Abstract
Diarrhea is a common manifestation of gastrointestinal disorders. Diarrhea-induced losses of fluid and electrolyte could lead to dehydration and electrolyte imbalances, resulting in significant morbidity and mortality, especially in children living in developing countries. Somatostatin, a peptide hormone secreted by D-cells, plays an important role in regulating motility and intestinal Na+ absorption. Although octreotide, a somatostatin analog, is used to treat diarrhea, its mechanisms of action are unclear. Here we showed that octreotide increased brush-border membrane Na+/H+ exchanger 8 (NHE8) expression in the small intestine to the exclusion of other NHEs that participate in Na+ absorption. The same effect also occurred in human intestinal cells (Caco-2). We found that the increase of NHE8 expression by somatostatin required p38 mitogen-activated protein kinase (MAPK) activation. Furthermore, the somatostatin receptor SSTR2 antagonist CYN154806 could abolish somatostatin-induced NHE8 expression and p38 MAPK phosphorylation. Thus our data provided the first concrete evidence indicating that somatostatin stimulates intestinal Na+ absorption by increasing intestinal NHE8 expression through the SSTR2-p38 MAPK pathway.
Keywords: diarrhea, D-cells, sodium/hydrogen exchanger
diarrhea affects millions of people worldwide each year. It is a common cause of death in developing countries and one of the leading causes of death for infants. The etiology and the types of diarrhea are diverse, but the disease pathology always points to an imbalance in the secretory and absorptive functions of the intestines. An essential component of these functions is sodium absorption. Several transporter families including the Na+/H+ exchanger (NHE) family mediate Na+ absorption. The members of the NHE family are plasma membrane-bound antiporters that move extracellular Na+ into cells in exchange for intracellular H+. They are widely expressed in mammalian cells and have broad physiological functions (i.e., intracellular pH homeostasis, cell volume regulation, acid-base regulation, and electroneutral NaCl transport). Five of nine NHEs (NHE1–4, NHE8) are located in intestinal enterocytes (32, 34), but only NHE2, NHE3, and NHE8 are expressed in the brush-border membrane (BBM) of the intestinal epithelial cells (7, 8, 14, 32). NHE2 is involved in gastric function. Disruption of NHE2 expression alters oxyntic mucosa, reduces parietal and zymogenic cell number, and impairs intestinal barrier recovery (3, 15, 17). NHE3 is an important player in Na+ absorption. NHE3 knockout mice have altered acid-base balance and Na+ homeostasis (24). NHE8 is another apically expressed NHE, and it is the newest member of the NHE family. The transporter kinetics of NHE8 is distinct when compared with other NHEs. NHE8 is believed to have important roles in sodium absorption during early development (31, 32).
Somatostatin, an important neuropeptide produced by D cells in the gastrointestinal tract, functions as neurotransmitter and hormone (13, 20). Somatostatin is also a pro-absorptive and anti-secretory molecule. Its analog, octreotide, has been used as an anti-diarrheal agent for decades (16). In the intestine, somatostatin has been shown to stimulate NaCl absorption and to inhibit chloride secretion (9, 18). However, the mechanisms responsible for these effects are not known. Whether the effect of somatostatin on NaCl absorption stimulation involves NHEs is also unknown.
The present study explored the effect of somatostatin on apical NHEs. Our results indicated that somatostatin stimulated NHE8 but not NHE3 expression in the mouse intestine. This stimulation could be duplicated in Caco-2 cells, and the stimulation of NHE8 expression by somatostatin involved the type 2 somatostatin receptor (SSTR2)-p38 mitogen-activated protein kinase (MAPK) pathway. These findings suggest that somatostatin might be an important regulator involved in modulating intestinal sodium and water absorption through its selective effect on NHE8 expression.
MATERIALS AND METHODS
Animals.
Female mice (6–8 wk old, 129S6; Taconic) received octreotide (Bachem Bioscience; King of Prussia, PA, Sigma) at dose of 50 μg/kg body wt or saline subcutaneously twice a day for 3 days. This dose is within the physiological range of somatostatin. Eighteen hours after the last injection, mice were euthanized, and jejunal mucosa were collected and used for BBM protein isolation and RNA purification. All of the animal work was approved by the University of Arizona Institutional Animal Care and Use Committee. The experiments were repeated in three groups with three mice in each group.
Cell culture.
Caco-2 cells were obtained from American Type Culture Collection (Manassas, VA). Cells were grown in MEM-NEAA medium (Irvine Scientific, Santa Ana, CA) supplemented with 50 U/ml penicillin, 50 μg/ml streptomycin, and 20% fetal bovine serum in 5% CO2 incubator at 37°C. Cells from passages 37 to 47 were used in this study. For dose-response studies, the common concentrations of somatostatin for in vitro studies (0.1 and 1 μM) were used. Caco-2 cells were exposed 0.1 and 1 μM somatostatin for 18 h. For time-course study, cells were exposed to 1 μM somatostatin for 1 or 18 h. For the NHE8 activity assay, cells were exposed to 1 μM somatostatin in serum-free cell culture medium for 30 min. For the signaling pathway study, cells were pretreated with 10 μM H7, 25 μM SB202190, or 25 μM PD098059 for 1 h before the addition of 1 μM somatostatin. For somatostatin agonist study, 1 μM of seglitide was used. For somatostatin antagonist study, 1 μM of CYN154806 was used. Somatostatin and seglitide were obtained from Sigma (St. Louis, MO). CYN154806 was obtained from Tocris Bioscience (Ellisville, MI). SB202190, PD98059, and H7 were obtained from CalBiochem (San Diego, CA).
