Abstract
We investigated the role of reactive oxygen species (ROS) in promoting cell survival during oxidative stress induced by the inflammatory mediator tumor necrosis factor-α (TNF-α) in cerebral microvascular endothelial cells (CMVEC) from newborn piglets. Nox4 is the major isoform of NADPH oxidase responsible for TNF-α-induced oxidative stress and apoptosis in CMVEC. We present novel data that Nox4 NADPH oxidase-derived ROS also initiate a cell survival mechanism by increasing production of a gaseous antioxidant mediator carbon monoxide (CO) by constitutive heme oxygenase-2 (HO-2). TNF-α rapidly enhanced endogenous CO production in a superoxide- and NADPH oxidase-dependent manner in CMVEC with innate, but not with small interfering RNA (siRNA)-downregulated Nox4 activity. CORM-A1, a CO-releasing compound, inhibited Nox4-mediated ROS production and enhanced cell survival in TNF-α-challenged CMVEC. The ROS-induced CO-mediated survival mechanism requires functional interactions between the protein kinase B/Akt and extracellular signal-related kinase (ERK)/p38 MAPK signaling pathways activated by TNF-α. In Akt siRNA-transfected CMVEC and in cells with pharmacologically inhibited Akt, Erk1/2, and p38 mitogen-activated protein kinase (MAPK) activities, CORM-A1 was no longer capable of blocking Nox4 activation and apoptosis caused by TNF-α. Overall, Nox4 NADPH oxidase-derived ROS initiate both death and survival pathways in TNF-α-challenged CMVEC. The ROS-dependent cell survival pathway is mediated by an endogenous antioxidant CO, which inhibits Nox4 activation via a mechanism that includes Akt, ERK1/2, and p38 MAPK signaling pathways. The ability of CO to inhibit TNF-α-induced ERK1/2 and p38 MAPK activities in an Akt-dependent manner appears to be the key element in ROS-dependent survival of endothelial cells during TNF-α-mediated brain inflammatory disease.
Keywords: cerebral vascular endothelial cells, inflammatory cytokines, heme oxygenase, HO-2, NOX1, NOX2, CO-releasing molecule, CORM-A1
reactive oxygen species (ROS) have recently emerged as signaling molecules involved in cell functioning and cell survival (16, 19, 35, 48). The regulated balance between detrimental and advantageous effects of ROS, termed “redox regulation,” has numerous implications in cell biology (16, 35). The redox state is controlled by the balance between ROS formation and elimination by the antioxidant enzymes. Elevation of ROS in excess of antioxidant capacity (oxidative stress) leads to cell damage. Constitutively expressed heme oxygenase-2 (HO-2) is an essential component of acute antioxidant protection in brain endothelial cells (42). The cytoprotective properties of HO are largely due to the antioxidant capacities of carbon monoxide (CO) and bilirubin, the end products of the HO-mediated heme degradation (3, 4, 42).
Tumor necrosis factor-α (TNF-α), a mediator of inflammation in the brain, rapidly increases ROS formation and elicits oxidative stress-mediated apoptosis in cerebral microvascular endothelial cells (CMVEC) (4). HO-2 is the key enzyme in cytoprotection against oxidative stress induced by TNF-α. HO-2 gene deletion or inhibition of constitutively expressed HO-2 greatly potentiates TNF-α-elicited apoptosis in CMVEC (3, 4, 42). Nox4 NADPH oxidase is a major source of death-inducing ROS in TNF-α-challenged CMVEC (4). Exogenous CO administered as a CO-releasing molecule CORM-A1 inhibits NADPH oxidase activation by TNF-α and prevents development of oxidative stress-induced apoptosis (3). However, it is not known whether TNF-α upregulates endogenous production of CO, thus initiating the antioxidant defense mechanism in CMVEC.
The role of phosphorylation-regulated signaling pathways in CO-mediated survival of brain endothelial cells during inflammatory insults has not been investigated. Serine/threonine protein kinase B (PKB/Akt), a major downstream effector of activated phosphoinositide-3 kinase (PI3K), is a critical component of the cell survival mechanism (7, 9, 13, 17, 20–23, 36, 38). Most notably, Akt has been implicated in inhibiting apoptosis during conditions of oxidative stress (9, 21, 27, 33, 38). Akt activated by phosphorylation at Thr308 and Ser473 translocates to various cell compartments to phosphorylate the key substrates of the apoptotic machinery, including proapoptotic proteins, transcription factors, caspases, and others (38). Interactions between the Akt and MAPK signaling pathways, including extracellular signal-related kinase (ERK) and p38 MAPK, are critical for balance between cell death and cell survival (6, 22, 46).
Therefore, the present study in CMVEC from newborn piglets was designed to investigate the CO-mediated mechanisms of cell survival during oxidative stress induced by TNF-α by addressing two major hypotheses: 1) ROS increase endogenous enzymatic CO production, and 2) PKB/Akt and MAPK signaling pathways are involved in the CO-mediated antioxidant defense mechanism.
METHODS
Cerebral microvessels.
All protocols and procedures involving animals were reviewed and approved by the Animal Care and Use Committee of the University of Tennessee Health Science Center, Memphis. Cerebral microvessels (60–300 μM) were obtained from the brain cortex by sequential filtration of the piglet cortex homogenate through 300- and 60-μm nylon mesh filters as we described previously (3); the filtrate contains neurons and astrocytes (brain parenchyma), whereas cerebral vessels (60–300 μm) are retained on the filters.
CMVEC cultures.
CMVEC were obtained by collagenase-dispase digestion of cerebral microvessels followed by density gradient centrifugation, plated on Matrigel-coated plates, and cultured in DMEM with 20% FBS, 30 μg/ml endothelial cell growth supplement, 1 U/ml heparin, and antibiotic-antimycotic mixture for 5–6 days until confluence. CMVEC identified by von Willebrand factor expression accounted for >90% of the cell population. For the experiments involving detection of CO production, confluent CMVEC were replated to Cytodex 3 microcarrier beads (Pharmacia; Piscataway, NJ) and grown for 3–4 days as we described previously (32). All experiments were performed on confluent quiescent cells in primary cultures. To achieve quiescence, CMVEC were incubated overnight in DMEM with 0.1% FBS.
siRNA-mediated gene silencing.
To downregulate Akt expression, CMVEC were transfected with Akt small interfering RNA (siRNA) that targets Akt1 and Akt2 (SignalSilence Akt siRNA I) or with control siRNA (Cell Signaling Technology; Beverly, MA). To downregulate the NADPH oxidase isoforms, we used Nox1 siRNA, Nox2 siRNA, Nox4 siRNA, and nontargeting control siRNA (Dharmacon RNA Technologies; Lafayette, CO) as we have described previously (3). Briefly, confluent CMVEC were replated to 12-well plates (3 × 105 cells/ml) and grown in antibiotic-free 20% FBS-DMEM for 24 h to 60–70% confluence. To prepare the transfection complex, DharmaFECT 1 transfection reagent (2 μl/well; Dharmacon RNA Technologies) was incubated with Akt siRNA (final concentration, 50 nM); Nox1, Nox2, or Nox4 siRNA (final concentration, 100 nM); or corresponding control siRNAs in antibiotic- and serum-free medium for 30 min at room temperature. For transfection, cells were incubated with the siRNA-DharmaFECT transfection complexes in antibiotic- and serum-free medium for 24 h at 37°C followed by a 48-h recovery period in 20% FBS-DMEM (antibiotic-free). Before the experiment, CMVEC were serum-deprived overnight in antibiotic-free 0.1% FBS-DMEM.
Detection of DNA fragmentation.
DNA fragmentation, a hallmark of apoptosis, was detected by quantitation of cytoplasmic histone-associated-DNA fragments using a cell death detection kit (Roche Applied Science; Indianapolis, IN) as described elsewhere (3). Quiescent CMVEC were treated with TNF-α (15 ng/ml) for 3 h at 37°C. Aliquots of the nuclei-free cytoplasmic fractions were placed in streptavidin-coated wells and incubated with anti-histone biotin-conjugated antibody and anti-DNA peroxidase-conjugated antibody. Immobilized DNA fragments were visualized with 2,2′-azino-di(3-ethylbenzthiazolin-sulfonate); the absorbance at 405 nm was measured by the Synergy HT multimode microplate reader (BioTek Instruments; Winooski, VT) and normalized to the protein amount. Protein was measured by the bicinchoninic acid method (Pierce; Rockford, IL).
