Abstract
Cyclic AMP produced from membrane receptor complex bound adenylyl cyclases is protective in corneal endothelial cells (CEC). CEC also express soluble adenylyl cyclase (sAC), which is localized throughout the cytoplasm. When activated by HCO3−, cAMP concentration ([cAMP]) increases by ∼50%. Here we ask if cAMP produced from sAC is also protective. We examined the effects of HCO3−, pH, phosphodiesterase 4 inhibition by rolipram, sAC inhibition by 2HE (2-hydroxyestradiol), and sAC small interfering RNA (siRNA) knockdown on basal and staurosporine-mediated apoptosis. HCO3− (40 mM) or 50 μM rolipram raised [cAMP] to similar levels and protected endothelial cells by 50% relative to a HCO3−-free control, whereas 2HE, which decreased [cAMP] by 40%, and H89 (PKA inhibitor) doubled the apoptotic rate. sAC expression was reduced by two-thirds in the absence of HCO3− and was reduced to 15% of control by sAC siRNA. Protection by HCO3− was eliminated in siRNA-treated cells. Similarly, caspase-3 activity and cytochrome c release were reduced by HCO3− and enhanced by 2HE or siRNA. Analysis of percent annexin V+ cells as a function of [cAMP] revealed an inverse, nonlinear relation, suggesting a protective threshold [cAMP] of 10 pmol/mg protein. Relative levels of phosphorylated cAMP response element binding protein and phosphorylated Bcl-2 were decreased in CEC treated with 2HE or siRNA, suggesting that HCO3−-dependent endogenous sAC activity can mobilize antiapoptotic signal transduction. Overall, our data suggest a new role for sAC in endogenous cellular protection.
Keywords: adenosine 3′,5′-cyclic monophosphate; 2-hydroxyestradiol; protein kinase A; phosphorylated Bcl-2
the barrier function and transport properties of the corneal endothelium are responsible for maintaining the hydration and transparency of the cornea. During aging, corneal endothelial cell (CEC) density progressively decreases by ∼0.5% per year; however, in most individuals, this cell loss does not result in functional deficits, i.e., corneal edema, because of a large functional reserve (10). Following surgery or trauma, or in specific corneal endothelial dystrophies, however, the endothelial cell density can drop to a threshold of 500–700 cells/mm2, and loss of hydration control occurs (27). Because the corneal endothelium does not undergo mitosis in vivo, slowing the loss of endothelial cells using protective strategies will be useful in these cases. Moreover, loss of CECs during eye banking (19, 24) and subsequent accelerated loss following corneal grafting are significant clinical problems (13).
Increasing cell cAMP concentration ([cAMP]) is often associated with cell protection; however, depending on cell type and the change in downstream signaling, increased [cAMP] can also be proapoptotic. For example, protection has been documented in many tissues via stimulation of membrane receptors that lead to increased [cAMP], such as cardiac β-adrenergic receptors (26), vascular adenosine A2b receptors (39), PGE2 receptors (21), and brain pituitary adenylate cyclase-activating peptide-38 (14) that typically produce protein kinase A (PKA)-dependent phosphorylation of Bcl-2 and other apoptotic factors, as well as phosphorylating the cAMP response element binding protein (CREB), leading to enhanced expression of antiapoptotic factors Bcl-2 and Bcl-xL (42). Conversely, increased [cAMP] promotes cell death in lymphoid cells by suppressing expression of antiapoptotic members of the Bcl-2 family or enhancement of proapoptotic factors (6, 28, 32, 41).
Exposing the corneal endothelium to vasoactive intestinal peptide (VIP), which increases [cAMP] by activation of VIP receptor Gs-linked transmembrane adenylyl cyclase (tmAC) (17), provides protection against H2O2-induced apoptosis (18). VIP increased expression of Bcl-2 and led to PKA-dependent Bcl-2 phosphorylation (16). Studies of graft survival have shown that viable donor corneas were found to highly express Bcl-2. Furthermore, overexpression of Bcl-xL prolongs graft survival (1), indicating that raising cAMP could be useful for protecting CECs.
Stimulation of VIP or adenosine A2b receptors in CECs can produce severalfold increases in [cAMP] (17, 35) through activation of tmACs. However, stimulation is generally transient, and receptors are often actively downregulated. Another source of cAMP in many cell types, including corneal endothelium, is from soluble adenylyl cyclase (sAC). sAC can be distributed throughout the cytoplasm and mitochondria, including the nucleus, and activation can lead to phosphorylation of CREB (43). sAC is not membrane bound and not activated by the tmAC activator forskolin (4). The main stimulatory ligand for sAC is HCO3− (38). Bathing CECs in DMEM medium containing 40 mM HCO3− raised [cAMP] by 50% relative to the absence of HCO3− (33). Thus sAC produces a relatively low, but steady, supply of cAMP within the cell. Here we ask if the cAMP produced by sAC is sufficient to protect CECs from staurosporine (SP)-induced apoptosis. We found that HCO3−/sAC/cAMP is protective and that the effect may be through activation of Bcl-2.
MATERIALS AND METHODS
Materials.
N-[2-(p-bromocinnamylamino)ethyl]-5-isoquinollnesulfonamide (H89; LC Laboratories) is a potent and selective inhibitor of PKA. 2-Hydroxyestradiol (2HE, Sigma) is an inhibitor of sAC (31). Staurosporine (SP) (Sigma) is (a nonspecific protein kinase inhibitor) a strong inducer of apoptosis (2). 4-(3-Cyclopentoxy-4-[11C]methoxy-phenyl)pyrrolidin-2-one (Rolipram, Sigma) is a cAMP-specific phosphodiesterase (PDE) inhibitor (36). Mouse anti-sAC antibody was from FabGennix (SAC-101-AP). Mouse anti-β-actin (Sigma) was used for loading control. Goat anti-mouse IgG (Sigma, 7074) was conjugated to horseradish peroxidase. Mouse anti-cytochrome c was from MitoScience (MSA06). CREB, phosphorylated CREB (pCREB), Bcl-2, and phosphorylated Bcl-2 (pBcl-2) antibodies were obtained from Cell Signaling. Direct cAMP detection kit was from Assay Designs (no. 900–066). Annexin V (AnV)-FITC apoptosis detection kit was from BioVision (K101). Caspase-3 activity detection kit (Caspase Assay System) was from Promega.
