Abstract
When supply arteries become occluded, blood is diverted through preexisting collateral vessels. Shear stress arising from this increase in blood flow provides the initial physiological stimulus for expansion of the collateral circulation, a process termed arteriogenesis. Endothelial cells (EC) respond to increased shear stress by releasing a variety of mediators that can act on underlying smooth muscle cells (SMC). Placenta growth factor (PLGF) is known to mediate certain aspects of arteriogenesis, such as recruitment of monocytes to the vessel wall. Therefore, we tested whether SMC PLGF expression is influenced by mediators released by EC. We used A10 SMC cultured with medium that had been conditioned by EOMA EC for 4 days as a model. We found that EC-conditioned medium is able to upregulate PLGF gene expression in A10 SMC. Further experiments identified hydrogen peroxide (H2O2) as a key mediator of this response. We confirmed the physiological relevance of this mechanism in primary human coronary artery SMCs by demonstrating that exogenous H2O2 specifically upregulates PLGF gene and protein expression. We also demonstrated that the physiological stimulus of shear stress raises endogenous H2O2 levels in media into the range found to increase PLGF expression. In this study, we demonstrate that EC-released H2O2 acts as a positive regulator of PLGF gene and protein expression in vascular SMC. To our knowledge, this is the first study to describe H2O2 as a regulator of PLGF expression and therefore an upstream mediator of PLGF-driven arteriogenesis.
Keywords: reactive oxygen species, arteriogenesis, angiogenesis, vascular endothelial growth factor
arteriogenesis, the process of expansion of collateral vessels in response to an arterial occlusion, improves tissue survival by restoring blood flow to the affected area (11). Placenta growth factor (PLGF) is a vascular endothelial growth factor (VEGF)-related protein. PLGF and VEGF-A are structurally similar; however, they have different receptor binding specificities and thus have marked functional differences. In particular, PLGF has been shown to cause arteriogenesis (15, 18, 28) via its ability to attract monocytes (7, 24, 30), whereas VEGF-A induces capillary proliferation (angiogenesis) via effects on endothelial cell proliferation and migration.
Early studies suggested that PLGF mainly mediates arteriogenesis in response to ischemia or tissue damage, whereas PLGF gene knockout appears to have little effect on vascular development (4). PLGF has also been linked with inflammatory angiogenesis in a variety of disease settings, including sickle cell anemia, atherosclerosis, cystic fibrosis, and rheumatoid arthritis (22, 23, 35, 38). In addition, PLGF has been implicated in tumor neovascularization; however, the role of PLGF in cancer remains controversial. PLGF expression is associated with reduced survival in patients with certain types of cancer, including malignant pleural mesothelioma, oral squamous cell carcinoma, and hepatocellular carcinoma (5, 25, 36). However, studies on the effect of PLGF inhibition on tumor growth in animal models and in vitro systems have yielded conflicting results (for example, see Refs. 2 and 34).
Although PLGF was first identified in placental tissue (16), we and others have shown that it is also expressed in adult vasculature (27). Within the vasculature, PLGF is expressed by both endothelial cells (EC) and smooth muscle cells (SMC) (28). As discussed above, the ability of PLGF to induce arteriogenesis is dependent on the recruitment of monocytes to the vascular wall. Since the vascular wall is largely composed of SMC, we hypothesized that SMC PLGF expression may have an important role in arteriogenesis.
As a vessel becomes occluded, blood is diverted through preexisting collaterals. Shear stress arising from this increase in blood flow is the initial physiological stimulus for arteriogenesis. EC respond to increased shear stress by releasing a variety of soluble mediators that could potentially transduce the shear signal to the underlying smooth muscle. Thus we further hypothesized that SMC PLGF expression is influenced by mediators released by EC. This hypothesis is supported by the observation that ligation of the femoral artery (which produces an increase in shear stress through downstream collaterals) can elevate PLGF expression in collateral arteries in the absence of any additional arteriogenic stimulus, such as exercise training (27). The putative shear-dependent regulatory mechanism influencing PLGF in this setting remains undefined. However, mediators known to be produced by EC in response to shear stress include nitric oxide (NO) [which relaxes smooth muscle (20) and functions as a prerequisite for arteriogenesis (11, 14)], prostacyclin (PGI2) (19), and hydrogen peroxide (H2O2) (8, 10).
Although our understanding of arteriogenesis and PLGF has increased over recent years, very little is known about the regulation of PLGF expression, particularly in vascular smooth muscle. To test our hypothesis that EC release mediators that influence SMC PLGF expression, we used A10 SMC cultured with medium that had been conditioned by EOMA EC for 4 days as a model. We found that EC-conditioned medium is able to upregulate PLGF gene expression in A10 SMC. Further experiments identified H2O2 as a key mediator of this response. We confirmed the physiological relevance of this mechanism in primary human coronary artery smooth muscle cells (CASMC), in which both PLGF mRNA and protein were specifically increased by H2O2 treatment. Finally, we showed that the physiological stimulus of shear stress elevates H2O2 in media to levels that can effectively induce PLGF expression.
In this study, we demonstrate that EC-released H2O2 acts as a positive regulator of PLGF gene and protein expression in vascular SMC. To our knowledge, this is the first study to describe H2O2 as a regulator of PLGF expression and therefore an upstream mediator of PLGF-driven arteriogenesis.
MATERIALS AND METHODS
Cell lines.
Vascular SMC (A10) and EC (EOMA) were purchased from American Type Culture Collection (Manassas, VA). Cells were grown in a humidified incubator (5% CO2) in Dulbecco's modified Eagle's medium (DMEM, Invitrogen, Carlsbad, CA) containing 5–10% fetal bovine serum (FBS, Invitrogen) and 1% penicillin-streptomycin (Invitrogen) until confluent. Culture medium was changed every 2–3 days. Cells were used for experiments between passages 6 and 13. Confluent A10 cells were serum starved (1% FBS, DMEM) for 48 h before treatments.
Primary cells.
