Abstract
Mechanisms regulating spontaneous physical activity remain poorly characterized despite evidence of influential genetic and acquired factors. We evaluated ambulatory activity and wheel running in leptin-deficient ob/ob mice and in wild-type mice rendered hypoleptinemic by fasting in both the presence and absence of subcutaneous leptin administration. In ob/ob mice, leptin treatment to plasma levels characteristic of wild-type mice acutely increased both ambulatory activity (by 4,000 ± 200 beam breaks/dark cycle, P < 0.05) and total energy expenditure (TEE; by 0.11 ± 0.01 kcal/h during the dark cycle, P < 0.05) in a dose-dependent manner and acutely increased wheel running (+350%, P < 0.05). Fasting potently increased ambulatory activity and wheel running in wild-type mice (AA: +25%, P < 0.05; wheel running: +80%, P < 0.05), and the effect of fasting was more pronounced in ob/ob mice (AA: +400%, P < 0.05; wheel running: +1,600%, P < 0.05). However, unlike what occurred in ad libitum-fed ob/ob mice, physiological leptin replacement attenuated or prevented fasting-induced increases of ambulatory activity and wheel running in both wild-type and ob/ob mice. Thus, plasma leptin is a physiological regulator of spontaneous physical activity, but the nature of leptin's effect on activity is dependent on food availability.
Keywords: locomotor activity, energy expenditure, metabolism, exercise
energy homeostasis is the biological process whereby energy intake and energy expenditure are linked, and this process is tightly regulated by endocrine signals (29). Recent evidence suggests that hormonal regulation of energy expenditure entails adjustments not only of resting energy expenditure (REE) but also of activity energy expenditure (AEE). Specifically, physiological leptin administration was able to increase AEE by decreasing muscle work efficiency in weight-reduced human subjects (34). However, whether spontaneous activity and voluntary exercise are biologically regulated in human subjects related to energy balance has been difficult to determine (21). In contrast, both spontaneous ambulatory activity (AA) and voluntary exercise (wheel running) are readily measured in mice, and numerous studies have suggested a role for AEE in genetic and acquired mouse models of obesity (3). Obese, leptin-deficient ob/ob mice are characterized by low AA (6, 8, 9) and even lower wheel running (17). Pharmacological leptin treatment causes weight loss and increases AA in leptin-deficient rodents (1, 33), whereas the effect of leptin treatment on wheel running has not been investigated. In contrast, in female mice selectively bred for high wheel running activity, plasma leptin levels were lower than predicted based on fat mass, and low leptin levels correlated with greater distance run and running speed (13). Thus, whether spontaneous activity and voluntary exercise are behaviors biologically regulated by physiological concentrations of plasma leptin remains unknown and provides the central focus for the current studies.
The finding that low spontaneous activity of obese mice that lack leptin receptors is corrected by selective restoration of leptin signaling to the medial hypothalamus or to proopiomelanocortin (POMC) neurons (7, 16) suggests that leptin regulates physical activity via actions on hypothalamic neurocircuits involved in energy homeostasis. This conclusion is further supported by a study in which intracerebroventricular (icv) leptin administration was shown to increase AA in rats (5) and by studies implicating various hypothalamic neurotransmitters, including brain-derived neurotrophic factor, neuromedin U, urocortin, orexin, and brain-specific homeobox transcriptional factor (Bsx) in regulation of physical activity and AEE (31, 38). The question of whether circulating leptin regulates hypothalamic neurotransmitters consistent with effects on physical activity remains unanswered and was a secondary focus for the current studies.
In this study we evaluated AA, wheel running, and energy expenditure in leptin-deficient ob/ob mice and in hypoleptinemic fasted wild-type mice. Mice were evaluated in both the presence and absence of continuous subcutaneous infusion of leptin at doses designed to achieve physiological replacement.
MATERIALS AND METHODS
Animals.
Adult male wild-type C57/Bl6 (average body weight 23.5 ± 0.2 g) and ob/ob mice on the C57/Bl6 background (average body weight 37.4 ± 0.2 g) (Jackson Laboratories, Bar Harbor, ME) were housed individually in a specific pathogen-free American Association for Accreditation of Laboratory Animal Care-accredited facility. All mice were maintained in a 12:12-h light-dark cycle in a temperature-controlled room with ad libitum access to water and standard laboratory chow (Purina 5053; Purina Mills, St. Louis, MO) unless otherwise stated. Calorimetry data from these animals was previously reported as part of a large University of Washington Mouse Metabolic Phenotyping Center study describing the optimal method to adjust total energy expenditure for body mass, lean mass, and fat mass in mice (18). All procedures were approved by the Institutional Animal Care and Use Committee of the University of Washington.
Body composition analysis.
Body composition was evaluated in live, conscious animals in triplicate by quantitative nuclear magnetic resonance spectroscopy (EchoMRI 3-in-1 Animal Tissue Composition Analyzer; Echo Medical Systems, Houston, TX) (39). A system test is performed routinely at the beginning of each measurement day, and the equipment is calibrated by scanning a calibration holder containing a known amount of fat to test the validity of measurement.
Indirect calorimetry.
Total oxygen consumption (V̇o2) and carbon dioxide production (V̇co2), measurements that are proportional to metabolic rate, were measured continuously by indirect calorimetry using an eight-chamber Oxymax Laboratory Animal Monitoring System (Columbus Instruments, Columbus, OH). V̇o2 was converted to individual total energy expenditure (TEE) in kilocalories per hour by Columbus software, which uses the standard Lusk formula [TEE in kcal/h = (3.815 + 1.232 × RQ) × V̇o2 in l/h, where RQ (respiratory quotient) is the ratio of V̇co2 to V̇o2] (24). RQ, an indicator of the proportions of fat and carbohydrate oxidation under specified conditions (24), ranges between ∼0.7 (complete reliance on fat oxidation) and 1.0 (complete reliance on carbohydrate oxidation).
Locomotor activity.
AA was determined simultaneously with the collection of indirect calorimetry data using an Opto-Varimetrix-3 sensor system (Columbus Instruments). Consecutive adjacent infrared beam breaks in either the x- or y-axes were scored as an activity count, and a tally was recorded every 15 min.
Running wheel activity.
Running wheel activity was measured in a separate group of experiments from studies measuring AA. Mouse activity wheels (model no. 80820F) hung from filtered cage lids in standard-sized mouse cages that contained regular bedding (Lafayette Instruments, Lafayette, IN). Running wheels were equipped with a Servo-Brake to permit the locking and/or unlocking of running wheels and an activity wheel control and counter (model no. 86070) to monitor the revolution of activity wheels (1 revolution = 0.40 m running distance). Activity Wheel Software (version 11.10, model no. 86065) was then used to record interval counts (5 min), average speed, total counts, and total distance.
