Abstract
Paracrine signaling between cholangiocytes and stromal cells regulates biliary remodeling. Cholangiocytes have neuroepithelial characteristics and serotonin receptor agonists inhibit their growth, but whether they are capable of serotonin biosynthesis is unknown. We hypothesized that cholangiocytes synthesize serotonin and that cross talk between liver myofibroblasts (MF) and cholangiocytes regulates this process to influence biliary remodeling. Transwell cultures of cholangiocytes ± MF, and tryptophan hydroxylase-2 knockin (TPH2KI) mice with an inactivating mutation of the neuronal tryptophan hydroxylase (TPH) isoform, TPH2, were evaluated. Results in the cell culture models confirm that cholangiocytes have serotonin receptors and demonstrate for the first time that these cells express TPH2 and produce serotonin, which autoinhibits their growth but stimulates MF production of TGF-β1. Increased TGF-β1, in turn, counteracts autocrine inhibition of cholangiocyte growth by repressing cholangiocyte TPH2 expression. Studies of TPH2KI mice confirm that TPH2-mediated production of serotonin plays an important role in remodeling damaged bile ducts because mice with decreased TPH2 function have reduced biliary serotonin levels and exhibit excessive cholangiocyte proliferation, accumulation of aberrant ductules and liver progenitors, and increased liver fibrosis after bile duct ligation. This new evidence that cholangiocytes express the so-called neuronal isoform of TPH, synthesize serotonin de novo, and deploy serotonin as an autocrine/paracrine signal to regulate regeneration of the biliary tree complements earlier work that revealed that passive release of serotonin from platelets stimulates hepatocyte proliferation. Given the prevalent use of serotonin-modulating drugs, these findings have potentially important implications for recovery from various types of liver damage.
Keywords: neuronal tryptophan hydroxylase, fibroductular reaction, biliary fibrosis, liver progenitors
various factors that obstruct large bile ducts (e.g., stones, strictures, cancers) inhibit bile flow and induce compensatory proliferation of intrahepatic ductular cells to generate alternative biliary conduits (14). In healthy adult livers, bile ducts are lined by cholangiocytes that are derived from small numbers of resident, bipotent liver epithelial progenitors (dubbed hepatoblasts in humans and oval cells in rodents) that are also capable of differentiating to form hepatocytes. Hepatoblasts/oval cells and their immediate progeny localize to the most proximal branches of the intrahepatic biliary tree (dubbed canals of Hering) (31, 32). During large bile duct obstruction, the ductular cells that accumulate in periportal areas express markers of immature liver epithelial cells, including keratin 7 (KRT7) and nestin, suggesting that bile duct obstruction promotes expansion of the progenitor pool (8, 28, 29). Myofibroblasts (MF) and fibrous matrix also accumulate along portal tracts, eventually leading to biliary cirrhosis (14).
Intrahepatic accumulation of proliferative, immature ductular cells and stromal elements also occurs transiently during the normal process of hepatobiliary development. As in adults, in developing embryos both the biliary tree and the liver parenchyma are formed from multipotent progenitors (30). Ductal plates form when hepatoblasts that rim portal vein branches begin cholangiocytic differentiation (30). Remodeling of the ductal plate structures is subsequently orchestrated via epithelial-mesenchymal interactions that eventually generate tubes that are incorporated into the perivenous mesenchyme (30). This phase of ductular morphogenesis begins with migration of the nascent ductular cells into the mesenchyme and is associated with transient accumulation of fibrous stroma and fibroblastic cells. Eventually, the fibroblastic cells disappear and excess matrix is reabsorbed, leaving small ducts embedded in well-defined portal tracts (30). Further maturation of the cells lining these remodeled tubular ducts ultimately completes the intrahepatic biliary tree (30). In humans, growth of the intrahepatic biliary system continues into early childhood and maturation is completed in adolescence (30).
The mechanisms that mediate remodeling of the adult biliary system in response to obstruction are less well understood. It is clear, however, that, as in development, in adulthood biliary morphogenesis involves paracrine cross talk between cholangiocytes and neighboring stromal cells, particularly MF (25, 27, 30). After large bile duct obstruction, intrahepatic ductular cell production of various profibrogenic factors, including certain developmental morphogens, increases (9, 12, 13, 25–27). This promotes the growth of MF populations and enhances fibrogenesis. The MF, in turn, generate soluble factors that promote the proliferation and viability of ductular cells and other liver progenitors, thereby fueling further accumulation of such cells (25, 27). Hence the microenvironment of the portal tracts in adult livers with bile duct obstruction becomes skewed to favor the growth of immature ductular cells and MF. Large bile duct obstruction in adults, therefore, may recapitulate some of the forces that promote bile duct formation during embryogenesis.
As detailed earlier, formation and remodeling of primitive ductal plates to generate mature bile ducts requires cross talk between liver epithelial progenitors and stromal elements (33). Cholangiocytic differentiation of hepatoblasts is promoted by the profibrogenic cytokine, transforming growth factor-β1 (TGF-β1) (3, 30). Formation of the ductal plate also involves angiopoietin 1 (Angpt1)-mediated induction of tyrosine kinase receptor (Tie2) signaling (6). TGF-β1 inhibits Angpt1 (23), however, suggesting that this cytokine may regulate ductulogenesis by differentially influencing various stages of the process. In endothelial cells, Angpt1 activation of Tie2 induces tryptophan hydroxylase (TPH) and 5-hydroxytryptamine (5-HT, serotonin) production (4, 5, 10, 35). It is not known whether 5-HT has any role in bile duct development during embryogenesis. In adults, however, platelet-derived 5-HT has been reported to promote hepatocyte growth (24), whereas type 1 5-HT receptor agonists have been shown to inhibit cholangiocyte proliferation (21). These findings suggest that 5-HT might exert opposing effects on the hepatocytic and cholangiocytic progeny of bipotent liver progenitors.
Cholangiocytes variably express markers of neuroendocrine cells, such as nestin, chromogranin A, and N-cam (7, 21, 30). In addition, they are known to release 5-HT (7, 21). This background information led us to hypothesize that cholangiocytes synthesize 5-HT and that the proliferation of cholangiocyte precursors and their progeny in adulthood is regulated by cross talk between liver MF and cholangiocytes that modulates cholangiocyte 5-HT biosynthesis. To address this issue, adult cholangiocytes were examined for expression of the enzymes, receptors, and transporters that control 5-HT homeostasis, as well as for their ability to produce 5-HT when cultured alone or with liver MF in a Transwell coculture system. The direct effects of 5-HT on cholangiocyte proliferation were characterized and a MF-derived factor that regulated cholangiocyte 5-HT production was identified. To determine whether cholangiocyte-derived 5-HT exerted pathophysiologically relevant effects on remodeling of the obstructed biliary system, bile duct ligation was then performed in adult WT mice and transgenic mice with an inactivating TPH mutation that reduces biliary 5-HT production. Data from the in vitro and in vivo studies support the hypothesis and identify 5-HT as an important mediator of biliary remodeling in adults.
MATERIALS AND METHODS
Human Subjects
Anonymized liver sections from three patients with primary biliary cirrhosis and two control healthy livers were obtained from the Duke University School of Medicine Tissue Bank Shared Resources. Samples were studied under a protocol approved by the Duke University Health System Institutional Review Board, in accordance with National Institutes of Health guidelines for human subject research.
In Vitro Studies
Cell lines and culture experiments.
Murine cholangiocyte 603B line was kindly provided by Yoshiyuki Ueno (Tohoku University, Sendai, Japan) and G. Gores (Mayo Clinic, Rochester, MN) and maintained as described (25, 27). Normal rat cholangiocyte line (NRC) was a gift of N. LaRusso (Mayo Clinic, Rochester, MN), and clonally derived rat hepatic stellate cell line (MF-8B) was obtained from M. Rojkind (George Washington University, Washington, DC) (25, 27). To perform a phenotypic analysis and comparison of cholangiocytes from mouse and rat, monocultures of 603B and NRC lines were performed and mRNA, protein extracts, and cytospins were collected.
To assess the effects of MF-derived factors on cholangiocytes, MF-8B and 603B cholangiocyte lines were cultured for 3 and 6 days alone or in a Transwell insert coculture system, as previously reported (25, 27). In each experiment, mRNA, protein, and conditioned medium were pooled from all six wells and used for subsequent quantitative real-time reverse transcription-polymerase chain reaction (QRT-PCR), Western blot analysis, or HPLC. All the experiments were repeated three times.
