Abstract
Extracellular ATP and ADP have been shown to exhibit potent angiogenic effects on pulmonary artery adventitial vasa vasorum endothelial cells (VVEC). However, the molecular signaling mechanisms of extracellular nucleotide-mediated angiogenesis remain not fully elucidated. Since elevation of intracellular Ca2+ concentration ([Ca2+]i) is required for cell proliferation and occurs in response to extracellular nucleotides, this study was undertaken to delineate the purinergic receptor subtypes involved in Ca2+ signaling and extracellular nucleotide-mediated mitogenic responses in VVEC. Our data indicate that stimulation of VVEC with extracellular ATP resulted in the elevation of [Ca2+]i via Ca2+ influx through plasma membrane channels as well as Ca2+ mobilization from intracellular stores. Moreover, extracellular ATP induced simultaneous Ca2+ responses in both cytosolic and nuclear compartments. An increase in [Ca2+]i was observed in response to a wide range of purinergic receptor agonists, including ATP, ADP, ATPγS, ADPβS, UTP, UDP, 2-methylthio-ATP (MeSATP), 2-methylthio-ADP (MeSADP), and BzATP, but not adenosine, AMP, diadenosine tetraphosphate, αβMeATP, and βγMeATP. Using RT-PCR, we identified mRNA for the P2Y1, P2Y2, P2Y4, P2Y13, P2Y14, P2X2, P2X5, P2X7, A1, A2b, and A3 purinergic receptors in VVEC. Preincubation of VVEC with the P2Y1 selective antagonist MRS2179 and the P2Y13 selective antagonist MRS2211, as well as with pertussis toxin, attenuated at varying degrees agonist-induced intracellular Ca2+ responses and activation of ERK1/2, Akt, and S6 ribosomal protein, indicating that P2Y1 and P2Y13 receptors play a major role in VVEC growth responses. Considering the broad physiological implications of purinergic signaling in the regulation of angiogenesis and vascular homeostasis, our findings suggest that P2Y1 and P2Y13 receptors may represent novel and specific targets for treatment of pathological vascular remodeling involving vasa vasorum expansion.
Keywords: angiogenesis, extracellular nucleotides, intracellular calcium, pulmonary hypertension
purine and pyrimidine nucleotides are released from both resident vascular and circulating blood cells as a result of fluid shear stress, changes in osmolarity, hypoxia, cell activation in response to various stimuli, and neurotransmission, and they play an important role in regulating vascular functions (5–7, 13, 21, 30, 45, 46). The effects of extracellular nucleotides and nucleosides are mediated by P2 and P1 purinergic receptors, respectively. Ubiquitous expression of purinergic receptors by the cells present in the vascular wall indicates that extracellular nucleotides are important signaling molecules involved in modulating vascular responses under physiological and pathological conditions. It has been demonstrated that endothelial cells from various vascular beds express multiple sets of purinergic receptor subtypes, including P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, P2X4, P2X5, and P2X7 depending on the vascular bed they originate from (7, 8, 37, 43, 50). Activation of P2 receptors in vascular endothelial cells induces Ca2+-dependent NO production and release of endothelium-dependent hyperpolarizing factor, prostacyclin, and tissue plasminogen activator (8, 30, 50). A number of studies have demonstrated a contribution of P2 receptors to cytokine release (5, 18), platelet aggregation (53), and immune cell activation and proliferation (5). In addition, involvement of P2 receptors in chemotactic, morphogenetic, and proliferative responses of endothelial cells also has been reported, generating considerable interest in the field of purinergic signaling and its relevance to angiogenic regulation (1, 9, 21, 29, 45, 52). However, the data on the role of individual P2Y receptor subtypes in angiogenic responses in endothelial cells remain very limited.
Ca2+ signaling plays an important role in regulation of physiological responses in endothelial cells (3, 4, 20, 38, 39). Angiogenic activation of endothelial cells in response to growth factors such as VEGF, basic FGF, and IGF2 promotes an elevation of intracellular Ca2+ followed by the activation of Ca2+-dependent signaling events (38). Ca2+-mediated signal transduction has been implicated in the endothelial and epithelial cell responses, notably the mechanically stimulated intercellular and intracellular calcium waves (20, 30), vesicular exocytosis, and cytoskeleton alterations (27, 39). In addition, a role of Ca2+ signaling has been demonstrated in airway ciliary activity (56), oxidative stress regulation (2, 27), and P2Y-induced (48), Zn2+-dependent (24), transient receptor potential (TRP) channel-regulated (31), and other types of cellular responses (4, 38). It also has been demonstrated that in epithelial cells, an ATP-induced increase in intracellular Ca2+ concentration ([Ca2+]i) may be linked to a store-operated Ca2+ release (36), may be regulated by Na+-K+-ATPase in an inositol 1,4,5-trisphosphate (IP3)-dependent fashion (12), and may originate from distinct sustained and transient Ca2+ influx via activated P2X receptors (57). Thus it can be concluded that the variety of mechanisms of Ca2+ responses and their role in endothelial cell physiology strongly suggest a fundamental importance of intracellular Ca2+ for regulation of angiogenesis (38).
A number of studies have addressed a possibility that the changes in [Ca2+]i can be observed in both cytosolic and nuclear compartments and that increases in nucleoplasmic Ca2+ are required for cell proliferation (4, 44). It also was reported that a mitogenic effect of hepatocyte growth factor observed in SkHep1 cells involves c-Met translocation to the nucleus and elevation of nucleoplasmic [Ca2+]i (22, 44). Moreover, Ca2+ signaling plays a role in VEGF- and Wnt5a-induced endothelial cell proliferation (11, 34) as well as in EGF signaling in embryonic stem cells (25). Accordingly, inhibition of VEGF- and FGF-induced angiogenesis by endostatin and angiostatin was associated with a diminished Ca2+ response (28). Thus it has been suggested that Ca2+ signaling plays an important role in endothelial cell physiology and may be involved in regulation of angiogenesis (38). Because the Ca2+ responses in the endothelium in the context of purinergic receptors have not been extensively characterized, we aimed to investigate a role of extracellular nucleotide-induced Ca2+ signaling in endothelial cells and its possible role in angiogenesis.
We recently reported that, unlike endothelial cells of large systemic and pulmonary vessels, vasa vasorum endothelial cells (VVEC) isolated from pulmonary arteries of chronically hypoxic calves exhibit a distinct endothelial phenotype characterized by increased mitogenic responses to extracellular ATP. Moreover, we found that even more dramatic VVEC mitogenic responses were observed to extracellular ADP, but not to extracellular UTP and UDP (21, 52). These observations suggested that P2Y1 and/or P2Y13 receptors responding to ADP may play a unique role in regulation of VVEC angiogenesis and in adventitial vasa vasorum expansion. To further delineate the role of purinergic receptors in VVEC angiogenesis, this study has been focused on extracellular nucleotide-induced intracellular Ca2+ signaling and proliferative responses, including DNA synthesis and phosphorylation of ERK1/2, Akt, and S6 ribosomal protein. Our data demonstrate that in VVEC, extracellular ATP induced Ca2+ influx through both the plasma membrane channels and Ca2+ mobilization from the intracellular stores. Ca2+ responses were simultaneously generated in the cytosolic and the nuclear compartments, consistent with the role of Ca2+ signaling in mediating VVEC proliferation. Furthermore, establishing a purinergic receptor expression profile by RT-PCR and pharmacological analysis, with the use of P2 receptor agonists and antagonists, revealed that mitogenic and Ca2+ responses in VVEC were mediated predominantly by P2Y1 and P2Y13 receptors. Together, our findings present new evidence for the role of P2Y1 and P2Y13 receptors in VVEC and suggest that these receptors may be potential therapeutic targets for modulating physiological and pathological angiogenesis.
MATERIALS AND METHODS
Culturing of VVEC from pulmonary artery adventitia.
Pulmonary arteries were obtained from male Holstein calves that had been exposed to hypobaric hypoxia for 2 wk (barometric pressure = 430 mmHg). All animal work was undertaken using standard veterinary care in the Department of Physiology, School of Veterinary Medicine, Colorado State University. The protocol was approved by the Institutional Animal Care and Use Committee at Colorado State University (no. 08-090A-02; animal welfare assurance no. A3572-01). Adventitia was dissected from the media and extensively washed in phosphate-buffered saline solution (PBS), and VVEC were isolated as previously described (21). Cells were cultured in DMEM (Cellgro Mediatech, Manassas, VA) supplemented with 20 mM l-glutamine (Cellgro), nonessential amino acids (1:100 vol/vol; Sigma, St. Louis, MO), 100 U/ml penicillin and 100 μg/ml streptomycin (Sigma), 10% fetal bovine serum (FBS; Gemini Bio-Products, Woodland, CA), and 15 μg/ml endothelial cell growth supplement (Upstate Biotechnology, Charlottesville, VA). VVEC were characterized by the expression of endothelial markers, including von Willebrand factor, endothelial nitric oxide synthase, and platelet endothelial cell adhesion molecule-1; binding of the lectin Licopercsicon esculentum; and incorporation of acetylated low-density lipoproteins labeled with 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (14). All studies were performed on cells between passages 2 and 7.
Intracellular Ca2+ measurements.
