Abstract
Gastrin is secreted from a subset of neuroendocrine cells residing in the gastric antrum known as G cells, but low levels are also expressed in fetal pancreas and intestine and in many solid malignancies. Although past studies have suggested that antral gastrin is transcriptionally regulated by inflammation, gastric pH, somatostatin, and neoplastic transformation, the transcriptional regulation of gastrin has not previously been demonstrated in vivo. Here, we describe the creation of an enhanced green fluorescent protein reporter (mGAS-EGFP) mouse using a bacterial artificial chromosome that contains the entire mouse gastrin gene. Three founder lines expressed GFP signals in the gastric antrum and the transitional zone to the corpus. In addition, GFP(+) cells could be detected in the fetal pancreatic islets and small intestinal villi, but not in these organs of the adult mice. The administration of acid-suppressive reagents such as proton pump inhibitor omeprazole and gastrin/CCK-2 receptor antagonist YF476 significantly increased GFP signal intensity and GFP(+) cell numbers in the antrum, whereas these parameters were decreased by overnight fasting, octreotide (long-lasting somatostatin ortholog) infusion, and Helicobacter felis infection. GFP(+) cells were also detected in the anterior lobe of the pituitary gland and importantly in the colonic tumor cells induced by administration with azoxymethane and dextran sulfate sodium salt. This transgenic mouse provides a useful tool to study the regulation of mouse gastrin gene in vivo, thus contributing to our understanding of the mechanisms involved in transcriptional control of the gastrin gene.
Keywords: BAC transgenic, mouse gastrin gene, EGFP, G cells
gastrin is a gastrointestinal hormone secreted from neuroendocrine cells termed “G cells” located in the antrum and the transitional zone to the corpus of the adult stomach (11). Gastrin has been identified as the circulating hormone primarily responsible for stimulation of acid secretion from the stomach. Gastrin is also a potent growth factor that has been implicated in a variety of normal and abnormal biological processes including maintenance of the gastric mucosa, proliferation of enterochromaffin-like (ECL) cells, and neoplastic transformation (28). Gastrin gene and protein expression in G cells is regulated by changes in gastric pH, fasting, and refeeding (41), and gastrin expression is suppressed by somatostatin, another gastrointestinal hormone secreted from neuroendocrine “D cells” located adjacent to G cells in the adult gastric antrum (11). Chronic gastritis caused by Helicobacter pylori (H. pylori) infection in the stomach also modulates gastrin expression (14, 35) and over time possibly suppresses G cell numbers (11). Studies in the past have clearly demonstrated that induction of achlorhydria by proton pump inhibitors results in elevations in both serum gastrin as well as antral gastrin mRNA levels (3). In addition, it was reported that gastrin gene expression was upregulated by oncogenic K-Ras mutation, abnormal Wnt/β-catenin stimulation, and activated TGF-β/Smad signals in the colon cancer cells (6, 17, 21, 25).
However, whether these changes in gene expression are due to transcriptional control or to other mechanisms has been difficult to specifically address. Thus, although administration to rodents of the proton pump inhibitor omeprazole can induce a fourfold increase in gastrin mRNA (3), the increase could in theory be due entirely to alterations in mRNA stability or turnover. The mechanisms involved in the transcriptional regulation of mammalian antral gastrin gene expression have been challenging to study in vitro because of the absence of G cell lines and in vivo because of the lack of appropriate transgenic models. In addition, although numerous reports have suggested that gastrin is upregulated in colon, liver, pancreatic, lung, and ovarian cancer (1, 4, 5, 8, 18, 39), studies of cell-specific expression of gastrin in the setting of neoplasia have been limited.
We and other groups previously reported that the use of rat and human gastrin gene promoters are able to drive the expression of human gastrin and other cDNAs in mice (24, 41, 43, 46), but to date none of these have been able to achieve authentic cell-specific expression in both of a tissue-specific and developmental-specific manner. In particular, with respect to antral G cell expression, some success has been achieved with a chimeric rat-human transgene that contained most of human gastrin intron I and all of intron II (41). Human gastrin promoters, of lengths less than 3 kb, resulted consistently in no detectable antral G cell expression (41), whereas 10.5 kb of human gastrin transgene (5 kb of 5′ flanking DNA, 4 kb of the entire transcribed region, and 1.5 kb of 3′ flanking sequence) was used to drive SV40 T antigen expression and resulted in antral hyperplasia of gastrin-expressing cells (24). These results supported the notion that tissue-specific expression of the gastrin promoter in the stomach may require enhancers presented in 5′, 3′, and intragenic region. More recently, a transgenic mouse containing a human gastrin BAC clone (hGasBAC) was generated and crossed to gastrin-deficient (GAS-KO) mouse (23). This double-mutant mouse successfully expressed human gastrin in G cells of the gastric antrum, but interestingly no gastrin was detected in the serum, suggesting some species differences in gastrin exportation and secretion.
The use of reporter genes such as GFP has been of great value for both tracking gene expression in vivo as well as for studies of transcriptional regulation. Green fluorescent protein (GFP) was originally discovered in the marine jellyfish Aequorea victoria as a side product after purification of aequorin, a chemiluminescent protein, from which emission of blue light leads to excitation of its companion protein GFP, thereby resulting in green fluorescence, and the enhanced version of GFP (EGFP) is now widely used in the biochemical studies (7, 32, 38). In view of the fact that prior gastrin-promoter transgene constructs were unable to completely recapitulate the native tissue specific expression pattern of the mouse gastrin gene in both adult and fetal tissues, we created a transgenic reporter mouse that expresses EGFP using a bacterial artificial chromosome (BAC) (9, 34). Moreover, we demonstrate that pharmacological stimulation as well as pathophysiological conditions regulates the transcription of the mouse gastrin gene by tracing GFP signals. We demonstrate in this study that the mouse gastrin gene is regulated in the stomach in a transcriptional manner by physiological stimuli and that the gene is transcriptionally upregulated in the setting of cancer.
MATERIALS AND METHODS
Creation of BAC transgene construct and mGAS-EGFP reporter mouse.
The Ensemble 129S7-derived mouse genomic BAC clone bMQ262-F1, which contains the entire mouse gastrin gene, was obtained from the Genome Research Limited, the Wellcome Trust Sanger Institute (Cambridge, UK). The open reading frame of the mouse gastrin gene after ATG start codon located in exon 2 and 3 was then replaced by the EGFP-PGK-neomycin cassette by the recombineering-base cloning strategy (9). The PGK-neomycin cassette was removed by arabinose-induced Flpe recombinase gene. The fertilized eggs of B6/CBAF1 hybrid mice were injected with the BAC transgenic construct and transplanted into pseudo-pregnant mothers. Potential F0 founders were genotyped by tail DNA PCR using following two different primer pairs: 1) universal EGFP primers: sense 5′′- gagctgaagggcatcgacttcaag-3′, antisense 5′-ggactgggtgctcaggtagtgg-3′ and 2) transgene-specific primers: sense 5′-ttagtgtgcgcatgcctctttgtt-3′, antisense 5′-cagctcctcgcccttgctca-3′. PCR amplification was performed by GeneAmp PCR System 2700 (Applied Biosystems) by using a PCR core kit (Roche) with the following conditions: 94°C for 2 min, followed by 35 cycles at 94°C for 30 s, 55°C for 30 s, and 72°C for 30 s, then 1 cycle of 72°C for 7 min. Three positive founders were obtained, and the offspring of these three lines were bred to congenic C57/BL6NTac mice (Taconic) in five generations to maintain the transgene.
Animal care and Helicobacter felis infection.
BAC transgenic mGAS-EGFP reporter mice, GAS-KO mice (19), and human progastrin-overexpressing (hGAS) transgenic mice (43) were maintained in individual sterile microisolator cages under specific pathogen-free housing conditions. All studies were performed with the approval of the Institutional Animal Care and Use Committee at Columbia University Medical Center, accredited by the Association for Assessment and Accreditation of Laboratory Animal Care. Helicobacter felis was cultured and administered as previously described (36, 42). Briefly, mice were infected by oral gavage with H. felis in 0.2 ml trypticase broth three times per week on every other day for a total dose of 100 million colony-forming units per mouse. H. felis-uninfected mice were sham dosed with 0.2 ml of broth. Mice were euthanized and analyzed at 6 or 32 wk postinfection.