Detection of ROS.
ROS generation in CMVEC was detected using dihydroethidium (DHE), a cell-permeable superoxide-sensitive probe, as we described previously (3). Ethidium, a DHE-oxidized derivative, intercalates with nuclear DNA and emits red fluorescence. For spectroscopic measurements of ROS, CMVEC were incubated with 15 ng/ml TNF-α (alone or with the inhibitors as indicated) for 1 h at 37°C. DHE (20 μM) was added to the incubation media for an additional 20 min. Pooled CMVEC were disrupted by sonication in ice-cold DPBS, and the lysates were cleared by centrifugation. Ethidium fluorescence (excitation/emission, 485/590 nm) was measured with a Synergy HT multimode microplate reader (BioTek Instruments).
NADPH oxidase activity.
NADPH oxidase activity in cell homogenates was measured by superoxide (O2·−) production from NADPH using the lucigenin-enhanced luminescence assay (3). CMVEC were lysed by sonication in 5 ml of ice-cold lysis buffer (20 mM KH2PO4, 1 mM EGTA) containing protease inhibitors. NADPH oxidase activity was measured in a reaction mixture containing 50 mM KH2PO4 (pH 7.0), 1 mM EGTA, 150 mM sucrose, and protease inhibitors (total volume, 300 μl). Cell homogenates (∼100 μg protein) were incubated with 100 μM NADPH in the absence or presence of TNF-α (15 ng/ml) and CORM-A1 for 40 min at 37°C. Lucigenin (50 μM) was added to the reaction mixture, and the incubation continued for 20 min. Enhanced lucigenin luminescence indicative of O2·− concentration was measured by a Synergy HT microplate reader (BioTek Instruments) and normalized to the protein amount. Stock solution of CORM-A1 was freshly prepared before the experiment by dissolving the compound in a physiological solution (pH 7.4). CO-depleted (inactivated) CORM-A1 was prepared by exposure of the CORM-A1 stock solution to open air for 20 h at room temperature to fully decompose the carrier compound sodium borate (40, 54).
Measurements of CO production by gas chromatography-mass spectrometry (GS-MS).
Freshly isolated cerebral microvessels (60–300 μm) or CMVEC grown on Cytodex beads were incubated for 60 min at 37°C in Krebs solution inside sealed amber vials (2.0 ml) that contained the internal standard of the isotopically labeled CO (13C18O) as previously described (32). The headspace gas CO concentration was determined by GC-MS. The components of the headspace gas were separated on a molecular sieve-coated capillary column [CP-Molsieve 5Å column (30 m, 0.32 mm ID); Varian; Palo Alto, CA], and the CO peak was quantitatively detected on the basis of the peak areas with the mass-to-charge ratios corresponding to 12C16O and 13C18O (m/z values, 28 and 31, respectively). The results were expressed as picomoles of CO released into the headspace gas per milligram of protein.
Western immunoblotting.
Proteins (20–50 μg/lane) were separated by 15% SDS-PAGE, transferred to polyvinylidene fluoride membranes, and blocked with 5% milk-0.1% Tween-20. Caspase-3 activation was detected by formation of the 17-kDa proteolytic fragments using anti-caspase-3-Asp175 polyclonal antibodies (Cell Signaling Technology). Total Akt (including Akt1, Akt2, and Akt3 proteins) and phospho-Akt (Ser473), total and phosphorylated ERK1/2, and p38 MAPK were detected using highly specific antibodies from Cell Signaling Technology. HO-1 and HO-2 were detected using isoform-specific polyclonal antibodies from StressGen Biotechnologies (Victoria, BC, Canada). To normalize the antigen expression to a housekeeping gene, the membranes were stripped and reprobed with monoclonal anti-actin (Roche Molecular Biochemicals; Indianapolis, IN). The immunocomplexes were visualized with a Western Lightning Chemiluminescence Kit (Perkin Elmer Life Sciences; Shelton, CT) and quantified by digital densitometry using Image J 1.33 software (National Institutes of Health; Bethesda, MD).
Immunofluorescence.
Quiescent CMVEC on Matrigel-coated coverslips were fixed with 3.7% paraformaldehyde (pH 7.4, 20 min), permeabilized by 0.1% Triton-X100 (20 min), and blocked with 5% BSA-PBS (1 h). The cells were incubated with monoclonal antibodies against phospho-Akt (Ser473) (1:30; Cell Signaling Technology), Nox1, Nox2, and Nox4 (1:30; Santa Cruz Biotechnology; Santa Cruz, CA), for 1 h at 37°C followed by FITC-conjugated secondary antibody (Vector Laboratories; Burlingame, CA) for 1 h at 37°C. Slides were viewed using a Nikon Diaphot fluorescence microscope. Images were processed using IPLab Spectrum software and Adobe Photoshop (Adobe Systems).
Statistical analysis.
Data are presented as means ± SE of absolute values or percentage of control. Data were analyzed by repeated measures ANOVA followed by Tukey's post test to isolate differences between groups. A level of P < 0.05 was considered significant.
Materials.
Cell culture reagents were purchased from Life Technologies (Gaithersburg, MD), Hyclone (South Logan, UT), Roche Diagnostics (Indianapolis, IN), and GE Healthcare Biosciences (Piscataway, NJ). Human TNF-α and Matrigel were from BD Biosciences (Bedford, MA). CORM A-1 was a generous gift from Tyco Healthcare/Mallinckrodt B.B. (Petten, Holland). DHE was from Invitrogen (Carlsbad, CA). Inhibitors of PI3K, Akt, and MAPKs were from Calbiochem (EMD Biosciences; Gibbstown, NJ). All other reagents were from Sigma (St. Louis, MO).
RESULTS
TNF-α enhances endogenous CO production by cerebral microvessels and CMVEC in a superoxide- and NADPH oxidase-dependent manner.
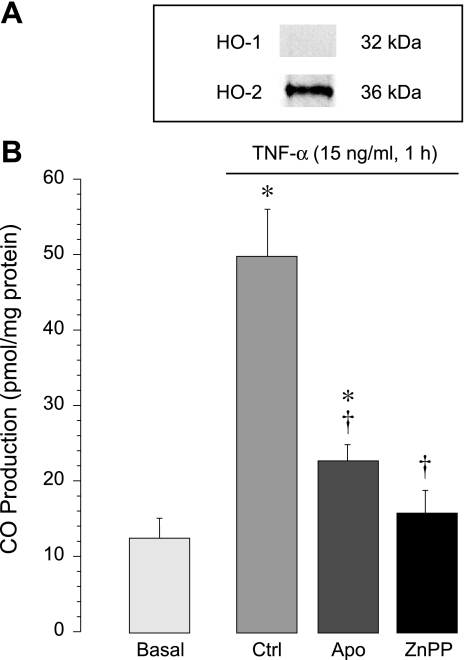
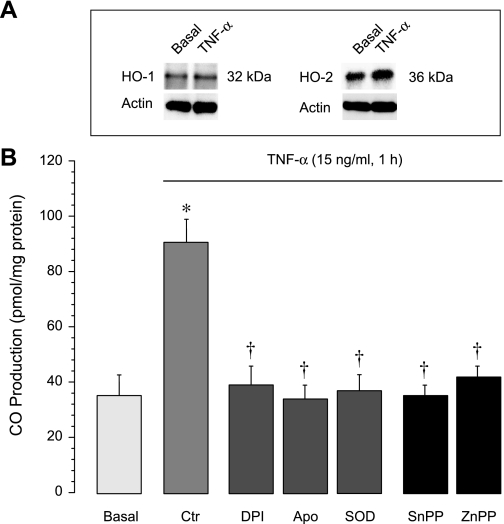
Endogenous CO is formed in cerebral vessels and in cultured CMVEC enzymatically via HO activity and is cytoprotective against oxidative inflammatory damage (4, 42). Constitutive HO-2 is the only HO isoform expressed in freshly isolated cerebral microvessels (Fig. 1; Refs. 4, 42). We investigated whether TNF-α is an immediate regulator of HO-2 activity, which accounts for overall CO production by cerebral microvessels. TNF-α (15 ng/ml, 1 h) rapidly increased CO production by cerebral microvessels; the CO response was blocked by the HO inhibitor Zinc protoporphyrin (ZnPP, 20 μM), consistent with HO-2 activation by the inflammatory mediator (Fig. 1). TNF-α (15 ng/ml, 1 h) also rapidly increased CO production by cultured CMVEC; the CO response was abolished in the presence of the inhibitors of HO, ZnPP (20 μM), and tin protoporphyrin (SnPP, 20 μM) (Fig. 2). Although CMVEC coexpress HO-2 and serum-inducible HO-1, TNF-α did not upregulate HO-1/HO-2 proteins (Fig. 2), indicating that the CO response is attributed to increased enzymatic activity of constitutive HO-2 (Fig. 2). TNF-α-enhanced CO production by CMVEC was blocked by superoxide dismutase (1,000 U/ml) and by the inhibitors of NADPH oxidase diphenyleneiodonium (5 μM) and apocynin (500 μM) (Fig. 2). These data demonstrate that TNF-α rapidly enhances endogenous CO production in cerebral vascular endothelium by stimulating HO-2 activity in a superoxide- and NADPH oxidase-dependent manner.
