimental procedures. Sitagliptin reduces the MAC2 immunostaining and crown-like structures in adipose tissue of high-fat-fed mice. B: flow cytometry of stromal vascular fraction obtained following collagenase digestion of epididymal adipose tissue. Macrophages were identified as double-positive CD11b/F4/80 cells gated for CD45. The lymphoid cells were determined as F4/80/I-Ab double-negative cells gated for CD45. Representative plots are shown. Quantification was performed on samples from n = 5 mice/group and expressed as relative percentage of cells normalized to adipose tissue weight. *Statistically significant (P < 0.05) compared with HF group.
Effect of sitagliptin on adipose tissue morphology.
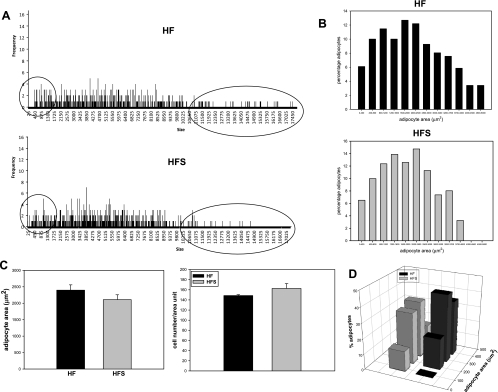
Adipocyte hyperplasia is a known response to high-fat feeding in rodents and is correlated with altered metabolic function such as reduced insulin sensitivity and elevated lipolysis. We analyzed adipocyte number and size to establish whether sitagliptin treatment was associated with changes in adipose tissue morphology (Fig. 9). There was a trend for a reduced mean adipocyte size in the sitagliptin-treated group that was borderline significant (P = 0.058; Fig. 9C). Adipocyte number was not significantly changed by sitagliptin treatment (Fig. 9C). Analysis of adipocyte size distribution revealed an evident reduction in the number of very large adipocytes (area >4,000 μm2) in the sitagliptin-treated mice compared with controls (Fig. 9, A and B). Also, the number of the very small adipocytes (area <500 μm2) was higher in the sitagliptin-treated group (Fig. 9D). As shown in Fig. 1, the total visceral adipose tissue was not changed, which suggests that sitagliptin induced remodeling of visceral fat with lower numbers of very large adipocytes and larger numbers of small adipocytes, which is suggestive of an improved adipocyte metabolic phenotype. Sitagliptin had no significant effect on adipose tissue morphometry in lean control mice (data not shown).
Fig. 9.
Effect of sitagliptin on adipocyte morphometry. Sitagliptin reduces the number of very large adipocytes, whereas it increases the number of small adipocytes. A: representative graphs indicating the size distribution of the measured cells. B: size distribution histograms from 3–5 mice/group. C: adipocyte area and cell number were measured in 3–5 mice/group. D: distribution of the very small adipocyte population measured in 3–5 mice/group.
Taken together, these results show for the first time that sitagliptin can prevent or attenuate inflammation in both pancreatic islets and adipose tissue, which may in part explain the improved glucose tolerance in diet-induced obese mice.
DISCUSSION
Our study shows that in a high-fat-diet model of obesity and insulin resistance, sitagliptin reduces pancreatic islet and adipose tissue inflammation, concurrent with improved glucose metabolism. Also, sitagliptin improves islet function and reduces inflammation in visceral adipose tissue. The data suggest that sitagliptin has anti-inflammatory effects that enhance positive effects for metabolic and vascular function.
Sitagliptin significantly reduced body weight in high-fat-fed mice compared with untreated controls. This effect was somewhat surprising since the majority of the diabetic patients treated with sitagliptin do not experience significant weight loss (2). However, one other report in a similar animal model on high-fat diet reported significant loss of weight following sitagliptin treatment (33). In the same report, the authors found no difference in the oxygen consumption, locomotor activity, or food intake in mice after prolonged DPP IV inhibition by sitagliptin (33). Interestingly, mice with genetic ablation of the Dpp4 gene are resistant to the development of obesity (14). In our study, the reduction in body weight is not accompanied by a reduction in adiposity, although there is a strong trend for reduced adipose mass in sitagliptin-treated mice. Importantly, the effect of sitagliptin is present only in mice fed a high-fat diet. In lean mice, sitagliptin has no effect on body weight, indicating that the drug specifically attenuates body weight gain in response to high-fat feeding.
