Abstract
Unilateral denervation (DNV) of rat diaphragm muscle increases protein synthesis at 3 days after DNV (DNV-3D) and degradation at DNV-5D, such that net protein breakdown is evident by DNV-5D. On the basis of existing models of protein balance, we examined DNV-induced changes in Akt, AMP-activated protein kinase (AMPK), and ERK½ activation, which can lead to increased protein synthesis via mammalian target of rapamycin (mTOR)/p70S6 kinase (p70S6K), glycogen synthase kinase-3β (GSK3β), or eukaryotic initiation factor 4E (eIF4E), and increased protein degradation via forkhead box protein O (FoxO). Protein phosphorylation was measured using Western analyses through DNV-5D. Akt phosphorylation decreased at 1 h and 6 h after DNV compared with sham despite decreased AMPK phosphorylation. Both Akt and AMPK phosphorylation returned to sham levels by DNV-1D. Phosphorylation of their downstream effector mTOR (Ser2481) did not change at any time point after DNV, and phosphorylated p70S6K and eIF4E-binding protein 1 (4EBP1) increased only by DNV-5D. In contrast, ERK½ phosphorylation and its downstream effector eIF4E increased 1.7-fold at DNV-1D and phosphorylated GSK3β increased 1.5-fold at DNV-3D (P < 0.05 for both comparisons). Thus, following DNV there are differential effects on protein synthetic pathways with preferential activation of GSK3β and eIF4E over p70S6K. FoxO1 nuclear translocation occurred by DNV-1D, consistent with its role in increasing expression of atrogenes necessary for subsequent ubiquitin-proteasome activation evident by DNV-5D. On the basis of our results, increased protein synthesis following DNV is associated with changes in ERK½-dependent pathways, but protein degradation results from downregulation of Akt and nuclear translocation of FoxO1. No single trigger is responsible for protein balance following DNV. Protein balance in skeletal muscle depends on multiple synthetic/degradation pathways that should be studied in concert.
Keywords: innervation, skeletal muscle, protein synthesis
the balance between protein synthesis and degradation determines whether there is a net loss (atrophy) or gain (hypertrophy) in muscle mass. Recent studies suggest that phosphoinositide 3-kinase (PI3K)-dependent activation of protein kinase B (Akt) is an important regulator of both protein synthesis [via mammalian target of rapamycin (mTOR) and glycogen synthase kinase-3β (GSK3β); Fig. 1] and degradation [via the transcription factor forkhead box protein O1 (FoxO1)] (6, 26, 54, 62). A previous study showed that unilateral denervation (DNV) of the diaphragm muscle (DIAm) increases protein synthesis at 3 days after DNV and protein degradation by 5 days after DNV, with these changes persisting through 14 days after DNV (3). There was a sustained increase in net protein breakdown at 5 days through 14 days after DNV. The underlying signaling pathways responsible for these time-dependent changes in protein balance are not known.
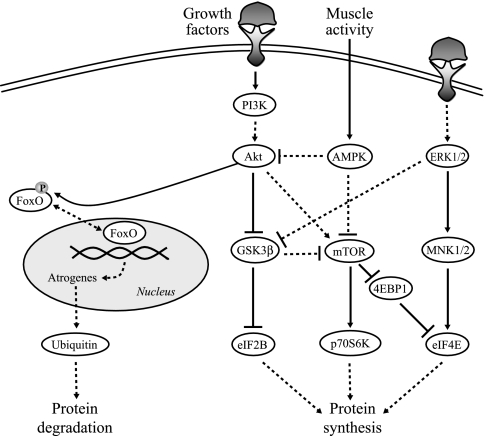
Fig. 1.
Simplified model for the intersection of signaling pathways regulating protein synthesis and degradation. Arrows indicate activating events, whereas perpendicular lines indicate inhibitory events. The solid lines represent direct activation. The dashed lines represent indirect activation, whereby intermediate steps are involved but are not specified in this schematic. Protein synthesis is regulated by protein kinase B (Akt), p44/42 MAPK (ERK½), and AMP-activated protein kinase (AMPK), leading to activation of the downstream targets mammalian target of rapamycin (mTOR), glycogen synthase kinase-3β (GSK3β), MAPK-interacting kinases ½ (MNK½), p70S6 kinase (p70S6K), eIF4E-binding protein 1 (4EBP1), and eukaryotic initiation factors 2B and 4E (eIF2B and eIF4E). Conversely, Akt is responsible for the phosphorylation status of forkhead box protein (FoxO). If FoxO is phosphorylated by Akt, it leaves the nucleus and becomes inactive, thus preventing protein degradation. If Akt activity is suppressed, FoxO becomes dephosphorylated, translocates to the nucleus, and exerts its transcriptional effects on atrogenes to induce protein degradation through the ubiquitin/proteasome pathway. PI3K, phosphoinositide 3-kinase.
Increased protein synthesis associated with muscle hypertrophy is thought to involve Akt signaling (19, 53), because 1) there is an increase in Akt phosphorylation (6) and 2) expression of an activated form of Akt in skeletal muscle is sufficient to cause hypertrophy (49, 50, 63) and prevent atrophy of denervated muscles (6). The downstream branches of the Akt pathway are GSK3β and mTOR. One of the effectors of mTOR is p70S6 kinase (p70S6K), which phosphorylates the ribosomal protein S6 and is thought of as a mTOR “activation mirror” (72) and marker of increased protein synthesis (4, 12, 29, 56). These upstream changes in signaling pathways affect the rate of protein synthesis through translation initiation factors such as eukaryotic initiation factor 2B (eIF2B), eukaryotic initiation factor 4E (eIF4E), and eIF4E-binding protein 1 (4EBP1) (36, 67). In addition to the Akt pathway, the p44/42 MAPK (ERK½) pathway affects translational control through eIF4E (59).
Aside from responding to changes in nerve-derived trophic factors that presumably occur after DNV, the DIAm responds to metabolic demands as well. In fact, mTOR is inhibited by AMP-activated protein kinase (AMPK), an energy sensor that responds to changes in the intracellular ratio of AMP to ATP (23, 37).
Protein degradation is also thought to involve Akt signaling. The FoxO family of transcription factors is negatively regulated by Akt activation. The role of FoxO in protein degradation has been shown in atrophy models by several studies, where FoxO was sufficient to increase protein degradation and promote net protein loss by directly increasing muscle-specific ubiquitin ligases (32, 54). In its Akt-dependent phosphorylated state, FoxO remains cytosolic and is unable to act on promoters responsible for transcription of atrogenes necessary for the production of ubiquitin ligases (9, 60). In the absence of Akt-induced FoxO phosphorylation, FoxO translocates to the nucleus where it can exert its transcriptional activity. Indeed, in a recent study (3), we showed that following DNV the majority of intracellular protein is degraded by the ubiquitin-proteasome pathway.
The present study was undertaken to determine the mechanisms responsible for the time-dependent changes in net protein balance observed after DIAm DNV. We hypothesized that DIAm DNV results in time-dependent changes in Akt activation, leading to activation of mTOR and GSK3β (increasing protein synthesis via translation initiation factors) and subsequently to FoxO1 activation (increasing protein degradation).
METHODS
Adult male Sprague-Dawley rats (body wt ∼300 g) were used in these experiments. Animals were assigned to one of five DNV groups with sham control surgeries performed in parallel. Aseptic conditions were maintained during all surgical procedures, and recovery from surgery was carefully monitored. All procedures were approved by the Institutional Animal Care and Use Committee.