Drugs and experimental design.
The proton pump inhibitor omeprazole (Sigma, MO) was dissolved in a 1:1 mixture of DMSO (Sigma) and polyethylene glycol (PEG) 300 (Fisher Scientific) at the concentration of 40 mg/ml, and injected intraperitoneally 6 days per week for 2 wk at the dose of 2 mg/mouse (=180 μmol/kg). The CCK2/gastrin receptor antagonist YF476 was a kind gift of Dr. Keiji Miyata and Dr. Hidenobu Yuki (Astellas Pharma, Tokyo, Japan). The drug was dissolved in PEG 300 at the concentration of 12 mg/ml and subcutaneously injected twice per week for 2 wk at the dose of 20 mg/kg (=40 μmol/kg) (42). Administration of octreotide (Bachem, Torrance, CA) was performed in the following way: Alzet microosmotic pumps (model 1007D, Durect) were inserted into the center of the back of animals after anesthesia with 2% isoflurane and oxygen inhalation, and the skin incisions were closed with staples. The pumps delivered PBS or octreotide at a rate of 3 μg·kg−1·h−1 (1.8 μg per mouse per day of peptide) for 14 days before euthanasia.
Tissue preparation for histopathology and immunohistochemistry.
GFP was detected by direct fluorescent observation of frozen slides or immunohistochemistry of paraffin slides with anti-GFP antibodies (see below) according to previously published protocols. Briefly, longitudinal sections of the stomach or other organs were fixed in 4% paraformaldehyde (USB) or 10% buffered formalin (VWR International) solutions for 4–16 h (depending on tissue size). To make frozen sections, tissues were soaked in 30% sucrose (Fisher Scientific) overnight, then embedded in TissueTek OCT compound (Sakura Finetek) and prepared into 10-μm sections. To make paraffin sections, tissues were soaked in 70% ethanol, then processed and embedded in paraffin and prepared into 5-μm sections. The slides were deparaffinized, blocked with 3% peroxidase, and heated to 100°C in 0.01 M sodium citrate buffer for antigen retrieval, and nonspecific antigenic sites were blocked with 5% serum containing PBS for 30 min. The slides were incubated for 1 h at room temperature or overnight at 4°C with following primary antibodies: rabbit anti-GFP antibody (1:500 dilution, Invitrogen) or chicken anti-GFP antibody (1:500, Aves Labs), goat anti-gastrin C-20 antibody (1:100, Santa Cruz), and rat anti-EpCAM antibody (1:100, Iowa State University Hybridoma Facility), then appropriate secondary antibodies conjugated with Texas red for frozen section or conjugated with horseradish peroxidase (1:200, Vector Laboratories) for paraffin section were used. Frozen sections were counterstained with 4′,6-diamidino-2-phenylindole (DAPI; 2 μg/ml, Sigma), and paraffin sections were counterstained with hematoxylin solution (Fisher Scientific). The number of GFP-positive cells was expressed as the average number of positive cells counted per ×300 magnification high-power field (5 fields per slide). Stomach sections from genotype-negative littermates were used as negative controls.
Administration with AOM and DSS.
hGAS mice had the accelerated development of colonic tumors by administration with azoxymethane (AOM) and dextran sodium sulfate (DSS) as previously described (26, 33). Therefore, hGAS/mGAS-EGFP double-mutant mice were injected once intraperitoneally with AOM of the concentration of 12.5 mg/kg, and 1 wk later, mice were administrated with DSS of the concentration of 2.5% in the drinking water for 1 wk. Three weeks later, 2.5% DSS water was administrated again for 1 wk, and mice were analyzed 4 mo after AOM injection.
Quantitative RT-PCR analysis.
cDNAs were reverse transcribed from 4 μg of total RNAs obtained from mouse tissues using an oligo(dT) primer according to the manufacturer's protocol of Superscript III cDNA synthesis kit (Invitrogen). To confirm the integrity of the RNA prepared, the cDNAs were also subjected to PCR amplification of GAPDH. The primer sequences are as follows: mouse gastrin: forward 5′-catcatctggaccagggaccaa-3′, reverse 5′-cctccattcgtggcctctgct-3′; GFP: forward 5′-aagttcatctgcaccaccg-3′, reverse 5′-tccttgaagaagatggtgcg-3′; GAPDH: forward 5′-gacatcaagaaggtggtgaagcag-3′, reverse 5′-ataccaggaaatgagcttgacaaa-3′. PCR amplification was performed in a total volume of 20 μl by use of a QuantiTect SYBR Green PCR kit (Qiagen). PCR amplification was performed by 7300 real-time PCR system (Applied Biosystems) under the following conditions: 94°C for 15 min, followed by 40 cycles at 94°C for 10 s, 55°C for 20 s, and 72°C for 30 s, then 1 cycle of 72°C for 10 min. The expression level of each gene was normalized to the expression level of internal control GAPDH by the ΔΔCt method as previously described (36, 37).
Evaluation of H. felis colonization by quantitative real-time PCR.
Small pieces (1–2 mm2) of tissue from the gastric antrum were digested with proteinase K at 55°C for 24–48 h, followed by genomic DNA extraction by use of a DNA isolation kit (Lamda Biotech) based on the manufacturer's instruction. H. felis colonization levels in gastric tissue were quantified by real-time PCR assay with H. felis flagellar filament B (flaB) primers using QuantiTect SYBR Green PCR kit (QIAGEN) and 7300 real-time PCR system (Applied Biosystems) as previously described (36, 37). The number of genomic copies of H. felis colonies was normalized by comparison to GAPDH level determined by quantitative PCR (qPCR), which was assumed to represent endogenous stomach genomic DNA quantity. Primer sequences used in this experiment as follows: H. felis flaB: forward; 5′-ttcgattggtcctacaggctcaga-3′, reverse; 5′-ttcttgttgatgacattgaccaacgca-3′, mouse GAPDH: 5′-gacatcaagaaggtggtgaagcag-3′, reverse; 5′-ataccaggaaatgagcttgacaaa-3′. PCR conditions are 95°C for 15 min followed by 40 cycles of 94°C for 10 s, 55°C for 20 s, and 72°C for 30 s. Any sample detecting <10 copies of the H. felis genome was considered negative for H. felis colonization.
Measurement of serum amidated gastrin levels.
Mouse serum was collected by bleeding from incised brachial artery on anesthetized mice into microcontainer serum separator tube (Becton Dickinson), followed by centrifugation of 5 min at 6,000 rpm. Separated serum was stored in −80°C until usage. Amidated gastrin levels were measured with antiserum 1296 as previously described (8, 15).
Statistical analysis.
Statistical analysis by Student's t-test was performed by using Microsoft Excel, with significance at P < 0.01 or 0.05.
RESULTS
Generation and characterization of mGAS-EGFP-BAC transgene.
We and other groups have previously reported the mouse gastrin gene structure, which consists of three short exons with the length of 53, 216, and 192 base pairs (bp), respectively, whereas the two introns contain 2,020 and 112 bp, respectively (Fig. 1A) (12, 20). By searching for BAC clone DNAs that contained the entire mouse gastrin gene through the Ensemble 129S7-derived mouse genomic BAC database, we found two suitable clones. One of them, BAC clone bMQ262-F1, was subsequently obtained from Genome Research Limited, the Wellcome Trust Sanger Institute (Cambridge, UK). The open reading frame of mouse gastrin gene, located in the second and third exons, was then replaced following the ATG start codon with the EGFP cDNA-PGK-neomycin cassette by the recombineering-base cloning strategy (9). To generate the final transgene construct, PGK-neomycin cassette was removed by arabinose-induced Flpe recombinase gene (Fig. 1A).