VVEC were cultured on glass-bottom dishes (MatTek, Ashland, MA) to 80% confluence and growth-arrested in DMEM without serum for 72 h. For intracellular Ca2+ measurements, cells were loaded with 1 μM fura-2 AM (Invitrogen, Carlsbad, CA) for 30 min at room temperature, washed with medium (or Ca2+-free Ringer's solution where indicated), and left to recover for 10 min to reduce spontaneous intrinsic Ca2+ activity. Time-lapse λ340/380-nm image acquisition was started, and cells were perfused with a solution containing a stimulus as indicated. Experiments were performed at room temperature. The imaging system was constructed by Intelligent Imaging Innovations (Denver, CO) utilizing a Nikon TE2000 microscope with a motorized Z-axis focusing mechanism, a Sutter Arc lamp with fiber optical light guide, and a Cooke SensiCam charge-coupled device camera. SlideBook software was used for image acquisition and analysis, and a ratiometric technique was used for monitoring intracellular Ca2+ over time in a single cell (32). Single-cell Ca2+ traces (typically from 5–10 cells) were averaged.
Analysis of purinergic receptor expression by RT-PCR.
Total cellular RNA was isolated from VVEC extracts using an RNA isolation kit (Qiagen, Santa Clarita, CA) and was treated with RQ1 DNase 1 (Promega, Madison, WI) to remove residual contaminating DNA. Two micrograms of RNA were reverse-transcribed into first-strand cDNA using the SuperScript III First Strand and Platinum PCR SuperMix kits (Invitrogen) and quantified by spectrophotometric analysis. PCR amplification was carried out on equal amounts of cDNA for 35 cycles using the following parameters: initial denaturation step, 5 min at 94°C; amplification steps, 30 s at 55°C and 2 min at 72°C; and final elongation step, 10 min at 72°C. Amplification products were resolved on 2% agarose gel and stained with ethidium bromide, and the fragment sizes were determined by comparison to known DNA standards. Receptor-specific oligonucleotide primers were chosen based on the published sequences for purinergic receptors and the GenBank database of the National Center for Biotechnology Information.
DNA synthesis measurement.
VVEC were seeded on 24-well plates at a density of 1.2 × 104 cells per well in DMEM supplemented with 10% FBS. The next day, cells were rinsed with PBS and growth-arrested by culturing in DMEM without serum for 72 h. Cells were stimulated with 100 μM ATP, ADP, ATPγS, ADPβS, 2-methylthio-ATP (MeSATP), or 2-methylthio-ADP (MeSADP) (Sigma) in the presence of 0.125 μCi of [methyl-3H]thymidine (NEN Life Science Products, Boston, MA) for 24 h. To evaluate a contribution of P2Y1 and P2Y13 receptors, cells were preincubated with the P2Y1 receptor antagonist MRS2179 (10 μM, 30 min) or the P2Y13 receptor antagonist MRS2211 (10 μM, 30 min) before stimulation with extracellular nucleotides. P2Y13 receptors were shown to be coupled to Gαi protein; therefore, in some experiments cells were preincubated with its inhibitor, pertussis toxin (PTx; 100 ng/ml, 18 h). In the experiments designed to evaluate a role of Ca2+ in DNA synthesis, cells were preincubated with cell-permeable BAPTA-AM (10 and 30 μM; Invitrogen) for 30 min. After pretreatment, cells were stimulated with ATP (100 μM) in the presence of 0.125 μCi of [methyl-3H]thymidine for 24 h. Incorporated radioactivity in total cell lysates was determined using a Beckman LS6500 counter.
Preparation of cell extracts and Western blot analysis.
VVEC were cultured to near confluence, growth-arrested in DMEM for 72 h, and stimulated with ATP, ADP, ATPγS, ADPβS, MeSATP, or MeSADP (100 μM) in serum-free medium for 10 min. To evaluate a role of P2Y1 and P2Y13 receptors in nucleotide-induced signaling, cells were preincubated with the P2Y1 receptor antagonist MRS2179 (10 μM, 30 min), the P2Y13 receptor antagonist MRS2211 (10 μM, 30 min), or PTx (100 ng/ml, 18 h). Total cell lysates were prepared as described previously (21). Equivalent amounts of total cell protein (20 μg) were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Proteins were transferred to polyvinylidene difluoride (PVDF) membranes and probed with rabbit polyclonal antibodies against phospho-ERK1/2 (Tyr202/Thr204), phospho-Akt (Ser473), phospho-S6 (Ser236), following conditions recommended by the manufacturer (Cell Signaling Technology, Danvers, MA). After being washed with Tris-buffered saline-Tween buffer, membranes were incubated with donkey anti-rabbit peroxidase-conjugated IgG at 1:20,000 dilution (Amersham Biosciences, Piscataway, NJ) for 1 h at room temperature. Immunoreactive bands were detected using the Renaissance ECL kit (NEN Life Science Product) followed by exposure to Hyperfilm. In all experiments, equal sample loading and transfer were verified by staining PVDF membranes with Ponseau and by immunoprobing with anti-GAPDH antibody (Cell Signaling Technology).
Statistical analysis.
For the analysis of variances between groups of data, one-way ANOVA was performed, followed by the Dunnett or Bonferroni test, using GraphPad Prism 3.0 (GraphPad Software). Density of protein bands from Western blot images was quantified using NIH ImageJ software.
RESULTS
Purinergic receptor agonists increase [Ca2+]i in VVEC.
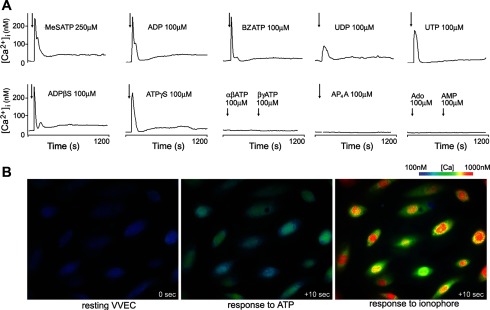
First, we screened selected nucleotides and their analogs for the ability to initiate Ca2+ signaling. VVEC exhibited robust Ca2+ flux within seconds after stimulation with MeSATP, ADP, ADPβS, ATPγS, 2′(3′)-O-(4-benzoylbenzoyl)adenosine 5′-triphosphate (BzATP), or UTP (Fig. 1A; concentrations as indicated). Similar Ca2+ responses were observed for ATP and MeSADP (as shown below), and smaller Ca2+ influx was induced by UDP. VVEC were unresponsive to αβ-methylene-ATP (αβMeATP), βγ-methylene-ATP (βγMeATP), diadenosine tetraphosphate (Ap4A), AMP, and adenosine (Ado), indicating that several P2 receptor subtypes, but not P1 (adenosine) receptors, are coupled to intracellular Ca2+ signaling in VVEC.
Fig. 1.
Extracellular nucleotides induce Ca2+ responses in vasa vasorum endothelial cells (VVEC). A: growth-arrested VVEC (72 h in serum-free DMEM) exhibited robust Ca2+ influx in response to stimulation with extracellular nucleotides (concentrations as indicated). Image acquisition began at time 0; after 100 s, cells were stimulated with nucleotides (arrows), and data acquisition continued in the presence of stimuli for an additional 1,100 s. [Ca2+]i, intracellular Ca2+ concentration; MeSATP, 2-methylthio-ATP; BzATP, 2′(3′)-O-(4-benzoylbenzoyl)adenosine 5′-triphosphate; αβATP, αβ-methylene-ATP; βγATP, βγ-methylene-ATP. B: representative images of Ca2+ responses in VVEC stimulated with ATP followed by ionomycin (images taken 10 s after each stimulation). Note that nuclear Ca2+ levels in response to both stimuli were higher than those in the cytoplasm.
Purinergic stimulation of VVEC induces cytosolic and nucleoplasmic [Ca2+]i increase via both influx of extracellular Ca2+ and Ca2+ mobilization from intracellular stores.
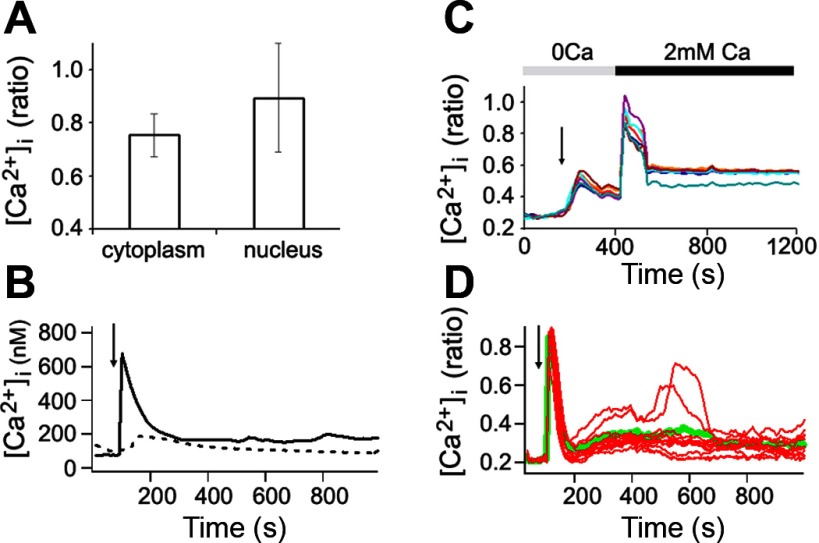
To further characterize Ca2+ responses in VVEC, we investigated localization and mechanisms of intracellular Ca2+ transients. The experiment shown in Fig. 1B demonstrates characteristic subcellular patterns of Ca2+ increases in VVEC 10 s after the addition of ATP. The data obtained from several (n = 3–5) distinct VVEC populations revealed the following nucleotide rank order of potency to induce Ca2+ response: MeSATP ≥ MeSADP ≥ ADP = ATP > ADPβS ≥ ATPγS = BzATP > UTP > UDP. The observed pharmacological profile suggests an involvement of several purinergic receptor subtypes in an increase in [Ca2+]i, with a predominant role of P2Y1 and P2Y13 receptors and, to a lesser extent, a role of P2Y2 and P2X7 receptors. Our data also showed that although subcellular Ca2+ levels were relatively homogeneous within resting cells, after the stimulation with extracellular ATP (100 μM), [Ca2+]i appeared to be higher in the nucleus than in the cytoplasm (Fig. 2A). These differences were further magnified by the addition of ionomycin (Fig. 2A). Temporal differences between increases in nuclear and cytoplasmic Ca2+ levels were undetectable (data not shown).
