Abstract
Anoikis or detachment-induced apoptosis plays an essential role in the regulation of cancer cell metastasis. Caveolin-1 (Cav-1) is a key protein involved in tumor metastasis, but its role in anoikis and its regulation during cell detachment are unclear. We report here that Cav-1 plays a key role as a negative regulator of anoikis through a reactive oxygen species (ROS)-dependent mechanism in human lung carcinoma H460 cells. During cell detachment, Cav-1 is downregulated, whereas ROS generation is upregulated. Hydrogen peroxide and hydroxyl radical are two key ROS produced by cells during detachment. Treatment of the cells with hydrogen peroxide scavengers, catalase and N-acetylcysteine, promoted Cav-1 downregulation and anoikis during cell detachment, indicating that produced hydrogen peroxide plays a primary role in preventing anoikis by stabilizing Cav-1 protein. Catalase and N-acetylcysteine promoted ubiquitination and proteasomal degradation of Cav-1, which is a major pathway of its downregulation during cell anoikis. Furthermore, addition of hydrogen peroxide exogenously to the cells inhibited Cav-1 downregulation by preventing the formation of Cav-1-ubiquitin complex, supporting the inhibitory role of endogenous hydrogen peroxide in Cav-1 degradation during cell detachment. Together, these results indicate a novel role of hydrogen peroxide as an endogenous suppressor of cell anoikis through its stabilizing effect on Cav-1.
Keywords: detachment, metastasis, reactive oxygen species
to approach the efficient prevention and treatment strategies for metastatic cancers, efforts have been made to identify key mechanisms involved in the metastatic process. Metastasis, a spread of cancer cells to secondary sites, is a major cause of cancer-related death which frequently occurs in lung cancer patients (20). To metastasize, tumor cells must have an ability to overcome anoikis or apoptosis induced by loss of cell adhesion (16). Although the mechanisms by which cancer cells resist anoikis are not well understood, it is generally accepted that defects in apoptosis machineries and/or stimulation of alternative survival signals [i.e., non- extracellular matrix (ECM)] are involved (17). Recently, the role of caveolin-1 (Cav-1) in the regulation of cancer progression and metastasis has gained increasing attention. Cav-1 is an essential protein constituent of the plasma membrane caveolae and a key regulator of caveolae-dependent signaling and endocytosis. It is a member of a gene family that includes three genes, namely caveolin-1, -2, and -3, all of which encode 20- to 24-kDa proteins (57). It has been proposed that caveolin family members function as scaffolding proteins (45) to organize and concentrate specific lipids (cholesterol and glycosphingolipids) (15, 33, 39) and lipid-modified signaling molecules (Src-like kinases, H-Ras, endothelial nitric oxide synthase, and G proteins within caveolae membranes) (18, 31, 32, 47, 48). In addition, Cav-1 interacts with and modulates multiple signaling pathways, suggesting that Cav-1 may exert a prosurvival action (12, 14, 43). Several investigators have reported that Cav-1 expression is upregulated in several human cancers, including lung, breast, prostate, and pancreas cancers, and that this upregulation is associated with a high degree of metastasis and cancer progression (22, 40, 49, 53, 58). Cav-1 expression was shown to be elevated in multidrug-resistant non-small cell lung cancer (NSCLC) (28, 29, 46). In lung carcinoma, Cav-1 expression was shown to be associated with metastatic and invasive capacity of the tumor (9, 22). Thus far, several mechanisms of Cav-1-mediated cancer progression and metastasis have been proposed, which include promotion of cancer cell survival (53), migration, and invasion (22). Under pathological conditions of cancer, many reactive oxygen species (ROS) are upregulated and are associated with the aggressive behavior of cancer cells. However, the role of ROS in cancer cell metastasis and its regulation are largely unknown. Previous studies have shown that, depending on the molecular background of cells and tissues, the location of ROS production, and the amount and persistence of individual ROS, these oxidative species can have both promoting (4, 23, 41) and inhibitory (35) effects on cancer cell metastasis. While ROS have been shown to induce apoptosis of various cell types, treatment of mammalian carcinoma cells with hydrogen peroxide (H2O2) before intravenous injection into mice resulted in enhanced lung metastasis (27), suggesting the prosurvival and prometastatic role of H2O2 in certain forms of cancer.
In this study, we investigated the role of ROS in anoikis and Cav-1 regulation in human lung carcinoma H460 cells. Using molecular and pharmacological approaches, we demonstrate that ROS play an important role in Cav-1 regulation and survival of lung cancer cells after detachment. Multiple ROS are generated during cell detachment with each playing a distinctive role in regulating Cav-1 expression and cell anoikis. H2O2 inhibits Cav-1 downregulation and anoikis induced by cell detachment, whereas superoxide anion and hydroxyl radical promote these effects. We also show that downregulation of Cav-1 by cell detachment is mediated primarily by proteasomal degradation and H2O2 inhibits this degradation by preventing protein ubiquitination. Our findings reveal the existence of a novel mechanism of anoikis regulation by ROS through Cav-1 stabilization, which could be important in the understanding of anoikis resistance in metastatic cancers.
MATERIALS AND METHODS
Cells and reagents.
NSCLC-H460 cells were obtained from the American Type Culture Collection (Manassas, VA). Cells were cultured in RPMI 1640 medium containing 5% fetal bovine serum (FBS), 2 mM l-glutamine, and 100 U/ml penicillin-streptomycin in a 5% CO2 environment at 37°C. N-acetylcysteine (NAC), H2O2, catalase (CAT), Mn(III)tetrakis(4-benzoic acid)porphyrin chloride (MnTBAP), sodium formate (NaF), ferrous sulfate (FeSO4), 2,3-dimethoxy-1-naphthoquinone (DMNQ), lactacystin (LAC), concanamycin A (CMA), 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide (XTT), and annexin V-fluorescein isothiocyanate (FITC) were obtained from Sigma Chemical (St. Louis, MO). Propidium iodide (PI), 2′,7′-dichlorofluorescein diacetate (DCFH-DA), and Hoechst 33342 were obtained from Molecular Probes (Eugene, OR). Antibody for Cav-1 and peroxidase-conjugated secondary antibody were obtained from Abcam (Cambridge, MA). Antibodies for ubiquitin, protein A-agarose bead, and anti-β-actin were from Santa Cruz Biotechnology (Santa Cruz, CA). The transfecting agent Lipofectamine 2000 was obtained from Invitrogen (Carlsbad, CA).
Plasmids and transfection.
The Cav-1 expression plasmid pEX_Cav-1 was acquired from the American Type Culture Collection, and Cav-1 knockdown plasmid short hairpin (sh)RNA-Cav-1 was obtained from Santa Cruz Biotechnology. Stable transfections of Cav-1 expression plasmid or Cav-1 knockdown plasmid were generated by culturing H460 cells in a six-well plate until they reached 60% confluence. Fifteen microliters of Lipofectamine reagent and 2 μg of Cav-1, shRNA-Cav-1, or mock pcDNA3 control plasmid were used to transfect the cells in the absence of serum. After 12 h the medium was replaced with culture medium containing 5% FBS. Approximately 36 h after the beginning of transfection, the cells were digested with 0.03% trypsin, and the cell suspensions were plated onto 75-ml culture flasks and cultured for 24 to 28 days with G418 selection (600 μg/ml). The stable transfectants were pooled, and the expression of Cav-1 protein in the transfectants was confirmed by Western blotting. The cells were cultured in antibiotic-free RPMI 1640 medium for at least two passages before use in each experiment.