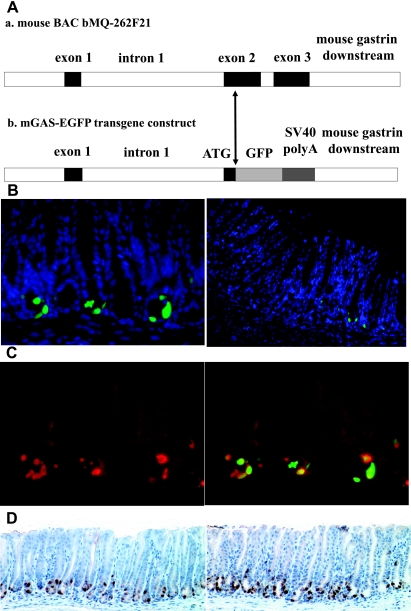
Fig. 1.
Characterization of the mouse gastrin gene and the transgene construct of bacterial artificial chromosome (BAC) transgenic murine gastrin-promoter enhanced green fluorescent protein (EGFP) reporter (mGAS-EGFP) mice and the expression of green fluorescent protein (GFP) signals in the antrum and the transitional zone of mGAS-EGFP mice. A: genomic segment encompassing the mouse gastrin gene locus is represented as a horizontal line. Exons are represented as filled boxes and introns are unshaded. The transgene construct of mGAS-EGFP mice is created by replacing the open reading frame of the mouse gastrin gene, located in the 2nd and 3rd exons, after the ATG start codon with the EGFP cDNA-SV40polyA-PGK-neomycin cassette by using the BAC recombineering technology, followed by the removal of PGK-neomycin cassette with arabinose-induced Flpe recombinase gene. B: GFP expression in the gastric antrum and corpus of mGAS-EGFP mice. Left: merged image of GFP signals and 4′,6-diamidino-2-phenylindole (DAPI) staining in the gastric antrum (magnification: ×400). Right: merged image of GFP signals and DAPI staining from the gastric corpus to the transitional zone (magnification: ×200). Many GFP-positive cells were detected in the gastric antrum, whereas there were few positive cells in the transitional zone and no positive cells in the corpus. C: immunohistochemical staining with anti-amidated gastrin antibody in the antrum. Anti-amidated gastrin immunostaining with Texas red (left) and merged image of GFP expression with anti-amidated gastrin immunostaining (right) (magnification: ×400). D: bright-field immunohistochemical staining with anti-GFP and anti-amidated gastrin antibodies in the antrum. Anti-GFP antibody immunostaining (left) and anti-amidated gastrin antibody immunostaining (right), both followed by peroxidase-conjugated secondary antibodies and diaminobenzidine staining (magnification: ×200). C and D: images indicated that most of the GFP-positive cells were also positive for gastrin immunostaining, which confirmed that these cells were indeed G cells.
BAC transgenic mGAS-EGFP reporter mice demonstrate antral G cell-specific expression.
Three potential founder lines were identified. We analyzed the stomachs of F1 offspring born from each of the three potential founders. As shown in Fig. 1B, we detected GFP-positive cells at the base of the gastric antrum, whereas there were few GFP-positive cells in the transitional zone and no positive cells were detected in the gastric corpus in all of the mice examined. To confirm that these GFP-positive cells were truly G cells, we performed fluorescence immunostaining with a gastrin antibody (goat polyclonal, Santa Cruz). Most of the GFP-positive cells were also positive for gastrin immunostaining, which confirmed that these cells were indeed G cells (Fig. 1, C and D). On the basis of these data, all three lines were then backcrossed to C57BL/6NTac mice, and F2 to F5 generations were used in this study.
Acid suppression upregulates mGAS-EGFP transgene expression.
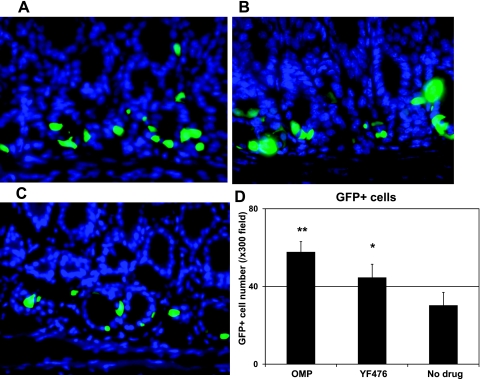
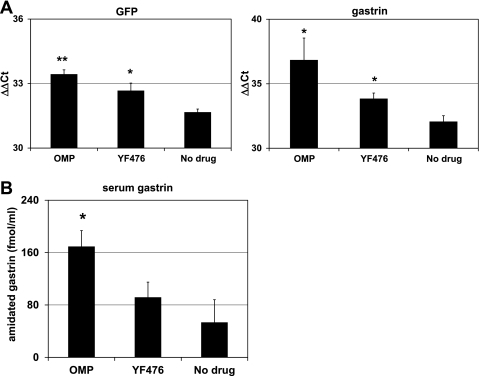
It is well established that suppression of acid secretion from parietal (3, 30) cells results in physiological stimulation of gastrin secretion and increased gastrin gene expression. However, whereas a number of previous studies have demonstrated increased gastrin mRNA abundance, it has not been determined whether this upregulation was a result of increased promoter activity and transcription, as opposed to increased mRNA stability. Thus a reporter gene that contained the murine gastrin promoter upstream of a reporter gene, such as EGFP, offers the ideal tool for addressing this question. Consequently, we administered two types of acid-suppressive drugs: the proton pump inhibitor omeprazole and the gastrin/CCK2 receptor antagonist YF476. As shown in Fig. 2, administration of either omeprazole or YF476 for 2 wk increased the intensity of GFP signals as well as the number of GFP(+) cells in the gastric antrum of the mGAS-EGFP mice. Interestingly, the increase in the number of GFP-expressing cells induced by omeprazole was greater than that induced by YF476. In addition, as shown in Fig. 3A, we confirmed that these treatments resulted in an increase of GFP mRNA as well as gastrin mRNA expression by quantitative real-time reverse transcription-PCR (qRT-PCR). Furthermore, we confirmed through gastrin immunoassay of serum samples that hypergastrinemia was indeed induced by these acid-suppressive reagents (Fig. 3B). Taken together, these data demonstrate that acid-suppressive regimens resulted in upregulation of murine gastrin promoter activity and transcription.
Fig. 2.
Increased intensity of GFP signals and GFP-positive cell numbers in the gastric antrum of mGAS-EGFP mice after administration of acid-suppressive reagents. A–C: merged images of GFP expression with DAPI staining in the antrum of mGAS-EGFP mice (magnification: ×600). A: mice were injected intraperitoneally with omeprazole (OMP; 5 days per week) for 2 wk. B: mice were injected intraperitoneally with gastrin/CCK2 receptor antagonist YF476 (twice per week) for 2 wk. C: mice were injected intraperitoneally with vehicle only for 2 wk. D: total numbers of GFP-positive cells in the antrum of mGAS-EGFP mice by administration with OMP or YF476 or vehicle only. The number of GFP-positive cells was expressed as the average number of positive cells counted per ×300 magnification high-power field. From each slide, cells were counted from at least 5 fields, and 3 slides (=3 mice) from each group were analyzed. The administration of either OMP or YF476 for 2 wk increased the intensity of GFP signals as well as the number of GFP(+) cells in the gastric antrum of the mGAS-EGFP mice. *P < 0.05, **P < 0.01; n = 3 for each group.
Fig. 3.
Quantitative real-time RT-PCR analysis of GFP and gastrin expression in the stomachs of mGAS-EGFP mice and serum gastrin levels after administration of acid-suppressive reagents. A: GFP and gastrin expression were analyzed by quantitative real-time RT-PCR (qRT-PCR) in the stomachs of mGAS-EGFP mice administered with either OMP (5 days per week) or YF476 (twice per week) or vehicle only for 2 wk; n = 3 for each group. Both GFP and gastrin expression in the stomachs of mGAS-EGFP mice treated with either OMP or YF476 were significantly higher than in mice treated with vehicle only. *P < 0.05, **P < 0.01; n = 3 for each group. B: total amidated gastrin levels were measured in serum from mGAS-EGFP mice administered with either OMP (5 days per week) or YF476 (twice per week) or vehicle only for 2 wk; n = 3 for each group. Hypergastrinemia was induced by these acid-suppressive reagents, especially OMP treatment resulted in significantly higher serum gastrin levels compared with vehicle only. *P < 0.05; n = 3 for each group.