Functional assay of NHE8 in Caco-2 cells.
Caco-2 cells were seeded on glass coverslips and were cultured for 16 h before pHi measurement was performed. The activity of NHE8 was monitored by measuring the rate of Na+-dependent recovery of intracellular pH (pHi) after acid load in HCO3−-free HBSS as described in previous publications (31, 33). pHi was assessed by monitoring the fluorescence emission of the pH-sensitive dye SNARF-1 AM (Invitrogen; Carlsbad, CA). An Olympus IX-70 microscope equipped with a 40×/1.4 numberical aperature objectives was used to acquire light emitted from the cells. A Multispectral Imager (Optical Insights; Albuquerque, NM) was used to split the emission beam, forming side-by-side images of emitted light at 640 nm (basic pH wavelength) and 570 nm (acidic pH wavelength) that were acquired on a single charge-coupled device imaging array (Photometrics; Tucson, AZ). The ratio of fluorescence intensity (640 nm/570 nm) was measured for individual cells, and these ratios were subsequently converted to pHi by means of an in situ-derived calibration curve. The relationship between the SNARF-1 fluorescence ratio and pHi was fitted by using the following equation: pH = pK + log[(R − Rmin)/(Rmax − R)], where R represents the experimentally measured ratio of 640 nm/570 nm, Rmin is the ratio measured at the most acidic pH, and Rmax is the ratio measured at the most basic pH.
Protein isolation and Western blot analysis.
BBM protein was prepared from mouse intestinal mucosa as previously described (32). Cell surface protein was isolated using Pierce Cell Surface Protein Isolation Kit (Thermo Scientific; Rockford, IL) following manufacture's instruction. Total protein and crude membrane protein were prepared with methods described previously (8). For Western blot detection the following were used: 1:3,000 dilution of NHE3 antibody (8) or NHE8 antibody (32), 1:1,000 dilution of pp38 antibody (Promega; Madison, WI), 1:1,000 dilution of p38 antibody (Santa Cruz Biotechnology; Santa Cruz, CA), and 1:5,000 of β-actin antiserum (Sigma). Western blot detection was performed with the BM Chemiluminescence Western Blotting kit (Roche Diagnostics; Indianapolis, IN). To quantitate protein expression level, a ratio of target protein intensity over β-actin protein intensity or phosphorylated p38 protein over total p38 protein was used.
RNA purification and RT-PCR analysis.
RNA was purified from mouse intestinal mucosa and Caco-2 cells using the TRIzol reagent (Invitrogen). Total RNA (500 ng) was reverse transcribed to cDNA by Moloney murine leukemia virus reverse transcriptase (Promega). For detecting NHE8 mRNA abundance, real-time PCR was performed using NHE8-specific primers (Applied Biosystems; Carlsbad, CA). For somatostatin receptor (SSTR) mRNA detection, RT-PCR was carried out with human and mouse SSTR gene specific primers (Table 1). PCR was performed as described: initial denaturation at 94°C for 10 min followed by 35 cycles of 1 min at 94°C, 1 min at 58°C, and 1 min at 72°C. PCR products were visualized by electrophoresis in 1.5% agarose gel. DNA sequencing was also used to confirm the identity of amplified sequence.
Table 1.
Primers for SSTR PCR
| Gene | Primer (Forward/Reverse) | Product Length (bp) | GenBank Accession No. |
|---|---|---|---|
| Human | |||
| SSTR1 | 5′-GCAACATGCTCATGC-3′ | 414 | NM001049 |
| 5′-GCGTTGTCCATCCAG-3′ | |||
| SSTR2 | 5′-AAGAGGGTCGAGGAGCCAGGAAC-3′ | 450 | NM001050 |
| 5′-GGCATAGCGGAGGATGACATAAA-3′ | |||
| SSTR3 | 5′-TGGGCACCCTCGTGCCAGCGG-3′ | 447 | NM001051 |
| 5′-GGGCGGCCGCTCCTGCCCGC-3′ | |||
| SSTR4 | 5′-CTGAACCTCTTCGTGACCAGCCTT-3′ | 278 | NM001052 |
| 5′-CTGGTTGCAGGGCTTCCTGCT-3′ | |||
| SSTR5 | 5′-GTGCAGGAGGGCGGTACC-3′ | 373 | NM001053 |
| 5′-TGGACGCGGCTCCGTGGC-3′ | |||
| Mouse | |||
| SSTR1 | 5′-CACCAGCATCTACTGTCTGACTG-3′ | 638 | NM009216 |
| 5′-CATAGTAGTCGACTGGTTCCTCC-3′ | |||
| SSTR2 | 5′-CTTGGCCATGCAGGTGGCGCTAGT-3′ | 1081, 742 | NM009217 |
| 5′-CAATGATGTCTTCCGCTCCGGATTGT-3′ | |||
| SSTR3 | 5′-AGTGTCTATATCCTCAACCTGGC-3′ | 627 | NM009218 |
| 5′-ATTGACGATGTTGAGCAGATAGA-3′ | |||
| SSTR4 | 5′-TCGGATTATGCTACCTGCTTATT-3′ | 483 | NM009219 |
| 5′-AAGTAGTGGTATTGGTGAAAGGG-3′ | |||
| SSTR5 | 5′-GGTATGCCAAGATGAAGACAGTT-3′ | 784 | U82697 |
| 5′-TATCCTCTACTGAGGCACAGAGC-3′ |
SSTR, somatostatin receptor.