Several human and animal studies showed that sitagliptin treatment improves glucose tolerance and stimulates pancreatic insulin secretion in response to a glucose load in humans and animal models of type 2 diabetes, insulin resistance, and dietary obesity (1, 20–22, 33, 39). In accord with previous data, our study shows that 12-wk sitagliptin treatment in mice on 60% kcal fat diet improved glucose tolerance, reduced fasted blood glucose, and lowered plasma insulin in randomly fed mice compared with untreated insulin-resistant obese mice. However, in contrast to the study by Lamont and Drucker (33), we did find a significant reduction in glucose excursion during an intraperitoneal glucose tolerance test. One reason for the discrepancy in the results may be the longer treatment time in our study of 12 wk vs. 8 wk in the study by Lamont and Drucker (33). Sitagliptin improves blood glucose control by potentiating the effects of GLP-1 and GIP (21). Continuous, sustained activation of the incretin axis appears to be critical for optimal blood glucose control (34). Therefore, the length of the treatment may be critical in achieving better glucose control by long-term persistence of circulating GLP-1 and explain sitagliptin effect to improve glucose excursion in the intraperitoneal glucose tolerance test in our study.
To determine whether sitagliptin improved glucose-stimulated insulin secretion, we measured insulin secretion in response to low and high glucose in isolated islets (Fig. 4). This result reflects preserved insulin secretory response to a glucose challenge in sitagliptin-treated mice, consistent with an insulinotropic effect of GLP-1, likely elevated by sitagliptin. Also, extensive and sustained sitagliptin treatment may improve pancreatic insulin secretion by preserving islet mass in type 1 and type 2 diabetes and preventing β-cell apoptosis (39, 44). Interestingly, we found a change in the islet size distribution showing a significantly higher percentage of the small islets and reduced relative percentage of the very large islets. Islet hyperplasia is induced by very high-fat diets and is associated with reduced insulin sensitivity (37). This result may explain the better insulin secretory response of the islets from sitagliptin-treated mice in response to an in vitro glucose challenge. It is also possible that improvement in glucose tolerance by sitagliptin is also the result of reduced glucagon production and reduced hepatic glucose production and outputs, as shown previously (22).
Obesity, insulin resistance, and type 2 diabetes are associated with the presence of an inflammatory phenotype in both pancreatic islets and insulin target tissues, notably adipose tissue, in animal models and humans (19, 54).
Accumulating evidence shows that inflammation in the pancreatic islets impairs function and reduces insulin secretion in type 2 diabetes (17, 23). Production of proinflammatory lipid mediators and cytokines has been shown to induce islet apoptosis, reduce insulin secretion, and lead to progressive islet loss (18, 24). Importantly, increased cytokine production was reported in human islets treated in vitro with 12/15-lipoxygenase products such as 12-hydroxyeicosatetraenoic acid generated via the 12-lipoxygenase pathway (36). Also, laser-captured β-cells from patients with type 2 diabetes compared with nondiabetic controls have elevated levels of various cytokines and chemokines (38). Previous findings from our group, as well as results from this study, showed increased production of inflammatory cytokines in the islets of C57Bl/6 mice on high-fat diets (13, 43). Our present results are the first ones to demonstrate that islets from mice treated with sitagliptin have reduced expression of several proinflammatory cytokines and chemokines compared with control untreated mice. Expression of both IL-12 subunits, as well as IL-6 expression, was reduced. IL-12 is a key cytokine driving Th1 lineage via production of IFNγ. Although IFNγ expression was not significantly decreased by sitagliptin, there was a strong trend showing reduced expression (data not shown). The effect of sitagliptin was selective in obese mice, with no effect in lean mice with lower levels of cytokine expression. Interestingly, a recent report shows that IL-12 is increased in serum of type 2 diabetic patients and is correlated with BMI and homeostatic model assessment of insulin resistance (52). Therefore, sitagliptin may have beneficial effects on insulin secretion by also reducing islet IL-12 expression and production. However, additional studies are needed to determine mechanisms that may impact on islet function due to increased expression of the IL-12 pathway in obesity and type 2 diabetes. IL-6 may have both positive and negative metabolic effects, and its role in obesity and type 2 diabetes remains controversial (4, 54). Although the majority of IL-6 is produced by the adipose tissue, pancreatic islets also express low levels of IL-6. We found a significant reduction of IL-6 in the pancreatic islets of sitagliptin-treated mice. In transgenic mice overexpressing IL-6 in pancreatic β-cells, a hyperplastic response and altered islet architecture was reported (10).