Human CASMC were purchased from Lonza (Walkersville, MD). Cells were grown in a humidified incubator containing 5% CO2 in Smooth Muscle Cell Basal Medium (SmBM, Lonza) plus Smooth Muscle Growth Medium (SmGM-2 SingleQuots, Lonza) until confluent. Culture medium was changed every other day. Cells were used for experiments between passages 4 and 8. Confluent CASMC were serum starved (1% FBS, DMEM) for 48 h before treatments.
Conditioned medium.
Culture medium was conditioned by allowing standard DMEM culture medium to remain in flasks containing confluent EOMA endothelial cells for 4 days.
Gene expression.
At the conclusion of experimental treatment, culture medium was aspirated and cells were rinsed briefly in Dulbecco's phosphate-buffered saline (D-PBS, Invitrogen). Total RNA was then extracted using TRIzol reagent (Invitrogen). RNA was assayed for quality and quantity in a Nanodrop spectrophotometer (Thermo Scientific, Waltham, MA), treated to remove genomic DNA (Turbo DNAFree, Ambion, Austin, TX), and converted to cDNA (qScript cDNA SuperMix, Quanta BioSciences, Gaithersburg, MD). Real-time PCR was performed on an ABI 7500 Fast instrument for quantification of gene expression. Primers for rat and human PLGF and β-actin were designed using Primer Express and custom synthesized (Invitrogen). Primer sequences: rat PLGF forward 5′-CTGCTGGGAACAACTCAACAGA-3′; rat PLGF reverse 5′-GCGGCCCCACACTTCATT-3′; human PLGF forward 5′-CCTACGTGGAGCTGACGTTCT-3′; human PLGF reverse 5′-TCCTTTCCGGCTTCA TCTTCT-3′; human VEGF-A forward 5′-ACGAGGGCCTGGAGTGTGT-3′; human VEGF-A reverse 5′-GATCCGCATAATCTGCATGGT-3′. PCR product formation was detected using SYBR Green chemistry (PerfeCTa SYBR Green FastMix, Low ROX) (Quanta BioSciences). PLGF and VEGF gene expression was normalized to β-actin.
Glucose assay.
Glucose concentration in culture medium was assayed enzymatically in a spectrophotometer. Ten microliters of culture medium were added to cuvettes containing 610 μl of glucose assay buffer (1 mM MgCl2, 25 mM Tris base, 0.5 mM dithiothreitol, 1.5 mM ATP, 0.3 U/ml glucose 6-phosphate dehydrogenase, 0.25 mM NADP, and 1 U/ml hexokinase; pH 8.1) (all reagents from Sigma, St. Louis, MO). Cuvettes were then incubated at 37°C for 1 h. Absorbance was measured at 340 nm and compared with a standard curve for calculation of glucose concentration.
Lactate assay.
Media lactate concentration was assayed enzymatically. Twenty microliters of culture media were added to cuvettes containing 980 μl of lactate assay buffer (160 mM hydrazine, 400 mM glycine, 0.625 mM NAD, and 20 U/ml lactate dehydrogenase; pH 9.0) (all reagents from Sigma). Cuvettes were then incubated at 37°C for 1 h. Absorbance was measured at 340 nm and compared with a standard curve for calculation of lactate concentration.
Exogenous glucose and lactate.
For studies on the effects of exogenous glucose and lactate, glucose-free DMEM (Sigma) was prepared and sterile-filtered. Stock solutions of glucose and lactate in H2O were prepared, syringe filtered, and added to DMEM to give final concentrations of glucose ranging from 0 to 20 mM and final concentrations of lactate ranging from 0 to 10 mM. A10 SMC were then exposed to the prepared media for 4 h, followed by RNA extraction and real-time PCR as described above.
NO donor treatment.
The NO donor sodium nitroprusside (SNP) (Sigma) was used to assess the effect of NO on PLGF expression in A10 SMC. A stock solution of SNP (100 mM) was made in PBS and then added to A10 culture vessels containing fresh culture medium to reach the final desired concentration. PBS alone was used as a vehicle control. Cells were incubated with vehicle or SNP for 4 h before total RNA was harvested for real-time PCR as above.
Prostacyclin treatment.
A stock solution of the prostacyclin analog beraprost (Cayman Chemical) was made in DMSO. Stock solution was then added to A10 culture vessels containing fresh culture medium to reach the final desired beraprost concentration (100 nM). DMSO alone was added to control culture vessels. Cells were incubated with vehicle or beraprost for 4 h before total RNA was harvested for real-time PCR as above.
Peroxide assay.
Peroxide in culture medium was assayed spectrophotometrically using the Peroxoquant kit (Pierce, Rockford, IL) according to manufacturer's instructions.
Peroxide treatment.
A10 SMC were serum starved for 48 h and then treated with varying doses of H2O2 (1–100 μM) for 4 h. Doses of >100 μM H2O2 were found to be cytotoxic (data not shown). RNA was harvested for real-time PCR as described above to determine whether upregulation of PLGF by H2O2 was dose dependent. A time course was performed on A10 SMC using 50 μM H2O2 (4 and 8 h data presented here). For treatment of primary cells, human CASMC were serum starved for 48 h followed by the addition of 50 μM H2O2 for 4–8 h.
ELISA.
Medium was collected from culture vessels containing human CASMC following treatment with 50 μM H2O2 for 8 h. To protect the secreted PLGF or VEGF-A protein from degradation during sample processing, protease inhibitor cocktail (Sigma) was added (1:250) at the end of the 8-h H2O2 treatment. Media was then concentrated using molecular weight cutoff filters (Icon, 7 ml/9 K, Pierce). Total protein was quantified using the BCA Protein Assay (Pierce). PLGF and VEGF-A ELISAs was performed on the concentrated media using the Quantikine Human PLGF and VEGF-A ELISA kits (R&D Systems, Minneapolis, MN) to measure levels of the secreted proteins.
Shear stress.
EOMA cells were grown until confluence in standard six-well culture plates, serum-starved for 48 h, and then exposed to shear stress in a custom-built dynamic cone and plate shearing device (37). The apparatus was designed to shear cells grown in a six-well culture plate. It consists of four high-density polyethylene cones attached to stepper motors. The motors were controlled by an Allegra two-axis programmable stepper motor control system (Optimal Engineering Systems, Los Angeles, CA). Shear stress applied to the cells was controlled by adjusting the speed of the cones. The center wells of the six-well plate were not fitted with cones and served as paired static control samples. Cells were exposed to either constant shear of 5 dyn/cm2 or oscillatory shear of ± 5 dyn/cm2 for 2 h, after which media was collected for analysis of H2O2 levels as described above.