ROS detection.
Intracellular ROS were determined by flow cytometry using DCFH-DA as a fluorescent probe. Briefly, cells were incubated with 10 μM DCFH-DA for 30 min at 37°C, after which they were washed, trypsinized, resuspended in phosphate-buffered saline, and immediately analyzed for fluorescence intensity by FACScan flow cytometer (Beckton Dickinson, Rutherford, NJ) using a 488-nm excitation beam and a 538-nm band-pass filter. Median fluorescence intensity was quantified by CellQuest software (Becton Dickinson) analysis of the recorded histograms.
Anoikis assay.
To prevent cell adhesion, tissue culture six-well plates were coated with 200 μl (6 mg/ml in 95% ethanol) of poly(2-hydroxyethylmethacrylate) (poly-HEMA; Sigma) and left to evaporate overnight in a laminar flow hood at room temperature. Adherent H460 cells in culture plates were trypsinized into a single-cell suspension in RPMI medium and then seeded in poly-HEMA-coated plates at a density of 1 × 105 cells/ml. Suspended cells were incubated at 37°C for various times up to 24 h. Cells were harvested, washed, and stained with annexin V-FITC and PI, then analyzed for fluorescence intensity by fluorescence microscopy. For Hoechst 33342 apoptosis assay, cells were incubated with 10 μM of the Hoechst dye for 30 min at 37°C. The apoptotic cells having condensed chromatin and/or fragmented nuclei were visualized and scored under a fluorescence microscope (Olympus IX51 with DP70). For cell survival assay, cells were similarly treated, harvested, washed, and incubated with 20 μM XTT for 4 h at 37°C. Optical density was then determined by V-max photometer (Molecular Devices, Menlo Park, CA) at a wavelength of 450 nm.
Western blot analysis.
After specific treatments, cells were incubated in lysis buffer containing 20 mM Tris·HCl (pH 7.5), 1% Triton X-100, 150 mM sodium chloride, 10% glycerol, 1 mM sodium orthovanadate, 50 mM sodium fluoride, 100 mM phenylmethylsulfonyl fluoride, and a commercial protease inhibitor cocktail (Roche Molecular Biochemicals) for 30 min on ice. Cell lysates were collected and determined for protein content using the Bradford method (Bio-Rad, Hercules, CA). Equal amount of proteins of each sample (40 μg) were denatured by heating at 95°C for 5 min with Laemmli loading buffer and were subsequently loaded on 10% SDS-polyacrylamide gel electrophoresis (PAGE). After separation, proteins were transferred onto 0.45-μm nitrocellulose membranes (Bio-Rad). The transferred membranes were blocked for 1 h in 5% nonfat dry milk in TBST [25 mM Tris·HCl (pH 7.5), 125 mM NaCl, 0.05% Tween 20] and incubated with the appropriate primary antibodies at 4°C overnight. Membranes were washed twice with TBST for 10 min and incubated with horseradish peroxidase-coupled isotype-specific secondary antibodies for 1 h at room temperature. The immune complexes were detected by enhanced chemiluminescence substrate (Supersignal West Pico; Pierce) and quantified using analyst/PC densitometry software (Bio-Rad).
Immunoprecipitation.
Cells were washed after treatments and lysed in lysis buffer at 4°C for 20 min. After centrifugation at 14,000 g for 15 min at 4°C, the supernatants were collected and determined for protein content. Cell lysates were normalized and equal amounts of protein per sample (60 μg) were incubated with anti-Cav-1 antibody conjugated to protein G plus-agarose beads (Santa Cruz) for 6 h at 4°C. The immune complexes were washed five times with ice-cold lysis buffer, resuspended in 2× Laemmli sample buffer, and boiled at 95°C for 5 min. Immune complexes were separated by 10% SDS-PAGE and analyzed by Western blotting as described above.
Colony formation assay.
Anchorage-independent growth was determined by colony formation assay in soft agar as described by Koleske et al. (26) with minor modifications. Briefly, H460 cells from six-well plate monolayer cultures were prepared into a single-cell suspension by treatment with a mixture of 350 μl trypsin and 1.5 mM EDTA. Cells were suspended in RPMI containing 10% FBS and 0.33% low melting temperature agarose, then 2 ml containing 2 × 104 cells were plated in a 35-mm dish over a 3-ml layer of solidified RPMI-10% FBS-0.6% agarose. The cells were fed every 3 days by adding 200 μl of RPMI-10% FBS. Colonies were assayed by Trypan blue staining and photographed at ×10 magnification after 2 wk.
Statistical analysis.
Mean densitometry data from independent experiments were normalized to the results in cells in the control. The data are presented as means ± SD from three or more independent experiments and were analyzed by the Student's t-test at a significance level of P < 0.05.
RESULTS
Cell detachment induces lung carcinoma H460 cell apoptosis.
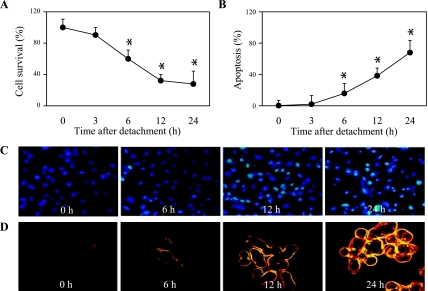
We first characterized detachment-induced apoptosis of human lung carcinoma H460 cells by detaching and incubating the cells in adhesion-resistant poly-HEMA-coated plates for various times (0–24 h) and analyzed for cell viability using XTT assay. Figure 1A shows that cell detachment caused a time-dependent decrease in cell survival with ∼60% and ∼30% of the cells remaining viable after 6 and 24 h, respectively. Control experiments showed that adherent cells in normal tissue culture plates exhibited no significant change in cell viability during the test period (data not shown). Analysis of cell apoptosis by Hoechst 33342 assay further showed that the decrease in cell survival after detachment was mainly due to anoikis, as indicated by the increase in number of cells with intense nuclear fluorescence and chromatin condensation. Apoptosis was detected as early as 6 h (∼15%) after cell detachment and reached ∼68% at 24 h (Fig. 1, B and C). Confirmation studies using annexin V-FITC and PI as probes for apoptosis and necrosis, respectively, further showed an increase in annexin V-positive cells over time after detachment, whereas PI-positive cells were not observed during this period (Fig. 1D). These results indicate anoikis as the primary mode of cell death after detachment of H460 cells.
Fig. 1.
Detachment-induced apoptosis of H460 non-small lung carcinoma cells. A: subconfluent (90%) monolayers of H460 cells were detached and suspended in poly(2-hydroxyethylmethacrylate) (poly-HEMA)-coated plates for various times (0–24 h). At the end of the indicated suspended periods, cells were collected and cell survival was determined by 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide (XTT) assay. Viability of detached cells at time 0 h was considered as 100%. B: percentage of cell detachment-induced apoptosis at indicated times was analyzed by Hoechst 33342 nuclear fluorescence staining assay. C: morphology of detached H460 cell anoikis nuclei stained with Hoechst 33342. D: effects of time dependence on detachment-induced apoptosis and necrosis. Detached cells were suspended at various time periods for 0–24 h, and cell apoptosis and necrosis were determined by annexin V-FITC and propidium iodide fluorescence microscopy. Data points represent means ± SD (n = 3). *P < 0.05 vs. control at detachment time = 0 h.