Fig. 2.
Purinergic stimulation of VVEC evokes an increase in cytosolic and nucleoplasmic Ca2+ levels via influx of extracellular Ca2+ and mobilization of Ca2+ from intracellular stores. A: comparison of averaged peak Ca2+ levels in response to ATP in the nucleus and cytoplasm. B: VVEC Ca2+ response to thapsigargin in Ca2+-free medium (dashed line) and to ATP in normal medium (solid line). The presence of a relatively small transient increase in cytoplasmic Ca2+ level is indicative of its release from intracellular stores and thus the existence of a store-derived component of Ca2+ signaling in VVEC. C: Ca2+ response to ATP in Ca2+-free medium followed by the addition of Ca2+. Each of the superimposed Ca2+ traces represents the recording from a single cell. D: multiphased Ca2+ fluctuations exhibited by an individual VVEC in response to ATP. Red traces represent individual cells; green trace is an average for a whole cell population. Results are representative of 3 experiments. Image acquisition began at time 0; after 100 s, cells were stimulated with ATP (arrows), and data acquisition continued in the presence of stimulus for an additional 1,100 s.
Although the store-operated Ca2+ entry (SOC) of ATP-mediated Ca2+ influx has been reported in epithelial cells (36), cardiac fibroblasts (17), and mesenteric microvessels (42), the presence of this mechanism in VVEC has not been established. To address this, we stimulated cells with 1 μM thapsigargin (TG) in Ca2+-free medium. TG is a specific inhibitor of sarco(endo)plasmic reticulum Ca2+-ATPases, known to induce depletion of intracellular Ca2+ stores. We observed a small transient increase of Ca2+ in response to TG that was severalfold lower than that induced by ATP in Ca2+-containing medium (Fig. 2B). This type of increase in Ca2+ concentration is indicative of the presence of a store-derived component of the Ca2+ pool in VVEC. Data presented in Fig. 2C provide additional evidence in support of this finding. Stimulation of VVEC with ATP in Ca2+-free medium (200 s after image acquisition was started, as indicated by arrow) resulted in the release of Ca2+ from intracellular stores, whereas cell perfusion with Ca2+-containing medium at 400 s resulted in a rapid influx of newly added Ca2+ through Ca2+ channels on the plasma membrane. Thus ATP-induced changes in [Ca2+]i include initial release of Ca2+ from intracellular stores followed by influx through membrane Ca2+ channels.
Moreover, we observed variations in multiphased Ca2+ fluctuations exhibited by some individual VVEC in response to ATP stimulation (Fig. 2D). Such Ca2+ fluctuation patterns or even stable oscillatory responses were observed in other types of endothelial cells and are believed to play a role in intercellular signaling that synchronizes regulatory mechanisms at the tissue level (16, 17, 54).
Overall, data presented in Figs. 1 and 2 demonstrate that the primary response of VVEC to nucleotides is Ca2+ release. The presence of a store-operated component in VVEC Ca2+ signaling is consistent with the G protein-coupled characteristics of metabotropic P2Y receptors.
VVEC express multiple purinergic receptor subtypes.
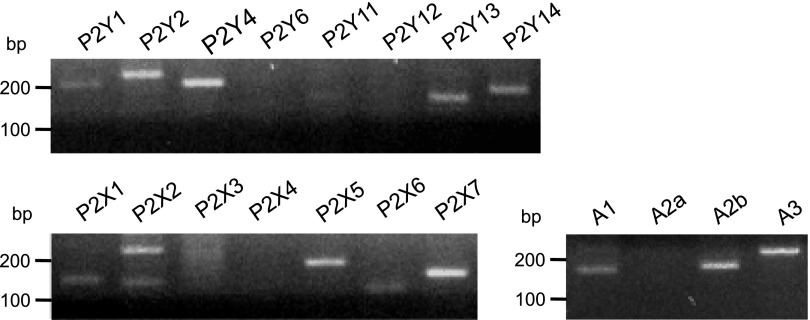
Expression of heterogenous purinergic receptors in endothelial cells has been previously reported (37, 50). The efficacy of various extracellular nucleotides to induce Ca2+ responses suggested that multiple purinergic receptors are expressed in VVEC. Our RT-PCR analysis revealed the expression of metabotropic P2Y1, P2Y2, P2Y4, P2Y13, and P2Y14 receptors and ionotropic P2X1, P2X2, P2X5, and P2X7 receptors in VVEC (Fig. 3 and Table 1). In addition, VVEC express A1, A2b, and A3 adenosine receptors. Considering potent mitogenic effects of extracellular ATP and ADP, but not UTP and UDP, in VVEC (21), these data strongly suggest that P2Y1 and P2Y13 receptors may play a predominant role in nucleotide-mediated angiogenic signaling in these cells.
Fig. 3.
PCR analysis of purinergic receptor expression in VVEC. PCR was performed as described in materials and methods using primers specific for P2 and P1 receptors (see Table 1). Amplification products were identified using a 2% agarose gel containing ethidium bromide and visualized under ultraviolet light. Sizes of the PCR products were verified by comparison to DNA standards.
Table 1.
Primers used for PCR analysis of purinergic receptor expression in VVEC
| Gene/Accession No. | Oligonucleotide | Sequence | Product Size, bp |
|---|---|---|---|
| P2Y1 | Sense primer | 5′-GCT GCA GAG GTT CAT CTT CC-3′ | 204 |
| NM 174410 | Antisense primer | 5′-CCG GTG CCA GAG TAG AAG AG-3′ | |
| P2Y2 | Sense primer | 5′-GGT AAG GAC TGA GCC AGC AG-3′ | 240 |
| XM 612432 | Antisense primer | 5′-GCC GTA ACC TCA TCC TCA AA-3′ | |
| P2Y4 | Sense primer | 5′-GCA AGT TCA TCC GCT TTC TC-3′ | 206 |
| XM 592537 | Antisense primer | 5′-GGT GGT GAC GAA GAA CAG GT-3′ | |
| P2Y6 | Sense primer | 5′-CCA ACA CAC TTG GGG CTA CT-3′ | 171 |
| XM 588870 | Antisense primer | 5′-CCT GGA CTC CAT CTT CCA AA-3′ | |
| P2Y11* | Sense primer | 5′-CGC TCA GGT GAG AGA AAC TG-3′ | 162 |
| Antisense primer | 5′-TCT TCA TCA CCT GCA TCA GC-3′ | ||
| P2Y12 | Sense primer | 5′-ACG GAA CAC GTT CTC CAA TC-3′ | 224 |
| NM 001001174 | Antisense primer | 5′-GGC TCG TGT CTC TCT CTG CT-3′ | |
| P2Y13* | Sense primer | 5′-TCA AGT CAG CCA CCA AAG TG-3′ | 163 |
| Antisense primer | 5′-CCC AGA GAC CTG AAG ACA GC-3′ | ||
| P2Y14* | Sense primer | 5′-TTC CAG GCA AGG ATA GGA TG-3′ | 193 |
| Antisense primer | 5′-CTC CTG CTG CTT CCC TAA TG-3′ | ||
| P2X1* | Sense primer | 5′-ACA GGT GGA TCC AGA GGT CA-3′ | 163 |
| Antisense primer | 5′-CCG CCC CCA TCT ATC TTT-3′ | ||
| P2X2* | Sense primer | 5′-AGA CAA GGG CCA GGG TCA C-3′ | 153 |
| Antisense primer | 5′-ATC TGT CTT GCT GGC CTC AG-3′ | ||
| P2X3* | Sense primer | 5′-GGA CCA TTG GGA TCA TCA AC-3′ | 188 |
| Antisense primer | 5′-TGG GGT CAC GTA GTC AGA CA-3′ | ||
| P2X4 | Sense primer | 5′-TGA CAC AGA AGT GCC AAA GC-3′ | 158 |
| NM 001034049 | Antisense primer | 5′-TCG CAT CAT AAA TGC ACG TT-3′ | |
| P2X5 | Sense primer | 5′-AGC AGG AAT CTG AGC ACG TT-3′ | 203 |
| XM 594012 | Antisense primer | 5′-GGT ACT CCA AAG GCA TGG AA-3′ | |
| P2X6* | Sense primer | 5′-CGG GAG CTC TTC TCT TAC CC-3′ | 170 |
| Antisense primer | 5′-GGG GGC TTC TGG ATT ACA A-3′ | ||
| P2X7 | Sense primer | 5′-TGC TTT GGT GAG TGA CAA GC-3′ | 165 |
| XM 591410 | Antisense primer | 5′-TGC AAG GGG AAG GTG TAA TC-3′ | |
| ADORA1 | Sense primer | 5′-GTT GTG TGT GTG CTG GGA AC-3′ | 185 |
| NM 174497 | Antisense primer | 5′-CCC CAC TTG GCA CAT CTA GT-3′ | |
| ADORA2a | Sense primer | 5′-TCA ACA GCA ACC TGC AGA AC-3′ | 154 |
| Antisense primer | 5′-ACA AAG CAG GCG AAG AAG AG-3′ | ||
| A2b | Sense primer | 5′-CCA GTC CAC ACC ATC AAC TG-3′ | 190 |
| NM 001075925 | Antisense primer | 5′-GGC AGA GGA TGT ACC TGG AA-3′ | |
| ADORA3 | Sense primer | 5′-TTT GTG CTG GAC ACA GCT TC-3′ | 242 |
| NM 001079645 | Antisense primer | 5′-TCA TCT CTG GCA AAG TCA CG-3′ |
Sense and antisense primers used for PCR analysis of purinergic receptor expression in vasa vasorum endothelial cells (VVEC) are shown with their product size in base pairs (bp). Primers were designed based on 100% homology sequence between human and mice cDNA.
