Abstract
Background
Integrity of the blood brain barrier (BBB) after cardiopulmonary bypass (CPB) with hypothermic circulatory arrest (HCA) is controversial in children. We tested the hypothesis that BBB is disrupted by HCA.
Methods
41 piglets (mean weight 11 kg) were randomly divided into acute and survival experiments. Five groups (25 piglets; 5 per group) underwent acute studies: anesthesia alone (control); CPB at 37°C with full-flow (FF); CPB at 25°C with very low flow (LF); HCA at 15°C and 25°C. Two groups (16 piglets; 8 per group) underwent survival studies: CPB at 25°C with LF and HCA. Acute; Evans blue dye (EBD) extravasation through the blood brain barrier into the brain was measured using 2 methods: EBD absorbance of homogenized brain and immunohistochemical localization of EBD linked albumin for cortex, caudate nucleus, thalamus, hippocampus and cerebellum. Survival; Cerebral histology was assessed with hematoxylin-eosin stain after sacrifice at 4 days post-surgery.
Results
BBB disruption was clearly observed around watershed areas for 25°C HCA compared to other conditions. Microscopic data showed that leakage of EBD in 25°C HCA was more severe than control in all brain areas (p < 0.05), EBD and albumin were co-localizing. Histological damage scores were significantly higher in watershed areas with 25°C HCA.
Conclusions
BBB was impaired around watershed areas by 25°C HCA for 1 hour according to both macro and microscopic data. An increase in permeability of the BBB may be both a sign and a mechanism of brain damage.
Keywords: Blood brain barrier, Cardiopulmonary bypass, Ischemia/reperfusion, Hypothermia/circulatory arrest
Introduction
The brain is a unique tissue being protected from free exchange with circulating blood by the blood brain barrier (BBB), located at the tight junctions and cell walls of the cerebral endothelium.1 The BBB plays an important role in homeostasis and is generally seen as a defense mechanism that shields the brain against various molecules such as inflammatory cytokines that might otherwise enter cerebral tissue. Damage to the BBB during CPB is one reason for neurological dysfunction after heart surgery.2,3 Studies of BBB during CPB are few and the effect of CPB on the BBB is controversial.4,5 Mechanisms underlying this susceptibility are not completely understood.
The present study sought to determine whether Evans blue dye, a widely used nonradioactive intravascular tracer, can be used as an effective alternative for BBB breakdown quantitation. Evans blue dye (EBD), a tetrasodium diazo salt (MW 980 Daltons), irreversibly binds to plasma albumin in a 10:1 molar ratio in vivo6,7 and in vitro.8 In quantitative studies of vascular permeability, Evans blue is injected into the bloodstream where it rapidly binds to plasma albumin. Whenever plasma extravasates from blood vessels according to vascular permeability, the Evans blue dye–albumin complex leaks into surrounding tissues. After variable circulation times animals are perfused with citrate-buffered paraformaldehyde9,10 or saline11 to clear the EBD from the bloodstream. The extravasated EBD can then be extracted from tissues by different solvents including formamide.8,12 The concentration of dye is then measured by routine spectrophotometry8.13 or fluorescence spectrophotometry.9 These properties have made Evans blue suitable for quantitation of plasma albumin leakage secondary to increased vascular permeability in the brain.14
In this study, we tested whether cardiopulmonary bypass with HCA affects the integrity of the BBB and whether this impairment is associated with brain damage.
Materials and Methods
Animals
Forty-one experiments (acute experiments; 25 animals, each group: n=5 and survival experiments; 16 animals, each group: n=8) were performed on 4 week-old Yorkshire piglets (Archer Farms, Inc., Darlington, MD) with average body weight of 11.2 ± 1.4 kg. All animals received humane care in accordance with the ‘‘Principles of Laboratory Animal Care’’ formulated by the National Society for Medical Research and the ‘‘Guide for the Care and Use of Laboratory Animals’’ prepared by the Institute of Laboratory Animal Resources, National Research Council, and published by the National Academy Press, revised in 1996. This study was approved by the Institutional Animal Care and Use Committee of Children’s National Medical Center.
Surgical Procedure
Piglets were sedated with intramuscular ketamine (20 mg/kg) and xylazine (4 mg/kg). After endotracheal intubation (cuffed 5-mm tube) and a bolus of fentanyl (25 µg/kg IV), piglets were ventilated with a pressure-controlled respirator using an inspired oxygen fraction of 21% pre-CPB and 100% post-CPB (rate 10–18 breaths per minute) to achieve arterial pCO2 between 38–42 mmHg. Anesthesia was maintained with fentanyl (25 µg/kg/h), midazolam (0.2 mg/kg/h), and pancuronium (0.2 mg/kg/h) using an infusion pump. Animals were placed supine on a water-circulating heating mat to prevent hypothermia. Esophageal and rectal temperature probes were placed. The left femoral artery was cannulated and the catheter was advanced into the descending aorta for monitoring blood pressure and blood gases. Blood pressure and body temperature were continuously monitored and recorded every 10 minutes. Blood gases were checked for pH, pO2, pCO2, sodium, potassium, calcium, glucose and lactate every 20 minutes during CPB in 0.5 mL samples using a blood-gas analyzer (Siemens, Rapidlab 1265, Erlangen, Germany). A catheter was placed through the femoral vein into vena cava for drug infusion. The right femoral artery was exposed for arterial CPB cannulation and an anterolateral thoracotomy was performed in the third intercostal space. After administration of heparin (300 IU/kg), an arterial cannula (8Fr, Biomedicus) was advanced through the femoral artery into the abdominal aorta. A 28F venous cannula (Harvey, MA) was inserted into the right atrium. The heart was not opened during the procedure.