Cav-1 inhibits detachment-induced cell death in H460 cells.
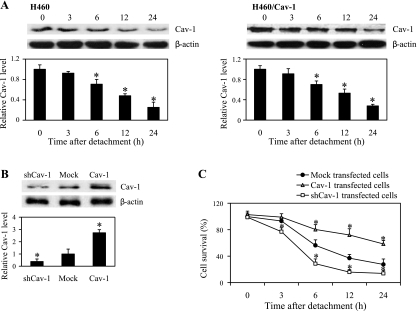
Cav-1 has been suggested to play a role in anoikis regulation in different cell types (6, 44). To provide supporting evidence for the role of Cav-1 in cell anoikis, we evaluated the expression profile of Cav-1 after cell detachment in H460 cells. Detached cells were suspended in poly-HEMA-coated plates for various times (0–24 h) and analyzed for Cav-1 expression by Western blotting. Figure 2A shows that detachment of the cells caused a gradual decrease in Cav-1 protein expression over time. To test whether Cav-1 expression might determine cell death and survival after detachment, we stably transfected the cells with Cav-1, shRNA Cav-1, or control plasmid and evaluated their effect on cell anoikis. Western blot analysis of Cav-1 expression showed a substantial increase in Cav-1 protein level in Cav-1-transfected cells, whereas a significant decrease in Cav-1 level was observed in shRNA-Cav-1-transfected cells as compared with control-transfected cells (Fig. 2B). The Cav-1-transfected cells exhibited less anoikis than the control cells as indicated by their higher survival rate after cell detachment, whereas shRNA-Cav-1-transfected cells showed a lower percentage of cell survival over time as compared with control cells (Fig. 2C). At 12 h postdetachment, Cav-1-transfected cells showed ∼20% reduction in cell survival compared with nondetached cells. In contrast, shRNA-Cav-1 cells exhibited ∼80% reduction in cell survival compared with control-transfected cells exhibiting ∼60% reduction. Western blot analysis of Cav-1 levels in H460 and its Cav-1-overexpressing counterpart was performed over time, and the results indicated that cell detachment resulted in a gradual decrease of Cav-1 in both cells. Notably, the remaining levels of Cav-1 in H460/Cav-1 cells were significantly higher than those of H460 cells, and the sustained protein level correlated well with the cell survival after detachment. These results indicate that Cav-1 plays a negative regulatory role in cell anoikis.
Fig. 2.
Caveolin-1 (Cav-1) overexpression increased anoikis resistance in detached H460 cells. A: H460 and H460/Cav-1 cells were detached and suspended in poly-HEMA-coated plates for various times (0–24 h). Cell extracts were prepared and separated on 10% polyacrylamide-SDS gels, transferred, and probed with Cav-1 antibody and then analyzed for Cav-1 protein expression by Western blotting. Blots were reprobed with β-actin antibody to confirm equal loading of samples. The immunoblot signals were quantified by densitometry, and mean data from independent experiments were normalized to the results. Columns are means ± SD (n = 3). *P < 0.05 vs. control of each cell type at time 0 h. B: mock (pcDNA3), Cav-1, or short hairpin (sh)RNA-Cav-1-transfected cells were grown in culture medium and analyzed for Cav-1 expression levels by Western blotting. C: cell survival analysis was done by XTT assay to compare the effects of Cav-1-overexpressing cells (H460/Cav-1) and Cav-1 knockdown cells (shCav-1) with mock-transfected cells at the indicated times after detachment (0–24 h). Data points represent means ± SD (n = 3). *P < 0.05 vs. control-transfected cells.
Cell detachment induces ROS generation.
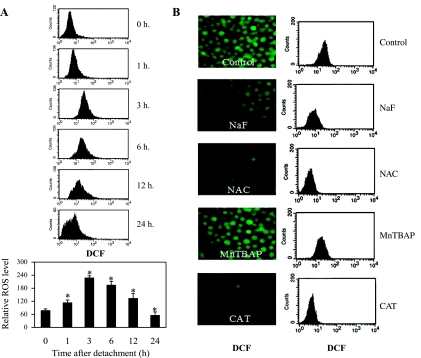
ROS have been shown to regulate apoptosis in many cell systems; however, their role in anoikis and Cav-1 regulation is largely unknown. We analyzed cellular ROS following cell detachment by flow cytometry using DCFH-DA as a fluorescent probe. Figure 3A shows that cell detachment induced an increase in cellular ROS in a time-dependent manner. A significant increase of cellular ROS level was observed as early as 1 h after detachment and peaked at 3 h, where it subsequently declined, which is likely due to cell death. To identify the specific ROS generated during cell detachment, detached cells were treated with various known ROS scavengers, including MnTBAP (superoxide anion scavenger), CAT (H2O2 scavenger), NaF (hydroxyl radical scavenger), and NAC (general antioxidant and precursor of glutathione), and cellular ROS levels were determined by flow cytometry and fluorescence microscopy using DCFH-DA as an oxidative probe. Figure 3B shows that treatment of the cells with CAT, NaF, or NAC inhibited the cellular fluorescence intensity, whereas MnTBAP had no inhibitory effect. These results suggest that H2O2 and hydroxyl radical are the primary ROS generated after cell detachment.
Fig. 3.
Effects of cell detachment on cellular reactive oxygen species (ROS) levels. A: H460 cells were detached, and intracellular ROS levels were then measured by flow cytometry analysis using 2′,7′-dichlorofluorescein diacetate (DCFH-DA) at indicated time points (0–24 h). B: detached H460 cells were left untreated or treated with the specific ROS scavengers sodium formate (NaF; 5 mM), N-acetylcysteine (NAC; 10 mM), Mn(III)tetrakis(4-benzoic acid)porphyrin chloride (MnTBAP; 100 μM), and catalase (CAT; 1,000 U/ml). Levels of intracellular ROS levels were analyzed at 3 h after detachment (time at peak ROS production) by fluorescence microscopy and flow cytometry using the fluorescent probe DCFH-DA. Columns are means ± SD (n = 3). *P < 0.05 vs. control at detachment time = 0 h.
Effect of ROS on cell anoikis.
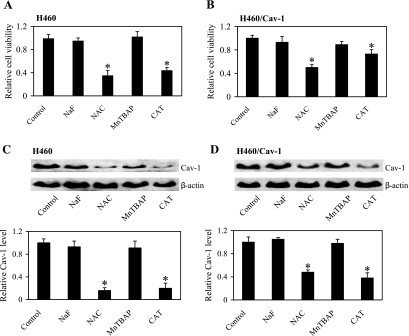
Having demonstrated the generation of ROS after cell detachment, we next determined the role of specific ROS in the regulation of cell anoikis. Detached cells were treated with specific ROS scavengers as earlier described, and cell viability was determined by XTT assay. Figure 4A shows that treatment of the cells with CAT or NAC decreased cell viability as compared with nontreated control, whereas treatment with MnTBAP or NaF had no significant effect. These results indicate that H2O2 is the primary oxidative species regulating anoikis of H460 cells after detachment, which is supported by our subsequent H2O2 exposure studies. The ability of NAC to enhance cell anoikis may be attributed to its antioxidant activity against H2O2 through glutathione-dependent mechanism (2). The regulatory role of H2O2 in anoikis was confirmed by studies using Cav-1 overexpressing (H460/Cav-1) cells earlier described. Figure 4B shows that treatment of the H460/Cav-1 cells with CAT or NAC, but not MnTBAP or NaF, had a similar inhibitory effect on cell viability, although the magnitude of reduction was less pronounced than that in normal H460 cells due to the increased expression of Cav-1.