RESULTS
Effect of octreotide on intestinal NHE8 and NHE3 expression in mice.
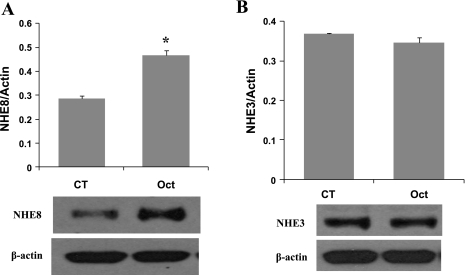
Female mice at age of 6–8 wk were given octreotide subcutaneously (50 μg/kg body wt twice a day for 3 days). Eighteen hours after the last octreotide administration, mice were euthanized, and BBM protein was isolated from intestinal mucosa. NHE8 and NHE3 protein abundance in BBM protein preps were determined by Western blot analysis. NHE8 and NHE3 immunoreactive protein abundance was calculated by dividing the optical densities of the NHE8 or NHE3 band to that of the β-actin band. As shown in Fig. 1, octreotide administration stimulated intestinal NHE8 protein expression by ∼62% (0.836 ± 0.038 in control mice; 1.357 ± 0.098 in octreotide mice. n = 3, P < 0.01) (Fig. 1A), but it has no effect on NHE3 expression (0.368 ± 0.002 in control mice; 0.345 ± 0.022 in octreotide mice. n = 3) (Fig. 1B).
Fig. 1.
Effect of octreotide on the intestinal Na+/H+ exchanger 8 (NHE8) expression in mice. Brush-border membrane (BBM) protein was isolated from the intestinal mucosa of control or octreotide-treated mice. BBM protein (30 μg) was separated on SDS-PAGE gels and immunoblotted. NHE8 antibody, NHE3 antibody, and β-actin antibody were used to detect NHE8, NHE3, and β-actin, respectively. NHE8 protein was detected at ∼65 kDa. NHE3 protein was detected at ∼85 kDa. β-Actin protein was detected at ∼42 kDa. The expression of NHE8 (A) and NHE3 (B) was calculated by dividing the optical density of the NHE8 and NHE3 band over that of the β-actin band. Bar chart shows the NHE8 and NHE3 protein expression indicated as means ± SE in the sum of 3 experiments. *P ≤ 0.01 for control mice vs. octreotide mice. Bottom, corresponding Western blot image. CT, control; Oct, octreotide.
Concentration-dependent effects of somatostatin on NHE8 expression in Caco-2 cells.
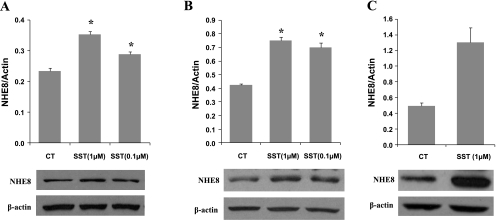
Caco-2 cells were exposed to different concentrations of somatostatin for 18 h. Total protein, crude membrane protein, and cell surface protein were extracted from Caco-2 cells and used for Western blot detection. As indicated in Fig. 2, when cells were exposed to 0.1 or 1 μM somatostatin, NHE8 protein abundance was increased in all protein preps. The total NHE8 protein was increased from 0.235 ± 0.009 in control to 0.354 ± 0.009 in 1 μM somatostatin and 0.290 ± 0.008 in 0.1 μM somatostatin (n = 3, P < 0.05) (Fig. 2A). The membrane NHE8 protein was increased from 0.422 ± 0.020 in control to 0.749 ± 0.049 in 1 μM somatostatin and 0.699 ± 0.060 in 0.1 μM somatostatin (n = 3, P < 0.01) (Fig. 2B). The biggest change on NHE8 protein expression was seen in cell surface protein extracts. Somatostatin increased cell surface NHE8 protein abundance by ∼2.64 fold from 0.494 ± 0.039 in control to 1.304 ± 0.187 in 1 μM somatostatin (Fig. 2C).
Fig. 2.
Effect of somatostatin concentration on the endogenous NHE8 expression in Caco-2 cells. Cells were cultured in standard medium or somatostatin-containing medium (1 or 0.1 μM) for 18 h. Total protein (A), crude membrane protein (B), and cell surface protein (C) were prepared and used for Western blot analysis. NHE8 protein was detected at ∼65 kDa. β-Actin protein was detected at ∼42 kDa. Results are means ± SE from 2 to 5 experiments. *P < 0.01 for somatostatin treatment vs. control. Bottom, corresponding Western blot image. SST, somatostatin.
Effect of somatostatin exposure duration on NHE8 expression in Caco-2 cells.