Effect of leptin replacement on spontaneous physical activity in ob/ob mice.
Adult male ob/ob mice (∼10 wk of age, body weight 37.4 ± 0.6 g) were housed individually and habituated to metabolic cages prior to the study. Animals had baseline body composition performed and were returned to metabolic cages just prior to dark cycle onset for continuous baseline measurement of AA and calorimetry for 3 days while having ad libitum access to food and water. Animals were separated into weight-matched groups and were implanted subcutaneously with an osmotic minipump (Alzet Model 1007D; Durect, Cupertino, CA) containing either vehicle (PBS, pH 7.9) or leptin at a dose of 50, 100, or 200 ng/h (Dr. A. F. Parlow, National Hormone and Peptide Program, Torrance, CA) (n = 7–8/group) and returned to the metabolic cages. Doses of leptin were designed to achieve leptin levels in the low physiological, high physiological, and supraphysiological range for wild-type mice based on previous studies (19, 27). A predetermined length of Tygon flexible plastic tubing filled with vehicle was attached to the end of each osmotic minipump such that vehicle was infused only for the 24-h recovery period following surgery. Approximately 30 h following surgery, a blood sample was taken from the tail vein for measurement of plasma leptin levels. Three hours following the blood sample, just prior to dark cycle onset, acquisition of continuous AA and indirect calorimetry measurements began for an additional 64 h while all animals had ad libitum access to food and water. Animals were then removed from the calorimeter, and body weight and body composition were again measured. Animals were then euthanized, and a block of mediobasal hypothalamus (MBH) defined caudally by the mamillary bodies, rostrally by the optic chiasm, laterally by the optic tract, and superiorly by the apex of the third ventricle was rapidly dissected and snap-frozen as described previously (41), and blood was collected in EDTA-treated tubes.
Effect of leptin replacement on spontaneous physical activity in fasted wild-type and ob/ob mice.
A similar protocol was used to examine the effect of physiological leptin replacement on spontaneous physical activity (SPA) in fasted wild-type mice. Following measurement of body composition and baseline continuous AA and indirect calorimetry in the fed state, weight-matched adult male C57Bl/6 mice (∼10 wk of age, body weight 23.5 ± 0.3 g) were implanted with an osmotic minipump containing either vehicle or leptin at a dose of 50, 100, or 200 ng/h (n = 8/group). All osmotic minipumps were attached to a predetermined length of Tygon flexible plastic tubing filled with vehicle, as described above, to deliver vehicle only for the 24-h recovery period following surgery. A blood sample was obtained from the tail vein ∼30 h after surgery for measurement of plasma leptin levels. Just prior to dark cycle onset, mice were returned to the indirect calorimeter for continuous AA and calorimetry measurements for a 24-h fasting period while having ad libitum access to water. Immediately following calorimetry, mice were studied for body composition before being euthanized with tissue and blood samples collected as described above. This entire experiment was then replicated with ob/ob mice being substituted for wild-type mice.
Effect of leptin replacement on wheel running in ob/ob mice.
Adult male ob/ob mice (∼10 wk of age, body weight 37.7 ± 0.7 g) were housed individually and habituated to the running wheels (Lafayette Instruments) while they were placed in the locked position for 4 days. Baseline body composition and body weight was performed before running wheels were unlocked, and wheel running was then recorded in the fed state for 4 days. Weight-matched animals were then subcutaneously implanted with an osmotic minipump containing either vehicle (PBS, pH 7.9) or leptin at a dose of 100 ng/h (n = 7/group) as described above. Animals were then returned to running wheel cages, and data were recorded for 6 days, during which food and water were available ad libitum. This experiment was repeated in a separate group of ob/ob mice, except that animals were euthanized 2 h prior to the second dark cycle. A wedge of MBH was taken and blood collected as noted above.
Effect of leptin replacement on wheel running in fasted wild-type mice.
A similar protocol was used to examine the effect of leptin replacement on wheel running in fasted wild-type mice (C57Bl/6 ∼10 wk of age, body weight 23.5 ± 0.2 g). Following habituation to the running wheels, wheel running was recorded in the fed state for 4 days. Weight-matched animals were then implanted with an osmotic minipump containing either vehicle or leptin at a dose of 100 ng/h (n = 8/group). Animals were returned to running wheel cages and data recorded for a fasting period of 24 h. This experimental paradigm was repeated again in both ob/ob mice and in a separate group of wild-type animals in which mice were euthanized 2 h prior to second dark cycle onset (i.e., 22 h into their fast), at which time a wedge of MBH was taken and blood collected as previously described.
Plasma analysis.
Whole blood was collected into EDTA-treated tubes and centrifuged, and plasma was removed for subsequent measurement of plasma immunoreactive leptin levels by ELISA (cat no. 90030; Crystal Chem, Chicago, IL) and corticosterone levels by EIA (Cayman Chemical, Ann Arbor, MI) according to the manufacturers' instructions.
RT-PCR.
Total RNA was extracted from hypothalami using TRIzol B according to manufacturer's instructions (MRC, Cincinnati, OH). RNA was quantitated by spectrophotometry at 260 nm (Nanodrop 1000; Thermo Scientific) and reverse-transcribed with AMV reverse transcriptase (1 μg) (Promega, Madison, WI), and real-time PCR was performed on a ABI Prism 7900 HT (Applied Biosystems) using the commercially available PCR master mix (SYBR Green, Applied 2.0; Applied Biosystems), as described previously (28). PCR data were analyzed using the Sequence Detection System software (SDS Version 2.2; Applied Biosystems). Expression levels of each gene were normalized to a housekeeping gene (18S RNA) and expressed as a percentahe of vehicle-vehicle controls. Nontemplate controls were incorporated into each PCR run.
Statistical analysis.
All results are expressed as means ± SE. For analyses that did not require adjustment for variation in body size, we used a one-way analysis of variance (ANOVA) for omnibus group-wide testing followed by a post hoc least significant difference between-subjects Student t-test for pairwise comparisons. To control for the influence of body size variation on TEE (18), group comparisons involving this outcome were adjusted for total body mass using analysis of covariance (ANCOVA) (2) in keeping with recent work from our group (18). ANOVA was performed using Statistica (version 7.1; StatSoft). ANCOVA was performed with the univariate general linear model module in PASW statistics (version 17, IBM, Chicago, IL). Significance was established at P < 0.05 (2-tailed Student's t-test).