Bypass Management
A roller pump (Polystan, Vaerlose, Denmark) was used to generate nonpulsatile pump flow at 100 mL/kg body weight in all experiments. The oxygenator gas mixture consisted of 5% carbon dioxide and 95% oxygen in all CPB groups. The pH-stat management strategy was used in all CPB groups. The cardiopulmonary bypass circuit consisted of 1000-mL filtered hard-shell venous reservoir (1361 Minimax, Medtronic, Minneapolis, MN), a membrane oxygenator (3381 Minimax Plus, Medtronic), a 40-µm arterial filter (Terumo, Tokyo, Japan) and 1/4-inch tubing. Venous drainage was by gravity. No cardiotomy suction was used. The venous line was left open during circulatory arrest. A sterile circuit was used in each experiment. The CPB circuit was primed with 800 mL blood. The blood used for priming of the CPB circuit to achieve a hematocrit of 30% on CPB was drawn on the morning of the experiment from an adult donor piglet. Before the start of CPB and just before reperfusion methylprednisolone (30 mg/kg), 10 mL sodium bicarbonate 7.4%, and furosemide (0.25 mg/kg) were added to the prime. After 10 minutes of normothermic bypass, piglets in 15°C, 25°C HCA group and 25°C LF group underwent 40 minutes cooling to an esophageal temperature of 15°C and 25°C, respectively. Topical cooling was applied for hypothermic temperature groups. After a 60-minute period of HCA at 15°C, 25°C and 10 mL/kg/min low-flow CPB at 25°C, animals were rewarmed on CPB to 37°C for 50 minutes. In the 37°C FF group, esophageal temperature was kept at 37°C during CPB. Esophageal temperature in the control group was maintained at 37°C without CPB.
I. Postoperative Management for acute experiments
Following weaning from CPB, animals were observed for 120 minutes for acute experiments. Evans Blue Dye (EBD; 100mg/kg) was intravenously administered via the femoral vein at two hours after terminating CPB. The brain was washed by saline and fixed by 4% paraformaldehyde one hour injection following EBD injection and was then removed.
II. Postoperative Management for Survival Experiments
Animals remained sedated and paralyzed with a continuous infusion of fentanyl (50 mg·kg−1·h−1),midazolam (0.2 mg·kg−1·h−1), and pancuronium (0.2 mg·kg−1·h−1) and were mechanically ventilated with a gradually decreasing oxygen fraction for survival experiments. They were monitored continuously for 12 hours after CPB. The chest tubes were removed, and animals were weaned from ventilation and extubated. On postoperative day 4, all surviving piglets were sedated with intramuscular ketamine (20 mg/kg) and xylazine (4 mg/kg) and anesthetized with intravenous fentanyl (50 µg/kg). After a midline sternotomy, heparin (300 IU/kg) was administered, and a cannula was inserted into the bovine trunk. Three liters of Plasmalyte solution (Baxter, Deerfield, Ill) were infused through the bovine trunk. Blood was suctioned from the superior vena cava until the perfusate was clear of blood. Then 3 L of 10% formalin solution was perfused through the brain in the same manner to accomplish perfusion fixation. The entire head of the piglet was immersed in 10% formalin for a week, and the brain was harvested and fixed with 10% formalin solution for histologic assessment.15,16,17,18
Physiologic Parameters and Bypass-Related Variables during Experiments
Data regarding pH, partial oxygen pressure (PO2), partial carbon dioxide pressure (PCO2) and mean arterial pressure (MAP) were measured using a blood gas machine (Rapid lab 1246; Bayer, Germany) and pressure monitoring system.
I. Estimation of BBB Leakage after Cardiopulmonary Bypass
Macro Photography
Photographs of the entire surface of the brain were taken after removal of the brain from the skull. Evans Blue dye leakage was apparent as a blue area.
Quantification of Evans Blue Dye (EBD) in Brain Tissue and Plasma
Brains were divided into two hemispheres. The cortex and cerebellum were removed. The brain tissue was mechanically homogenized using formamide with a ratio of 1g tissue to 2mL formamide. The solution was warmed to 50°C for 72 hrs. The extract was ultracentrifuged (TLX; Beckman) at a speed of 60,000 rpm for 45 minutes at a temperature of 4°C to precipitate any proteins that had an absorbance at 620 nm. Absorbance of the supernatant at 620 nm was measured. The concentration of dye in the extract was calculated from a standard curve of EBD in formamide. The ratio of EBD absorbance in brain tissue to EBD absorbance in plasma was calculated as a measure of BBB breakdown.
Microscopic Analysis
Immunohistochemistry for identifying extravasation
Another hemisphere was frozen using 2-methyl butane with dry ice bath in order to detect extravasation of fluorescence albumin-binding EBD. Frozen tissues were sectioned on a cryostat at 20µm and sagittal sections of the following areas were mounted on slides: cortex, caudate nucleus, thalamus, hippocampus, and cerebellum. EBD was detected using a microscope with appropriate filter settings (EBD excitation 546 nm, emission 611 nm). For detection of blood vessel endothelia, slides were incubated with the isolectin, GS-IB4 from Griffonia simplicifolia conjugated to biotin (1:500; Molecular Probes), over night at 4°C after PBS rinse. Slides were then washed again in PBS before being incubated with an Alexa Fluor 488-conjugated streptavidin (1:500; Molecular Probes) for 60 minutes at room temperature. Images were made using Zeiss software (Axio Vision).
Pixel Intensity of EBD using Image Analysis
Images were made using Zeiss software (Axio Vision) and analyzed using ImageJ software (NIH). EBD intensity was analyzed in each slide of 5 random sections for each brain area (cortex, caudate nucleus, thalamus, hippocampus, and cerebellum).
Albumin Co-localized Study using Immunohistochemistry19
Albumin in brain tissue was studied by fluorescent albumin immunocytochemistry. Brain sections were washed in phosphate buffered saline (PBS) and then incubated with FITC-conjugated polyclonal rabbit anti -albumin (rabbit anti-albumin, 1:500, DakoCytomation, Glostrup, Denmark). After 24 h, the sections were washed in PBS (3 × 5 min). Following three additional washes in PBS, sections were coverslipped with mounting medium for fluorescence, containing 40,6-diamidino-2-phenylindole, which labels cell nuclei (Vectashield with DAPI, Vector Laboratories, Burlingame, CA, USA). Images were acquired using Zeiss software with Axio Vision system.