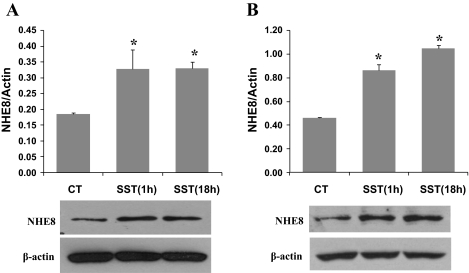
Caco-2 cells were exposed to 1 μM somatostatin for 1 or 18 h. Membrane protein and total protein were isolated from cells and used for Western blot detection. As shown in Fig. 3, the increase of NHE8 by somatostatin occurred as early as 60 min after somatostatin treatment. Somatostatin stimulated total NHE8 protein expression from 0.185 ± 0.004 in control to 0.328 ± 0.061 at 1 h and 0.331 ± 0.020 at 18 h (n = 3, P < 0.05) (Fig. 3A). Similar results were observed in membrane protein preparations. Somatostatin significantly increased membrane NHE8 protein expression from 0.463 ± 0.003 in control to 0.863 ± 0.089 at 1 h and to 1.051 ± 0.046 at 18 h (n = 3, P < 0.01) (Fig. 3B).
Fig. 3.
Effect of SST exposure time on the endogenous NHE8 expression in Caco-2 cells. Cells were cultured in standard medium or SST-containing medium (1 μM) for 1 or 18 h. Total protein (A) and crude membrane protein (B) were prepared and used for Western blot analysis. Results are means ± SE from 3 to 5 experiments. *P < 0.01 for SST treatment vs. CT. Bottom, corresponding Western blot image.
Effect of somatostatin on NHE8 function in Caco-2 cells.
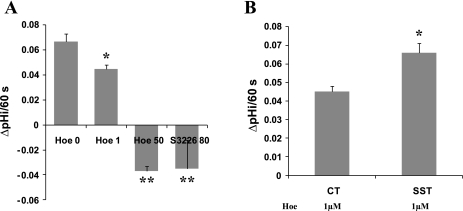
pHi measurement was performed in Caco-2 cells grown on glass coverslips. Using NHE specific inhibitors, we identified that only NHE1 and NHE8 activity were detected at a functional level in Caco-2 cells. The rate of pHi recovery in Caco-2 cells was 0.067 ± 0.006 pH/min in the absence of HOE694 and 0.045 ± 0.003 pH/min in the presence of 1 μM HOE694. Caco-2 cells failed recover from acid load in the presence of 50 μM HOE694 or 80 μM S3226 (Fig. 4A). After cells were exposed to somatostatin (1 μM, 30 min), the rate of pHi recovery in the presence of 1 μM HOE694 was increased by ∼1.5-fold, from 0.045 ± 0.003 pH/min in control cells to 0.066 ± 0.006 pH/min in somatostatin-treated cells (Fig. 4B).
Fig. 4.
Effect of SST on NHE8 activity in Caco-2 cells. A: charcterization of functional NHEs in Caco-2 cells. Cells were loaded with 40 mM NH4Cl for 5 min and then washed with sodium-free HBSS for 2 min before addition of sodium-containing HBSS. The rate of pHi recovery was analyzed during the initial 60 s following addition of sodium-containing HBSS in the presence or absence of NHE inhibitors HOE694 (0, 1, and 50 μM) or S3226 (80 μM). HOE694 or S3226 was included both during the NH4Cl washout phase and the recovery phase. Data were collected from 7 to 10 cells for each group. *P < 0.01 for 1 μM HOE694 vs. others; **P ≤ 0.001 for 50 μM HOE694 and 80 μM S3226 vs. others. B: effect of SST on NHE8 activity. Cells treated with or without SST (1 μM, 30 min) were loaded with 40 mM NH4Cl in HBSS for 5 min and then washed with sodium-free HBSS for 2 min before addition of sodium-containing HBSS. The rate of pHi recovery was analyzed during the initial 60 s following addition of sodium-containing HBSS in the presence of 1 μM HOE694. Data were collected from 7 to 10 cells for each group. *P < 0.01 for SST treatment vs. CT.
SSTR expression in mouse intestine and in Caco-2 cells.
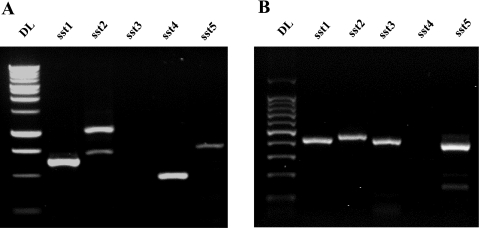
RNA was isolated from mouse intestinal mucosa and from Caco-2 cells. Conventional RT-PCR assay was used to determine SSTR expression with mouse- and human-specific SSRT primers. SSTR1, SSTR2, SSTR4, and SSTR5, but not SSTR3, were amplified from mouse intestine (Fig. 5A). SSTR1, SSTR2, SSTR3, and SSTR5, but not SSTR4, were amplified from Caco-2 cells (Fig. 5B). DNA sequencing further confirmed that these PCR products were indeed the human and the mouse SSTRs.
Fig. 5.
Expression of intestinal SST receptors (SSTRs) in mice and Caco-2 cells. Total RNA was isolated from mouse intestinal mucosa and Caco-2 cells. Reverse transcription reaction was performed with Moloney murine leukemia virus reverse transcriptase and random primers. RT-PCR was used to amplify SSTR cDNAs with SSRT gene-specific primers. PCR products were separated on 1.5% agarose gel and visualized after ethidium bromide staining. A: PCR amplification of SSTRs from mouse intestine. B: PCR amplification of SSTRs from Caco-2 cells.
Effect of SSTR2 agonist and antagonist on NHE8 expression in Caco-2 cells.