Bos Taurus gene accession numbers are not available.
P2Y1 and P2Y13 receptors mediate elevation of intracellular Ca2+ in VVEC.
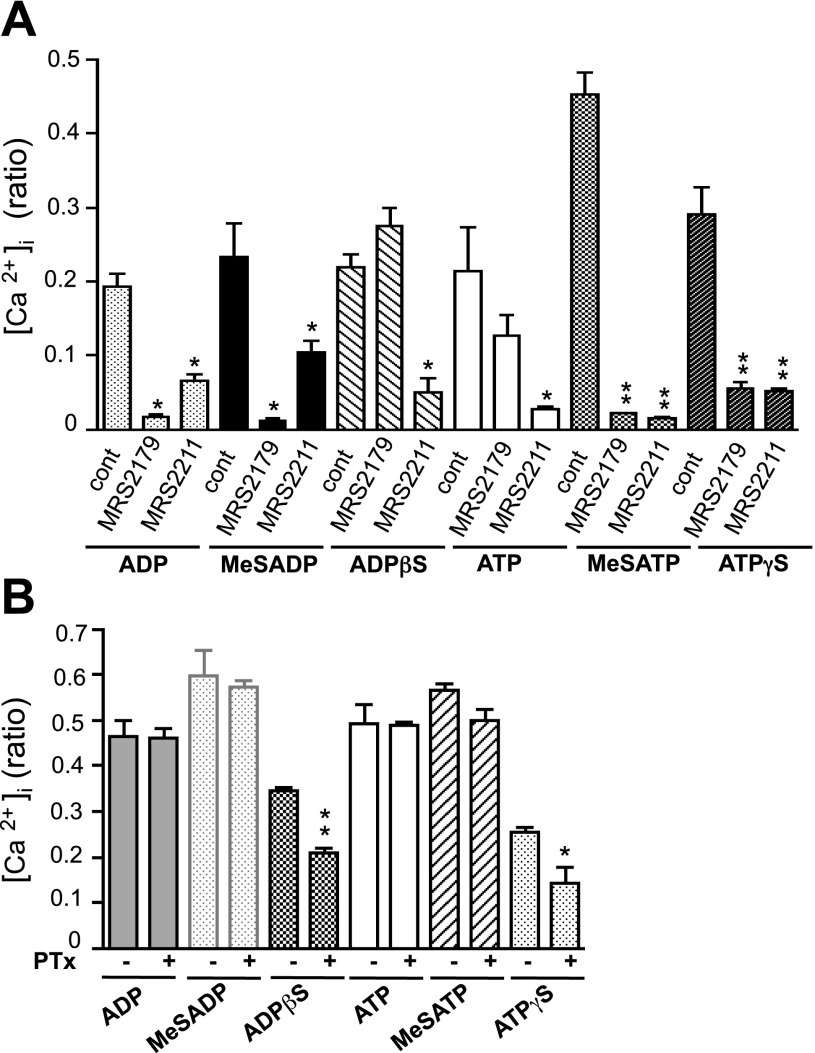
To determine whether P2Y1 and P2Y13 receptors mediate Ca2+ responses in VVEC, we used a pharmacological approach that involves P2Y1 and P2Y13 agonists and antagonists. The agonists were chosen based on the published pharmacological profile of purinergic receptors (7, 49). Stimulation of VVEC with extracellular nucleotides ADP, MeSADP, ADPβS, ATP, MeSATP, and ATPγS (100 μM each) resulted in significant elevation of intracellular Ca2+ (Fig. 4A). The strongest responses were observed when VVEC were stimulated with MeSATP. Preincubation with the P2Y1 receptor-specific antagonist MRS2179 (10 μM, 30 min) suppressed Ca2+ responses to all these nucleotides, with the exception of ADPβS. ADPβS is a less potent P2Y1/P2Y2/P2Y13 receptor agonist; therefore, it may act on P2Y2 and/or P2Y13 receptors. Preincubation with the P2Y13 receptor-specific antagonist MRS2211 resulted in significant inhibition of Ca2+ responses to ATP, MeSATP, ATPγS, ADP, and ADPβS. The response to MeSADP was less sensitive to MRS2211, suggesting the possibility that in bovine VVEC, MeSADP may act preferentially on P2Y1 receptors. In human and rat tissues and some cell lines, P2Y13 was characterized as a Gαi protein-coupled receptor (7, 49, 55). Therefore, to further distinguish a contribution of P2Y1 and P2Y13 to Ca2+ responses, we examined the effects of nucleotides on PTx-treated cells (Fig. 4B). Preincubation of VVEC with PTx (100 ng/ml, 18 h) decreased the responses to ADPβS (by 38%) and ATPγS (by 43%). A small but not significant inhibitory effect of PTx was observed in cells stimulated with MeSADP (by 4%) and MeSATP (by 13%). The responses to ADP and ATP remained unaffected. Given that ADPβS and ATPγS can activate Gαi/αq-coupled P2Y2 receptors, our results indicate the involvement of both Gαi-dependent (P2Y13 or P2Y2) and Gαi-independent (P2Y1, P2Y2 and P2Y13) receptors in nucleotide-induced Ca2+ signaling in VVEC.
Fig. 4.
P2Y1 and P2Y13 receptors are involved in nucleotide-induced Ca2+ responses in VVEC. Fura-2-loaded growth-arrested VVEC were pretreated for 30 min with 10 μM MRS2179, 10 μM MRS2211, or a vehicle control (A) or were pretreated for 18 h with 100 ng/ml PTx before fura-2 loading (B). Cells were stimulated with ATP, ADP, MeSATP, MeSADP, ADPβS, and ATPγS (100 μM). Ca2+ responses from 5–10 single cells in the field were monitored as described in materials and methods. *P < 0.05; **P < 0.01 vs. nucleotide-stimulated cells. cont, Control.
Intracellular Ca2+ is required for VVEC proliferation.
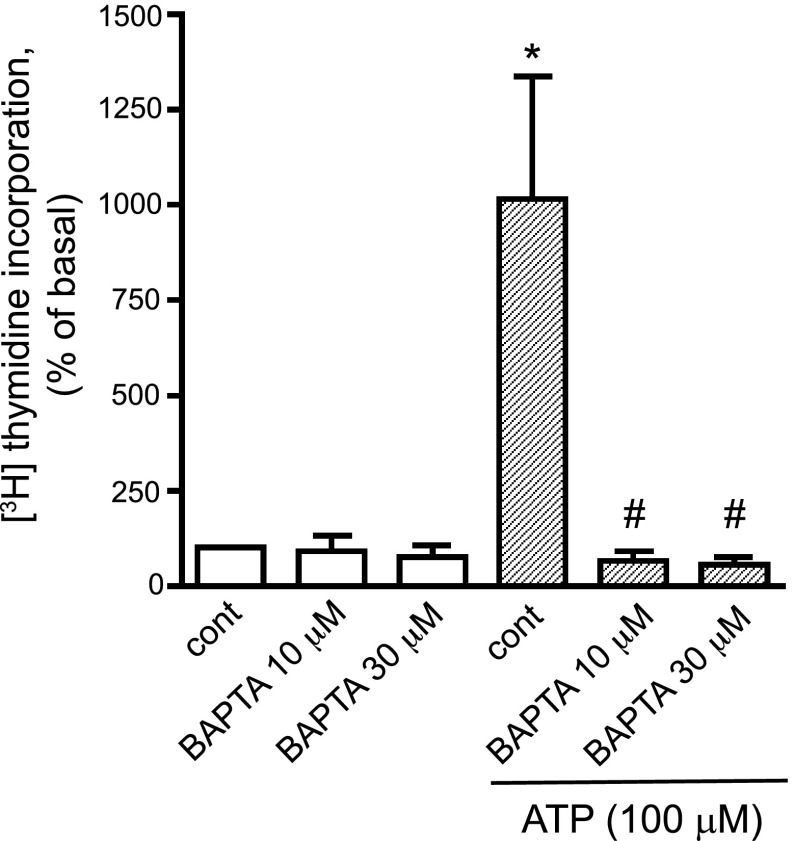
Because elevated nucleoplasmic Ca2+ might be required for mitogenic responses in VVEC, we next examined whether nucleotide-induced Ca2+ responses are functionally important to VVEC proliferation. The cell-permeable Ca2+ chelator BAPTA-AM (10 and 30 μM) completely inhibited extracellular ATP-induced DNA synthesis in VVEC (Fig. 5). A similar inhibitory effect was observed when cells were stimulated with extracellular ADP and selected nonhydrolyzable nucleotide analogs (data not shown).