In addition to reduced cytokine expression, we also found a significant decrease in expression of the chemokines MCP-1 and IP-10 in islets from sitagliptin-treated mice compared with controls. Islets from patients with type 2 diabetes are infiltrated with macrophages, and human islets exposed to metabolic stress (elevated glucose and palmitate) release increased levels of cytokines and chemokines (25). An increased macrophage infiltration was also reported for the C57Bl/6 mice on high-fat diets (25). MCP-1 is a potent macrophage chemoattractant and contributes to increased inflammation via macrophage islet homing and further cytokine production. MCP-1 can be produced by both the β-cells and the immune cells infiltrating the islets. Also, IP-10 is produced by the β-cell and by immune and vascular cells and is a potent chemoattractant for the T cells. Interestingly, IP-10 is elevated in diabetes (40, 46), and high glucose levels increase IP-10 production by human monocytes (16). Therefore, sitagliptin may reduce IP-10 islet expression indirectly via reducing blood glucose, attenuation of islet immune infiltration, or more direct effects on the β-cell. Consequently, multiple pathways may contribute to reduced chemokine production by sitagliptin in pancreatic islets. Additional studies would be important in elucidating the mechanisms accounting for the effects of sitagliptin on cytokine and chemokine production in pancreatic islets.
Adipose tissue inflammation is a major contributor to insulin resistance seen in central obesity. In accord with previous findings, we showed that proinflammatory cytokines as well as the 12/15-lipoxygenase pathway are increased in C57Bl/6J mice on high-fat diets (13, 43). In this study, we found that sitagliptin treatment reduces adipocyte expression of proinflammatory cytokines IL-6, TNFα, IL-12(p35), and IL-12(p40). These cytokines are known to act locally to impair adipocyte insulin sensitivity as well as systemically affect whole body insulin sensitivity (47, 58). Unlike the effect of sitagliptin in pancreatic islets, expression of MCP-1 and IP-10 was not changed in adipocytes by sitagliptin treatment. However, MCP-1 expression was reduced significantly in total adipose tissue (data not shown), suggesting that nonadipose cells, most likely immune cells, present in adipose tissue are a likely target for sitagliptin. Further studies will be needed to evaluate whether sitagliptin has incretin-dependent or -independent effects on the adipocytes or on the immune cells infiltrating the adipose tissue. DPP IV (CD26) is known to modulate immune function, T cell proliferation, and macrophage migration (28, 45), and DPP IV inhibition reportedly reduced inflammatory cytokine production by the T cells and inhibited transendothelial migration of the latter (27, 45). Interestingly, a recent study showed that sitagliptin decreases NOD mice CD4+ T cell migration through incretin-dependent and -independent pathways (30). We showed that sitagliptin reduced macrophage infiltration in adipose tissue. Reduction of adipose tissue macrophages in obese rodents is positively associated with improved glucose tolerance and insulin sensitivity (47). Importantly, a recent study showed that DDP IV expression is increased in blood monocytes of obese human subjects (35). Therefore, DPP IV inhibition may have an additional therapeutic effect by limiting monocyte migration into adipose tissue and reducing local inflammation.