Statistical analyses.
All data are expressed as means ± SE. A Mann-Whitney rank sum test was used to identify significant differences in gene expression and lactate concentration between control and conditioned-media treated cells and to identify significant effects of beraprost treatment. Differences in glucose concentration between control and conditioned media were analyzed by t-test. The effects of glucose and lactate on PLGF were analyzed by one-way ANOVA. The effect of SNP on PLGF and the effect of shear stress on peroxide levels in EOMA cell media were analyzed by one-way ANOVA on ranks. Differences in peroxide levels between control and conditioned media were analyzed by t-test as well as differences between A10 and CASMC PLGF and VEGF-A gene expression and CASMC PLGF and VEGF-A protein expression following peroxide treatment. SigmaStat 3.5 (Systat) was used for all statistical analyses. Differences were considered significant at P < 0.05.
RESULTS
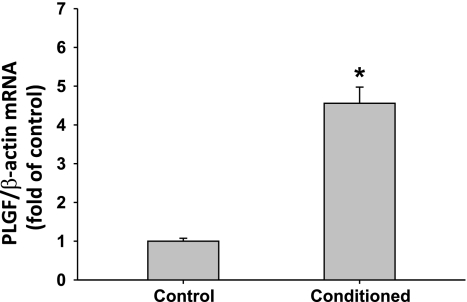
Treatment of A10 SMC for 4 h with medium preconditioned for 4 days by EOMA EC strongly upregulated PLGF gene expression to 4.56 ± 0.42-fold of the level in untreated cells (Fig. 1; P < 0.001). The increase in PLGF expression was transient and returned to baseline by 24 h (data not shown).
Fig. 1.
Treatment of A10 smooth muscle cells (SMC) with EOMA-conditioned medium increases placenta growth factor (PLGF) mRNA expression. PLGF mRNA expression is increased 4.56 ± 0.42-fold after 4 h of exposure to media conditioned by EOMA endothelial cells (EC, n = 9) when compared with expression in A10 cells receiving fresh media (n = 8). *P < 0.001 (Mann-Whitney rank sum test).
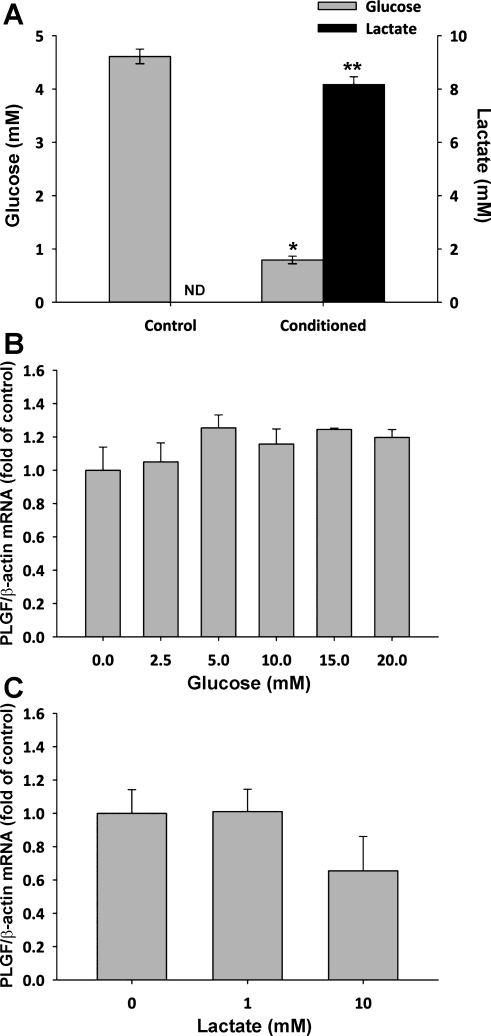
Analysis of the EOMA-conditioned medium showed that glucose was depleted from a starting concentration of 4.61 ± 0.14 to 0.79 ± 0.07 mM during the conditioning phase. There was a concomitant increase in lactate from undetectable levels to 8.17 ± 0.29 mM (Fig. 2A; P < 0.01 for both). To determine whether glucose depletion contributed to increased PLGF expression following conditioned medium treatment, cultured A10 SMC were exposed to medium containing 0–20 mM glucose for 4 h. Glucose concentration had no significant effect on PLGF mRNA levels over this range (Fig. 2B). Likewise, treatment of A10 SMC with exogenous lactate (0–10 mM) also had no significant effect on PLGF gene expression (Fig. 2C).
Fig. 2.
Glucose is reduced and lactate is elevated in EOMA-conditioned medium, but exogenous glucose or lactate have no effect on PLGF mRNA expression. A: glucose was consumed and lactate was produced by EOMA cells during the conditioning phase, resulting in decreased glucose levels and increased lactate levels in conditioned media (n = 7) relative to fresh media (n = 5). *P < 0.001 (t-test); **P < 0.01 (Mann-Whitney rank sum test). ND, not detected. B: exogenous glucose (0–20 mM, 4 h) had no effect on PLGF gene expression by A10 SMC (n = 3/group). P = NS for all (ANOVA). C: exogenous lactate (0–10 mM, 4 h) had no effect on PLGF gene expression by A10 SMC (n = 3–5/group). P = NS for all (ANOVA).