Unilateral Denervation
The procedure for unilateral DIAm DNV has been described previously (3, 20, 41, 74, 75). Briefly, ketamine (60 mg/kg) and xylazine (2.5 mg/kg) was injected intramuscularly to anesthetize the animals. The right phrenic nerve was exposed at a point beneath the sternomastoid muscle in the lower neck. The nerve was transected and a portion (∼10–20 mm) of the distal end was removed. Sham surgery animals underwent the same surgical procedure without nerve transection. The wound was sutured and treated with topical antibiotics. Periods of DNV were maintained for 1 h, 6 h, 1 day, 3 days, or 5 days (n = 6 animals for each time point). After the appropriate DNV period, animals were euthanized by exsanguination under anesthesia as above. The right hemi-diaphragm was excised and cut into eight strips. Samples from sham control surgery animals were collected at 1 day, 3 days, and 5 days postsurgery (n = 6 animals for each time point).
Western Blot Analyses
Two DIAm strips from each animal were immediately frozen in liquid nitrogen. DIAm strips were subsequently homogenized in lysis buffer (Cell Signaling Technology, Beverly, MA) with 5 mM sodium fluoride, 5 mM sodium orthovanadate, 2.5 mM sodium pyrophosphate, and complete mini protease inhibitor (Roche, Indianapolis, IN). The homogenate was centrifuged (10,000 g for 10 min), and the protein concentration of the supernatant was determined using the Bio-Rad DC protein assay according to manufacturer's protocol (Bio-Rad, Hercules, CA).
Samples were diluted 1:1 in Laemmli sample buffer (Bio-Rad) and electrophoretically separated under denaturing conditions on 7.5% or 10% SDS-PAGE Criterion gels (Bio-Rad), followed by transfer to polyvinylidene difluoride membranes (Bio-Rad). Membranes were blocked in Tris-buffered saline (TBS; pH 7.5) with 5% milk and 0.1% Tween 20 (Sigma-Aldrich, St. Louis, MO), followed by an overnight incubation with primary antibodies for phospho-Akt (Ser473), Akt, phospho-ERK½ (Thr202/Tyr204), ERK½, phospho-mTOR (Ser2481), mTOR, phospho-AMPKα (α1 and α2; Thr172), AMPKα, phospho-p70S6K (Thr389), p70S6K, 4EBP1, phospho-eIF4E (Ser209), eIF4E, phospho-GSK3α/β (Ser21/9), GSK3β, and FoxO1 (all from Cell Signaling). Antibodies were diluted 1:1,000 in TBS containing 5% milk and 0.1% Tween 20. After three washes, membranes were incubated for 1 h with appropriate horseradish peroxidase-conjugated secondary antibodies (Santa Cruz Biotechnology, Santa Cruz, CA). Immunodetection was performed using enhanced chemiluminescence consistent with the manufacturer's protocol (Pierce Biotechnology, Rockford, IL). Membranes were reprobed with an antibody for β-tubulin (Santa Cruz) to serve as a loading control. Protein expression was semiquantitated by measuring the density of autoradiographs obtained with Kodak MM4000 Image Station software (Kodak Molecular Imaging Systems, New Haven, CT). Density measurements included measurement of background intensity, identification of bands, and measurement of net intensity of bands. For each measurement, the density of the band for each specific protein was divided by the density of the band for β-tubulin for each lane. This provided normalization for variable protein loading across lanes.
Cell Fractionation
For FoxO1 analysis, the nuclear and cytoplasmic fractions were separated using NE-PER Nuclear and Cytoplasmic Extraction Kit (Pierce Biotechnology). Protein concentrations of both the nuclear and cytoplasmic fractions were determined, and Western blot analyses for FoxO1 were performed as described above. Expression of lamin A/C was used to assess the purity of the fractions, confirming that the cytoplasmic fractions had no detectable lamin A/C while the nuclear fractions displayed lamin A/C expression. In our fractions that were verified to be nuclear fractions, β-tubulin was present. β-Tubulin was used as a loading control to normalize FoxO1 expression, because its presence in both the cytoplasm and nucleus has been previously verified (1, 66, 71).
Statistical Analyses
Statistical analyses were performed with JMP software (SAS Institute, Cary, NC). Protein expression was normalized to loading control (β-tubulin) for each sample. Expression of all proteins studied (both phosphorylated and total) was not different across time points in the sham controls. Thus, all sham controls were averaged for the purposes of comparison to the DNV groups. Protein expression for both total and phosphorylated proteins and the ratio of phosphorylated protein to total protein was compared in the sham control and DNV DIAm using two-way ANOVA for each time point based on experimental group and protein state (total, phosphorylated, ratio) variables. Significant differences between individual treatment conditions were determined post hoc using Student's t-test. For FoxO1 analyses, each DNV time point was compared with the sham control using one-way ANOVA. Statistical significance was indicated by P < 0.05. All values are reported as means ± SE, unless otherwise specified.
RESULTS
Phosphorylation of Akt After DIAm DNV
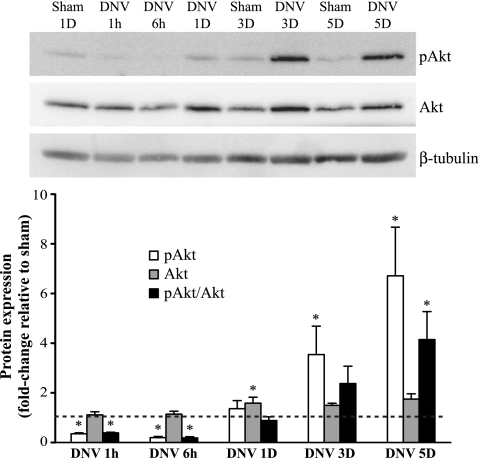
DNV caused time-dependent changes in the relative phosphorylation of Akt (pAkt-to-Akt ratio; Fig. 2). At 1 h and 6 h after DNV, pAkt/Akt decreased significantly by 61% and 82% of sham, respectively. Relative phosphorylation returned to sham levels at 1 and 3 days after DNV. By 5 days after DNV, pAkt/Akt was significantly increased to 4.1 times that of sham. In addition, expression of total Akt was significantly different from sham only at 1 day after DNV (58% increase compared with sham). Although relative phosphorylation was not significantly changed at 3 days after DNV, pAkt expression increased to 3.5 times that of sham (P < 0.05).
Fig. 2.
Unilateral denervation (DNV)-induced changes in phosphorylation of Akt, as detected by Western analyses. Top: representative immunoblot of phospho-Akt, Akt, and β-tubulin for each DNV time point [in hours (h) or days (D)]. Bottom: relative expression (means ± SE) of phospho-Akt, Akt, and the ratio of phospho-Akt to total Akt, compared with sham control after normalization to β-tubulin. *Significantly different (P < 0.05; n = 6 animals per time point) from the average of all sham controls.
In summary, the immediate effect of DIAm DNV was to decrease Akt phosphorylation (both absolute and relative), followed by a return to sham levels by 1 day after DNV and a subsequent progressive increase in Akt phosphorylation that became significant at 5 days after DNV.