To evaluate how 5-HT affects cholangiocyte growth, murine cell line monocultures were performed in both chamber glass slide systems (NUNC Lab-Tek II, Nalge Nunc International) and 96-well plates (Corning). Cells were treated with increasing doses of exogenous 5-HT (0–6-60–180 ng/ml, 5-HT hydrochloride H9523 Sigma) in medium supplemented with 5% FBS to reproduce coculture conditions. After 48 h, cholangiocyte proliferation was assessed by evaluating 5-bromo-2′-deoxyuridine (BrdU) incorporation by both immunocytochemistry and ELISA assays.
Cholangiocyte monocultures were also treated with vehicle or recombinant TGF-β1 (2 ng/ml, 240-B002 R&D Systems) for 24 h. The effects of this treatment on the Angpt1/Tie2/TPH2 axis were evaluated by QRT-PCR.
In the attempt to reproduce the effect of exposure to cholangiocyte-derived 5-HT, MF monocultures were also treated with exogenous 5-HT (5-HT hydrochloride H9523 Sigma), and TGF-β1 mRNA induction was quantified by QRT-PCR.
mRNA extraction and QRT-PCR.
Total RNA was extracted by using TRIzol (Invitrogen) combined with RNeasy columns (Qiagen). Samples were reverse transcribed to cDNA templates after RNase-free DNase I treatment (Qiagen) and amplified by using a SYBR Green PCR Master Mix (Bio-Rad Laboratories) as previously described (25, 27). Amplicon products were separated by electrophoresis on a 2.0% agarose gel buffered with 0.5 × Tris-borate-EDTA and representative products were then visualized via an AlphaImager 3400 gel analysis system. Target gene expression was normalized to housekeeping gene expression and presented as a ratio to levels detected in corresponding experimental control according to the ΔΔCt method (25, 27).
Western blotting.
Western blot analysis was performed by use of standard techniques. 603B and NRC cells were homogenized in RIPA buffer (Sigma) supplemented with protease inhibitors (Complete Mini 11 836 153 001, Roche). Whole cell lysates (40 mg) were separated by polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes. Primary antibodies against KRT19 were rat anti-mouse (Troma-III, Hybridoma Bank) and mouse anti-human (1:1,000, Dako) for mouse and rat cell lines, respectively. Appropriate secondary antibodies (1:2,000) were used with antigens demonstrated by enhanced chemiluminescence (Pierce Biotechnology).
Immunocytochemistry.
To characterize the cholangiocyte cell lines used in this study, 603B or NRC lines were also cytospun onto VWR superfrost plus microslides (VWR International) by using the Shandon Cytospin 4 (Thermo Scientific) at 600 rpm for 5 min. Slides were air dried for 24 h and then fixed with cold (−20°C) acetone for 5 min. Cells were permeabilized with TBS-Tween 0.2% for 10 min followed by TBS-Triton-X 0.1% for an additional 10 min at room temperature (RT). Cells were then washed in PBS twice and incubated with Peroxidase Block reagent (Dako) for 10 min to inhibit endogenous peroxidase activity, and nonspecific binding was blocked by use of DakoCytomation serum-free protein block (Dako) for 30 min. Slides were then incubated overnight at 4°C with primary antibody (1:100 dilution) against KRT19 (rat anti-mouse, Troma-III, Hybridoma Bank) or type 1A 5-HT receptor (HTR1A, ab79230, Abcam). Goat anti-rat IgG horseradish peroxidase (HRP)-conjugated (1:100, sc-2006, Santa Cruz, CA) or Dako EnVision-HRP labeled polymer anti-mouse were used as secondary antibodies and incubated for 1 h at RT. Counterstaining with diaminobenzidine (DAB) (Dako) was then performed.
603B and NRC were then analyzed by immunofluorescence to assess the presence of enzymes involved in 5-HT biosynthesis. Cells were fixed with cold (−20°C) acetone for 5 min and then permeabilized with TBS-Tween 0.2% for 10 min followed by TBS-Triton-X 0.1% for additional 10 min at RT. Cells were then washed in PBS twice, primary antibodies against TPH1 (rabbit polyclonal TPH-H60, sc-30079, Santa Cruz) or TPH2 (goat polyclonal, TPH2-C12, sc48955) were applied overnight in 4°C. After a washing with TBS-Tween 20 0.01%, secondary antibodies were added for 30 min. Isotype-matched antibodies (R&D Systems) were used as negative controls. Alexa Fluor 488 goat anti-rabbit (Molecular Probes) and Alexa Fluor 594 donkey anti-goat were used as secondary antibodies (1:100 dilution). Coimmunofluorescence for TPH2 and the biliary marker γ-glutamyl transferase (γGT) (GGT1/2 sc-20639, Santa Cruz) was also performed in both cell lines. Counterstaining with 4′,6-diamidino-2-phenylindole (Vectashield H-1200, Vector Laboratories) was employed.
To assess BrdU labeling index in vitro, 603B were seeded onto glass chamber slides (Lab-Tek II, Nalge Nunc International) and incubated with increasing doses of 5-HT (0–6-60–180 ng/ml, 5-HT hydrochloride, H9523 Sigma) in medium containing 5% FBS for 48 h. At 4 h before the incubation end time, BrdU was added to allow proliferating cholangiocytes to incorporate it into nuclei. Cells were then washed in PBS twice, fixed in 80% acetone for 10 min, and then denaturated with 1 N HCl for 10 min. Incubation with Peroxidase Block reagent (Dako) was used to inhibit endogenous peroxidase activity and nonspecific binding of antibodies were blocked with DakoCytomation serum-free protein block (Dako) for the following 30 min. Slides were then incubated with primary antibody (1:100 dilution) against BrdU (M0744 anti-BrdU, clone Bu20a Dako) for 1 h at room temperature. Dako EnVision-HRP labeled polymer anti-mouse was used as detection system and standard DAB (Dako) counterstaining was performed.
Cell proliferation assay.
Incubation of 603B cells with exogenous 5-HT was repeated in 96-well plates, and ELISA BrdU immunoassay (Roche, Mannheim, Germany) was performed, as per the manufacturer's instructions.
Soluble TGF-β1 protein quantification.
Conditioned medium was collected from mono/cocultures. The amount of TGF-β1 released by cholangiocytes and MF into culture medium was quantified by using the Quantikine mouse, rat, porcine, canine TGF-β1 immunoassay (MAB100B, R&D Systems) according to the manufacturer's instructions.
In Vivo Studies
Animals and surgery.
Age-matched knockin (KI) mice carrying a TPH2 functional mutation in R439H allele (n = 16) (1) and wild-type (WT) littermates (n = 12) were obtained and maintained in a temperature- and light-controlled facility. To induce biliary fibrosis, animals underwent bile duct ligation (BDL) or sham surgery (Sham) and were euthanized 2 wk after surgical procedure. In each animal, liver and body weight were annotated and blood, bile, and liver samples were obtained. To assess cholangiocyte proliferating index in vivo, BrdU (50 μg/g of body wt) was injected intraperitoneally 2 h before euthanasia as described. All animal care and procedures performed were approved by the Duke University Medical Center Institutional Animal Care and Use Committee.
Immunohistochemistry.
Formalin-fixed, paraffin-embedded liver sections were stained with standard hematoxylin and eosin (H&E) to assess general histology. Cholangiocyte DNA replication index was assessed by in vivo nuclear incorporation of BrdU (Sigma-Aldrich). Sections were processed by using mouse anti-BrdU (M0744, Clone Bu20a, Dako) as described. Briefly, slides were fixed, permeabilized, and incubated with Peroxidase Block reagent (Dako) for 10 min. Tissues were pretreated for 10 min with Citraplus buffer (BioGenex) as heat-induced epitope retrieval. Slides were subjected to a 10-min denaturation process with 1 N HCl to permit anti-BrdU antibody to bind and blocked with DakoCytomation serum-free protein block (Dako) for the following 30 min. Slides were then incubated with primary antibody (1:100 dilution) against BrdU (M0744, clone Bu20a Dako) overnight at 4°C and Dako EnVision-HRP labeled polymer anti-mouse was used as detection system with standard DAB (Dako) counterstaining. Randomly selected, 20 × portal tract fields were evaluated for BrdU-positive nuclei, and the BrdU labeling index was calculated separately for ductular and hepatocytic cells. To better evaluate proliferating cholangiocytes within areas of ductular reaction, colocalization of BrdU with KRT19 was also assessed. Namely, BrdU immunohistochemistry was performed as aforementioned. Slides were incubated with DakoCytomation serum-free protein block (Dako) for the following 30 min and rat anti-mouse KRT19 antibody (TROMA-III, Developmental Studies Hybridoma Bank) was then applied overnight at 4°C (1:500 dilution). Rat on mouse polymer (PROMARK, Biocare Medical) and Vulcan Fast Red Chromogen Kit2 (Biocare Medical) were used as a secondary detection system, following the manufacturer's instructions.