Fig. 4.
Effect of ROS scavengers on cell anoikis and Cav-1 regulation. H460 and H460/Cav-1 cells were detached and left untreated or treated with the specific ROS scavengers NaF (5 mM), NAC (10 mM), MnTBAP (100 μM), and CAT (1,000 U/ml). A and B: viability of detached H460 cells and H460/Cav-1 cells was determined by XTT assay, respectively. C and D: Western blot analysis of Cav-1 expression of detached H460 cells and H460/Cav-1 cells, respectively. Cells were left untreated or treated with specific ROS scavengers NaF, NAC, MnTBAP, and CAT for 6 h after detachment. Cell extracts were prepared and separated on 10% polyacrylamide-SDS gels, transferred, and probed with Cav-1 antibody. β-Actin was used as a loading control. Columns are means ± SD (n = 3). *P < 0.05 vs. nontreated control of each cell type.
Effect of ROS on caveolin-1 expression.
Because both ROS and Cav-1 affected cell anoikis, we tested whether ROS regulate anoikis through Cav-1. H460 and H460/Cav-1 cells were detached and suspended in poly-HEMA-coated plates in the presence or absence of specific ROS scavengers, and Cav-1 expression was determined by Western blotting. Figure 4, C and D, shows that CAT and NAC were able to decrease Cav-1 expression in both H460 and H460/Cav-1 cells, whereas NaF and MnTBAP had minimal effect. The downregulating effect of CAT and NAC was more pronounced in H460 cells than in H460/Cav-1 cells. These results indicate the role of H2O2 as a key regulator of Cav-1 expression which presents a key mechanism of anoikis regulation after cell detachment.
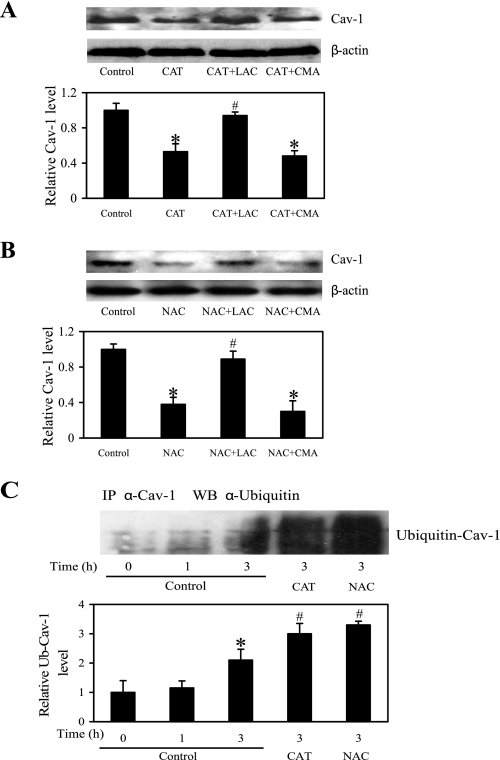
H2O2 attenuates detachment-induced caveolin-1 proteasomal degradation.
Degradation of Cav-1 by proteasomes has been shown to be the primary mechanism of Cav-1 downregulation by cell detachment (9). We tested whether CAT and NAC downregulated Cav-1 expression through this pathway. Detached H460 cells were treated with CAT or NAC in the presence or absence of a specific proteasome inhibitor, LAC, or lysosome inhibitor, CMA, and Cav-1 protein expression was determined by Western blotting. Figure 5, A and B, shows that the downregulating effect of CAT and NAC on Cav-1 expression was inhibited by LAC but not CMA, suggesting that the effect of CAT and NAC was through proteasomal degradation and not lysosomal degradation. Since proteasomal degradation of a protein is triggered by protein ubiquitination, we studied the effect of CAT and NAC on Cav-1 ubiquitination after cell detachment. Cells were detached and suspended in poly-HEMA-coated plates in the presence or absence of CAT or NAC for various times, and cell lysates were prepared and immunoprecipitated with anti-Cav-1 antibody. The resulting immune complexes were then analyzed for ubiquitination by Western blotting using anti-ubiquitin antibody. The results showed that Cav-1 was rapidly ubiquitinated as early as 1 h and peaked at about 3 h after cell detachment (Fig. 5C). In the presence of CAT or NAC, Cav-1 ubiquitination was substantially enhanced, suggesting the inhibitory role of H2O2 in Cav-1 ubiquitination.
Fig. 5.
Effects of NAC and CAT on Cav-1 downregulation through ubiquitin proteasome degradation pathway. A and B: H460 cells were detached and maintained in poly-HEMA-coated plates, then left untreated or treated for 6 h with CAT (1,000 U/ml) and NAC (10 mM), respectively, in the presence or absence of lactacystin (LAC; 20 μM) and concanamycin A (CMA; 0.5 μM). After the treatment period, cell extracts were prepared and analyzed for Cav-1 expression level by Western blotting and normalized to β-actin. Columns are means ± SD (n = 3). *P < 0.05 vs. nontreated control cells; #P < 0.05 vs. CAT- or NAC-treated control cells. C: detached H460 cells were untreated or treated for 3 h [time at high ubiquitin (Ub)-Cav-1 complex] with CAT (1,000 U/ml) or NAC (10 mM) in poly-HEMA-coated plates. Cell lysates were prepared and immunoprecipitated (IP) with anti-Cav-1 antibody. The resulting immune complexes were then analyzed for ubiquitin by Western blotting (WB). Maximum ubiquitination of Cav-1 was observed at ∼3 h after cell detachment. Lysate input was determined by probing with β-actin. The immunoblot signals were quantified by densitometry, and mean data from independent experiments were normalized to the results obtained in cells in the absence of treatment (control). Columns are means ± SD (n = 3). *P < 0.05 vs. nontreated control cells; #P < 0.05 vs. nontreated control at detachment time = 3 h.
Exogenous H2O2 inhibits Cav-1 degradation and cell anoikis.