II. Histological Assessment for Survival experiments
Twenty-four areas, including the neocortex, hippocampus, dentate gyrus, caudate nucleus, thalamus, and cerebellum, were examined. Histologic damage was scored by using the following criteria: 5, cavitated lesions with necrosis; 4, significant damage to neurons; 3, large clusters of injured neurons; 2, small clusters of damaged neurons; 1, isolated neuronal damage; and 0, normal. Scores were summed to determine the total HS (range, 0–120). A single neuropathologist examined all specimens in a blinded fashion.
Statistical Analysis
Continuous variables are expressed as mean ± standard deviation (SD). Baseline and intraoperative data were compared for acute and survival experiments using one-way analysis of variance (ANOVA) with Bonferroni adjustment for post-hoc comparisons and two-sample Student t-test, respectively. Pixel intensity and albumin data were compared between experimental groups ANOVA with the Fisher least significant difference procedure. Since histologic damage scores did not follow a normal distribution, median scores for each brain region were compared between the 25°C, HCA and 25°C, LF groups by the nonparametric Mann-Whitney U-test. Statistical analysis was performed by SPSS 16.0 software (SPSS Inc, Chicago, IL). Two-tailed values of p < 0.05 were considered statistically significant.
Results
Physiologic Parameters and Bypass-Related Variables
Baseline and post operative variables were compared between the five experimental groups undergoing acute experiments and the two experimental groups undergoing survival experiments (Table 1). There were no significant group differences detected with respect to age, weight, pH, arterial PO2, arterial PCO2, hematocrit level, and MAP (all p > 0.20). Hematocrit levels between baseline and just after the end of CPB for each group were not significantly different within each group (p > 0.20).
Table 1.
Baseline Characteristics for the Experimental Groups
| Acute Experiments (5 per group) | Survival Experiments (8 per group) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | 37°C, OFF | 37°C, FF | 25°C, LF | 25°C, HCA | 15°C, HCA | p Value† | 25°C, LF | 25°C, HCA | p Value‡ |
| Age (d) | 23.6 ± 1.1 | 24.6 ± 1.5 | 23.0 ± 2.3 | 24.8 ± 1.5 | 26.0 ± 2.0 | 0.11 | 24.8 ± 3.5 | 28.0 ± 6.4 | 0.23 |
| Weight (kg) | 11.1 ± 0.7 | 11.7 ± 1.2 | 11.8 ± 1.6 | 11.5 ± 1.6 | 11.8 ± 1.4 | 0.90 | 10.3 ± 1.2 | 10.7 ± 1.6 | 0.55 |
| pH | 7.51 ± 0.04 | 7.51 ± 0.05 | 7.49 ± 0.04 | 7.50 ± 0.07 | 7.50 ± 0.03 | 0.98 | 7.52 ± 0.03 | 7.52 ± 0.03 | 0.80 |
| pO2 (mm Hg) | 81.0 ± 6.9 | 88.0 ± 1.2 | 84.6 ± 4.6 | 80.2 ± 7.8 | 83.4 ± 11.7 | 0.48 | 85.1 ± 6.8 | 83.1 ± 6.3 | 0.54 |
| pCO2 (mm Hg) | 40.3 ± 1.9 | 40.6 ± 2.2 | 40.5 ± 0.8 | 40.7 ± 1.6 | 39.9 ± 2.1 | 0.97 | 38.3 ± 3.5 | 38.6 ± 3.1 | 0.85 |
| Hematocrit (%) | 27.8 ± 1.9 | 26.4 ± 3.4 | 25.9 ± 2.5 | 26.8 ± 3.1 | 27.8 ± 2.1 | 0.75 | 28.7 ± 3.3 | 27.2 ± 1.9 | 0.29 |
| MAP (mm Hg) | 84.8 ± 10.4 | 77.6 ± 10.1 | 96.6 ± 14.0 | 79.2 ± 8.6 | 90.2 ± 17.1 | 0.13 | 68.9 ± 11.6 | 70.3 ± 15.3 | 0.84 |
Data are presented as means ± standard deviation. HCA, hypothermic circulatory arrest; FF, full flow; LF, low flow; MAP, mean arterial pressure.
Based on F-test in ANOVA, post-hoc comparisons indicated no significant group differences for any of the variables (all p>0.05).
Based on two-sample Student t-test with no group mean differences for any variable.
I. ACUTE EXPERINENTS
Macro photography of brain from acute experiments (Figure 1-a, b, c)
Figure 1.

Whole brain photographs of sagittal view for 25°C HCA, 15°C HCA and control groups. BBB disruption around watershed area at 25 °C HCA clearly apparent. The leakages of Evans Blue Dye (blue area on 25°C HCA) as a disruption tracer of BBB was not observed under other conditions: 15°C HCA, 25 °C LF, 37 °C FF and control.
Brain photographs of sagittal views for each group undergoing acute experiments clearly demonstrated BBB disruption around watershed areas in the 25 °C HCA group. Leakage of EBD was less obvious under other experimental conditions.
Quantification of Ratio of EBD Absorbance in Brain Tissue of Cortex and Cerebellum vs Plasma EBD (Figure 2)
Figure 2.
