Abstract
MEP1A, which encodes the α subunit of meprin metalloproteinases, is a susceptibility gene for inflammatory bowel disease (IBD), and decreased intestinal meprin-α expression is associated with enhanced IBD in humans. Mice lacking meprin α (α knockout, αKO) have more severe colitis induced by dextran sulfate sodium (DSS) than wild-type (WT) mice, indicating an anti-inflammatory role for meprin A. Previous studies and those herein indicate the meprin B has proinflammatory activities. Therefore, mice lacking both meprin A and B (dKO mice) were generated to determine how their combined absence alters the inflammatory response to DSS. Unchallenged dKO mice grow and reproduce normally and have no obvious abnormal phenotype, except for a slightly elevated plasma albumin in both males and females and a lower urine creatinine level in dKO males. Upon oral administration of 3.5% DSS, the dKO mice have more severe colitis than the WT and βKO mice but significantly less than the αKO mice. The dKO mice lose more weight and have elevated MPO and IL-6 activities in the colon compared with WT mice. Systemic inflammation, monitored by plasma nitric oxide levels, is absent in DSS-treated dKO mice, unlike WT mice. The severity of experimental IBD in dKO mice is intermediate between αKO and WT mice. The data indicate that the absence of meprin A aggravates chronic inflammation and the lack of meprin B affords some protection from injury. Manipulation of the expression of meprin gene products may have therapeutic potential.
Keywords: metalloproteinase, knockout mice, intestine
meprins are tissue-specific zinc metalloproteases, composed of two multidomain subunits α and β (36), which are abundantly expressed in mammalian epithelial cells of intestine, kidney, lung, and epidermis (5, 6, 10). In addition, they are found in human intestinal lamina propria leukocytes and mouse mesenteric lymph nodes in the presence as well as absence of intestinal inflammation (14, 29, 30). Meprin α and meprin β mRNA are also expressed in the mononuclear cell lineages in both human and mouse peripheral blood (37). The evolutionarily related meprin subunits are encoded on different chromosomes (α on human chromosome 6 and mouse chromosome 17; β on human and mouse chromosome 18) and share ∼40% amino acid identity (11, 18, 19, 25, 26).
The meprin subunits form disulfide-linked homo- or heteromeric dimers that constitute the functional unit of the enzyme (24). Although homomeric meprin A (consisting of dimers of the α subunit that form high-molecular-mass oligomers) is secreted into the extracellular space, heteromeric meprin A (dimers of disulfide-linked α and β subunits, that form tetramers) and meprin B (dimers of β subunits) are membrane-bound type 1 transmembrane proteins, bound through the β subunit (20, 36). The α and β subunits show distinct substrate specificities; meprin β has a preference for cleaving amino acids flanking negatively charged residues, whereas meprin α prefers small or hydrophobic residues (7). Nevertheless, both meprin A and meprin B can cleave a wide variety of proteins including extracellular matrix (ECM) proteins, such as laminin, fibrinogen, nidogen, and collagen IV and V, as well as various cytokines such as pro-IL-1β (1, 21, 22, 28). However, in line with the differences at their active sites, differences in substrate specificities have been documented. For example, bradykinin, regulated on activation, normal T-cell expressed, and presumably secreted (RANTES), macrophage inflammatory protein (MIP)-1, and monocyte chemotactic protein (MCP)-1 are substrates for homomeric meprin A and not meprin B, whereas gastrin 17, orcokinin, and pro-IL-18 are cleaved by meprin B and not homomeric meprin A (3, 7, 32).
Recently, several polymorphisms in the MEP1A gene have been found to be significantly associated with ulcerative colitis (UC) (4). Along with these studies, meprin α mRNA was found to be lower in the colon of patients with inflammatory bowel disease (IBD) compared with healthy controls. Furthermore, mice lacking meprin α (αKO) are more severely affected by dextran sulfate sodium (DSS)-induced colitis than WT mice (4). These observations document an association of decreased meprin α expression with intestinal inflammation in mice and humans and imply an anti-inflammatory role for meprin A in chronic intestinal disease. In contrast, initial experiments with meprin β knockout (βKO) mice indicated that mice of this genotype are less vulnerable to DSS-induced colitis than WT mice (9) and to inflammation induced by transitory renal ischemia (12). Because the αKO and βKO mice display distinctly different responses to DSS, we have undertaken an examination of the responses of mice lacking both meprin α and β (dKO) to this experimental model of IBD.
Although there is strong evidence indicating that meprin αKO mice develop more severe inflammation than WT controls in reaction to DSS-induced colitis, there are only data on older mice (7 mo old), indicating that meprin βKO mice are less vulnerable than WT in this model of experimental colitis [only body weight loss in older mice was reported for the βKO mice previously (9)]. Therefore, the reaction of younger meprin βKO mice (8 to 9 wk old) to DSS administration was investigated. In addition, dKO mice have not been available until recently; thus the general characteristics of these mice were determined and their vulnerability to oral administration of DSS was assessed. The aim of these studies was to determine whether the α and β subunits of meprins do indeed have opposing effects on the severity of inflammation in this model of experimental IBD and whether the meprin dKO results in a similar phenotype to the WT. Our studies of the four genotypes confirm that the αKO mice develop a more severe inflammatory response than WT mice and show that the βKO mice are less vulnerable to chronic inflammation in this model than WT mice and that the dKO mice develop a more severe inflammatory response than WT, but less severe than the αKO mice. Thus the results indicate that the balance of meprin isoforms affects the progression of IBD. The lack of the protective effect of meprin α is a dominant factor in the phenotype, but the lack of meprin β results in less severe inflammation.
MATERIALS AND METHODS
Mice.
All experiments were performed with 8- to 9-wk-old male WT and knockout (KO) littermates. The meprin αβKO (dKO) mice were generated by crossing meprin αKO (on a C57BL/6 × 129/Sv background) (4) mice and congenic meprin βKO (31) C57BL/6 mice. The single KO mice were generated by targeted disruption of the Mep1a gene on chromosome 17 or the Mep1b gene on chromosome 18. No mRNA or protein for meprin was expressed in tissues of the individual KO mice. The resulting heterozygous mice were mated to produce progeny lacking both meprin A and meprin B. The mice were housed under conventional (23°C, 12:12 h light-dark cycle) conditions and given free access to food and liquid. The experimental and control groups of each genotype were caged separately, with no more than five mice per cage. All animal protocols were approved by Institutional Animal Care and Use Committee.
Immunohistochemistry and Western blotting.
Tissues were fixed in methyl Carnoy's solution (60% methanol, 30% chloroform, and 10% acetic acid), dehydrated in ethanol, embedded in paraffin, and thin sectioned (5 μm), and sections were probed by use of anti-meprin α or β antibodies (31). Sections were counterstained with hematoxylin-eosin, rinsed in 100% ethanol, submerged in xylene, and mounted with Permount (Fisher). Digital photographs were taken with a Nikon Eclipse E600 microscope. Western blot analyses of intestinal tissue were performed as previously described (30).