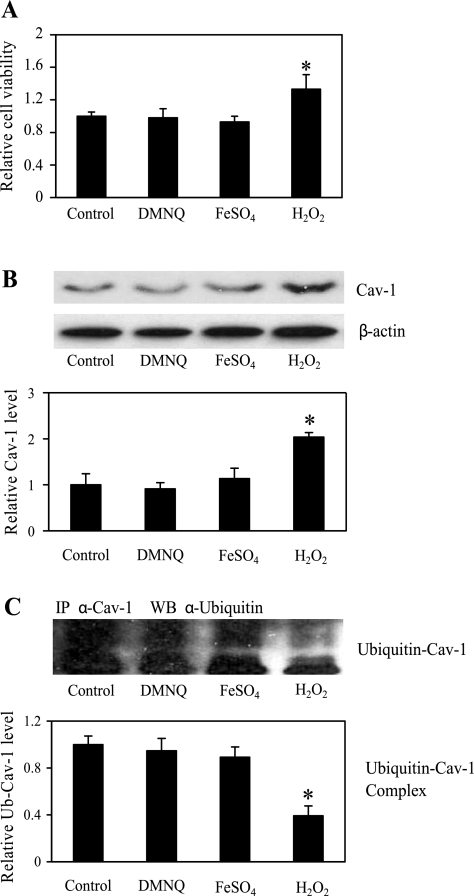
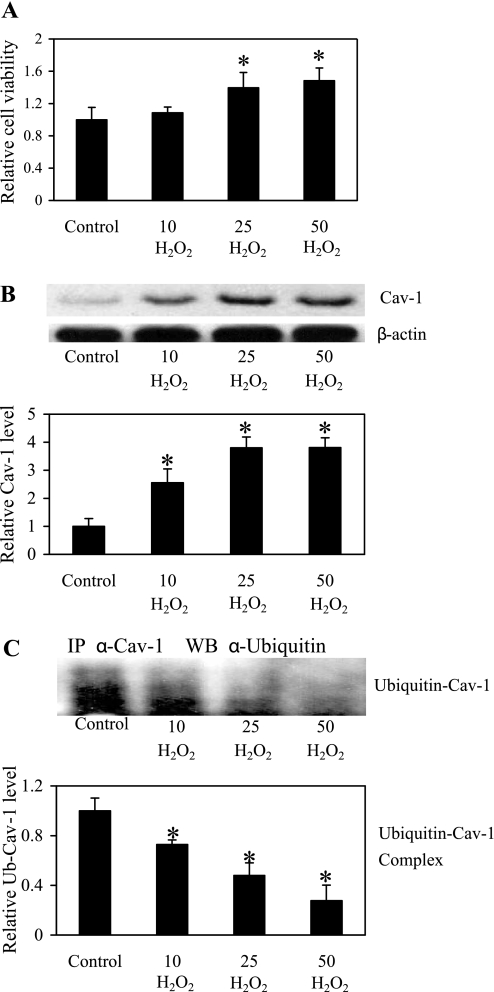
To substantiate the role of H2O2 in the regulation of Cav-1 expression and cell anoikis, cells were directly treated with exogenous H2O2, DMNQ (superoxide anion generator), or FeSO4 (hydroxyl radical generator), and their effect on cell anoikis was determined by XTT assay. Figure 6A shows that treatment of the cells with DMNQ or FeSO4 had no effect on cell viability, whereas treatment with H2O2 significantly increased cell viability. We also investigated the effect of ROS generators on Cav-1 expression. Figure 6B shows that H2O2 was able to increase the expression of Cav-1, whereas DMNQ and FeSO4 had no significant effect. These results are consistent with the anoikis data and indicate the role of H2O2 as a positive regulator of Cav-1 and suppressor of cell anoikis. To determine whether exogenous H2O2 increased Cav-1 expression through inhibition of ubiquitin-proteasomal degradation, cells were treated with H2O2 and analyzed for Cav-1 ubiquitination by immunoprecipitation and blotting. Parallel studies were also performed with DMNQ and FeSO4. The results show that H2O2, but not DMNQ or FeSO4, inhibited Cav-1 ubiquitination (Fig. 6C). The role of H2O2 as a regulator of anoikis and Cav-1 expression was further demonstrated by the dose-dependent effects of H2O2 on cell viability (Fig. 7A), Cav-1 expression (Fig. 7B), and Cav-1 ubiquitination (Fig. 7C). Together, these results strongly support the earlier findings on the inhibitory role of H2O2 in cell anoikis through inhibition of Cav-1 ubiquitination and degradation.
Fig. 6.
Effect of ROS on cell anoikis and Cav-1 regulation through ubiquitination pathway. H460 cells were detached and left untreated or treated with the specific ROS generators 2,3-dimethoxy-1-naphthoquinone (DMNQ; 5 μM), ferrous sulfate (FeSO4; 50 μM), and hydrogen peroxide (H2O2; 25 μM) for 6 h. A: viability of detached H-460 cells was determined by XTT assay. B: Western blot analysis of Cav-1 expression of detached H460 cells after cells were left untreated or treated with specific ROS scavengers DMNQ, FeSO4, and H2O2. Cell extracts were prepared and analyzed for Cav-1 protein expression by Western blotting. Blots were reprobed with β-actin antibody to confirm equal loading of samples. The immunoblot signals were quantified by densitometry, and mean data from independent experiments were normalized to the results in control cells. C: detached H460 cells were untreated or treated with DMNQ, FeSO4, or H2O2 in poly-HEMA-coated plates for 3 h (time at high Ub-Cav-1 complex), and cells lysates were then prepared and immunoprecipitated with anti-Cav-1 antibody. The resulting immune complexes were then analyzed for ubiquitin by Western blotting. Maximum ubiquitination of Cav-1 was observed at ∼3 h after cell detachment. Lysate input was determined by probing with β-actin. The immunoblot signals were quantified by densitometry, and mean data from independent experiments were normalized to the results obtained in cells in the absence of treatment (control). Columns are means ± SD (n = 3). *P < 0.05 vs. nontreated control cells.
Fig. 7.
H2O2 inhibits detachment-induced anoikis and Cav-1 downregulation through ubiquitination pathway. The cells were detached and untreated or treated with H2O2 (10, 25, and 50 μM) in poly-HEMA-coated plates for 6 h. A: cell survival was determined by XTT assay. B: cell lysates were prepared and analyzed for Cav-1 expression. Blots were reprobed with β-actin antibody to confirm equal loading of samples. Densitometry was performed to determine the relative level of Cav-1 protein. C: cell lysates were immunoprecipitated with Cav-1 antibody, and the immune complexes were analyzed for ubiquitin by Western blotting. Analysis of ubiquitin was performed after 3 h treatment of H2O2, where ubiquitin was found to be maximal. The immunoblot signals were quantified by densitometry, and mean data from independent experiments were normalized to the results obtained in cells in the absence of H2O2 (control). Columns are means ± SD (n = 3). *P < 0.05 vs. nontreated control cells.
H2O2 and Cav-1 expression enhance anchorage-independent growth of H460 cells.
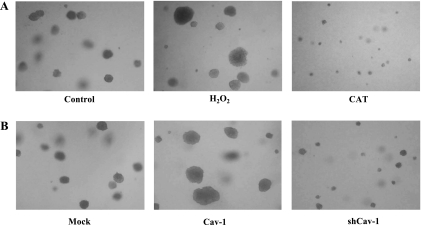
Anchorage-independent growth is a key characteristic of metastatic cancer cells. To test whether H2O2 could promote anchorage-independent growth, H460 cells were subjected to soft agar assay as described in materials and methods for 2 wk and cell colony was determined by microscopy. Figure 8 shows that H460 cells have the ability to survive and grow under anchorage-independent conditions and that treatment of the cells with H2O2 resulted in increased cell growth as indicated by the formation of large cell colonies. In contrast, treatment of the cells with CAT resulted in much smaller colonies, and most of the cells in the colonies were dead as determined by Trypan blue exclusion assay. These results indicate that H2O2 is required for anchorage-independent growth of H460 cells. In addition, the effect of Cav-1 on anchorage-independent growth of H460 cells was determined. Our results indicate that Cav-1 overexpressing cells have a higher ability to survive and grow in anchorage-independent condition as compared with H460 control cells, whereas the shRNA-Cav-1 cells exhibited a substantial decrease in the size and number of colonies formed.
Fig. 8.