Fig. 5.
Ca2+ is required for nucleotide-induced DNA synthesis in VVEC. Growth-arrested VVEC (72 h in serum-free DMEM) were preincubated with or without BAPTA-AM (10 and 30 μM) for 30 min and then stimulated with ATP (100 μM) in the presence of 0.125 μCi of [3H]thymidine for 24 h. Incorporated radioactivity was measured in total cell lysates using a beta counter. Data are means ± SE from 4 experiments conducted on 2 distinct VVEC populations. *P < 0.001 vs. nonstimulated control. #P < 0.001 vs. ATP-stimulated cells.
P2Y1 and P2Y13 receptors are involved in mitogenic responses in VVEC.
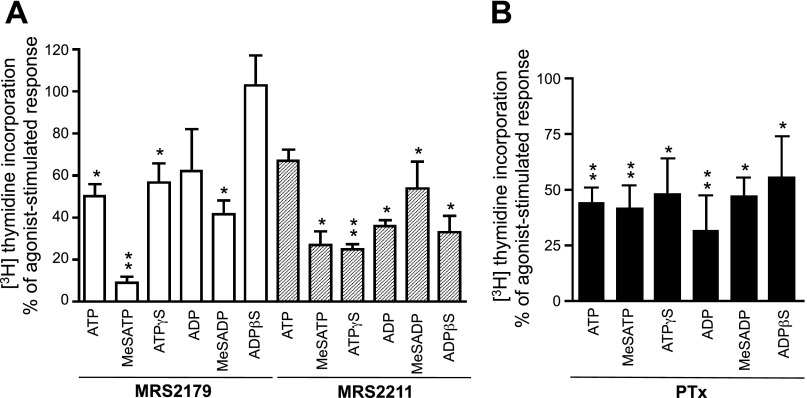
To further explore the involvement of P2Y1 and P2Y13 receptors in VVEC mitogenesis, we examined the role of these receptors in DNA synthesis. We found that pretreatment with the P2Y1 receptor antagonist MRS2179 resulted in a significant >50% inhibition of DNA synthesis in response to ATP, MeSATP, and MeSADP. A lesser inhibitory effect was observed in ATPγS- and ADP-stimulated cells and was not observed in ADPβS-stimulated cells (Fig. 6A). Pretreatment of VVEC with MRS2211 had an even larger effect on nucleotide-mediated DNA synthesis. The most pronounced inhibitory effects were observed in VVEC stimulated with MeSATP, ATPγS, ADP, and ADPβS (>50%). MRS2211 also suppressed the effects of ATP and MeSADP. Furthermore, PTx treatment had an inhibitory effect on DNA synthesis (by 32–68%) in responses to all nucleotides and on activation of the intracellular kinases ERK1/2 (by 28–57%), Akt (by 22–73%), and S6 (by 10–38%), indicating a substantial contribution of Gαi-coupled P2Y13 receptors in the integrated growth response in VVEC (Fig. 6B; see Supplemental Fig. 2). (Supplemental data for this article is available online at the American Journal of Physiology-Cell Physiology website.)
Fig. 6.
P2Y1 and P2Y13 receptor agonists induce DNA synthesis in VVEC in a Gαi-dependent and -independent manner. Growth-arrested VVEC (72 h in serum-free DMEM) were preincubated with MRS2179 (10 μM, 30 min; A), MRS2211 (10 μM, 30 min; A), or PTx (100 ng/ml, 18 h; B) or remained untreated and were then stimulated with the indicated nucleotides (100 μM) in the presence of 0.125 μCi of [3H]thymidine for 24 h. Incorporated radioactivity was determined in total cell lysates as described in materials and methods. Data are means ± SE from 4–6 independent experiments conducted on 3 distinct VVEC populations. *P < 0.05; **P < 0.01 vs. nucleotide-stimulated cells.
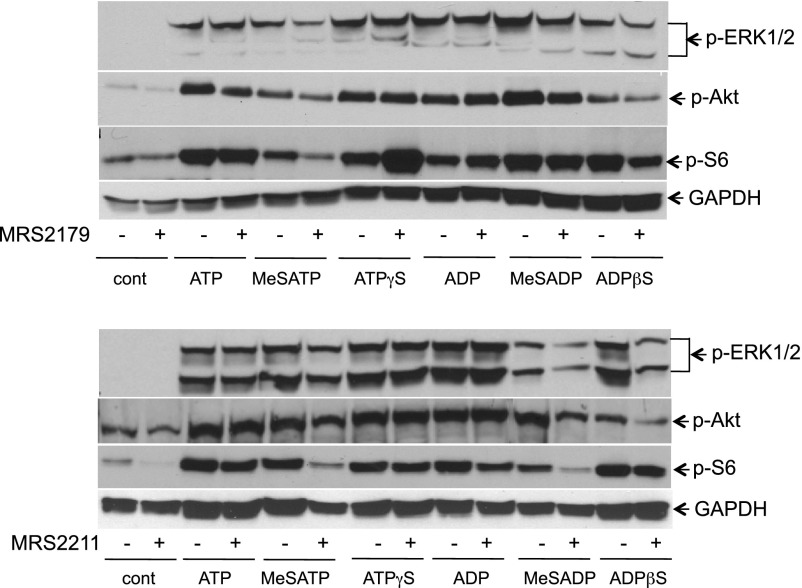
Finally, a role of P2Y1 and P2Y13 receptors in mitogenic activation of VVEC was examined by agonist-dependent activation of intracellular mitogenic signaling pathways in the presence and absence of MRS2179 and MRS2211. Western blot analysis of phospho-ERK1/2, phospho-Akt, and phospho-S6 ribosomal protein (Fig. 7 and Table 2) showed that P2Y1 and P2Y13 purinergic receptor antagonists MRS2179 and MRS2211 exhibited an overlapping but slightly different inhibitory effect on nucleotide-mediated responses in VVEC. MRS2179 (10 μM) had the most dramatic inhibitory effect on MeSATP-induced ERK1/2 phosphorylation; but also had an effect on ATP-, MeSATP-, and MeSADP-induced Akt phosphorylation and MeSATP-, ATPγS-, MeSADP-induced S6 ribosomal protein phosphorylation. Less significant inhibition of ERK1/2 and Akt phosphorylation was observed in VVEC stimulated with ADPβS. MRS2211 (10 μM) effectively inhibited ERK1/2 and Akt phosphorylation in VVEC stimulated with MeSADP and ADPβS, and S6 protein phosphorylation in cells stimulated with MeSATP and MeSADP. Because MRS2179 and MRS2211 act as competitive antagonists at P2Y1 and P2Y13 receptors, we examined the effects of these compounds under conditions when cells were stimulated at the lower (1 μM) concentration of some agonists (see Table 2, values in parentheses). The data showed that MRS2179 had a more potent inhibitory effect on ATP- and MeSATP-induced ERK1/2 and Akt phosphorylation and a lesser effect on ATP- and MeSATP-induced S6 phosphorylation. MeSADP-induced responses remained without significant changes. MRS2211 exhibited a more potent antagonistic effect on ATP- and MeSATP-induced ERK1/2 phosphorylation and MeSATP-induced Akt and S6 phosphorylation. The responses to MeSADP also remained without significant changes. Collectively, the inhibitory effects of MRS2179 and MRS2211 indicate that both P2Y1 and P2Y13 receptors are involved in VVEC mitogenesis to some extent, through the activation of ERK1/2 and Akt/mTOR/S6 signaling pathways.
Fig. 7.
P2Y1 and P2Y13 receptors are involved in activation of mitogenic signaling pathways in VVEC. Growth-arrested VVEC were preincubated with MRS2179 (10 μM, 30 min) or MRS2211 (10 μM, 30 min) or remained untreated and were then stimulated with selected agonists (100 μM, 30 min) for 10 min. Phoshoproteins in total cell lysates (20 μg) were determined with antibodies to phospho-ERK1/2 (Tyr202/Thr204), phospho-Akt (Ser473), and phospho-S6 (Ser236). Data are representative of at least 3 independent experiments.
Table 2.