Plasma and urine analyses.
Mice were anesthetized by isoflurane and blood collected by cardiac puncture into heparin-coated microcuvettes (Sarstedt). Blood cells were removed by centrifugation at 10,000 g for 10 min and plasma was used for analysis of blood urea nitrogen (BUN), total plasma protein, plasma albumin, plasma creatinine, and plasma Na+ and plasma K+ levels. Urine was collected from WT and KO mice by lower abdomen massage. Urine and plasma creatinine levels were measured by using the Jaffe reaction (Infinity Creatinine Assay, Sigma Diagnostics). Plasma albumin was determined using the bromocresol green method, Sigma Diagnostic albumin reagent. BUN was measured by using the BUN rate reagent, based on the Talke and Schubert method (Infinity Sigma Diagnostics).
Induction of experimental colitis by DSS.
The experimental groups were given 3.5% (wt/vol) DSS (molecular weight 44,000; TDB Consultancy, Uppsala, Sweden) in their drinking water for 4 days, followed by normal drinking water for 3 days. For recovery studies, a lower dosage of DSS was used: the experimental groups were given 2.5% DSS for 4 days and the study was carried on for 10 days. Administration of DSS leads to a UC-like colitis (8). The controls were given normal drinking water during the study period. Stool formation and rectal bleeding were monitored to calculate the disease activity index (DAI) scores (13). The mice were euthanized on different days by inhalation of isoflurane followed by cervical dislocation. The entire colon was removed by standard surgical procedures and measured for length before being dissected for sample preparation. Blood was collected by cardiac puncture and processed for further studies.
To calculate the DAI, equal weight was given to the scores for stool formation, rectal bleeding, and weight loss. Rectal bleeding was scored visually and by testing for blood on rectal swabs, by using hemoccult slides (Beckman Coulter). The scores were expressed on a scale of 1 to 4, with 0 indicating no disease and 4 corresponding to maximum disease activity.
In vivo permeability studies.
To measure intestinal barrier permeability, mice were gavaged with a permeability tracer, fluorescein isothiocyanate (FITC)-labeled dextran (60 mg/100 g body wt of FITC-labeled dextran, molecular weight 4,000; Sigma-Aldrich) as described (16). Food and water were withdrawn 4 h before the gavage. After 4 more h, blood was withdrawn by cardiac puncture. Fluorescence intensity of the serum samples was measured by using a Hitachi F-2000 spectrophotometer (excitation 490 nm; emission 525 nm). FITC-dextran concentrations were determined from a FITC-dextran standard curve.
Colon MPO assay.
Colon samples were longitudinally opened and washed gently with PBS to remove the fecal matter, weighed, and homogenized in 10 volumes of phosphate buffer (50 mM, pH 7.4) and centrifuged at 20,000 g for 20 min. The resulting sediment was suspended in an equivalent volume of phosphate buffer (50 mM, pH 6.0) containing 0.5% hexadecyltrimethyl-ammonium bromide and 10 mM EDTA, sonicated thrice for 5 s each, freeze-thawed thrice, and incubated at 4°C for 20 min. The samples were centrifuged at 4,000 g for 15 min, and 0.1 ml of the supernatant fluid was added to 2.9 ml of phosphate buffer (50 mM, pH 6.0) containing o-dianisidine dihydrochloride (0.167 mg/ml) and H2O2 (0.0005%), and absorbance was measured at 460 nm for 5 min. One unit of enzyme activity has been defined as the amount of MPO that degrades 1 mmol of H2O2 per minute at 25°C.
Cytokine and chemokine levels.
Colon cytokines (IL-1α, IL-1β, IL-3, IL-4, IL-5, IL-6, IL-9, IL-10, IL-12, MCP-1, IFN-γ, MIP-1α, granulocyte-macrophage colony-stimulating factor (GM-CSF), RANTES) in control and DSS-treated mice were measured by using mouse Inflammation Cytokine Array (Biolegend). Colons were homogenized in DMEM containing 10% FBS (0.1 gm/ml). Imaging of the array was performed using Biochemi EC3 imaging system (UVP). Intensities of the spots were analyzed using the software from Quansys Biosciences. Plasma IL-18 was measured using a murine ELISA system (MBL) that detected only the active form.
Plasma nitrite measurement.
Blood was collected by cardiac puncture, transferred to EDTA-coated microcuvette (Sarstedt), and centrifuged at 10,000 g for 10 min to obtain the plasma. The plasma, ultrafiltered with a 10,000 molecular weight cutoff Microcon (Millipore), was used to measure total nitrite levels by the Greiss assay (Cayman Chemicals).
Statistical analysis.
GraphPad PRISM was used for data analysis. Statistical analysis was done by Student's t-test and ANOVA followed by Bonferroni posttests; P values of <0.05 at a confidence interval of 95% were considered significant.
RESULTS
Meprin dKO mice are overtly normal.
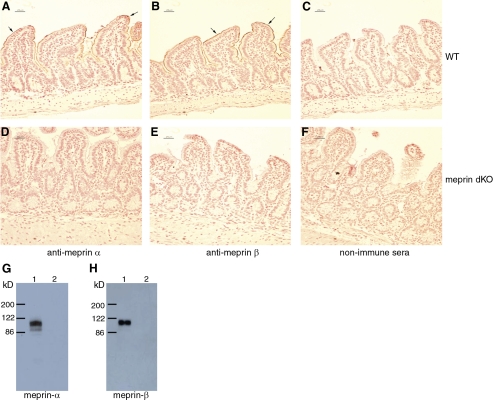
Immunohistochemical staining of intestinal tissue showed the presence of meprin α (Fig. 1A) and meprin β (Fig. 1B) in WT mice and the lack of meprin α (Fig. 1D) and meprin β (Fig. 1E) in the dKO mice. The absence of meprin α and of meprin β in the dKO mice was further confirmed by Western analyses (Fig. 1, G and H), in addition to both Southern analysis as well as PCR from tail biopsies (37).
Fig. 1.
Meprin α and meprin β proteins are absent in intestinal villi of mice lacking both meprin A and B (dKO mice). Immunohistochemical staining (arrows) with anti-meprin α (A and D) and anti-meprin β (B and E) antibody on wild-type (WT; A and B) and dKO (D and E) intestinal sections. The nonimmune sera did not show any positive staining (C and F). Scale markers denote 25 μm. Representative results of Western blots are shown for meprin α (G) and meprin β (H); lane 1 contains WT intestinal tissue and lane 2 contains dKO tissue.