RESULTS
Effect of leptin replacement on AA and TEE in ob/ob mice.
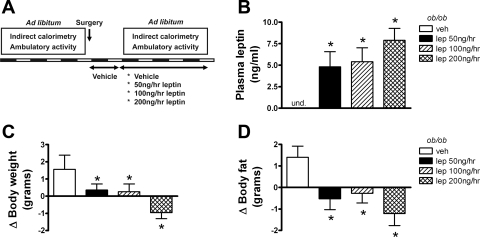
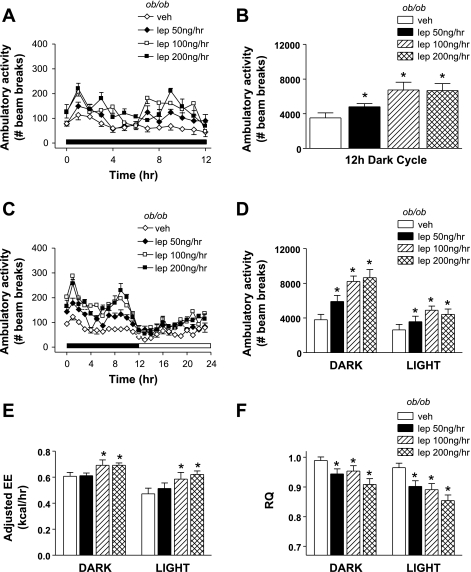
Vehicle- and leptin-treated ob/ob mice were housed in metabolic cages to continuously measure AA and TEE as depicted in Fig. 1A. Based on plasma sampled at the end of the study, systemic leptin replacement achieved our intended goal of restoring circulating leptin to low physiological (50 ng/h), midphysiological (100 ng/h), and high physiological (200 ng/h) levels (Fig. 1B). As expected, systemic leptin replacement was accompanied by significant reductions in body weight (Fig. 1C) and body fat (Fig. 1D). Leptin-deficient mice exhibit low baseline AA, as shown previously (33). Relative to vehicle-treated ob/ob mice, physiological leptin administration acutely increased AA in a dose-dependent manner during the first dark cycle (Fig. 2, A and B), and this effect was maintained throughout both dark and light cycles for the duration of the study (Fig. 2, C and D). Importantly, although dark cycle AA doubled in ob/ob mice receiving the highest leptin dose, this maximal value represents only ∼33% of values characteristic of wild-type mice. Leptin infusion also raised energy expenditure adjusted for total body mass using ANCOVA (Fig. 2E) and reduced RQ (Fig. 2F) in a dose-dependent manner. Plasma corticosterone levels were also modestly reduced by leptin administration [vehicle: 119 ± 8 ng/ml; Lep50: 92 ± 6 ng/ml (P = 0.05); Lep100: 101 ± 7 ng/ml (P = 0.15); Lep200: 86 ± 11 ng/ml (P = 0.01)].
Fig. 1.
Effect of acute physiological leptin (lep) replacement in ob/ob mice. Adult male ob/ob mice were treated with one of 4 doses (n = 8/group) of continuous subcutaneous leptin replacement: 1) 0 [vehicle (veh); open bar], 2) 50 (black bars), 3) 100 (hatched bars), or 4) 200 ng/h (cross-hatched bars). Study design (A) and plasma leptin (B), change in body weight (C), and change in body fat [by quantitative magnetic resonance (QMR); D] at the end of the study. Data shown are means ± SE. Significance, *P < 0.05 by 1-way ANOVA vs. veh group. und., Undetectable.
Fig. 2.
Effect of acute physiological lep replacement on ambulatory activity and energy expenditure (EE) in ob/ob mice. Adult male ob/ob mice were treated with one of 4 doses (n = 8/group) of continuous subcutaneous leptin replacement: 1) 0 (veh; ◊), 2) 50 (♦), 3) 100 (□), or 4) 200 ng/h (■). Hourly (A) and cumulative (B) ambulatory activity during the first dark cycle of lep treatment. Mean hourly ambulatory activity (C) and mean dark and light cycle cumulative ambulatory activity (D), EE adjusted for total body mass (E), and respiratory quotient (RQ; F) throughout the study period. Data shown are means ± SE. Significance, *P < 0.05 by 1-way ANOVA or ANCOVA vs. veh group.
Effect of leptin replacement on AA and TEE in fasted wild-type mice.
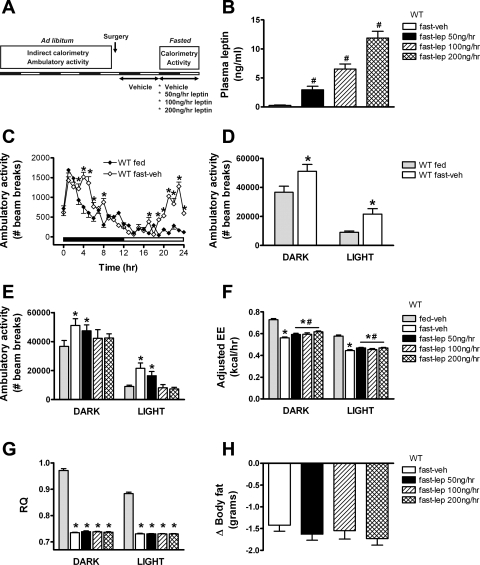
AA was measured in fed and fasted wild-type mice receiving continuous sc infusion of either vehicle or leptin at the same doses previously administered to ob/ob mice (Fig. 3A). As expected, plasma leptin levels were markedly reduced in vehicle-treated fasted animals and were dose-dependently increased with progressively greater concentrations of leptin replacement (Fig. 3B). Fasting induced a significant increase in AA relative to mice fed ad libitum, an effect observed during both the dark and light cycles (Fig. 3, C and D). Restoring plasma leptin concentrations into the physiological range attenuated the fasting-induced stimulation of AA in a dose-dependent manner (Fig. 3E), identifying leptin deficiency as a contributory factor in this response. Despite increased AA, fasting significantly decreased adjusted TEE (Fig. 3F) relative to wild-type mice fed ad libitum. Consistent with a previous report (10), the highest dose of leptin significantly increased TEE of fasted wild-type mice compared with vehicle (Fig. 3F) despite preventing fasting-induced increased AA. The effect of fasting to induce a dramatic decrease of RQ (reflecting a marked increase of lipid oxidation) was not altered by leptin replacement (Fig. 3G). Because of the short duration of the study and the potent effect of fasting, both loss of body fat (Fig. 3H) and body weight loss (data not shown) were equivalent between all fasted groups, irrespective of plasma leptin level.