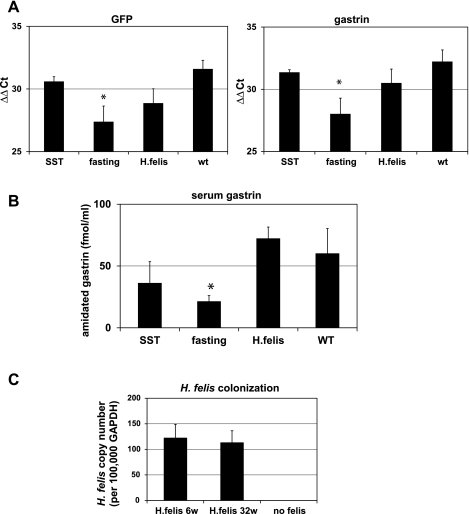
Fasting, somatostatin infusion, and Helicobacter felis infection suppressed mGAS-EGFP transgene expression.
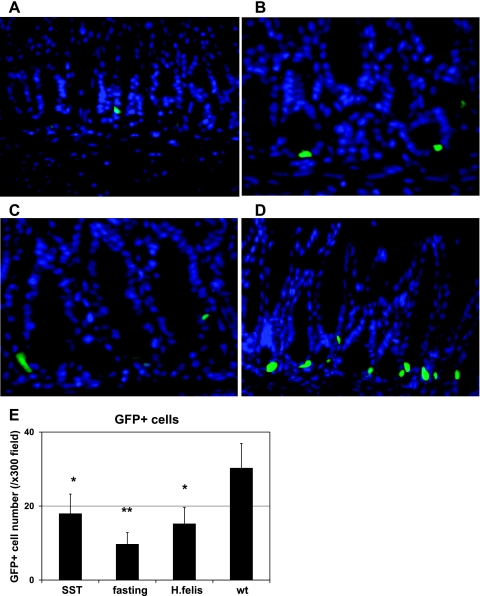
Next, we examined the possible suppression of gastrin promoter activity using several regimens that have been reported to downregulate gastrin gene expression in vivo, including overnight fasting (44), systemic infusion of octreotide (a long-lasting analog of somatostatin) (45), and H. felis infection (14, 37). As shown in Fig. 4, we confirmed that the intensity of GFP signals was significantly decreased in these three conditions (Fig. 4, A–C) compared with the untreated, fed control (Fig. 4D). Moreover, the overall number of GFP(+) cells was decreased with the three treatments compared with the control (Fig. 4E). In addition, downregulation of the level of GFP mRNA in these experiments was also examined by qRT-PCR. Overall, the gastrin promoter and GFP mRNA was clearly suppressed in parallel with endogenous gastrin gene expression by fasting, consistent with transcriptional regulation (Fig. 5A), but not significantly repressed by octreotide or H. felis infection. Serum gastrin levels were markedly reduced in the mice by fasting, whereas octreotide treatment and H. felis infection did not result in a significant change of serum gastrin levels (Fig. 5B) despite successful H. felis colonization in the stomach, as confirmed by qPCR (Fig. 5C) and hematoxylin-eosin histology (data not shown).
Fig. 4.
Decreased GFP signal intensity and GFP-positive cell numbers in the gastric antrum of mGAS-EGFP mice following administration of somatostatin, overnight fasting, and Helicobacter felis infection. A–D: merged image of GFP expression with DAPI staining in the antrum of mGAS-EGFP mice (magnification: ×600). A: mice were systemically infused with octreotide (long-lasting somatostatin ortholog) for 2 wk by use of osmotic minipumps. B: mice were fasted overnight. C: mice were infected with H. felis for 6 wk. D: mice without treatment (wt) or infection. E: total numbers of GFP-positive cells in the antrum of mGAS-EGFP mice by systemic infusion with the somatostatin ortholog octreotide (SST) for 2 wk, overnight fasting, and H. felis infection for 6 wk. The number of GFP-positive cells were expressed as the average number of positive cells counted per ×300 magnification high-power field. From each slide, cells were counted from at least 5 fields, and 3 slides (=3 mice) from each group were analyzed. The systemic infusion with somatostatin for 2 wk or overnight fasting or H. felis infection for 6 wk decreased the intensity of GFP signals as well as the number of GFP(+) cells in the gastric antrum of the mGAS-EGFP mice. *P < 0.05, **P < 0.01; n = 3 for each group.
Fig. 5.
Quantitative real-time RT-PCR analysis of GFP and gastrin expression in the stomachs of mGAS-EGFP mice and serum gastrin levels after systemic infusion of octreotide, overnight fasting, and Helicobacter felis infection. A: GFP and gastrin mRNA expression were analyzed by quantitative real-time RT-PCR in the stomachs of mGAS-EGFP mice after systemic infusion of octreotide, long-lasting somatostatin (SST) homolog, for 2 wk or overnight fasting or Helicobacter felis infection for 6 wk; n = 3 for each group. Both of GFP and gastrin expression in the stomachs of mGAS-EGFP mice after these treatments showed lower expression level, especially overnight fasting resulted in significantly lower expression compared with mice without treatment. *P < 0.05; n = 3 for each group. B: total amidated gastrin levels were measured in serum from mGAS-EGFP mice after systemic infusion of octreotide for 2 wk or overnight fasting or H. felis infection for 6 wk; n = 3 for each group. Hypogastrinemia was induced by octreotide infusion or overnight fasting, especially overnight fasting resulted in significantly lower serum gastrin levels compared with vehicle only, whereas H. felis infection did not change serum gastrin levels. *P < 0.05; n = 3 for each group. C: H. felis colonization in the stomachs of H. felis-infected mGAS-EGFP transgenic mice at 6 and 32 wk (6w and 32w) postinfection as measured by quantitative real-time PCR of genomic DNAs; n = 3 for each group. DNA copy numbers of H. felis normalized to GAPDH (per 100,000 copies) in the stomachs were approximately the same among the 2 time points. Of note, no H. felis DNAs were detected in the stomachs of mGAS-EGFP mice without infection.
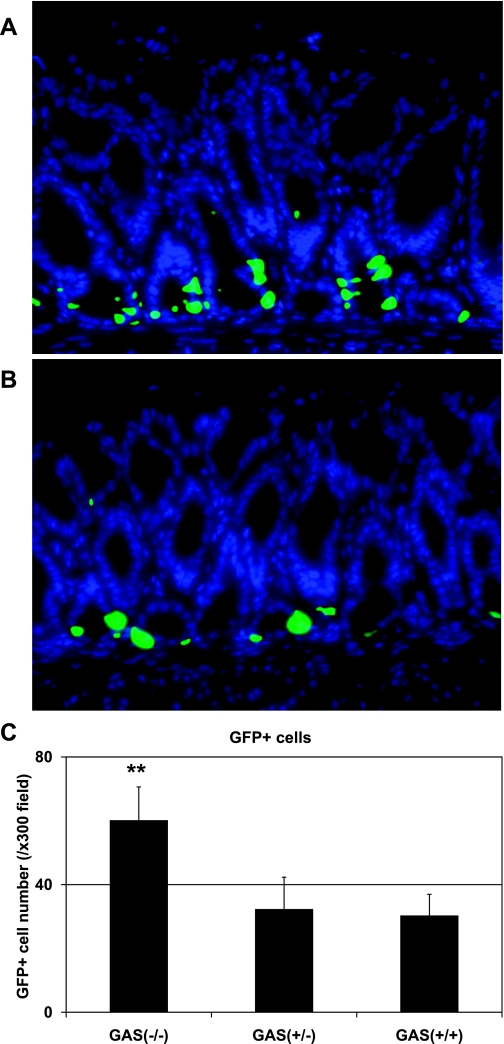
Increased GFP expression in the antrum of gastrin-deficient mice.