Fig. 1.
Acute stimulation of endogenous carbon monoxide (CO) production by tumor necrosis factor (TNF)-α in freshly isolated cerebral microvessels. A: expression of heme oxygenase (HO)-1 and HO-2 (representative immunoblots). B: cerebral microvessels, untreated or pretreated for 30 min with the NADPH oxidase inhibitor apocynin (Apo, 500 μM) or with the HO inhibitor zinc protoporphyrin (ZnPP, 20 μM), were exposed to TNF-α (15 ng/ml) in sealed vials for 1 h at 37°C. CO in the headspace gas was quantitatively detected by gas chromatography-mass spectrometry (GC-MS). *P < 0.05 compared with the baseline control value. †P < 0.05 compared with TNF-α alone.
Fig. 2.
Acute stimulation of endogenous CO production by TNF-α in cerebral microvascular endothelial cells (CMVEC). A: expression of HO-1 and HO-2 under baseline conditions and after exposure to TNF-α (15 ng/ml, 2 h) (representative immunoblots). B: CMVEC, untreated or pretreated for 30 min with NADPH oxidase inhibitors diphenyleneiodonium (DPI, 5 μM), apocynin (Apo, 500 μM), superoxide dismutase (SOD, 1,000 U/ml), or with the HO inhibitors tin protoporphyrin (SnPP, 20 μM) and ZnPP (20 μM), were exposed to TNF-α (15 ng/ml) in sealed vials for 1 h at 37°C. CO in the headspace gas was quantitatively detected by GC-MS. *P < 0.05 compared with the baseline control value. †P < 0.05 compared with TNF-α alone.
Contributions of Nox1, Nox2, and Nox4 NADPH oxidases to TNF-α-evoked oxidative stress.
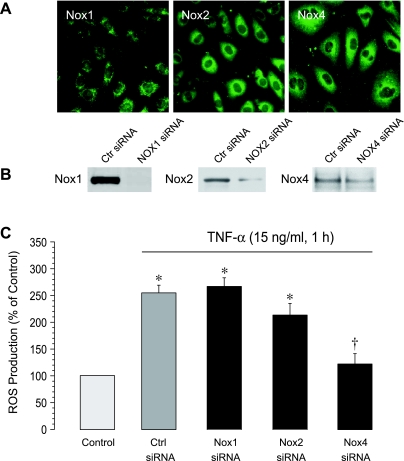
NADPH oxidase is the major source of oxidative stress in CMVEC exposed to TNF-α (3). CMVEC express Nox1, Nox2, and Nox4 isoforms of NADPH oxidase (Fig. 3, A and B). Using siRNA-mediated gene silencing, we determined contributions of these isoforms to oxidative stress caused by the inflammatory mediator. CMVEC transfected with control siRNA responded to TNF-α (15 ng/ml, 1 h) by increasing ROS production 2.5- to 3-fold (Fig. 3C). Downregulation of Nox1 and Nox2 expression by siRNAs (Fig. 3B) had little or no effect on TNF-α-induced ROS generation (Fig. 3C). In contrast, Nox4 siRNA completely abolished the oxidative stress response of CMVEC to TNF-α (Fig. 3C). These data demonstrate that Nox4 is the major isoform of NADPH oxidase responsible for TNF-α-stimulated ROS generation in brain endothelial cells.
Fig. 3.
Expression of NADPH oxidase isoforms (A, B) and their functional contribution to TNF-α-induced oxidative stress (C). A: immunostaining for Nox1, Nox2, and Nox4 NADPH oxidase isoforms. B: knockdown of NADPH oxidase using isoform-specific small interfering RNAs (siRNAs). C: reactive oxygen species (ROS) production. CMVEC transfected with control siRNA (control), Nox1-siRNA, Nox2-siRNA, or Nox4 siRNA were treated with TNF-α (15 ng/ml, 1 h). ROS production was measured by ethidium fluorescence generated from DHE and expressed as percentage of the baseline control. Values are means ± SE. *P < 0.05 compared with the baseline control value. †P < 0.05 compared with the TNF-α-induced value in control siRNA-transfected cells.
Nox-4-derived ROS mediate TNF-α-stimulated CO production.
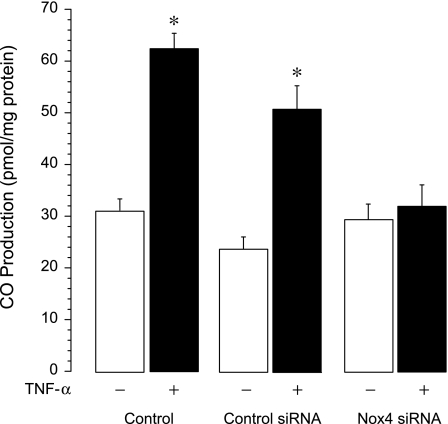
We investigated whether TNF-α-induced Nox4 activation contributes to enhanced CO production. In contrast to its actions in innate and control siRNA-transfected cells, TNF-α failed to enhance CO production in CMVEC with downregulated Nox4 expression (Nox4 siRNA-transfected cells) (Fig. 4). These data demonstrate that Nox-4-derived ROS are critical mediators of TNF-α-stimulated endogenous CO production.
Fig. 4.
Effects of TNF-α on CO production in Nox4-downregulated cells. CMVEC, nontransfected (control) and transfected with control siRNA or Nox4 siRNA, were exposed to TNF-α (15 ng/ml) in sealed vials for 1 h at 37°C. CO in the headspace gas was quantitatively detected by GC-MS. Values are means ± SE. *P < 0.05 compared with the baseline control value.
CORM-A1 inhibits Nox4 NADPH oxidase activity via Akt and MAPKs signaling.