Standard immunohistochemistry was also performed to evaluate the expansion of KRT19-, AE1/AE3 (Zymex)-, and α-fetoprotein (A0008 Dako)-positive populations in response to Sham or BDL in transgenic mice and WT littermates. For KRT19 quantification, ×20 portal tract fields (excluding the major bile duct in each portal tract from consideration) were analyzed with the Metaview software (Universal Imaging) as described (26). Detailed retrieval techniques and antibodies used are presented in Table 1.
Table 1.
Antibodies and retrieval techniques used for immunohistochemistry
| Target Protein | Antibody | Retrieval Technique | Dilution | Incubation |
|---|---|---|---|---|
| HTR1A | ab79230 | Citraplus | 200 | Overnight 4°C |
| KRT-19 | TROMA-III | Citraplus | 1,000 | Overnight 4°C |
| BrdU | M0744, clone Bu20a | 0.1 M Citrate | 500 | Overnight 4°C |
| BrdU/KRT-19 | M0744/TROMA-III | Citraplus | 500/1,000 | Overnight 4°C/2 h room temperature |
| AE1/AE3 | Zymex | Citraplus | 500 | Overnight 4°C |
| AFP | AA008 | 400 | Overnight 4°C |
Morphometry.
To quantify fibrosis, 5-μm sections (N = 5 per group) were stained with picrosirius red (Sigma) and counterstained with fast green (Sigma) (30). Morphometric analysis and quantification were then performed by using Meta View software as previously described (27).
Statistical analysis.
Results are expressed as means with SE. Comparisons between groups were performed using the Student's t-test. Significance was accepted at the 5% level.
RESULTS
Human, Murine, and Rat Cholangiocytes Express Type 1A 5-HT Receptors and Respond to 5-HT Treatment by Reducing Proliferative Activity
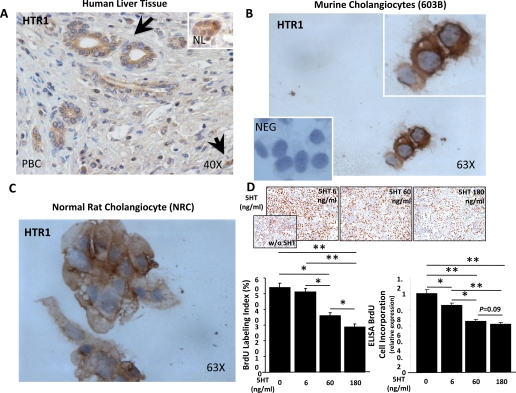
Immunostaining was used to localize expression of HTR1A in human liver biopsies. We found that HTR1A was expressed by hepatocytes (Supplemental Fig. S1; the online version of this article contains supplemental data), consistent with earlier reports (15, 34). In addition, immunohistochemistry demonstrated expression of 5-HTR1A protein by cholangiocytes in bile ducts within portal tracts in healthy human livers. In livers with primary biliary cirrhosis, bile ducts, bile ductules, and scattered stromal cells in fibrotic portal tracts also expressed HTR1A (Fig. 1A). Because this immunohistochemical evidence of HTR1A on cholangiocytes in healthy and diseased livers was novel, the specificity of HTR1A staining was verified by exposing cytospins of well-established mouse and rat cholangiocyte cell lines to HRP-conjugated secondary antibody after incubation with primary antisera to HTR1A or nonimmune sera. Staining was demonstrated only after preexposure to anti-HTR1A; both murine cholangiocytes (603B cell line) and rat cholangiocytes (NRC line) uniformly demonstrated strong HTR1A expression (Fig. 1, B and C). The immunostaining data, therefore, complement and extend an earlier report that agonists of HTR1 inhibited ductular proliferation in BDL rats (20). That earlier study did not, however, directly examine the effect of 5-HT itself on ductular proliferation. Therefore, we treated the 5-HTR1-expressing mouse cholangiocytes with 5-HT. 5-HT induced dose-dependent reductions in cholangiocyte BrdU incorporation (Fig. 1D), establishing definitively that 5-HT is a cholangiocyte growth inhibitor.
Fig. 1.
Cholangiocytes express HTR1A and respond to 5-HT treatment by reducing proliferative activity. A: type 1A 5-HT receptor (HTR1A) immunostaining in representative sections from patient with primary biliary cirrhosis (PBC) or healthy control subject (NL; inset). Arrows indicate HRT1A-positive bile ducts/ductules and point at HTR1A-positive stromal cell. Original magnification ×40. B and C: immunocytochemistry of murine 603B line (B) and rat normal rat cholangiocyte (NRC) line (C) demonstrating that cholangiocytes uniformly express HT1RA. Inset in B shows 603B cholangiocytes exposed to nonimmune sera as negative (NEG) control. Original magnification ×63. D: treatment of murine cholangiocytes with exogenous 5-HT caused inhibition of cell proliferation, as demonstrated by the a dose-dependent decrease of 5-bromo-2′-deoxyuridine (BrdU) labeling index (top, and bottom left) and ELISA BrdU incorporation assay (bottom right). Representative pictures of chamber slides of cholangiocytes exposed to exogenous 5-HT (0–6-60–180 ng/ml) for 48 h are shown at ×10 original magnification. Quantitative data are expressed as means ± SE and differences between groups are evaluated by 2-tailed Student's t-test. *P < 0.05, **P < 0.001.
Cholangiocytes express enzymes for 5-HT biosynthesis, catabolism, and transport.
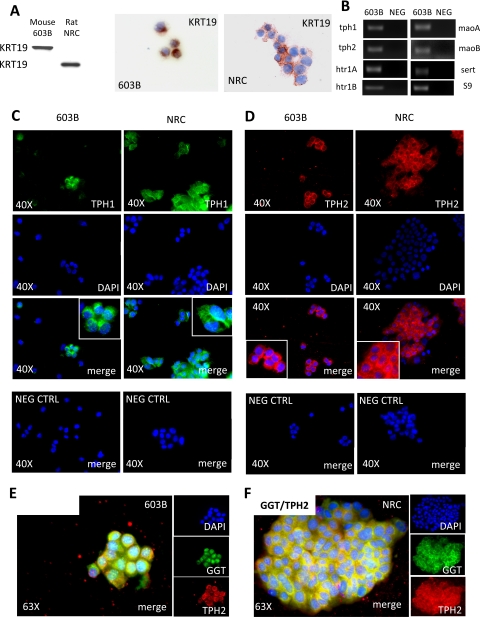
Next, to determine whether cholangiocytes themselves might be a source of 5-HT, we used QRT-PCR and immunocytochemistry to examine mRNA and protein expression of TPH, the rate-limiting enzyme for 5-HT biosynthesis. There are two isoforms of TPH: TPH1 has been localized to nonneuronal (peripheral) cells, whereas TPH2 is expressed mainly in neural cells (38–40). We assessed expression of both TPH1 and TPH2 in the mouse and rat cholangiocyte lines, after verifying that the cells retained expression of cholangiocyte markers (KRT19 and γGT) in vitro (Fig. 2, A, E, and F). Mouse and rat cholangiocytes expressed both isoforms of TPH (Fig. 2, B–F). As predicted by HTR1 immunostaining, cholangiocytes also expressed HTR1A mRNA. In addition, transcripts of HTR1B, monoamine oxidase (mao) A and maoB (which degrade 5-HT), and the 5-HT transporter (sert) (which mediates cellular uptake of 5-HT from the extracellular space) were easily demonstrated (Fig. 2B). Thus cholangiocytes have the enzymatic machinery to produce, degrade, respond to, and transport 5-HT.
Fig. 2.
