Abstract
Chronic inflammatory disorders such as inflammatory bowel diseases (IBDs) affect bone metabolism and are frequently associated with the presence of osteopenia, osteoporosis, and increased risk of fractures. Although several mechanisms may contribute to skeletal abnormalities in IBD patients, inflammation and inflammatory mediators such as TNF, IL-1β, and IL-6 may be the most critical. It is not clear whether the changes in bone metabolism leading to decreased mineral density are the result of decreased bone formation, increased bone resorption, or both, with varying results reported in experimental models of IBD and in pediatric and adult IBD patients. New data, including our own, challenge the conventional views, and contributes to the unraveling of an increasingly complex network of interactions leading to the inflammation-associated bone loss. Since nutritional interventions (dietary calcium and vitamin D supplementation) are of limited efficacy in IBD patients, understanding the pathophysiology of osteopenia and osteoporosis in Crohn's disease and ulcerative colitis is critical for the correct choice of available treatments or the development of new targeted therapies. In this review, we discuss current concepts explaining the effects of inflammation, inflammatory mediators and their signaling effectors on calcium and phosphate homeostasis, osteoblast and osteoclast function, and the potential limitations of vitamin D used as an immunomodulator and anabolic hormone in IBD.
Keywords: bone mineral density, Crohn's disease, osteopenia, osteoporosis, ulcerative colitis
inflammatory bowel diseases (IBDs) represent a group of chronic inflammatory disorders of the intestinal tract that to this date remains idiopathic. More recent basic and clinical research indicates that the chronic inflammatory reaction of the intestinal mucosa is directed against the gut microbiota in individuals with genetic and/or environmental susceptibilities. Disease onset occurs typically during young adulthood (25–35 yr), although ∼20–25% of cases are diagnosed during childhood (93). On a clinical basis, two major subtypes of IBD are defined as Crohn's disease (CD), which potentially affects any part of the gastrointestinal tract from the mouth to the anus, and ulcerative colitis (UC), an inflammatory condition limited to the colonic mucosa. However, IBD does not affect just a single organ and should be considered a systemic disease with several extraintestinal manifestations, which occur in a large proportion of IBD patients (60). Osteopenia and osteoporosis are two of the more common extraintestinal symptoms with a general consensus that IBD patients are at a significantly higher risk of developing metabolic bone disease and low bone mineral density (BMD) than the healthy subjects. The relative risk of fracture in IBD patients has been estimated to be 40% higher than in general population (6), with the prevalence of osteopenia and osteoporosis varying significantly depending on the study populations, geographic location, and study design, with the reported range of osteopenia at 22–77% and osteoporosis at 17–41% (6). Among the many risk factors predisposing for the loss of BMD in general population, several are more pertinent in IBD patient population. Some of them are listed in Table 1.
Table 1.
Risk factors for osteopenia/osteoporosis in inflammatory bowel disease
| ∙ Malnutrition |
| ∙ Malabsorption of vitamin D, calcium, and vitamin K |
| ∙ Low body mass index |
| ∙ Low bone mineral intensity peak in IBD patients with pediatric onset |
| ∙ Chronic inflammatory state |
| ∙ Type of IBD (CD vs. UC; small intestinal involvement) |
| ∙ Increasing age |
| ∙ Female gender |
| ∙ Immobilization |
| ∙ Use of corticosteroids |
| ∙ Previous fragility fracture |
| ∙ Hypogonadism |
| ∙ Smoking |
| ∙ Family history of osteoporosis |
IBD, inflammatory bowel disease; CD, Crohn's disease; UC, ulcerative colitis.
Although the data regarding patients with early (pediatric) onset IBD are limited, a recent study clearly demonstrated low BMD associated with IBD in children (especially in CD) (98). Pediatric IBD patients form a separate group not only because of distinct disease characteristics and perhaps distinct genetic predispositions (93), but also because of differences in their bone metabolism. During the prepubescent and early pubescent period, bone modeling is the predominant form of skeletal growth, and the activities of osteoblasts and osteoclasts are not coupled. Therefore, the principles of bone loss observed in adult patients with chronic inflammation or during postmenopausal osteopenia/osteoporosis are not directly applicable (97). Although the risk of fracture in pediatric patients with IBD is not well established and not without controversy (44), a more important question is whether chronic, even subclinical inflammation predisposes the child to increased risk of future fractures later in life, as a result of low bone accrual and lower peak BMD reached during early adulthood. Although it has been long known that poor bone health in childhood often leads into osteopenia/osteoporosis and increased risk of fracture in adulthood (50), longitudinal studies with pediatric IBD patients are yet to be performed.