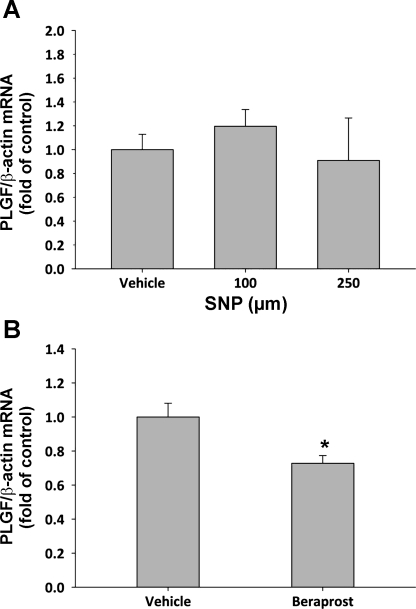
Since the effect of EOMA-conditioned medium on PLGF expression in A10 cells suggested that soluble EC-derived mediators could regulate PLGF, we next tested the effect of the endothelial-derived mediators NO and prostaglandin I2 (PGI2; prostacyclin) on PLGF expression. A10 SMC were treated with the NO donor sodium nitroprusside (SNP; 100 and 250 μM). SNP treatment did not significantly affect PLGF gene expression in A10 SMC at either concentration tested (Fig. 3A). Interestingly, the prostacyclin analog beraprost (100 nM) significantly inhibited PLGF gene expression (Fig. 3B; P < 0.05). Thus neither NO nor prostacyclin appears to mediate the increased PLGF expression seen following treatment of A10 SMC with EOMA-conditioned medium.
Fig. 3.
Neither nitric oxide (NO) nor prostacyclin upregulate PLGF mRNA expression. A: NO donor sodium nitroprusside (SNP) had no effect on PLGF gene expression at either 100 or 250 mM. n = 6 (vehicle, 100 μM), n = 3 (250 μM). P = NS for all (ANOVA on ranks). B: prostacyclin analog beraprost (100 nM) significantly inhibited PLGF gene expression relative to untreated cells (n = 6 for both groups). *P < 0.01 (ANOVA).
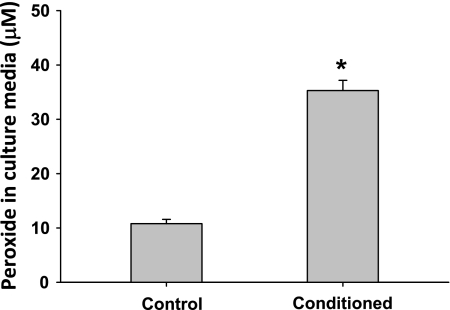
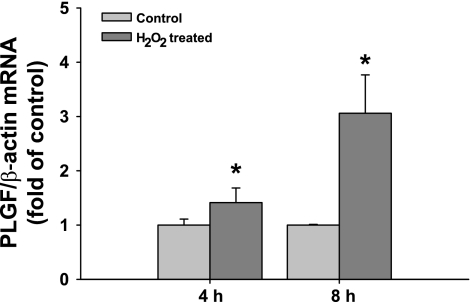
Further analysis of EOMA-conditioned medium revealed that H2O2 levels were significantly increased following the conditioning phase, relative to fresh medium (Fig. 4, P < 0.001). To assess whether H2O2 regulates PLGF gene expression, exogenous H2O2 (50 μM) was added to culture vessels containing A10 cells. RNA was isolated following 4 and 8 h H2O2 treatment for real-time PCR. Peroxide treatment increased PLGF gene expression to 1.53 ± 0.18-fold of control at 4 h and 3.08 ± 0.90-fold of control at 8 h. (Fig. 5, P < 0.05). The upregulation of PLGF was transient and returned to baseline by 24 h (data not shown).
Fig. 4.
H2O2 levels are significantly elevated in EOMA-conditioned medium. EOMA-conditioned medium was collected, and H2O2 levels were measured. EOMA-conditioned medium contained significantly elevated levels of H2O2 relative to fresh medium (n = 3). *P < 0.001 (t-test).
Fig. 5.
PLGF mRNA expression is significantly increased in A10 SMC following treatment with exogenous H2O2. Serum-starved A10 SMC were treated with 50 μM H2O2 for 4 or 8 h. PLGF gene expression increased to 1.53 ± 0.18-fold of control at 4 h and 3.08 ± 0.90-fold of control at 8 h (n = 3) *P < 0.05 (t-test).
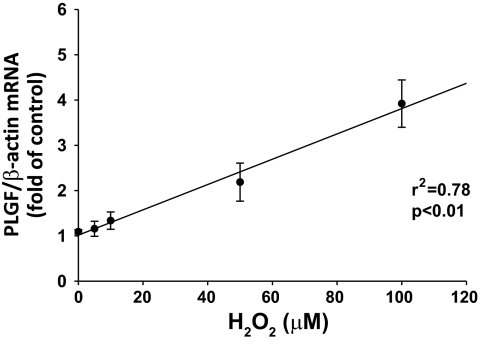
To determine whether the response of SMC to H2O2 was dose dependent, SMC were treated with exogenous H2O2 at concentrations ranging from 1 to 100 μM. PLGF gene expression was dose-dependently upregulated by H2O2 treatment (Fig. 6, R2 = 0.78, P < 0.01). Doses of >100 μM H2O2 were found to be cytotoxic (data not shown), consistent with other studies (9).
Fig. 6.
Upregulation of PLGF by H2O2 in A10 SMC is dose dependent. A10 SMC were treated with exogenous H2O2 at concentrations ranging from 1 to 100 μM. PLGF gene expression was dose dependently upregulated by H2O2 treatment (n = 4).