Fig. 3.
Effect of acute physiological lep replacement in fasting wild-type (WT) mice. The study design (A) and plasma lep levels (B) in adult male C57Bl/6 mice that were fasted for 24 h at the onset of the dark cycle and treated with one of 4 doses (n = 8/group) of continuous subcutaneous lep replacement: 1) 0 (veh; open bars), 2) 50 (black bars), 3) 100 (hatched bars), or 4) 200 ng/h (cross-hatched bars). Baseline (ad libitum fed) state shown in the gray bar. Ambulatory activity (C–E), EE adjusted for total body mass (F), and RQ (G) during the 24-h fast. Changes in body fat (by QMR; H) were determined at the end of the study. Data shown are means ± SE. Significance, *P < 0.05 by 1-way ANOVA or ANCOVA vs. WT-fed group; #P < 0.05 vs. WT-fasted group.
Effect of leptin replacement on AA and TEE in fasted ob/ob mice.
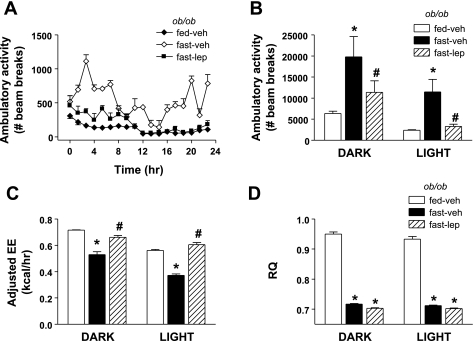
To determine whether a decline of leptin signaling is required for the effect of fasting to increase AA, we performed an additional study in which fasted ob/ob mice received either a continuous sc infusion of vehicle or a physiological dose of leptin (100 ng/h). AA was increased significantly in fasted ob/ob mice (+400%) relative to ob/ob controls fed ad libitum during both dark and light cycles, and this fasting-induced increase of ambulatory activity was prevented by leptin treatment (Fig. 4, A and B). Despite elevated AA, TEE adjusted for total body mass using ANCOVA (Fig. 4C) was reduced in fasted ob/ob mice receiving sc vehicle (compared with fed ob/ob mice), and physiological leptin administration increased TEE in ob/ob mice beyond baseline values observed in fed ob/ob mice, in contrast to what was observed in wild-type mice, despite a complete reversal of the fasting-induced increase of AA (Fig. 4, B and C). In comparison, RQ levels were significantly reduced with fasting and even slightly further reduced with leptin treatment (Fig. 4D). Body weight loss was equivalent in both vehicle- and leptin-treated fasting ob/ob mice (data not shown).
Fig. 4.
Effect of acute physiological lep replacement in fasting ob/ob mice. Adult male ob/ob mice were fasted for 24 h at the onset of the dark cycle. Fasting mice were treated with one of 2 doses (n = 8/group) of continuous subcutaneous leptin replacement, 1) 0 (veh; ◊) or 2) 100 ng/h (■), and compared with fed controls (♦). Ambulatory activity (A and B), EE adjusted for total body mass (C), and RQ during the 24-h fast. Data shown are means ± SE. Significance, *P < 0.05 by 1-way ANOVA or ANCOVA vs. fed-veh group; #P < 0.05 vs. fast-veh group.
Effect of leptin replacement on hypothalamic neuropeptide gene expression.
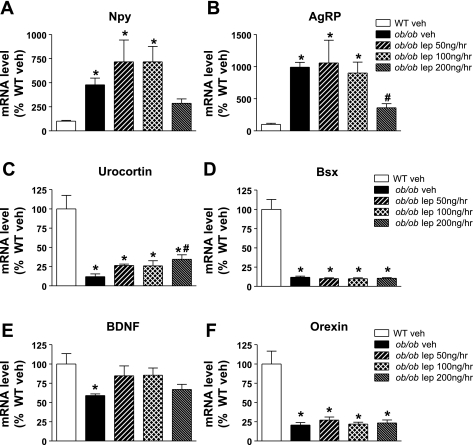
To gain insight into the central nervous system (CNS) mechanism whereby leptin regulates SPA, we measured levels of mRNA encoding hypothalamic peptides implicated in the control of energy homeostasis and activity in a mediobasal wedge of whole hypothalamus from animals in a subset of the above studies. As expected, hypothalamic levels of Npy and Agrp mRNA were increased significantly in ob/ob relative to wild-type controls (Fig. 5, A and B). These increases in both hypothalamic Npy and Agrp mRNA levels were attenuated by leptin at the high physiological dose (200 ng/h; Fig. 5, A and B), whereas hypothalamic melanin-concentrating hormone mRNA levels were not affected (data not shown). Similarly, we observed that fasting-induced decreases of Pomc and Cart mRNA levels were partially reversed by leptin treatment without a clear dose-dependent effect (data not shown).
Fig. 5.
Hypothalamic mRNA expression following acute physiological leptin replacement in ob/ob mice. Adult male ob/ob mice were treated with one of 4 doses (n = 8/group) of continuous subcutaneous leptin replacement, 1) 0 (veh; black bars), 2) 50 (black and white hatched bars), 3) 100 (cross-hatched bars), or 4) 200 ng/h (black and gray hatched bars), and compared with WT controls (open bars). At the end of the study, a mediobasal wedge of hypothalamus was harvested and mRNA expression of neuropeptide Y (NPY; A), agouti-related protein (AgRP; B), urocortin (C), brain-specific homeobox transcriptional factor (Bsx; D), brain-derived neurotrophic factor (BDNF; E), and orexin (F) evaluated. Data shown are means ± SE. Significance, *P < 0.05 by 1-way ANOVA vs. WT fed; #P < 0.05 vs. ob/ob veh groups.
Among peptides known to regulate AA, levels of hypothalamic mRNA encoding urocortin, Bsx, brain-derived neurotrophic factor (BDNF), and orexin were reduced in ob/ob mice relative to wild-type controls, but of these, only urocortin expression was increased by exogenous leptin in a dose-dependent manner (similar to the effect on AA) (Fig. 5, C–F).