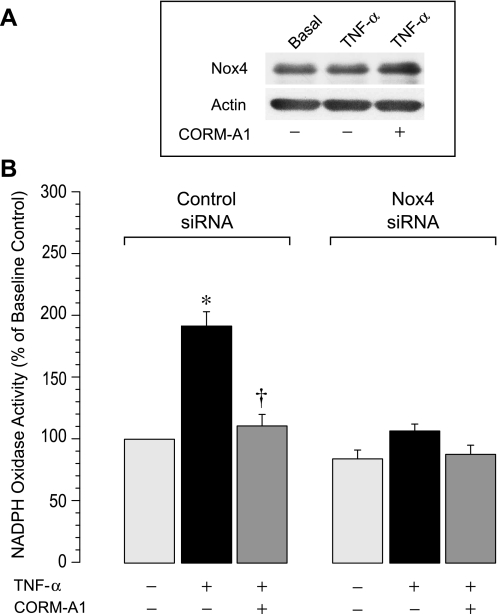
We demonstrated previously that CO, endogenously produced via HO-2 activation or administered exogenously as a CO-releasing molecule CORM-A1, prevents NADPH oxidase- and ROS-mediated apoptosis in TNF-α-stimulated CMVEC (3). We investigated whether CORM-A1 directly inhibits Nox4 NADPH oxidase activity. NADPH oxidase activity in CMVEC transfected with control siRNA and Nox4 siRNA was detected as O2·− production by the cell homogenates from 100 μM NADPH. TNF-α (15 ng/ml, 1 h) rapidly increased NADPH oxidase activity in control but not in Nox4 siRNA-transfected cells (Fig. 5B). As anticipated, acute changes in NADPH oxidase activity in innate CMVEC did not involve increased Nox4 protein expression (Fig. 5A). These data confirm that Nox4 NADPH oxidase is the major contributor to oxidative stress in TNF-α-challenged brain endothelial cells (3). Furthermore, CORM-A1 inhibited NADPH oxidase activity in control CMVEC but not in the cells with downregulated Nox4 expression (Fig. 5), suggesting that CO selectively inhibits Nox4 NADPH oxidase. CO-depleted inactivated CORM-A1 was not capable of releasing gaseous CO (54) and had no effects on ROS production in TNF-α-stimulated CMVEC (ROS, 250 ± 20% and 220 ± 30% of the baseline value in the absence and the presence of CO-depleted CORM-A1, respectively). These control experiments eliminate potential CO-independent effects of the carrier molecule on NADPH oxidase activation by TNF-α.
Fig. 5.
Effects of TNF-α and CORM-A1 on NADPH oxidase activity. CMVEC, transfected with control siRNA or Nox4 siRNA, were exposed to TNF-α (15 ng/ml, 1 h) in the absence or presence of CORM-A1 (50 μM). A: Nox4 protein expression in CMVEC. B: NADPH oxidase activity was determined as O2·− production from NADPH (100 μM), measured by enhanced lucigenin luminescence, normalized to the protein amount, and expressed as percentage of the baseline control. Values are means ± SE. *P < 0.05 compared with the baseline activity. †P < 0.05 compared with TNF-α alone.
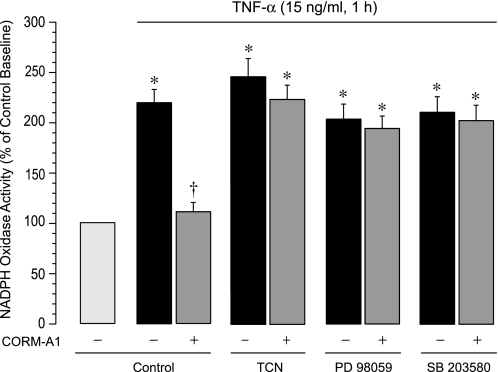
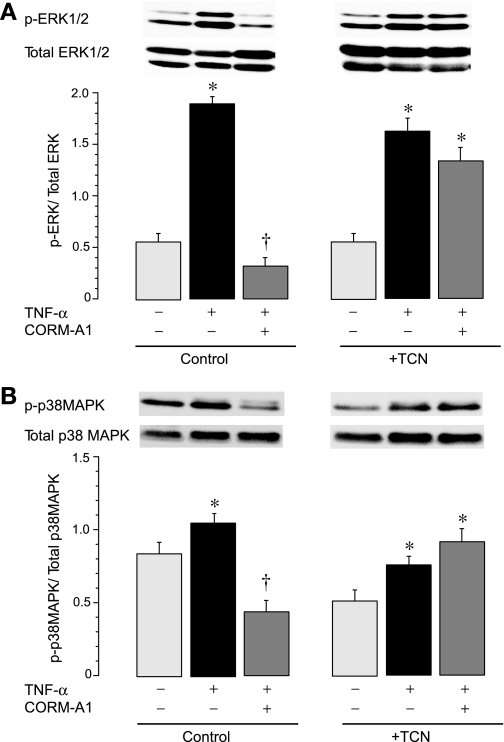
We addressed the question of whether intracellular phosphorylation signaling pathways are involved in the inhibitory effect of CO on NADPH oxidase activation by TNF-α. Pharmacological inhibitors of Akt (triciribine, 1 μM), ERK1/2 (PD 98059, 20 μM), or p38 MAPK (SB203580, 5 μM) completely abolished the ability of CORM-A1 to suppress NADPH oxidase activation by TNF-α (Fig. 6). Conversely, neither inhibitor altered the ability of TNF-α to increase the NADPH oxidase activity (Fig. 6), suggesting that TNF-α increases Nox4 activity independently of the Akt and MAPK signaling. These data indicate that phosphorylation-dependent signaling pathways involving Akt, Erk1/2, and p38 MAPK are required for the ability of CORM-A1 to inhibit TNF-α-induced Nox4-mediated oxidative stress.
Fig. 6.
Contributions of Akt, extracellular signal-related kinase (ERK)1/2, and p38 mitogen-activated protein kinase (MAPK) to the CO inhibition of NADPH oxidase activity. Fractionated confluent quiescent CMVEC, untreated or pretreated with triciribine (TCN; Akt inhibitor, 1 μM), PD98059 (ERK1/2 inhibitor, 20 μM), and SB203580 (p38 MAPK inhibitor, 5 μM) were exposed to TNF-α (15 ng/ml, 1 h). NADPH oxidase activity was determined as O2·− production from NADPH (100 μM), normalized to the protein amount, and expressed as percentage of the baseline control. Values are means ± SE. *P < 0.05 compared with the baseline activity. †P < 0.05 compared with TNF-α alone.
Akt and MAPKs are required for antiapoptotic effects of CO.
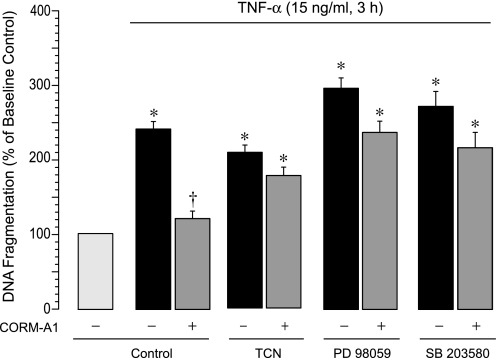
We then determined whether Akt and MAPKs are functionally involved in antiapoptotic effects of CO. Triciribine (1 μM), PD 98059 (20 μM), and SB 203580 (5 μM) did not affect TNF-α-induced DNA fragmentation but completely abolished the antiapoptotic effects of CORM-A1 in TNF-α-stimulated CMVEC (Fig. 7). These data suggest that TNF-α causes apoptosis independently of the Akt and MAPK signaling. However, Akt, Erk1/2, and p38 MAPK signaling pathways are absolutely critical for the antiapoptotic cytoprotective effects of CO in TNF-α-challenged CMVEC.
Fig. 7.
Contributions of Akt, ERK1/2, and p38 MAPK to antiapoptotic effects of CO in TNF-α-challenged CMVEC. CMVEC, untreated or pretreated with TCN (Akt inhibitor, 1 μM), PD98059 (ERK1/2 inhibitor, 20 μM), or SB203580 (p38 MAPK inhibitor, 5 μM), were exposed to TNF-α (15 ng/ml, 3 h) in the absence or presence of CORM-A1 (50 μM). Apoptosis was detected by DNA fragmentation, normalized to the protein amount and expressed as percentage of the baseline control. Values are means ± SE. *P < 0.05 compared with the baseline activity. †P < 0.05 compared with TNF-α alone.
Effects of TNF-α on Akt, Erk1/2, and p38 MAPK signaling.