Effect of H2O2 and Cav-1 expression on anchorage-independent growth of H460 cells in soft agar. A: H460 cells were detached and left untreated (control) or treated with H2O2 or CAT under suspending conditions in soft agar containing growth medium (RPMI), 10% FBS, and 0.33% agarose as described in materials and methods. B: H460-transfected cells including pcDNA3 control, Cav-1, and shCav-1 were detached and suspended in soft agar using the same conditions as in A. Cells were fed twice a week and photographed after 2 wk for their ability to proliferate and form colonies. The representative fields were photographed at ×10 magnification. The results are representative of three independent experiments.
DISCUSSION
Cellular interaction with adjacent cells or ECM provides an important signaling mechanism that regulates and maintains cell survival. Under normal conditions, detachment of cells from the ECM initiates an apoptotic process; however, some cancer cells possess an ability to overcome this process, resulting in sustaining their survival until they reach the new sites and form secondary tumors. Understanding the mechanisms by which cancer cells evade anoikis could lead to the development of novel therapeutic strategies for metastatic cancers. Among a number of factors affecting cancer cell behaviors, the ROS presenting in the tumor microenvironment have garnered increasing attention not only because they are reported to be upregulated in tumor cells and their microenvironment (5, 50) but also because they have been shown to be crucial regulators of cell growth and survival in many systems (7, 24, 25). The specific cellular responses to ROS are dependent on the type of ROS generated as well as their source, concentration, localization, and kinetics of production and elimination (13, 52). The alteration in cellular ROS during cell detachment is still largely unknown and we report here that several ROS are upregulated after cell detachment in lung carcinoma H460 cells. Two of the key ROS generated in these cells after their detachment are H2O2 and hydroxyl radical, with the former playing a major role in anoikis.
Increased ROS generation and oxidative stress has been implicated in the development of many human metastatic cancers including lung (10, 37), breast (4), prostate (34), colon (50), and ovary (21). Likewise, increased expression of Cav-1 has been associated with the advanced stage of metastatic tumors and poor survival of cancer patients (8, 38, 59). In lung adenocarcinoma, Cav-1 upregulation has been reported to enhance metastasis and invasive capacity of the cells (22). In human breast cancer, Cav-1 expression was shown to enhance matrix-independent cell survival through the upregulation of insulin-like growth factor receptor and signaling (14, 44). Moreover, Cav-1 overexpression has been demonstrated to mediate cell survival by sustaining Akt activation (30). In addition, prostate cancer cells produce Cav-1, which promotes cancer cell viability and clonal growth under serum-free conditions by inhibiting apoptosis (51). Cav-1 has been proposed to promote cancer progression and metastasis through multiple mechanisms, including promotion of cell survival (53) and migration (22). Cav-1 also mediates integrin-dependent activation of extracellular signal-regulated kinase and focal adhesion kinase and promotes cell cycle procession (55, 56). However, additional studies are required to clarify the role of Cav-1 in regulation of downstream apoptotic or survival pathways.
The present study provides evidence for the role of Cav-1 in cell anoikis and its regulation by ROS that has not previously been demonstrated. We found that, during cell anoikis, Cav-1 is downregulated and that such downregulation is crucial to the anoikis since ectopic overexpression of Cav-1 significantly inhibited cell anoikis (Fig. 2). Furthermore, downregulation of Cav-1 by shRNA significantly increased cell anoikis (Fig. 2C), supporting the above finding. Similarly, gene overexpression and knockdown experiments indicated the promoting role of Cav-1 in anchorage-independent cell growth (Fig. 8B). The downregulation of Cav-1 in H460 cells is dependent on the oxidative status of cells. Inhibition of H2O2 generation by CAT or NAC promoted the downregulation of Cav-1 as well as cell anoikis, whereas inhibition of hydroxyl radical and superoxide anion by NaF and MnTBAP had minimal effects (Fig. 4). These results indicate the dominant role of H2O2 in regulating Cav-1 expression and cell anoikis, which is substantiated by studies using exogenous ROS agents (Fig. 7). To our knowledge, this is the first demonstration of the role H2O2 in Cav-1 and anoikis regulation which may be important in the understanding of cancer progression and metastasis. H2O2 is a rapidly diffuse molecule and known mediator of many cellular events including mitochondrial metabolism, inflammation, and cell growth and differentiation (3, 19, 50, 54). Our finding adds to the growing list of the many diverse functions of this molecule in regulating cellular physiologic and pathologic events.
The mechanism by which H2O2 regulates Cav-1 expression and cell anoikis was shown to involve inhibition of Cav-1 degradation via ubiquitin-proteasome pathway. Proteasomal degradation was previously shown to be the primary mechanism of Cav-1 downregulation after cell detachment (9). In this study, we further demonstrated that such downregulation is dependent on the cellular level of H2O2. Inhibition of cellular H2O2 by CAT or NAC increased ubiquitination of Cav-1 and reduced its expression (Fig. 5). Furthermore, addition of H2O2, but not other ROS-generating agents, inhibited the ubiquitination of Cav-1 and increased its expression after cell detachment (Fig. 6).
Finally, we showed that H2O2 also plays a role in anchorage-independent growth and colony formation of lung carcinoma H460 cells, which may contribute to their metastatic capacity. Inhibition of H2O2 by CAT strongly inhibited anchorage-independent growth, whereas addition of H2O2 promoted colony growth (Fig. 8A). This result is consistent with previous studies showing the prosurvival role of subcytotoxic concentrations of H2O2 and superoxide anion (11, 13, 42). Since H2O2 can be inhibited by compounds like NAC, which has been used in human mucolytic therapy and in many dietary supplements, this compound may potentially be used as a preventive or therapeutic agent against cancer. Indeed, anticarcinogenic and antimutagenic effects of NAC have been reported (36). In addition, activities of NAC against cancer cell metastasis and invasion have been reported (1).
In summary, we report a novel finding on the anoikis-inhibitory effect of H2O2 in human lung carcinoma H460 cells that is associated with the ability of H2O2 to stabilize Cav-1 protein by preventing its ubiquitination and proteasomal degradation after cell detachment. Because sustained or elevated Cav-1 expression has been associated with anoikis resistance which is crucial to the survival of metastatic cancer cells, the results of this study could be beneficial to the understanding of cancer progression and metastasis.