Cell culture and stimulation.
Bovine CECs (BCEC) were isolated from fresh bovine eyes, obtained from a local slaughterhouse, as previously described (34). In brief, corneal endothelium was digested with 2.5% trypsin-EDTA at 37°C for 15 min. The cells were collected by centrifugation at 6,000 g for 5 min and transferred into a T-25 flask that contained 5 ml of DMEM medium supplemented with 10% bovine calf serum and 1% antibiotic-antimycotic (100 U/ml penicillin, 100 μg/ml streptomycin, and 0.25 μg/ml Fungizone). The cells were incubated at 37°C in a humidified incubator with 5% CO2. The cells were split twice. When the third-passage BCEC grew to 90% confluent, they were seeded onto six-well plates or coverslips at a density of 1,000 cells/mm2, and grown to 60 ∼ 90% confluence before changing to either bicarbonate-rich (BR) or bicarbonate-free (BF) culture. In BR culture, the cells were grown in DMEM medium containing 40 mM HCO3−, 25 mM HEPES, pH 7.5, and incubated in a 5% CO2 atmosphere. In BF culture, the cells were grown in DMEM medium containing 25 mM HEPES, but 0 HCO3−, pH 7.5, and placed in an air-equilibrated incubator. BF and BR incubation was in serum-free DMEM for 48 h.
For cell treatments, BCEC grown in either BF or BR media for 48 h were incubated an additional 17 h with either 10 μM 2HE, 10 μM H89, 0.1 μM SP, or 50 μM rolipram. After treatment, cells were dissociated using trypsin for flow cytometry, or lysed for caspase assay, cytochrome c fractionation, cAMP assay, and Western analysis.
sAC small interfering RNA synthesis and transfection.
Four potential sAC small interfering RNA (siRNA) primers were selected using the Ambion siRNA design tool (http://www.ambion.com/techlib/misc/siRNA_finder.html). The primer oligos were synthesized by Invitrogen. The sequences are listed below. siRNAs were generated using the silencer siRNA construction kit (Ambion, 1620): I: sense AAATCATTCATCTGAGCCATGCCTGTCTC, antisense AACATGGCTCAGATGAATGATCCTGTCTC; II: sense AATGGTGACAGAATAACATCACCTGTCTC, antisense AATGATGTTATTCTGTCACCACCTGTCTC; III: sense AAGTATGAATTCCAAACATTCCCTGTCTC, antisense AAGAATGTTTGGAATTCATACCCTGTCTC; IV: sense AATGTTTGGAATTCATACACCCCTGTCTC, antisense AAGGTGTATGAATTCCAAACACCCTGTCTC.
Sequence I was the most efficient, resulting in 70–80% sAC silencing.
In siRNA transfection experiments, BCEC were grown in six-well plates. At 50% confluence, the cells were washed twice with Opti-MEM (Invitrogen) to remove serum and antibiotics. For a 2-ml culture, 2 μl of lipofectamine 2000 were used to transfer either 10 nM of scrambled siRNA (siCon, Dharmacon) or 10 nM of sAC siRNA. The transfection was maintained at 37°C for 4 h. The transfection medium was then replaced with either BR or BF medium. Maximum sAC knockdown was achieved at 48 h posttransfection.
Direct cAMP enzyme immunoassay.
BCEC were grown in BR or BF in six-well plates. After specific treatment, culture media was removed. Cellular cAMP was extracted by incubating the cells with 0.1 N HCl (100 μl/well) at room temperature for 30 min. The solution was collected by centrifugation at 6,000 g for 10 min. Clear supernatant was used for measuring [cAMP] with an ELISA kit (Assay Designs) following the manufacturer's instructions.
Apoptosis assessment with 4,6-diamidino-2-phenylindole staining.
BCEC grown on coverslips were incubated in either BF or BR medium for 48 h (∼90% confluent). The cells were then treated with DMSO or 0.1 μM SP for 17 h. After being washed twice with PBS, the cells were immediately fixed in 4% paraformaldehyde-lysine-periodate buffer for 10 min. The nuclei were stained with 300 nM 4,6-diamidino-2-phenylindole for 2 min. The coverslips were washed twice with nanopure water, air-dried, and mounted with Prolong anti-fade solution (Invitrogen). The coverslips were examined the next day for condensed nuclei using a Nikon E800 microscope using a ×20 objective.
Apoptosis assessment by flow cytometry.
BCEC grown in six-well plates at 90% confluence were treated with 10 μM 2HE, 10 μM H89, 0.1 μM SP, or 50 μM rolipram for 17 h. The cells were dissociated using trypsin and collected by centrifugation at 6,000 g for 5 min. The cells were labeled using the AnV-FITC apoptosis kit (BioVision). In brief, cell pellets were washed twice with AnV buffer and suspended in the same buffer. Five microliters of AnV-FITC and 5 μl of propidium iodide were added to 5 × 105 cells in 500 μl AnV buffer and incubated at room temperature for 5 min in the dark, and flow cytometry was performed immediately with a counting threshold of 1 × 104 cells.
Western blot analysis.
Cells were washed twice with PBS and lysed with RIPA buffer containing 1× protease inhibitor cocktail (Sigma), 1% SDS, 1% Nonidet P-40, 0.5% deoxycholic acid, 150 mM NaCl, 50 mM Tris-base, and 3 nM calyculin A (Sigma). Cell lysate was briefly sonicated on ice (10 pulses, pulse duration 0.2 s) and then pelleted at 13,000 g for 10 min. The supernatant (20 μg) was used for protein separation by 10% SDS-PAGE. Blots were probed with sAC and cytochrome c antibodies and β-actin as a loading control. Films were scanned and band density determined using UN-SCAN-IT software.
Cytochrome c analysis.