Prevalence, risk factors, clinical evaluation, and prevention and treatment of bone loss associated with IBD were reviewed very well by Lichtenstein et al. (62) and Mascarenhas and Thayu (68). It has to be acknowledged, however, that the majority of knowledge on pathogenesis and pharmacological interventions into bone metabolism in IBD is not tailored specifically to unique aspects of this group of diseases. The mechanisms and contribution of altered osteoblast and/or osteoclast function in IBD have not been clearly defined and many of the proposed mechanisms and the proposed clinical approaches are not the result of direct experimentation, but rather of an extrapolation of the growing field of osteoimmunology and rheumatoid arthritis (73) to the known cellular and soluble inflammatory mediators involved in the pathogenesis of IBD. The goal of this review is to focus on recent preclinical studies with animal models of IBD as well as findings derived directly from IBD patients. Studies addressing specific issues related to mineral metabolism and bone health in IBD will likely point to mechanisms common with other disorders as well as unique to IBD (perhaps unique to their different forms and time of onset) that may be explored in tailoring therapies aimed at bone loss prevention.
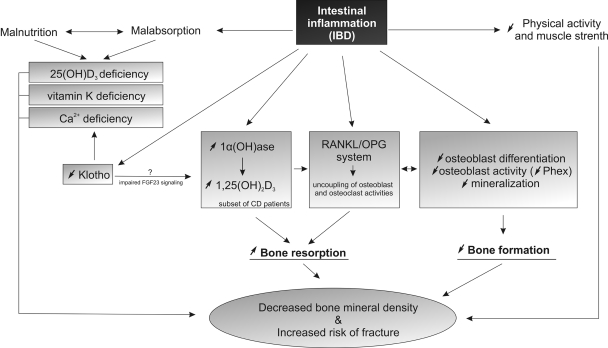
The pathogenesis of osteopenia and osteoporosis in IBD has been suggested to result from altered rates of bone formation and resorption secondary to multifactorial but still poorly characterized set of mechanisms. These are typically sorted into two major groups: poor nutritional status and malabsorption (particularly affecting vitamin D, vitamin K, and Ca2+ homeostasis), and inflammation with IBD-associated inflammatory infiltrate and soluble mediators (IL-1, IL-6, TNF) affecting bone more directly. More recent studies in animal models of IBD indicate that malabsorption and inflammation are not two independent contributors to IBD-associated BMD loss and that inflammation and inflammatory cytokines actively contribute to the abnormalities in the intestinal and renal mineral and vitamin absorption. In this review, we will outline current knowledge about the effects of intestinal inflammation on epithelial mineral handling, vitamin D and K absorption and metabolism, and the role of inflammation in bone modeling and remodeling. The described relationships are schematically illustrated in Fig. 1.
Fig. 1.
Factors and mechanisms associated with bone mass loss and increased risk of fractures in patients with inflammatory bowel diseases. IBD, inflammatory bowel disease; RANKL, receptor activator of NF-κB ligand; OPG, osteoprotegerin.
SUPPLY OF Ca2+ AND PO42−: THE BUILDING BLOCKS OF THE INORGANIC COMPONENT OF THE BONE MINERAL MATRIX
The inorganic component makes up 60% of the dry weight of bone and is composed primarily of calcium hydroxyapatite, Ca10(PO4)6(OH)2. Calcium hydroxyapatite crystals are arranged parallel to collagen fibers, which maximizes the bone's resistance to both tensile (stretch) and compressive forces. Bone is highly dynamic and it undergoes constant remodeling throughout life. The remodeling involves coupled resorption of existing bone and the formation of new bone. The supply of calcium and inorganic phosphate available for bone formation is a result of highly regulated and complex homeostatic mechanism involving paracellular and transcellular intestinal and renal (re)absorption. In this review, we provide only a brief overview of those physiological mechanisms and focus on their regulation in inflammatory states as pertinent to bone loss in IBD patients. For more detailed description of intestinal and renal calcium and phosphate transport mechanism, the reader is referred to recent literature reviews and references therein (20, 30, 51, 66, 81).
Intestinal and Renal Ca2+ (Re)absorption
Intestinal malabsorption has been intuitively linked to the pathogenesis of bone loss in IBD and celiac disease patients. Several factors likely contribute to this aspect of diminished Ca2+ supply. Avoidance of milk and dairy food consumption and relatively common lactose intolerance may prevent the achievement of an adequate peak bone mass and may predispose to osteoporosis (31). In the intestinal tract, calcium is primarily absorbed in the duodenum and proximal jejunum. Depending on the segment and the luminal Ca2+ concentration, it is absorbed through a weakly regulated paracellular route, or by highly regulated and vitamin D3-sensitive transcellular pathway. When dietary Ca2+ intake and luminal concentration is low (<20 mM), active transcellular transport in the duodenum predominates and accounts for ∼80% of the total Ca2+ absorption. At higher Ca2+ supply (≥50 mM), the contribution of active transport diminishes to below 10%, largely because of the short duodenal transit time and because of downregulation of the key molecular components of the transcellular Ca2+ transport proteins.