Cholangiocytes express enzymes for 5-HT biosynthesis, catabolism, and transport. A: Western blot (left) and immunocytochemical (middle and right) analyses show that both murine 603B and rat NRC cholangiocytes homogeneously express the ductular marker keratin-19 (KRT19). Original magnification ×63. B: 2% agarose gel of quantitative real-time reverse transcription-polymerase chain reaction (QRT-PCR) amplicons demonstrates expression of tryptophan hydroxylase (tph)-1 and -2, htr1A and B, monoamine oxidase (mao) A and B and serotonin (5-HT) transporter (sert) in 603B cholangiocytes. C and D: immunofluorescence cell staining for TPH1 in green (C) and TPH2 in red (D) showing that both murine (603B, C and D, left) and rat (NRC, D and E, right) express the rate-limiting enzymes for 5-HT biosynthesis. 4′,6-Diamidino-2-phenylindole (DAPI) demonstrates nuclei. 603B and NRC incubated with nonimmune sera plus secondary antibodies are shown as negative controls. Original magnification ×40 (C and D). Coimmunofluorescence of TPH2 in red with the ductular marker γ-glutamyl transpeptidase (GGT) in green is displayed in 603B (E) and NRC (F) cholangiocytes. Original magnification ×63.
Cholangiocytes Produce 5-HT
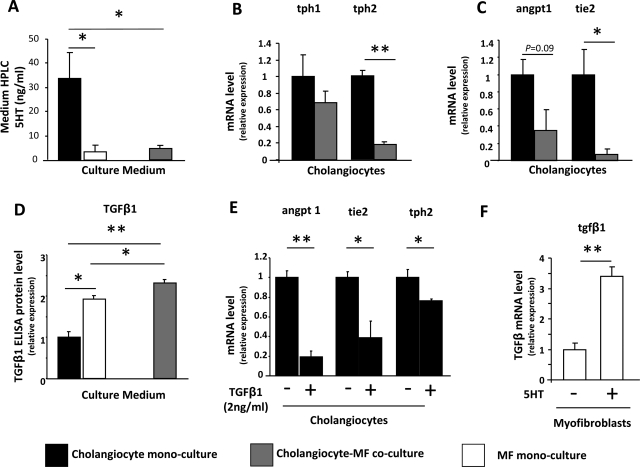
HPLC was then used to compare the 5-HT content of conditioned medium from 603B-cholangiocyte monocultures, Transwell cocultures of 603B cholangiocytes with liver MF and MF monocultures (Fig. 3A). 5-HT was detected in all culture medium. Medium harvested from monocultures of MF, and Transwell cocultures of cholangiocytes plus MF contained comparable levels of 5-HT. However, the 5-HT content of medium from cholangiocyte monocultures was sixfold higher. These findings suggested that soluble MF-derived factors might have inhibited cholangiocyte production of 5-HT.
Fig. 3.
Myofibroblast (MF)-derived TGF-β inhibits Angpt1/Tie2/TPH2 axis and 5-HT production in neighboring cholangiocytes. A: HPLC was employed to detect 5-HT content in the culture medium from monocultures of 603B cholangiocytes (solid bars) or MF (open bars), and from cholangiocyte/MF cocultures (shaded bars). Data are expressed as means ± SE of the absolute concentration (ng/ml). B and C: QRT-PCR of monocultured and cocultured cholangiocytes (solid and shaded bars, respectively) shows differential expression of tph-1 and tph-2 (B) and angiopoietin 1 (angpt1) and tie-2 (C). Data are normalized to monocultured cholangiocytes and displayed as means ± SE. D: ELISA assay demonstrated TGF-β1 content in the culture medium from monocultured 603B cholangiocytes (solid bars) or MF (open bars), and in conditioned medium of cocultures (shaded bars). Results are normalized to values in cholangiocytes monocultures and expressed as means ± SE. E: 603B-cholangiocyte monocultures were exposed to recombinant TGF-β1 (2 ng/ml) or vehicle for 24 h in serum-deprived medium, and QRT-PCR analysis was performed to evaluate the expression of angpt1, tie2, and tph2. Data are normalized to vehicle-treated controls and displayed as means ± SE. F: MF monocultures were treated with exogenous 5-HT for 48 h and TGF-β1 cell induction was assessed by QRT-PCR. Results are normalized to vehicle-treated MF and expressed as means ± SE. For all data sets 2-tailed Student's t-test was employed to evaluate differences between groups. *P < 0.05, **P < 0.001.
To evaluate this possibility further, TPH1 and TPH2 expression were compared in mono- and cocultured cholangiocytes. TPH1 mRNA levels were fairly similar in monocultured cholangiocytes and cholangiocytes that had been cocultured with MF. In contrast, coculture with MF reduced cholangiocyte expression of TPH2 by 80% (Fig. 3B). Because Angpt1 interacts with Tie2 to regulate 5-HT synthesis in endothelial cells (4, 35), we next determined whether coculturing cholangiocytes with MF influenced expression of either of these regulators of 5-HT production. Compared with monocultured cholangiocytes, cholangiocytes that had been cocultured with MF expressed significantly lower levels of both Angpt1 and Tie2 mRNA (Fig. 3C). Together, these results suggest that MF-derived factors may disrupt cholangiocyte Angpt1-Tie2 signaling that promotes TPH2 expression and 5-HT synthesis, but further research is required to prove this definitively.
TGF-β1 has been reported to inhibit Angpt1 (23), and MF provide a rich source of TGF-β1 in injured livers. Therefore, we compared the TGF-β1 content of conditioned medium from our culture systems to assess whether or not differences in TGF-β1 exposure might have contributed to the differences in Angpt1/Tie2/TPH2 expression and 5-HT content that we noted in mono and cocultured cholangiocytes. Medium from MF monocultures contained about twice as much TGF-β1 as cholangiocyte monocultures. Coculturing MF with cholangiocytes further increased TGF-β1 levels. Hence cholangiocytes that were cocultured with MF were exposed to significantly higher concentrations of TGF-β1 than monocultures of cholangiocytes (Fig. 3D). To determine whether or not the higher levels of TGF-β1 might have directly impacted cholangiocyte 5-HT synthesis, monocultures of 603B cholangiocytes were treated with TGF-β1 (or vehicle) for 24 h. Compared with vehicle-treated controls, cholangiocytes that were exposed to TGF-β1 expressed significantly reduced levels of Angpt1, Tie2, and TPH2 mRNAs. These findings support the concept that MF-derived TGF-β1 suppresses cholangiocyte production of 5-HT. The inhibited de novo biosynthesis of 5-HT in cholangiocytes, in turn, likely contributes to the heightened proliferative activity of cholangiocytes that are cocultured with liver MF (27).
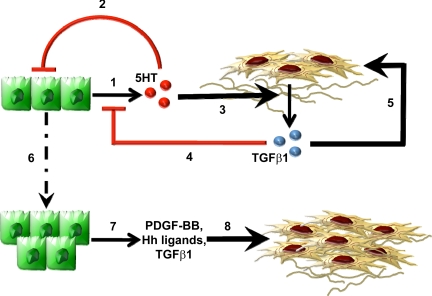
Because levels of TGF-β1 increased when MF were cocultured with cholangiocytes (Fig. 3D), we next asked whether cholangiocytes might produce factors that promote MF production of TGF-β1. Since the 5-HT content of monocultured cholangiocytes was significantly higher than that of cocultured cholangiocytes (Fig. 3A), we treated MF monocultures with 5-HT to determine whether it directly influenced MF production of TGF-β1. Indeed, 5-HT directly acted upon MF to upregulate their expression of TGF-β1 mRNA by threefold (Fig. 3E). These results suggest that 5-HT and TGF-β1 are among the various paracrine signals that liver MF and cholangiocytes exchange to control their growth. According to this model (Fig. 4), cholangiocyte-derived 5-HT acts via autocrine mechanisms to limit cholangiocyte growth, but it functions in a paracrine fashion to stimulate production of TGF-β1 by neighboring MF. The MF-derived TGF-β1, in turn, provides an autocrine stimulus for MF growth but acts in a paracrine fashion to downregulate cholangiocyte 5-HT production. Reductions in 5-HT derepress cholangiocyte growth, permitting expansion of the ductular cell population. Proliferating ductular cells, in turn, produce Hedgehog ligands, PDGF-BB, and other MF growth factors that sustain MF growth (7, 9, 12–13, 25–27) despite reductions in local 5-HT.
Fig. 4.