Phosphorylation of ERK½ After DIAm DNV
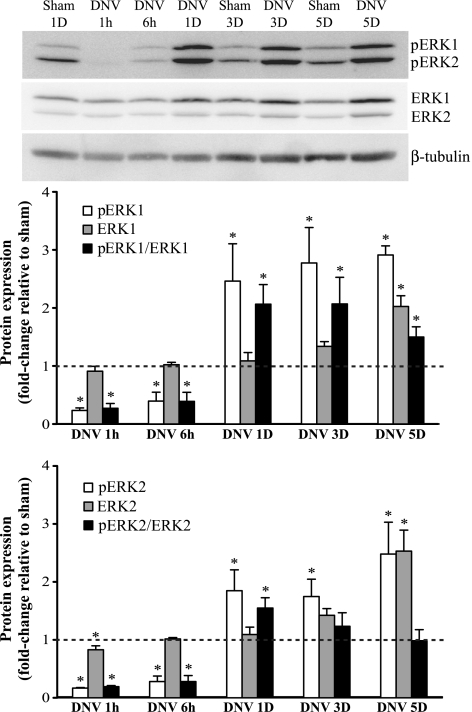
Protein expression of p44/42 MAPK (ERK½) consists of two bands, at 44 kDa and at 42 kDa, which were analyzed separately (Fig. 3). DNV caused time-dependent changes in the relative phosphorylation of both ERK1 (pERK1-to-ERK1 ratio) and ERK 2 (pERK2-to-ERK2 ratio). At 1 h and 6 h after DNV, pERK1/ERK1 decreased significantly by 73% and 61% of sham, respectively. Relative phosphorylation of ERK2 decreased significantly at 1 h and 6 h after DNV as well (81% and 72% of sham, respectively). By 1 day after DNV, however, this response reversed and there was a significant increase in both pERK1/ERK1 and pERK2/ERK2 (2.1- and 1.6-fold change relative to sham, respectively). This increase in relative phosphorylation persisted for ERK1 at 3 and 5 days after DNV, with a 2.1- and 1.5-fold change relative to sham, respectively (P < 0.05). Relative phosphorylation of ERK2 returned to sham levels at 3 and 5 days after DNV. Expression of phospho-ERK2 was significantly increased at 3 and 5 days; however, expression of ERK2 was increased as well at 5 days after DNV, leading to no change in the ratio of pERK2/ERK2.
Fig. 3.
DNV-induced changes in phosphorylation of ERK½, as detected by Western analyses. Top: representative immunoblot of phospho-ERK½, ERK½, and β-tubulin for each DNV time point. Bottom: relative expression (means ± SE) of phospho-ERK½, ERK½, and the ratio of phospho-ERK½ to total ERK½, compared with sham control after normalization to β-tubulin. *Significantly different (P < 0.05; n = 6) from the average of all sham controls.
In summary, the immediate effect of DIAm DNV was to decrease both ERK1 and ERK2 phosphorylation (both absolute and relative). At 1 day after DNV, there was an increase in relative phosphorylation of both ERK1 and ERK2, with ERK1 relative phosphorylation remaining elevated through 5 days after DNV.
Phosphorylation of AMPK After DIAm DNV
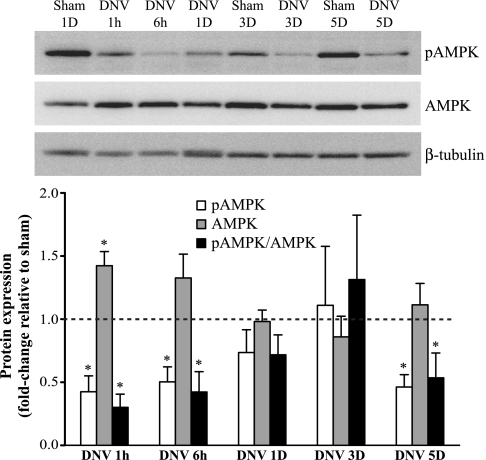
Following DNV, there was a general reduction in relative AMPK phosphorylation (pAMPK-to-AMPK ratio), although the reduction varied over time (Fig. 4). pAMPK/AMPK was significantly less than control at 1 h and 6 h after DNV (70% and 58% less, respectively), returned to sham levels at 1 and 3 days after DNV, and by 5 days after DNV had significantly decreased again (47% less than sham). In addition, expression of total AMPK increased only at 1 h after DNV (40% increase compared with sham; P < 0.05).
Fig. 4.
DNV-induced changes in phosphorylation of AMPK, as detected by Western analyses. Top: representative immunoblot of phospho-AMPK, AMPK, and β-tubulin for each DNV time point. Bottom: relative expression (means ± SE) of phospho-AMPK, AMPK, and the ratio of phospho-AMPK to total AMPK, compared with sham control after normalization to β-tubulin. *Significantly different (P < 0.05; n = 6) from the average of all sham controls.
Phosphorylation of mTOR After DIAm DNV
Relative phosphorylation of mTOR (pmTOR-to-mTOR ratio) did not change significantly at any time point after DNV (Fig. 5A). There were changes that occurred in pmTOR or mTOR expression. The expression of pmTOR was significantly decreased compared with sham at 6 h after DNV (25% decrease). This response was subsequently reversed, and by 5 days after DNV, phospho-mTOR increased by 3.6-fold compared with sham (P < 0.05). Since mTOR protein expression increased as well at 5 days after DNV (2.4-fold increase compared with sham, P < 0.05), there was no significant increase in relative phosphorylation.
Fig. 5.
DNV-induced changes in phosphorylation of mTOR (A) and p70S6K (B), as detected by Western analyses. A, top: representative immunoblot of phospho-mTOR, mTOR, and β-tubulin for each DNV time point. Bottom: relative expression (means ± SE) of phospho-mTOR, mTOR, and the ratio of phospho-mTOR to total mTOR, compared with sham control after normalization to β-tubulin. *Significantly different (P < 0.05; n = 5) from the average of all sham controls. B, top: representative immunoblot of phospho-p70S6K, p70S6K, and β-tubulin for each DNV time point. Bottom: relative expression (means ± SE) of phospho-p70S6K and p70S6K, compared with sham control after normalization to β-tubulin. *Significantly different (P < 0.05; n = 4) from the average of all sham controls.
Phosphorylation of p70S6K After DIAm DNV
Protein expression of phospho-p70S6K was minimal at all sham time points and at 1 h, 6 h, and 1 day after DNV (Fig. 5B). The low levels of protein expression preclude meaningful interpretation of relative expression levels (i.e., phospho-p70S6K to p70S6K ratio) for sham animals and up to 3 days after DNV. Nevertheless, there was a significant increase in phospho-p70S6K at 5 days after DNV, when it increased by 12.1-fold compared with sham. There was no significant change in total p70S6K protein expression at any time after DNV.
Phosphorylation of GSK3β After DIAm DNV
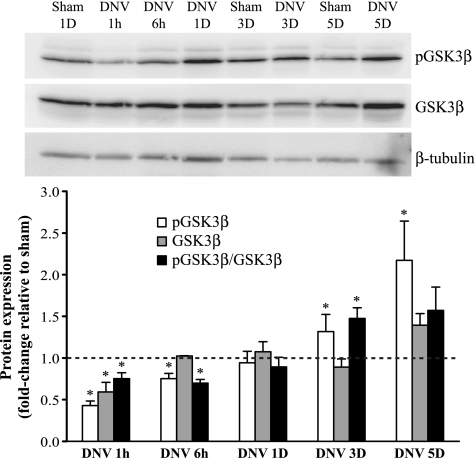
Relative phosphorylation of GSK3β (pGSK3β-to-GSK3β ratio) was significantly decreased at 1 and 6 h after DNV (25% and 30% decrease, respectively; Fig. 6). GSK3β relative phosphorylation returned to sham levels at 1 day after DNV (P > 0.05), but by 3 days after DNV it had significantly increased to 50% greater than sham. Expression of pGSK3β remained elevated at 5 days after DNV (2.2-fold increase compared with sham, P < 0.05); however, relative phosphorylation was not changed. Expression of GSK3β changed only at 1 h after DNV, when it decreased by 40% compared with sham (P < 0.05).