Apical Ca2+ entry.
Calcium enters the epithelial cells via selective non-voltage-gated Ca2+ channels at the luminal membrane under the influence of a steep, inwardly directed electrochemical gradient. This facilitated diffusion is mediated by two major members of the TRPV subfamily (transient receptor potential vanilloid channels), TRPV5 (predominant in the kidney) and TRPV6 (predominant in the duodenum). Both channels are extensively regulated by transcriptional mechanisms [calciotropic hormones including 1,25-dihydroxyvitamin D3 (1,25(OH)2D3), parathyroid hormone, and estrogens], by pH- and Ca2+-dependent regulatory mechanisms, by trafficking of TRPV5 and TRPV6 to the luminal membrane, and by physical interactions with associated proteins. Several comprehensive reviews on this subject have been recently published (30, 51). Results from knockout mice highlight the importance of these two transport proteins in Ca2+ homeostasis. TRPV5−/− mice show robust renal Ca2+ wasting, with active Ca2+ reabsorption in distal convoluted tubules and connecting tubules effectively abolished, and with a reduced bone thickness despite the compensatory intestinal Ca2+ hyperabsorption accompanied by significantly enhanced duodenal TRPV6 expression (82). TRPV6−/− mice display a consistent decrease in Ca2+ absorption over time, increased urinary excretion, and decreased BMD (15).
Transcellular Ca2+ movement.
Free ionized cytoplasmic Ca2+ concentration has to be tightly controlled because of its cytotoxic effects and because of its inhibitory effects on TRPV5 and TRPV6-mediated Ca2+ currents. Some of these effects are mediated by associated proteins like calmodulin and protein kinase C substrate 80K-H. There is a large body of literature on the role of calbindins D9K and D28K acting both as Ca2+ buffers and ferries facilitating transcytoplasmic movement of Ca2+ to the basolateral site. Despite extensive research, the role of the two calbindins in both functions remains controversial. Calbindin D9K knockout mice have normal plasma Ca2+, the same increase in the efficiency of Ca2+ absorption when fed low Ca2+ diet, and display no difference in 1,25(OH)2D3-induced transcellular Ca2+ transport (4, 58). The phenotype of calbindin D28K knockout mice is also negligible. These mice are not different from the wild-type littermates (growth, life span, fertility), have normal plasma Ca2+ and Pi levels, and display only mild impairment in motor coordination/motor learning (abnormal Ca2+ oscillations in Purkinje cells of the cerebellum) (2). Moreover, D28K knockout mice had a measurable increase in bone volume and stiffness, indicating that calbindin-D28K plays an important role in bone remodeling (65).
An alternative vesicular transport model has also been proposed that may partially explain the lack of a significant phenotype in calbindin-deficient mice. According to this model, apical formation of Ca2+-loaded vesicles is initiated upon TRPV-mediated Ca2+ uptake. These vesicles can then be transported vectorially by microtubules, or they can fuse with endoplasmic reticulum (ER) followed by passive diffusion with the ER. Ca2+ is then released from the ER at the basolateral membrane through calcium release channels and extruded by NCX1 and PMCA1b (see Basolateral Ca2+ exit). Alternatively, these vesicles may fuse with the lysosomes, which move laterally to eventually coalesce with the lateral membrane and release the content through exocytosis. This route is also stimulated by 1,25(OH)2D3. Net epithelial Ca2+ is inhibited by chloroquine, suggesting that this may be the main route of transcellular Ca2+ trafficking (51).
Basolateral Ca2+ exit.
Two transport proteins have been implicated in the cellular exit of Ca2+. One of them is a bidirectional Na+/Ca2+ exchanger, NCX1, which under physiological electrochemical gradients is responsible for basolateral Ca2+ extrusion. It is widely expressed in the absorptive epithelia, although it is considered to be of primary importance in the renal distal convoluted tubules (16) and of minor significance in the intestinal epithelium (45). The physiological importance of this exchanger is highlighted by the embryonic lethality of NCX1-null knockout mice (55). In heterozygous mice, expression of NCX1 protein in the tubular epithelial cells and Ca2+ influx via NCX1 in renal tubules are markedly attenuated. The second transporter involved in basolateral Ca2+ extrusion is a P-type ATPase, a high-affinity Ca2+ efflux pump PMCA1b, abundantly expressed in the intestine (45). Calcium is expelled through a channel-like opening formed by the transmembrane elements, and phosphorylation is believed to bring about the necessary conformational change such that Ca2+ bound to the enzyme is propelled through the opening. Similarly to NCX1, PMCA1b-null knockout is embryonically lethal (75).