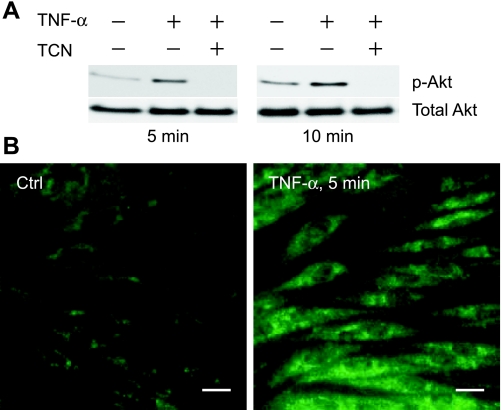
We investigated whether TNF-α activates Akt and MAPK signaling pathways in CMVEC. Akt is constitutively active as indicated by a detectable amount of p-Akt Ser473 during baseline control conditions (Fig. 8A). TNF-α (15 ng/ml) induced a rapid phosphorylation of Akt on Ser473 in 5–10 min as detected by Western immunoblotting using the Akt1/2 (phospho-Ser473) antibodies (Fig. 8A) and indirect immunofluorescence (Fig. 8B) that returned to the baseline level in 1 h (not shown). Triciribine (1 μM) blocked Akt phosphorylation, confirming its effectiveness as the Akt inhibitor in CMVEC (Fig. 8A). In the control cells, constitutively active Akt (p-Akt Ser473) accumulated in the perinuclear area, with no staining at the plasma membrane or the nucleus (Fig. 8B). In TNF-α-stimulated cells, p-Akt Ser473 fluorescence was distributed throughout the cytoplasm, including the plasma membrane area, whereas no nuclear signal was detected (Fig. 8B).
Fig. 8.
TNF-α stimulates Akt phosphorylation in CMVEC. Akt activation was detected by formation of phosphorylated Akt (p-Akt) using phospho-Akt (Ser473) antibodies by immunoblotting (A) and immunostaining (B). A: CMVEC, untreated or pretreated with TCN (1 μM, 1 h) were exposed to TNF-α (15 ng/ml) for 5 or 10 min. B: CMVEC were untreated (Ctrl) or treated with TNF-α (15 ng/ml, 10 min) and processed for phospho-Akt immunostaining. Scale bar, 20 μm.
TNF-α (15 ng/ml) triggered sustained (1–3 h) phosphorylation and activation of ERK1/2 (Fig. 9A) and, to a lesser extent, p38 MAPK (Fig. 9B). Triciribine (1 μM) did not affect the phosphorylation pattern (Fig. 9, A and B), suggesting that TNF-α activates ERK1/2 and p38 MAPK in an Akt-independent manner. Conversely, we found that CO causes Akt-dependent inhibition of activated MAPKs in TNF-α-stimulated CMVEC. CORM-A1 (50 μM) completely blocked TNF-α-elicited phosphorylation of ERK1/2 and p38 MAPK in cells with intact Akt activity but not in those with inhibited Akt activity (Fig. 9, A and B). Overall, these data suggest that Akt-dependent inhibition of MAPK signaling is involved in the mechanism of CO-mediated cytoprotection against TNF-α-induced apoptosis.
Fig. 9.
Effects of CORM-A1 on TNF-α-induced MAPK signaling in CMVEC with active and inhibited Akt. CMVEC, untreated (control) or pretreated with TCN (1 μM), were exposed to TNF-α (15 ng/ml, 3 h) in the absence or presence of a CO-releasing compound CORM-A1 (50 μM). Phosphorylated and total ERK1/2 (A) and p38 MAPK (B) were immunodetected. Values are means ± SE. *P < 0.05 compared with the corresponding baseline values. †P < 0.05 compared with TNF-α alone.
Akt activation is critical for the CO cytoprotection against TNF-α-induced oxidative stress and apoptosis.
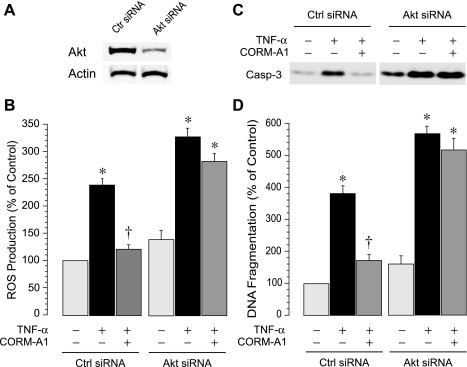
To further investigate the functional contribution of Akt signaling to the CO-mediated cytoprotection during inflammatory stress, we downregulated the expression of endogenous Akt using the SignalSilence Akt siRNA I, which targets Akt 1 and 2 (Cell Signaling Technology). Transfection of CMVEC with Akt siRNA I (50 nM, 72 h) resulted in downregulation of Akt protein expression by ∼70% (Fig. 10A). We investigated whether Akt signaling is involved in generation of oxidative stress and apoptosis in response to TNF-α. As anticipated, in control siRNA-transfected CMVEC, TNF-α (15 ng/ml) greatly increased ROS production (Fig. 10B) and caused apoptosis-related caspase-3 activation and DNA fragmentation (Fig. 10, C and D). Akt downregulation by siRNA potentiated oxidative stress and apoptosis under basal and TNF-α-stimulated conditions (Fig. 10, B–D). When Akt expression/activity was downregulated, CORM-A1 failed to protect CMVEC from TNF-α-induced oxidative stress (Fig. 10B), caspase-3 activation (Fig. 10C), and DNA fragmentation (Fig. 10D). Similarly, when PI3K was inhibited by LY294002 (10 μM), CORM-A1 lost the ability to prevent apoptosis in TNF-α-challenged CMVEC (DNA fragmentation, 160 ± 30% and 630 ± 60% of the baseline values in control and LY294002-treated CMVEC, respectively, P > 0.05). Overall, these data suggest that the Akt signaling pathway is cytoprotective against oxidative stress and apoptosis caused by TNF-α in CMVEC. Furthermore, PI3K/Akt is the key component in the mechanism of CO cytoprotection against TNF-α-induced oxidative stress and apoptosis.
Fig. 10.
Effects of CORM-A1 on TNF-α-induced ROS formation and apoptosis in CMVEC with innate and downregulated Akt. CMVEC transfected with control siRNA or Akt siRNA were exposed to TNF-a (15 ng/ml, 1–3 h) in the absence or presence of CORM-A1 (50 μM) (n = 3 independent transfections). A: Akt expression detected by immunoblotting (representative blot). B: ROS production (1 h) in CMVEC as measured using the dihydroethidium fluorescence probe. C: caspase-3 activity (3 h) immunodetected by formation of the active 17 kDa-proteolytic fragments of caspase-3 (representative blot). D: DNA fragmentation (3 h) normalized to the protein amount and expressed as percentage of the baseline control. Values are expressed as percentage of the baseline control. Values are means ± SE. *P < 0.05 compared with the corresponding control values. †P < 0.05 compared with TNF-α alone.
DISCUSSION
ROS are signaling molecules that may participate in regulation of cell death and survival. Previously, we demonstrated that ROS formed via activation of Nox4 NADPH oxidase in brain endothelial cells exposed to the inflammatory mediator TNF-α cause oxidative stress-related apoptosis (3, 4). Here we explored the hypothesis that ROS simultaneously initiate acute CO-mediated antioxidant defense mechanism that promotes cell survival during oxidative stress. We present major novel findings regarding ROS-dependent cell survival: 1) TNF-α acutely increases endogenous production of CO by activation of constitutive HO-2; 2) increase in CO production involves ROS-dependent mechanism; 3) Nox4 NADPH oxidase-derived ROS that initiate apoptosis also act as the survival signals to increase endogenous CO; 4) CO inhibits Nox4 activation by TNF-α; 5) the ability of CO to inhibit Nox4 is Akt dependent; and 6) functional interaction between Akt and MAPK signaling is required for the CO inhibition of Nox4 activation, reducing oxidative stress and promoting cell survival during inflammatory conditions.