BCEC were lysed for separating the cytosol fraction from mitochondria using a cytosol/mitochondria kit (Calbiochem, QIA88). Briefly, 5 × 106 cells were washed with ice-cold PBS, trypsinized, pelleted, and incubated with 100 μl cytosol extraction buffer on ice for 10 min. The cells were homogenized with 30 passes of a dounce tissue homogenizer. The homogenate was centrifuged at 10,000 g for 30 min at 4°C. A 20-μg protein sample of the cytosolic (supernatant) fraction was loaded on a 16% SDS-PAGE to perform Western blotting as described above. A monoclonal anti-cytochrome c antibody developed from mouse was used for detection. β-Actin was checked as a loading control. Protein density was measured using UN-SCAN-IT software.
Assessment of caspase-3 activity.
BCEC were grown in six-well plates as two sets of cultures. Both sets were treated the same, except one set received the caspase-3 inhibitor Z-VAD-FMK (5 μM). Cell extracts were made and analyzed using the CaspACE Assay System (Promega), following the manufacturer's instructions. Caspase-3 activity was indicated by absorbance at 405 nm.
Statistical analysis.
Student's t-test was employed to analyze differences between BF and BR culture, and between control and treated groups. P ≤ 0.05 was considered statistically significant. All data were from three or more independent experiments and expressed as means ± SE.
RESULTS
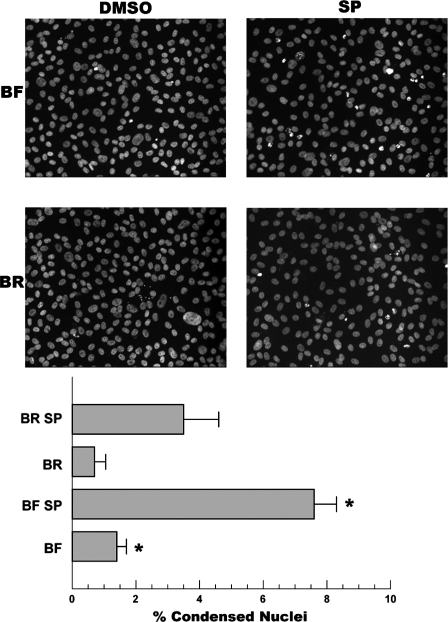
We first asked if the presence of HCO3−, which activates sAC and increases [cAMP] in CEC by ∼50% (33), was protective relative to a BF control. Cells were preincubated in BR or BF DMEM for 48 h before application of 0.1 μM SP, a general protein kinase C inhibitor and known apoptosis initiator, for 17 h. Cells were fixed and examined for apoptosis by staining for condensed/fragmented nuclei. Figure 1 shows a representative set of images and analysis, indicating that incubation in BR was protective against SP-induced apoptosis.
Fig. 1.
HCO3− protects corneal endothelial cells. Confluent cultured bovine corneal endothelium cells (BCEC) seeded on coverslips were incubated with either bicarbonate-rich (BR; 40 mM HCO3−) medium or bicarbonate-free (BF; 0 mM HCO3−) DMEM for 48 h. Cells were then treated with 50 nM staurosporine (SP) for 17 h and fixed, and the nuclei was stained with 4,6-diamidino-2-phenylindole. Representative micrographs (×20 objective) show fewer condensed nuclei in BR-incubated cells. Percent condensed nuclei per field were counted. Values are means ± SE; n = 3 separate experiments. *Significantly different from BR or BR + SP, respectively; P < 0.05.
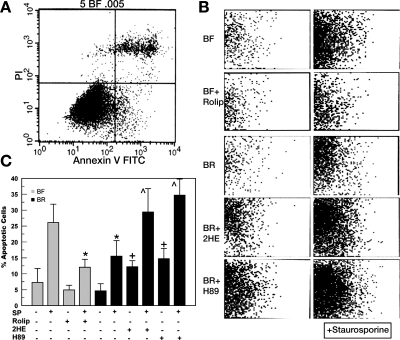
To provide a quantitative assessment of protection of an earlier phase of apoptosis, cells incubated in BF and BR were examined for AnV (AnV+) staining by flow cytometric analysis. Figure 2A shows a representative flow cytometry result for cells incubated in BF media, where the bottom right quadrant represents apoptotic cells, i.e., AnV+ and propidium iodide negative. Figure 2B shows this apoptosis quadrant for the various treatments, and the data are summarized in Fig. 2C. There was a small increase in AnV+ cells in BF vs. BR, but this was not significant. However, SP-induced apoptosis was reduced 43% (P < 0.01) by incubation in BR relative to BF. Next, we tested a specific sAC inhibitor, 2HE, and found that 10 μM 2HE was optimum in reducing [cAMP] (see Fig. 3). Figure 2C shows that inhibition of sAC activity by 2HE significantly increased %AnV+ cells in BR, with or without SP treatment. To test if this protective effect of sAC was through cAMP-dependent activation of PKA, we incubated cells in the PKA inhibitor H89. Similar to 2HE, we found that H89 significantly increased AnV+ cells, with or without staurosporine treatment. If protection of CEC in BR medium is through stimulation of sAC and increased [cAMP], then increasing [cAMP] to a similar level by other means should also protect cells. Cell [cAMP] was increased in cells bathed in BF by inhibiting PDE4 with 50 μM rolipram. This increases basal levels of cAMP approximately to 32.5 pmol/mg protein, which is similar to the cellular cAMP level in BR (34). Figure 2C shows that bathing cells in BF with rolipram significantly decreased SP-induced apoptosis (n = 5, P < 0.02). This level of cell viability was very similar to that seen with cells incubated in BR and stressed with SP and suggests that protection of CEC in BR is by a HCO3−-induced increase in intracellular [cAMP].
Fig. 2.