Ca2+ Transport in IBD
Surprisingly little is known on the effects of acute and chronic inflammation on intestinal and renal calcium handling. In recent comprehensive reviews of the mechanism and regulation of epithelial Ca2+ absorption in health and disease, the role of inflammation and inflammatory mediators is not even mentioned (71, 96). On the other hand, malnutrition and negative systemic Ca2+ balance in conjunction with vitamin D insufficiency are widely considered as important factors in the pathogenesis of bone loss in IBD patients. However, in a randomized, placebo-controlled trial in glucocorticoid-treated patients with IBD, the intake of 250 IU of vitamin D and 1,000 mg/day calcium had no significant benefit in bone density at 1 year of follow-up (14). Similarly, a study with pediatric IBD patients supplemented with calcium with or without vitamin D failed to demonstrate any effect on BMD accrual (12). It appears, therefore, that the supplementation with calcium and vitamin D, although accepted as a cost-effective medication, may be insufficient in the prevention and treatment of IBD-associated osteoporosis. It is not inconceivable that changes in expression and activity of key Ca2+ transport proteins in the gut and kidney, induced by associated inflammatory mediators, are the main reason for failure of dietary Ca2+ and vitamin D supplementation in IBD. Indeed, a very recent report (47) from the group of Joost Hoenderop in the Netherlands demonstrated for the first time that in mouse model of Crohn's-like ileitis (TNF-α-overexpressing TNFΔARE mice), both duodenal and renal Ca2+ absorptive epithelia displayed significant downregulation of TRPV6, calbindin D9K, PMCA1b, as well as calbindin D28K and NCX1, respectively (47). In this report, changes in expression of these Ca2+ transport genes were accompanied with increased bone resorption, as documented by tomography scanning (reduced trabecular and cortical bone thickness and volume) and increased total deoxypyridinoline in serum of the TNFΔARE mice.
Contrary to the initially widespread notion, loss of bone density in IBD patients poorly correlates with cumulative steroid exposure and is frequently observed in newly diagnosed steroid-naive patients. However, corticosteroids undoubtedly contribute to bone loss in chronically ill IBD patients by directly influencing the balance of bone formation and resorption. Additional likely mechanism pertinent to intestinal Ca2+ absorption was described by Huybers et al. (48), who demonstrated that in mice orally administered with methylprednisolone, duodenal absorption of Ca2+ was significantly decreased. This decrease coincided with inhibited expression of TRPV6 and calbindin D9K mRNA and protein, with unaltered serum Ca2+ or 1,25(OH)2D3 levels (48).
These murine observations are yet to be translated to the clinical settings. Kumari et al. (56) recently demonstrated in a small pilot study with four CD patients in remission that fractional intestinal Ca2+ absorption at baseline is similar to that of control subjects. Moreover, both groups of patients responded similarly to oral 1,25(OH)2D3 (0.25 or 0.5 μg twice daily) given for 7 days (56). Keeping the small sample size in mind, these findings suggest that in patients with mild CD or in patients in remission intestinal Ca2+ is not impaired and that there may not be a need for high-dose calcitriol supplementation. Similar well-designed studies with higher numbers of patients with both active and quiescent IBD are needed to verify these preliminary findings. It is also plausible that supraphysiological vitamin D3 supplementation may be detrimental for the overall bone health in active inflammation (see discussion in Vitamin D Status in IBD below).
Intestinal and Renal Pi (Re)absorption
Phosphate is one the most abundant dietary constituents and one of the most abundant minerals in the organism. Complex homeostatic mechanism evolved in mammals to maintain the extracellular and intracellular Pi levels within a narrow range necessary for many biological processes, including skeletal development and BMD. Prolonged phosphate deficiency and hypophosphatemia resulting from malnutrition or malabsorption may, among other complications, lead to bone demineralization, leading to skeletal defects such as rickets in children and osteomalacia in adults. Phosphate homeostasis is maintained through a concerted action of sodium-dependent phosphate transporters forming the SLC34 solute carrier family. Most of the active renal Pi reabsorption occurs in the proximal convoluted tubules via NaPi-IIa (SLC34A1) and NaPi-IIc (SLC34A3), whereas NaPi-IIb (SLC34A2) is the predominant intestinal isoform. More recent data also implicate members of the SLC20 family, or type III transporters, specifically PiT1 and PiT2, initially identified as retroviral receptors, in the intestinal and renal Pi transport (26). Contrary to initial findings, they are now believed to be expressed at the intestinal (PiT1) (40) and renal brush border membrane (BBM) (PiT2) (106).