We and other group have previously reported that gastrin deficiency altered gastric mucosal differentiation and homeostasis (13, 19). However, so far no data have been described about the status of transcriptional machinery for gastrin gene expression in the setting of gastrin deficiency. Thus, to further investigate, we next crossed mGAS-EGFP mice with GAS-KO mice to determine whether gastrin deficiency resulted in any changes for gastrin gene transcription. As shown in Fig. 6, A–C, the number of GFP(+) cells in the gastric antrum were significantly increased in homozygous GAS-KO/mGAS-EGFP double-mutant mice compared with heterozygous GAS-KO/mGAS-EGFP double-mutant or mGAS-EGFP single-transgenic mice. These results indicate that gastrin deficiency is able to stimulate gastrin transcription and that gastrin gene regulation is indeed regulated in vivo.
Fig. 6.
Increase in the intensity of GFP signals and GFP-positive cell numbers in the gastric antrum of gastrin-deficient (GAS-KO)/mGAS-EGFP double-mutant mice. A: merged image of GFP expression with DAPI staining in the antrum of homozygous GAS-KO/mGAS-EGFP double-mutant mice. B: merged image of GFP expression with DAPI staining in the antrum of heterozygous GAS-KO/mGAS-EGFP double-mutant mice (magnification for A and B: ×400). C: total numbers of GFP-positive cells in the antrum of homozygous (GAS−/−) and heterozygous (GAS+/−) GAS-KO/mGAS-EGFP double-mutant mice and mGAS-EGFP single-transgenic mice (GAS+/+). The number of GFP-positive cells were expressed as the average number of positive cells counted per ×300 magnification high-power field. From each slide, cells were counted from at least 5 fields, and 3 slides (=3 mice) from each group were analyzed. GFP(+) cell numbers in the gastric antrum were significantly increased in homozygous GAS-KO/mGAS-EGFP double-mutant mice compared with heterozygous GAS-KO/mGAS-EGFP double-mutant or mGAS-EGFP single-transgenic mice. **P < 0.01; n = 3 for each group.
Developmental and tissue-specific regulation of the transgene in mGAS-EGFP mice.
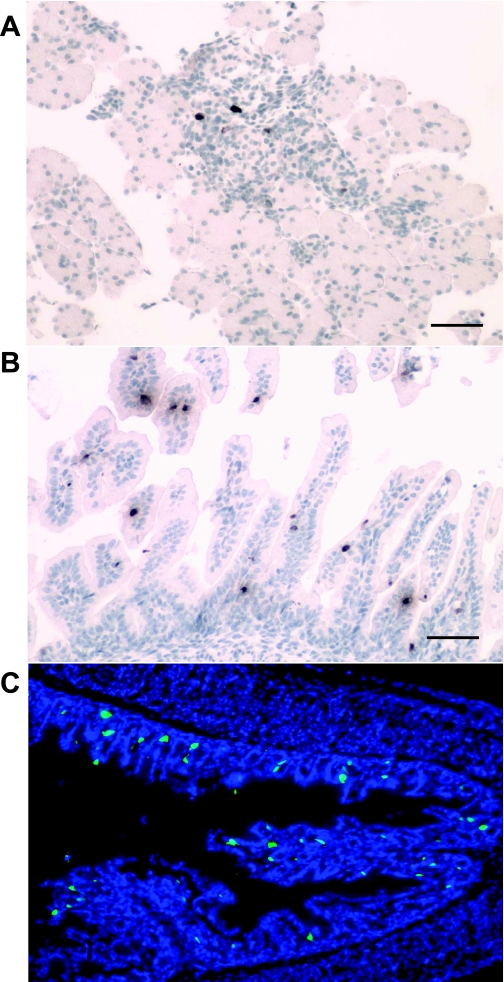
In addition to physiological regulation, we sought to determine whether the transgene expression of mGAS-EGFP mice was correctly regulated in both a developmental- and tissue-specific manner. A number of previous reports had shown that the gastrin gene is upregulated in late fetal development, primarily in the pancreatic islets (2). As shown in Fig. 7, A and B, we observed a few GFP weakly positive cells in the pancreatic islets and the small intestinal villi of the transgenic murine fetus at 18.5 days postconception (dpc). We employed immunostaining with an anti-GFP antibody for these studies of the fetal pancreas and small intestine, primarily because the intensity of the GFP signal was too weak to be evident under direct fluorescence. Of note, these GFP signals disappeared in these organs at ∼2 days after birth, indicating appropriate developmental regulation. We detected the appearance of GFP(+) cells in the gastric antrum at 2 days after birth, but no GFP signals were detected in this location at the fetal stage (data not shown). As shown in Fig. 7C, some of GFP(+) cells in the neonatal antrum were located not at the base of the antral glands but higher up in the middle to the top third of the glands.
Fig. 7.
GFP expression in the fetal and neonatal organs of mGAS-EGFP mice. A and B: immunohistochemical staining with anti-GFP antibody in the fetal pancreas (A) and small intestinal villi (B) of mGAS-EGFP mice at 18.5 days postconception (dpc). C: merged image of GFP expression with DAPI staining in the gastric antrum of neonatal mGAS-EGFP mice at 2 days after birth (magnification for A–C: ×200; scale bar for A and B: 50 μm). GFP(+) cells were detected in the pancreatic islets and the small intestinal villi of the fetus of mGAS-EGFP mice at 18.5 dpc, whereas GFP(+) cells appeared in the gastric antrum of neonatal mGAS-EGFP mice at 2 days after birth. Of note, GFP(+) cells in the neonatal antrum were located at not only the base of the antral glands but also higher up in the middle to the top third of the glands.
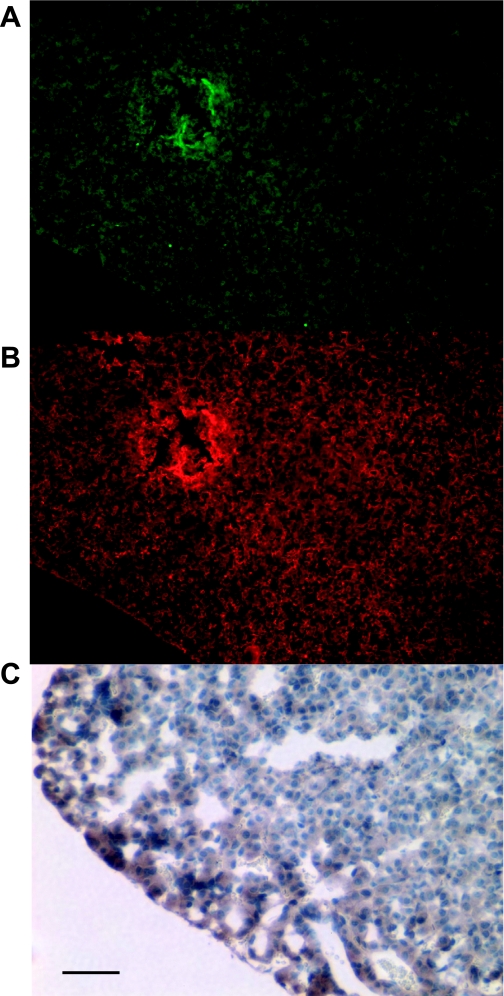
Studies in the past that have relied primarily on radioimmunoassay of tissue extracts have reported gastrin expression in the organs other than stomach, such as pituitary gland, brain, testis, and ovary (11, 12, 27, 29). Therefore, we investigated the possible presence of GFP expression in these tissues. As shown in Fig. 8A, weak GFP signals could be detected in the anterior lobe of the pituitary gland, and immunofluorescence staining with anti-amidated gastrin antibody or immunohistochemistry with anti-GFP antibody confirmed both endogenous gastrin as well as EGFP expression in this tissue (Fig. 8, B and C). In contrast, we were unable to detect GFP signals or endogenous gastrin expression in the cerebral cortex, testis, or ovary (data not shown).
Fig. 8.
GFP expression in the anterior lobe of the pituitary gland of mGAS-EGFP mice. A: GFP expression in the anterior lobe of the pituitary gland of mGAS-EGFP mice. B: immunofluorescence staining with anti-amidated gastrin antibody followed by Texas red-conjugated anti-goat secondary antibody in the same slides as A (magnification for A–B: ×100). C: immunohistochemistry with anti-GFP antibody in the anterior lobe of the pituitary gland of mGAS-EGFP mice (scale bar: 50 μm, magnification: ×300). Weak GFP signals were observed in the anterior lobe of the pituitary gland of mGAS-EGFP mice, and immunofluorescence staining with anti-amidated gastrin antibody confirmed that these GFP-positive cells in the anterior lobe of the pituitary gland were also positive for amidated gastrin.