Previous data showed that 12-lipoxygenase expression is increased in adipose tissue of high-fat-fed, obese, insulin-resistant C57Bl/6J mice, and global deletion of the 12-lipoxygenase reduced adipose tissue inflammation and macrophage infiltration, concurrent with improved glucose tolerance and insulin sensitivity (43). In this study, we showed reduced immunopositive cells for 12/15-lipoxygenase in adipose tissue in sitagliptin-treated obese mice localized mainly in the crown-like structures but also in adipocytes, as further confirmed by mRNA expression data in purified adipocytes. 12-Lipoxygenase impairs insulin signaling in both adipocytes and macrophages (11) and enhances proinflammatory cytokine production (55). Therefore, it is conceivable that the 12-lipoxygenase pathway is one of the targets of sitagliptin and may in part explain the reduction of cytokine production in adipocytes and macrophage infiltration in adipose tissue.
We also found that in sitagliptin-treated mice the relative number of very large hypertrophic adipocytes is reduced, whereas the very small adipocyte population is relatively increased compared with control mice. The functional characteristics of adipose tissue apparently depend on the finer details of the adipose cell distribution (29), with a prevalence of large adipocytes conducive to increased proinflammatory cytokine production (49) and reduced adipocyte insulin sensitivity (5). A recent report also showed that sitagliptin reduces adipocyte hypertrophy in C57Bl/6J mice on high-fat diets (50). The remodeling of adipose tissue in obese mice treated with sitagliptin suggests improved insulin sensitivity and correlates well with the reduced adipose tissue inflammation.
Collectively, our results indicate that sitagliptin treatment in obese insulin-resistant mice is associated with an improved metabolic phenotype and concurrent reduction of inflammation in pancreatic islets and adipose tissue. Therefore, DPP IV reduction may emerge as a beneficial treatment not only for improving insulin sensitivity but also for associated complications of type 2 diabetes characterized by chronic inflammation in adipose tissue and β-cells.
GRANTS
This study was supported in part by National Heart, Lung, and Blood Institute Grant PO1-HL-55798 to J. L. Nadler, A. D. Dobrian, and E. V. Galkina and National Institute of Diabetes and Digestive and Kidney Diseases Grant NIH-RO1-DK-55240 to J. L. Nadler.
DISCLOSURES
This study was also supported in part by an investigator-initiated research grant from Merck (Whitehouse Station, NJ) to J. L. Nadler.
Supplementary Material
ACKNOWLEDGMENTS
We thank Drs. David Taylor-Fishwick and Yumi Imai for their valuable advice on pancreatic islet morphometry and the glucose-stimulated insulin secretion in isolated pancreatic islets.
REFERENCES
- 1. Aaboe K, Knop FK, Vilsboll T, Deacon CF, Holst JJ, Madsbad S, Krarup T. Twelve weeks treatment with the DPP-4 inhibitor, sitagliptin, prevents degradation of peptide YY and improves glucose and non-glucose induced insulin secretion in patients with type 2 diabetes mellitus. Diabetes Obes Metab 12: 323–333, 2010 [DOI] [PubMed] [Google Scholar]
- 2. Ahren B. Dipeptidyl peptidase-4 inhibitors: clinical data and clinical implications. Diabetes Care 30: 1344–1350, 2007 [DOI] [PubMed] [Google Scholar]
- 3. Ahren B, Holst JJ, Martensson H, Balkan B. Improved glucose tolerance and insulin secretion by inhibition of dipeptidyl peptidase IV in mice. Eur J Pharmacol 404: 239–245, 2000 [DOI] [PubMed] [Google Scholar]