GRANTS
This research is supported by National Institutes of Health (Grant R01-HL076340 to Y. Rojanasakul), grants from the Thailand Research Fund (MG5080134, to P. Chanvorachote), and The 90th Anniversary of Chulalongkorn University Fund (Ratchadaphiseksomphot Endowment Fund) (F-31-GS-ES13, to U. Nimmannit).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Albini A, D'Agostini F, Giunciuglio D, Paglieri I, Balansky R, De Flora S. Inhibition of invasion, gelatinase activity, tumor take and metastasis of malignant cells by N-acetylcysteine. Int J Cancer 29: 121–129, 1995 [DOI] [PubMed] [Google Scholar]
- 2. Aruoma OI, Halliwell B, Hoey BM, Butler J. The antioxidant action of N-acetylcysteine: its reaction with hydrogen peroxide, hydroxyl radical, superoxide, and hypochlorous acid. Free Radic Biol Med 6: 593–597, 1989 [DOI] [PubMed] [Google Scholar]
- 3. Barja QG, Gil P, Lopez-Torres M. Physiological significance of catalase and glutathione peroxidases, and in vivo peroxidation, in selected tissues of the toad Discoglossus pictus (Amphibia) during acclimation to normobaric hyperoxia. J Comp Physiol [B] 158: 583–590, 1988 [DOI] [PubMed] [Google Scholar]
- 4. Brown NS, Bicknell R. Hypoxia and oxidative stress in breast cancer: oxidative stress - its effects on the growth, metastatic potential and response to therapy of breast cancer. Breast Cancer Res 3: 323–327, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Burdon RH. Superoxide and hydrogen peroxide in relation to mammalian cell proliferation. Free Radic Biol Med 18: 775–794, 1995 [DOI] [PubMed] [Google Scholar]
- 6. Burgermeister E, Xing X, Röcken C, Juhasz M, Chen J, Hiber M, Mair K, Shatz M, Liscovitch M, Schmid RM, Ebert MPA. Differential expression and function of caveolin-1 in human gastric cancer progression. Cancer Res 67: 8519–8526, 2007 [DOI] [PubMed] [Google Scholar]
- 7. Cai J, Jones DP. Mitochondrial redox signaling during apoptosis. J Bioenerg Biomembr 31: 327–334, 1999 [DOI] [PubMed] [Google Scholar]
- 8. Cassoni P, Daniele L, Maldi E, Righi L, Tavaglione V, Novello S, Volante M, Scagliotti GV, Papotti M. Caveolin-1 expression in lung carcinoma varies according to tumor histotype and is acquired de novo in brain metastases. Histopathology 55: 20–27, 2009 [DOI] [PubMed] [Google Scholar]
- 9. Chanvorachote P, Nimmannit U, Lu Y, Talbott S, Jiang BH, Rojanasakul Y. Nitric oxide regulates lung carcinoma cell anoikis through inhibition of ubiquitin-proteasomal degradation of caveolin-1. J Biol Chem 284: 28476–28484, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chung-man HJ, Zheung S, Comhair SA, Farver C, Erzurm SC. Differential expression of manganese superoxide dismutase and catalase in lung cancer. Cancer Res 61: 8578–8585, 2001 [PubMed] [Google Scholar]
- 11. Dunning S, Hannivoort RA, de Boer JF, Buist-Homan M, Faber KN, Moshage H. Superoxide anions and hydrogen peroxide inhibit proliferation of activated rat stellate cells and induce different modes of cell death. Liver Int 29: 922–932, 2009 [DOI] [PubMed] [Google Scholar]
- 12. Engelman JA, Wykoff CC, Yasuhara S, Song KS, Okamoto T, Lisanti MP. Recombinant expression of caveolin-1 in oncogenically transformed cells abrogates anchorage-independent growth. J Biol Chem 272: 16374–16381, 1997 [DOI] [PubMed] [Google Scholar]
- 13. Feinendegen LE. Reactive oxygen species in cell responses to toxic agents. Hum Exp Toxicol 21: 85–90, 2002 [DOI] [PubMed] [Google Scholar]
- 14. Fiucci G, Ravid D, Reich R, Liscovitch M. Caveolin-1 inhibits anchorage-independent growth, anoikis and invasiveness in MCF-7 human breast cancer cells. Oncogene 21: 2365–2375, 2002 [DOI] [PubMed] [Google Scholar]
- 15. Fra AM, Masserini M, Palestini P, Sonnino S, Simons K. A photo-reactive derivative of ganglioside GM1 specifically cross-links VIP21-caveolin on the cell surface. FEBS Lett 375: 11–14, 1995 [DOI] [PubMed] [Google Scholar]
- 16. Frisch SM, Francis H. Disruption of epithelial cell-matrix interactions induces apoptosis. J Cell Biol 124: 619–626, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Frisch SM, Screaton RA. Anoikis mechanisms. Curr Opin Cell Biol 13: 555–562, 2001 [DOI] [PubMed] [Google Scholar]
- 18. Garcia-Cardena G, Oh P, Liu J, Schnitzer JE, Sessa WC. Targeting of nitric oxide synthase to endothelial cell caveolae via palmitoylation: implications for nitric oxide signaling. Proc Natl Acad Sci USA 93: 6448–6453, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hampton MB, Orrenius S. Dual regulation of caspase activity by hydrogen peroxide: implications for apoptosis. FEBS Lett 414: 552–556, 1997 [DOI] [PubMed] [Google Scholar]
- 20. Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 100: 57–70, 2000 [DOI] [PubMed] [Google Scholar]
- 21. Hileman OE, Liu J, Albitar M, Keating MJ, Huang P. Intrinsic oxidative stress in cancer cells: a biochemical basis for therapeutic selectivity. Cancer Chemother Pharmacol 53: 209–219, 2004 [DOI] [PubMed] [Google Scholar]
- 22. Ho CC, Huang PH, Huang HY, Chen YH, Yang PC, Hsu SM. Up-regulated caveolin-1 accentuates the metastasis capability of lung adenocarcinoma by inducing filopodia formation. Am J Pathol 161: 1647–1656, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ishikawa K, Takenaga K, Akimoto M, Koshikawa N, Yamaguchi A, Imanishi H, Nakada K, Honma Y, Hayashi J. ROS-generating mitochondrial DNA mutations can regulate tumor cell metastasis. Science 320: 661–664, 2008 [DOI] [PubMed] [Google Scholar]
- 24. Jabs T. Reactive oxygen intermediates as mediators of programmed cell death in plants and animals. Biochem Pharmacol 57: 231–245, 1999 [DOI] [PubMed] [Google Scholar]
- 25. Kamata H, Hirata H. Redox regulation of cellular signalling. Cell Signal 11: 1–14, 1999 [DOI] [PubMed] [Google Scholar]
- 26. Koleske AJ, Baltimore D, Lisanti MP. Reduction of caveolin and caveolae in oncogenically transformed cells. Proc Natl Acad Sci USA 92: 1381–1385, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kundu N, Zhang S, Fulton AM. Sublethal oxidative stress inhibits tumor cell adhesion and enhances experimental metastasis of murine mammary carcinoma. Clin Exp Metastasis 13: 16–22, 1995 [DOI] [PubMed] [Google Scholar]
- 28. Lavie Y, Fiucci G, Liscovitch M. Up-regulation of caveolae and caveolar constituents in multidrug resistant cancer cells. J Biol Chem 273: 32380–32383, 1998 [DOI] [PubMed] [Google Scholar]
- 29. Lavie Y, Liscovitch M. A role for caveolae in multidrug resistance of cancer cells (Abstract). Mol Biol Cell 8: 207, 1997. [Google Scholar]