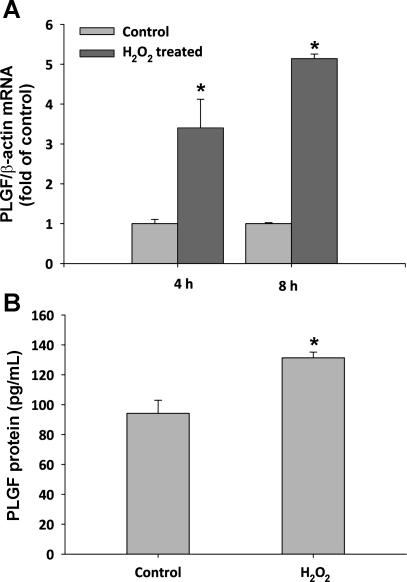
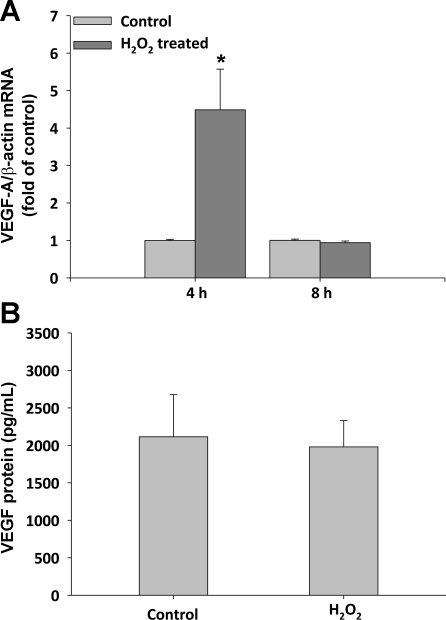
Since the A10 SMC line is well-established in culture and has a less-differentiated SMC phenotype than primary SMC, we next treated primary CASMC with H2O2 to determine whether this regulatory mechanism is operative in differentiated SMC. Human CASMC were treated with 50 μM exogenous H2O2 for either 4 or 8 h. PLGF gene expression increased to 3.4 ± 0.7-fold of control (at 4 h) and 5.1 ± 0.1-fold of control (at 8 h) in H2O2-treated CASMC, compared with untreated CASMC (Fig. 7A, P < 0.05 for both time points). As observed in A10 SMC, PLGF upregulation was transient and returned to baseline by 24 h (data not shown). To determine whether the effect of H2O2 on PLGF gene expression was specific, we also measured VEGF-A mRNA levels. Peroxide increased VEGF-A gene expression to 4.5 ± 1.1-fold of control at 4 h; however, VEGF-A expression had returned to control by 8 h of treatment (1.0 ± 0.4-fold of control), whereas PLGF expression remained elevated at the 8-h time point (Fig. 8A). To determine whether the upregulation of PLGF gene transcription produces a corresponding increase in PLGF protein, we measured PLGF protein levels in culture medium from flasks containing human CASMC that had been treated with 50 μM exogenous H2O2 for 8 h. PLGF protein expression was slightly, but significantly increased by H2O2 treatment, from 91 to 131 pg/ml (Fig. 7B, P < 0.05). ELISA confirmed that VEGF-A protein was not significantly increased 8 h post-H2O2 (Fig. 8B).
Fig. 7.
PLGF gene and protein expression is increased in response to H2O2 in primary human CASMC. A: serum-starved human coronary artery SMC (CASMC) were treated with 50 μM H2O2 for 4 and 8 h. PLGF gene expression increased to 3.4 ± 0.7-fold of control (4 h) and 5.1 ± 0.1-fold of control (8 h) (n = 3). *P < 0.05 vs. time-matched control (t-test). B: human CASMC were treated with 50 μM exogenous H2O2 for 8 h. PLGF protein levels in media increased from 91 to 131 pg/ml following H2O2 treatment (n = 3). *P < 0.05 (t-test).
Fig. 8.
Vascular endothelial growth factor (VEGF)-A gene and protein expression is not different from control 8 h post-H2O2 treatment. A: serum-starved human CASMC were treated with 50 μM H2O2 for 4 or 8 h. VEGF-A gene expression increased to 4.5 ± 1.1-fold of control at 4 h but was not significantly different from control at 8 h (1.0 ± 0.04) (n = 3). *P < 0.05 vs. time-matched control (t-test). B: human CASMC were treated with 50 μM exogenous H2O2 for 8 h. VEGF-A protein levels in media were not significantly different between treatment groups (n = 3).
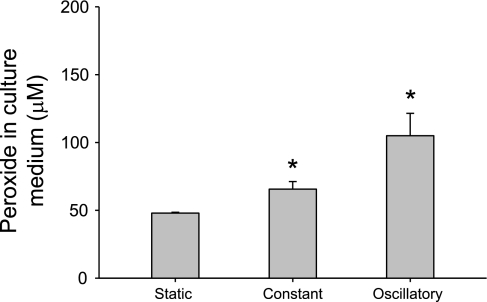
Finally, to determine whether a physiological stimulus (shear stress) could potentially activate this signaling mechanism, we exposed EOMA endothelial cells to two different shear stress patterns using a cone and plate shearing device. After 2 h of exposure, both constant shear of 5 dyn/cm2 and oscillatory shear of ± 5 dyn/cm2 significantly increased peroxide levels in EOMA cell media (static control, 47.9 ± 0.6 μM; constant shear, 65.6 ± 5.6 μM; oscillatory shear, 105.0 ± 16.5 μM, N = 8/group) (Fig. 9, P < 0.05 vs. static control). Thus brief exposures to low levels of shear stress increased H2O2 production by endothelial cells to levels that our data show are sufficient to elevate PLGF expression in vascular smooth muscle cells.
Fig. 9.
Shear stress-stimulated EC H2O2 production is within the range of concentrations capable of inducing SMC PLGF expression in vitro. EOMA EC were exposed to static conditions, constant shear stress (5 dyn/cm2), or oscillatory shear stress (± 5 dyn/cm2) in a cone and plate apparatus for 2 h, followed by measurement of H2O2 in culture medium. H2O2 was significantly increased by both constant and oscillatory shear, relative to static control (static control, 47.9 ± 0.6 μM; constant shear, 65.6 ± 5.6 μM; oscillatory shear, 105.0 ± 16.5 μM, n = 8/group). *P < 0.05 vs. static control (ANOVA on ranks followed by Tukey's t-test).
DISCUSSION
In this report we present the first evidence identifying H2O2 as a novel EC-released, positive regulator of PLGF expression in vascular SMCs. Physiological concentrations of H2O2 in the vasculature in vivo are estimated to range between 0.1 and 60 μM (13, 26, 31). Our data suggest that H2O2, at a level that reflects mild oxidative stress, functions as a physiological signaling molecule that can transduce a shear signal from the endothelium to the underlying smooth muscle to promote arteriogenesis via upregulation of PLGF. Our data show an acute, early response of PLGF expression to H2O2. The response was specific, as shown by continued upregulation of PLGF, but not the related protein VEGF-A, at 8 h post-H2O2 treatment. PLGF levels were no longer elevated 24 h post-H2O2 (data not shown). There are two potential explanations for this lack of sustained response. First, it is possible that H2O2 has a transient effect on PLGF levels and that this mechanism contributes to acute regulation of PLGF expression. Transient upregulation of PLGF could set the arteriogenic sequence of events in motion by favoring recruitment of monocytes to the vessel wall, where they can release additional arteriogenic mediators. As-yet-unknown negative feedback mechanisms may then act to return PLGF to normal levels in the absence of additional stimulatory inputs. If upregulation of PLGF by H2O2 is transient, sustained reactive oxygen species generation may not enhance PLGF expression.