Working model of paracrine mechanisms that modulate 5-HT-TGF-β1 production in liver MF and cholangiocytes. Cholangiocyte-derived 5-HT (red spheres) acts in an autocrine fashion to limit cholangiocyte growth (1) and functions in a paracrine fashion to stimulate production of TGF-β1 (blue spheres) by neighboring MF (2). The MF-derived TGF-β1, in turn, provides an autocrine stimulus for MF growth (3) but acts in a paracrine fashion to downregulate cholangiocyte 5-HT production (4). Reduction of 5-HT derepress cholangiocyte growth, permitting expansion of the ductular cell population (5). Proliferating cholangiocytes, in turn, produce soluble factors (e.g., Hedgehog ligands, PDGF-BB, TGF-β1) that sustain MF growth despite reductions in local 5-HT (6).
Mice With Functional Inactivation of TPH2 Exhibited Decreased Biliary 5-HT and Exaggerated Ductular Proliferation After BDL
To determine the pathophysiological relevance of this model that was derived from our cell culture data, we compared responses to biliary injury induced by BDL in transgenic mice with an inactivating mutation of TPH2 (1) and WT littermate controls. TPH2 knockin mice (TPH2KI) carry a functional mutation in the murine TPH2 R439H allele that is equivalent to the human TPH2 R441H allele mutation that was identified in a cohort of patients with major unipolar depression (1). Previous characterization of TPH2KI mice demonstrated that 5-HT synthesis is reduced by ∼80% in TPH2 expressing cells, whereas activities of TPH1 and SERT are unaffected (1).
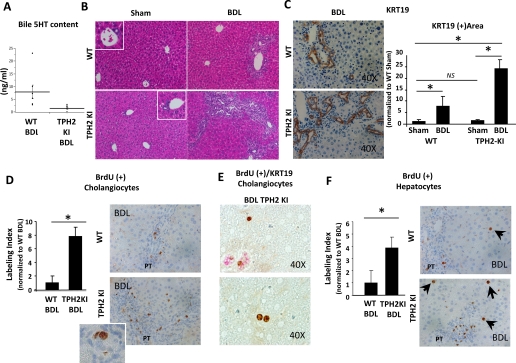
The effects of TPH2 inhibition on cholangiocyte production of 5-HT were evaluated by comparing biliary 5-HT content of WT and TPH2KI mice via HPLC. Because healthy mice generated insufficient quantities of bile for analysis, bile was collected from WT and TPH2KI mice 2 wk after BDL. Biliary 5-HT content was consistently reduced in TPH2KI mice (Fig. 5A). Reduced production of 5-HT by cholangiocytes in the TPH2KI mice was likely responsible for their decreased biliary 5-HT content because 1) primary hepatocytes expressed neither TPH1 nor TPH2 mRNAs (Supplemental Fig. S2) and 2) SERT function is known to be preserved in TPH2KI mice (1). Thus evidence for altered 5-HT production and/or release by hepatocytes was lacking.
Fig. 5.
Mice with functional inactivation of TPH2 (TPH2KI) displayed exaggerated proliferation of ductular and hepatocytic cells in response to bile duct ligation (BDL). A: HPLC was performed to assess 5-HT content in bile from TPH2KI (n = 10) and wild-type (WT; n = 8) mice 2 wk after BDL surgery. Dots demonstrate the absolute biliary concentration of 5-HT (ng/ml) in each animal. Black bars show means ± SE of values for each group. B: hematoxylin and eosin (H&E) staining of liver sections from representative mice that were euthanized 2 wk after either Sham (left) or BDL (right) surgery. Original magnification ×20. C: representative pictures of keratin (KRT)-19 staining in WT (top) or TPH2KI (bottom) mice 2 wk after BDL surgery. Original magnification ×40. The graph on the right shows results of the computer-assisted morphometric evaluation of KRT19+ area in WT and TPH2KI mice 2 wk after Sham or BDL surgery. Data are normalized to WT Sham controls and displayed as means ± SE. D: 2 wk after surgery, mice were injected with BrdU and euthanized 2 h later; cholangiocyte BrdU labeling index was quantified in portal tracts (PT) from WT (top) and TPH2KI (bottom). Data are normalized to WT BDL and expressed as means ± SE. Original magnification ×40. Inset shows magnified image of small duct with BrdU positive nucleus. E: representative pictures of section from BDL TPH2KI mice display KRT19/BrdU costaining with BrdU localizing to KRT19+ ducts (top) and to KRT19− hepatocytic cells in periportal areas (top and bottom). F: hepatocytic cell BrdU labeling index was quantified in periportal areas from WT (top) and TPH2KI (bottom). Data are normalized to WT BDL and expressed as means ± SE. Original magnification ×40. All quantitative data are expressed as means ± SE and differences between groups are evaluated by 2-tailed Student's t-test. *P < 0.05, **P < 0.001.
Next, the effects of reduced cholangiocyte-derived 5-HT on biliary remodeling responses were evaluated by exposing WT and TPH2KI mice to BDL. BDL induced ductular proliferation in both groups (Fig. 5B). However, examination of H&E-stained liver sections suggested that the ductular response to biliary injury was much more florid in the TPH2KI mice, which exhibited large numbers of aberrant ductular structures in periportal areas following BDL. Computer-assisted morphometric assessment of liver sections that were stained with the cholangiocyte marker, KRT19, confirmed this impression. By 2 wk after BDL, TPH2KI mice accumulation of KRT19+ cells had increased almost 25-fold in TPH2KI mice compared with a sevenfold increase in WT mice (Fig. 5C and Supplemental Fig. S3A). BrdU labeling demonstrated that expansion of KRT19-expressing cells in the TPH2KI mice reflected increased cholangiocyte proliferation (Fig. 5, D and E). Interestingly, some small hepatocytic cells in periportal areas also incorporated BrdU after BDL (Fig. 5F). Post-BDL accumulation of BrdU-positive small hepatocytic cells was greater in TPH2KI mice than WT mice, and this was accompanied by significantly increased liver to body weight ratios in the TPH2KI group (Table 2).
Table 2.
Effects of BDL on Liver and Body Weights in WT and TPH2KI mice
| WT BDL | TPH2 BDL | P Value | |
|---|---|---|---|
| Body weight, g | 20.46 ± 0.077 | 24.78 ± 1.17 | 0.021 |
| Liver weight, g | 1.12 ± 0.042 | 1.59 ± 0.08 | 0.001 |
| Liver/body weight, % | 5.5 ± 0.20 | 6.4 ± 0.07 | 0.0004 |
Values are means ± SE.
BDL, bile duct ligation; WT, wild-type; TPH2KI, tryptophan hydroxylase-2 knockin.
Reduced TPH2 Activity Promotes the Outgrowth of Liver Progenitors in BDL Mice
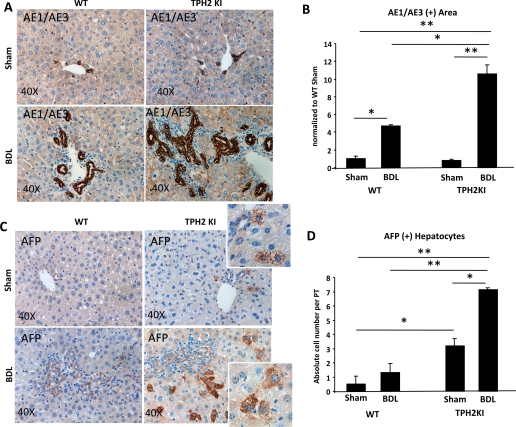
5-HT has been shown to stimulate proliferation of mature hepatocytes (7). Thus evidence of increased hepatocyte proliferative activity in TPH2KI mice [in which 5-HT production is reduced and SERT function is preserved (1)] was unanticipated. Because the biliary tree provides a niche for bipotent progenitors that are capable of differentiating along either the hepatocytic or cholangiocytic lineages (31–32) and reduced biliary 5-HT production stimulated the growth of ductular cells (Fig. 5, B–E), we examined the effect of reduced TPH2 activity on progenitor accumulation after BDL. Compared with WT-BDL mice, TPH2KI-BDL mice expressed significantly higher mRNA levels of various liver progenitor markers, including markers of immature hepatocytes [α-fetoprotein (afp) and krt7], as well as markers of immature cholangiocytes (krt19, krt7, and nestin) (Supplemental Fig. S4). These findings were validated by immunohistochemistry, which also showed significant expansion of cells that expressed progenitor-associated keratins (Fig. 6, A and B, and Supplemental Fig. S3B) and AFP (Fig. 6, C and D, and Supplemental Fig. S3C). Interestingly, AFP-expressing hepatocytic cells were easily demonstrated in TPH2KI mice (but were rarely, if ever, noted in WT mice) following sham surgery (Fig. 6, C and D). The aggregate data suggest that biliary production of 5-HT regulates the proliferative activity of the population of bipotent liver progenitors that give rise to both cholangiocytes and hepatocytes.