- 4. Allen TL, Febbraio MA. IL6 as a mediator of insulin resistance: fat or fiction? Diabetologia 53: 399–402, 2010 [DOI] [PubMed] [Google Scholar]
- 5. Andersson CX, Gustafson B, Hammarstedt A, Hedjazifar S, Smith U. Inflamed adipose tissue, insulin resistance and vascular injury. Diabetes Metab Res Rev 24: 595–603, 2008 [DOI] [PubMed] [Google Scholar]
- 6. Bahrami J, Longuet C, Baggio LL, Li K, Drucker DJ. Glucagon-like peptide-2 receptor modulates islet adaptation to metabolic stress in the ob/ob mouse. Gastroenterology 139: 857–868, 2010 [DOI] [PubMed] [Google Scholar]
- 7. Blondeau B, Garofano A, Czernichow P, Breant B. Age-dependent inability of the endocrine pancreas to adapt to pregnancy: a long-term consequence of perinatal malnutrition in the rat. Endocrinology 140: 4208–4213, 1999 [DOI] [PubMed] [Google Scholar]
- 8. Bouloumie A, Casteilla L, Lafontan M. Adipose tissue lymphocytes and macrophages in obesity and insulin resistance: makers or markers, and which comes first? Arterioscler Thromb Vasc Biol 28: 1211–1213, 2008 [DOI] [PubMed] [Google Scholar]
- 9. Bruning JC, Winnay J, Bonner-Weir S, Taylor SI, Accili D, Kahn CR. Development of a novel polygenic model of NIDDM in mice heterozygous for IR and IRS-1 null alleles. Cell 88: 561–572, 1997 [DOI] [PubMed] [Google Scholar]
- 10. Campbell IL, Hobbs MV, Dockter J, Oldstone MB, Allison J. Islet inflammation and hyperplasia induced by the pancreatic islet-specific overexpression of interleukin-6 in transgenic mice. Am J Pathol 145: 157–166, 1994 [PMC free article] [PubMed] [Google Scholar]
- 11. Chakrabarti SK, Cole BK, Wen Y, Keller SR, Nadler JL. 12/15-lipoxygenase products induce inflammation and impair insulin signaling in 3T3-L1 adipocytes. Obesity (Silver Spring) 17: 1657–1663, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen M, Yang ZD, Smith KM, Carter JD, Nadler JL. Activation of 12-lipoxygenase in proinflammatory cytokine-mediated beta cell toxicity. Diabetologia 48: 486–495, 2005 [DOI] [PubMed] [Google Scholar]
- 13. Cole BK, Keller SR, Wu R, Carter JD, Nadler JL, Nunemaker CS. Valsartan protects pancreatic islets and adipose tissue from the inflammatory and metabolic consequences of a high-fat diet in mice. Hypertension 55: 715–721, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Conarello SL, Li Z, Ronan J, Roy RS, Zhu L, Jiang G, Liu F, Woods J, Zycband E, Moller DE, Thornberry NA, Zhang BB. Mice lacking dipeptidyl peptidase IV are protected against obesity and insulin resistance. Proc Natl Acad Sci USA 100: 6825–6830, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Deacon CF, Danielsen P, Klarskov L, Olesen M, Holst JJ. Dipeptidyl peptidase IV inhibition reduces the degradation and clearance of GIP and potentiates its insulinotropic and antihyperglycemic effects in anesthetized pigs. Diabetes 50: 1588–1597, 2001 [DOI] [PubMed] [Google Scholar]
- 16. Devaraj S, Jialal I. Increased secretion of IP-10 from monocytes under hyperglycemia is via the TLR2 and TLR4 pathway. Cytokine 47: 6–10, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Donath MY, Boni-Schnetzler M, Ellingsgaard H, Ehses JA. Islet inflammation impairs the pancreatic beta-cell in type 2 diabetes. Physiology (Bethesda) 24: 325–331, 2009 [DOI] [PubMed] [Google Scholar]
- 18. Donath MY, Boni-Schnetzler M, Ellingsgaard H, Halban PA, Ehses JA. Cytokine production by islets in health and diabetes: cellular origin, regulation and function. Trends Endocrinol Metab 21: 261–267, 2010 [DOI] [PubMed] [Google Scholar]