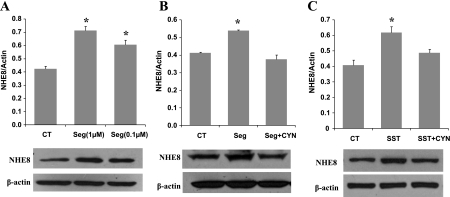
SSTR2 has been shown to be involved in stimulating butyrate uptake in Caco-2 cells (23). To test whether SSTR2 participates in somatostatin-mediated NHE8 activation, selective SSTR2 agonist (seglitide) and antagonist (CYN154806) were used. Like somatostatin, seglitide increased NHE8 expression from 0.424 ± 0.019 in control cells to 0.713 ± 0.32 in 1 μM seglitide-treated cells or 0.608 ± 0.033 in 0.1 μM seglitide-treated cells (Fig. 6A). This increase was completely abolished by CYN154806 (Fig. 6B). Moreover, somatostatin-induced NHE8 expression was also abolished by CYN154806 (Fig. 6C).
Fig. 6.
Effect of SSTR2 agonist and antagonist on NHE8 expression in Caco-2 cells. Caco-2 cells were preincubated with 1 μM SSTR2 antagonist CYN154806 (CYN) for 1 h before addition of 1 μM SST or SSTR2 agonist seglitide (Seg). After 18 h, total protein was prepared from cells and used for Western blot analysis. Results are means ± SE from 3 to 5 experiments. *P < 0.01 for treatment vs. control. Bottom, corresponding Western blot image. A: effect of Seg on NHE8 expression in Caco-2 cells. B: effect of CYN on Seg-mediated NHE8 activation in Caco-2 cells. C: effect of CYN on SST-mediated NHE8 activation in Caco-2 cells.
Role of p38 MAPK in SST-induced NHE8 expression in Caco-2 cells.
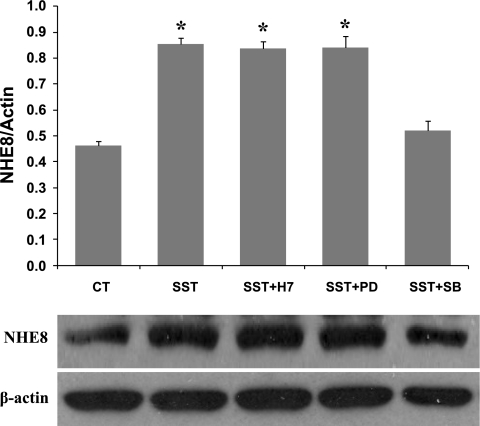
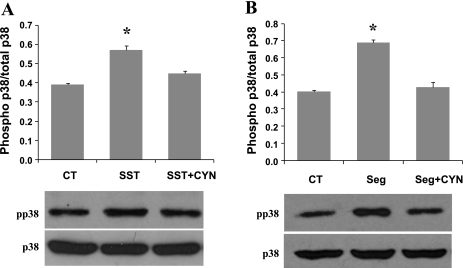
Somatostatin has been shown to activate PKC and MAPK signal transduction pathways through interacting with its receptors. In this experiment, we wanted to test whether PKC and/or MAPK participate in somatostatin-mediated NHE8 expression stimulation in Caco-2 cells. As shown in Fig. 7, SB202190, a p38 MAPK-specific inhibitor, blocked somatostatin-stimulated NHE8 expression in Caco-2 cells, neither the PKC inhibitor (H7) nor the MEK inhibitor (PD98059) blocked this stimulation. Therefore, p38 MAPK is necessary for somatostatin-mediated NHE8 stimulation. To study if p38MAPK activation was a pathway downstream of SSTR2 signaling, Western blot was used to compare p38 phosphorylation in Caco-2 cells in the absence or presence of SSTR2 agonist and antagonist. As shown in Fig. 8, somatostatin induced p38 MAPK phosphorylation in Caco-2 cells, and CYN154806 inhibited the effect of somatostatin on p38 MAPK phosphorylation (Fig. 8A). Similarly, seglitide also induced p38MAPK phosphorylation, and this effect was abolished by CYN154806 (Fig. 8B).
Fig. 7.
Signaling pathway of SST-mediated NHE8 activation in Caco-2 cells. Caco-2 cells were preincubated with H7 (10 μM), PD98059 (PD, 25 μM), and SB202190 (SB, 25 μM) for 1 h before addition of SST (1 μM). After 18 h, total protein was prepared from cells and used for Western blot analysis. Results are means ± SE from 3 to 5 experiments. *P < 0.01 for treatment vs. control. Bottom, corresponding Western blot image.
Fig. 8.
p38 mitogen-activated protein kinase (MAPK) phosphorylation involvement in response to SSTR2 activation. Caco-2 cells were treated with 1 μM SST (A) or 1 μM Seg (B) for 18 h in the absence or presence of 1 μM CYN. Total protein was prepared from cells and used for Western blot analysis. p38 MAPK and pp38 MAPK protein was detected at ∼38 kDa. Results are means ± SE from 3 experiments. *P < 0.05 for treatment vs. control. Bottom, corresponding Western blot image.
DISCUSSION
The intestinal epithelium is important for ion transport and barrier functions (2). Transepithelial movement of a solute across the entire epithelium is either absorptive or secretory. Disruption of absorption or secretion function results in diarrheal illness. In fact, reduced NHE function has been observed in diarrheal diseases (11, 22, 30).