GFP-positive cells in the colonic tumors induced by administration of AOM and DSS.
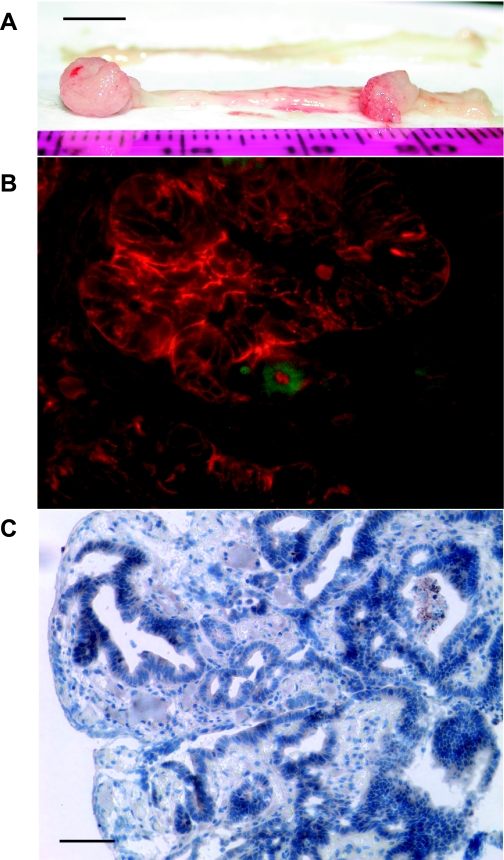
Next we analyzed GFP expression in a mouse model of colon cancer induced by administration with AOM and/or DSS in hGAS mice crossed to mGAS-EGFP mice. We chose to conduct this study in hGAS background because we previously found that hGAS mice had the more rapid and more advanced tumors by AOM and/or DSS treatment (33). As shown in Fig. 9A, we successfully obtained colonic tumors in the distal colon and the rectum of hGAS/mGAS-EGFP mice at ∼16 wk after initial treatment with AOM/DSS protocol (26). GFP-positive cells were detected in these colon tumors (Fig. 9B). We also performed fluorescence immunostaining with anti-EpCAM antibody, and we observed most of GFP(+) cells in the tumors were EpCAM positive; i.e., these cells were epithelial cancer cells (Fig. 9B). Furthermore, we performed immunostaining with anti-GFP antibody, and we observed most of GFP(+) cells were detected in the neoplastic glands (Fig. 9C). Finally, we performed immunostaining with anti-amidated gastrin antibody, and none of GFP(+) cells were positive, suggesting that these cells expressed nonamidated form of gastrin (data not shown).
Fig. 9.
GFP expression in the cancer cells of the colon tumors induced by administration with azoxymethane (AOM) and dextran sodium sulfate (DSS) in hyperprogastrinemic hGAS/mGAS-EGFP double-transgenic mice. A: macroimage of the colorectal tumors of hGAS/mGAS-EGFP double-transgenic mice after 4 mo of administration with AOM and DSS (scale bar: 5 mm). B: merged image of GFP expression with fluorescence immunostaining with anti-EpCAM antibody followed by Texas red-conjugated anti-rat secondary antibody in the colorectal tumors of hGAS/mGAS-EGFP mice after 4 mo of administration with AOM and DSS (magnification: ×600). C: immunohistochemistry with anti-GFP antibody in the colon tumors of hGAS/mGAS-EGFP mice after 4 mo of administration with AOM and DSS (scale bar: 50 μm, magnification: ×200). Most of the GFP(+) cells were detected in the neoplastic colonic glands, and they were EpCAM positive, which indicated they were epithelial cancer cells.
DISCUSSION
In this study, we have generated EGFP reporter mice under the entire murine gastrin promoter by BAC transgenic technology. All three founders showed strong GFP expression in the gastric antrum as well as the transitional zone to the corpus. In addition, GFP(+) cells could be detected in the pancreatic islets and the small intestinal villi of the fetus, but not in these organs of the adult mice. These results indicate that the transgene was expressed in an appropriate developmental and tissue-specific manner. However, importantly, the transgene was regulated in a correct physiology and pathophysiological manner, with increases in the stomach with acid suppression and decreases with fasting and octreotide, showing for the first time that such regulation is likely transcriptional. Finally, studies with the AOM/DSS model of colonic neoplasia show upregulation in a small subset of colonic epithelial cells, in the absence of expression of amidated gastrin, confirming previous reports that the gastrin gene is activated at early stages of colonic carcinogenesis.
Our data point to the inherent advantages of the use of BAC transgenic technology in view of the lower rate of success the traditional plasmid-based transgenic technology (40) and lack of ideal cell-specific expression with most of our previous gastrin promoter constructs (24, 41, 43, 46). Of note, we performed qPCR to evaluate the copy numbers of BAC transgenic construct in each line of mGAS-EGFP mice using tail DNAs, and we found that all three founders had one copy of the transgene integrated into their genome (data not shown). These data indicated one copy of the EGFP transgene in the mouse genome was enough for the analysis of tissue-specific EGFP expression as previously described (22).
The main site of gastrin expression in rodents is in the distal stomach, and our mGAS-EGFP transgenic mice showed appropriate GFP expression in the gastric antral G cells, a finding confirmed by fluorescence immunostaining with an anti-gastrin antibody. It should be noted, though, that some GFP(+) cells were completely negative for gastrin immunostaining. One possible reason for this discrepancy is that these GFP(+) cells in the gastric antrum might produce only nonamidated forms of gastrin, since the antibody used in this study was specific to amidated gastrin. However, immunofluorescent staining of the gastric antrum with antibodies specific for human progastrin and glycine-extended gastrin was unsuccessful (data not shown), which could be due to specific differences or the very low level of peptides in these cells. Another possibility is that the translation of gastrin mRNA might be suppressed in these cells by the novel mechanism such as noncoding RNAs as was recently reported in other endocrine cells (10, 31).
It has been well described that an increase in gastric pH by pharmacological or physiological conditions regulates gastrin gene expression (11, 30). We confirmed that the intensity of GFP(+) signals and also the number of GFP(+) cells were significantly increased in the gastric antrum by administration with proton pump inhibitor omeprazole or gastrin/CCK2 receptor antagonist YF476. These data were compatible with our previous finding that treatment with either of omeprazole or YF476 of human amidated-gastrin-overexpressing transgenic (INS-GAS) mice significantly increased serum gastrin levels (36). In contrast, we found that overnight fasting led to the significant decreases in the intensity of GFP signals and GFP(+) cell numbers, which was consistent with previous reports (44). In addition, the systemic infusion of octreotide, a long-lasting analog of somatostatin, showed a tendency (that was not statistically significant) toward suppression of GFP signal intensity as well as GFP(+) cell numbers (45). Thus regulation of the mGAS-EGFP BAC transgene reproduced the patterns of endogenous gastrin gene expression, pointing to a mechanism of transcriptional regulation.
H. pylori infection has also been reported to regulate gastrin gene expression as well as G cell numbers in the antrum. Previous papers reported that the numbers of G cells and D cells in the stomachs of H. pylori-infected patients both showed significant decreases compared with those in noninfected healthy subjects, whereas a significant increase of G-to-D cell ratio could explain the hypergastrinemia observed in H. pylori-infected duodenal ulcer patients (14, 16). In the present study, H. felis infection of mGAS-EGFP mice resulted in a significant decrease in GFP signal intensity as well as GFP(+) cells numbers (Fig. 4, C and E), which were consistent with the (insignificant) trend toward a suppression of GFP and gastrin gene expression (Fig. 5A), although these findings were not consistent with the serum gastrin levels of mGAS-EGFP mice infected with H. felis for 6 wk (Fig. 5B). This discrepancy might reflect differing mechanisms for the regulation of transcription, secretion, or G cell number. We also analyzed gastrin transcriptional changes in gastrin deficiency using GAS-KO/mGAS-EGFP double-mutant mice and found that the GFP signal intensity as well as GFP-positive cell numbers in the gastric antrum were significantly increased. Taken together, these data indicate that the gastrin transcriptional machinery is activated by gastrin deficiency in vivo, and that gastrin may therefore repress its own transcription. It seems likely that the upregulation of gastrin gene expression by gastrin deficiency might occur through an indirect mechanism, which might include a decrease in gastric acid output and/or an increase in gastric pH, both of which might have stimulated gastrin gene description (19).