Fig. 6.
Reduced TPH2 activity and biliary 5-HT content promote the outgrowth of liver progenitors. Immunohistochemical analysis of AE1/AE3 (which marks progenitor cell-associated cytokeratins) (A and B) and α-fetoprotein (AFP) (C and D) in representative sections of WT (left) and TPH2KI mice (right) 2 wk after sham (top) or BDL (bottom) surgery. Representative pictures are displayed at ×40 original magnification. B: AE1/AE3+ area was quantified by computer-assisted morphometric evaluation. Data are normalized to WT Sham controls and displayed as means ± SE. D: AFP+ hepatocytic cells near portal tracts were counted in immunostained liver sections (n = 5/group) under ×40 magnification. Mean ± SE results are shown. *P < 0.05, **P < 0.001.
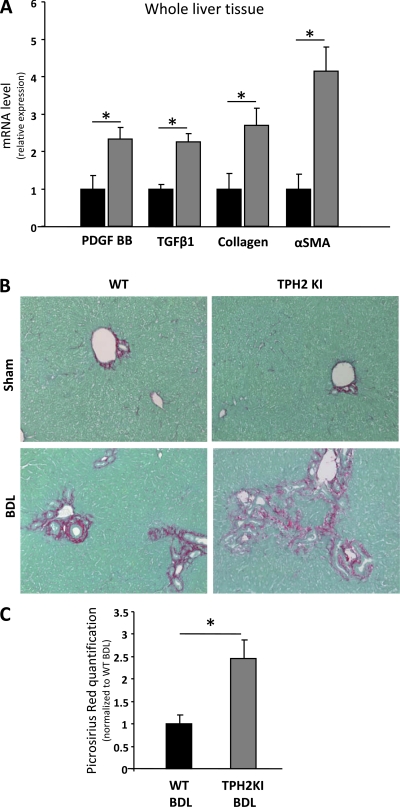
Increased Liver Fibrosis in Mice With Reduced Biliary 5-HT Production
During chronic liver injury, expansion of immature ductular cells and other progenitors, including bipotent liver progenitors, generally parallels MF accumulation and fibrosis progression (31). Therefore, we compared fibrogenesis in the WT and TPH2KI mice following BDL. TPH2KI mice exhibited increased mRNA expression of fibrogenic factors (e.g., tgfβ, pfgf-BB), αsma (a MF marker), and collagen 1(α). I (Fig. 7A). Sirius red staining also demonstrated consistently more liver fibrosis in BDL-TPH2KI mice than BDL-WT (Fig. 7, B and C). Hence reduced biliary 5-HT content in adult TPH2KI mice (which harbor an inactivating mutation of TPH2) exhibit increased proliferation of immature ductular cells, formation of aberrant intrahepatic bile ductules, accumulation of AFP+ progenitors, and enhanced collagen deposition when subjected to a challenge that stimulated biliary remodeling.
Fig. 7.
Increased hepatic fibrogenesis in TPH2KI mice after BDL. A: whole liver tissues from TPH2KI (shaded bars) and WT (solid bars) mice 2 wk after BDL were analyzed by QRT-PCR to assess changes in fibrogenic markers [platelet-derived growth factor BB (PDGFBB), TGF-β1, collagen 1α(I), α-smooth muscle actin (αSMA)]. Data are normalized to WT-BDL values and displayed as means ± SE. Differences between groups are evaluated by 2-tailed Student's t-test. *P < 0.05. B: Sirius red staining was done to demonstrate differences in fibrous matrix. Representative photomicrographs from each group are shown. Original magnification ×20. C: computer-assisted quantification of picrosirius red+ area in liver sections from WT (solid bars) and TPH2KI (shaded bars) mice (N = 5 per group), 2 wk BDL surgery. Results are normalized to WT-BDL values and displayed as means ± SE. Differences between groups are evaluated by 2-tailed Student's t-test. *P < 0.05.
DISCUSSION
Chronic obstruction of the extrahepatic biliary system stimulates proliferation of ductular cells within the liver, as well as progressive periportal accumulation of fibrous matrix that eventually bridges adjacent portal tracts, resulting in secondary biliary cirrhosis (13). This study identifies 5-HT as an important modulator of this fibroductular response by demonstrating that BDL-induced ductular cell proliferation and liver fibrosis are both exacerbated significantly in TPH2KI mice that have an inactivating mutation of TPH2 and reduced biliary 5-HT content.
Accompanying in vitro studies show that cholangiocytes express both TPH1 (the typical TPH isoform that is expressed by nonneuronal cells) and TPH2 (the so-called neuron-specific TPH isoform) and prove that cholangiocytes are capable of synthesizing 5-HT de novo. The results also indicate that 5-HT directly represses cholangiocyte proliferation and demonstrate that cholangiocyte production of this autocrine growth inhibitor is significantly influenced by TGF-β1, a MF-derived factor that selectively downregulates cholangiocyte expression of TPH2.
Earlier studies showed that ductular cells and liver MF typically aggregate during cholestatic liver injury (12, 13, 37) and proved that the liver MF release soluble factors that promote ductular cell growth (27). Therefore, the new findings reveal a novel paracrine mechanism by which liver MF promote the growth of neighboring cholangiocytes. The cell culture data also demonstrate that 5-HT directly stimulates liver MF production of TGF-β, a profibrogenic factor that supports MF growth. The latter information expands the list of cholangiocyte-derived profibrogenic factors (7), which is already known to include PDGF-BB, a potent MF mitogen (7, 9). The aggregate data, therefore, provide further support for the concept that cross talk between cholangiocytes and liver MF orchestrates biliary remodeling by modulating cellular exposure to growth regulators.
Additional research will be required to clarify the specific mechanisms by which MF-derived TGF-β1 results in downregulation of cholangiocyte TPH2 expression. Because TPH2 was not previously known to be expressed by cholangiocytes, its regulation in such cells has not been explored. However, in other cell types, interactions between Angpt1, Tie2, and TGF-β1 were shown to modulate 5-HT production (4, 5, 10, 23, 35). Similar mechanisms may be operative in cholangiocytes because we found that coculturing cholangiocytes with liver MF significantly repressed cholangiocyte expression of Angpt1 and Tie2 mRNAs. Further work is also needed to tease apart the relative contributions and cumulative effects of various cholangiocyte-derived factors on MF growth. Our studies show that 5-HT stimulates such cells to produce TGF-β1, an acknowledged MF growth factor. However, it is also evident that other autocrine/paracrine factors are able to drive MF growth when cholangiocyte production of 5-HT declines because MF populations generally expand as a net effect of cholangiocyte-liver MF paracrine interactions (12, 13, 25, 27, 37). Indeed, the latter was demonstrated to occur in the present studies of BDL-TPH2KI mice, which accumulated more MF and developed worse liver fibrosis than WT mice that maintained higher levels of 5-HT.
Many types of chronic liver injury are capable of triggering fibrosis and ductular cell accumulation (dubbed the ductular reaction). This response is often accompanied by expansion of liver progenitor populations, including hepatoblasts/oval cells, bipotent liver epithelial progenitors which reside along canals of Hering, the proximal branches of the biliary tree (30, 32). The present study reveals a role for 5-HT in this process because TPH2KI mice, which are deficient in 5-HT, exhibit increased numbers of liver progenitors during chronic cholestatic injury. Interestingly, such populations included cells that expressed markers of immature hepatocytes (e.g., α-fetoprotein), as well as cells with features of immature cholangiocytes (e.g., nestin expression). These new findings, therefore, suggest that suppression of biliary 5-HT production promotes the outgrowth of hepatoblasts/oval cells and their progeny. This possibility is further supported by our finding that small AFP-positive hepatocytic cells in periportal areas accumulated BrdU, indicating enhanced proliferative activity in this compartment. Such results are particularly provocative in light of published evidence that exposing mature hepatocytes to platelet-derived 5-HT stimulates their proliferation (15). Thus 5-HT appears to exert differential effects on the growth of immature and mature hepatocytes. The aggregate data, therefore, support the concept that progenitor populations, rather than mature hepatocytes, fueled regenerative responses that caused hepatomegaly to occur in 5-HT-deficient TPH2KI mice following BDL.