Very little is known about the effects of intestinal inflammation on intestinal or renal Pi (re)absorption. Similar to Ca2+, serum level of Pi is not a good indicator of modest changes in active mineral transport, unless more severe malabsorption and cachexia are present. Constant serum level of Ca2+ and Pi are the primary determinants of the homeostatic mechanisms controlling both minerals, with bone being the disposable reservoir for the systemic demand for these two minerals. It is therefore not surprising that little to no changes in serum Ca2+ or Pi are observed in IBD patients. Urinary excretion is rarely reported and mostly limited to patients at risk for or diagnosed with uro- or nephrolithiasis. In a recent study, our group has demonstrated that trinitrobenzene sulfonic acid (TNBS)-induced colitis resulted in significant inhibition of NaPi-IIb-mediated intestinal Pi absorption in isolated BBM vesicles (23). This was accompanied by an ∼50% decrease in NaPi-IIb mRNA and protein expression. This inhibition could be recapitulated in TNF-treated Caco-2 cells, with the transcriptional mechanism potentially involving epidermal growth factor receptor, and ERK1/2-dependent decrease in NF1 transcription factor interaction with the basal region of the human NaPi-IIb gene promoter (23). The extent to which this inhibition contributes to the limited supply of Pi for the skeletal system in IBD patients remains unknown. One report questioned the significance of Na+-dependent Pi absorption in the gut (108), an observation seemingly in agreement with a lack of significant changes in serum Pi levels in patients with inactivating mutations of NaPi-IIb gene (27). However, pharmacological inhibition of Na+-dependent Pi absorption and genetic ablation of the murine NaPi-IIb gene argue to the contrary (35, 88). Interestingly, consistently with our comments above, NaPi-IIb−/− mice had increased fecal phosphate excretion and hypophosphaturia, with no change in serum phosphate concentration. Renal Pi reabsorption has not been systematically studied in clinical or preclinical settings. Our unpublished observations with TNBS model of colitis suggest significant downregulation of NaPi-IIa and NaPi-IIc renal transcript level (50 and 90% decrease, respectively). However, neither in TNBS colitis nor in the model of adoptive naive T-cell transfer colitis were we able to demonstrate a change in fractional Pi excretion and phosphaturia (F. K. Ghishan and P. R. Kiela, unpublished observations). This may indicate that compensatory mechanisms, perhaps involving type III phosphate transporter (s), counteract the observed changes in the expression of NaPi-IIa and NaPi-IIc.
Role of Klotho in Epithelial Ca2+ and Pi Transport
In 1997, Kuro-o et al. (57) described transgenic mice obtained by accidental mutational insertion, which exhibited symptoms typical of human aging: short lifespan, infertility, arteriosclerosis, skin atrophy, severe osteoporosis, and emphysema. A then-unknown gene was disrupted or mutated in its 5′-flanking promoter region by the random insertion of an exogenously introduced nonfunctional gene, the rabbit Na/H exchanger under the control of the human elongation factor promoter. The coding region of this mutated gene termed Klotho was still preserved, but its expression was markedly reduced, generating a mouse strain with a strong hypomorphic allele. Klotho gene encodes a 130-kDa single pass transmembrane protein with β-glucuronidase activity and is primarily expressed in the epithelial cells of renal distal tubules, in the choroid plexus, and in the parathyroid gland.
Involvement of the Klotho gene in bone homeostasis was suggested by recent genetic studies showing that certain allelic variants of Klotho constitute one of the genetic factors influencing BMD in male adults (110) and in postmenopausal women (83). All this evidence implies that Klotho is a multifunctional protein that regulates phosphate/calcium metabolism as well as aging. Klotho has also been directly implicated in the regulation of transepithelial Ca2+ transport through regulation of both apical entry as well as basolateral Ca2+ exit.
The existing homeostatic mechanisms provide for a fast and active Ca2+ reabsorption when the extracellular Ca2+ concentration in the circulation decreases. A key component to create such elevated Ca2+ influx is to increase TRPV5 and TRPV6 channels' abundance at the epithelial cell surface. Shuttling of TRPV5 to and from a subapical pool is a key component of this regulation, and high Ca2+ influx may be obtained by prolonging the channel's durability at the cell surface before inactivation or internalization. Klotho, through its glucuronidase activity, modulates N-glycosylation of TRPV5 at Asn358 by removal of the capping sialic acid, which enables physical interaction of galectin 1 with TRPV5 on the cell surface and promotes its apical retention (89). Similar activating effects of Klotho on TRPV6-mediated Ca2+ flux have also been shown (63), although intestinal Ca2+ absorption has not been shown to date to be controlled by Klotho. Moreover, under decreased extracellular Ca2+ concentration, intracellular Klotho physically interacts with endosomal pool of Na+/K+ ATPase and enables active recruitment of the sodium pump to the basolateral membrane. This, in turn, promotes the maintenance of the electrochemical Na+ gradient indispensable for the Na+/Ca2+ exchanger NCX1 to operate as a Ca2+ extrusion mechanism to complete the transepithelial calcium transport.