Brain endothelial cells produce O2·− and other ROS in response to the pro-inflammatory cytokine TNF-α (4, 42). Endothelium-derived ROS cause oxidative stress, loss of cell-cell, and cell-matrix contacts, and, finally, apoptosis when the endogenous antioxidant potency of the cell is decreased. NADPH oxidase (Nox family) is a key source of ROS in CMVEC exposed to TNF-α (3). Among a multitude of genetically distinct isoforms of the NADPH oxidase catalytic subunit, Nox1, Nox2, and Nox4 are most essential in vascular functions (1, 2, 28, 44, 49). CMVEC from newborn piglets constitutively express Nox1, Nox2, and Nox4. We found that only Nox4 NADPH oxidase is rapidly activated by TNF-α, whereas Nox1 and Nox2 do not respond to the cytokine. Nox4 siRNA-mediated gene silencing prevented the development of oxidative stress and remarkably improved the survival of CMVEC exposed to TNF-α. Conversely, Nox1 or Nox2 gene silencing did not alter ROS production and apoptosis caused by TNF-α. Overall, Nox4-derived ROS are the leading signals responsible for TNF-α-induced oxidative stress and apoptosis in CMVEC.
Recent data suggest that ROS may also act as signaling molecules that participate in the regulation of proliferation, differentiation, angiogenesis, cell shape, and cell contacts (16, 19, 35, 48). ROS regulation of physiological functions involves modulating key proteins by reversible oxidation of their transition metals components or redox-reactive cysteine residues (8, 16). Redox-sensitive key enzymes include tyrosine- and serine/threonine phosphatases and kinases (15, 24, 37). Oxidative inhibition of protein phosphatases leads to activation of corresponding protein kinases, most prominently, the MAPK cascade enzymes, including p38 MAPK and ERK1/2 (30, 37, 48, 50). Furthermore, ROS may directly activate MAPKs, PI3 kinase, protein kinase C, and protein tyrosine kinases, thus generating a wide variety of biological responses (31, 37).
We explored the hypothesis that ROS initiate an antioxidant defense mechanism that promotes cell survival. HO-2 has recently emerged as an essential component of a brain endothelial cell survival mechanism. In addition to a well-recognized role of HO-1 in a long-term cytoprotection, constitutive HO-2 is critical in acute antioxidant cell defense mechanism. HO-2 gene deletion or pharmacological inhibition of HO-2 in CMVEC exacerbated, whereas exogenous CO blocked oxidative stress-mediated apoptosis caused by excitotoxic and inflammatory mediators (4, 42). However, it was not known whether endogenous production of CO is upregulated during inflammatory stress. We now provide evidence that TNF-α rapidly enhances CO production in cerebral vessels and CMVEC by activating HO-2 via a ROS-mediated mechanism; Nox4 NADPH oxidase is the major source of ROS that activate CO production. Acute changes in CO production by cerebral microvessels and cultured CMVEC that occur in 1–3 h of the stimulation with TNF-α do not involve upregulation of HO-1/HO-2 or Nox4 proteins. These findings indicate that HO-2 is a redox-sensitive enzyme, which is posttranslationally activated by ROS. The molecular mechanism by which ROS modulate HO-2 activity may involve a thiol/disulfide redox molecular switch that controls the heme substrate binding to the HO-2 protein (51). Overall, these observations suggest that ROS derived from TNF-α-activated Nox4 have dual functions in CMVEC during inflammatory conditions. First, they cause cell death via oxidative stress-mediated apoptosis. Second, they initiate a cell survival pathway via enhancing production of an endogenous antioxidant CO. In the negative feedback mechanism, endogenous CO produced via a Nox4- and ROS-dependent mechanism selectively inhibits Nox4 activity, reduces oxidative stress, and promotes cell survival under inflammatory conditions.
We investigated the role of phosphorylation signaling pathways in the mechanism of ROS- and CO-mediated survival of brain endothelial cells during inflammatory insult. PKB/Akt has been shown to promote cell survival by inhibiting apoptosis induced by starvation, oxidative stress, excitotoxic neuromediators, and cytokines (7, 18, 20, 23, 26, 30). Constitutively active Akt accounts for resistance of endothelial cells from systemic vascular beds against TNF-α-induced cell death (11, 12, 52, 53). Although brain endothelial cells are vulnerable to TNF-α damage despite constitutively active Akt, siRNA-mediated Akt gene silencing exacerbated TNF-α-induced ROS and apoptosis in CMVEC. TNF-α caused a rapid transient activation of PKB/Akt signaling along with sustained phosphorylation of ERK1/2 and p38 MAPK in CMVEC. These MAPKs have been implicated in the control of apoptosis and cell survival via interaction with the PKB/Akt signaling cascade (6). However, activation of ERK1/2 and p38 MAPK signaling by TNF-α was not affected by triciribine and, therefore, was Akt independent. Furthermore, PD98059 and SB203580 did not alter the TNF-α-elicited ROS formation or apoptosis. Overall, these findings suggest that Akt, but not ERK1/2 or p38 MAPK, is essential for promoting cell survival during TNF-α-induced oxidative stress. Because Nox4 is the major source of ROS in CMVEC during TNF-α stimulation, it appears that Akt may negatively regulate Nox4 NADPH oxidase activity.
Our novel data demonstrate that CO inhibits activation of Nox4 NADPH oxidase by TNF-α and promotes cell survival via a mechanism that requires Akt activity. In contrast to innate CMVEC, in cells with inhibited or siRNA-downregulated Akt, CORM-A1 was unable to inhibit NADPH oxidase activity. Therefore, CO inhibits Nox4 activation by TNF-α via an Akt-dependent mechanism that may include the posttranslational modification of the Nox4 molecule that prevents CO binding to a specific CO-binding pocket of the heme protein. Nox4 NADPH oxidase is a target for Akt-dependent regulation. Although some reports indicate that PKB/Akt phosphorylates and regulates leukocytic Nox p47 (29), ours is the first report indicating that Akt regulates Nox4 activity in endothelial cells. Akt activity is required for promoting antioxidant and antiapoptotic effects of CO and, overall, for ROS-induced CO-mediated survival of cerebral vascular endothelial cells during inflammation.
The antiapoptotic effects of CORM-A1 against TNF-α-induced apoptosis were concomitant with the inhibition of ERK1/2 and p38 MAPK phosphorylation that occurred in an Akt-dependent manner. Furthermore, the inhibitors of ERK1/2 and p38 MAPK abrogated the ability of CORM-A1 to block TNF-α-induced NADPH oxidase activation and apoptosis. These data, for the first time, demonstrate the requirement of Akt-dependent MAPK signaling for antioxidant and antiapoptotic effects of CO during inflammatory conditions. Recent reports indicate involvement of Akt and MAPK signaling in long-term cytoprotective effects associated with HO-1 induction in various cells (13, 39, 43, 46). Akt and p38 MAPK are involved in the HO-1-mediated antiapoptotic mechanisms (11–14, 34, 39, 43, 45, 47). The involvement of p38 MAPK in CO-mediated cytoprotection has been shown in the vasculature (47), heart (25), and lung (41). HO-1 overexpression, in turn, enhances Akt phosphorylation and activation (14), although Batzlsperger (5) reported downregulation of Akt activity in HO-1 overepressing human umbilical vein endothelial cells.
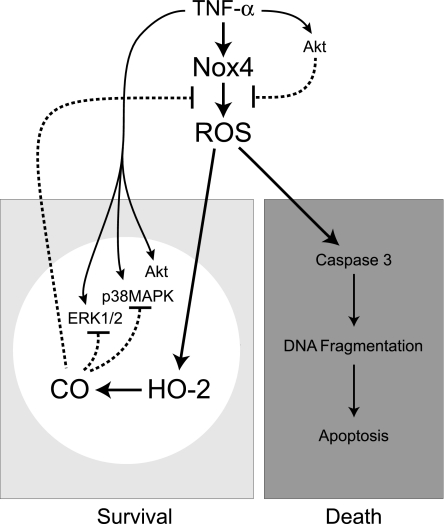
In conclusion, Nox4 NADPH oxidase-derived ROS initiate both death and survival pathways in TNF-α-challenged CMVEC (Fig. 11). Nox4 activation by TNF-α causes oxidative stress-related cell death. Simultaneously, Nox4-derived ROS initiate a CO-mediated survival pathway via acute activation of constitutive HO-2. CO inhibits Nox4 activation, reduces oxidative stress, and promotes survival of brain endothelial cells via a mechanism that requires Akt signaling. The ability of CO to inhibit TNF-α-induced ERK1/2 and p38 MAPK activities in an Akt-dependent manner appears to be the key element in ROS-dependent survival of endothelial cells during TNF-α-mediated brain inflammatory disease.