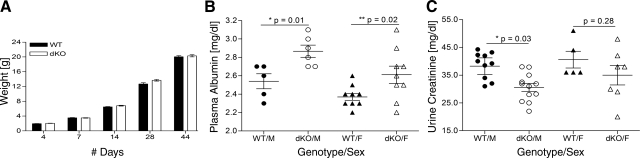
Absence of the meprin α and meprin β expression in the dKO mice did not alter survival and growth compared with the wild type (Fig. 2A). Litter sizes from meprin dKO (6.1 ± 0.4 pups/litter, 57 litters) and WT (6.4 ± 0.3 pups/litter, 55 litters) mating were comparable. The male-female ratios of both the genotypes were also similar. Conventional plasma and urine chemistries were determined for adult mice of both the sexes; values for dKO and WT mice were generally similar (Table 1). Plasma albumin values were consistently higher in the meprin dKO mice than the WT mice. Although the difference was significant in both males and females, it was more marked among the males (Fig. 2B). The urine creatinine values were similar between the female WT and meprin dKO mice; however, the male meprin dKO mice had significantly lower creatinine levels in the urine than the WT male mice (Fig. 2C).
Fig. 2.
WT and meprin dKO mice grow normally but differ in plasma albumin levels. A: the WT and dKO males showed comparable body weights at different stages of growth. B: plasma albumin levels were significantly elevated in both male (M) and female (F) dKO mice compared the to WT counterparts. *P = 0.01; **P = 0.02, n = 5–9 mice per group. C: urine creatinine levels were significantly lower in male dKO than WT, but there was no difference between the females of the 2 genotypes. *P = 0.03, n = 5–12 mice per group.
Table 1.
Plasma and urine chemistry for WT and dKO mice
| WT (αβ+/+) | dKO (αβ−/−) | P Value | |
|---|---|---|---|
| Plasma glucose, mg/dl | 248 ± 15.2 | 214. ± 15.2 | 0.16 |
| Plasma BUN, mg/dl | 20.8 ± 1.85 | 19.8 ± 0.05 | 0.62 |
| Plasma creatinine, mg/dl | 0.22 ± 0.03 | 0.22 ± 0.02 | 0.93 |
| Total plasma protein, mg/dl | 4.12 ± 0.15 | 4.08 ± 0.23 | 0.89 |
| Plasma Na+, mmol/l | 154 ± 1.47 | 150 ± 3.67 | 0.30 |
| Plasma K+, mmol/l | 6.04 ± 2.66 | 5.54 ± 0.45 | 0.37 |
| Total plasma bilirubin, mg/dl | 0.55 ± 0.08 | 0.48 ± 0.03 | 0.42 |
| Plasma albumin, mg/dl | 2.44 ± 0.04 | 2.7 ± 0.07 | 0.002 |
| Urine creatinine, mg/dl | 38.89 ± 2.02 | 32.55 ± 2.59 | 0.07 |
Values are means ± SD. Plasma and urine was collected from adult wild-type (WT) and double knockout (dKO) mice of both sexes (n = 5–14) and analyzed for the above parameters. BUN, blood urea nitrogen. All values, with the exception of albumin (shown in bold), were comparable between the 2 genotypes.
Meprin dKO mice show intermediate susceptibility to DSS-induced colitis whereas meprin βKO mice are less susceptible than WT mice.
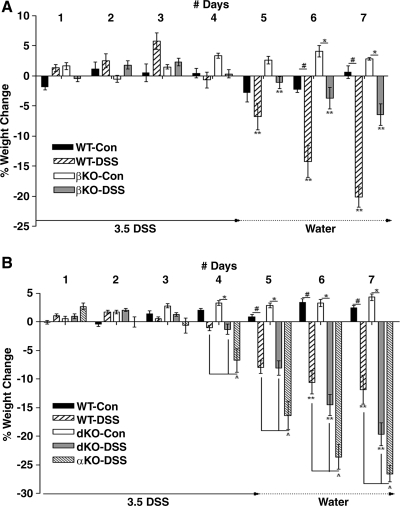
Colitis was induced in WT, meprin βKO mice, meprin αKO, and meprin dKO mice by oral administration of DSS (Fig. 3). The WT experimental group, by day 5, had lost over 5% of their starting weights, whereas the corresponding βKO mice only showed a slight weight loss (**P < 0.05). By the end of the study, DSS-treated WT mice showed significantly greater weight loss than the treated βKO mice (DSS-treated WT vs. βKO, **P < 0.001) (Fig. 3A). DSS-treated dKO mice weight loss on day 5 was comparable to the DSS-treated WT mice (Fig. 3B). On day 7, however, the meprin dKO mice had lost more weight than the corresponding WT group (**P < 0.001) (Fig. 3B). The DSS-treated meprin αKO mice were maximally affected by the DSS treatment; they began to lose weight by day 3 and lost significantly more weight than DSS-treated WT and meprin βKO or meprin dKO mice over days 4 to 7 (^P < 0.001).
Fig. 3.
Body weight loss of dKO mice after dextran sulfate sodium (DSS) administration was intermediate between αKO and WT or βKO mice. Body weight loss in WT, meprin αKO, meprin βKO, and meprin dKO mice was monitored over a 7-day period. DSS (3.5%) was administered in the drinking water for 4 days, then replaced with water. Controls (Con) received water only. All DSS groups showed significant weight loss by day 6. A: meprin βKO mice administered DSS group lost less weight at all time points than treated WT mice (**P < 0.05) (n = 7 mice per group; WT DSS-treated vs. control, #P < 0.001; βKO DSS-treated vs. control, *P < 0.05). B: the meprin dKO DSS group lost a greater percent of body weight than the WT DSS group (**P < 0.05) but less compared with meprin αKO DSS group (^P < 0.01) (n = 17 mice per group; WT DSS-treated vs. control, #P < 0.001; dKO DSS-treated vs. control, *P < 0.01).
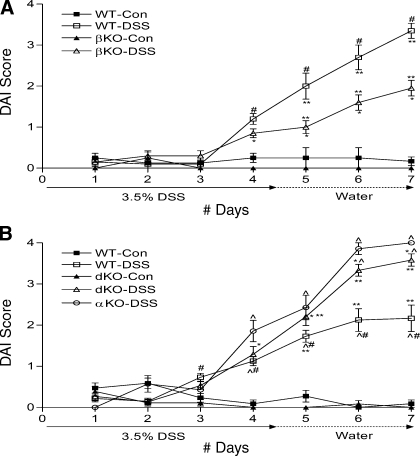
DAI scores were calculated giving equal importance to body weight loss, stool formation and rectal bleeding (Fig. 4). All the DSS-treated groups had significantly higher DAI scores than their control groups by day 4. Although the DSS-treated βKO mice had significantly lower DAI scores than WT mice day 5 onward (**P < 0.001) (Fig. 4A), the DSS-treated meprin dKO mice had significantly higher scores than WT mice from day 5 (**P < 0.05) (Fig. 4B). The DAI scores for the DSS-treated meprin αKO mice were significantly higher than the DSS-treated WT mice day 4 onward (^P < 0.05) (Fig. 4B). When the DSS-treated meprin αKO mice were compared with the DSS-treated meprin dKO mice, significant differences were observed on days 6 and 7 (^P < 0.05) (Fig. 4B). When percentage of weight loss and DAI scores of meprin αKO and dKO mice were compared, there was a consistent mild attenuation in the meprin dKO (Figs. 3B and 4B). This indicates that the presence of the meprin β subunit, which is expressed in αKO mice but not dKO mice, contributes to the pathology associated with DSS-induced colitis.