A second, alternative explanation is that the H2O2 signal may have been lost at time points >8 h. Indeed, analysis of culture media 8 h post-H2O2 treatment showed that H2O2 levels in media had returned to control (data not shown). It is clear that an endothelium-dependent mechanism for sustained upregulation of PLGF in remodeling collateral arteries must exist, since PLGF gene expression remains elevated in collateral arteries downstream of an occlusion for up to 24 days in rats with hindlimb ischemia (27). In this model, an early spike in PLGF was followed by a lower, but nevertheless sustained, increase. Additional experiments with repeated H2O2 administration or H2O2-generating systems are needed to determine whether the upregulation of PLGF in vascular SMC by H2O2 is transient, or whether it can be sustained in the presence of continued low levels of reactive oxygen species.
It also remains to be seen whether pathologically high levels of reactive oxygen species (oxidative stress) stimulate or inhibit PLGF expression. Rocic et al. (29) demonstrated that coronary collateral growth is dependent on optimal levels of reactive oxygen species, with either abnormally low or abnormally high levels causing inhibition of arteriogenesis. We speculate that PLGF expression in vascular SMC existing in an environment of high oxidative stress (e.g., diabetes or hyperlipidemia) may be refractory to further activation of this novel H2O2 signaling pathway, consistent with several studies in animal models linking elevated oxidative stress to impaired arteriogenesis (3, 29). Furthermore, PLGF expression has been shown to be reduced in wounds of streptozotocin-treated diabetic mice (6) and in the placenta of streptozotocin-treated rats (12). In contrast, mounting evidence shows that lower, physiological concentrations of reactive oxygen species contribute to pro-arteriogenic signaling (1, 29, 32, 33).
In these studies we also examined other potential factors that could influence PLGF expression in our culture system and/or in vivo. There was a sharp decrease in glucose and a concomitant increase in lactate in EC-conditioned media. Thus we tested the hypothesis that glucose deprivation or an increase in lactate could mimic the onset of ischemia and induce PLGF expression. However, we found that neither glucose nor lactate (across a wide range of concentrations consistent with those found in conditioned media) had any effect on PLGF expression. Thus we conclude that changes in glucose and lactate levels are not involved in the specific regulatory pathway operating in our model system.
Likewise, and contrary to our initial expectations, neither NO nor prostacyclin appear to be involved in EC-dependent upregulation of PLGF expression in SMC in our experimental model. NO and prostacyclin are key mediators released from the endothelium in response to shear stress that work via the cGMP or cAMP pathways, respectively, to drive relaxation of vascular smooth muscle. NO and prostacyclin also have pro-arteriogenic effects (14, 17). The apparent lack of involvement of NO or prostacyclin in upregulating PLGF supports the specific nature of the signaling pathway activated by H2O2. However, it is important to note that the studies described here were done in cultured vascular cells grown under static conditions. It is possible that NO and prostacyclin may contribute to PLGF regulation in a more physiological setting, such as an intact vessel experiencing flow. Indeed, Mohammed et al. (17) found that cyclic stretch upregulated PLGF expression in bronchial airway epithelial cells via a NO-dependent mechanism. The possibility that other EC-derived mediators such as NO and prostacyclin contribute to the putative PLGF regulatory cascade induced by shear stress is an important area for future research.
Although H2O2 strongly and reproducibly upregulated PLGF gene expression in SMC, PLGF protein expression was only slightly elevated by H2O2 treatment. One possible explanation for this observation is that PLGF protein expression is only partially regulated at the transcriptional level. Posttranscriptional mechanisms such as micro-RNA regulation of PLGF mRNA may also be involved. Thus H2O2 may be necessary, but not entirely sufficient, to fully upregulate PLGF. Further studies are underway to investigate the possibility that PLGF protein expression is subject to posttranscriptional control.
We are only aware of one other study to date that identifies an EC-derived molecule that acts on vascular SMC to increase PLGF expression. Recent work by Pan et al. (21) showed an increase in PLGF expression in vascular SMC following treatment with exogenous angiotensin II via an ERK (½) and phosphoinositol-3-kinase-dependent pathway.
Finally, we assessed H2O2 production by EOMA cells exposed to constant and oscillatory shear stress to determine whether this physiological stimulus for arteriogenesis can elevate H2O2 concentrations to the range shown to be effective at upregulating PLGF expression. These experiments confirmed that the concentrations of H2O2 tested in this study are physiological.
Continued unraveling of the details of the molecular mechanism by which H2O2 elicits upregulation of PLGF expression will provide important new insights into the understanding of PLGF-driven arteriogenesis. Such insights may suggest ways in which expression of this important arteriogenic signaling protein can be stimulated or inhibited in patients who could benefit from pro-arteriogenic (ischemic cardiovascular disease) or anti-angiogenic (cancer) therapies.
GRANTS
This work was supported by The National Institutes of Health Grant R01 HL-084494 (to P. G. Lloyd).
ACKNOWLEDGMENTS
The authors thank Desiree Poore for technical contributions in this study.