Gastrin has been reported to be expressed not only in the antrum but other organs such as the duodenum, pancreas, brain, pituitary glands, testis, and ovary in variety of species (11, 12, 27, 29). In this study, we detected weak GFP signals in the anterior lobe of the pituitary gland, but not in the other organs such as duodenum, pancreas, testis, or ovary of the adult mouse. Instead, we observed rare GFP-positive cells in the small intestinal epithelium as well as in the pancreatic islet cells of the fetus (18.5 dpc). Mensah-Osman et al. (23) recently reported that in their hGasBAC/GAS-KO double-mutant mice, they observed human gastrin expression in adult duodenum, whereas they confirmed that no gastrin was expressed in adult mouse duodenum. These data point to important species-specific differences in the tissue-specific regulation of gastrin gene expression.
Finally we analyzed GFP expression in colonic tumors induced by administration of AOM and/or DSS. We found GFP(+) cells in tumors of the distal colon as well as the rectum, but not in the nontumorous proximal colon, and most of these GFP+ cells were detected in the colonic neoplastic glands. The human gastrin gene is a known downstream target of oncogenic signals such as mutant K-Ras, Wnt/β-catenin, and TGF-β/Smad, and its expression is consistently upregulated in the colon cancer cells (6, 17, 21, 25). Furthermore, we have previously reported that gastrin deficiency in ApcMin mice significantly reduced the size and numbers of intestinal polyps (19). Therefore, we would speculate that the presence of GFP(+) cells points to the upregulation of gastrin gene expression in a subset of colonic tumor cells. Whether these cells represent partially differentiated tumor cells or cancer stem cells will require further investigation.
In conclusion, the mGAS-EGFP BAC transgene showed expression of GFP signals in G cells of the gastric antrum as well as the fetal pancreatic islets and small intestinal villi and was regulated in a correct physiological manner, pointing to the importance of transcriptional control in the achievement of developmental and tissue-specific regulation. Furthermore, we identified GFP(+) cells in the anterior lobe of pituitary glands and the colonic cancer cells induced by AOM and/or DSS treatment. Although the precise characterization of these GFP(+) cells in the setting of cancer will require additional work, in view of the complicated posttranslation processing of gastrin gene product, these mice may provide a useful opportunity to study the regulation of mouse gastrin gene in vivo and may contribute to our understanding of the mechanisms involved in transcriptional control of this gene.
GRANTS
This work was supported by National Institutes of Health Grant R01-DK052778 (T. C. Wang) and the National Health and Medical Research Council of Australia fund (A. Shulkes).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
The authors sincerely thank Dr. James E. Goldman and his laboratory members, Columbia University Medical Center, for help with tissue preparations of mouse brain; Dr. Sharon L. Wardlaw and Dr. Manu Sebastian, Columbia University Medical Center, for help with tissue preparations of mouse pituitary glands; Dr. Juanita L. Merchant, University of Michigan Medical School, for helpful suggestions of G cell analysis; and Dr. Walden Ai, University of South Carolina, and Dr. Abhijit Chakladar, Duke University Medical Center, for help with creation of BAC transgene construct.
Present address of S. Takaishi: Department of 1st Internal Medicine and Biosystemic Science, Kyushu University Hospital, Fukuoka, Japan.
REFERENCES
- 1. Aly A, Shulkes A, Baldwin GS. Gastrins, cholecystokinins and gastrointestinal cancer. Biochim Biophys Acta 1704: 1– 10, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Brand SJ, Fuller PJ. Differential gastrin gene expression in rat gastrointestinal tract and pancreas during neonatal development. J Biol Chem 263: 5341– 5347, 1988 [PubMed] [Google Scholar]
- 3. Brand SJ, Stone D. Reciprocal regulation of antral gastrin and somatostatin gene expression by omeprazole-induced achlorhydria. J Clin Invest 82: 1059– 1066, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Caplin M, Khan K, Savage K, Rode J, Varro A, Michaeli D, Grimes S, Brett B, Pounder R, Dhillon A. Expression and processing of gastrin in hepatocellular carcinoma, fibrolamellar carcinoma and cholangiocarcinoma. J Hepatol 30: 519– 526, 1999 [DOI] [PubMed] [Google Scholar]
- 5. Caplin M, Savage K, Khan K, Brett B, Rode J, Varro A, Dhillon A. Expression and processing of gastrin in pancreatic adenocarcinoma. Br J Surg 87: 1035– 1040, 2000 [DOI] [PubMed] [Google Scholar]
- 6. Chakladar A, Dubeykovskiy A, Wojtukiewicz LJ, Pratap J, Lei S, Wang TC. Synergistic activation of the murine gastrin promoter by oncogenic Ras and beta-catenin involves SMAD recruitment. Biochem Biophys Res Commun 336: 190– 196, 2005 [DOI] [PubMed] [Google Scholar]
- 7. Chalfie M, Tu Y, Euskirchen G, Ward WW, Prasher DC. Green fluorescent protein as a marker for gene expression. Science 263: 802– 805, 1994 [DOI] [PubMed] [Google Scholar]
- 8. Ciccotosto GD, McLeish A, Hardy KJ, Shulkes A. Expression, processing, and secretion of gastrin in patients with colorectal carcinoma. Gastroenterology 109: 1142– 1153, 1995 [DOI] [PubMed] [Google Scholar]
- 9. Copeland NG, Jenkins NA, Court DL. Recombineering: a powerful new tool for mouse functional genomics. Nat Rev Genet 2: 769– 779, 2001 [DOI] [PubMed] [Google Scholar]
- 10. Correa-Medina M, Bravo-Egana V, Rosero S, Ricordi C, Edlund H, Diez J, Pastori RL. MicroRNA miR-7 is preferentially expressed in endocrine cells of the developing and adult human pancreas. Gene Expr Patterns 9: 193– 199, 2009 [DOI] [PubMed] [Google Scholar]
- 11. Dockray GJ, Varro A, Dimaline R, Wang T. The gastrins: their production and biological activities. Annu Rev Physiol 63: 119– 139, 2001 [DOI] [PubMed] [Google Scholar]
- 12. Friis-Hansen L, Rourke IJ, Bundgaard JR, Rehfeld JF, Samuelson LC. Molecular structure and genetic mapping of the mouse gastrin gene. FEBS Lett 386: 128– 132, 1996 [DOI] [PubMed] [Google Scholar]
- 13. Friis-Hansen L, Sundler F, Li Y, Gillespie PJ, Saunders TL, Greenson JK, Owyang C, Rehfeld JF, Samuelson LC. Impaired gastric acid secretion in gastrin-deficient mice. Am J Physiol Gastrointest Liver Physiol 274: G561– G568, 1998 [DOI] [PubMed] [Google Scholar]
- 14. Graham DY, Lew GM, Lechago J. Antral G-cell and D-cell numbers in Helicobacter pylori infection: effect of H. pylori eradication. Gastroenterology 104: 1655– 1660, 1993 [DOI] [PubMed] [Google Scholar]
- 15. Jin G, Ramanathan V, Quante M, Baik GH, Yang X, Wang SS, Tu S, Gordon SA, Pritchard DM, Varro A, Shulkes A, Wang TC. Inactivating cholecystokinin-2 receptor inhibits progastrin-dependent colonic crypt fission, proliferation, and colorectal cancer in mice. J Clin Invest 119: 2691– 2701, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kamada T, Haruma K, Kawaguchi H, Yoshihara M, Sumii K, Kajiyama G. The association between antral G and D cells and mucosal inflammation, atrophy, and Helicobacter pylori infection in subjects with normal mucosa, chronic gastritis, and duodenal ulcer. Am J Gastroenterol 93: 748– 752, 1998 [DOI] [PubMed] [Google Scholar]