Further research will be required to delineate the specific growth-modulatory mechanisms that are influenced by 5-HT. Previous publications identify interleukin (IL)-6 and factors such as Foxa1 and Foxa2 that regulate IL-6 expression as attractive targets for 5-HT regulation. In humans, chronic cholestatic liver diseases have been associated with decreased hepatic expression of Foxa2 (2), a factor that interacts with Foxa1 to repress IL-6 transcription (17). Enhanced proliferative activity of ductular cells following BDL has generally been attributed to IL-6 because BDL induces cholangiocyte production of IL-6, and various strategies that reduce IL-6 activity attenuate BDL-induced proliferation of ductular cells (19, 20). Our preliminary comparison of IL-6 mRNA levels in livers of WT and TPH2KI mice post-BDL demonstrated a sixfold greater induction of IL-6 expression in TPH2KI mice than WT mice (Supplemental Fig. S5). To date, however, we have detected few, if any, differences in expression of Foxa1 or Foxa2 between WT and TPH2KI mice post-BDL (data not shown). Thus additional research is needed to determine how reducing 5-HT amplifies IL-6 production. Such work will be important given a recent report that overexpression of IL-6 occurred in the livers of mice with targeted deletion of both Foxa1 and Foxa2 in liver progenitors and drove expansion of AFP-positive progenitor populations, ductular proliferation, and liver fibrosis in such animals (17).
In summary, the new evidence that liver ductular cells are capable of synthesizing 5-HT de novo and that autocrine/paracrine mechanisms that suppress biliary 5-HT production stimulate proliferation of ductular cells, liver MF, and progenitors demonstrates that liver cell-derived 5-HT normally functions as one of the “brakes” that restrains the outgrowth of such cells. This new information complements existing knowledge that 5-HT is an important mitogenic factor for mature hepatocytes (15). Unlike cholangiocytes, mature hepatocytes lack the enzymatic machinery that is necessary for 5-HT biosynthesis and, thus, their exposure to 5-HT depends on 5-HT release from other cells. In addition to cholangiocytes, platelets and neurons are capable of releasing 5-HT within the hepatic microenvironment (15, 21, 33). Hence concentrations of 5-HT may differ considerably across various liver microdomains, thereby permitting cell-specific regulation of proliferative activity. The net effects of 5-HT may also differ across the spectrum of liver epithelial cell differentiation, in accordance with variations in cellular repertoires of 5-HT receptors and expression of enzymes that synthesize and degrade 5-HT.
Although relatively little is known about such issues in adult livers, it has already been shown that depletion of platelet-derived 5-HT inhibited liver regeneration after partial hepatectomy in rodents (15). In humans, at least one episode of idiosyncratic hepatotoxicity and progressive liver failure has been attributed to the antiplatelet drug clopidogrel (11). Millions of individuals take drugs that alter 5-HT homeostasis as treatments for depression and other neuropsychiatric disorders. Conversely, fibroductular responses that occur in most types of fibrosing and cholestatic liver diseases would be predicted to reduce hepatic production of 5-HT. Whether or not this contributes to any of the neuropsychiatric complications of advanced liver disease, however, has not been examined. Nonetheless, the aggregate observations raise the intriguing possibility that serotonergic signaling influences various outcomes of liver injury and identify this area as worthy of future research.
GRANTS
This work was supported by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases RO1 DK077794 (A. M. Diehl).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to W. C. Stone for administrative support to this study and to H. M. Vandongen for technical advice. The TROMA-III antibody developed by Rolf Kemler was obtained from the Developmental Studies Hybridoma Bank, developed under the auspices of the National Institute of Child Health and Human Development and maintained by The University of Iowa, Department of Biology, Iowa City, IA 52242.
REFERENCES
- 1. Beaulieu JM, Zhang X, Rodriguiz RM, Sotnikova TD, Cools MJ, Wetsel WC, Gainetdinov RR, Caron MG. Role of GSK3 beta in behavioral abnormalities induced by serotonin deficiency. Proc Natl Acad Sci USA 105: 1333–1338, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bochkis IM, Rubins NE, White P, Furth EE, Friedman JR, Kaestner KH. Hepatocyte-specific ablation of Foxa2 alters bile acid homeostasis and results in endoplasmic reticulum stress. Nat Med 14: 828–836, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Clotman F, Jacquemin P, Plumb-Rudewiez N, Pierreux CE, Van der Smissen P, Dietz HC, Courtoy PJ, Rousseau GG, Lemaigre FP. Control of liver cell fate decision by a gradient of TGF beta signaling modulated by Onecut transcription factors. Genes Dev 19: 1849–1854, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dewachter L, Adnot S, Fadel E, Humbert M, Maitre B, Barlier-Mur AM, Simonneau G, Hamon M, Naeije R, Eddahibi S. Angiopoietin/Tie2 pathway influences smooth muscle hyperplasia in idiopathic pulmonary hypertension. Am J Respir Crit Care Med 174: 1025–1033, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Eddahibi S, Guignabert C, Barlier-Mur AM, Dewachter L, Fadel E, Dartevelle P, Humbert M, Simonneau G, Hanoun N, Saurini F, Hamon M, Adnot S. Cross talk between endothelial and smooth muscle cells in pulmonary hypertension: critical role for serotonin-induced smooth muscle hyperplasia. Circulation 113: 1857–1864, 2006 [DOI] [PubMed] [Google Scholar]
- 6. Fabris L, Cadamuro M, Fiorotto R, Roskams T, Spirli C, Melero S, Sonzogni A, Joplin RE, Okolicsanyi L. 2006. Effects of angiogenic factor overexpression by human and rodent cholangiocytes in polycystic liver diseases. Hepatology 43: 1001–1012 [DOI] [PubMed] [Google Scholar]
- 7. Glaser SS, Gaudio E, Miller T, Alvaro D, Alpini G. Cholangiocyte proliferation and liver fibrosis. Expert Rev Mol Med 11: e7, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gleiberman AS, Encinas JM, Mignone JL, Michurina T, Rosenfeld MG, Enikolopov G. Expression of nestin-green fluorescent protein transgene marks oval cells in the adult liver. Dev Dyn 234: 413–421, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Grappone C, Pinzani M, Parola M, Pellegrini G, Caligiuri A, DeFranco R, Marra F, Herbst H, Alpini G, Milani S. Expression of platelet-derived growth factor in newly formed cholangiocytes during experimental biliary fibrosis in rats. J Hepatol 31: 100–109, 1999 [DOI] [PubMed] [Google Scholar]
- 10. Izikki M, Hanoun N, Marcos E, Savale L, Barlier-Mur AM, Saurini F, Eddahibi S, Hamon M, Adnot S. Tryptophan hydroxylase 1 knockout and tryptophan hydroxylase 2 polymorphism: effects on hypoxic pulmonary hypertension in mice. Am J Physiol Lung Cell Mol Physiol 293: L1045–L1052, 2007 [DOI] [PubMed] [Google Scholar]
- 11. Kastalli S, El Aidli S, Zaiem A, Ben Abdallah H, Daghfous R. Fatal liver injury associated with clopidogrel. Fundam Clin Pharmacol 24: 433–435, 2010 [DOI] [PubMed] [Google Scholar]