Fig. 6.
DNV-induced changes in phosphorylation of GSK3β, as detected by Western analyses. Top: representative immunoblot of phospho-GSK3β, GSK3β, and β-tubulin for each DNV time point. Bottom: relative expression (means ± SE) of phospho-GSK3β, GSK3β, and the ratio of phospho-GSK3β to total GSK3β, compared with sham control after normalization to β-tubulin. *Significantly different (P < 0.05; n = 6) from the average of all sham controls.
Phosphorylation of 4EBP1 After DIAm DNV
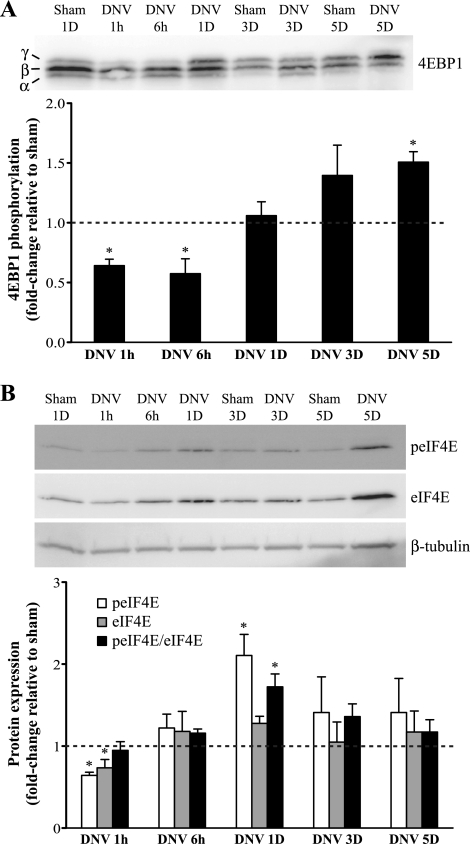
Three isoforms of 4EBP1 were detected: α-, β-, and γ-isoforms (Fig. 7A). The slow moving form, the γ-isoform, represents highly phosphorylated 4EBP1 (2, 34); thus phosphorylation of 4EBP1 was represented as the γ-form relative to the sum of all three isoforms. Phosphorylation of 4EBP1 was significantly decreased at 1 and 6 h after DNV (36% and 43% decrease, respectively). This returned to sham levels at 1 and 3 days after DNV (P > 0.05) but subsequently increased at 5 days after DNV (50% greater than sham control; P < 0.05).
Fig. 7.
DNV-induced changes in phosphorylation of 4EBP1 (A) and eIF4E (B), as detected by Western analyses. A, top: representative immunoblot of 4EBP1 for each DNV time point. Bottom: relative expression (means ± SE) of 4EBP1 phosphorylation, calculated by the ratio of the hyperphosphorylated γ-band relative to the sum of all bands (γ + β + α). *Significantly different (P < 0.05; n = 6) from the average of all sham controls. B, top: representative immunoblot of phospho-eIF4E, eIF4E, and β-tubulin for each DNV time point. Bottom: relative expression (means ± SE) of phospho-eIF4E, eIF4E, and the ratio of phospho-eIF4E to eIF4E, compared with sham control after normalization to β-tubulin. *Significantly different (P < 0.05; n = 5) from the average of all sham controls.
Phosphorylation of eIF4E After DIAm DNV
Relative phosphorylation of eIF4E (peIF4E-to-eIF4E ratio) did not change until 1 day after DNV, when it was 72% greater than sham control (P < 0.05; Fig. 7B). Relative phosphorylation of eIF4E returned to sham levels at 3 and 5 days after DNV (P > 0.05). Expression of peIF4E and eIF4E decreased significantly only at 1 h after DNV (36% and 27% decrease, respectively).
FoxO1 Protein Expression After DIAm DNV
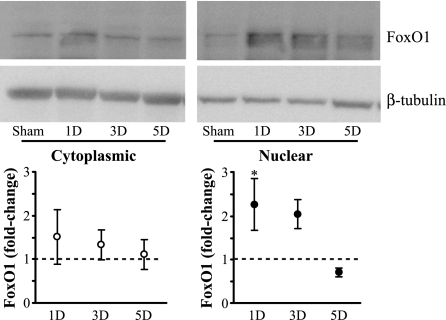
FoxO1 protein expression in the cytoplasmic fraction did not significantly change at 1, 3, or 5 days after DNV compared with sham controls (Fig. 8). FoxO1 expression in the nuclear fraction increased by 2.1-fold compared with sham at 1 day after DNV (P < 0.05) but was no longer significantly different from sham at 3 and 5 days after DNV.
Fig. 8.
DNV-induced changes in FoxO1 protein expression in cytoplasmic and nuclear cell fractions, as detected by Western analyses. Top: representative immunoblot of cytoplasmic and nuclear FoxO1 in sham control and at 1, 3, and 5 days after DNV. Bottom: relative expression (means ± SE) of cytoplasmic FoxO1 (left) and nuclear FoxO1 protein expression (right), each compared with sham control for the same cell fraction after normalization to β-tubulin. *Significantly different (P < 0.05; n = 3) from the sham control.
DISCUSSION
The results of this study support the hypothesis that DIAm DNV results in time-dependent changes in Akt activation. Akt was not, however, the single trigger responsible for the changes in both protein synthesis and degradation. Rather, increased protein synthesis following DNV was temporally associated with changes in ERK½-dependent pathways. In fact, there were differential effects on protein synthetic pathways with preferential activation of GSK3β and eIF4E over p70S6K. Protein degradation was associated with downregulation of Akt and nuclear translocation of FoxO1.
Physiological Changes Triggered by DNV
DNV is one of many conditions that affect net protein balance, and the use of this dynamic model provides insight into other conditions that similarly affect muscle mass, such as exercise and aging. All too often the changes induced by such conditions are oversimplified. Although DNV is a relatively simple model, the changes induced by DNV are time dependent and highly complex. In fact, the effect of DNV on net protein balance in the DIAm could be triggered by removal of nerve-derived trophic influence, reduced muscle activity, and/or decreased load. Adding to the complexity, these physiologic triggers are not independent of each other, nor are they temporally linked. It is not possible to point to any one physiologic trigger as being responsible for changes in net protein balance at each time point, but we can look at signaling pathways associated with protein synthesis and degradation to begin to understand this complex regulation.
After DNV, removal of nerve-derived trophic influence takes time. Using an estimate of anterograde axoplasmic flow rate of 2 mm/day (8), it is likely that trophic factors from the distal phrenic nerve stump are not completely removed until ∼24 h after DNV. One possible nerve-derived growth factor that could be removed after DNV is neuregulin (NRG). Neuregulin contributes to the regulation of muscle growth by increasing protein synthesis via a PI3K-dependent mechanism (25), so changes in PI3K-Akt signaling may result from a reduction in NRG. In addition, the MAPK/ERK½ pathway is also activated by growth factor signaling (59).
In contrast, changes in muscle activity occur immediately after DNV, but even these changes in activity are complex. With the nerve injury induced by DNV, there is a spontaneous short-term (5 min) increase in muscle activity (41) likely due to muscle fasciculations (61). The fasciculations do not last long, leading to an expected decrease in muscle activity after DNV (41). Changes in muscle activity are reflected by a decrease in energy consumption which may result in altered AMPK signaling (31).