Fig. 4.
Disease activity indexes (DAI) for DSS-treated βKO mice were less severe than for WT mice, whereas those of αKO and dKO mice were more severe than for WT. The DAI score was the summation of the extent of intestinal bleeding, consistency of the fecal pellet, and weight loss. A: DAI scores for the WT and meprin βKO DSS groups significantly increased from day 4 onward (n = 7 mice per group; WT DSS-treated vs. control, #P < 0.01; βKO DSS-treated vs. control, *P < 0.05). DSS-treated meprin βKO mice had significantly lower DAIs than the corresponding WT mice over days 5 to 7 (**P < 0.001). B: the DAI for the WT and meprin dKO DSS group significantly increased from day 4 onward (n = 17 mice per group; WT DSS-treated vs. control, #P < 0.05; dKO DSS-treated vs. control, *P < 0.001). DSS-treated dKO mice had higher DAI scores than the corresponding WT mice on days 6 and 7 (**P < 0.05). DAI score for αKO mice treated with DSS was significantly higher than WT from day 4 onward and dKO mice on days 6 and 7 (^P < 0.05).
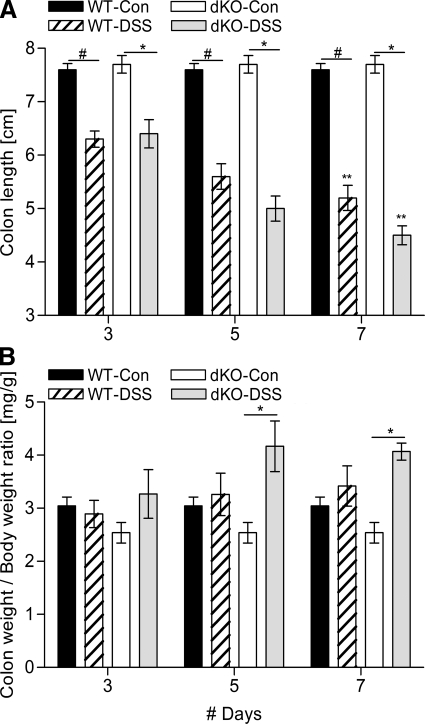
Colon lengths and weights in meprin dKO and WT mice were also altered in this experimental model of IBD elicited by DSS-induced colitis (Fig. 5). Both the DSS-treated groups showed progressive shortening of colon. Comparing the two DSS-treated groups, by day 7 the meprin dKO mice had significantly greater colon shortening compared with the WT (**P < 0.05) (Fig. 5A). The colon-to-body weight ratios of the control WT and meprin dKO mice were similar throughout the course of study. Although the colons shortened markedly with DSS exposure, the weights of the colons were relatively constant, indicating that the change in length primarily reflected contraction or fibrosis rather than loss of mass. The weight of DSS-treated WT and meprin dKO colons did not change noticeably during the 7 days, but the ratio of weight of control to treated meprin dKO colon was statistically significant at days 5 and 7 (Fig. 5B). Taken together, the data indicate that the meprin dKO mice are more affected by DSS treatment than WT mice.
Fig. 5.
Meprin dKO mice exhibit greater colon edema after DSS administration than WT mice. A: colon shortening was evident in the DSS-treated mice of both the genotypes (n = 6 mice per group; WT DSS-treated vs. control, #P < 0.001; dKO DSS-treated vs. control, *P < 0.001). On day 7, DSS-treated dKO mice had significantly shorter colons than the WT mice (**P < 0.05). B: colon weights, normalized to initial body weights, were measured as an indication of edema after DSS induction. The DSS-treated WT mice had slight increase in their colon weights, but it did not reach significance. The dKO mice had significantly higher edematous increase, compared with their control populations on days 5 and 7 (n = 6 per group; dKO DSS-treated vs. control, *P < 0.001).
Colon inflammation in dKO mice was greater than in WT mice.
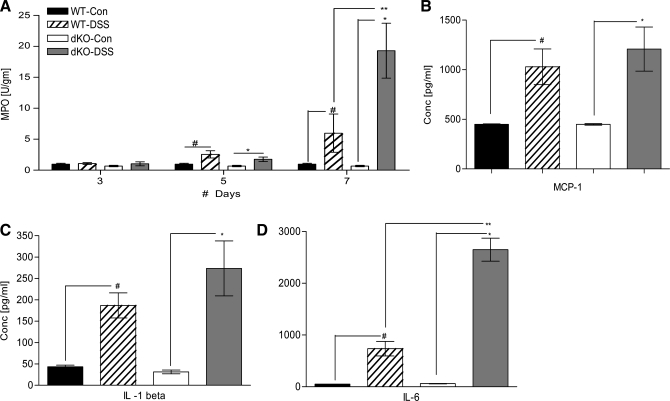
Colon leukocytic infiltration and inflammation were investigated by measuring MPO activity and cytokine concentrations (Fig. 6). On day 3, the colons of both the control groups had negligible MPO activity and no elevation of MPO activity in the DSS-treated groups was evident on day 3. By day 5, both the DSS-treated groups had significantly higher MPO activities than their corresponding control populations (n = 6 to 12 per group, WT DSS-treated vs. control, #P < 0.05, dKO DSS-treated vs. control, *P < 0.05). By day 7, however, compared with DSS-treated WT mice, the DSS-treated meprin dKO mice had a greater infiltration of neutrophils as evidenced by a significant elevation in their MPO activity (DSS-treated, WT vs. dKO, **P < 0.001) (Fig. 6A). This was consistent with the fact that meprin dKO mice had significantly higher DAI scores during the later phase of colitis induction.
Fig. 6.
DSS-induced colitis causes greater inflammation in colons of dKO mice than in WT mice. A: inflammation and leukocytic infiltration was measured by MPO activity in the colons of the WT and dKO mice treated with or without DSS. The MPO activity in DSS-treated dKO mice was significantly higher than that in their WT counterparts on day 7 (**P < 0.001). Colon cytokine and chemokine levels of WT and dKO mice treated with or without DSS were measured on day 5 (n = 6 per group). B: MCP-1 levels showed 2-fold elevation upon DSS treatment in both WT and dKO mice. C: IL-1β showed 4- to 8-fold elevation in DSS-treated WT mice and dKO mice. D: IL-6 levels were greatly elevated upon DSS treatment in both WT and dKO mice, to significantly higher levels in the latter (**P < 0.01), (#P < 0.05; *P < 0.05).