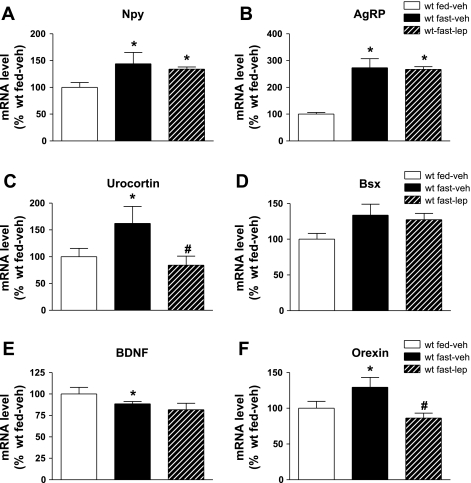
As expected, fasting increased hypothalamic expression of both Npy and Agrp mRNA in wild-type mice, and unlike what was observed in ob/ob mice, this increase was not reversed by leptin replacement (100 ng/h; Fig. 6, A and B). By contrast, exogenous leptin completely blocked the fasting-induced increase of hypothalamic urocortin and orexin (Fig. 6, C and D) mRNA expression despite having no effect on hypothalamic Bsx or Bdnf mRNA levels (Fig. 6, E and F).
Fig. 6.
Hypothalamic mRNA expression following acute physiological lep replacement in fasted WT mice. Fasted adult male C57Bl/6 mice were treated with one of 2 doses (n = 6/group) of continuous subcutaneous leptin replacement, 1) 0 (veh; black bars) or 2) 100 ng/h (hatched bars), and compared with fed WT controls (white bars). At the end of the study, a mediobasal wedge of hypothalamus was harvested and mRNA expression of NPY (A), AgRP (B), urocortin (C), Bsx (D), BDNF (E), and orexin (F) evaluated. Data shown are means ± SE. Significance, *P < 0.05 by 1-way ANOVA vs. fed WT control; #P < 0.05 vs. fast-veh group.
Effect of leptin replacement on wheel running in fed and fasted ob/ob and wild-type mice.
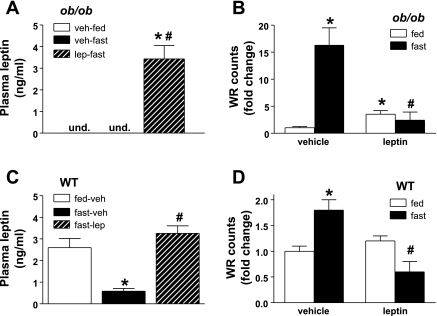
In all fed animals, wheel running occurred almost exclusively during the dark cycle, and consequently, only dark cycle wheel running activity data are presented below. Analysis of data generated in vehicle-treated controls revealed that ob/ob mice display markedly reduced wheel running activity compared with wild-type mice (<5% of wheel-running distance, P < 0.001). Leptin administration at doses that achieve physiological plasma concentrations (Fig. 7A) increased wheel running (3.5 ± 0.5-fold, P < 0.05; Fig. 7B) in ob/ob mice relative to vehicle-treated controls. However, peak leptin-induced wheel running was still <10% of wheel running in wild-type mice. As was observed for AA, fasting caused a dramatic increase of wheel running activity in ob/ob mice (16.3 ± 3.2 fold, P < 0.05; Fig. 7B), and physiological leptin treatment prevented the fasting-induced increase (2.4 ± 1.5-fold; Fig. 7B).
Fig. 7.
Wheel running (WR) activity in fed and fasted ob/ob and WT mice. Plasma lep concentrations and WR counts in adult male ob/ob (A and B) and C57Bl/6 (C and D) mice, respectively, treated with one of 2 doses (n = 6/group) of continuous subcutaneous lep replacement, 1) 0 or 2) 100 ng/h, in the fed (open bars) and fasted (black bars) state. Data shown are means ± SE of fold change over veh-fed groups for both genotypes. Significance, *P < 0.05 by 1-way ANOVA vs. veh-fed; #P < 0.05 vs. fast-veh.
Fasting also elicited an increase in wheel running activity in vehicle-treated wild-type mice, and this increase was blocked by physiological leptin replacement (Fig. 7, C and D). The relative increase of wheel running induced by fasting in wild-type mice was smaller than in ob/ob mice (50 vs. 600%), similar to the effect of fasting on AA between the two genotypes. Interestingly, wheel running in fasting wild-type mice was reduced during leptin replacement to values below their baseline counts (Fig. 7B) despite equivalent plasma leptin levels. Importantly, whereas 24-h fasting induced an expected increase of plasma corticosterone levels, leptin replacement had no effect to reduce corticosterone in fasting mice (fed vehicle: 120 ± 20 ng/ml; fasted vehicle: 225 ± 27 ng/ml; fasted leptin: 260 ± 15 ng/ml, P < 0.01 for both fasting groups vs. fed, P = 0.3 for fasted vehicle vs. fasted leptin).
DISCUSSION
Leptin is a key hormonal regulator of energy homeostasis and body fat stores. Although physiological roles for leptin in the control of energy intake (25, 29) and TEE (18) are well described, information about its role in the regulation of SPA and AEE is comparatively limited. Previous studies have shown that physical activity is increased by chronic administration of leptin to ob/ob mice at a dose that also causes weight loss (1, 33), and similar effects were observed in response to acute icv leptin treatment in rats (5), demonstrating that leptin can alter physical activity when administered at pharmacological doses. The current studies were undertaken to determine whether physiological variation in plasma leptin levels regulates SPA in mice using an established paradigm in which mice with genetic or acquired hypoleptinemia receive exogenous leptin at doses that raise circulating leptin levels into the physiological range for wild-type mice (19, 27). Our results demonstrate potent effects of leptin on physical activity, both ambulatory activity and wheel running (voluntary exercise), and that the nature of the leptin effect is dependent on food availability.
Specifically, we found that, in wild-type mice, physiological leptin replacement ameliorates fasting-induced increases of both ambulatory activity and wheel running, indicating a role for leptin deficiency in the potent effect of fasting to increase physical activity. This conclusion is consistent with a theoretical model in which a fasting-induced increase of physical activity is a manifestation of increased food-seeking behavior (32, 36) that is driven in part by a reduced leptin signal. Interestingly, fasting also increases ambulatory activity and wheel running in ob/ob mice, demonstrating that factors other than hypoleptinemia can also drive this response. Yet, even in these mice, the effect of fasting to stimulate physical activity is nonetheless prevented by leptin administration at doses that achieve physiological plasma levels, as was observed in fasting wild-type mice.