- 17. Koh TJ, Bulitta CJ, Fleming JV, Dockray GJ, Varro A, Wang TC. Gastrin is a target of the beta-catenin/TCF-4 growth-signaling pathway in a model of intestinal polyposis. J Clin Invest 106: 533– 539, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Koh TJ, Field JK, Varro A, Liloglou T, Fielding P, Cui G, Houghton J, Dockray GJ, Wang TC. Glycine-extended gastrin promotes the growth of lung cancer. Cancer Res 64: 196– 201, 2004 [DOI] [PubMed] [Google Scholar]
- 19. Koh TJ, Goldenring JR, Ito S, Mashimo H, Kopin AS, Varro A, Dockray GJ, Wang TC. Gastrin deficiency results in altered gastric differentiation and decreased colonic proliferation in mice. Gastroenterology 113: 1015– 1025, 1997 [DOI] [PubMed] [Google Scholar]
- 20. Koh TJ, Wang TC. Molecular cloning and sequencing of the murine gastrin gene. Biochem Biophys Res Commun 216: 34– 41, 1995 [DOI] [PubMed] [Google Scholar]
- 21. Lei S, Dubeykovskiy A, Chakladar A, Wojtukiewicz L, Wang TC. The murine gastrin promoter is synergistically activated by transforming growth factor-beta/Smad and Wnt signaling pathways. J Biol Chem 279: 42492– 42502, 2004 [DOI] [PubMed] [Google Scholar]
- 22. Lin T, Yasumoto H, Tsai RY. BAC transgenic expression efficiency: bicistronic versus ATG-fusion strategies. Genesis 45: 647– 652, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mensah-Osman E, Labut E, Zavros Y, El-Zaatari M, Law DJ, Merchant JL. Regulated expression of the human gastrin gene in mice. Regul Pept 151: 115– 122, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Montag AG, Oka T, Baek KH, Choi CS, Jay G, Agarwal K. Tumors in hepatobiliary tract and pancreatic islet tissues of transgenic mice harboring gastrin simian virus 40 large tumor antigen fusion gene. Proc Natl Acad Sci USA 90: 6696– 6700, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nakata H, Wang SL, Chung DC, Westwick JK, Tillotson LG. Oncogenic ras induces gastrin gene expression in colon cancer. Gastroenterology 115: 1144– 1153, 1998 [DOI] [PubMed] [Google Scholar]
- 26. Neufert C, Becker C, Neurath MF. An inducible mouse model of colon carcinogenesis for the analysis of sporadic and inflammation-driven tumor progression. Nat Protoc 2: 1998– 2004, 2007 [DOI] [PubMed] [Google Scholar]
- 27. Rourke IJ, Rehfeld JF, Moller M, Johnsen AH. Characterization of the cholecystokinin and gastrin genes from the bullfrog, Rana catesbeiana: evolutionary conservation of primary and secondary sites of gene expression. Endocrinology 138: 1719– 1727, 1997 [DOI] [PubMed] [Google Scholar]
- 28. Rozengurt E, Walsh JH. Gastrin, CCK, signaling, and cancer. Annu Rev Physiol 63: 49– 76, 2001 [DOI] [PubMed] [Google Scholar]
- 29. Schalling M, Persson H, Pelto-Huikko M, Odum L, Ekman P, Gottlieb C, Hokfelt T, Rehfeld JF. Expression and localization of gastrin messenger RNA and peptide in spermatogenic cells. J Clin Invest 86: 660– 669, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schubert ML, Peura DA. Control of gastric acid secretion in health and disease. Gastroenterology 134: 1842– 1860, 2008 [DOI] [PubMed] [Google Scholar]
- 31. Seim I, Carter SL, Herington AC, Chopin LK. Complex organisation and structure of the ghrelin antisense strand gene GHRLOS, a candidate non-coding RNA gene. BMC Mol Biol 9: 95, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shimomura O, Johnson FH, Saiga Y. Extraction, purification and properties of aequorin, a bioluminescent protein from the luminous hydromedusan, Aequorea. J Cell Comp Physiol 59: 223– 239, 1962 [DOI] [PubMed] [Google Scholar]
- 33. Singh P, Velasco M, Given R, Varro A, Wang TC. Progastrin expression predisposes mice to colon carcinomas and adenomas in response to a chemical carcinogen. Gastroenterology 119: 162– 171, 2000 [DOI] [PubMed] [Google Scholar]
- 34. Sparwasser T, Eberl G. BAC to immunology—bacterial artificial chromosome-mediated transgenesis for targeting of immune cells. Immunology 121: 308– 313, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sumii M, Sumii K, Tari A, Kawaguchi H, Yamamoto G, Takehara Y, Fukino Y, Kamiyasu T, Hamada M, Tsuda T, et al. Expression of antral gastrin and somatostatin mRNA in Helicobacter pylori-infected subjects. Am J Gastroenterol 89: 1515– 1519, 1994 [PubMed] [Google Scholar]
- 36. Takaishi S, Cui G, Frederick DM, Carlson JE, Houghton J, Varro A, Dockray GJ, Ge Z, Whary MT, Rogers AB, Fox JG, Wang TC. Synergistic inhibitory effects of gastrin and histamine receptor antagonists on Helicobacter-induced gastric cancer. Gastroenterology 128: 1965– 1983, 2005 [DOI] [PubMed] [Google Scholar]
- 37. Takaishi S, Tu S, Dubeykovskaya ZA, Whary MT, Muthupalani S, Rickman BH, Rogers AB, Lertkowit N, Varro A, Fox JG, Wang TC. Gastrin is an essential cofactor for helicobacter-associated gastric corpus carcinogenesis in C57BL/6 mice. Am J Pathol 175: 365– 375, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tsien RY. The green fluorescent protein. Annu Rev Biochem 67: 509– 544, 1998 [DOI] [PubMed] [Google Scholar]
- 39. Van Solinge WW, Odum L, Rehfeld JF. Ovarian cancers express and process progastrin. Cancer Res 53: 1823– 1828, 1993 [PubMed] [Google Scholar]
- 40. Vintersten K, Testa G, Naumann R, Anastassiadis K, Stewart AF. Bacterial artificial chromosome transgenesis through pronuclear injection of fertilized mouse oocytes. Methods Mol Biol 415: 83– 100, 2008 [DOI] [PubMed] [Google Scholar]
- 41. Wang TC, Babyatsky MW, Oates PS, Zhang Z, Tillotson L, Chulak M, Brand SJ, Schmidt EV. A rat gastrin-human gastrin chimeric transgene directs antral G cell-specific expression in transgenic mice. Am J Physiol Gastrointest Liver Physiol 268: G1025– G1036, 1995 [DOI] [PubMed] [Google Scholar]
- 42. Wang TC, Dangler CA, Chen D, Goldenring JR, Koh T, Raychowdhury R, Coffey RJ, Ito S, Varro A, Dockray GJ, Fox JG. Synergistic interaction between hypergastrinemia and Helicobacter infection in a mouse model of gastric cancer. Gastroenterology 118: 36– 47, 2000 [DOI] [PubMed] [Google Scholar]
- 43. Wang TC, Koh TJ, Varro A, Cahill RJ, Dangler CA, Fox JG, Dockray GJ. Processing and proliferative effects of human progastrin in transgenic mice. J Clin Invest 98: 1918– 1929, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wu V, Sumii K, Tari A, Sumii M, Walsh JH. Regulation of rat antral gastrin and somatostatin gene expression during starvation and after refeeding. Gastroenterology 101: 1552– 1558, 1991 [DOI] [PubMed] [Google Scholar]
- 45. Zavros Y, Kao JY, Merchant JL. Inflammation and cancer. III. Somatostatin and the innate immune system. Am J Physiol Gastrointest Liver Physiol 286: G698– G701, 2004 [DOI] [PubMed] [Google Scholar]
- 46. Zhukova E, Afshar A, Ko J, Popper P, Pham T, Sternini C, Walsh JH. Expression of the human insulin gene in the gastric G cells of transgenic mice. Transgenic Res 10: 329– 341, 2001 [DOI] [PubMed] [Google Scholar]