Somatostatin is widely distributed in the central nervous system, gastrointestinal tract, and endocrine cells. It modulates many physiological processes, such as pro-absorption, anti-secretion, anti-proliferation, immunological modulation, neuronal excitability, and vascular smooth muscle contractility (4–6, 10, 28). In the intestine, somatostatin is known to affect intestinal motility, to reduce secretion, and to stimulate water and sodium absorption (1, 27, 29). Octreotide, a somatostatin analog, is often used to treat diarrheal disorders such as short bowel syndrome and dumping syndrome (1, 26). Although studies showed that somatostatin could stimulate net sodium and chloride absorption (21), it was unclear if this stimulation is due to somatostatin action on apically expressed NHEs in the intestine.
In our presented studies, we used the mouse as the in vivo model to study the effect of octreotide on intestinal NHE expression. In our preliminary study, we found out that NHE8 expression is higher in female mice than in male mice and that male mice barely responsed to somatostatin treatment. Therefore, we chose female mice for somatostatin study. Our study demonstrated that octreotide stimulated NHE8 but not NHE3 expression in the mouse intestine. Octreotide increased the intestinal NHE8 protein expression by ∼1.6-fold when compared with control mice, whereas NHE8 mRNA expression remained no change (data not shown). This observation suggested that somatostatin specifically stimulated NHE8 expression in the intestine, and this stimulation likely occurred at the protein level by increasing protein translation and/or increasing protein stability.
To study the mechanisms of somatostatin-mediated NHE8 activation, we chose a human intestinal cell line Caco-2 as the in vitro model. In Caco-2 cells, somatostatin stimulated NHE8 expression in a concentration- and time-dependent manner. Low concentration of somatostatin (0.1 μM) or short time exposure to somatostatin (1 h) was sufficient to stimulate NHE8 expression in Caco-2 cells. The stimulation on NHE8 protein expression occurred at all levels from total protein, crude membrane protein, to cell surface protein extracts. This observation suggested that somatostatin enhanced NHE8 expression by increasing NHE8 protein abundance through increasing protein synthesis and/or increasing protein stability.
Since somatostatin increased surface NHE8 protein abundance, we would expect that somatostatin would increase NHE8 functional activity in Caco-2 cells. To study the activity of NHE8 in Caco-2 cells, we needed to know how many NHE isforms were expressed in these undifferentiated cells.By using NHE-specific inhibitors HOE694 and S3226, we were able to identify functional NHE exchanger in Caco-2 cells. Low concentration of S3226 has been shown to inhibit NHE3 function and NHE8 function but not NHE2 function. Low concentration of HOE694 had no effect on NHE3 activity but it inhibited NHE2 and NHE8 (31). In our study, the pHi recovery after acid load was completely blocked by 80 μM S3226, indicating no functional NHE2 was expressed in Caco-2 cells. In the presence of 50 μM HOE694, Caco-2 cells failed to recover from acid load, suggesting that the functional NHE3 was absent. In the presence of 1 μM HOE694, a concentration inhibiting NHE1 activity, Caco-2 cells retained ∼60% of NHE activity, indicating there was functional NHE8 expression. These observations suggested that only NHE1 and NHE8 were functional NHEs in these undifferentiated Caco-2 cells. When Caco-2 cells were exposed to somatostatin for 30 min, the 1 μM HOE694 insensitive pHi recovery rate was increased by ∼80% compared with control cells, indicating that NHE8 activity could be stimulated by somatostatin.
Somatostatin induces biological responses by interacting with somatostatin receptors (SSTRs) expressed on the cell membrane. Previous studies showed that multiple SSTRs (SSTR1∼SSTR5) are heterogeneously distributed in a variety of tissues (19). In the present study, we identified four SSRTs that are expressed in mouse intestine and Caco-2 cells: SSTR1, SSTR2, SSTR4, and SSTR5 were expressed in mouse intestine, and SSTR1, SSTR2, SSTR3, and SSTR5 were expressed in Caco-2 cells. Since studies showed that SSTR2 is involved in somatostain-mediated butyrate absorption and SSTR2 activation stimulated p38 MAPK phosphorylation in Caco-2 cells (12, 23, 25), we wanted to explore if SSRT2 signaling participate in somatostatin-induced NHE8 stimulation using the SSTR2-specific agonist seglitide and the SSTR2 specific antagonist CYN154806. Seglitide increased NHE8 expression in the similar pattern observed with somatostatin, and CYN154806 abolished the stimulatory effect of somatostatin and seglitide on NHE8 expression. These findings strongly supported that somatostatin directly stimulated NHE8 expression via interacting with SSTR2 in the intestinal epithelial cells. To address the molecular mechanisms of somatostain-mediated NHE8 activation, signal transduction pathway studies were carried out in Caco-2 cells. H7, a potent protein kinase inhibitor, failed to block the effect of somatostatin on NHE8 expression, suggesting that protein kinase pathways were not involved. PD058052, a specific ERK inhibitor, also failed to block somatostatin-stimulated NHE8 expression, suggesting that ERK pathway was not involved. However, SB026038, a p38 MAPK-specific inhibitor, completely abolished the effect of somatostatin on NHE8 expression in Caco-2 cells, indicating the involvement of p38 MAPK pathway in somatostatin-mediated NHE8 stimulation. Further studies also showed that seglitide could increase NHE8 expression and p38 phosphorylation in Caco-2 cells, and CYN154806 abolished both somatostatin- and seglitide-induced NHE8 expression and p38 phosphorylation. Taken together, these results suggested that SSTR2-p38MAPK phosphorylation was activated in somatostatin-mediated NHE8 stimulation.