In contrast, we found that, in leptin-deficient ob/ob mice that have ad libitum access to food, low-dose leptin replacement acutely increased both ambulatory activity and wheel running. This effect occurred within the first 12 h of leptin treatment and is, therefore, independent of the effect of leptin to reduce body weight. These observations suggest that, unlike what is observed in fasted animals, leptin deficiency inhibits physical activity when food is available, and therefore, this effect may contribute to the obese phenotype of mice that lack a leptin signal. Finally, analysis of hypothalamic neuropeptide mRNA expression from a subset of these mice identifies urocortin and orexin as candidate mediators of the effect of physiological leptin signaling to acutely regulate physical activity, and additional studies are warranted to investigate this hypothesis.
Previous work has shown that, relative to wild-type controls, ob/ob mice are characterized by low ambulatory activity (6, 8, 33) and even lower wheel running (17) and that 3 wk of exogenous leptin administration at a pharmacological dose (10 mg/kg ip daily) increased observed activity scores in ob/ob mice to values similar to those recorded in control mice despite significantly reducing body weight, percent body fat, and food intake (33). In a second study, 1 wk of leptin treatment with a lower but still pharmacological dose (1 mg/kg ip daily) improved observed locomotor activity of ob/ob mice in an open-field testing paradigm (1). A limitation of these studies is that one cannot separate the effect of leptin to reduce body weight from its effect on activity levels and that brief testing paradigms were used that are better at testing anxiety and exploration rather than physical activity. Our study paradigm addresses this issue, since we used continuous computerized measurement of ambulatory activity to show not only that continuous sc leptin treatment acutely increases ambulatory activity in ob/ob mice prior to any change of body weight but that this effect occurred with doses of leptin that achieved physiological plasma levels. Unlike previous reports showing that chronic pharmacological leptin administration nearly normalizes activity measurements, we observed leptin-mediated increases of ambulatory activity amounting to approximately one-third of wild-type values. This quantitative difference in outcome suggests that in ob/ob mice, reduced ambulatory activity results from both a reduced leptin signal and other consequences of lifelong leptin deficiency (e.g., obesity, neuronal pathway development).
We also report that, at physiological concentrations, leptin increases wheel running in ob/ob mice, an effect that has not been reported previously and which occurred rapidly and was sustained over the 4-day treatment period. However, peak leptin-induced wheel running was only <10% of wild-type wheel running activity, and whether more prolonged or pharmacological leptin treatment can normalize wheel running activity awaits further study. Nonetheless, increased wheel running in leptin-treated ob/ob mice is crucial evidence that voluntary exercise behavior is regulated by physiological hormone signaling.
Although fasting clearly increases ambulatory activity in wild-type mice (36), the role of acquired hypoleptinemia to drive this response has not previously been investigated. We report that the fasting-induced increase of ambulatory activity in wild-type mice is dose-dependently blunted by acute, physiological leptin replacement, and this effect is even more dramatic with fasting-induced wheel running, which was prevented entirely by physiological leptin replacement. The mechanism underlying fasting-induced activity is uncertain. It could be related to a foraging response, an aspect of food-seeking behavior that is greatly augmented in fasted animals (40), or alternately, dopaminergic reward signaling induced by running (23) could be increased by fasting, as has been shown for other “reward” signals (12). Increased plasma corticosterone levels are associated with increased activity in fasting rodents, and adrenalectomy prevents increased wheel running in food-restricted rats (11). Our data show that the effect of leptin to decrease fasting-induced activity is not mediated through decreased plasma corticosterone levels.
Interestingly, decreased leptin signaling cannot be the sole mediator of fasting-induced activity, since fasting also dramatically increases ambulatory activity and wheel running in ob/ob mice that lack leptin, also proving that baseline physical activity in these mice is not limited purely by obesity. Nonetheless, leptin treatment prevented the acute increase of both ambulatory activity and wheel running induced by fasting in ob/ob mice. Although this seemingly paradoxical finding, that leptin increases physical activity in ob/ob mice when they are fed ad libitum but reduces it when they are fasted, awaits further explanation, a similar paradoxical effect has been described previously for the regulation of body temperature by the CNS leptin-melanocortin pathway, increasing body temperature in hypothermic ob/ob mice (33) but reducing lipopolysaccharide-induced fever (4, 15, 22). Thus, the overall effect of leptin on physical activity is highly dependent on whether food is available. This finding may represent an effect of leptin signaling to redirect physical activity behavior away from food seeking toward other activities such as exploration, socialization, or play. Alternatively, reward signaling induced by physical activity may be enhanced by fasting in a leptin-dependent manner, and further studies examining distinct components of physical activity as well as specific reward paradigms will be needed to address these hypotheses.
Recent work from many groups has identified a large number of hypothalamic peptides capable of altering ambulatory activity, including BDNF, urocortin, Bsx, orexin, and neuromedin U, among others (20, 30, 31, 37, 38), and many of these mediators are thought to have effects on energy balance beyond those on physical activity (31, 38). The connections among these hypothalamic neuronal groups are still being defined, but importantly, effects on ambulatory activity induced by leptin signaling can be separated from effects of leptin on food intake (7, 16, 26), suggesting that distinct neuronal pathways are involved.
From our evaluation of several candidate hypothalamic mediators, urocortin and orexin emerged as potential mediators of the effects of leptin on physical activity. In leptin-treated ob/ob mice fed ad libitum, we showed that hypothalamic levels of mRNA encoding urocortin were increased by leptin in a dose-dependent manner and that changes in urocortin mRNA expression were in turn correlated with leptin effects on ambulatory activity. Moreover, urocortin and orexin were the only candidate hypothalamic mediators for which mRNA expression increased with fasting and was reduced by leptin replacement in fasting animals, a complex pattern that also characterized changes of physical activity. For all the other candidate neuropeptide genes examined, correlations were not detected between hypothalamic mRNA levels, leptin treatment condition, and activity outcomes. One caveat to the interpretation of these data pertains to the method of tissue collection. We evaluated mRNA expression within a mediobasal hypothalamic wedge, which captures many discrete hypothalamic nuclei, and this approach differs from that employed in previous studies. For example, fasting reportedly increased Bsx mRNA expression selectively in the arcuate nucleus, and this effect was reversed by leptin treatment, with no effects observed in the dorsomedial nucleus or lateral hypothalamic area (30). Future studies, including selective inhibition of leptin signaling, are warranted to determine whether hypothalamic urocortin or orexin signaling is necessary or sufficient to explain acute leptin-mediated regulation of physical activity.