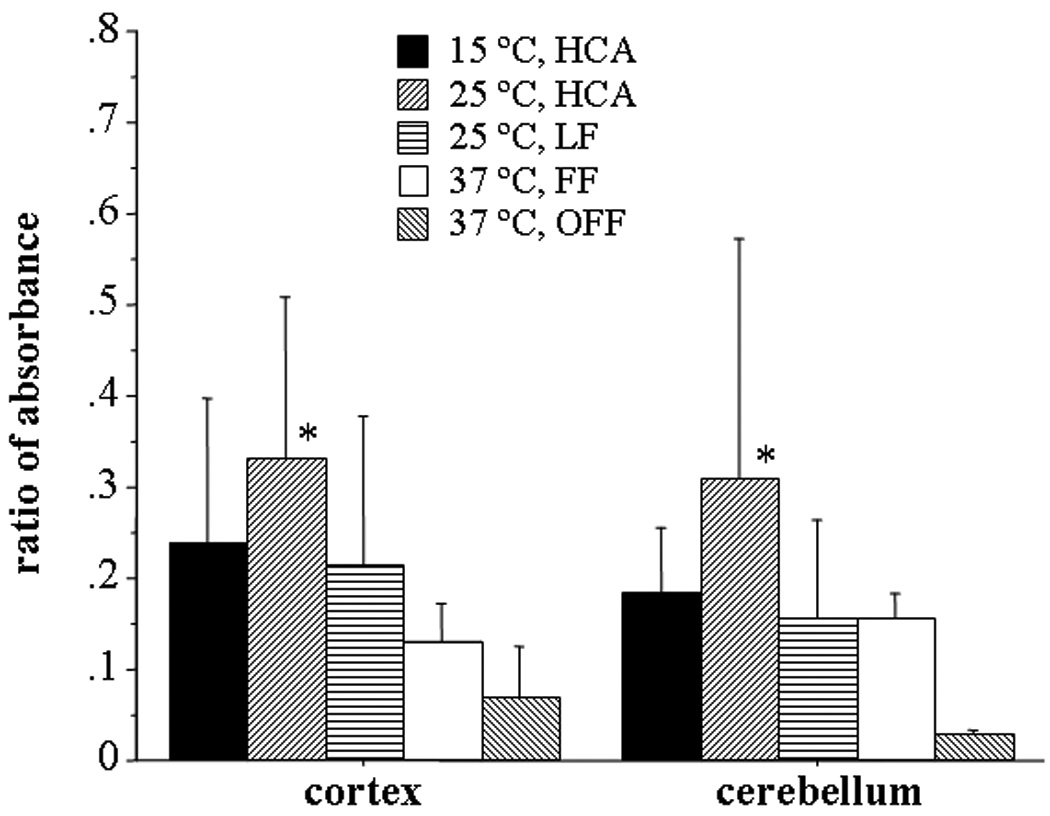
Ratio of absorbance of Evans blue dye in cortical and cerebellar tissue vs plasma EBD level three hours after end of bypass. The cortex/plasma ratio was significantly higher for 25°C, HCA than 37°C FF (p = 0.03) and 37 °C OFF (p = 0.005). Cerebellar tissue/plasma ratio was significantly higher for 25°C, HCA than 37 °C OFF (p = 0.005).
As tested by one-way ANOVA, the ratio of EBD in cortex vs plasma EBD at three hours after the end of bypass was significantly higher for 25°C, HCA versus 37°C FF (p = 0.03) and 37°C OFF (p = 0.005) and marginally significantly higher for 15°C HCA versus 37 °C OFF (p = 0.05). No differences were detected between: 37°C, OFF vs 37°C FF (p = 0.47) and vs. 25°C LF (p = 0.10), or between 37°C FF vs. 25°C LF (p = 0.34) or 15 °C HCA (p = 0.22), or between 25 °C LF vs. 25°C HCA (p = 0.18) or 15°C HCA (p = 0.77), and no significant difference between 25°C HCA vs. 15°C HCA (p = 0.28). For cerebellum to plasma ratio, one-way ANOVA indicated a significantly higher ratio only for 25°C HCA compared to 37 °C OFF (p = 0.003). There were no other significant differences detected, including 25°C HCA versus 37°C FF (p = 0.08), vs. 25°C LF (p = 0.08), and vs. 15°C HCA (p = 0.15). In addition, no differences were observed between 37°C FF vs. 25°C LF (p = 0.99) or 15°C HCA (p = 0.76), or between 25°C LF vs. 15°C HCA (p = 0.75), and no significant differences between 37°C compared to 37 °C FF (p = 0.14) vs. 25°C LF (p = 0.15), and 15°C HCA (p = 0.09).
Extravasation of EBD by Immunohistochemistry
In immunohistochemistry slides EBD was red, lectin on endothelial cells was green, and nuclei were blue. EBD was confirmed by immunohistochemistry to be present in extravasation areas. (Figure 3-a,b)
Figure 3.
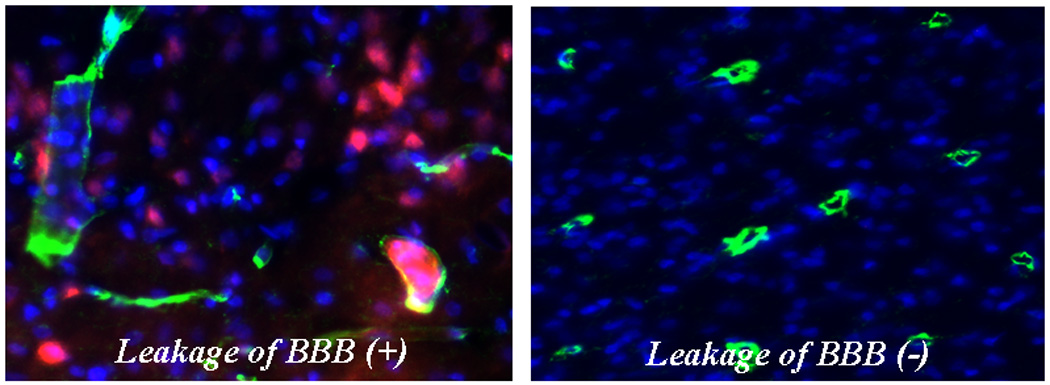
Immunohistochemistry in Brain tissue; Extravasation of Evans Blue dye (red) was observed in left photograph. The lectin (green) on endothelial cells was stained as a marker of vascularity. The nucleus (blue) was stained using 4',6'-diamidino-2-phenylindole hydrochloride (DAPI). Leakage of BBB was not found in right photograph.
Evaluating Pixel Intensity of EBD using Fluorescence Microscopy (Figure 4)
Figure 4.