The local inflammatory environment was further characterized by measuring a panel of 14 cytokines in the WT and meprin dKO colons on day 5 (Table 2). Seven of the 14 cytokines were significantly elevated in DSS-treated mice of both the genotypes compared with their water-treated controls (n = 10 per group, WT DSS-treated vs. control, #P < 0.05, dKO DSS-treated vs. control, *P < 0.05). Cytokines and chemokines such as IL-1α, IL-10, MCP-1, and RANTES showed similar levels of increase in both the DSS-treated groups (Table 2). For example, MCP-1 increased twofold in both the genotypes (Fig. 6B). Although IL-1β levels in the WT mice increased fourfold upon DSS treatment (#P < 0.05), the fold increase in meprin dKO mice was eightfold (*P < 0.05) (Fig. 6C). IL-6 levels between the two DSS-treated groups were significantly different (**P < 0.01). Compared with their water-treated controls, IL-6 was nearly 14-fold elevated in the DSS-treated WT colons (#P < 0.05), as opposed to a 40-fold increase in DSS-treated meprin dKO colons (*P < 0.05) (Fig. 6D). These data demonstrated that marked differences in IL-6 values had developed by day 5 in the inflammatory environment between the two genotypes.
Table 2.
Colon cytokines are elevated upon DSS treatment
| WT |
dKO |
||||||
|---|---|---|---|---|---|---|---|
| Cytokine, pg/ml | Con (n = 5) | DSS (n = 10) | P value | Con (n = 5) | DSS (n = 10) | P value | P Value (DSS; WT vs. dKO) |
| IL-1α | 164 ± 5.4 | 1,165 ± 213 | * | 157 ± 0.01 | 1,472 ± 259 | * | ns |
| IL-1β | 43.3 ± 3.9 | 186.9 ± 29.3 | * | 32.1 ± 3.8 | 273.5 ± 64.3 | * | ns |
| IL-3 | 12.6 ± 6.1 | 5.6 ± 2.3 | ns | 6.56 ± 0.01 | 65.3 ± 27 | ns | ns |
| IL-4 | 103 ± 12.2 | 154 ± 20.0 | ns | 103 ± 7.5 | 154 ± 18.2 | ns | ns |
| IL-5 | 46.4 ± 5.7 | 62.0 ± 3.0 | * | 39.9 ± 4.1 | 65.8 ± 3.5 | † | ns |
| IL-6 | 52.0 ± 0.1 | 735.8 ± 139.8 | * | 60.4 ± 4.2 | 2,649.1 ± 611.4 | † | * |
| IL-9 | 2,584 ± 245 | 3,730 ± 221 | ns | 2,678 ± 178 | 7,105 ± 1,265 | ns | ns |
| IL-10 | 8.56 ± 0.6 | 139.7 ± 6.2 | ‡ | 102.5 ± 14.6 | 172.2 ± 12.9 | * | ns |
| IL-12 | 17.6 ± 1.8 | 88.0 ± 16.1 | * | 32.8 ± 7.9 | 55.6 ± 4.7 | ns | ns |
| GM-CSF | 38.2 ± 3.4 | 50.4 ± 4.8 | ns | 23.6 ± 7.0 | 65.3 ± 6.4 | † | ns |
| IFN-γ | 407 ± 138 | 664 ± 72 | ns | 358 ± 62 | 971 ± 141 | * | ns |
| MIP-1α | 5.8 ± 0.3 | 66.3 ± 32.0 | ns | 4.7 ± 0.3 | 236 ± 125 | ns | ns |
| MCP-1 | 444 ± 2.2 | 1,029 ± 179 | * | 445 ± 2.1 | 1,208 ± 222 | * | ns |
| RANTES | 8.7 ± 0.6 | 372 ± 56 | † | 6.6 ± 0.7 | 218 ± 34 | ns | ns |
Values are expressed as means ± SD; ns, not significant;
P < 0.05;
P < 0.01;
P < 0.001. Fourteen cytokines were tested in WT and dKO mice on day 5 (n = 5–10 per group). Cytokines from both the dextran sulfate sodium (DSS)-treated groups show significant elevation. Comparing the WT and dKO mice treated with DSS (last column), only the IL-6 level (shown in bold) is significantly higher in the dKO mice.
Systemic inflammation is less in dKO mice than in WT mice after colitis induction.
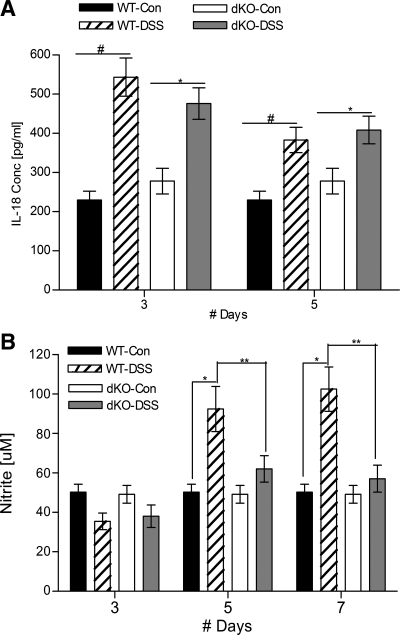
To characterize the inflammatory environment further, plasma IL-18 and nitrite levels were measured as indicators of systemic inflammation (Fig. 7). Earlier studies had shown that, compared with DSS-treated WT mice, plasma IL-18 levels were higher in the corresponding αKO mice and lower in βKO mice (3). This warranted a similar comparison in the dKO mice with their WT counterparts (Fig. 7A). Both the DSS-treated groups showed significantly higher levels of IL-18, compared with their controls; however, there was no difference between the two DSS-treated genotypes (Fig. 7A). WT mice treated with DSS had elevated nitric oxide values on days 5 and 7. By contrast, meprin dKO mice treated with DSS had no increase in nitric oxide levels at any time point, making this a noteworthy difference between the two genotypes (**P < 0.01) (Fig. 7B).
Fig. 7.
Plasma IL-18 and nitric oxide levels in meprin dKO vs. WT mice after DSS treatment. A: levels of active IL-18 are significantly elevated in both WT and dKO mice after DSS treatment (n = 5 per group; #P < 0.01, *P < 0.05), but comparable between the genotypes. B: nitric oxide levels were elevated in DSS-treated WT mice but not in treated dKO mice. DSS-treated WT mouse levels were elevated on days 5 and 7 (n = 12 per group, WT DSS-treated vs. control, #P < 0.001). The dKO mice did not show any nitric oxide level elevation even on day 7, a difference that was significant (**P < 0.01).
Lack of the meprin α subunit does not affect intestinal permeability or epithelial reconstitution.