In summary, we have shown that octreotide stimulated the intestinal NHE8 expression and that somatostatin stimulated NHE8 function in Caco-2 cells. We also identified that SSTR2-p38 MAPK activation was a key signal transduction pathway involved in somatostatin-mediated NHE8 upregulation in Caco-2 cells. Our studies uncovered for the first time the mechanisms of somatostatin as an anti-diarrheal agent by enhancing intestinal solute absorption through its effect on NHE8 expression.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
This investigation was funded by National Institutes of Health grant R01-DK073638 (F. K. Ghishan and H. Xu) and Natural Scientific Fund of China grant 81070294 (C. Wang).
REFERENCES
- 1. Anthone GJ, Bastidas JA, Orandle MS, Yeo CJ. Direct proabsorptive effect of octreotide on ionic transport in the small intestine. Surgery 108: 1136–1141; discussion 1141–1132, 1990 [PubMed] [Google Scholar]
- 2. Barrett KE. New ways of thinking about (and teaching about) intestinal epithelial function. Adv Physiol Educ 32: 25–34, 2008 [DOI] [PubMed] [Google Scholar]
- 3. Boivin GP, Schultheis PJ, Shull GE, Stemmermann GN. Variant form of diffuse corporal gastritis in NHE2 knockout mice. Comp Med 50: 511–515, 2000. [PubMed] [Google Scholar]
- 4. Buscail L, Vernejoul F, Faure P, Torrisani J, Susini C. [Regulation of cell proliferation by somatostatin]. Ann Endocrinol (Paris) 63: 2S13–2S18, 2002 [PubMed] [Google Scholar]
- 5. Calabresi E, Ishikawa E, Bartolini L, Delitala G, Fanciulli G, Oliva O, Veldhuis JD, Serio M. Somatostatin infusion suppresses GH secretory burst frequency and mass in normal men. Am J Physiol Endocrinol Metab 270: E975–E979, 1996 [DOI] [PubMed] [Google Scholar]
- 6. Cammalleri M, Martini D, Timperio AM, Bagnoli P. Functional effects of somatostatin receptor 1 activation on synaptic transmission in the mouse hippocampus. J Neurochem 111: 1466–1477, 2009 [DOI] [PubMed] [Google Scholar]
- 7. Collins JF, Honda T, Knobel S, Bulus NM, Conary J, DuBois R, Ghishan FK. Molecular cloning, sequencing, tissue distribution, and functional expression of a Na+/H+ exchanger (NHE-2). Proc Natl Acad Sci USA 90: 3938–3942, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Collins JF, Xu H, Kiela PR, Zeng J, Ghishan FK. Functional and molecular characterization of NHE3 expression during ontogeny in rat jejunal epithelium. Am J Physiol Cell Physiol 273: C1937–C1946, 1997 [DOI] [PubMed] [Google Scholar]
- 9. Csaba Z, Dournaud P. Cellular biology of somatostatin receptors. Neuropeptides 35: 1–23, 2001 [DOI] [PubMed] [Google Scholar]
- 10. Ferone D, Resmini E, Boschetti M, Arvigo M, Albanese V, Ceresola E, Pivonello R, Albertelli M, Bianchi F, Giusti M, Minuto F. Potential indications for somatostatin analogues: immune system and limphoproliferative disorders. J Endocrinol Invest 28: 111–117, 2005 [PubMed] [Google Scholar]
- 11. Gill RK, Saksena S, Tyagi S, Alrefai WA, Malakooti J, Sarwar Z, Turner JR, Ramaswamy K, Dudeja PK. Serotonin inhibits Na+/H+ exchange activity via 5-HT4 receptors and activation of PKC alpha in human intestinal epithelial cells. Gastroenterology 128: 962–974, 2005 [DOI] [PubMed] [Google Scholar]
- 12. Gong AY, Tietz PS, Muff MA, Splinter PL, Huebert RC, Strowski MZ, Chen XM, LaRusso NF. Somatostatin stimulates ductal bile absorption and inhibits ductal bile secretion in mice via SSTR2 on cholangiocytes. Am J Physiol Cell Physiol 284: C1205–C1214, 2003 [DOI] [PubMed] [Google Scholar]
- 13. Gyr KE, Meier R. Pharmacodynamic effects of Sandostatin in the gastrointestinal tract. Digestion 54, Suppl 1: 14–19, 1993 [DOI] [PubMed] [Google Scholar]
- 14. Hoogerwerf WA, Tsao SC, Devuyst O, Levine SA, Yun CH, Yip JW, Cohen ME, Wilson PD, Lazenby AJ, Tse CM, Donowitz M. NHE2 and NHE3 are human and rabbit intestinal brush-border proteins. Am J Physiol Gastrointest Liver Physiol 270: G29–G41, 1996 [DOI] [PubMed] [Google Scholar]
- 15. Ledoussal C, Woo AL, Miller ML, Shull GE. Loss of the NHE2 Na+/H+ exchanger has no apparent effect on diarrheal state of NHE3-deficient mice. Am J Physiol Gastrointest Liver Physiol 281: G1385–G1396, 2001 [DOI] [PubMed] [Google Scholar]