REFERENCES
- 1. Angermayr B, Fernandez M, Mejias M, Gracia-Sancho J, Garcia-Pagan JC, Bosch J. NAD(P)H oxidase modulates angiogenesis and the development of portosystemic collaterals and splanchnic hyperaemia in portal hypertensive rats. Gut 56: 560–564, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bais C, Wu X, Yao J, Yang S, Crawford Y, McCutcheon K, Tan C, Kolumam G, Vernes JM, Eastham-Anderson J, Haughney P, Kowanetz M, Hagenbeek T, Kasman I, Reslan HB, Ross J, Van BN, Carano RA, Meng YJ, Hongo JA, Stephan JP, Shibuya M, Ferrara N. PlGF blockade does not inhibit angiogenesis during primary tumor growth. Cell 141: 166–177, 2010 [DOI] [PubMed] [Google Scholar]
- 3. Boodhwani M, Nakai Y, Mieno S, Voisine P, Bianchi C, Araujo EG, Feng J, Michael K, Li J, Sellke FW. Hypercholesterolemia impairs the myocardial angiogenic response in a swine model of chronic ischemia: role of endostatin and oxidative stress. Ann Thorac Surg 81: 634–641, 2006 [DOI] [PubMed] [Google Scholar]
- 4. Carmeliet P, Moons L, Luttun A, Vincenti V, Compernolle V, De Mol M, Wu Y, Bono F, Devy L, Beck H, Scholz D, Acker T, DiPalma T, Dewerchin M, Noel A, Stalmans I, Barra A, Blacher S, Vandendriessche T, Ponten A, Eriksson U, Plate KH, Foidart JM, Schaper W, Charnock-Jones DS, Hicklin DJ, Herbert JM, Collen D, Persico MG. Synergism between vascular endothelial growth factor and placental growth factor contributes to angiogenesis and plasma extravasation in pathological conditions. Nature Med 7: 575–583, 2001 [DOI] [PubMed] [Google Scholar]
- 5. Cheng SJ, Lee JJ, Kok SH, Chou CH, Chang HH, Chiang ML, Chen HM, Kuo MY, Chiang CP. Expression of placenta growth factor: an independent factor for prediction of progression and prognosis of oral cancer. Head Neck 32: 1363–1369, 2010 [DOI] [PubMed] [Google Scholar]
- 6. Cianfarani F, Zambruno G, Brogelli L, Sera F, Lacal PM, Pesce M, Capogrossi MC, Failla CM, Napolitano M, Odorisio T. Placenta growth factor in diabetic wound healing: altered expression and therapeutic potential. Am J Pathol 169: 1167–1182, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Clauss M, Weich H, Breier G, Knies U, Rockl W, Waltenberger J, Risau W. The vascular endothelial growth factor receptor Flt-1 mediates biological activities. Implications for a functional role of placenta growth factor in monocyte activation and chemotaxis. J Biol Chem 271: 17629–17634, 1996 [DOI] [PubMed] [Google Scholar]
- 8. Coyle CH, Kader KN. Mechanisms of H2O2-induced oxidative stress in endothelial cells exposed to physiologic shear stress. ASAIO J 53: 17–22, 2007 [DOI] [PubMed] [Google Scholar]
- 9. Droge W. Free radicals in the physiological control of cell function. Physiol Rev 82: 47–95, 2002 [DOI] [PubMed] [Google Scholar]
- 10. Frey RS, Ushio-Fukai M, Malik AB. NADPH oxidase-dependent signaling in endothelial cells: role in physiology and pathophysiology. Antioxid Redox Signal 11: 791–810, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Heil M, Schaper W. Influence of mechanical, cellular, and molecular factors on collateral artery growth (arteriogenesis). Circ Res 95: 449–458, 2004 [DOI] [PubMed] [Google Scholar]
- 12. Koh PO, Sung JH, Won CK, Cho JH, Moon JG, Park OS, Kim MO. Streptozotocin-induced diabetes decreases placenta growth factor (PlGF) levels in rat placenta. J Vet Med Sci 69: 877–880, 2007 [DOI] [PubMed] [Google Scholar]
- 13. Leung FP, Yung LM, Laher I, Yao X, Chen ZY, Huang Y. Exercise, vascular wall and cardiovascular diseases: an update (Part 1). Sports Med 38: 1009–1024, 2008 [DOI] [PubMed] [Google Scholar]
- 14. Lloyd PG, Yang HT, Terjung RL. Arteriogenesis and angiogenesis in rat ischemic hindlimb: role of nitric oxide. Am J Physiol Heart Circ Physiol 281: H2528–H2538, 2001 [DOI] [PubMed] [Google Scholar]
- 15. Luttun A, Tjwa M, Moons L, Wu Y, Angelillo-Scherrer A, Liao F, Nagy JA, Hooper A, Priller J, De Klerck B, Compernolle V, Daci E, Bohlen P, Dewerchin M, Herbert JM, Fava R, Matthys P, Carmeliet G, Collen D, Dvorak HF, Hicklin DJ, Carmeliet P. Revascularization of ischemic tissues by PlGF treatment, and inhibition of tumor angiogenesis, arthritis and atherosclerosis by anti-Flt1. Nature Med 8: 831–840, 2002 [DOI] [PubMed] [Google Scholar]
- 16. Maglione D, Guerriero V, Viglietto G, li-Bovi P, Persico MG. Isolation of a human placenta cDNA coding for a protein related to the vascular permeability factor. Proc Natl Acad Sci USA 88: 9267–9271, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mohammed KA, Nasreen N, Tepper RS, Antony VB. Cyclical stretch induces PlGF expression in bronchial epithelial cells via nitric oxide release. Am J Physiol Lung Cell Mol Physiol 292: L559–L566, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Odorisio T, Schietroma C, Zaccaria ML, Cianfarani F, Tiveron C, Tatangelo L, Failla CM, Zambruno G. Mice overexpressing placenta growth factor exhibit increased vascularization and vessel permeability. J Cell Sci 115: 2559–2567, 2002 [DOI] [PubMed] [Google Scholar]
- 19. Osanai T, Fujita N, Fujiwara N, Nakano T, Takahashi K, Guan W, Okumura K. Cross talk of shear-induced production of prostacyclin and nitric oxide in endothelial cells. Am J Physiol Heart Circ Physiol 278: H233–H238, 2000 [DOI] [PubMed] [Google Scholar]