Lastly, muscle load decreases immediately after DNV, as the DIAm is paralyzed. With unilateral DNV, the contralateral side of the DIAm still contracts; however, in a previous study we showed that the resulting passive length changes on the DNV side do not result in appreciable mechanical stress. Thus, passive mechanical stress is not likely to be responsible for muscle fiber adaptations induced by DNV (73). However, unloading a chronically active muscle such as the DIAm may remove a trophic signal such as IGF-1, which exerts its effects via Akt activation (5).
Intracellular Pathways Involved in Regulation of Protein Synthesis
p70S6K pathway.
It has been widely accepted that Akt-induced activation of mTOR is a key regulator of protein synthesis (24, 67), since 1) phosphorylation of mTOR is increased during muscle hypertrophy (48) and 2) inhibiting mTOR with rapamycin blocks overload hypertrophy and growth in regenerating muscles (6, 44). In contrast, mTOR is inhibited by AMPK, and activation of AMPK with the drug 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside (AICAR) inhibits protein synthesis by downregulating both Akt and mTOR (7, 35, 70).
mTOR is part of two multiprotein complexes: mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2). mTORC1 involves phosphorylation at Ser2448, and mTORC2 involves phosphorylation at Ser2481 (14). The effect of mTOR on translation and ultimately protein synthesis is generally mediated by mTORC1-induced phosphorylation of p70S6K (24, 29, 67) and 4EBP1 (17, 59). While most previous research focused on mTORC1, mTORC2 also appears to play a role in regulating the actin cytoskeleton and in activating Akt (46, 55, 57). There is also cross talk between mTORC1 and mTORC2, so activation of mTORC2 may activate mTORC1 and cause increased protein synthesis (39).
Treatment with IGF-1, a growth factor known to respond to changes in mechanical loading by increasing protein synthesis through PI3K/Akt pathway (49, 65), activates mTORC2 (46, 64). NRG, representing the neurotrophic influence, increases protein synthesis in both cultured C2C12 myotubes and DIAm to the same degree as IGF-1 (25). Since NRG is implicated in maintaining protein synthesis in DIAm, and its effects are similar to IGF-1, we chose to evaluate mTORC2 activation after DNV. Mechanical stimulation is known to stimulate mTOR activation, but in three studies examining effects on phosphorylation of mTOR at Ser2481, no changes were found (16, 27, 40). However, as pointed out, it is unlikely that DNV induced significant passive mechanical stress on the affected side of the DIAm. Unfortunately, the effects of NRG and/or AMPK activation on mTORC2 activation have not been fully explored.
One mechanism by which mTOR increases protein synthesis is through its downstream effector p70S6K (4, 18, 24, 67, 72). A study of the plantaris muscle showed that a compensatory hypertrophy model resulted in phosphorylation of Akt and downstream targets p70S6K and 4EBP1 (6). In this study, phosphorylation of Akt increased significantly only by 5 days after DNV, despite decreased AMPK phosphorylation. Thus, Akt is not responsible for the initial increase in protein synthesis at 3 days after DNV.
Phosphorylation of mTOR at Ser2481 did not change at any time point after DNV. However, by 5 days after DNV, phosphorylation of p70S6K was increased compared with sham. It is possible that p70S6K is activated by mTORC1 activation rather than mTORC2 following DNV. Although we attempted to examine mTORC1 phosphorylation (Ser2448), we could not verify an antibody that consistently worked to provide unambiguous definitive results. In any case, its downstream effector p70S6K is not responsible for the initial increase in protein synthesis at 3 days after DNV.
GSK3β/eIF2B pathway.
Another way in which Akt promotes protein synthesis is through GSK3β. Akt phosphorylates GSK3β, leading to its inactivation and diminishing its inhibitory effect on the initiation factor eIF2B (30, 59). The compensatory hypertrophy model described earlier caused GSK3β phosphorylation and inactivation (6), and expressing a dominant-negative form of GSK3β caused hypertrophy in cultured C2C12 differentiated myotubes (49). In addition, ERK½ phosphorylates and inactivates GSK3β through MAP kinase-activated protein kinase-l (52).
In this study, GSK3β phosphorylation initially decreased likely due to the concomitant decrease in both Akt and ERK½ phosphorylation. By 3 days after DNV, GSK3β phosphorylation increased. Akt was not activated until 5 days after DNV; thus ERK½ was responsible for the increase in GSK3β phosphorylation. This increase in phosphorylation of GSK3β may contribute to the increase in protein synthesis through its release of eIF2B or diminished inhibition of mTOR (28), in concert with eIF4E (see below).
eIF4E pathway.
The other downstream effector of mTOR, 4EBP1, contributes to increases in protein synthesis (51, 59). 4EBP1 is a translation repressor protein, which binds to the eIF4E initiation factor and inhibits cap-dependent translation. Phosphorylation of 4EBP1 by the Akt/mTOR pathway (10, 17) disrupts this interaction and results in initiation of cap-dependent translation through eIF4E (45, 48). Phosphorylation of 4EBP1 was significantly increased only by 5 days after DNV. Since protein synthesis was already elevated by 3 days after DNV, activation of 4EBP1 likely does not contribute to the initial increase but possibly contributes to the sustained increase in protein synthesis.
Like the PI3K/Akt pathway, the MAPK/ERK½ pathway is also activated by growth factor signaling and modulates translational control (59). ERK½ activates MAPK-interacting kinases ½ (MNK½), which subsequently phosphorylate eIF4E independently of 4EBP1 phosphorylation (47, 68, 69).
In the current study, phosphorylation of ERK½ decreased immediately after DNV, presumably because of the loss of nerve-derived growth factor or the change in mechanical load. By 1 day after DNV, however, ERK½ phosphorylation increased and remained elevated. In contrast to the delayed increase of 4EBP1, phosphorylation of eIF4E increased by 1 day after DNV. Thus, the increased phosphorylation of ERK½ likely positively affected translation initiation via eIF4E and was mainly responsible for both the initial increase and the sustained elevation in protein synthesis.
Intracellular pathways involved in regulation of protein degradation
Eventually, DIAm DNV leads to net protein loss due to an overwhelming increase in protein degradation (3). We have previously shown that total protein ubiquitination increased by 5 days after DNV, which is when protein degradation increased (3). Neither activation of the caspase-3 pathway nor actin fragmentation was necessary for this increase in degradation. The present study provides novel information regarding the signaling pathways involved in the time-dependent changes in protein degradation after DIAm DNV, namely, FoxO1-induced protein ubiquitination. The role of FoxO in protein degradation has been previously studied in atrophy models. Myotube atrophy induced by fasting or treatment with the glucocorticoid dexamethasone results in upregulation of atrogin-1/muscle atrophy F-box (MAFbx) mRNA, dephosphorylation of Akt, FoxO1, and FoxO3a (54). In addition, FoxO1 transgenic mice display reduced muscle mass, suggesting that FoxO is sufficient to blunt postnatal growth (32).
Most studies support the view that FoxO is negatively regulated by Akt activation (9, 53, 60, 62) (Fig. 1). In the absence of Akt-induced FoxO phosphorylation, FoxO translocates to the nucleus where it can exert its transcriptional activity. The importance of Akt in protein degradation is highlighted by a study using overexpression of a constitutively active form of Akt, where the PI3K/Akt pathway prevents activation of atrophy pathways induced by glucocorticoid treatment of C2C12 myotubes by inhibiting FoxO transcription factors muscle RING finger-1 (MuRF1) and MAFbx (62). In the present study, phosphorylation of Akt was significantly less than sham control at 1 and 6 h after DNV. By 1 and 3 days after DNV, this phosphorylation ratio was back to sham levels. Accordingly, the expression of FoxO1 in the nuclear fraction at 1 day after DNV was significantly increased compared with sham. Taken together, we propose that DNV caused an immediate decrease in Akt activation followed by subsequent translocation of FoxO1 protein to the nucleus at 1 day after DNV, which initiated the transcriptional activity of FoxO1 and initiating atrogene expression (3). Even though the nuclear content of FoxO returned back to sham levels, FoxO1 had likely already accomplished its function: increasing ubiquitin ligase expression, thus, subsequently leading to increased total protein ubiquitination (3). It is possible that another FoxO, e.g., FoxO3a, could have contributed to the increase in protein degradation (38, 54, 76). Unfortunately, we were unable to obtain consistent results with the FoxO3a antibody.