Fig. 11.
Proposed mechanisms of ROS-dependent CO-mediated survival pathway in brain endothelial cells stimulated by TNF-α. TNF-α activates Nox4 resulting in ROS-mediated cell death by apoptosis. Simultaneously, TNF-α initiates ROS-mediated survival pathways that attenuate oxidative stress: 1) PKB/Akt signaling negatively regulates Nox4 activity; 2) Nox4-derived ROS activate constitutive HO-2 and increase endogenous CO production, 3) CO inhibits Nox4 and reduces oxidative stress; 4) Akt, ERK1/2, and p38 MAPK linked to the Nox4 inhibition by CO. The key element of the ROS-dependent CO-mediated cytoprotection against TNF-α-induced apoptosis involves the CO inhibition of Nox4 that requires an Akt-dependent suppression of ERK1/2 and p38 MAPK.
GRANTS
This work was supported by National Institutes of Health Grants R01-NS-63936 and R01-HL-099655 (to H. Parfenova) and R01-HL034059 and R01-HL042851 (to C. W. Leffler), and by American Heart Association Grant R073037263 (to S. Basuroy).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the American Heart Association.
ACKNOWLEDGMENTS
We are deeply grateful to Hector Knight (Tyco-Mallinckrodt Medical; Petten, Holland) for the generous gift of CORM-A1. The authors thank Danny Morse for helping with preparation of the figures. We thank Prof. Kafait U. Malik and Dr. Ramesh Ray for fruitful discussions.
REFERENCES
- 1. Abid MR, Spokes KC, Shih SC, Aird WC. NADPH oxidase activity selectively modulates vascular endothelial growth factor signaling pathways. J Biol Chem 282: 35373–35385, 2007 [DOI] [PubMed] [Google Scholar]
- 2. Ago T, Kitazono T, Ooboshi H, Iyama T, Han YH, Takada J, Wakisaka M, Ibayashi S, Utsumi H, Iida M. Nox4 as the major catalytic component of an endothelial NAD(P)H oxidase. Circulation 109: 227–233, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Basuroy S, Bhattacharya S, Leffler CW, Parfenova H. Nox4 NADPH oxidase mediates oxidative stress and apoptosis caused by TNF-α in cerebral vascular endothelial cells. Am J Physiol Cell Physiol 296: C422–C432, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Basuroy S, Bhattacharya S, Tcheranova D, Qu Y, Regan RF, Leffler CW, Parfenova H. HO-2 provides endogenous protection against oxidative stress and apoptosis caused by TNF-α in cerebral vascular endothelial cells. Am J Physiol Cell Physiol 291: C897–C908, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Batzlsperger CA, Achatz S, Spreng J, Riegger GA, Griese DP. Evidence for a possible inhibitory interaction between the HO-1/CO- and Akt/NO-pathways in human endothelial cells. Cardiovasc Drugs Ther 21: 347–355, 2007 [DOI] [PubMed] [Google Scholar]
- 6. Berra E, Diaz-Meco MT, Moscat J. The activation of p38 and apoptosis by the inhibition of Erk is antagonized by the phosphoinositide 3-kinase/Akt pathway. J Biol Chem 273: 10792–10797, 1998 [DOI] [PubMed] [Google Scholar]
- 7. Bhattacharya S, Ray RM, Johnson LR. Decreased apoptosis in polyamine depleted IEC-6 cells depends on Akt-mediated NF-kB activation but not GSK3beta activity. Apoptosis 10: 759–776, 2005 [DOI] [PubMed] [Google Scholar]
- 8. Biswas S, Chida AS, Rahman I. Redox modifications of protein-thiols: emerging roles in cell signaling. Biochem Pharmacol 71: 551–564, 2006 [DOI] [PubMed] [Google Scholar]
- 9. Bozulic L, Hemmings BA. PIKKing on PKB: regulation of PKB activity by phosphorylation. Curr Opin Cell Biol 21: 256–261, 2009 [DOI] [PubMed] [Google Scholar]
- 10. Brazil DP. Advances in protein kinase B signalling: AKTion on multiple fronts. Trends Biochem Sci 29: 233–242, 2004 [DOI] [PubMed] [Google Scholar]
- 11. Brouard S, Otterbein LE, Anrather J, Tobiasch E, Bach FH, Choi AM, Soares MP. Carbon monoxide generated by heme oxygenase 1 suppresses endothelial cell apoptosis. J Exp Med 192: 1015–1026, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brouard S, Berberat PO, Tobiash E, Seldon MP, Bach FH, Soares MP. Heme oxygenase-1-derived carbon monoxide requires the activation of transcription factor NF-kB to protect endothelial cells from tumor necrosis factor-α-mediated apoptosis. J Biol Chem 277: 17950–17961, 2002 [DOI] [PubMed] [Google Scholar]
- 13. Brunt KR, Fenrich KK, Kiani G, Yat Tse M, Pang SC, Ward CA, Melo LG. Protection of human vascular smooth muscle cells from H2O2-induced apoptosis through functional codependence between HO-1 and AKT. Arterioscler Thromb Vasc Biol 26: 2027–2034, 2006 [DOI] [PubMed] [Google Scholar]
- 14. Busserolles J, Megias J, Terencio MC, Alcaraz MJ. Heme oxygenase-1 inhibits apoptosis in Caco-2 cells via activation of Akt pathway. Int J Biochem Cell Biol 38: 1510–1517, 2006 [DOI] [PubMed] [Google Scholar]
- 15. Chiarugi P, Fiaschi T. Redox signalling in anchorage-dependent cell growth. Cell Signal 19: 672–682, 2007 [DOI] [PubMed] [Google Scholar]
- 16. D'Autréaux B, Toledano MB. ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nat Rev Mol Cell Biol 8: 813–824, 2007 [DOI] [PubMed] [Google Scholar]
- 17. Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev 13: 2905–2927, 1999 [DOI] [PubMed] [Google Scholar]
- 18. Downward J. Mechanisms and consequences of activation of protein kinase B/Akt. Curr Opin Cell Biol 10: 262–267, 1998 [DOI] [PubMed] [Google Scholar]
- 19. Dröge W. Free radicals in the physiological control of cell function. Physiol Rev 82: 47–95, 2002 [DOI] [PubMed] [Google Scholar]
- 20. Dummler B, Hemmings BA. Physiological roles of PKB/Akt isoforms in development and disease. Biochem Soc Trans 35: 231–235, 2007 [DOI] [PubMed] [Google Scholar]
- 21. Duronio V. The life of a cell: apoptosis regulation by the PI3K/PKB pathway. Biochem J 415: 333–344, 2008 [DOI] [PubMed] [Google Scholar]
- 22. Franke TF. PI3K/Akt: getting it right matters. Oncogene 27: 6473–6488, 2008 [DOI] [PubMed] [Google Scholar]
- 23. Franke TF, Hornik CP, Segev L, Shostak GA, Sugimoto C. PI3K/Akt and apoptosis: size matters. Oncogene 22: 8983–8998, 2003 [DOI] [PubMed] [Google Scholar]
- 24. Forman HJ, Fukuto JM, Torres M. Redox signaling: thiol chemistry defines which reactive oxygen and nitrogen species can act as second messengers. Am J Physiol Cell Physiol 287: C246–C256, 2004 [DOI] [PubMed] [Google Scholar]
- 25. Fujimoto H, Ohno M, Ayabe S, Kobayashi H, Ishizaka N, Kimura H, Yoshida K, Nagai R. Carbon monoxide protects against cardiac ischemia-reperfusion injury in vivo via MAPK and Akt-eNOS pathways. Arterioscler Thromb Vasc Biol 24: 1848–1853, 2004 [DOI] [PubMed] [Google Scholar]