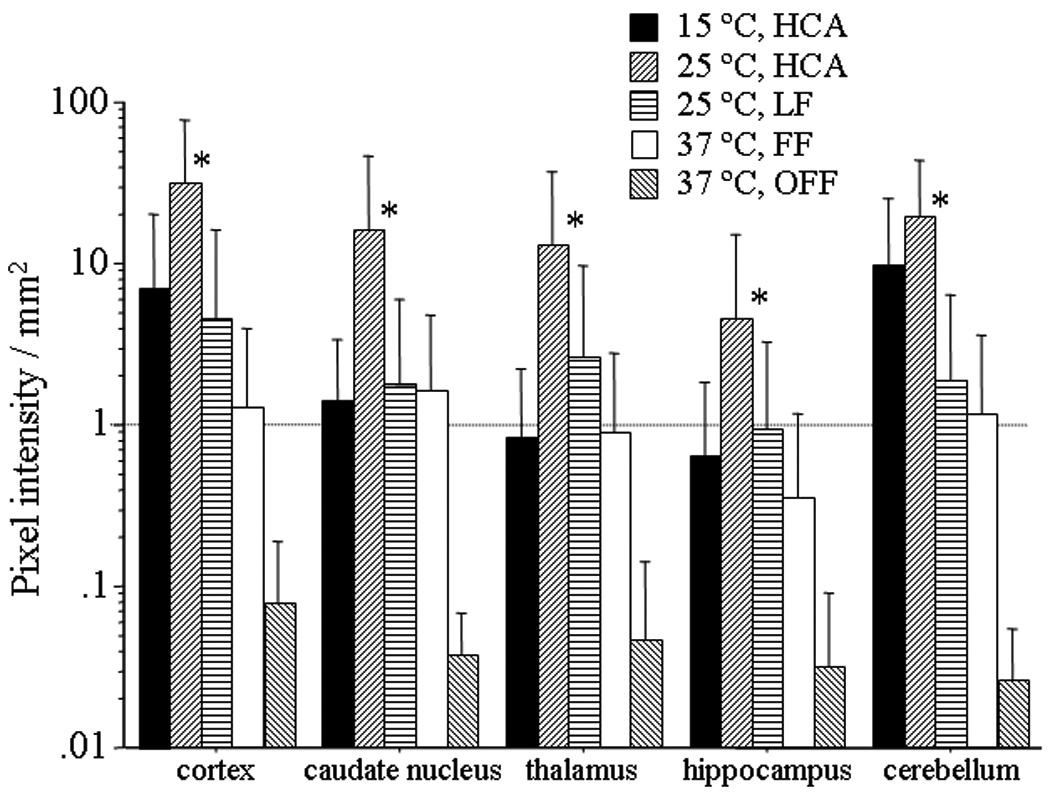
Blood brain barrier leakage for cortex, caudate nucleus, thalamus, hippocampus and cerebellum using Evans blue dye at three hours after cardiopulmonary bypass. Pixel intensity in 25°C, HCA group was significant higher than control for each brain area (*p<0.05 vs control; one-way ANOVA).
Blood brain barrier leakage was assessed for cortex, caudate nucleus, thalamus, hippocampus and cerebellum using Evans blue dye at three hours after end of cardiopulmonary bypass. The pixel intensity in the 25°C HCA group was significantly greater than 37°C OFF group for every brain area. (*p < 0.01; the nonparametric Mann-Whitney U-test), The pixel intensity in the 25°C HCA group was significantly higher than in the 25 °C LF in the cortex and cerebellum. (*p <0.01; the nonparametric Mann-Whitney U-test) Even simple full-flow bypass at 37°C was associated with greater pixel intensity than control in the cortex, thalamus and hippocampus (*p<0.03).
Albumin co-localization study using immunohistochemistry (Figure 5-a, b, c, d)
Figure 5.
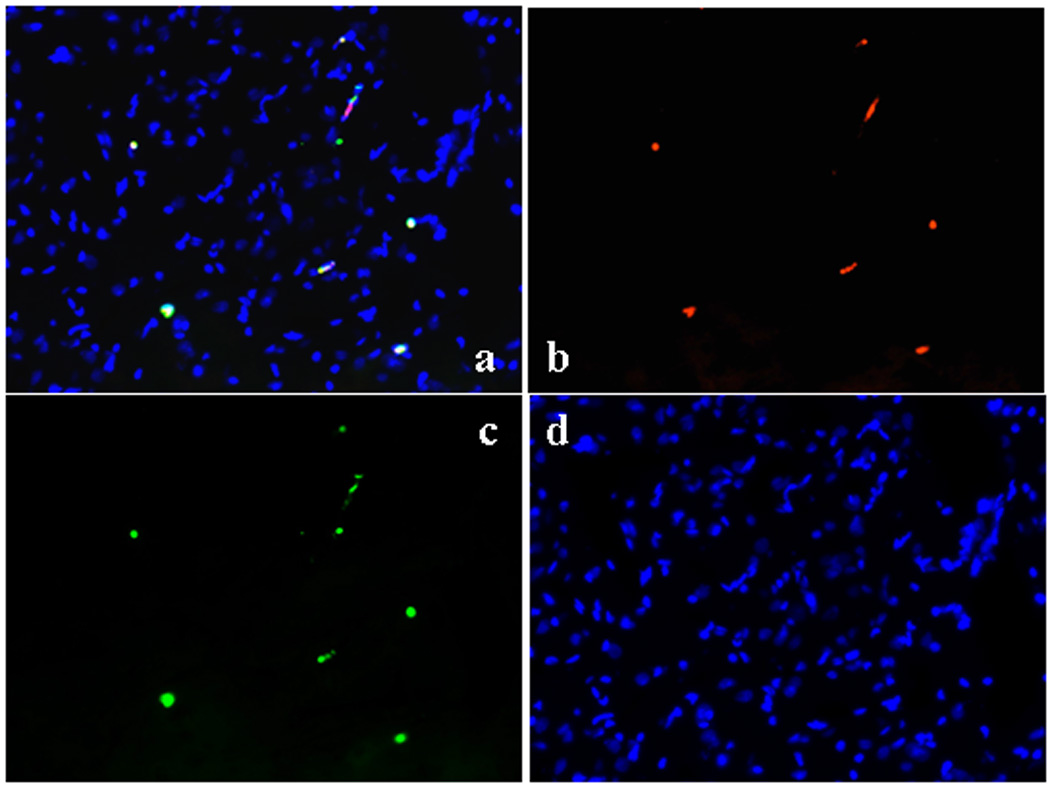
Immunohistochemistry of cortex to demonstrate colocalization of EBD and albumin; (a) Evans Blue dye shows red (b). Albumin is green with FITC-conjugated polyclonal rabbit anti –albumin. (c) Nuclei are blue using 4',6-diamidino-2-phenylindole. (d)
Evans blue dye bound exclusively with albumin in the blood stream. These images show that EBD is co-localized with albumin. EBD shows as red, albumin was green with FITC-conjugated polyclonal rabbit anti -albumin (rabbit anti-albumin, 1:500, DakoCytomation, Glostrup, Denmark), and nuclei were blue with 4',6-diamidino-2-phenylindole, which labels cell nuclei (Vectashield with DAPI, Vector Laboratories, Burlingame, CA, USA). Each picture shows similar conditions for all groups including control.