Although AMPK is generally considered to regulate protein synthesis, it may also contribute to regulation of protein degradation. In vitro studies using myotubes showed that activation of AMPK with AICAR treatment (15) stimulated myofibrillar protein degradation and increased atrogin-1/MAFbx expression through FoxO1 and FoxO3a activation (43). Furthermore, AMPK phosphorylation enhanced FoxO3a-dependent transcriptional activity of atrogenes without affecting FoxO3a subcellular localization (21, 22). In the present study, no increase in AMPK phosphorylation was observed after DNV; in fact, AMPK phosphorylation decreased. Future studies could directly activate AMPK by treating DIAm with AICAR to perform direct protein degradation measurements and examine FoxO1 activation. AMPK may play a larger role in protein balance by altering protein synthesis regulation through Akt or mTOR inhibition, but this study suggests that AMPK plays a minor role, if any, in regulating protein degradation following DNV.
It is unknown whether the initial increase in nuclear FoxO1 was sufficient to subsequently activate the ubiquitin-proteasome pathway, even after FoxO returned to baseline. The mechanism for FoxO-induced activation of atrogenes and their subsequent induction of ubiquitin deserves future study. Most studies of the induction of atrogenes after muscle atrophy have focused on mRNA levels (13, 33, 58), which unfortunately may not reflect protein expression. A few recent studies have examined atrogene protein expression (11, 42) although not after DNV. These studies support the importance of examining protein, not mRNA expression levels, when assessing downstream effects on protein degradation. Nevertheless, Akt, FoxO1, and ubiquitin play an important role in upregulating protein degradation after DIAm DNV.
Regulation of Net Protein Balance
This study built on previous work that detailed the significant effects of DIAm DNV on protein synthesis and protein degradation. Perhaps the most important message is that highly contextual changes in signaling pathways are the result of several confounding and time-dependent triggers involved in the regulation of net protein balance. While the focus here has been on DNV, changes in nerve-derived trophic influence, muscle activity, and load are relevant to other conditions such as exercise and aging. No single molecular trigger is responsible for changes in net protein balance. Protein balance in skeletal muscle depends on multiple synthetic/degradation pathways that should be studied in concert.
GRANTS
This work was supported by National Institutes of Health Grant R01 AR-51173 and the Mayo Foundation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Akoumianaki T, Kardassis D, Polioudaki H, Georgatos SD, Theodoropoulos PA. Nucleocytoplasmic shuttling of soluble tubulin in mammalian cells. J Cell Sci 122: 1111–1118, 2009 [DOI] [PubMed] [Google Scholar]
- 2. Anthony JC, Yoshizawa F, Anthony TG, Vary TC, Jefferson LS, Kimball SR. Leucine stimulates translation initiation in skeletal muscle of postabsorptive rats via a rapamycin-sensitive pathway. J Nutr 130: 2413–2419, 2000 [DOI] [PubMed] [Google Scholar]
- 3. Argadine HM, Hellyer NJ, Mantilla CB, Zhan WZ, Sieck GC. The effect of denervation on protein synthesis and degradation in adult rat diaphragm muscle. J Appl Physiol 107: 438–444, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Baar K, Esser K. Phosphorylation of p70S6k correlates with increased skeletal muscle mass following resistance exercise. Am J Physiol Cell Physiol 276: C120–C127, 1999 [DOI] [PubMed] [Google Scholar]
- 5. Bamman MM, Shipp JR, Jiang J, Gower BA, Hunter GR, Goodman A, McLafferty CL, Jr, Urban RJ. Mechanical load increases muscle IGF-I and androgen receptor mRNA concentrations in humans. Am J Physiol Endocrinol Metab 280: E383–E390, 2001 [DOI] [PubMed] [Google Scholar]
- 6. Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, Zlotchenko E, Scrimgeour A, Lawrence JC, Glass DJ, Yancopoulos GD. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol 3: 1014–1019, 2001 [DOI] [PubMed] [Google Scholar]
- 7. Bolster DR, Crozier SJ, Kimball SR, Jefferson LS. AMP-activated protein kinase suppresses protein synthesis in rat skeletal muscle through down-regulated mammalian target of rapamycin (mTOR) signaling. J Biol Chem 277: 23977–23980, 2002 [DOI] [PubMed] [Google Scholar]
- 8. Bray JJ, Harris AJ. Dissociation between nerve-muscle transmission and nerve trophic effects on rat diaphragm using type D botulinum toxin. J Physiol 253: 53–77, 1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 96: 857–868, 1999 [DOI] [PubMed] [Google Scholar]
- 10. Brunn GJ, Hudson CC, Sekulic A, Williams JM, Hosoi H, Houghton PJ, Lawrence JC, Jr, Abraham RT. Phosphorylation of the translational repressor PHAS-I by the mammalian target of rapamycin. Science 277: 99–101, 1997 [DOI] [PubMed] [Google Scholar]
- 11. Cao P, Hanai J, Tanksale P, Imamura S, Sukhatme VP, Lecker SH. Statin-induced muscle damage and atrogin-1 induction is the result of a geranylgeranylation defect. FASEB J 23: 2844–2854, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen J. Novel regulatory mechanisms of mTOR signaling. Curr Top Microbiol Immunol 279: 245–257, 2004 [DOI] [PubMed] [Google Scholar]
- 13. Cho JE, Fournier M, Da X, Lewis MI. Time course expression of Foxo transcription factors in skeletal muscle following corticosteroid administration. J Appl Physiol 108: 137–145, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Copp J, Manning G, Hunter T. TORC-specific phosphorylation of mammalian target of rapamycin (mTOR): phospho-Ser2481 is a marker for intact mTOR signaling complex 2. Cancer Res 69: 1821–1827, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Corton JM, Gillespie JG, Hawley SA, Hardie DG. 5-Aminoimidazole-4-carboxamide ribonucleoside. A specific method for activating AMP-activated protein kinase in intact cells? Eur J Biochem 229: 558–565, 1995 [DOI] [PubMed] [Google Scholar]
- 16. Eliasson J, Elfegoun T, Nilsson J, Kohnke R, Ekblom B, Blomstrand E. Maximal lengthening contractions increase p70 S6 kinase phosphorylation in human skeletal muscle in the absence of nutritional supply. Am J Physiol Endocrinol Metab 291: E1197–E1205, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Gingras AC, Kennedy SG, O'Leary MA, Sonenberg N, Hay N. 4E-BP1, a repressor of mRNA translation, is phosphorylated and inactivated by the Akt(PKB) signaling pathway. Genes Dev 12: 502–513, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Glass DJ. Molecular mechanisms modulating muscle mass. Trends Mol Med 9: 344–350, 2003 [DOI] [PubMed] [Google Scholar]