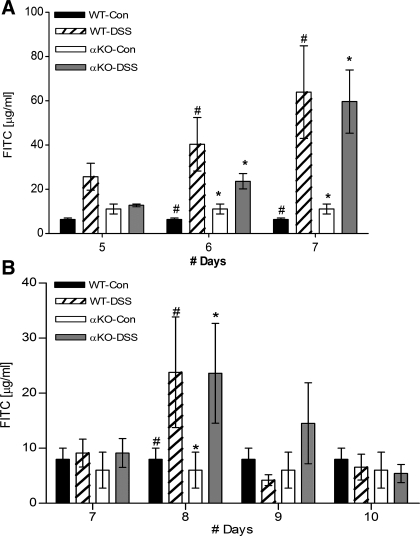
Because the data showed that mice lacking meprin α have the most severe inflammatory reaction in response to DSS treatments, the question arose as to whether the meprin α subunit affects the barrier function of the intestine. To address this question, intestinal permeability was monitored on days 5, 6, and 7 after DSS treatment. FITC-dextran was administered to the mice by oral gavage and FITC levels in the plasma were quantified after 4 h (Fig. 8A). Compared with the water-treated controls, the experimental IBD groups had increased FITC levels in their plasma, indicating compromised epithelium. However, there was no difference between the two DSS-treated genotypes, indicating that meprin α does not play a role in affecting intestinal permeability.
Fig. 8.
Meprin α does not affect intestinal permeability or reconstitution. WT and meprin αKO mice were given FITC-labeled dextran by oral gavage and FITC levels were determined in the plasma after 4 h, as an indicator of in vivo intestinal permeability. The water-treated mice (WT-Con, αKO-Con) as well as DSS-treated mice were monitored after administration of FITC-dextran. A: experimental inflammatory bowel disease groups (WT-DSS, αKO-DSS) were treated with 3.5% DSS for 4 days and permeability was measured from day 5 to 7. Compared with the control groups, both the DSS-treated groups had increased FITC levels in their plasma, indicating a leaky intestinal membrane (n = 6 per group; WT DSS-treated vs. control #P < 0.01; αKO DSS-treated vs. control *P < 0.05). There was no difference between the 2 DSS-treated genotypes. B: WT and meprin αKO mice were given 2.5% DSS for 4 days, and plasma FITC levels were measured from days 7 to 10. On day 8 both the experimental groups had significantly high FITC levels (n = 10 per group; WT DSS-treated vs. control #P < 0.05; αKO DSS-treated vs. control *P < 0.01). On days 9 and 10, there were no significant difference between the control and DSS-treated values.
To test whether epithelial reconstitution was affected in mice lacking the meprin α subunit, mice were administered a lower dose of DSS (2.5% instead of 3.5% DSS) to engender less intestinal damage, and permeability of the intestine to FITC-dextran was monitored from days 7 to 10 (Fig. 8B). The lower dosage of DSS caused intestinal damage sufficient to generate visible pathology, but at the same time was mild enough for the mice to recover following withdrawal of DSS. The DSS-treated WT mice lost weight steadily from days 4 to 7, after which a reversal was seen by day 9. The αKO mice also showed a similar trend, but the recovery process was slower and weight gain was seen only on day 10 (data not shown). When barrier function was tested, compared with the controls, both the DSS-treated groups had significantly higher FITC values on day 8. The FITC-dextran values for the DSS-treated WT and αKO mice returned to control levels by days 9 and 10, although there was a tendency for the WT mice to recover slightly faster. Overall, the permeability and reconstitution data do not support a role for meprin α in the protection or reconstitution of the membrane barrier after injury.
DISCUSSION
The studies herein demonstrate that mice lacking both meprin α and β proteins develop more severe inflammation than WT or meprin βKO mice, but less severe than the meprin αKO mice. Therefore, the absence of the meprin β subunit affords protection for both βKO mice and dKO mice. Although the KO mice react quite differently to the DSS challenge, with meprin βKO mice least vulnerable to the chronic inflammation and meprin αKO mice most susceptible, the dKO phenotype is more like the αKO, indicating that meprin A is the major meprin isoform that determines the response to chronic injury in this experimental model of IBD. Considering the data in terms of which meprin isoforms are present in the different genotypes, the studies indicate that meprin A protects and meprin B promotes intestinal injury.
One of the factors that likely underlies these observations is the effect of meprins on cytokine activation and degradation. Previous work has shown that meprin B (but not homomeric meprin A) can activate the proinflammatory cytokine IL-18, whereas both meprin A and B activate IL-1β (3, 21, 22). By contrast, homomeric meprin A (but not meprin B) can inactivate certain other cytokines (MCP-1, MIP-1, and RANTES) (31). In the αKO mice several colonic cytokines were elevated compared with the WT in response to DSS, including MCP-1, RANTES, IL-6 and IL-12, consistent with the ability of meprin A to inactivate some of these cytokines (4). In the dKO mice, however, only IL-6 was significantly elevated in the colon compared with the WT mice. IL-6 is a well-known myokine released by skeletal and smooth muscles as well as by immunocytes (33). Changes in intestinal smooth muscle after administration of DSS might contribute to the changes in the IL-6 levels. IL-6 is also known to be involved in the pathogenesis of IBD as well as in promoting inflammation and neutrophil influx (2, 15), thus setting the stage for exacerbation of the localized damage that is evident at later stages of disease, as seen by heightened MPO activity at the molecular level, and greater weight loss and bleeding at the macroscopic level. Thus the balance of meprin α and β contributes to the colonic cytokine profile; the decrease in proinflammatory cytokines in mice lacking meprin β (βKO and dKO mice) could contribute to the decreased inflammation. Indeed systemic inflammation, as indicated by plasma nitrite levels, was not apparent in the dKO in contrast WT mice, whereas in the αKO mice this marker indicated greater inflammation (4). The lack of a systemic nitric oxide response in dKO mice indicates that the inflammatory process is not disseminating to the same extent as in WT mice and remains localized, perhaps because of less damage (or the lack of an exaggerated cytokine gradient) in the DSS-treated dKO mice. Of note, previous studies showed that βKO mice also have decreased inflammation and decreased production of proinflammatory cytokines such as IL-6 compared with WT mice in response to kidney ischemia reperfusion, a model of acute renal injury (12). Thus two different experimental models of inflammation show meprin β to have proinflammatory activities.
The opposing effects of meprin A and B on the plasma levels of IL-18 was demonstrated previously (3). The early elevation of active IL-18 is prognostic for the severity of the colonic inflammation in humans as well as in mice (34, 35). Meprin B produces a biologically active form of IL-18 from pro-IL-18 that has a different NH2-terminus than caspase-1-activated IL-18. The latter protease acts intracellularly to activate this cytokine, whereas meprin B acts extracellularly under most conditions. In the DSS experimental model of IBD, there is a significant decrease in the plasma levels of IL-18 in βKO mice compared with WT mice (presumably due to a lack of meprin B), whereas there is a significant increase in the plasma levels of the cytokine in the αKO mice compared with WT mice, possibly due to the action of meprin B unopposed by meprin A. In the work herein it was found that the dKO mice have similar levels of plasma IL-18 to the WT mice, supporting the contention that the balance of the meprin subunits is an important factor in determining cytokine levels and inflammation.
This is the first general characterization of the meprin dKO mouse. The dKO mice appeared normal with respect to growth rate, litter size, gender distribution, and a panel of plasma and urinary markers except for urine creatinine and plasma albumin. Urine creatinine was lower only in male mice whereas plasma albumin was elevated in both male and female mice, perhaps indicating a genetically determined difference in the filtration or reabsorption of tissue fluids and blood components (27). There is also evidence that leukocytes behave differently in dKO mice compared with WT mice (37). Monocytes and natural killer cells have been shown to express meprins, and their movement from bone marrow to blood appears to be affected by meprin expression. These observations can be attributed to the ability of meprins to degrade ECM proteins.