- 16. Lewin MJ. The somatostatin receptor in the GI tract. Annu Rev Physiol 54: 455–468, 1992 [DOI] [PubMed] [Google Scholar]
- 17. Moeser AJ, Nighot PK, Ryan KA, Simpson JE, Clarke LL, Blikslager AT. Mice lacking the Na+/H+ exchanger 2 have impaired recovery of intestinal barrier function. Am J Physiol Gastrointest Liver Physiol 295: G791–G797, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Patel YC. Somatostatin and its receptor family. Front Neuroendocrinol 20: 157–198, 1999 [DOI] [PubMed] [Google Scholar]
- 19. Patel YC, Srikant CB. Somatostatin receptors. Trends Endocrinol Metab 8: 398–405, 1997 [DOI] [PubMed] [Google Scholar]
- 20. Pless J, Bauer W, Briner U, Doepfner W, Marbach P, Maurer R, Petcher TJ, Reubi JC, Vonderscher J. Chemistry and pharmacology of SMS 201–995, a long-acting octapeptide analogue of somatostatin. Scand J Gastroenterol Suppl 119: 54–64, 1986 [DOI] [PubMed] [Google Scholar]
- 21. Roberts WG, Fedorak RN, Chang EB. In vitro effects of the long-acting somatostatin analogue SMS 201–995 on electrolyte transport by the rabbit ileum. Gastroenterology 94: 1343–1350, 1988 [DOI] [PubMed] [Google Scholar]
- 22. Rocha F, Musch MW, Lishanskiy L, Bookstein C, Sugi K, Xie Y, Chang EB. IFN-gamma downregulates expression of Na+/H+ exchangers NHE2 and NHE3 in rat intestine and human Caco-2/bbe cells. Am J Physiol Cell Physiol 280: C1224–C1232, 2001 [DOI] [PubMed] [Google Scholar]
- 23. Saksena S, Theegala S, Bansal N, Gill RK, Tyagi S, Alrefai WA, Ramaswamy K, Dudeja PK. Mechanisms underlying modulation of monocarboxylate transporter 1 (MCT1) by somatostatin in human intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol 297: G878–G885, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schultheis PJ, Clarke LL, Meneton P, Miller ML, Soleimani M, Gawenis LR, Riddle TM, Duffy JJ, Doetschman T, Wang T, Giebisch G, Aronson PS, Lorenz JN, Shull GE. Renal and intestinal absorptive defects in mice lacking the NHE3 Na+/H+ exchanger. Nat Genet 19: 282–285, 1998 [DOI] [PubMed] [Google Scholar]
- 25. Sellers LA, Alderton F, Carruthers AM, Schindler M, Humphrey PP. Receptor isoforms mediate opposing proliferative effects through gbetagamma-activated p38 or Akt pathways. Mol Cell Biol 20: 5974–5985, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tack J, Arts J, Caenepeel P, De Wulf D, Bisschops R. Pathophysiology, diagnosis and management of postoperative dumping syndrome. Nat Rev Gastroenterol Hepatol 6: 583–590, 2009 [DOI] [PubMed] [Google Scholar]
- 27. Tomita R. Relationship between interdigestive migrating motor complex and gut hormones in human. Hepatogastroenterology 56: 714–717, 2009 [PubMed] [Google Scholar]
- 28. Torrecillas G, Medina J, Diez-Marques ML, Rodriguez-Puyol D, Rodriguez-Puyol M. Mechanisms involved in the somatostatin-induced contraction of vascular smooth muscle cells. Peptides 20: 929–935, 1999 [DOI] [PubMed] [Google Scholar]
- 29. Warhurst G, Barbezat GO, Higgs NB, Reyl-Desmars F, Lewin MJ, Coy DH, Ross I, Grigor MR. Expression of somatostatin receptor genes and their role in inhibiting Cl− secretion in HT-29cl.19A colonocytes. Am J Physiol Gastrointest Liver Physiol 269: G729–G736, 1995 [DOI] [PubMed] [Google Scholar]
- 30. Xu H, Chen H, Dong J, Li J, Chen R, Uno JK, Ghishan FK. Tumor necrosis factor-α downregulates intestinal NHE8 expression by reducing basal promoter activity. Am J Physiol Cell Physiol 296: C489–C497, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Xu H, Chen H, Dong J, Lynch R, Ghishan FK. Gastrointestinal distribution and kinetic characterization of the sodium-hydrogen exchanger isoform 8 (NHE8). Cell Physiol Biochem 21: 109–116, 2008 [DOI] [PubMed] [Google Scholar]
- 32. Xu H, Chen R, Ghishan FK. Subcloning, localization, and expression of the rat intestinal sodium-hydrogen exchanger isoform 8. Am J Physiol Gastrointest Liver Physiol 289: G36–G41, 2005 [DOI] [PubMed] [Google Scholar]
- 33. Xu H, Collins JF, Bai L, Kiela PR, Lynch RM, Ghishan FK. Epidermal growth factor regulation of rat NHE2 gene expression. Am J Physiol Cell Physiol 281: C504–C513, 2001 [DOI] [PubMed] [Google Scholar]
- 34. Zachos NC, Tse M, Donowitz M. Molecular physiology of intestinal Na+/H+ exchange. Annu Rev Physiol 67: 411–443, 2005 [DOI] [PubMed] [Google Scholar]