As we have shown (18), physiological leptin replacement increases TEE, in agreement with studies involving either pharmacological leptin administration in normal mice or physiological leptin replacement in fasting mice (10, 14, 27). This effect is similar to that observed in clinical studies of weight-reduced human subjects, where physiological leptin replacement has been shown to increase TEE (35). In ob/ob mice, ambulatory activity was increased by leptin treatment at all doses, suggesting that both increased AEE and increased REE contribute to leptin-mediated increases of TEE. The degree to which increased AEE contributed to increased TEE is difficult to determine in this study, because estimating REE is confounded by episodes of profound, torpor-like hypometabolism observed during the light cycle (10, 32). Determining the relative contribution of REE and AEE to changes in TEE in fasted wild-type and ob/ob mice is even more challenging, since leptin replacement increased TEE in all groups of fasted mice despite consistently decreasing ambulatory activity. Thus, although the increase in TEE may have been greater had activity not decreased, our data clearly suggest that the effect of leptin to increase REE trumps the effect to decrease AEE. However, further work is needed to precisely define the relationship between the effect of leptin to regulate physical activity and the effect on AEE.
In conclusion, we report that physiological variation in leptin levels regulates both ambulatory activity and wheel running in mice. In fed ob/ob mice, leptin acutely increases wheel running and increases ambulatory activity in a dose-dependent manner, and this effect is not dependent on reduced body weight. In both wild-type and ob/ob mice, fasting increases ambulatory activity and wheel running, and physiological leptin replacement acutely decreases this response in both genotypes. Taken together, our data show that spontaneous physical activity, like food intake and thermogenesis, is a key energy homeostasis variable regulated acutely by physiological variation in leptin signaling. This finding suggests that as-yet-unidentified leptin signaling pathways may offer pharmacological targets to alter physical activity in ways that are beneficial to the control of energy balance.
GRANTS
This work was supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases to B. E. Wisse [DK-71784 and MicroMouse 5-R01-DK-074758-02 (subcontract)] and M. W. Schwartz (DK-068384, DK-052989. and DK-083042), the Nutrition Obesity Research Center (P30-DK-035816, B. E. Wisse), and the Seattle Mouse Metabolic Phenotyping Center (U24-DK-076126) at the University of Washington. G. J. Morton is supported by an American Heart Association Scientist Development Grant.
DISCLOSURES
The authors have nothing to disclose.
ACKNOWLEDGMENTS
We acknowledge the excellent technical assistance provided by Alex Cubelo, Miles Matsen, and Iaela David.
REFERENCES
- 1. Ahima RS, Bjorbaek C, Osei S, Flier JS. Regulation of neuronal and glial proteins by leptin: implications for brain development. Endocrinology 140: 2755–2762, 1999 [DOI] [PubMed] [Google Scholar]
- 2. Armitage P, Berry G, Matthews JN. Statistical Methods in Medical Research. New York: Blackwell Science, 2002 [Google Scholar]
- 3. Castañeda TR, Jürgens H, Wiedmer P, Pfluger P, Diano S, Horvath TL, Tang-Christensen M, Tschöp MH. Obesity and the neuroendocrine control of energy homeostasis: the role of spontaneous locomotor activity. J Nutr 135: 1314–1319, 2005 [DOI] [PubMed] [Google Scholar]
- 4. Catania A, Delgado R, Airaghi L, Cutuli M, Garofalo L, Carlin A, Demitri MT, Lipton JM. alpha-MSH in systemic inflammation. Central and peripheral actions. Ann NY Acad Sci 885: 183–187, 1999 [DOI] [PubMed] [Google Scholar]
- 5. Choi YH, Li C, Hartzell DL, Little DE, Della-Fera MA, Baile CA. ICV leptin effects on spontaneous physical activity and feeding behavior in rats. Behav Brain Res 188: 100–108, 2008 [DOI] [PubMed] [Google Scholar]
- 6. Clark LD, Gay PE. Activity and body-weight relationships in genetically obese animals. Biol Psychiatry 4: 247–250, 1972 [PubMed] [Google Scholar]
- 7. Coppari R, Ichinose M, Lee CE, Pullen AE, Kenny CD, McGovern RA, Tang V, Liu SM, Ludwig T, Chua SC, Jr, Lowell BB, Elmquist JK. The hypothalamic arcuate nucleus: a key site for mediating leptin's effects on glucose homeostasis and locomotor activity. Cell Metab 1: 63–72, 2005 [DOI] [PubMed] [Google Scholar]
- 8. Dauncey MJ. Activity-induced thermogenesis in lean and genetically obese (ob/ob) mice. Experientia 42: 547–549, 1986 [DOI] [PubMed] [Google Scholar]
- 9. Dauncey MJ, Brown D. Role of activity-induced thermogenesis in twenty-four hour energy expenditure of lean and genetically obese (ob/ob) mice. Q J Exp Physiol 72: 549–559, 1987 [DOI] [PubMed] [Google Scholar]
- 10. Döring H, Schwarzer K, Nuesslein-Hildesheim B, Schmidt I. Leptin selectively increases energy expenditure of food-restricted lean mice. Int J Obes Relat Metab Disord 22: 83–88, 1998 [DOI] [PubMed] [Google Scholar]
- 11. Duclos M, Gatti C, Bessiere B, Mormede P. Tonic and phasic effects of corticosterone on food restriction-induced hyperactivity in rats. Psychoneuroendocrinology 34: 436–445, 2009 [DOI] [PubMed] [Google Scholar]
- 12. Fulton S, Woodside B, Shizgal P. Modulation of brain reward circuitry by leptin. Science 287: 125–128, 2000 [DOI] [PubMed] [Google Scholar]
- 13. Girard I, Rezende EL, Garland T., Jr Leptin levels and body composition of mice selectively bred for high voluntary locomotor activity. Physiol Biochem Zool 80: 568–579, 2007 [DOI] [PubMed] [Google Scholar]