II. SURVIVAL EXPERIMENTS
Histological assessments
Histologic damage was found predominantly in the watershed areas of the cortex, caudate nucleus, and cerebellum. Nonparametric testing indicated significantly greater damage with significantly higher median histologic scores in the 25°C, HCA group compared to 25°C, LF with respect to the cortex (p = 0.038), caudate nucleus (p < 0.001), and cerebellum (p = 0.021), as well as the total score summed across all five regions (p < 0.001) (Table 2).
Table 2.
Intraoperative Data for the Experimental Groups
| Acute Experiments (5 per group) | Survival Experiments (8 per group) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | 37°C, OFF | 37°C, FF | 25°C, LF | 25°C, HCA | 15°C, HCA | Overall p Value† |
25°C, LF | 25°C, HCA | Overall p Value‡ |
| pH | 7.53 ± 0.03 | 7.52 ± 0.06 | 7.56 ± 0.02 | 7.56 ± 0.05 | 7.57 ± 0.04 | 0.35 | 7.55 ± 0.07 | 7.51 ± 0.05 | 0.17 |
| pO2 (mm Hg) | 80 ± 7 | 324 ± 80 | 357 ± 109 | 317 ± 174 | 414 ± 122 | <0.01* | 486 ± 96 | 294 ± 198 | 0.03* |
| pCO2 (mm Hg) | 37.1 ± 2.3 | 42.7 ± 6.7 | 40.3 ± 4.5 | 34.7 ± 3.3 | 35.8 ± 3.5 | 0.06 | 37.3 ± 5.9 | 39.0 ± 5.5 | 0.55 |
| Hematocrit (%) | 22.0 ± 1.6 | 28.6 ± 2.1 | 27.9 ± 1.5 | 29.6 ± 3.2 | 27.9 ± 1.8 | <0.01* | 30.3 ± 2.6 | 30.5 ± 2.1 | 0.88 |
| MAP (mm Hg) | 52.2 ± 7.4 | 68.4 ± 11.7 | 74.0 ± 14.9 | 75.2 ± 13.4 | 87.0 ± 13.7 | <0.01* | 80.0 ± 12.4 | 79.3 ± 14.1 | 0.75 |
Data are presented as means ± standard deviation. HCA, hypothermic circulatory arrest; FF, full flow; LF, low flow; MAP, mean arterial pressure.
Statistically significant.
Based on F-test in ANOVA; post-hoc comparisons with Bonferroni adjustment indicated no significant group differences for pH or pCO2. For pO2 and hematocrit, 37°C, OFF was lower than each of the other groups (p < 0.01); for MAP, 37°C, OFF was lower than 15°C, HCA (p < 0.01).
Based on two-sample Student t-test.
Discussion
There are a few systematic research studies evaluating the effects of CPB on BBB. It remains controversial whether the BBB is disrupted by CPB.4, 5 Furthermore BBB damage in previous studies did not always involve neuronal damage. In the current study, we have examined the integrity of the BBB with various CPB techniques in a piglet model.
Evans Blue dye (EBD) is a traditional tracer used to detect changes in BBB permeability.14 Our data show increased permeability throughout watershed areas with a specific CPB condition. Moreover we found evidence of neuronal damage in the same watershed areas where there was evidence of severe leakage of EBD. However our histological assessment in Survival studies showed that disruption of BBB was not always associated with neuronal damage. However with 25°C LF and 25°C HCA we found both BBB permeability increase and neuronal histological damage both in acute and survival experiments. We believe these results suggest a close relationship between vascular permeability impairment and neuronal injury consistent with the hypothesis that injury to the BBB may be a mechanism of brain injury in addition to being a sign of injury.
Watershed-distribution strokes are seen more frequently in adult patients who suffer a stroke postcardiac surgery than in the general stroke population (over 40% versus 2% to 5%, respectively).20,21 In adults the mechanism underlying postcardiac surgery watershed stroke probably involves a combination of hypoperfusion and embolization,22 though the role of hypoperfusion has not been well described. Watershed strokes in the general population are usually secondary to global hypoperfusion, such as during cardiac arrest, or can be attributed to stenosis of the carotid artery or other major vessels, leading to local hypoperfusion.23 In cardiac surgery patients, global systemic hypoperfusion, caused by severe intraoperative hypotension, is known to be associated with poor outcomes.24,25
We chose a three hour interval after CPB for removing the brains to investigate the increase of BBB permeability. Previous papers have suggested that promotion of capillary leak due to systemic inflammation after CPB is more pronounced following such a period.26,27 Another paper reported that the BBB was disrupted at initiation of CPB.5 However it was unknown whether CPB for a very short time would be associated with neuronal damage in a survival model. We selected one hour of HCA because previous work performed in our laboratory indicated that at least this duration was required to observe neuronal damage.28 We felt it was appropriate that this model should include both an ischemic insult (HCA at 25°C) as well as the inflammatory effects of CPB.
In this study, EBD was injected into the central vein alone. We confirmed that albumin coexisted with EBD by the co-localization study. Anesthesia alone did not affect the BBB. Our study has specific limitations. The increase in BBB permeability was confirmed three hours following CPB at 25°C with one hour HCA. Given the single time point of assessment it is not possible to pinpoint precisely when disruption of the BBB actually started.
Modifications of our model are needed to better understand the mechanism of disruption of the BBB, timing of BBB permeability and its associated effects.
In summary, 25°C HCA causes brain damage and BBB dysfunction in infant piglets. BBB leakage seems to result from severe ischemia in the setting of CPB with HCA in the piglet. BBB leakage may contribute to the neuronal dysfunction observed after CPB. Anesthesia was not associated with BBB disruption. However even full-flow bypass can be associated with BBB disruption. Protection of the BBB may improve neurological outcome after cardiac surgery. These data will be useful for understanding the relationship between vascular permeability changes and neuronal damage in brain tissue,
Table 3.