- 19. Donath MY, Schumann DM, Faulenbach M, Ellingsgaard H, Perren A, Ehses JA. Islet inflammation in type 2 diabetes: from metabolic stress to therapy. Diabetes Care 31, Suppl 2: S161–S164, 2008 [DOI] [PubMed] [Google Scholar]
- 20. Drab SR. Incretin-based therapies for type 2 diabetes mellitus: current status and future prospects. Pharmacotherapy 30: 609–624, 2010 [DOI] [PubMed] [Google Scholar]
- 21. Drucker DJ. Dipeptidyl peptidase-4 inhibition and the treatment of type 2 diabetes: preclinical biology and mechanisms of action. Diabetes Care 30: 1335–1343, 2007 [DOI] [PubMed] [Google Scholar]
- 22. Duez H, Smith AC, Xiao C, Giacca A, Szeto L, Drucker DJ, Lewis GF. Acute dipeptidyl peptidase-4 inhibition rapidly enhances insulin-mediated suppression of endogenous glucose production in mice. Endocrinology 150: 56–62, 2009 [DOI] [PubMed] [Google Scholar]
- 23. Ehses JA, Ellingsgaard H, Boni-Schnetzler M, Donath MY. Pancreatic islet inflammation in type 2 diabetes: from alpha and beta cell compensation to dysfunction. Arch Physiol Biochem 115: 240–247, 2009 [DOI] [PubMed] [Google Scholar]
- 24. Ehses JA, Meier DT, Wueest S, Rytka J, Boller S, Wielinga PY, Schraenen A, Lemaire K, Debray S, Van Lommel L, Pospisilik JA, Tschopp O, Schultze SM, Malipiero U, Esterbauer H, Ellingsgaard H, Rutti S, Schuit FC, Lutz TA, Boni-Schnetzler M, Konrad D, Donath MY. Toll-like receptor 2-deficient mice are protected from insulin resistance and beta cell dysfunction induced by a high-fat diet. Diabetologia 53: 1795–1806, 2010 [DOI] [PubMed] [Google Scholar]
- 25. Ehses JA, Perren A, Eppler E, Ribaux P, Pospisilik JA, Maor-Cahn R, Gueripel X, Ellingsgaard H, Schneider MK, Biollaz G, Fontana A, Reinecke M, Homo-Delarche F, Donath MY. Increased number of islet-associated macrophages in type 2 diabetes. Diabetes 56: 2356–2370, 2007 [DOI] [PubMed] [Google Scholar]
- 26. Feuerer M, Herrero L, Cipolletta D, Naaz A, Wong J, Nayer A, Lee J, Goldfine AB, Benoist C, Shoelson S, Mathis D. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med 15: 930–939, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ikushima H, Munakata Y, Iwata S, Ohnuma K, Kobayashi S, Dang NH, Morimoto C. Soluble CD26/dipeptidyl peptidase IV enhances transendothelial migration via its interaction with mannose 6-phosphate/insulin-like growth factor II receptor. Cell Immunol 215: 106–110, 2002 [DOI] [PubMed] [Google Scholar]
- 28. Iwata S, Yamaguchi N, Munakata Y, Ikushima H, Lee JF, Hosono O, Schlossman SF, Morimoto C. CD26/dipeptidyl peptidase IV differentially regulates the chemotaxis of T cells and monocytes toward RANTES: possible mechanism for the switch from innate to acquired immune response. Int Immunol 11: 417–426, 1999 [DOI] [PubMed] [Google Scholar]
- 29. Jo J, Gavrilova O, Pack S, Jou W, Mullen S, Sumner AE, Cushman SW, Periwal V. Hypertrophy and/or Hyperplasia: Dynamics of Adipose Tissue Growth. PLoS Comput Biol 5: e1000324, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kim SJ, Nian C, McIntosh CH. Sitagliptin (MK0431) inhibition of dipeptidyl peptidase IV decreases nonobese diabetic mouse CD4+ T-cell migration through incretin-dependent and -independent pathways. Diabetes 59: 1739–1750, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kintscher U, Hartge M, Hess K, Foryst-Ludwig A, Clemenz M, Wabitsch M, Fischer-Posovszky P, Barth TF, Dragun D, Skurk T, Hauner H, Bluher M, Unger T, Wolf AM, Knippschild U, Hombach V, Marx N. T-lymphocyte infiltration in visceral adipose tissue: a primary event in adipose tissue inflammation and the development of obesity-mediated insulin resistance. Arterioscler Thromb Vasc Biol 28: 1304–1310, 2008 [DOI] [PubMed] [Google Scholar]