Contribution of P2Y1 and P2Y13 receptors to activation of mitogenic pathways in VVEC
| Agonist | −MRS2179 | +MRS2179 | −MRS2211 | +MRS2211 |
|---|---|---|---|---|
| p-ERK1/2 | ||||
| Control | 1 (1) | 0.8 (1.4) | 1 (1) | 1 (1.3) |
| ATP | 35 (8.6) | 40 (2.8) | 14.7 (8.6) | 15 (3.6) |
| MeSATP | 33.4 (10.2) | 21 (2.5) | 18.7 (10.2) | 22.4 (2.4) |
| ATPγS | 48 | 57 | 23.7 | 22 |
| ADP | 56.2 | 56.2 | 28.7 | 32.1 |
| MeSADP | 65 (11.9) | 60.2 (10.3) | 11.1 (11.9) | 5 (7) |
| ADPβS | 46 | 39 | 24.1 | 8 |
| p-Akt | ||||
| Control | 1 (1) | 0.5 (1.3) | 1 (1) | 0.4 (1.2) |
| ATP | 7 (1.9) | 4.2 (1.4) | 2.3 (1.9) | 2.6 (1.65) |
| MeSATP | 4 (2.4) | 2.4 (1.7) | 2.2 (2.4) | 3.3 (1.1) |
| ATPγS | 6.2 | 6.6 | 3 | 3.1 |
| ADP | 6.2 | 7.1 | 3.3 | 4.1 |
| MeSADP | 4.1 (1.8) | 3.1 (1.7) | 4.5 (1.8) | 2.3 (1.3) |
| ADPβS | 11 | 8.5 | 1.6 | 0.7 |
| p-S6 | ||||
| Control | 1 (1) | 0.73 (1.3) | 1 (1) | 0.2 (1.2) |
| ATP | 2 (1.9) | 1.7(1.7) | 4.1 (1.9) | 3.5 (1.8) |
| MeSATP | 1.6 (2.4) | 0.2 (1.7) | 3.5 | 1.8 (1.1) |
| ATPγS | 2.4 | 1.2 | 3.7 | 3.5 |
| ADP | 1.8 | 1.5 | 4.7 | 3.8 |
| MeSADP | 2.4 (1.8) | 1.4 (1.7) | 3.5 | 0.8 (1.3) |
| ADPβS | 2.1 | 2.1 | 4.4 | 3.7 |
Values indicate the relative increase in ERK1/2, Akt, and S6 phosphorylation in response to selected agonists with (+) and without (−) the P2Y1 and P2Y13 specific antagonists MRS2179 and MRS2211, respectively. Cells were treated with P2Y1 and P2Y13 receptor antagonists and agonists as described in materials and methods. The activation of ERK1/2, Akt, and S6 was determined by Western blot analysis. Values without parentheses represent a 100 μM nucleotide concentration; values given in parentheses represent a 1 μM nucleotide concentration. The calculated values represent the degree of phospho-ERK1/2, phospho-Akt, and phospho-S6 increase in nucleotide-stimulated cells compared with that in nonstimulated cells. The densities of protein bands were normalized to GAPDH; normalized basal levels (control) were taken as having a value of 1.
DISCUSSION
Our previous studies demonstrated potent angiogenic effects of extracellular ATP in pulmonary artery VVEC (21, 52). In this study, we further investigated the role of purinergic signaling and intracellular Ca2+ in VVEC mitogenesis. Data obtained in different endothelial cell models demonstrate that, as a second messenger, Ca2+ is involved in the regulation of various physiological and pathological cell responses. Purinergic receptor-mediated increase in [Ca2+]i has been recognized as the intracellular response leading to NO production in the vascular endothelium and regulation of vasorelaxation and vascular permeability (15, 26, 30, 43). However, the cellular mechanisms and the role of Ca2+ signaling in angiogenic activation of endothelial cells in response to extracellular nucleotides remain largely unexplored.
Ca2+-mediated activation of several signal transduction pathways has been described in endothelial and epithelial cells (2, 4, 17, 20, 27, 48, 52). The importance of Ca2+ signaling in angiogenesis is supported by evidence that inhibitors of Ca2+ entry and inhibitors of TRP channels suppress cell proliferation and angiogenesis in vitro and in vivo (reviewed in Ref. 38). It also has been demonstrated that ATP-induced Ca2+ influx involves a store-operated Ca2+ entry, or capacitative Ca2+ entry (36). This mechanism is a dominant signaling pathway in nonexcitable cells (47) and involves the opening of Ca2+-permeable channels in the plasma membrane in response to the depletion of intracellular Ca2+ stores (reviewed in Ref. 41). The results of our study demonstrate that nucleotide-induced elevation in [Ca2+]i in VVEC includes both the release from intracellular stores and Ca2+ influx from the extracellular milieu. Most likely, this process involves IP3 receptor-coupled Ca2+ channels in the endoplasmic reticulum, as described in other nonexcitable cells featuring store-operated Ca2+ entry (12, 33). However, this is probably not the only mechanism of ATP-mediated Ca2+ signaling in VVEC, since an increase in nuclear [Ca2+]i observed in our experiments (Figs. 1B and 2A) indicates the presence of additional Ca2+ pathways. These possibilities are currently under study in our laboratory.
Our study revealed multiphased Ca2+ fluctuations exhibited by individual VVEC following ATP stimulation. Such Ca2+ fluctuation patterns or even more stable oscillatory responses at a single-cell level have been shown in several epithelial and endothelial cell types. These responses are believed to play a role in the generation of intercellular Ca2+ signaling “waves” providing an intercellular communication to synchronize regulatory mechanisms at the tissue level (16, 17). Importantly, a recent study demonstrated that extracellular ATP-induced Ca2+ cytosolic oscillations in lung epithelial and endothelial cells mediate paracrine effects, including NO production and lung expansion in the model of lung mechanical ventilation (30). However, the fact that in our experimental setting only a part of VVEC responded in this manner may be attributed to different differentiation stages or individual cell phenotypes, as well as to variations in confluence of the cell culture, which affects the number of gap junctions responsible for intercellular signaling. Nevertheless, this interesting phenomenon in VVEC certainly deserves additional investigation in specifically optimized cell culture conditions.
Real-time measurements of intracellular Ca2+ in VVEC performed in our study demonstrated increases in Ca2+ levels in both cytoplasm and nucleus on ATP stimulation. Moreover, our results indicate that Ca2+ signaling is required for nucleotide-mediated VVEC proliferation. These findings are in agreement with the reports showing that elevation of nucleoplasmic Ca2+ is involved in growth factor-induced proliferative responses (4, 44), necessary for activation of the nuclear PLC/PKC pathway and transcription factors, and in centrosome separation during early prophase (22, 23, 40, 44). The importance of nucleoplasmic Ca2+ in cell proliferation is also supported by reports showing bradykinin B2 and metabotropic glutamate mGluR5 receptor expression in hepatocyte nuclei and in nuclei of primary neurons, respectively (reviewed in Ref. 4). Our future studies need to explore a possibility of nuclear localization of purinergic receptors and their role in mediating VVEC nucleoplasmic Ca2+ responses and proliferation.
The sensitivity of VVEC to stimulation with extracellular nucleotides suggests that multiple purinergic receptors contribute to the angiogenic phenotype of these cells. Indeed, RT-PCR analysis revealed mRNA for several metabotropic P2Y receptors (PY1, P2Y2, P2Y4, P2Y13, and P2Y14), ionotropic P2X receptors (P2X2, P2X5, and P2X7), and P1 adenosine receptors (A1, A2b, and A3). In line with this observation, expression of P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, P2X4, P2X5, and P2X7 has been previously reported in vascular endothelium (8, 37). The rank order of potency (MeSATP ≥ MeSADP ≥ ADP = ATP > ADPβS ≥ ATPγS = BzATP > UTP > UDP) of extracellular nucleotides to induce Ca2+ responses suggests a contribution of more than one receptor with the overlapping pharmacological profile. The application of MRS2179, a selective antagonist of P2Y1 receptor, and MRS2211, a selective antagonist of P2Y13 receptor, strongly blocked the agonist-induced Ca2+ responses in VVEC. In addition, treatment with PTx demonstrated an involvement of Gαi-coupled P2 receptors in mediating Ca2+ responses. Together, these studies revealed a predominant role of P2Y1 and P2Y13 receptors and, to a lesser degree, contribution of P2Y2 and P2X7 receptors in observed changes in [Ca2+]i. Interestingly, we observed some differences in relative efficacy of MeSATP, MeSADP, ATP, and ADP on an increase of [Ca2+]i in distinct VVEC populations, pointing to possible variations in a relative expression, conformation state, and/or functional involvement of P2Y1, P2Y2, and P2Y13 receptors. In contrast to nucleotide di- and triphosphates, AMP and Ado did not elevate [Ca2+]i and were much less effective in activating selected kinases and proteins belonging to mitogenic pathways (Supplemental Fig. 1).
Although our results demonstrate that UTP, a ligand for P2Y2 receptors, is capable of raising intracellular Ca2+ levels in VVEC, it did not stimulate VVEC proliferation (21). Importantly, in contrast to extracellular ATP and ADP, extracellular UTP and UDP are less hydrolysable by ectonucleotidases/NTPDases, implying their possible role in longer term effects in endothelial cells. The involvement of ATP and UTP, and hence P2Y2 and, possibly, P2Y4 receptors, in endothelial cell migration has been previously reported (29). The role of extracellular UTP and UDP in VVEC angiogenic responses such as migration, invasion, and tube formation needs to be further investigated. In addition, our study demonstrated that bradykinin, but not endothelin, 5-HT, angiotensin II, or norepinephrine, elevated [Ca2+]i in VVEC (data not shown). These observations suggest that VVEC exhibit a distinct endothelial phenotype with selective reliance on extracellular nucleotides as angiogenic stimuli. Complementary to our study, ATP-mediated Ca2+ responses were detected in tumor endothelial cells and were associated with an in vitro tubulogenic effect (19).