In our laboratory's recent study (101), we described that renal Klotho is significantly downregulated in experimental colitis. This was consistently observed in three distinct models of murine IBD: TNBS colitis, SPF-associated germ-free IL-10−/− mice, and CD4+CD45RBHi T-cell transfer colitis. In vitro, Klotho mRNA and protein expression was greatly and synergistically reduced in immortalized distal convoluted tubule cells by TNF and IFN-γ. The latter cytokine contributed to the reduction of Klotho by induction of NOS2 expression and the production of NOx, which by itself significantly affected Klotho expression. The effects of both cytokines were transcriptionally mediated as determined by unaltered transcript stability and decreased gene promoter activity (101). Consistent with the role of Klotho in preventing tubular Ca2+ loss (5), colitis mice exhibit increased urinary fractional Ca2+ excretion accompanied with dramatically decreased TRPV5 protein, but not mRNA expression (P. R. Kiela and F. K. Ghishan; unpublished observations). This is consistent with earlier observations by Fries et al. (36), who observed increased urinary calcium excretion in rats with TNBS colitis associated with decreased bone density.
The relationship between Klotho and renal Pi reabsorption is also quite complex. Klotho functions as a coreceptor for fibroblast growth factor (FGF) 23, a phosphaturic hormone produced primarily by osteoblasts under the control of 1,25(OH)2D3 (54). FGF23 inhibits renal phosphate reabsorption by activating FGF receptor (FGFR) 1c in a Klotho-dependent fashion (105), leading to decreased expression of NaPi-2a and NaPi-2c proteins and phosphate wasting (38). A recent paper by Hu et al. (46) also shows that Klotho directly, and independently of FGF23, inhibits Na+-dependent Pi reabsorption by acutely inhibiting NaPi-2a-mediated Pi transport activity followed by decreased cell surface protein expression. Whether downregulation of renal Klotho expression in colitis (101) represents a compensatory mechanism for the observed changes in NaPi-2a and NaPi-2c mRNA levels and contributes to maintenance of renal and systemic Pi homeostasis remains to be determined.
Vitamin D Status in IBD
The biochemistry, nutritional aspects, metabolism, and physiological and therapeutic potential of vitamin D and its analogs have been the subject of countless reviews and monographs. Vitamin D3 (cholecalciferol) is supplied from the diet, mostly from fortified dairy products and fish oils, or is synthesized in the skin as previtamin D3 from 7-dehydrocholesterol by ultraviolet irradiation. Previtamin D3 spontaneously but slowly isomerizes to vitamin D3 (cholecalciferol), which is then transported in the blood by the vitamin D binding protein (DBP) to the liver, where it is hydroxylated at C-25 by one or more cytochrome P-450 vitamin D 25-hydroxylases (CYP2R1, CYP2D11, and CYP2D25), resulting in the formation of 25-hydroxyvitamin D3 [25(OH)D3]. Due to its stability and ease of assay, 25(OH)D3 is almost exclusively used clinically as a measure of vitamin D status. 25(OH)D3 is transported by DBP to the kidney, where it is endocytosed in the proximal tubules and is further hydroxylated at position C-1 by 1α(OH)ase, resulting in the hormonally active form of vitamin D, 1,25(OH)2D3, which is responsible for most, if not all of the biological effects of vitamin D (24). 24-hydroxylase [CYP24, 24(OH)ase], also a renal mitochondrial P-450 enzyme, can hydroxylate both 25(OH)D3 and 1,25(OH)2D3, with preference for the latter. Catabolism of 1,25(OH)2D3 to 1,24,25(OH)3D3 decreases its biological activity, whereas hydroxylation of 25(OH)D3 to 24,25(OH)2D3 leads to a reduction in the pool of 25(OH)D3 available for 1α-hydroxylation. Therefore, 24(OH)ase functions to inactivate vitamin D.