- 32. Kos K, Baker AR, Jernas M, Harte AL, Clapham JC, O'Hare JP, Carlsson L, Kumar S, McTernan PG. DPP-IV inhibition enhances the antilipolytic action of NPY in human adipose tissue. Diabetes Obes Metab 11: 285–292, 2009 [DOI] [PubMed] [Google Scholar]
- 33. Lamont BJ, Drucker DJ. Differential antidiabetic efficacy of incretin agonists versus DPP-4 inhibition in high fat fed mice. Diabetes 57: 190–198, 2008 [DOI] [PubMed] [Google Scholar]
- 34. Larsen J, Hylleberg B, Ng K, Damsbo P. Glucagon-like peptide-1 infusion must be maintained for 24 h/day to obtain acceptable glycemia in type 2 diabetic patients who are poorly controlled on sulphonylurea treatment. Diabetes Care 24: 1416–1421, 2001 [DOI] [PubMed] [Google Scholar]
- 35. Laudes M, Oberhauser F, Schulte DM, Schilbach K, Freude S, Bilkovski R, Schulz O, Faust M, Krone W. Dipeptidyl-peptidase 4 and attractin expression is increased in circulating blood monocytes of obese human subjects. Exp Clin Endocrinol Diabetes 118: 473–477, 2010 [DOI] [PubMed] [Google Scholar]
- 36. Ma K, Nunemaker CS, Wu R, Chakrabarti SK, Taylor-Fishwick DA, Nadler JL. 12-Lipoxygenase Products Reduce Insulin Secretion and {beta}-Cell Viability in Human Islets. J Clin Endocrinol Metab 95: 887–893, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mahler RJ. The relationship between the hyperplastic pancreatic islet and insulin insensitivity in obesity. Acta Diabetol Lat 18: 1–17, 1981 [DOI] [PubMed] [Google Scholar]
- 38. Marselli L, Thorne J, Ahn YB, Omer A, Sgroi DC, Libermann T, Otu HH, Sharma A, Bonner-Weir S, Weir GC. Gene expression of purified beta-cell tissue obtained from human pancreas with laser capture microdissection. J Clin Endocrinol Metab 93: 1046–1053, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mu J, Petrov A, Eiermann GJ, Woods J, Zhou YP, Li Z, Zycband E, Feng Y, Zhu L, Roy RS, Howard AD, Li C, Thornberry NA, Zhang BB. Inhibition of DPP-4 with sitagliptin improves glycemic control and restores islet cell mass and function in a rodent model of type 2 diabetes. Eur J Pharmacol 623: 148–154, 2009 [DOI] [PubMed] [Google Scholar]
- 40. Nicoletti F, Conget I, DiMauro M, DiMarco R, Mazzarino MC, Bendtzen K, Messina A, Gomis R. Serum concentrations of the interferon-gamma-inducible chemokine IP-10/CXCL10 are augmented in both newly diagnosed Type I diabetes mellitus patients and subjects at risk of developing the disease. Diabetologia 45: 1107–1110, 2002 [DOI] [PubMed] [Google Scholar]
- 41. Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M, Otsu M, Hara K, Ueki K, Sugiura S, Yoshimura K, Kadowaki T, Nagai R. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med 15: 914–920, 2009 [DOI] [PubMed] [Google Scholar]
- 42. Novelli EL, Diniz YS, Galhardi CM, Ebaid GM, Rodrigues HG, Mani F, Fernandes AA, Cicogna AC, NovelliFilho JL. Anthropometrical parameters and markers of obesity in rats. Lab Anim 41: 111–119, 2007 [DOI] [PubMed] [Google Scholar]