- 14. Halaas JL, Boozer C, Blair-West J, Fidahusein N, Denton DA, Friedman JM. Physiological response to long-term peripheral and central leptin infusion in lean and obese mice. Proc Natl Acad Sci USA 94: 8878–8883, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Huang QH, Entwistle ML, Alvaro JD, Duman RS, Hruby VJ, Tatro JB. Antipyretic role of endogenous melanocortins mediated by central melanocortin receptors during endotoxin-induced fever. J Neurosci 17: 3343–3351, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Huo L, Gamber K, Greeley S, Silva J, Huntoon N, Leng XH, Bjørbaek C. Leptin-dependent control of glucose balance and locomotor activity by POMC neurons. Cell Metab 9: 537–547, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jürgens HS, Schürmann A, Kluge R, Ortmann S, Klaus S, Joost HG, Tschöp MH. Hyperphagia, lower body temperature, and reduced running wheel activity precede development of morbid obesity in New Zealand obese mice. Physiol Genomics 25: 234–241, 2006 [DOI] [PubMed] [Google Scholar]
- 18. Kaiyala KJ, Morton GJ, Leroux BG, Ogimoto K, Wisse B, Schwartz MW. Identification of body fat mass as a major determinant of metabolic rate in mice. Diabetes 59: 1657–1666, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Knight ZA, Hannan KS, Greenberg ML, Friedman JM. Hyperleptinemia is required for the development of leptin resistance. PLoS One 5: e11376, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kotz CM. Integration of feeding and spontaneous physical activity: role for orexin. Physiol Behav 88: 294–301, 2006 [DOI] [PubMed] [Google Scholar]
- 21. Levine JA, Lanningham-Foster LM, McCrady SK, Krizan AC, Olson LR, Kane PH, Jensen MD, Clark MM. Interindividual variation in posture allocation: possible role in human obesity. Science 307: 584–586, 2005 [DOI] [PubMed] [Google Scholar]
- 22. Lipton JM, Catania A. Mechanisms of antiinflammatory action of the neuroimmunomodulatory peptide alpha-MSH. Ann NY Acad Sci 840: 373–380, 1998 [DOI] [PubMed] [Google Scholar]
- 23. Mathes WF, Nehrenberg DL, Gordon R, Hua K, Garland T, Jr, Pomp D. Dopaminergic dysregulation in mice selectively bred for excessive exercise or obesity. Behav Brain Res 210: 155–163, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McLean J, Tobin G. Animal and Human Calorimetry. New York: Cambridge University, 1987 [Google Scholar]
- 25. McMinn JE, Baskin DG, Schwartz MW. Neuroendocrine mechanisms regulating food intake and body weight. Obes Rev 1: 37–46, 2000 [DOI] [PubMed] [Google Scholar]
- 26. Mesaros A, Koralov SB, Rother E, Wunderlich FT, Ernst MB, Barsh GS, Rajewsky K, Bruning JC. Activation of Stat3 signaling in AgRP neurons promotes locomotor activity. Cell Metab 7: 236–248, 2008 [DOI] [PubMed] [Google Scholar]
- 27. Montez JM, Soukas A, Asilmaz E, Fayzikhodjaeva G, Fantuzzi G, Friedman JM. Acute leptin deficiency, leptin resistance, and the physiologic response to leptin withdrawal. Proc Natl Acad Sci USA 102: 2537–2542, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Morrison CD, Morton GJ, Niswender KD, Gelling RW, Schwartz MW. Leptin inhibits hypothalamic Npy and Agrp gene expression via a mechanism that requires phosphatidylinositol 3-OH-kinase signaling. Am J Physiol Endocrinol Metab 289: E1051–E1057, 2005 [DOI] [PubMed] [Google Scholar]
- 29. Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature 443: 289–295, 2006 [DOI] [PubMed] [Google Scholar]
- 30. Nogueiras R, López M, Lage R, Perez-Tilve D, Pfluger P, Mendieta-Zerón H, Sakkou M, Wiedmer P, Benoit SC, Datta R, Dong JZ, Culler M, Sleeman M, Vidal-Puig A, Horvath T, Treier M, Diéguez C, Tschöp MH. Bsx, a novel hypothalamic factor linking feeding with locomotor activity, is regulated by energy availability. Endocrinology 149: 3009–3015, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Novak CM, Levine JA. Central neural and endocrine mechanisms of non-exercise activity thermogenesis and their potential impact on obesity. J Neuroendocrinol 19: 923–940, 2007 [DOI] [PubMed] [Google Scholar]
- 32. Overton JM, Williams TD. Behavioral and physiologic responses to caloric restriction in mice. Physiol Behav 81: 749–754, 2004 [DOI] [PubMed] [Google Scholar]
- 33. Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T, Collins F. Effects of the obese gene product on body weight regulation in ob/ob mice. Science 269: 540–543, 1995 [DOI] [PubMed] [Google Scholar]
- 34. Rosenbaum M, Goldsmith R, Bloomfield D, Magnano A, Weimer L, Heymsfield S, Gallagher D, Mayer L, Murphy E, Leibel RL. Low-dose leptin reverses skeletal muscle, autonomic, and neuroendocrine adaptations to maintenance of reduced weight. J Clin Invest 115: 3579–3586, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rosenbaum M, Nicolson M, Hirsch J, Murphy E, Chu F, Leibel RL. Effects of weight change on plasma leptin concentrations and energy expenditure. J Clin Endocrinol Metab 82: 3647–3654, 1997 [DOI] [PubMed] [Google Scholar]
- 36. Sakkou M, Wiedmer P, Anlag K, Hamm A, Seuntjens E, Ettwiller L, Tschöp MH, Treier M. A role for brain-specific homeobox factor Bsx in the control of hyperphagia and locomotory behavior. Cell Metab 5: 450–463, 2007 [DOI] [PubMed] [Google Scholar]
- 37. Slawecki CJ, Somes C, Rivier JE, Ehlers CL. Neurophysiological effects of intracerebroventricular administration of urocortin. Peptides 20: 211–218, 1999 [DOI] [PubMed] [Google Scholar]
- 38. Teske JA, Billington CJ, Kotz CM. Neuropeptidergic mediators of spontaneous physical activity and non-exercise activity thermogenesis. Neuroendocrinology 87: 71–90, 2008 [DOI] [PubMed] [Google Scholar]
- 39. Tinsley FC, Taicher GZ, Heiman ML. Evaluation of a quantitative magnetic resonance method for mouse whole body composition analysis. Obes Res 12: 150–160, 2004 [DOI] [PubMed] [Google Scholar]
- 40. Williams G, Cai XJ, Elliott JC, Harrold JA. Anabolic neuropeptides. Physiol Behav 81: 211–222, 2004 [DOI] [PubMed] [Google Scholar]
- 41. Wisse BE, Ogimoto K, Morton GJ, Wilkinson CW, Frayo RS, Cummings DE, Schwartz MW. Physiological regulation of hypothalamic IL-1β gene expression by leptin and glucocorticoids: implications for energy homeostasis. Am J Physiol Endocrinol Metab 287: E1107–E1113, 2004 [DOI] [PubMed] [Google Scholar]