Potent and selective purinergic receptor agonists and antagonists represent an advanced pharmacological approach to establish a functionality of individual P2 receptor subtypes. Our study demonstrates the ability of P2Y1 and P2Y13 agonists to activate ERK1/2, Akt, and S6 ribosomal protein signaling pathways in VVEC. Furthermore, using newly developed selective P2Y1 and P2Y13 antagonists, MRS2179 and MRS2211, respectively, we confirmed a role of these receptors in nucleotide-induced signaling in VVEC. Although the association of some P2Y receptor subtypes with ERK, phosphatidylinositol 3-kinase/PDK/PKCζ, and Ca2+/PLC pathways and cross talk between P2Y1 and VEGF receptor 2 signaling in endothelial cells have been reported (1, 9, 29, 35, 45), specific signaling functions of P2Y13 receptors have not been investigated. The expression of the P2Y13 receptors was found to be the highest in spleen, brain, and lymph nodes, as well as in hematopoietic, neuronal, and red blood cells. Nucleotides such as ADP and MeSADP are the most potent ligands for human and rat P2Y13, whereas MeSATP is the most potent ligand for the bovine P2Y13 receptors (7, 49). In red blood cells, ADP-induced activation of P2Y13 receptors decreases ATP release and diminishes the vasodilatory response to hypoxia (51). In mouse and human tissues, P2Y13 receptor was identified as a Gαi-coupled orphan receptor, SP174, pointing out a possible role for this receptor in cell proliferation (55). The expression and function of P2Y13 receptors in endothelial cells, as well as in bovine tissue, have not been documented yet. The results of our study present new evidence for the expression of the P2Y13 receptors in bovine pulmonary artery VVEC and their functional role in Ca2+ and mitogenic signaling responses. As outlined above, PTx was used to distinguish between a contribution of Gαi-coupled receptors vs. Gαq-coupled receptors to nucleotide-induced Ca2+ increase, DNA synthesis, and protein phosphorylation. Our results suggest that Gαq-coupled P2Y1 receptors are predominantly involved in acute Ca2+ responses, whereas both Gαq-coupled P2Y1 (and possibly P2Y2) receptors and Gαi-coupled P2Y13 receptors are involved in VVEC growth responses. Keeping in mind that isolated VVEC exhibit highly proliferative potential and that these cells may be enriched with endothelial progenitor cells, which may contribute to a neovascularization process, future studies are needed to validate the expression patterns of P2Y13 receptors within VVEC populations, identify G protein α-subunits coupled to P2Y13 receptors, and unveil signal transduction pathways leading to angiogenic activation of VVEC. Given that elevated levels of extracellular ATP and ADP are observed under various pathological conditions associated with hypoxia and inflammation, our findings indicate that P2Y1 and P2Y13 receptors may be targeted by these nucleotides and therefore may play a role in regulating angiogenic expansion of the vasa vasorum and, potentially, other microvascular endothelial cell types.
GRANTS
This work was funded by American Heart Association Grant 0665464Z (to E. V. Gerasimovskaya) and National Heart, Lung, and Blood Grants R01 HL086783 (to E. V. Gerasimovskaya), PPG HL014985 (to K. R. Stenmark), and SCCOR HL084923 (to K. R. Stenmark).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Michael Holers (University of Colorado Denver, SOM, Division of Rheumatology) for providing the Nikon TE2000 microscope for intracellular Ca2+ measurements and Dr. Elzbieta Kaczmarek (Harvard Medical School, Beth Israel Deaconess Medical Center, Boston, MA) for critical comments.
REFERENCES
- 1. Albert JL, Boyle JP, Roberts JA, Challiss RA, Gubby SE, Boarder MR. Regulation of brain capillary endothelial cells by P2Y receptors coupled to Ca2+, phospholipase C and mitogen-activated protein kinase. Br J Pharmacol 122: 935–941, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aley PK, Porter KE, Boyle JP, Kemp PJ, Peers C. Hypoxic modulation of Ca2+ signaling in human venous endothelial cells. Multiple roles for reactive oxygen species. J Biol Chem 280: 13349–13354, 2005. [DOI] [PubMed] [Google Scholar]
- 3. Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodeling. Nat Rev Mol Cell Biol 4: 517–529, 2003. [DOI] [PubMed] [Google Scholar]
- 4. Bootman MD, Fearnley C, Smyrnias I, MacDonald F, Roderick HL. An update on nuclear calcium signalling. J Cell Sci 122: 2337–2350, 2009. [DOI] [PubMed] [Google Scholar]
- 5. Bours MJ, Swennen EL, Di Virgilio F, Cronstein BN, Dagnelie PC. Adenosine 5′-triphosphate and adenosine as endogenous signaling molecules in immunity and inflammation. Pharmacol Ther 112: 358–404, 2006. [DOI] [PubMed] [Google Scholar]
- 6. Burnstock G. Pathophysiology and therapeutic potential of purinergic signaling. Pharmacol Rev 58: 58–86, 2006. [DOI] [PubMed] [Google Scholar]
- 7. Burnstock G. Purine and pyrimidine receptors. Cell Mol Life Sci 64: 1471–1483, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Buvinic S, Briones R, Huidobro-Toro JP. P2Y1 and P2Y2 receptors are coupled to the NO/cGMP pathway to vasodilate the rat arterial mesenteric bed. Br J Pharmacol 136: 847–856, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cha SH, Hahn TW, Sekine T, Lee KH, Endou H. Purinoceptor-mediated calcium mobilization, and cellular proliferation in cultured bovine corneal endothelial cells. Jpn J Pharmacol 82: 181–187, 2000. [DOI] [PubMed] [Google Scholar]
- 10. Chen JB, Tao R, Sun HY, Tse HF, Lau CP, Li GR. Multiple Ca2+ signaling pathways regulate intracellular Ca2+ activity in human cardiac fibroblasts. J Cell Physiol 223: 68–75, 2010. [DOI] [PubMed] [Google Scholar]
- 11. Cheng CW, Yeh JC, Fan TP, Smith SK, Charnock-Jones DS. Wnt5a-mediated non-canonical Wnt signalling regulates human endothelial cell proliferation and migration. Biochem Biophys Res Commun 365: 285–290, 2008. [DOI] [PubMed] [Google Scholar]
- 12. Chen Y, Cai T, Yang C, Turner DA, Giovannucci DR, Xie Z. Regulation of inositol 1,4,5-trisphosphate receptor-mediated calcium release by the Na/K-ATPase in cultured renal epithelial cells. J Biol Chem 283: 1128–1136, 2008. [DOI] [PubMed] [Google Scholar]
- 13. Corriden R, Insel PA. Basal release of ATP: an autocrine-paracrine mechanism for cell regulation. Sci Signal 3: re1, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Davie NJ, Gerasimovskaya EV, Hofmeister SE, Richman AP, Jones PL, Reeves JT, Stenmark KR. Pulmonary artery adventitial fibroblasts cooperate with vasa vasorum endothelial cells to regulate vasa vasorum neovascularization: a process mediated by hypoxia and endothelin-1. Am J Pathol 168: 1793–1807, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. De Mey JG, Vanhoutte PM. Role of the intima in cholinergic and purinergic relaxation of isolated canine femoral arteries. J Physiol 316: 347–355, 1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. D'hondt C, Ponsaerts R, Srinivas SP, Vereecke J, Himpens B. Reduced intercellular communication and altered morphology of bovine corneal endothelial cells with prolonged time in cell culture. Curr Eye Res 34: 454–465, 2009. [DOI] [PubMed] [Google Scholar]
- 17. Evans JH, Sanderson MJ. Intracellular calcium oscillations induced by ATP in airway epithelial cells. Am J Physiol Lung Cell Mol Physiol 277: L30–L41, 1999. [DOI] [PubMed] [Google Scholar]
- 18. Ferrari D, La Sala A, Chiozzi P, Morelli A, Falzoni S, Girolomoni G, Idzko M, Dichmann S, Norgauer J, Di Virgilio F. The P2 purinergic receptors of human dendritic cells: identification and coupling to cytokine release. FASEB J 14: 2466–2476, 2000. [DOI] [PubMed] [Google Scholar]
- 19. Fiorio Pla A, Grange C, Antoniotti S, Tomatis C, Merlino A, Bussolati B, Munaron L. Arachidonic acid-induced Ca2+ entry is involved in early steps of tumor angiogenesis. Mol Cancer Res 6: 535–545, 2008. [DOI] [PubMed] [Google Scholar]
- 20. Frame MK, de Feijter AW. Propagation of mechanically induced intercellular calcium waves via gap junctions and ATP receptors in rat liver epithelial cells. Exp Cell Res 230: 197–207, 1997. [DOI] [PubMed] [Google Scholar]