- 30. Li L, Ren CH, Tahir SA, Ren C, Thompson TC. Caveolin-1 maintains activated Akt in prostate cancer cells through scaffolding domain binding site interactions with and inhibition of serine/threonine protein phosphatases PP1 and PP2A. Mol Cell Biol 23: 9389–9404, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Li S, Couet J, Lisanti MP. Src tyrosine kinases, G alpha subunits and H-Ras share a common membrane-anchored scaffolding protein, caveolin. Caveolin binding negatively regulates the auto-activation of Src tyrosine kinases. J Biol Chem 271: 29182–29190, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Li S, Okamoto T, Chun M, Sargiacomo M, Casanova JE, Hansen SH, Nishimoto I, Lisanti MP. Evidence for a regulated interaction of hetero-trimeric G proteins with caveolin. J Biol Chem 270: 15693–15701, 1995 [DOI] [PubMed] [Google Scholar]
- 33. Li S, Song KS, Lisanti MP. Expression and characterization of recombinant caveolin. Purification by polyhistidine tagging and cholesterol-dependent incorporation into defined lipid membranes. J Biol Chem 271: 568–573, 1996 [PubMed] [Google Scholar]
- 34. Lim SD, Sun C, Lambeth JD, Marshall F, Amin M, Chung L, Petros JA, Arnold RS. Increased Nox1 and hydrogen peroxide in prostate cancer. Prostate 62: 200–207, 2004 [DOI] [PubMed] [Google Scholar]
- 35. Maeda H, Hori S, Nishitoh H, Ichijo H, Ogawa O, Kakehi Y, Kakizuka A. Tumor growth inhibition by arsenic trioxide (As2O3) in the orthotopic metastasis model of androgen-independent prostate cancer. Cancer Res 61: 5432–5440, 2001 [PubMed] [Google Scholar]
- 36. Martinez E, Domingo P. N-acetylcysteine as chemoprotectant in cancer chemotherapy. Lancet 338: 249, 1991 [DOI] [PubMed] [Google Scholar]
- 37. Misthos P, Katsaragakis S, Milingos N, Kakaris S, Sepsas E, Athasnassiadi K, Theodorou D, Skottis T. Postresectional pulmonary oxidative stress in lung cancer patient. The role of one-lung ventilation. Eur J Cardiothorac Surg 27: 379–383, 2005 [DOI] [PubMed] [Google Scholar]
- 38. Moon KC, Lee GK, Yoo SH, Jeon YK, Chung JH, Han J, Chung DH. Expression of caveolin-1 in pleomorphic carcinoma of the lung is correlated with poor prognosis. Anticancer Res 25: 4631–4637, 2005 [PubMed] [Google Scholar]
- 39. Murata M, Peranen J, Schreiner R, Wieland F, Kurzchalia TV, Simons K. VIP21/caveolin is a cholesterol-binding protein. Proc Natl Acad Sci USA 92: 10339–10343, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nasu Y, Timme TL, Yang G. Suppression of caveolin expression induces androgen sensitivity in metastatic androgen-insensitive mouse prostate cancer cells. Nat Med 4: 1062–1064, 1998 [DOI] [PubMed] [Google Scholar]
- 41. Nishikawa M, Hashida M, Takakura Y. Catalase delivery for inhibiting ROS-mediated tissue injury and tumor metastasis. Adv Drug Deliv Rev 61: 319–326, 2009 [DOI] [PubMed] [Google Scholar]
- 42. Novo E, Marra F, Zamara E, Valfrè di BL, Caligiuri A, Cannito S, Antonaci C, Colombatto S, Pinzani M, Parola M. Dose dependent and divergent effects of superoxide anion on cell death, proliferation, and migration of activated human hepatic stellate cells. Liver 55: 90–97, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ravid D, Maor S, Werner H, Liscovitch M. Caveolin-1 inhibits anoikis and promotes survival signaling in cancer cells. Adv Enzyme Regul 46: 163–175, 2006 [DOI] [PubMed] [Google Scholar]
- 44. Ravid D, Maor S, Werner H, Liscovitch M. Caveolin-1 inhibits cell detachment-induced p53 activation and anoikis by up-regulation of insulin-like growth factor-I receptors and signaling. Oncogene 24: 1338–1347, 2005 [DOI] [PubMed] [Google Scholar]
- 45. Sargiacomo M, Scherer PE, Tang ZL, Kubler E, Song KS, Sanders MC, Lisanti MP. Oligomeric structure of caveolin: implications for caveolae membrane organization. Proc Natl Acad Sci USA 92: 9407–9411, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Shatz M, Liscovitch M. Caveolin-1 and cancer multidrug resistance: coordinate regulation of pro-survival proteins?. Leuk Res 28: 907–908, 2004 [DOI] [PubMed] [Google Scholar]
- 47. Shaul PW, Smart EJ, Robinson LJ, German Z, Yuhanna IS, Ying Y, Anderson RGW, Michel T. Acylation targets endothelial nitric-oxide synthase to plasmalemmal caveolae. J Biol Chem 271: 6518–6522, 1996 [DOI] [PubMed] [Google Scholar]
- 48. Song KS, Li S, Okamoto T, Quilliam L, Sargiacomo M, Lisanti MP. Copurification and direct interaction of Ras with caveolin, an integral membrane protein of caveolae microdomains. Detergent free purification of caveolae membranes. J Biol Chem 271: 9690–9697, 1996 [DOI] [PubMed] [Google Scholar]
- 49. Suzuoki M, Miyamoto M, Kato K. Impact of caveolin-1 expression on prognosis of pancreatic ductal adenocarcinoma. Br J Cancer 87: 1140–1144, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Szatrowski TP, Nathan CF. Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Res 51: 794–798, 1991 [PubMed] [Google Scholar]
- 51. Tahir SA, Yang G, Ebara S, Timme TL, Satoh T, Li L, Goltsov A, Ittmann M, Morrisett JD, Thompson TC. Secreted caveolin-1 stimulates cell survival/clonal growth and contributes to metastasis in androgen-insensitive prostate cancer. Cancer Res 61: 3882–3885, 2001 [PubMed] [Google Scholar]
- 52. Temple MD, Perrone GG, Dawes IW. Complex cellular responses to reactive oxygen species. Trends Cell Biol 15: 319–326, 2005 [DOI] [PubMed] [Google Scholar]
- 53. Thompson TC. Metastasis-related genes in prostate cancer: the role of caveolin-1. Cancer Metastasis Rev 17: 439–442, 1999 [DOI] [PubMed] [Google Scholar]
- 54. Veal EA, Day AM, Morgan BA. Hydrogen peroxide sensing and signaling. Mol Cell 26: 1–14, 2007 [DOI] [PubMed] [Google Scholar]
- 55. Wary KK, Mariotti A, Zurzolo C, Giancotti FG. A requirement for caveolin-1 and associated kinase Fyn in integrin signaling and anchorage-dependent cell growth. Cell 94: 625–634, 1998 [DOI] [PubMed] [Google Scholar]
- 56. Wei Y, Yang X, Liu Q, Wilkins JA, Chapman HA. A role for caveolin and the urokinase receptor in integrin-mediated adhesion and signaling. J Cell Biol 144: 1285–1294, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Williams TM, Lisanti MP. Caveolin-1 in oncogenic transformation, cancer, and metastasis. Am J Physiol Cell Physiol 288: C494–C506, 2005 [DOI] [PubMed] [Google Scholar]
- 58. Yang G, Truong LD, Timme TL, Ren C, Wheeler TM, Park SH, Nasu Y, Bangma CH, Kattan MW, Scardino PT, Thompson TC. Elevated expression of caveolin is associated with prostate and breast cancer. Clin Cancer Res 4: 1873–1880, 1998 [PubMed] [Google Scholar]
- 59. Yoo SH, Park YS, Kim HR, Sung SW, Kim JH, Shim YS, Lee SD, Choi YL, Kim MK, Chung DH. Expression of caveolin-1 is associated with poor prognosis of patients with squamous cell carcinoma of the lung. Lung Cancer 42: 195–202, 2003 [DOI] [PubMed] [Google Scholar]