Quantitative assessment of apoptosis by flow cytometry. A: representative result from cells incubated in BF media; y-axis, propidium iodide (PI) fluorescence; x-axis, annexin V FITC fluorescence. Bottom right quadrant represents apoptotic portion of population. B: bottom right quadrants from representative experiments of cells incubated in BF or BR media and treated with 50 μM rolipram (Rolip), 10 μM 2-hydroxyestradiol (2HE), or 10 μM N-[2-(p-bromocinnamylamino)ethyl]-5-isoquinollnesulfonamide (H89), ± 10 nM SP. C: summary data. Values are means ± SE; n = 5. *Significantly lower than BF + SP; + significantly higher than BR; ^ significantly higher than BR + SP: P < 0.05.
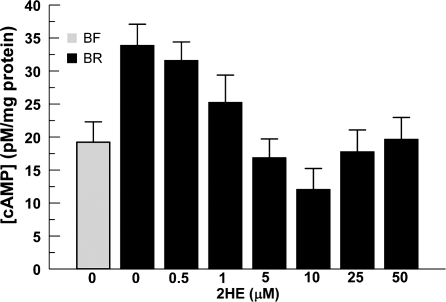
Fig. 3.
Quantitative assessment of cyclic AMP (cAMP). Cellular cAMP was extracted from BCEC with 0.1 N HCl. BCEC were cultured either in BF or BR. Increasing concentrations of 2HE were applied for 17 h to the cells that were grown in BR before extracting cAMP. The data were an average of at least three independent experiments and expressed as means ± SE.
Previous studies have shown that CEC intracellular pH (pHi) is somewhat higher in BR medium (pHi 7.32) than in BF (pHi 7.15) (3). To determine whether the protection of CEC observed in BR could be due to these differences in pHi, we preincubated cells in BF medium with bath pH adjusted to 7.0, 7.5, and 8.0 for 48 h. These extracellular pH values corresponded to pHi of 6.88, 7.12, and 7.32, respectively (34). This incubation was followed by 50 nM SP (17 h) treatment. Flow cytometry quantification of AnV+ cells indicated no significant difference (P > 0.05, n = 3) at the three pH levels, either in control or SP-treated cultures (data not shown), which is consistent with previous studies showing that sAC expression or [cAMP] did not significantly change with pH (33, 34). These data indicate that the protection in BR seen in Figs. 1 and 2 is not simply due to differences in pHi.
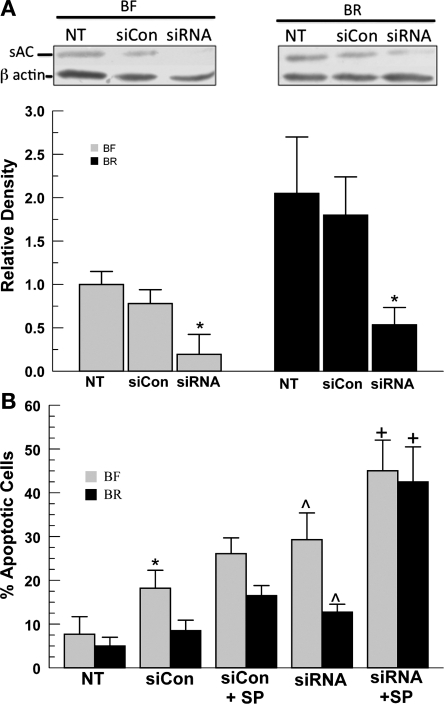
To definitively determine that the protection of CEC in BR solutions is due to sAC activity, we used a siRNA approach to knockdown sAC expression. Expression of sAC was examined in cells incubated in BR or BF, each under three conditions: no treatment, scrambled sequence control siRNA (siCon), and sAC-specific siRNA transfection. A representative Western blot and summary data are shown in Fig. 4A. First, the absence of HCO3− reduces sAC expression by at least twofold relative to BR. This differential expression was also reported in a previous study (33). The cause is not known, but is not due to differences in pHi or [cAMP] between the two conditions (33). Second, siCon transfection (lipofectamine + scrambled sequence siRNA) produced a small drop in sAC expression in BF and a slightly larger decrease in BR; however, neither change was statistically significant. Last, treatment with the sAC-specific siRNA produced significant decreases in sAC expression in BR (−80%, P < 0.03) and BF (−85%, P < 0.01) relative to no treatment, whereas the decrease in sAC expression relative to the scrambled control was 71 and 75% (P < 0.05), respectively, in BR and BF. In summary, the sAC-specific siRNA is effective; however, sAC expression is somewhat sensitive to the transfection process.
Fig. 4.
Effect of soluble adenylyl cyclase (sAC) small interfering RNA (siRNA) on basal and SP-induced apoptosis. A, top: sAC expression and siRNA transfection under BF and BR conditions. Nontransfected cells (NT), cells transfected with scrambled control siRNA sequences (siCon), and cells transfected with sAC-specific siRNA (siRNA) were analyzed for sAC expression (50 kDa) relative to β-actin by Western blot 48 h following transfection. Bottom: densitometric analysis of sAC expression normalized to β-actin and plotted relative to BF no treatment. Values are means ± SE; n = 3. *Significantly less sAC expression (P < 0.05 by paired t-test). B: flow cytometric analysis (annexin V+ cells) of endothelial cells incubated in BF or BR media for 48 h following transfection with siCon or sAC siRNA and treated with SP. Values are means ± SE; n = 7. *Significantly different from NT; ^ significantly different from siCon; + significantly different from siCon + SP: P < 0.05.