- 21. Gerasimovskaya EV, Woodward HN, Tucker DA, Stenmark KR. Extracellular ATP is a pro-angiogenic factor for pulmonary artery vasa vasorum endothelial cells. Angiogenesis 11: 169–182, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gomes DA, Rodrigues MA, Leite MF, Gomez MV, Varnai P, Balla T, Bennett AM, Nathanson MH. c-Met must translocate to the nucleus to initiate calcium signals. J Biol Chem 283: 4344–4351, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hardingham GE, Chawla S, Johnson CM, Bading H. Distinct functions of nuclear and cytoplasmic calcium in the control of gene expression. Nature 385: 260–265, 1997. [DOI] [PubMed] [Google Scholar]
- 24. Hargitai D, Pataki A, Raffai G, Fuzi M, Danko T, Csernoch L, Varnai P, Szigeti GP, Zsembery A. Calcium entry is regulated by Zn2+ in relation to extracellular ionic environment in human airway epithelial cells. Respir Physiol Neurobiol 170: 67–75, 2010. [DOI] [PubMed] [Google Scholar]
- 25. Heo JS, Lee YJ, Han HJ. EGF stimulates proliferation of mouse embryonic stem cells: involvement of Ca2+ influx and p44/42 MAPKs. Am J Physiol Cell Physiol 290: C123–C133, 2006. [DOI] [PubMed] [Google Scholar]
- 26. Hirakawa M, Oike M, Karashima Y, Ito Y. Sequential activation of RhoA and FAK/paxillin leads to ATP release and actin reorganization in human endothelium. J Physiol 558: 479–488. 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hoffmann G, Gollnick F, Meyer R. Neopterin inhibits ATP-induced calcium release in alveolar epithelial cells in vitro. Mediators Inflamm 11: 181–185, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jiang L, Jha V, Dhanabal M, Sukhatme VP, Alper SL. Intracellular Ca2+ signaling in endothelial cells by the angiogenesis inhibitors endostatin and angiostatin. Am J Physiol Cell Physiol 280: C1140–C1150, 2001. [DOI] [PubMed] [Google Scholar]
- 29. Kaczmarek E, Erb L, Koziak K, Jarzyna R, Wink MR, Guckelberger O, Blusztajn JK, Trinkaus-Randall V, Weisman GA, Robson SC. Modulation of endothelial cell migration by extracellular nucleotides: involvement of focal adhesion kinase and phosphatidylinositol 3-kinase-mediated pathways. Thromb Haemost 93: 735–742, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kiefmann R, Islam MN, Lindert J, Parthasarathi K, Bhattacharya J. Paracrine purinergic signaling determines lung endothelial nitric oxide production. Am J Physiol Lung Cell Mol Physiol 296: L901–L910, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lorenzo IM, Liedtke W, Sanderson MJ, Valverde MA. TRPV4 channel participates in receptor-operated calcium entry and ciliary beat frequency regulation in mouse airway epithelial cells. Proc Natl Acad Sci USA 105: 12611–12616, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lyubchenko TA, Nielsen JP, Miller SM, Liubchenko GA, Holers VM. Role of initial protein phosphorylation events and localized release-activated calcium influx in B cell antigen receptor signaling. J Leukoc Biol 85: 298–309, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lyubchenko TA, Wurth GA, Zweifach A. Role of calcium influx in cytotoxic T lymphocyte lytic granule exocytosis during target cell killing. Immunity 15: 847–859, 2001. [DOI] [PubMed] [Google Scholar]
- 34. McLaughlin AP, De Vries GW. Role of PLCγ and Ca2+ in VEGF- and FGF-induced choroidal endothelial cell proliferation. Am J Physiol Cell Physiol 281: C1448–C1456, 2001. [DOI] [PubMed] [Google Scholar]
- 35. Montiel M, de la Blanca EP, Jiménez E. P2Y receptors activate MAPK/ERK through a pathway involving PI3K/PDK1/PKC-zeta in human vein endothelial cells. Cell Physiol Biochem 18: 123–134, 2006. [DOI] [PubMed] [Google Scholar]
- 36. Mori M, Hosomi H, Nishizaki T, Kawahara K, Okada Y. Calcium release from intracellular stores evoked by extracellular ATP in a Xenopus renal epithelial cell line. J Physiol 502: 365–573, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Motte S, Pirotton S, Boeynaems JM. Evidence that a form of ATP uncomplexed with divalent cations is the ligand of P2y and nucleotide/P2u receptors on aortic endothelial cells. Br J Pharmacol 109: 967–971, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Munaron L, Fiorio Pla A. Endothelial calcium machinery and angiogenesis: understanding physiology to interfere with pathology. Curr Med Chem 16: 4691–4703, 2009. [DOI] [PubMed] [Google Scholar]
- 39. Pubill D, Dayanithi G, Siatka C, Andrés M, Dufour MN, Guillon G, Mendre C. ATP induces intracellular calcium increases and actin cytoskeleton disaggregation via P2x receptors. Cell Calcium 295: 299–309, 2001. [DOI] [PubMed] [Google Scholar]
- 40. Pusl T, Wu JJ, Zimmerman TL, Zhang L, Ehrlich BE, Berchtold MW, Hoek JB, Karpen SJ, Nathanson MH, Bennett AM. Epidermal growth factor-mediated activation of the ETS domain transcription factor Elk-1 requires nuclear calcium. J Biol Chem 277: 27517–27527, 2002. [DOI] [PubMed] [Google Scholar]
- 41. Putney JW., Jr New molecular players in capacitative Ca2+ entry. J Cell Sci 120: 1959–1965, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pocock TM, Williams B, Curry FE, Bates DO. VEGF and ATP act by different mechanisms to increase microvascular permeability and endothelial [Ca2+]i. Am J Physiol Heart Circ Physiol 279: H1625–H1634, 2000. [DOI] [PubMed] [Google Scholar]
- 43. Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev 50: 413–492, 1998. [PubMed] [Google Scholar]
- 44. Rodrigues MA, Gomes DA, Leite MF, Grant W, Zhang L, Lam W, Cheng YC, Bennett AM, Nathanson MH. Nucleoplasmic calcium is required for cell proliferation. J Biol Chem 282: 17061–17068, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rumjahn SM, Yokdang N, Baldwin KA, Thai J, Buxton IL. Purinergic regulation of vascular endothelial growth factor signaling in angiogenesis. Br J Cancer 100: 1465–1470, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Seiffert K, Ding W, Wagner JA, Granstein RD. ATPgammaS enhances the production of inflammatory mediators by a human dermal endothelial cell line via purinergic receptor signaling. J Invest Dermatol 126: 1017–1027, 2006. [DOI] [PubMed] [Google Scholar]
- 47. Taylor CW, Broad LM. Pharmacological analysis of intracellular Ca2+ signalling: problems and pitfalls. Trends Pharmacol Sci 19: 370–375, 1998. [DOI] [PubMed] [Google Scholar]
- 48. Tovell VE, Sanderson J. Distinct P2Y receptor subtypes regulate calcium signaling in human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci 49: 350–357, 2008. [DOI] [PubMed] [Google Scholar]
- 49. von Kügelgen I. Pharmacological profiles of cloned mammalian P2Y-receptor subtypes. Pharmacol Ther 110: 415–432, 2006. [DOI] [PubMed] [Google Scholar]
- 50. Wang L, Karlsson L, Moses S, Hultgårdh-Nilsson A, Andersson M, Borna C, Gudbjartsson T, Jern S, Erlinge D. P2 receptor expression profiles in human vascular smooth muscle and endothelial cells. J Cardiovasc Pharmacol 40: 841–853, 2002. [DOI] [PubMed] [Google Scholar]
- 51. Wang L, Olivecrona G, Götberg M, Olsson ML, Winzell MS, Erlinge D. ADP acting on P2Y13 receptors is a negative feedback pathway for ATP release from human red blood cells. Circ Res 96: 189–196, 2005. [DOI] [PubMed] [Google Scholar]
- 52. Woodward HN, Anwar A, Riddle S, Taraseviciene-Stewart L, Fragoso M, Stenmark KR, Gerasimovskaya EV. PI3K, Rho, and ROCK play a key role in hypoxia-induced ATP release and ATP-stimulated angiogenic responses in pulmonary artery vasa vasorum endothelial cells. Am J Physiol Lung Cell Mol Physiol 297: L954–L964, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Woulfe D, Yang J, Brass L. ADP and platelets: the end of the beginning. J Clin Invest 107: 1503–1535, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Yi FX, Boeldt DS, Gifford SM, Sullivan JA, Grummer MA, Magness RR, Bird IM. Pregnancy enhances sustained Ca2+ bursts and endothelial nitric oxide synthase activation in ovine uterine artery endothelial cells through increased connexin 43 function. Biol Reprod 82: 66–75, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zhang FL, Luo L, Gustafson E, Palmer K, Qiao X, Fan X, Yang S, Laz TM, Bayne M, Monsma F., Jr P2Y13: identification and characterization of a novel Gαi-coupled ADP receptor from human and mouse. J Pharmacol Exp Ther 301: 705–713, 2002. [DOI] [PubMed] [Google Scholar]
- 56. Zhang L, Sanderson MJ. Oscillations in ciliary beat frequency and intracellular calcium concentration in rabbit tracheal epithelial cells induced by ATP. J Physiol 546: 733–749, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zsembery A, Boyce AT, Liang L, Peti-Peterdi J, Bell PD, Schwiebert EM. Sustained calcium entry through P2X nucleotide receptor channels in human airway epithelial cells. J Biol Chem 278: 13398–13408, 2003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.