- 26. Gary DS, Mattson MP. Integrin signaling via the PI3-kinase-Akt pathway increases neuronal resistance to glutamate-induced apoptosis. J Neurochem 76: 1485–1496, 2001 [DOI] [PubMed] [Google Scholar]
- 27. Gorin Y, Ricono JM, Kim NH, Bhandari B, Choudhury GG, Abboud HE. Nox4 mediates angiotensin II-induced activation of Akt/protein kinase B in mesangial cells. Am J Physiol Renal Physiol 285: F219–F229, 2003 [DOI] [PubMed] [Google Scholar]
- 28. Hilenski LL, Clempus RE, Quinn MT, Lambeth JD, Griendling KK. Distinct subcellular localizations of Nox1 and Nox4 in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 24: 677–683, 2004 [DOI] [PubMed] [Google Scholar]
- 29. Hoyal CR, Gutierrez A, Young BM, Catz SD, Lin JH, Tsichlis PN, Babior BM. Modulation of p47PHOX activity by site-specific phosphorylation: Akt-dependent activation of the NADPH oxidase. Proc Natl Acad Sci USA 100: 5130–5135, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kamata H, Honda S, Maeda S, Chang L, Hirata H, Karin M. Reactive oxygen species promote TNF-α-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell 120: 649–661, 2005 [DOI] [PubMed] [Google Scholar]
- 31. Kohli R, Pan X, Malladi P, Wainwright MS, Whitington PF. Mitochondrial reactive oxygen species signal hepatocyte steatosis by regulating the phosphatidylinositol 3-kinase cell survival pathway. J Biol Chem 282: 21327–21336, 2007 [DOI] [PubMed] [Google Scholar]
- 32. Leffler CW, Balabanova L, Fedinec AL, Waters CM, Parfenova H. Mechanism of glutamate stimulation of CO production in cerebral microvessels. Am J Physiol Heart Circ Physiol 285: H74–H80, 2003 [DOI] [PubMed] [Google Scholar]
- 33. Leslie NR. The redox regulation of PI 3-kinase-dependent signaling. Antioxid Redox Signal 8: 1765–1774, 2006 [DOI] [PubMed] [Google Scholar]
- 34. Li MH, Jang JH, Na HK, Cha YN, Surh YJ. Carbon monoxide produced by upregulated heme oxygenase-1 in response to nitrosative stress induces expression of glutamate cysteine ligase in PC12 cells via activation of PI3K-Akt and Nrf2-ARE signaling. J Biol Chem 282: 28577–28586, 2007 [DOI] [PubMed] [Google Scholar]
- 35. Madamanchi NR, Vendrov A, Runge MS. Oxidative stress and vascular disease. Arterioscler Thromb Vasc Biol 25: 29–38, 2005 [DOI] [PubMed] [Google Scholar]
- 36. Maiuri T, Ho J, Stambolic V. Regulation of adipocyte differentiation by distinct subcellular pools of protein kinase B (PKB/Akt). J Biol Chem 285: 15038–15047, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Matsuzawa A, Ichijo H. Redox control of cell fate by MAP kinase: physiological roles of ASK1-MAP kinase pathway in stress signaling. Biochim Biophys Acta 1780: 1325–1336, 2008 [DOI] [PubMed] [Google Scholar]
- 38. Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell 129: 1261–1274, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Morse D, Lin L, Choi AM, Ryter SW. Heme oxygenase-1, a critical arbitrator of cell death pathways in lung injury and disease. Free Radic Biol Med 47: 1–12, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Motterlini R. Carbon monoxide-releasing molecules (CO-RMs): vasodilatory, anti-ischaemic and anti-inflammatory activities. Biochem Soc Trans 35: 1142–1146, 2007 [DOI] [PubMed] [Google Scholar]
- 41. Otterbein LE, Otterbein SL, Ifedigbo E, Liu F, Morse DE, Fearns C, Ulevitch RJ, Knickelbein R, Flavell RA, Choi AM. MKK3 mitogen-activated protein kinase pathway mediates carbon monoxide-induced protection against oxidant-induced lung injury. Am J Pathol 163: 2555–2563, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Parfenova H, Leffler CW. Cerebroprotective functions of HO-2. Curr Pharm Design 14: 443–453, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Park MK, Kim CH, Kim YM, Kang YJ, Kim HJ, Kim HJ, Seo HG, Lee JH, Chang KC. Akt-dependent heme oxygenase-1 induction by NS-398 in C6 glial cells: a potential role for CO in prevention of oxidative damage from hypoxia. Neuropharmacology 53: 542–551, 2007 [DOI] [PubMed] [Google Scholar]
- 44. Petry A, Djordjevic T, Weitnauer M, Kietzmann T, Hess J, Görlach A. NOX2 and NOX4 mediate proliferative response in endothelial cells. Antioxid Redox Signal 8: 1473–1484, 2006 [DOI] [PubMed] [Google Scholar]
- 45. Rane MJ, Klein JB. Regulation of neutrophil apoptosis by modulation of PKB/Akt activation. Front Biosci 14: 2400–2412, 2009 [DOI] [PubMed] [Google Scholar]
- 46. Salinas M, Wang J, Rosa de Sagarra M, Martín D, Rojo AI, Martin-Perez J, Ortiz de Montellano PR, Cuadrado A. Protein kinase Akt/PKB phosphorylates heme oxygenase-1 in vitro and in vivo. FEBS Lett 578: 90–94, 2004 [DOI] [PubMed] [Google Scholar]
- 47. Soares MP, Usheva A, Brouard S, Berberat PO, Gunther L, Tobiasch E, Bach FH. Modulation of endothelial cell apoptosis by heme oxygenase-1-derived carbon monoxide. Antioxid Redox Signal 4: 321–329, 2002 [DOI] [PubMed] [Google Scholar]
- 48. Torres M. Mitogen-activated protein kinase pathways in redox signaling. Front Biosci 8: d369–d391, 2003 [DOI] [PubMed] [Google Scholar]
- 49. Ushio-Fukai M, Tang Y, Fukai T, Dikalov SI, Ma Y, Fujimoto M, Quinn MT, Pagano PJ, Johnson C, Alexander RW. Novel role of gp91(phox)-containing NAD(P)H oxidase in vascular endothelial growth factor-induced signaling and angiogenesis. Circ Res 91: 1160–1167, 2002 [DOI] [PubMed] [Google Scholar]
- 50. Wolin MS, Gupte SA, Oeckler RA. Superoxide in the vascular system. J Vasc Res 39: 191–207, 2002 [DOI] [PubMed] [Google Scholar]
- 51. Yi L, Jenkins PM, Leichert LI, Jakob U, Martens JR, Ragsdale SW. The heme regulatory motifs in heme oxygenase-2 form a thiol/disulfide redox switch that responds to the cellular redox state. J Biol Chem 284: 20556–20561, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhang L, Himi T, Morita I, Murota S. Inhibition of phosphatidylinositol-3 kinase/Akt or mitogen-activated protein kinase signaling sensitizes endothelial cells to TNF-α cytotoxicity. Cell Death Differ 8: 528–536, 2001 [DOI] [PubMed] [Google Scholar]
- 53. Zhang X, Shan P, Alam J, Fu XY, Lee PJ. Carbon monoxide differentially modulates STAT1 and STAT3 and inhibits apoptosis via a phosphatidylinositol 3-kinase/Akt and p38 kinase-dependent STAT3 pathway during anoxia-reoxygenation injury. J Biol Chem 280: 8714–8121, 2005 [DOI] [PubMed] [Google Scholar]
- 54. Zimmermann A, Leffler CW, Tcheranova D, Fedinec AL, Parfenova H. Cerebroprotective effects of the CO-releasing molecule CORM-A1 against seizure-induced neonatal vascular injury. Am J Physiol Heart Circ Physiol 293: H2501–H2507, 2007 [DOI] [PubMed] [Google Scholar]