All isoforms of meprin degrade extracellular matrix proteins, and it has been shown that leukocytes from meprin βKO mice have impaired capability to move through matrix and Matrigel (14, 36). However, when intestinal leukocytes (CD45+ cells) were examined on day 7 in the DSS model of IBD in the ileum, proximal colon, and distal colon and quantified after immunohistochemical staining of fixed tissue, there was no statistical difference between the meprin genotypes (data not shown). Nonetheless, the lack of systemic inflammation that is evident in the dKO mice upon colitis induction might arise as a result of defective leukocyte movement between the different hematological compartments in response to the intestinal challenge, thereby resulting in greater susceptibility during the progressive phase of the disease. It has also been demonstrated that meprins alter the integrity of epithelial membrane barriers (38). Meprin B cleaves E-cadherin in the adherens junction and disrupts the integrity of the ability of confluent MDCK cells to exclude dextran (23). The ability of meprins to damage the integrity of epithelial barriers may also be important for the infiltration of monocytes into injured tissue.
Meprins are members of a superfamily of metalloproteinases called “metzincins” that includes matrix metalloproteinases (MMPs) (36). A similar balance between different MMPs has been shown to modulate the severity of colitis as we have proposed for meprins. For example, although MMP-2 and MMP-9 show opposing effects to intestinal challenge, the proinflammatory role of MMP-9 overrides the regenerative function of MMP-2 (17). The meprins are somewhat more complex in that the two meprin subunits α and β combine as homo- and heterooligomers to give rise to the different functional meprin isoforms that have overlapping but distinct proteolytic profiles, which contribute to a counterpoised system for maintaining homeostasis. In addition, because meprin β is transmembrane protein whereas meprin α is an extracellular protein, meprin B and heteromeric meprin A are membrane-bound enzymes and homomeric meprin A is secreted. Thus mice deficient in one meprin subunit will have altered distribution of all three isoforms. Our studies with the meprin dKO mice show that although the lack of protective meprin A dominates the phenotype, an added deficiency of meprin B results in significant abatement in inflammation and injury. Previous studies of DSS-induced IBD in WT mice indicated that meprin α mRNA is downregulated in intestinal lymph nodes, whereas meprin β mRNA is unchanged by the inflammatory status (14). In addition, there was no evidence that meprin β protein decreased in epithelial cells in inflammation. The present studies imply that inhibiting meprin β would attenuate inflammation. Because patients with active colitis downregulate meprin α expression, agents that inhibit meprin β activity may have therapeutic potential.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases grant NIH DK19691 to J. S. Bond, a Broad Medical Foundation grant to J. M. Crisman, and the Carlino Gift Fund from the Penn State College of Medicine to G. L. Matters.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
Present affiliations: S. Banerjee, Institute for Genetics, University of Cologne, Cologne, Germany; R. D. Gailey, Diagnostic, Malvern, PA; J. M. Crisman, Jamestown Community College, State University of New York, Jamestown, NY.
REFERENCES
- 1. Ambort D, Stalder D, Lottaz D, Huguenin M, Oneda B, Heller M, Sterchi EE. A novel 2D-based approach to the discovery of candidate substrates for the metalloendopeptidase meprin. FEBS J 275: 4490–4509, 2008 [DOI] [PubMed] [Google Scholar]
- 2. Atreya R, Neurath MF. Involvement of IL-6 in the pathogenesis of inflammatory bowel disease and colon cancer. Clin Rev Allergy Immunol 28: 187–196, 2005 [DOI] [PubMed] [Google Scholar]
- 3. Banerjee S, Bond JS. Prointerleukin-18 is activated by meprin beta in vitro and in vivo in intestinal inflammation. J Biol Chem 283: 31371–31377, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Banerjee S, Oneda B, Yap LM, Jewell DP, Matters GL, Fitzpatrick LR, Seibold F, Sterchi EE, Ahmad T, Lottaz D, Bond JS. MEP1A allele for meprin A metalloprotease is a susceptibility gene for inflammatory bowel disease. Mucosal Immunol 2: 220–231, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Becker-Pauly C, Howel M, Walker T, Vlad A, Aufenvenne K, Oji V, Lottaz D, Sterchi EE, Debela M, Magdolen V, Traupe H, Stocker W. The alpha and beta subunits of the metalloprotease meprin are expressed in separate layers of human epidermis, revealing different functions in keratinocyte proliferation and differentiation. J Invest Dermatol 127: 1115–1125, 2007 [DOI] [PubMed] [Google Scholar]
- 6. Bergin DA, Greene CM, Sterchi EE, Kenna C, Geraghty P, Taggart CC, O'Neill SJ, McElvaney NG. Activation of EGFR by a novel metalloprotease pathway. J Biol Chem 283: 31736–31744, 2008 [DOI] [PubMed] [Google Scholar]
- 7. Bertenshaw GP, Turk BE, Hubbard SJ, Matters GL, Bylander JE, Crisman JM, Cantley LC, Bond JS. Marked differences between metalloproteases meprin A and B in substrate and peptide bond specificity. J Biol Chem 276: 13248–13255, 2001 [DOI] [PubMed] [Google Scholar]
- 8. Boismenu R, Chen Y. Insights from mouse models of colitis. J Leukoc Biol 67: 267–278, 2000 [DOI] [PubMed] [Google Scholar]
- 9. Bond JS, Gailey R, Bradley G. Meprin metalloproteases in intestinal disease. In: The Brush Border Membrane; From Molecular Cell Biology to Clinical Pathology (1st ed.), edited by Naim HY, Zimmer KP. Heilbronn, Germany: SPS Publications, 2006, p. 122–135. [Google Scholar]
- 10. Bond JS, Matters GL, Banerjee S, Dusheck RE. Meprin metalloprotease expression and regulation in kidney, intestine, urinary tract infections and cancer. FEBS Lett 579: 3317–3322, 2005 [DOI] [PubMed] [Google Scholar]
- 11. Bond JS, Rojas K, Overhauser J, Zoghbi HY, Jiang W. The structural genes, MEP1A and MEP1B, for the alpha and beta subunits of the metalloendopeptidase meprin map to human chromosomes 6p and 18q, respectively. Genomics 25: 300–303, 1995 [DOI] [PubMed] [Google Scholar]
- 12. Bylander J, Li Q, Ramesh G, Zhang B, Reeves WB, Bond JS. Targeted disruption of the meprin metalloproteinase beta gene protects against renal ischemia-reperfusion injury in mice. Am J Physiol Renal Physiol 294: F480–F490, 2008 [DOI] [PubMed] [Google Scholar]