- 19. Glass DJ. Signalling pathways that mediate skeletal muscle hypertrophy and atrophy. Nat Cell Biol 5: 87–90, 2003 [DOI] [PubMed] [Google Scholar]
- 20. Gosselin LE, Brice G, Carlson B, Prakash YS, Sieck GC. Changes in satellite cell mitotic activity during acute period of unilateral diaphragm denervation. J Appl Physiol 77: 1128–1134, 1994 [DOI] [PubMed] [Google Scholar]
- 21. Greer EL, Dowlatshahi D, Banko MR, Villen J, Hoang K, Blanchard D, Gygi SP, Brunet A. An AMPK-FOXO pathway mediates longevity induced by a novel method of dietary restriction in C. elegans. Curr Biol 17: 1646–1656, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Greer EL, Oskoui PR, Banko MR, Maniar JM, Gygi MP, Gygi SP, Brunet A. The energy sensor AMP-activated protein kinase directly regulates the mammalian FOXO3 transcription factor. J Biol Chem 282: 30107–30119, 2007 [DOI] [PubMed] [Google Scholar]
- 23. Hardie DG, Carling D. The AMP-activated protein kinase–fuel gauge of the mammalian cell? Eur J Biochem 246: 259–273, 1997 [DOI] [PubMed] [Google Scholar]
- 24. Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev 18: 1926–1945, 2004 [DOI] [PubMed] [Google Scholar]
- 25. Hellyer NJ, Mantilla CB, Park EW, Zhan WZ, Sieck GC. Neuregulin-dependent protein synthesis in C2C12 myotubes and rat diaphragm muscle. Am J Physiol Cell Physiol 291: C1056–C1061, 2006 [DOI] [PubMed] [Google Scholar]
- 26. Hoffman EP, Nader GA. Balancing muscle hypertrophy and atrophy. Nat Med 10: 584–585, 2004 [DOI] [PubMed] [Google Scholar]
- 27. Hornberger TA, Chu WK, Mak YW, Hsiung JW, Huang SA, Chien S. The role of phospholipase D and phosphatidic acid in the mechanical activation of mTOR signaling in skeletal muscle. Proc Natl Acad Sci USA 103: 4741–4746, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Inoki K, Ouyang H, Zhu T, Lindvall C, Wang Y, Zhang X, Yang Q, Bennett C, Harada Y, Stankunas K, Wang CY, He X, MacDougald OA, You M, Williams BO, Guan KL. TSC2 integrates Wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth. Cell 126: 955–968, 2006 [DOI] [PubMed] [Google Scholar]
- 29. Jacinto E, Hall MN. Tor signalling in bugs, brain and brawn. Nat Rev Mol Cell Biol 4: 117–126, 2003 [DOI] [PubMed] [Google Scholar]
- 30. Jefferson LS, Fabian JR, Kimball SR. Glycogen synthase kinase-3 is the predominant insulin-regulated eukaryotic initiation factor 2B kinase in skeletal muscle. Int J Biochem Cell Biol 31: 191–200, 1999 [DOI] [PubMed] [Google Scholar]
- 31. Jorgensen SB, Richter EA, Wojtaszewski JF. Role of AMPK in skeletal muscle metabolic regulation and adaptation in relation to exercise. J Physiol 574: 17–31, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kamei Y, Miura S, Suzuki M, Kai Y, Mizukami J, Taniguchi T, Mochida K, Hata T, Matsuda J, Aburatani H, Nishino I, Ezaki O. Skeletal muscle FOXO1 (FKHR) transgenic mice have less skeletal muscle mass, down-regulated Type I (slow twitch/red muscle) fiber genes, and impaired glycemic control. J Biol Chem 279: 41114–41123, 2004 [DOI] [PubMed] [Google Scholar]
- 33. Kim SJ, Roy RR, Kim JA, Zhong H, Haddad F, Baldwin KM, Edgerton VR. Gene expression during inactivity-induced muscle atrophy: effects of brief bouts of a forceful contraction countermeasure. J Appl Physiol 105: 1246–1254, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kimball SR, Jurasinski CV, Lawrence JC, Jr, Jefferson LS. Insulin stimulates protein synthesis in skeletal muscle by enhancing the association of eIF-4E and eIF-4G. Am J Physiol Cell Physiol 272: C754–C759, 1997 [DOI] [PubMed] [Google Scholar]
- 35. King TD, Song L, Jope RS. AMP-activated protein kinase (AMPK) activating agents cause dephosphorylation of Akt and glycogen synthase kinase-3. Biochem Pharmacol 71: 1637–1647, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kleijn M, Scheper GC, Voorma HO, Thomas AA. Regulation of translation initiation factors by signal transduction. Eur J Biochem 253: 531–544, 1998 [DOI] [PubMed] [Google Scholar]
- 37. Lantier L, Mounier R, Leclerc J, Pende M, Foretz M, Viollet B. Coordinated maintenance of muscle cell size control by AMP-activated protein kinase. FASEB J 24: 3555–3561 [DOI] [PubMed] [Google Scholar]
- 38. Mammucari C, Milan G, Romanello V, Masiero E, Rudolf R, Del Piccolo P, Burden SJ, Di Lisi R, Sandri C, Zhao J, Goldberg AL, Schiaffino S, Sandri M. FoxO3 controls autophagy in skeletal muscle in vivo. Cell Metab 6: 458–471, 2007 [DOI] [PubMed] [Google Scholar]
- 39. Martelli AM, Tazzari PL, Evangelisti C, Chiarini F, Blalock WL, Billi AM, Manzoli L, McCubrey JA, Cocco L. Targeting the phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin module for acute myelogenous leukemia therapy: from bench to bedside. Curr Med Chem 14: 2009–2023, 2007 [DOI] [PubMed] [Google Scholar]
- 40. Mascher H, Andersson H, Nilsson PA, Ekblom B, Blomstrand E. Changes in signalling pathways regulating protein synthesis in human muscle in the recovery period after endurance exercise. Acta Physiol (Oxf) 191: 67–75, 2007 [DOI] [PubMed] [Google Scholar]
- 41. Miyata H, Zhan WZ, Prakash YS, Sieck GC. Myoneural interactions affect diaphragm muscle adaptations to inactivity. J Appl Physiol 79: 1640–1649, 1995 [DOI] [PubMed] [Google Scholar]
- 42. Moylan JS, Smith JD, Chambers MA, McLoughlin TJ, Reid MB. TNF induction of atrogin-1/MAFbx mRNA depends on Foxo4 expression but not AKT-Foxo1/3 signaling. Am J Physiol Cell Physiol 295: C986–C993, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nakashima K, Yakabe Y. AMPK activation stimulates myofibrillar protein degradation and expression of atrophy-related ubiquitin ligases by increasing FOXO transcription factors in C2C12 myotubes. Biosci Biotechnol Biochem 71: 1650–1656, 2007 [DOI] [PubMed] [Google Scholar]
- 44. Pallafacchina G, Calabria E, Serrano AL, Kalhovde JM, Schiaffino S. A protein kinase B-dependent and rapamycin-sensitive pathway controls skeletal muscle growth but not fiber type specification. Proc Natl Acad Sci USA 99: 9213–9218, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pause A, Belsham GJ, Gingras AC, Donze O, Lin TA, Lawrence JC, Jr, Sonenberg N. Insulin-dependent stimulation of protein synthesis by phosphorylation of a regulator of 5′-cap function. Nature 371: 762–767, 1994 [DOI] [PubMed] [Google Scholar]