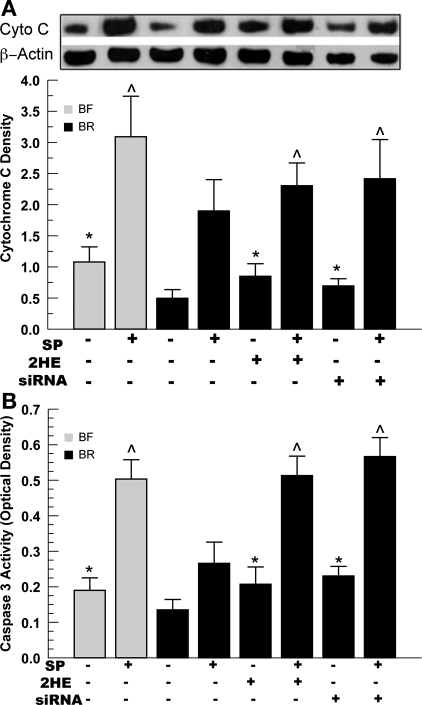
Using cells treated under the same three conditions, we examined the proportion of AnV+ cells by flow cytometry. Figure 4B shows that the transfection process with the scrambled control (siCon) produced a significant increase in apoptosis in cells bathed in BF, but not BR. However, sAC-specific siRNA treatment significantly increased apoptosis relative to siCon in both BF and BR incubated cells. Figure 4B also shows that, in the presence of the apoptosis inducer SP, siCon-treated cells were still significantly protected in BR (16.5% AnV+) relative to BF (26.1%, n = 5, P < 0.05). However, SP-induced apoptosis of sAC siRNA-transfected cells in BF and BR showed a very similar level of AnV+ staining (45% for BF vs. 42.5% for BR, n = 5, P > 0.05). Analyses using two other markers of apoptosis, cytochrome c release and caspase-3 activity, showed similar results. Figure 5 shows that 2HE and sAC siRNA significantly increased caspase-3 activity and cytochrome c release in both untreated and SP-treated cells. Elimination of the protective effect of HCO3− in sAC siRNA-treated cells indicates that sAC provided significant protection from apoptosis in CECs.
Fig. 5.
Examination of cytchrome c (Cyto C) release and caspase-3 activity. BCECs (5 × 106) were incubated in BF or BR media for 48 h. Cells were treated with 2HE (10 μM) or transfected with 10 nM sAC siRNA ± 0.1 μM SP. A: cytoplasmic extracts were made using a cytosol/mitochondria fractionation kit, as described in materials and methods. Anti-β-actin served as a loading control. Values are means ± SE; n = 3. *Significantly higher than BR; ^ significantly higher than BR + SP: P < 0.05. B: caspase-3 activity (see materials and methods for details). Values are means ± SE; n = 3. *Significantly higher than BR; ^ significantly higher than BR + SP: P < 0.05.
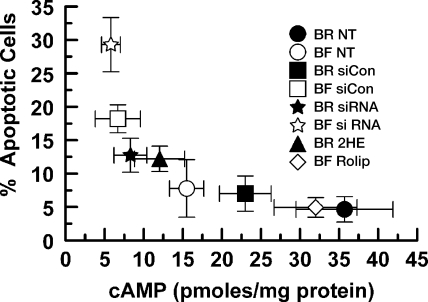
Protection by sAC should be related to the [cAMP]. We measured [cAMP] of cells incubated in BR, BF, cells exposed to siCon or sAC-specific siRNA transfection, as well as rolipram, and 2HE treatments. These data are shown in Fig. 6 and are plotted against the corresponding apoptotic rate (from Figs. 2, 4, and 5). We found a nonlinear relation between %apoptotic cells and [cAMP], suggesting that there is a threshold increase in apoptosis when [cAMP] drops below ∼10 pmol/mg.
Fig. 6.
Relation between apoptotic rate and cAMP concentration ([cAMP]). Apoptotic rates (shown in Figs. 2, 4, and 5) are plotted against [cAMP] for cells incubated in BR or BF media and treated with or without 50 μM rolipram, 10 μM 2HE, or transfected with siCon or sAC siRNA. [cAMP] is from triplicate experiments. Values are means ± SE.
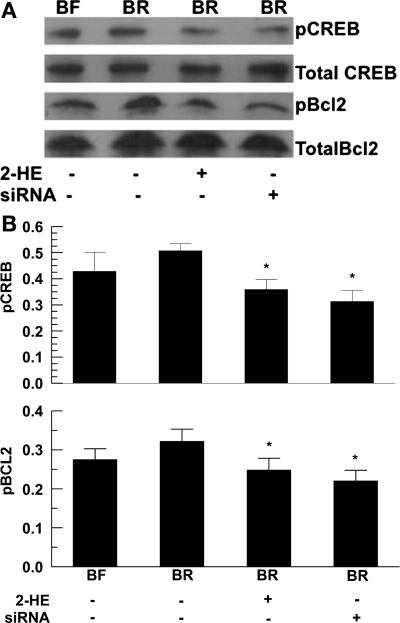
Last, protection that is associated with increasing [cAMP] suggests that the antiapoptotic factor Bcl-2 is being activated (16, 17). We explored this by examining CREB and Bcl-2 phosphorylation in CEC incubated in BF or BR and treated with 2HE or siRNA. Figure 7 shows that, in BR-incubated cells, both pCREB and pBcl-2 were elevated; however, this was not statistically significant. However, treatment with 2HE or siRNA significantly reduced pCREB and pBcl-2. This result, together with our finding that the PKA inhibitor H89 significantly increased apoptosis (Fig. 2), suggests that the sAC-derived cAMP protects CECs in a PKA-dependent manner.
Fig. 7.
Effect of HCO3− and sAC inhibition on phosphorylated cAMP response element binding protein (pCREB) and phosphorylated Bcl-2 (pBcl-2) levels. Confluent cells were incubated in either BR or BF for 48 h. Total CREB, pCREB, total Bcl-2, and pBcl-2 were analyzed by Western blot. A: pCREB and pBcl-2 were examined by Western blotting. 30 μg of BXE cell lysate were taken from BF culture (lane 1), BR culture (lane 2), treated with 10 μM 2HE (lane 3), or 10 nM siRNA (lane 4). B: semiquantitative assessment of protein density. The relative level of pCREB and pBcl-2 were normalized to totalized by total CREB and Bcl-2, respectively. The data are representative of three independent experiments and expressed as means ± SE. *P < 0.05.