- 13. Cooper HS, Murthy SN, Shah RS, Sedergran DJ. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest 69: 238–249, 1993 [PubMed] [Google Scholar]
- 14. Crisman JM, Zhang B, Norman LP, Bond JS. Deletion of the mouse meprin beta metalloprotease gene diminishes the ability of leukocytes to disseminate through extracellular matrix. J Immunol 172: 4510–4519, 2004 [DOI] [PubMed] [Google Scholar]
- 15. Fielding CA, McLoughlin RM, McLeod L, Colmont CS, Najdovska M, Grail D, Ernst M, Jones SA, Topley N, Jenkins BJ. IL-6 regulates neutrophil trafficking during acute inflammation via STAT3. J Immunol 181: 2189–2195, 2008 [DOI] [PubMed] [Google Scholar]
- 16. Garg P, Rojas M, Ravi A, Bockbrader K, Epstein S, Vijay-Kumar M, Gewirtz AT, Merlin D, Sitaraman SV. Selective ablation of matrix metalloproteinase-2 exacerbates experimental colitis: contrasting role of gelatinases in the pathogenesis of colitis. J Immunol 177: 4103–4112, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Garg P, Vijay-Kumar M, Wang L, Gewirtz AT, Merlin D, Sitaraman SV. Matrix metalloproteinase-9-mediated tissue injury overrides the protective effect of matrix metalloproteinase-2 during colitis. Am J Physiol Gastrointest Liver Physiol 296: G175–G184, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gorbea CM, Marchand P, Jiang W, Copeland NG, Gilbert DJ, Jenkins NA, Bond JS. Cloning, expression, and chromosomal localization of the mouse meprin beta subunit. J Biol Chem 268: 21035–21043, 1993 [PubMed] [Google Scholar]
- 19. Hahn D, Illisson R, Metspalu A, Sterchi EE. Human N-benzoyl-l-tyrosyl-p-aminobenzoic acid hydrolase (human meprin): genomic structure of the alpha and beta subunits. Biochem J 346: 83–91, 2000 [PMC free article] [PubMed] [Google Scholar]
- 20. Hengst JA, Bond JS. Transport of meprin subunits through the secretory pathway: role of the transmembrane and cytoplasmic domains and oligomerization. J Biol Chem 279: 34856–34864, 2004 [DOI] [PubMed] [Google Scholar]
- 21. Herzog C, Haun RS, Kaushal V, Mayeux PR, Shah SV, Kaushal GP. Meprin A and meprin alpha generate biologically functional IL-1beta from pro-IL-1beta. Biochem Biophys Res Commun 379: 904–908, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Herzog C, Kaushal GP, Haun RS. Generation of biologically active interleukin-1beta by meprin B. Cytokine 31: 394–403, 2005 [DOI] [PubMed] [Google Scholar]
- 23. Huguenin M, Muller EJ, Trachsel-Rosmann S, Oneda B, Ambort D, Sterchi EE, Lottaz D. The metalloprotease meprinbeta processes E-cadherin and weakens intercellular adhesion. PLoS One 3: e2153, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ishmael FT, Shier VK, Ishmael SS, Bond JS. Intersubunit and domain interactions of the meprin B metalloproteinase. Disulfide bonds and protein-protein interactions in the MAM and TRAF domains. J Biol Chem 280: 13895–13901, 2005 [DOI] [PubMed] [Google Scholar]
- 25. Jiang W, Beatty BG. Assignment of Mep 1a to mouse chromosome band 17C1-D1 by in situ hybridization. Cytogenet Cell Genet 76: 206–207, 1997 [DOI] [PubMed] [Google Scholar]
- 26. Jiang W, Dewald G, Brundage E, Mucher G, Schildhaus HU, Zerres K, Bond JS. Fine mapping of MEP1A, the gene encoding the alpha subunit of the metalloendopeptidase meprin, to human chromosome 6P21. Biochem Biophys Res Commun 216: 630–635, 1995 [DOI] [PubMed] [Google Scholar]
- 27. Koltun M, Nikolovski J, Strong K, Nikolic-Paterson D, Comper WD. Mechanism of hypoalbuminemia in rodents. Am J Physiol Heart Circ Physiol 288: H1604–H1610, 2005 [DOI] [PubMed] [Google Scholar]
- 28. Kruse MN, Becker C, Lottaz D, Kohler D, Yiallouros I, Krell HW, Sterchi EE, Stocker W. Human meprin alpha and beta homo-oligomers: cleavage of basement membrane proteins and sensitivity to metalloprotease inhibitors. Biochem J 378: 383–389, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lottaz D, Buri C, Monteleone G, Rosmann S, Macdonald TT, Sanderson IR, Sterchi EE. Compartmentalised expression of meprin in small intestinal mucosa: enhanced expression in lamina propria in coeliac disease. Biol Chem 388: 337–341, 2007 [DOI] [PubMed] [Google Scholar]
- 30. Lottaz D, Maurer CA, Hahn D, Buchler MW, Sterchi EE. Nonpolarized secretion of human meprin alpha in colorectal cancer generates an increased proteolytic potential in the stroma. Cancer Res 59: 1127–1133, 1999 [PubMed] [Google Scholar]
- 31. Norman LP, Jiang W, Han X, Saunders TL, Bond JS. Targeted disruption of the meprin beta gene in mice leads to underrepresentation of knockout mice and changes in renal gene expression profiles. Mol Cell Biol 23: 1221–1230, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Norman LP, Matters GL, Crisman JM, Bond JS. Expression of meprins in health and disease. Curr Top Dev Biol 54: 145–166, 2003 [DOI] [PubMed] [Google Scholar]
- 33. Pedersen BK, Febbraio MA. Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiol Rev 88: 1379–1406, 2008 [DOI] [PubMed] [Google Scholar]
- 34. Pizarro TT, Michie MH, Bentz M, Woraratanadharm J, Smith MF, Jr, Foley E, Moskaluk CA, Bickston SJ, Cominelli F. IL-18, a novel immunoregulatory cytokine, is up-regulated in Crohn's disease: expression and localization in intestinal mucosal cells. J Immunol 162: 6829–6835, 1999 [PubMed] [Google Scholar]
- 35. Sivakumar PV, Westrich GM, Kanaly S, Garka K, Born TL, Derry JM, Viney JL. Interleukin 18 is a primary mediator of the inflammation associated with dextran sulphate sodium induced colitis: blocking interleukin 18 attenuates intestinal damage. Gut 50: 812–820, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sterchi EE, Stocker W, Bond JS. Meprins, membrane-bound and secreted astacin metalloproteinases. Mol Aspects Med 29: 309–328, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sun Q, Jin HJ, Bond JS. Disruption of the meprin alpha and beta genes in mice alters homeostasis of monocytes and natural killer cells. Exp Hematol 37: 346–356, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yura RE, Bradley SG, Ramesh G, Reeves WB, Bond JS. Meprin A metalloproteases enhance renal damage and bladder inflammation after LPS challenge. Am J Physiol Renal Physiol 296: F135–F144, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]