- 46. Peterson RT, Beal PA, Comb MJ, Schreiber SL. FKBP12-rapamycin-associated protein (FRAP) autophosphorylates at serine 2481 under translationally repressive conditions. J Biol Chem 275: 7416–7423, 2000 [DOI] [PubMed] [Google Scholar]
- 47. Raught B, Gingras AC. eIF4E activity is regulated at multiple levels. Int J Biochem Cell Biol 31: 43–57, 1999 [DOI] [PubMed] [Google Scholar]
- 48. Reynolds TH, 4th, Bodine SC, Lawrence JC., Jr Control of Ser2448 phosphorylation in the mammalian target of rapamycin by insulin and skeletal muscle load. J Biol Chem 277: 17657–17662, 2002 [DOI] [PubMed] [Google Scholar]
- 49. Rommel C, Bodine SC, Clarke BA, Rossman R, Nunez L, Stitt TN, Yancopoulos GD, Glass DJ. Mediation of IGF-1-induced skeletal myotube hypertrophy by PI(3)K/Akt/mTOR and PI(3)K/Akt/GSK3 pathways. Nat Cell Biol 3: 1009–1013, 2001 [DOI] [PubMed] [Google Scholar]
- 50. Rommel C, Clarke BA, Zimmermann S, Nunez L, Rossman R, Reid K, Moelling K, Yancopoulos GD, Glass DJ. Differentiation stage-specific inhibition of the Raf-MEK-ERK pathway by Akt. Science 286: 1738–1741, 1999 [DOI] [PubMed] [Google Scholar]
- 51. Rose AJ, Bisiani B, Vistisen B, Kiens B, Richter EA. Skeletal muscle eEF2 and 4EBP1 phosphorylation during endurance exercise is dependent on intensity and muscle fiber type. Am J Physiol Regul Integr Comp Physiol 296: R326–R333, 2009 [DOI] [PubMed] [Google Scholar]
- 52. Saito Y, Vandenheede JR, Cohen P. The mechanism by which epidermal growth factor inhibits glycogen synthase kinase 3 in A431 cells. Biochem J 303: 27–31, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sandri M. Signaling in muscle atrophy and hypertrophy. Physiology (Bethesda) 23: 160–170, 2008 [DOI] [PubMed] [Google Scholar]
- 54. Sandri M, Sandri C, Gilbert A, Skurk C, Calabria E, Picard A, Walsh K, Schiaffino S, Lecker SH, Goldberg AL. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell 117: 399–412, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sarbassov DD, Ali SM, Kim DH, Guertin DA, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol 14: 1296–1302, 2004 [DOI] [PubMed] [Google Scholar]
- 56. Sarbassov DD, Ali SM, Sabatini DM. Growing roles for the mTOR pathway. Curr Opin Cell Biol 17: 596–603, 2005 [DOI] [PubMed] [Google Scholar]
- 57. Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 307: 1098–1101, 2005 [DOI] [PubMed] [Google Scholar]
- 58. Senf SM, Dodd SL, Judge AR. FOXO signaling is required for disuse muscle atrophy and is directly regulated by Hsp70. Am J Physiol Cell Physiol 298: C38–C45, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Shah OJ, Anthony JC, Kimball SR, Jefferson LS. 4E-BP1 and S6K1: translational integration sites for nutritional and hormonal information in muscle. Am J Physiol Endocrinol Metab 279: E715–E729, 2000 [DOI] [PubMed] [Google Scholar]
- 60. Skurk C, Maatz H, Kim HS, Yang J, Abid MR, Aird WC, Walsh K. The Akt-regulated forkhead transcription factor FOXO3a controls endothelial cell viability through modulation of the caspase-8 inhibitor FLIP. J Biol Chem 279: 1513–1525, 2004 [DOI] [PubMed] [Google Scholar]
- 61. Smith JW, Thesleff S. Spontaneous activity in denervated mouse diaphragm muscle. J Physiol 257: 171–186, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Stitt TN, Drujan D, Clarke BA, Panaro F, Timofeyva Y, Kline WO, Gonzalez M, Yancopoulos GD, Glass DJ. The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol Cell 14: 395–403, 2004 [DOI] [PubMed] [Google Scholar]
- 63. Takahashi A, Kureishi Y, Yang J, Luo Z, Guo K, Mukhopadhyay D, Ivashchenko Y, Branellec D, Walsh K. Myogenic Akt signaling regulates blood vessel recruitment during myofiber growth. Mol Cell Biol 22: 4803–4814, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Thimmaiah KN, Easton JB, Germain GS, Morton CL, Kamath S, Buolamwini JK, Houghton PJ. Identification of N10-substituted phenoxazines as potent and specific inhibitors of Akt signaling. J Biol Chem 280: 31924–31935, 2005 [DOI] [PubMed] [Google Scholar]
- 65. Vandenburgh HH, Karlisch P, Shansky J, Feldstein R. Insulin and IGF-I induce pronounced hypertrophy of skeletal myofibers in tissue culture. Am J Physiol Cell Physiol 260: C475–C484, 1991 [DOI] [PubMed] [Google Scholar]
- 66. Walss C, Kreisberg JI, Luduena RF. Presence of the betaII isotype of tubulin in the nuclei of cultured mesangial cells from rat kidney. Cell Motil Cytoskeleton 42: 274–284, 1999 [DOI] [PubMed] [Google Scholar]
- 67. Wang X, Proud CG. The mTOR pathway in the control of protein synthesis. Physiology (Bethesda) 21: 362–369, 2006 [DOI] [PubMed] [Google Scholar]
- 68. Waskiewicz AJ, Flynn A, Proud CG, Cooper JA. Mitogen-activated protein kinases activate the serine/threonine kinases Mnk1 and Mnk2. EMBO J 16: 1909–1920, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Waskiewicz AJ, Johnson JC, Penn B, Mahalingam M, Kimball SR, Cooper JA. Phosphorylation of the cap-binding protein eukaryotic translation initiation factor 4E by protein kinase Mnk1 in vivo. Mol Cell Biol 19: 1871–1880, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Williamson DL, Bolster DR, Kimball SR, Jefferson LS. Time course changes in signaling pathways and protein synthesis in C2C12 myotubes following AMPK activation by AICAR. Am J Physiol Endocrinol Metab 291: E80–E89, 2006 [DOI] [PubMed] [Google Scholar]
- 71. Xu K, Luduena RF. Characterization of nuclear betaII-tubulin in tumor cells: a possible novel target for taxol. Cell Motil Cytoskeleton 53: 39–52, 2002 [DOI] [PubMed] [Google Scholar]
- 72. Zanchi NE, Lancha AH., Jr Mechanical stimuli of skeletal muscle: implications on mTOR/p70s6k and protein synthesis. Eur J Appl Physiol 102: 253–263, 2008 [DOI] [PubMed] [Google Scholar]
- 73. Zhan WZ, Farkas GA, Schroeder MA, Gosselin LE, Sieck GC. Regional adaptations of rabbit diaphragm muscle fibers to unilateral denervation. J Appl Physiol 79: 941–950, 1995 [DOI] [PubMed] [Google Scholar]
- 74. Zhan WZ, Miyata H, Prakash YS, Sieck GC. Metabolic and phenotypic adaptations of diaphragm muscle fibers with inactivation. J Appl Physiol 82: 1145–1153, 1997 [DOI] [PubMed] [Google Scholar]
- 75. Zhan WZ, Sieck GC. Adaptations of diaphragm and medial gastrocnemius muscles to inactivity. J Appl Physiol 72: 1445–1453, 1992 [DOI] [PubMed] [Google Scholar]
- 76. Zhao J, Brault JJ, Schild A, Cao P, Sandri M, Schiaffino S, Lecker SH, Goldberg AL. FoxO3 coordinately activates protein degradation by the autophagic/lysosomal and proteasomal pathways in atrophying muscle cells. Cell Metab 6: 472–483, 2007 [DOI] [PubMed] [Google Scholar]