DISCUSSION
Increased cellular [cAMP] is protective in many cell types (11, 12, 23, 30), and this has also been demonstrated for corneal endothelium (16, 17). All of these studies, however, have used exogenous agonists that act through membrane receptors that activate membrane-associated adenylyl cyclase to produce substantial and relatively transient increases in cAMP. Here we show that endogenous activity of a nonmembrane associated adenylyl cyclase (sAC) activated by a ubiquitous agonist (HCO3−) can also produce sufficient cAMP that is protective. The corneal endothelium has a robust 1Na+-2HCO3− cotransporter (NBCe1) that maintains a high level of intracellular [HCO3−] (22). Therefore, sAC produces a relatively steady, but small, amount of cAMP (33) that contributes to the basal cAMP pool and maintains cell homeostasis. Our results suggest that modulation of sAC activity could significantly influence the balance between pro- and antiapoptotic forces, especially when cells are under stress.
Our findings are in contrast to a recent study showing that mitochondria-associated sAC is proapoptotic in an ischemia-acidosis model of cell death in rat coronary vascular endothelial cells (20). In this model, however, tmAC-derived cAMP was protective, suggesting that the mitochondria-specific sAC is responsible for promoting apoptosis. Moreover, in hypoxia-acidosis, mitochondria sAC in the vascular endothelial cells is activated by Ca2+ overload and not HCO3−. The mechanism by which mitochondrial cAMP promotes apoptosis in those cells is obscure. In BCEC, tmAC or sAC activation has similar protective actions. These differences suggest that BCEC mitochondria may lack sAC, may lack the signaling pathways that are provoked by cAMP in the coronary endothelial cells, or that apoptosis via ischemia-acidosis uniquely requires Ca2+ activation of mitochondrial sAC.
For cultured BCECs, BR ringer is protective against SP-induced apoptosis, as evident from fewer condensed nuclei and a reduced proportion of AnV+ cells (Figs. 1 and 2). This protective effect is not due to differences in pHi between BR and BF conditions. Moreover, in BF, the addition of the PDE4 inhibitor rolipram raised [cAMP] to levels comparable to BR and increased cell survival to a similar level. This rescue effect by rolipram strongly suggests that cAMP, a direct product of sAC activity, is an intermediate for cell protection.
To confirm that sAC is directly linked to the differential cell survival produced by HCO3−, sAC activity was reduced by 2HE, and sAC expression was silenced with siRNA. In BCEC, 10 μM 2HE reduced [cAMP] by >60%. This had a profound effect on both basal and SP-stimulated apoptosis rates (Figs. 2, 5, and 6). sAC-specific siRNA greatly reduced sAC expression in BR and BF incubated cells, increased the %AnV+ cells in BF and BR, and eliminated the protective effect of BR in SP-treated cells (Fig. 4). The protective effect of BR was also tested using two other markers of apoptosis, cytochrome c release and caspase-3 activity. The sensitivity to 2HE or siRNA was consistent with sAC as the source of the protection in BR.
In nontransfected cells, the absence of HCO3− reduces sAC expression relative to BR (Fig. 4). Previous studies (33) indicated that it is not due to differences in pHi or [cAMP] between the two conditions. Possibly it is a direct effect of HCO3−. Modulation of transcription or translation by CO2/HCO3− has been demonstrated in bacteria (5, 15, 40), but not eukaryotic cells. However, given the close homology between the catalytic domains of mammalian and cyanobacterial sACs (4), which are also HCO3− responsive, it is conceivable that mammalian sAC transcription/translation is HCO3− dependent.
Interestingly, lipofectamine, together with the scrambled sequence siCon, also decreased sAC expression and increased the %AnV+ cells under both BR and BF conditions (Fig. 4). Consequently, there was a decrease in basal and HCO3−-stimulated [cAMP] (Fig. 6). Although unlikely, we cannot exclude that this is due to off-target silencing of sAC. Another possibility is that sAC expression is very sensitive to stress, suggesting that regulation of sAC expression (in addition to sAC activity) could be a component of the overall cellular pro- and antiapoptotic forces (7).
If [cAMP] is modulating entry into apoptosis, then both the specific and nonspecific reductions in sAC expression under BR and BF conditions that alter [cAMP] should correlate with %AV+ cells. Indeed, we found an inverse, but nonlinear, relation between %AnV+ cells and [cAMP] (Fig. 6). There was an apparent threshold [cAMP] of ∼ 10 pmol/mg protein, below which there was an acceleration in %AnV+ cells. These results demonstrate that the small changes in [cAMP] produced by changing sAC expression and activity can have profound effects on cell survival.
CREB phosphorylation is a key step in the transcription of cAMP-sensitive genes like Bcl-2 and has been shown to be an integral part of cAMP-induced cell protection (8, 25, 37). The small increase in cAMP generated in BR is sufficient to increase phosphorylation of CREB and Bcl-2 and was significantly reduced by the sAC inhibitor 2HE or treatment with sAC-specific siRNA (Fig. 7). The sAC-dependent phosphorylation of Bcl-2 probably contributes to the antiapoptotic effects of increased cAMP by sAC activity, but we cannot exclude other contributors, e.g., cellular inhibitor of apoptosis-2 (29) which is induced by CREB phosphorylation, or phosphorylation of BAD by PKA (9). While further studies are needed to determine the downstream contributors to protection, what is clear is that the phosphorylation of CREB in response to the relatively small changes in [cAMP] from sAC activity is consistent with the protective effects of BR.
In summary, our results support a role for sAC in protection of CECs, which is consistent with the known protective effects of cAMP in CEC and other cell types. Modest changes in basal cell [cAMP] as a result of changes in sAC activity and expression can have profound effects on cell survival and protection from apoptotic stress. The sensitivity of sAC expression to the stress of siCon transfection, as well as cellular [HCO3−], suggests that sAC is a component of a stress signaling pathway that will modulate the balance between pro- and antiapoptotic forces. Further studies are needed to understand the mechanisms for regulating sAC expression and the possible role of sAC in stress detection and regulation of apoptosis.
GRANTS
This project was funded by National Eye Institute EY08834.
ACKNOWLEDGMENTS
We thank Dr. Xing Cai Sun for designing the sAC siRNA, and Michael Calvin for culturing the corneal endothelial cells used in this report. We also acknowledge Christiane Hassel for technical advice with flow cytometry.