- 12. Kinnman N, Francoz C, Barbu V, Wendum D, Rey C, Hultcrantz R, Poupon R, Housset C. The myofibroblastic conversion of peribiliary fibrogenic cells distinct from hepatic stellate cells is stimulated by platelet-derived growth factor during liver fibrogenesis. Lab Invest 83: 163–173, 2003 [DOI] [PubMed] [Google Scholar]
- 13. Kinnman N, Hultcrantz R, Barbu V, Rey C, Wendum D, Poupon R, Housset C. PDGF-mediated chemoattraction of hepatic stellate cells by bile duct segments in cholestatic liver injury. Lab Invest 80: 697–707, 2000 [DOI] [PubMed] [Google Scholar]
- 14. Lazaridis KN, Strazzabosco M, Larusso NF. The cholangiopathies: disorders of biliary epithelia. Gastroenterology 127: 1565–1577, 2004 [DOI] [PubMed] [Google Scholar]
- 15. Lesurtel M, Graf R, Aleil B, Walther DJ, Tian Y, Jochum W, Gachet C, Bader M, Clavien PA. Platelet-derived serotonin mediates liver regeneration. Science 312: 104–107, 2006 [DOI] [PubMed] [Google Scholar]
- 16. Li R, Liang J, Ni S, Zhou T, Qing X, Li H, He W, Chen J, Li F, Zhuang Q, Qin B, Xu J, Li W, Yang J, Gan Y, Qin D, Feng S, Song H, Yang D, Zhang B, Zeng L, Lai L, Esteban MA, Pei D. A mesenchymal-to-epithelial transition initiates and is required for the nuclear reprogramming of mouse fibroblasts. Cell Stem Cell 7: 51–63, 2010 [DOI] [PubMed] [Google Scholar]
- 17. Li Z, White P, Tuteja G, Rubins N, Sackett S, Kaestner KH. Foxa1 and Foxa2 regulate bile duct development in mice. J Clin Invest 119: 1537–1545, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lin W, Metzakopian E, Mavromatakis YE, Gao N, Balaskas N, Sasaki H, Briscoe J, Whitsett JA, Goulding M, Kaestner KH, Ang SL. Foxa1 and Foxa2 function both upstream of and cooperatively with Lmx1a and Lmx1b in a feedforward loop promoting mesodiencephalic dopaminergic neuron development. Dev Biol 333: 386–396, 2009 [DOI] [PubMed] [Google Scholar]
- 19. Liu Z, Sakamoto T, Ezure T, Yokomuro S, Murase N, Michalopoulos G, Demetris AJ. Interleukin-6, hepatocyte growth factor, and their receptors in biliary epithelial cells during a type I ductular reaction in mice: interactions between the periductal inflammatory and stromal cells and the biliary epithelium. Hepatology 28: 1260–1268, 1998 [DOI] [PubMed] [Google Scholar]
- 20. Liu Z, Sakamoto T, Yokomuro S, Ezure T, Subbotin V, Murase N, Contrucci S, Demetris AJ. Acute obstructive cholangiopathy in interleukin-6 deficient mice: compensation by leukemia inhibitory factor (LIF) suggests importance of gp-130 signaling in the ductular reaction. Liver 20: 114–124, 2000 [DOI] [PubMed] [Google Scholar]
- 21. Marzioni M, Glaser S, Francis H, Marucci L, Benedetti A, Alvaro D, Taffetani S, Ueno Y, Roskams T, Phinizy JL, Venter J, Fava G, Lesage GD, Alpini G. Autocrine/paracrine regulation of the growth of the biliary tree by the neuroendocrine hormone serotonin. Gastroenterology 128: 121–137, 2005 [DOI] [PubMed] [Google Scholar]
- 22. Matsumoto K, Fujii H, Michalopoulos G, Fung JJ, Demetris AJ. Human biliary epithelial cells secrete and respond to cytokines and hepatocyte growth factors in vitro: interleukin-6, hepatocyte growth factor and epidermal growth factor promote DNA synthesis in vitro. Hepatology 20: 376–382, 1994 [PubMed] [Google Scholar]
- 23. Nishishita T, Lin PC. Angiopoietin 1, PDGF-B, and TGF-beta gene regulation in endothelial cell and smooth muscle cell interaction. J Cell Biochem 91: 584–593, 2004 [DOI] [PubMed] [Google Scholar]
- 24. Nocito A, Georgiev P, Dahm F, Jochum W, Bader M, Graf R, Clavien PA. Platelets and platelet-derived serotonin promote tissue repair after normothermic hepatic ischemia in mice. Hepatology 45: 369–376, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Omenetti A, Porrello A, Jung Y, Yang L, Popov Y, Choi SS, Witek RP, Alpini G, Venter J, Vandongen HM, Syn WK, Baroni GS, Benedetti A, Schuppan D, Diehl AM. Hedgehog signaling regulates epithelial-mesenchymal transition during biliary fibrosis in rodents and humans. J Clin Invest 118: 3331–3342, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Omenetti A, Popov Y, Jung Y, Choi SS, Witek RP, Yang L, Brown KD, Schuppan D, Diehl AM. The hedgehog pathway regulates remodelling responses to biliary obstruction in rats. Gut 57: 1275–1282, 2008 [DOI] [PubMed] [Google Scholar]
- 27. Omenetti A, Yang L, Li YX, McCall SJ, Jung Y, Sicklick JK, Huang J, Choi S, Suzuki A, Diehl AM. Hedgehog-mediated mesenchymal-epithelial interactions modulate hepatic response to bile duct ligation. Lab Invest 87: 499–514, 2007 [DOI] [PubMed] [Google Scholar]
- 28. Omori M, Evarts RP, Omori N, Hu Z, Marsden ER, Thorgeirsson SS. Expression of alpha-fetoprotein and stem cell factor/c-kit system in bile duct ligated young rats. Hepatology 25: 1115–1122, 1997 [DOI] [PubMed] [Google Scholar]
- 29. Omori M, Omori N, Evarts RP, Teramoto T, Thorgeirsson SS. Coexpression of flt-3 ligand/flt-3 and SCF/c-kit signal transduction system in bile-duct-ligated SI and W mice. Am J Pathol 150: 1179–1187, 1997 [PMC free article] [PubMed] [Google Scholar]
- 30. Roskams T, Desmet V. Embryology of extra- and intrahepatic bile ducts, the ductal plate. Anat Rec (Hoboken) 291: 628–635, 2008 [DOI] [PubMed] [Google Scholar]
- 31. Roskams TA, Libbrecht L, Desmet VJ. Progenitor cells in diseased human liver. Semin Liver Dis 23: 385–396, 2003 [DOI] [PubMed] [Google Scholar]
- 32. Roskams TA, Theise ND, Balabaud C, Bhagat G, Bhathal PS, Bioulac-Sage P, Brunt EM, Crawford JM, Crosby HA, Desmet V, Finegold MJ, Geller SA, Gouw AS, Hytiroglou P, Knisely AS, Kojiro M, Lefkowitch JH, Nakanuma Y, Olynyk JK, Park YN, Portmann B, Saxena R, Scheuer PJ, Strain AJ, Thung SN, Wanless IR, West AB. Nomenclature of the finer branches of the biliary tree: canals, ductules, and ductular reactions in human livers. Hepatology 39: 1739–1745, 2004 [DOI] [PubMed] [Google Scholar]
- 33. Ruddell RG, Mann DA, Ramm GA. The function of serotonin within the liver. J Hepatol 48: 666–675, 2008 [DOI] [PubMed] [Google Scholar]
- 34. Sulaiman P, Joseph B, Kaimal SB, Paulose CS. Decreased hepatic 5-HT1A receptors during liver regeneration and neoplasia in rats. Neurochem Res 33: 444–449, 2008 [DOI] [PubMed] [Google Scholar]
- 35. Sullivan CC, Du L, Chu D, Cho AJ, Kido M, Wolf PL, Jamieson SW, Thistlethwaite PA. Induction of pulmonary hypertension by an angiopoietin 1/TIE2/serotonin pathway. Proc Natl Acad Sci USA 100: 12331–12336, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tan CE, Moscoso GJ. The developing human biliary system at the porta hepatis level between 11 and 25 weeks of gestation: a way to understanding biliary atresia. Part 2. Pathol Int 44: 600–610, 1994 [DOI] [PubMed] [Google Scholar]
- 37. Tuchweber B, Desmouliere A, Bochaton-Piallat ML, Rubbia-Brandt L, Gabbiani G. Proliferation and phenotypic modulation of portal fibroblasts in the early stages of cholestatic fibrosis in the rat. Lab Invest 74: 265–278, 1996 [PubMed] [Google Scholar]
- 38. Walther DJ, Bader M. A unique central tryptophan hydroxylase isoform. Biochem Pharmacol 66: 1673–1680, 2003 [DOI] [PubMed] [Google Scholar]
- 39. Walther DJ, Peter JU, Bashammakh S, Hortnagl H, Voits M, Fink H, Bader M. Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science 299: 76, 2003 [DOI] [PubMed] [Google Scholar]
- 40. Zhang X, Beaulieu JM, Sotnikova TD, Gainetdinov RR, Caron MG. Tryptophan hydroxylase-2 controls brain serotonin synthesis. Science 305: 217, 2004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.