- 20. Palmer RM, Rees DD, Ashton DS, Moncada S. l-Arginine is the physiological precursor for the formation of nitric oxide in endothelium-dependent relaxation. Biochem Biophys Res Commun 153: 1251–1256, 1988 [DOI] [PubMed] [Google Scholar]
- 21. Pan P, Fu H, Zhang L, Huang H, Luo F, Wu W, Guo Y, Liu X. Angiotensin II upregulates the expression of placental growth factor in human vascular endothelial cells and smooth muscle cells. BMC Cell Biol 11: 36, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Perelman N, Selvaraj SK, Batra S, Luck LR, Erdreich-Epstein A, Coates TD, Kalra VK, Malik P. Placenta growth factor activates monocytes and correlates with sickle cell disease severity. Blood 102: 1506–1514, 2003 [DOI] [PubMed] [Google Scholar]
- 23. Pilarczyk K, Sattler KJ, Galili O, Versari D, Olson ML, Meyer FB, Zhu XY, Lerman LO, Lerman A. Placenta growth factor expression in human atherosclerotic carotid plaques is related to plaque destabilization. Atherosclerosis 196: 333–340, 2008 [DOI] [PubMed] [Google Scholar]
- 24. Pipp F, Heil M, Issbrucker K, Ziegelhoeffer T, Martin S, van den Heuvel J, Weich H, Fernandez B, Golomb G, Carmeliet P, Schaper W, Clauss M. VEGFR-1-selective VEGF homologue PlGF is arteriogenic: evidence for a monocyte-mediated mechanism. Circ Res 92: 378–385, 2003 [DOI] [PubMed] [Google Scholar]
- 25. Pompeo E, Albonici L, Doldo E, Orlandi A, Manzari V, Modesti A, Mineo TC. Placenta growth factor expression has prognostic value in malignant pleural mesothelioma. Ann Thorac Surg 88: 426–431, 2009 [DOI] [PubMed] [Google Scholar]
- 26. Powers SK, Jackson MJ. Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol Rev 88: 1243–1276, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Prior BM, Lloyd PG, Ren J, Li H, Yang HT, Laughlin MH, Terjung RL. Time course of changes in collateral blood flow and isolated vessel size and gene expression after femoral artery occlusion in rats. Am J Physiol Heart Circ Physiol 287: H2434–H2447, 2004 [DOI] [PubMed] [Google Scholar]
- 28. Ribatti D. The discovery of the placental growth factor and its role in angiogenesis: a historical review. Angiogenesis 11: 215–221, 2008 [DOI] [PubMed] [Google Scholar]
- 29. Rocic P, Kolz C, Reed R, Potter B, Chilian WM. Optimal reactive oxygen species concentration and p38 MAP kinase are required for coronary collateral growth. Am J Physiol Heart Circ Physiol 292: H2729–H2736, 2007 [DOI] [PubMed] [Google Scholar]
- 30. Scholz D, Elsaesser H, Sauer A, Friedrich C, Luttun A, Carmeliet P, Schaper W. Bone marrow transplantation abolishes inhibition of arteriogenesis in placenta growth factor (PlGF)-/- mice. J Mol Cell Cardiol 35: 177–184, 2003 [DOI] [PubMed] [Google Scholar]
- 31. Tian J, Hou Y, Lu Q, Wiseman DA, Vasconcelos FF, Elms S, Fulton DJ, Black SM. A novel role for caveolin-1 in regulating endothelial nitric oxide synthase activation in response to H(2)O(2) and shear stress. Free Radic Biol Med 49: 159–170, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tojo T, Ushio-Fukai M, Yamaoka-Tojo M, Ikeda S, Patrushev N, Alexander RW. Role of gp91phox (Nox2)-containing NAD(P)H oxidase in angiogenesis in response to hindlimb ischemia. Circulation 111: 2347–2355, 2005 [DOI] [PubMed] [Google Scholar]
- 33. Ushio-Fukai M, Alexander RW. Reactive oxygen species as mediators of angiogenesis signaling: role of NAD(P)H oxidase. Mol Cell Biochem 264: 85–97, 2004 [DOI] [PubMed] [Google Scholar]
- 34. Van de Viere S, Stalmans I, Heindryckx F, Oura H, Tijeras-Raballand A, Schmidt T, Loges S, Albrecht I, Jonckx B, Vinckier S, Van SC, Tugues S, Rolny C, De MM, Dettori D, Hainaud P, Coenegrachts L, Contreres JO, Van BT, Cuervo H, Xiao WH, Le HC, Buysschaert I, Kharabi MB, Geerts A, Schomber T, Bonnin P, Lambert V, Haustraete J, Zacchigna S, Rakic JM, Jimenez W, Noel A, Giacca M, Colle I, Foidart JM, Tobelem G, Morales-Ruiz M, Vilar J, Maxwell P, Vinores SA, Carmeliet G, Dewerchin M, Claesson-Welsh L, Dupuy E, Van VH, Christofori G, Mazzone M, Detmar M, Collen D, Carmeliet P. Further pharmacological and genetic evidence for the efficacy of PlGF inhibition in cancer and eye disease. Cell 141: 178–190, 2010 [DOI] [PubMed] [Google Scholar]
- 35. Verhaeghe C, Tabruyn SP, Oury C, Bours V, Griffioen AW. Intrinsic pro-angiogenic status of cystic fibrosis airway epithelial cells. Biochem Biophys Res Commun 356: 745–749, 2007 [DOI] [PubMed] [Google Scholar]
- 36. Xu HX, Zhu XD, Zhuang PY, Zhang JB, Zhang W, Kong LQ, Wang WQ, Liang Y, Wu WZ, Wang L, Fan J, Tang ZY, Sun HC. Expression and prognostic significance of placental growth factor in hepatocellular carcinoma and peritumoral liver tissue. Int J Cancer 2010. June 2, (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 37. Yin W, Rubenstein DA. Dose effect of shear stress on platelet complement activation in a cone and plate shearing device. Cell Mol Bioeng 2: 274–280, 2009 [Google Scholar]
- 38. Yoo SA, Yoon HJ, Kim HS, Chae CB, De FS, Cho CS, Kim WU. Role of placenta growth factor and its receptor flt-1 in rheumatoid inflammation: a link between angiogenesis and inflammation. Arthritis Rheum 60: 345–354, 2009 [DOI] [PubMed] [Google Scholar]