REFERENCES
- 1. Barcia RN, Dana MR, Kazlauskas A. Corneal graft rejection is accompanied by apoptosis of the endothelium and is prevented by gene therapy with bcl-xL. Am J Transplant 7: 2082–2089, 2007 [DOI] [PubMed] [Google Scholar]
- 2. Bertrand R, Solary E, O'Connor P, Kohn KW, Pommier Y. Induction of a common pathway of apoptosis by staurosporine. Exp Cell Res 211: 314–321, 1994 [DOI] [PubMed] [Google Scholar]
- 3. Bonanno JA, Giasson C. Intracellular pH regulation in fresh and cultured bovine corneal endothelium. II. Na+:HCO3− cotransport and Cl-/HCO3− exchange. Invest Ophthalmol Vis Sci 33: 3068–3079, 1992 [PubMed] [Google Scholar]
- 4. Buck J, Sinclair ML, Schapal L, Cann MJ, Levin LR. Cytosolic adenylyl cyclase defines a unique signaling molecule in mammals. Proc Natl Acad Sci U S A 96: 79–84, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Caparon MG, Geist RT, Perez-Casal J, Scott JR. Environmental regulation of virulence in group A streptococci: transcription of the gene encoding M protein is stimulated by carbon dioxide. J Bacteriol 174: 5693–5701, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Caraglia M, Marra M, Giuberti G, D'Alessandro AM, Beninati S, Lentini A, Pepe S, Boccellino M, Abbruzzese A. Theophylline-induced apoptosis is paralleled by protein kinase A-dependent tissue transglutaminase activation in cancer cells. J Biochem 132: 45–52, 2002 [DOI] [PubMed] [Google Scholar]
- 7. Carlucci A, Lignitto L, Feliciello A. Control of mitochondria dynamics and oxidative metabolism by cAMP, AKAPs and the proteasome. Trends Cell Biol 18: 604–613, 2008 [DOI] [PubMed] [Google Scholar]
- 8. Costes S, Broca C, Bertrand G, Lajoix AD, Bataille D, Bockaert J, Dalle S. ERK1/2 control phosphorylation and protein level of cAMP-responsive element-binding protein: a key role in glucose-mediated pancreatic beta-cell survival. Diabetes 55: 2220–2230, 2006 [DOI] [PubMed] [Google Scholar]
- 9. Downward J. How BAD phosphorylation is good for survival. Nat Cell Biol 1: E33–E35, 1999 [DOI] [PubMed] [Google Scholar]
- 10. Edelhauser HF. The balance between corneal transparency and edema: the Proctor Lecture. Invest Ophthalmol Vis Sci 47: 1754–1767, 2006 [DOI] [PubMed] [Google Scholar]
- 11. Hoshino T, Tsutsumi S, Tomisato W, Hwang HJ, Tsuchiya T, Mizushima T. Prostaglandin E2 protects gastric mucosal cells from apoptosis via EP2 and EP4 receptor activation. J Biol Chem 278: 12752–12758, 2003 [DOI] [PubMed] [Google Scholar]
- 12. Hui H, Nourparvar A, Zhao X, Perfetti R. Glucagon-like peptide-1 inhibits apoptosis of insulin-secreting cells via a cyclic 5′-adenosine monophosphate-dependent protein kinase A- and a phosphatidylinositol 3-kinase-dependent pathway. Endocrinology 144: 1444–1455, 2003 [DOI] [PubMed] [Google Scholar]
- 13. Ing JJ, Ing HH, Nelson LR, Hodge DO, Bourne WM. Ten-year postoperative results of penetrating keratoplasty. Ophthalmology 105: 1855–1865, 1998 [DOI] [PubMed] [Google Scholar]
- 14. Journot L, Villalba M, Bockaert J. PACAP-38 protects cerebellar granule cells from apoptosis. Ann N Y Acad Sci 865: 100–110, 1998 [DOI] [PubMed] [Google Scholar]
- 15. Koehler TM, Dai Z, Kaufman-Yarbray M. Regulation of the Bacillus anthracis protective antigen gene: CO2 and a trans-acting element activate transcription from one of two promoters. J Bacteriol 176: 586–595, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Koh SW, Cheng J, Dodson RM, Ku CY, Abbondandolo CJ. VIP down-regulates the inflammatory potential and promotes survival of dying (neural crest-derived) corneal endothelial cells ex vivo: necrosis to apoptosis switch and up-regulation of Bcl-2 and N-cadherin. J Neurochem 109: 792–806, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Koh SW, Waschek JA. Corneal endothelial cell survival in organ cultures under acute oxidative stress: effect of VIP. Invest Ophthalmol Vis Sci 41: 4085–4092, 2000 [PubMed] [Google Scholar]
- 18. Koh SW, Yue BY. VIP stimulation of cAMP production in corneal endothelial cells in tissue and organ cultures. Cornea 21: 270–274, 2002 [DOI] [PubMed] [Google Scholar]
- 19. Komuro A, Hodge DO, Gores GJ, Bourne WM. Cell death during corneal storage at 4°C. Invest Ophthalmol Vis Sci 40: 2827–2832, 1999 [PubMed] [Google Scholar]
- 20. Kumar S, Kostin S, Flacke JP, Reusch HP, Ladilov Y. Soluble adenylyl cyclase controls mitochondria-dependent apoptosis in coronary endothelial cells. J Biol Chem 284: 14760–14768, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Leone V, di Palma A, Ricchi P, Acquaviva F, Giannouli M, Di Prisco AM, Iuliano F, Acquaviva AM. PGE2 inhibits apoptosis in human adenocarcinoma Caco-2 cell line through Ras-PI3K association and cAMP-dependent kinase A activation. Am J Physiol Gastrointest Liver Physiol 293: G673–G681, 2007 [DOI] [PubMed] [Google Scholar]