Vitamin D plays a key role in skeletal and mineral homeostasis. It is frequently viewed as a pro-bone anabolic hormone because of its positive effects on intestinal and renal Ca2+ and Pi (re)absorption and through its positive effects on osteoblast differentiation and bone matrix synthesis. However, a more appropriate view of vitamin D in bone metabolism is as a regulator of bone turnover. Indeed, 1,25(OH)2D3 can also increase the release of receptor activator of NF-κB ligand (RANKL) and decrease osteoprotegerin (OPG) release from osteoblastic cells and stimulate osteoclastogenesis, resulting in bone resorption. One example of such effect was provided by 24(OH)ase knockout mice, which demonstrated that defective catabolic clearance of 1,25(OH)2D3 leads to impaired intramembranous bone mineralization (95). Another example comes from Klotho-deficient mice. Klotho acts as the FGF23 coreceptor and contributes to FGF23-mediated negative feedback thus leading to suppression of 1α(OH)ase expression and decreased 1,25(OH)2D3 synthesis. In the absence of Klotho, mice develop severe defects in mineral homeostasis accompanied with very high levels of 1,25(OH)2D3 and osteoporosis (57). This defect in bone mineralization, as well as other phenotypic elements of aging, could be eliminated by crossing Klotho knockouts with 1α(OH)ase-deficient mice (74). These findings highlight the pleiotropic role of 1,25(OH)2D3 in mineral homeostasis and question the simplistic views of the vitamin often promoted by science and product marketing as “the fountain of youth” (17).
The 2002 report of the American Gastroenterological Association Committee on Osteoporosis in Gastrointestinal Disease stated that “osteomalacia and vitamin D deficiency are not common in IBD (including Crohn's disease) and are unlikely to be important causes of most cases of diminished bone mineral density (BMD) in IBD” (7). Since then, numerous studies demonstrated evidence for an increased prevalence of vitamin D deficiency among IBD patients. 25(OH)D3 deficiency or insufficiency has been reported more frequently in patients with ulcerative colitis and Crohn's disease compared with either control subjects or a healthy population. This was documented for both children with IBD (e.g., Ref. 34) as well as in many studies with adult IBD patients (e.g., Ref. 61). Among the many suggested reasons for the impaired vitamin status in IBD patients are reduced efficiency of intestinal absorption of vitamin D, primarily in patients with small intestinal involvement or ileal resection; a disrupted enterohepatic circulation of vitamin D; renal insufficiency; reduced dietary intake; and reduced exposure to sunshine. Vitamin D deficiency has also been postulated to contribute to the overall inflammatory state because of its diverse role in modulating the immune response (43). For these reasons, vitamin D3 (and Ca2+) supplementation has become the mainstream intuitive approach to prevent inflammation-associated bone loss and is widely recommended by gastroenterologists and promoted by the American Gastroenterological Association and by the British Society of Gastroenterology. However, the clinical efficacy of this approach in relationship with BMD loss or accrual in patients with active IBD remains controversial, with several published reports that failed to identify any clinical benefits (12, 14). Obviously, more systematic studies in pediatric and adult IBD patients need to be conducted to identify factors determining the clinical response to vitamin D and Ca2+ supplementation, the form and route of administered vitamin D, with careful monitoring of the parameters of bone mass, bone turnover, and mineral homeostasis. The need for such systematic approach is further justified by identification of at least a subset of IBD patients with inappropriate hypercalcitriolemia [elevated serum 1,25(OH)2D3] (1, 87). The earlier report by Rudnicki et al. (87) demonstrated high 1,25(OH)2D3 levels in CD patients undergoing ileal resection despite reduced levels of 25(OH)D3 and speculated that extrarenal 1α-hydroxylation, similar to that found in sarcoidosis, may contribute to this paradoxical elevation of the bioactive vitamin D form. Indeed, Abreu et al. (1) reproduced these findings in 42% of CD patients who had abnormally high (>60 pg/ml) 1,25(OH)2D3 levels, which inversely correlated with BMD irrespective of glucocorticoid use. Consistent with the hypothesis postulated by Rudnicki et al., the latter study showed elevated expression of 1α-hydroxylase in colon from patients with active CD, with the protein overexpressed primarily by macrophages and multinucleated giant cells, similar to that observed in sarcoidosis-associated granulomas (1). On the basis of these studies, it is not inconceivable that, paradoxically, high levels of 1,25(OH)2D3 are an important risk factor for the development of osteoporosis in patients with CD. In such scenario, elevated 1,25(OH)2D3 superimposed on limited Ca2+ and Pi supply and direct effect of proinflammatory mediators on bone turnover could skew the ultimate effects of 1,25(OH)2D3 toward a proresorptive mechanism. Since in clinical settings analysis of serum 1,25(OH)2D3 is rarely performed (because of limited stability, assay cost, and reliability), these findings beg the question whether indiscriminate use of vitamin D supplementation in patients with active CD is beneficial or destructive and further emphasize the need for more comprehensive clinical studies. It also raises concerns for potential use of large doses of vitamin D as an immunomodulator in IBD as proposed by some preclinical studies without careful monitoring of the bone metabolism.