- 43. Nunemaker CS, Chen M, Pei H, Kimble SD, Keller SR, Carter JD, Yang Z, Smith KM, Wu R, Bevard MH, Garmey JC, Nadler JL. 12-Lipoxygenase-knockout mice are resistant to inflammatory effects of obesity induced by western diet. Am J Physiol Endocrinol Metab 295: E1065–E1075, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pospisilik JA, Martin J, Doty T, Ehses JA, Pamir N, Lynn FC, Piteau S, Demuth HU, McIntosh CH, Pederson RA. Dipeptidyl peptidase IV inhibitor treatment stimulates beta-cell survival and islet neogenesis in streptozotocin-induced diabetic rats. Diabetes 52: 741–750, 2003 [DOI] [PubMed] [Google Scholar]
- 45. Reinhold D, Biton A, Goihl A, Pieper S, Lendeckel U, Faust J, Neubert K, Bank U, Tager M, Ansorge S, Brocke S. Dual inhibition of dipeptidyl peptidase IV and aminopeptidase N suppresses inflammatory immune responses. Ann NY Acad Sci 1110: 402–409, 2007 [DOI] [PubMed] [Google Scholar]
- 46. Ruster C, Wolf G. The role of chemokines and chemokine receptors in diabetic nephropathy. Front Biosci 13: 944–955, 2008 [DOI] [PubMed] [Google Scholar]
- 47. Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest 116: 1793–1801, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Simson EL, Gold RM. The Lee Obesity Index vindicated? Physiol Behav 29: 371–376, 1982 [DOI] [PubMed] [Google Scholar]
- 49. Skurk T, Alberti-Huber C, Herder C, Hauner H. Relationship between adipocyte size and adipokine expression and secretion. J Clin Endocrinol Metab 92: 1023–1033, 2007 [DOI] [PubMed] [Google Scholar]
- 50. Souza-Mello V, Gregório BM, Cardoso-de-Lemos FS, de Carvalho L, Aguila MB, Mandarim-de-Lacerda CA. Comparative effects of telmisartan, sitagliptin and metformin alone or in combination on obesity, insulin resistance, and liver and pancreas remodelling in C57BL/6 mice fed on a very high-fat diet. Clin Sci (Lond) 119: 239–250, 2010 [DOI] [PubMed] [Google Scholar]
- 51. Valverde I, Mérida E, Delgado E, Trapote MA, Villanueva-Peñacarrillo ML. Presence and characterization of glucagon-like peptide-1(7–36) amide receptors in solubilized membranes of rat adipose tissue. Endocrinology 132: 75–79, 1993 [DOI] [PubMed] [Google Scholar]
- 52. Wegner M, Winiarska H, Bobkiewicz-Kozlowska T, Dworacka M. IL-12 serum levels in patients with type 2 diabetes treated with sulphonylureas. Cytokine 42: 312–316, 2008 [DOI] [PubMed] [Google Scholar]
- 53. Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 112: 1796–1808, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest 115: 1111–1119, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wen Y, Gu J, Chakrabarti SK, Aylor K, Marshall J, Takahashi Y, Yoshimoto T, Nadler JL. The role of 12/15-lipoxygenase in the expression of interleukin-6 and tumor necrosis factor-alpha in macrophages. Endocrinology 148: 1313–1322, 2007 [DOI] [PubMed] [Google Scholar]
- 56. Winer S, Chan Y, Paltser G, Truong D, Tsui H, Bahrami J, Dorfman R, Wang Y, Zielenski J, Mastronardi F, Maezawa Y, Drucker DJ, Engleman E, Winer D, Dosch HM. Normalization of obesity-associated insulin resistance through immunotherapy. Nat Med 15: 921–929, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Winzell MS, Magnusson C, Ahren B. Temporal and dietary fat content-dependent islet adaptation to high-fat feeding-induced glucose intolerance in mice. Metabolism 56: 122–128, 2007 [DOI] [PubMed] [Google Scholar]
- 58. Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 112: 1821–1830, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Yip RG, Boylan MO, Kieffer TJ, Wolfe MM. Functional GIP receptors are present on adipocytes. Endocrinology 139: 4004–4007, 1998 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.