Blood Brain Barrier Leakage Determined by Pixel Intensity of Evans Blue Dye Using Fluorescence Microscopy: Comparison between Study Groups*
| Brain Region | 37°C, OFF | 37°C, FF | 25°C, LF | 25°C, HCA | 15°C, HCA |
|---|---|---|---|---|---|
| Cortex | 0.07 (0.04–0.12) |
0.57 (0.34–2.52) |
0.57 (0.42–10.75) |
10.71 (3.03–69.68) |
3.89 (1.13–14.48) |
| Caudate | 0.04 (0.03–0.05) |
0.62 (0.12–3.61) |
0.79 (0.10–3.94) |
3.61 (0.86–38.07) |
1.12 (0.45–2.52) |
| Hippocampus | 0.03 (0.01–0.05) |
0.06 (0.03–0.86) |
0.11 (0.02–2.24) |
5.02 0.60–8.17) |
0.17 (0.00–1.54) |
| Thalamus | 0.02 (0.01–0.10) |
0.26 (0.15–2.01) |
0.54 (0.21–6.15) |
5.29 (0.20–29.92) |
0.59 (0.00–1.77) |
| Cerebellum | 0.03 (0.02–0.04) |
1.42 (0.27–1.94) |
0.35 (0.03–4.51) |
11.7 (5.43–37.26) |
1.21 (0.14–23.67) |
Data represent median pixel intensity with interquartile range shown in parentheses.
Analysis was performed between groups with the nonparametric Mann-Whitney U-test.
Cortex: each group was significantly greater than 37°C, OFF (all p<0.01). No other group differences were detected, except 25°C, LF vs. 25°C, HCA (p<0.01).
Caudate: 25°C, HCA and 15°C, HCA both greater than 37°C, OFF (both p<0.01); no other group differences were observed (all p>0.10).
Hippocampus: the only significant difference was between 25°C, HCA vs. 37°C, OFF (p<0.01); no other group differences were detected (p>0.10).
Thalamus: 37°C, FF (p=0.03), 25°C, LF (p=0.02), and 25°C, HCA (p=0.02) greater than 37°C, OFF; no other group differences were found (all p>0.20).
Cerebellum: 37°C, FF (p<0.01) and 25°C, HCA (p<0.01) were greater than 37°C, OFF. In addition, 25°C, HCA was significantly greater than 25°C, LF (p<0.01) but no other group differences (all p>0.20).
Table 4.
Histologic Damage Scores by Brain Region for 25°C LF and HCA Bypass Conditions
| Brain Region | 25°C, LF | 25°C, HCA | p Value |
|---|---|---|---|
| Cortex | 0 (0 – 0) | 2 (0 – 10) | 0.03* |
| Caudate Nucleus | 0 (0 – 0) | 3 (1 – 4) | <0.001* |
| Hippocampus | 0 (0 – 0) | 0 (0 – 1) | 0.44 |
| Thalamus | 0 (0 – 0) | 0 (0 – 0) | 0.99 |
| Cerebellum | 1 (0 – 2) | 2 (1 – 4) | 0.02* |
| Total Sum | 1 (0 – 2) | 9 (4 – 19) | <0.001* |
Data are median scores with ranges shown in parentheses; n = 8 animals per group.
Statistically significant. p values are based on the Mann-Whitney U-test since histologic scores do not follow a normal distribution. LF = low flow; HCA = hypothermic circulatory arrest.
Acknowledgments
Disclosure statements
This study was supported by grant RO1-HL060922 from the National Institutes of Health.
Footnotes
Presented at the STS 46th Annual Meeting, January 24–27, 2010, Broward County Convention Center, Fort Lauderdale, Florida
References
- 1.Davson H, Segal MB. Physiology of the CSF and Blood-Brain Barriers. Boca Raton, FL: CRC Press; 1996. pp. 46–87. [Google Scholar]
- 2.Moody D. The blood-brain barrier and blood-cerebral spinal fluid barrier. Semin Cardiothorac Vasc Anesth. 2006;10:128–131. doi: 10.1177/1089253206288992. [DOI] [PubMed] [Google Scholar]
- 3.Westaby S, Johnsson P, Parry A, Blomqvist S, Solem J, Alling C, et al. Serum S100 protein: a potential marker for cerebral events during cardiopulmonary bypass. Ann Thorac Surg. 1996;61:88–92. doi: 10.1016/0003-4975(95)00904-3. [DOI] [PubMed] [Google Scholar]
- 4.Gillinov AM, Davis EA, Curtis WE, et al. Cardiopulmonary bypass and the blood-brain barrier. An experimental study. J Thorac Cardiovasc Surg. 1992;104:1110–1115. [PubMed] [Google Scholar]
- 5.Cavaglia M, Seshadri SG, Marchand JE, Ochocki CL, Mee RB, Bokesch PM. Increased transcription factor expression and permeability of the blood brain barrier associated with cardiopulmonary bypass in lambs. Ann Thorac Surg. 2004;78:1418–1425. doi: 10.1016/j.athoracsur.2004.04.036. [DOI] [PubMed] [Google Scholar]
- 6.Rawson RA. The binding of T-1824 and structurally related diazo dyes by plasma proteins. Am J Physiol. 1943;138:708–717. [Google Scholar]
- 7.Allen TH, Dralpvats PD. Spectrophotometric measurement of traces of the dye T-1824 by extraction with cellophane from both blood serum and urine of normal dogs. Am J Physiol. 1948;154:27–38. doi: 10.1152/ajplegacy.1948.154.1.27. [DOI] [PubMed] [Google Scholar]
- 8.Steinwall O, Klatzo I. Selective vulnerability of the blood-brain barrier in chemically induced lesions. J Neuropathol Exp Neurol. 1966;25:542–559. doi: 10.1097/00005072-196610000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Bolton PB, Lefevre P, McDonald DM. Salmeterol reduces early- and late-phase plasma leakage and leukocyte adhesion in rat airways. Am J Respir Crit Care Med. 1997;155:1428–1435. doi: 10.1164/ajrccm.155.4.9105089. [DOI] [PubMed] [Google Scholar]