- 22. Li J, Sun XC, Bonanno JA. Role of NBC1 in apical and basolateral HCO3− permeabilities and transendothelial HCO3− fluxes in bovine corneal endothelium. Am J Physiol Cell Physiol 288: C739–C746, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li M, Wang X, Meintzer MK, Laessig T, Birnbaum MJ, Heidenreich KA. Cyclic AMP promotes neuronal survival by phosphorylation of glycogen synthase kinase 3beta. Mol Cell Biol 20: 9356–9363, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li XY, De Marco BM, Mayhew ES, Niederkorn JY. Aqueous humor-borne factor upregulates Bcl-2 expression in corneal endothelial cells. Curr Eye Res 17: 970–978, 1998 [DOI] [PubMed] [Google Scholar]
- 25. Meller R, Minami M, Cameron JA, Impey S, Chen D, Lan JQ, Henshall DC, Simon RP. CREB-mediated Bcl-2 protein expression after ischemic preconditioning. J Cereb Blood Flow Metab 25: 234–246, 2005 [DOI] [PubMed] [Google Scholar]
- 26. Milano CA, Allen LF, Rockman HA, Dolber PC, McMinn TR, Chien KR, Johnson TD, Bond RA, Lefkowitz RJ. Enhanced myocardial function in transgenic mice overexpressing the beta 2-adrenergic receptor. Science 264: 582–586, 1994 [DOI] [PubMed] [Google Scholar]
- 27. Mishima S. Clinical investigations on the corneal endothelium-XXXVIII Edward Jackson Memorial Lecture. Am J Ophthalmol 93: 1–29, 1982 [DOI] [PubMed] [Google Scholar]
- 28. Naderi S, Blomhoff HK. Activation of cAMP signaling enhances Fas-mediated apoptosis and activation-induced cell death through potentiation of caspase 8 activation. Hum Immunol 69: 833–836, 2008 [DOI] [PubMed] [Google Scholar]
- 29. Nishihara H, Hwang M, Kizaka-Kondoh S, Eckmann L, Insel PA. Cyclic AMP promotes cAMP-responsive element-binding protein-dependent induction of cellular inhibitor of apoptosis protein-2 and suppresses apoptosis of colon cancer cells through ERK1/2 and p38 MAPK. J Biol Chem 279: 26176–26183, 2004 [DOI] [PubMed] [Google Scholar]
- 30. Nishihara H, Kizaka-Kondoh S, Insel PA, Eckmann L. Inhibition of apoptosis in normal and transformed intestinal epithelial cells by cAMP through induction of inhibitor of apoptosis protein (IAP)-2. Proc Natl Acad Sci U S A 100: 8921–8926, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schlicker C, Rauch A, Hess KC, Kachholz B, Levin LR, Buck J, Steegborn C. Structure-based development of novel adenylyl cyclase inhibitors. J Med Chem 51: 4456–4464, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Siegmund B, Welsch J, Loher F, Meinhardt G, Emmerich B, Endres S, Eigler A. Phosphodiesterase type 4 inhibitor suppresses expression of anti-apoptotic members of the Bcl-2 family in B-CLL cells and induces caspase-dependent apoptosis. Leukemia 15: 1564–1571, 2001 [DOI] [PubMed] [Google Scholar]
- 33. Sun XC, Cui M, Bonanno JA. [HCO3−]-regulated expression and activity of soluble adenylyl cyclase in corneal endothelial and Calu-3 cells. BMC Physiol 4: 8, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sun XC, Zhai CB, Cui M, Chen Y, Levin LR, Buck J, Bonanno JA. HCO3−-dependent soluble adenylyl cyclase activates cystic fibrosis transmembrane conductance regulator in corneal endothelium. Am J Physiol Cell Physiol 284: C1114–C1122, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tan-Allen KY, Sun XC, Bonanno JA. Characterization of adenosine receptors in bovine corneal endothelium. Exp Eye Res 80: 687–696, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Waldkirch E, Uckert S, Sigl K, Langnaese K, Richter K, Stief CG, Kuczyk MA, Hedlund P. Expression of cAMP-dependent protein kinase isoforms in the human prostate: functional significance and relation to PDE4. Urology 515: e8–e14, 2010 [DOI] [PubMed] [Google Scholar]
- 37. Wang P, Yan H, Li JC. CREB-mediated Bcl-2 expression in trichosanthin-induced Hela cell apoptosis. Biochem Biophys Res Commun 363: 101–105, 2007 [DOI] [PubMed] [Google Scholar]
- 38. Wuttke MS, Buck J, Levin LR. Bicarbonate-regulated soluble adenylyl cyclase. JOP 2: 154–158, 2001 [PubMed] [Google Scholar]
- 39. Yang D, Koupenova M, McCrann DJ, Kopeikina KJ, Kagan HM, Schreiber BM, Ravid K. The A2b adenosine receptor protects against vascular injury. Proc Natl Acad Sci U S A 105: 792–796, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yang J, Hart E, Tauschek M, Price GD, Hartland EL, Strugnell RA, Robins-Browne RM. Bicarbonate-mediated transcriptional activation of divergent operons by the virulence regulatory protein, RegA, from Citrobacter rodentium. Mol Microbiol 68: 314–327, 2008 [DOI] [PubMed] [Google Scholar]
- 41. Zhang L, Insel PA. The pro-apoptotic protein Bim is a convergence point for cAMP/protein kinase A- and glucocorticoid-promoted apoptosis of lymphoid cells. J Biol Chem 279: 20858–20865, 2004 [DOI] [PubMed] [Google Scholar]
- 42. Zhang X, Odom DT, Koo SH, Conkright MD, Canettieri G, Best J, Chen H, Jenner R, Herbolsheimer E, Jacobsen E, Kadam S, Ecker JR, Emerson B, Hogenesch JB, Unterman T, Young RA, Montminy M. Genome-wide analysis of cAMP-response element binding protein occupancy, phosphorylation, and target gene activation in human tissues. Proc Natl Acad Sci U S A 102: 4459–4464, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zippin JH, Chen Y, Nahirney P, Kamenetsky M, Wuttke MS, Fischman DA, Levin LR, Buck J. Compartmentalization of bicarbonate-sensitive adenylyl cyclase in distinct signaling microdomains. FASEB J 17: 82–84, 2003 [DOI] [PubMed] [Google Scholar]