- 10.Brokaw JJ, McDonald DM. Neurally mediated increase in vascular permeability in the rat trachea: onset, duration, and tachyphylaxis. Exp Lung Res. 1988;14:757–767. doi: 10.3109/01902148809087842. [DOI] [PubMed] [Google Scholar]
- 11.Saria A, Lundberg JM. Evans blue fluorescence: quantitative and morphological evaluation of vascular permeability in animal tissues. J Neurosci Methods. 1983;8:41–49. doi: 10.1016/0165-0270(83)90050-x. [DOI] [PubMed] [Google Scholar]
- 12.Thurston G, Murphy TJ, Baluk P, Lindsey JR, McDonald DM. Angiogenesis in mice with chronic airway inflammation: straindependent differences. Am J Pathol. 1998;153:1099–1112. doi: 10.1016/S0002-9440(10)65654-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stock EL, Roth SI, Kim ED, Walsh MK, Thamman R. The effect of platelet-activating factor (PAF), histamine, and ethanol on vascular permeability of the guinea pig conjunctiva. Invest Ophthalmol Vis Sci. 1990;31:987–992. [PubMed] [Google Scholar]
- 14.Vaz R, Sarmento A, Borges N, Cruz C, Azevedo T. Experimental traumatic cerebral contusion: morphological study of brain microvessels and characterization of the oedema. Acta Neurochir (Wien) 1998;140:76–81. doi: 10.1007/s007010050061. [DOI] [PubMed] [Google Scholar]
- 15.Anttila V, Hagino I, Iwata Y, Mettler BA, Lidov HG, Zurakowski D, et al. Aprotinin improves cerebral protection: evidence from a survival porcine model. J Thorac Cardiovasc Surg. 2006;132:948–953. doi: 10.1016/j.jtcvs.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 16.Forbess JM, Ibla JC, Lidov HG, Cioffi MA, Hiramatsu T, Laussen P, et al. University of Wisconsin cerebroplegia in a piglet survival model of circulatory arrest. Ann Thorac Surg. 1995;60 suppl:S494–S500. doi: 10.1016/0003-4975(95)00876-4. [DOI] [PubMed] [Google Scholar]
- 17.Sakamoto T, Hatsuoka S, Stock UA, Duebener LF, Lidov HG, Holmes GL, et al. Prediction of safe duration of hypothermic circulatory arrest by near-infrared spectroscopy. J Thorac Cardiovasc Surg. 2001;122:339–350. doi: 10.1067/mtc.2001.115242. [DOI] [PubMed] [Google Scholar]
- 18.Hagino I, Anttila V, Zurakowski D, Duebener LF, Lidov HG, Jonas RA. Tissue oxygenation index is a useful monitor of histologic and neurologic outcome after cardiopulmonary bypass in piglets. J Thorac Cardiovasc Surg. 2005;130:384–392. doi: 10.1016/j.jtcvs.2005.02.058. [DOI] [PubMed] [Google Scholar]
- 19.Van Vliet EA, da Costa Araújo S, Redeker S, van Schaik R, Aronica E, Gorter JA. Blood-brain barrier leakage may lead to progression of temporal lobe epilepsy. Brain. 2007;130(Pt 2):521–534. doi: 10.1093/brain/awl318. [DOI] [PubMed] [Google Scholar]
- 20.Yamamoto Y, Georgiadis AL, Chang H-M, Caplan LR. Posterior cerebral artery territory infarcts in the New England medical center posterior circulation registry. Arch Neurol. 1999;56:824–832. doi: 10.1001/archneur.56.7.824. [DOI] [PubMed] [Google Scholar]
- 21.Rankin JM, Silbert PL, Yadava OP, Hankey GJ, Stewart-Wynne EG. Mechanism of stroke complicating cardiopulmonary bypass surgery. Aust N Z J Med. 1994;24:154–160. doi: 10.1111/j.1445-5994.1994.tb00551.x. [DOI] [PubMed] [Google Scholar]
- 22.Caplan LR, Hennerici M. Impaired clearance of emboli (washout) is an important link between hypoperfusion, embolism, and ischemic stroke. Arch Neurol. 1998;55:1475–1482. doi: 10.1001/archneur.55.11.1475. [DOI] [PubMed] [Google Scholar]
- 23.Ringelstein EB, Stogbauer F. Border zone infarcts. In: Bogousslavsky J, Caplan L, editors. Stroke syndromes. New York, NY: Cambridge University Press; 2001. pp. 564–582. [Google Scholar]
- 24.Tufo HM, Oltfeld AM, Shekelle R. Central nervous system dysfunction following open-heart surgery. JAMA. 1970;212:1333–1340. [PubMed] [Google Scholar]
- 25.Gardner TJ, Horneffer PJ, Manolio TA, et al. Stroke following coronary artery bypass grafting: A ten-year study. Ann Thorac Surg. 1985;40:574–581. doi: 10.1016/s0003-4975(10)60352-9. [DOI] [PubMed] [Google Scholar]
- 26.Gessler P, Pfenninger J, Pfammatter JP, Carrel T, Baenziger O, Dahinden C. Plasma levels of interleukin-8 and expression of interleukin-8 receptors on circulating neutrophils and monocytes after cardiopulmonary bypass in children. J Thorac Cardiovasc Surg. 2003;126:718–725. doi: 10.1016/s0022-5223(03)00685-8. [DOI] [PubMed] [Google Scholar]
- 27.Zhang S, Wang S, Li Q, et al. Capillary leak syndrome in children with C4A-deficiency undergoing cardiac surgery with cardiopulmonary bypass: a double-blind, randomised controlled study. Lancet. 2005;366(9485):556–562. doi: 10.1016/S0140-6736(05)67099-7. [DOI] [PubMed] [Google Scholar]
- 28.Iwata Y, Okamura T, Ishibashi N, Zurakowski D, Lidov HG, Jonas RA. Optimal dose of aprotinin for neuroprotection and renal function in a piglet survival model. J Thorac Cardiovasc Surg. 2009;137:1521–1529. doi: 10.1016/j.jtcvs.2008.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]