Vitamin K Status in IBD Patients
Vitamin K family members phylloquinone (vitamin K1) and the menaquinones (vitamin K2) play prominent roles in bone metabolism and have been considered beneficial in preventing bone loss associated with menopause, Parkinson's disease, chronic steroid treatment, biliary cirrhosis, and other disorders (106a). The primary mechanism of vitamin K action is through γ-carboxylation of vitamin K-dependent proteins collectively called Gla proteins. Bone contains three known targets of vitamin K-mediated γ-carboxylation: osteocalcin (77), matrix Gla protein (78), and the more recently described periostin expressed by the bone mesenchymal stem cells (28). These three Gla proteins upon γ-carboxylation have dramatically enhanced capacity to bind calcium, effecting mineralizing nodule formation and play important roles in normal bone development and repair (29). Vitamin K has also been shown to have direct and γ-carboxylation-independent effects on gene expression in osteoblastic cells through activation of the steroid and xenobiotic receptor SXR (49, 99). Moreover, vitamin K2 inhibits RANKL mRNA expression and inhibits 1,25(OH)2D3-induced osteoclastogenesis (100). A recent paper by Atkins et al. (11) describes γ-carboxylation-dependent and -independent effects of vitamin K promoting bone mineralization, osteoblast-to-osteocyte transition, and an anticatabolic bone phenotype.
In IBD patients, vitamin K status has not received as much attention as vitamin D. Schoon et al. (91) demonstrated a significant decrease in serum vitamin K levels and free (non-γ-carboxylated) osteocalcin in a cohort of 32 stable CD patients with small intestinal involvement receiving prednisolone. In a more recent analysis in Japanese IBD patients, similar vitamin K deficiency was documented and shown to be more prominent in CD that in UC (59). Undercarboxylated osteocalcin was also elevated in CD patients. There was no significant difference in vitamin K intake between CD and UC; therefore, malabsorption of vitamin K was postulated to be the likely contributor (59). Although vitamin K absorption in inflammatory conditions has not been studied, one case report with a small bowel CD patient treated with warfarin for deep vein thrombosis showed resistance to oral vitamin K for reversal of overanticoagulation (37). Subsequent subcutaneous administration of vitamin K was, however, effective, suggesting that indeed chronic inflammation may affect intestinal vitamin K absorption. More systematic studies are needed to evaluate the effects of inflammation and inflammatory mediators on intestinal vitamin K absorption and the role of vitamin K deficiency in IBD-associated bone loss.
INFLAMMATORY MEDIATORS AND BONE MODELING AND REMODELING
Bone modeling (construction) is the process by which bone is formed by osteoblasts without prior bone resorption and is predominant during growth, resulting in changes in bone size and shape. Bone remodeling (reconstruction) occurs throughout life and involves a coordinated action of bone resorbing osteoclasts followed by bone formation at the same location by osteoblasts. Both of these processes are critical to achieve strength and lightness for mobility by strategically depositing bone in locations where it is needed and by removing bone to avoid bulk in places where it is unnecessary. Osteoblasts and osteoclasts form the basic metabolic unit and are engaged in a complex cross talk network that can be easily disrupted by both the physiological process of aging, as well as by hormonal and inflammatory mediators in numerous clinical disorders (79). One important example of such interaction is the osteoprotegerin-RANK-RANKL axis. RANKL is a member of the TNF family. It is produced by osteoblasts and by activated T cells and it interacts with its receptor (RANK) on osteoclast precursor cells and mature osteoclasts. The ligation of RANK with RANKL results in the commitment of monocyte/macrophage precursor cells to the osteoclast lineage and the activation of mature osteoclasts and preventing their apoptosis, thus leading to increased bone resorption. RANKL signaling is accelerated by inflammatory cytokines abundant in rheumatoid arthritis and IBD such as IL-1, IL-6, and TNF-α. These mediators potently induce RANKL on osteoblasts and synovial fibroblasts and are believed to directly contribute to the bone destruction process. OPG, also known as osteoclastogenesis inhibitory factor, is an osteoblast-derived soluble cytokine receptor from the TNF receptor superfamily. OPG acts as a decoy receptor for RANKL and inhibits osteoclast differentiation, blocks activation of mature osteoclasts, and permits osteoclast apoptosis. OPG is therefore a natural antagonist for RANKL and a powerful inhibitor of bone resorption. This pathway has been under intense scrutiny for the development of therapeutic approaches to prevent osteopenia and osteoporosis in postmenopausal women, in primary hyperparathyroidism, Paget's disease, rheumatoid arthritis, periodontal bone loss, and several other disorders (85). RANKL/OPG system is known to be activated in IBD patients (13, 72) and can be normalized with infliximab (69). Exogenous recombinant OPG (OPG-Fc) reverses skeletal abnormalities and reduces colitis in IL-2 knockout mice (10). OPG-Fc, soluble RANK-Fc IgG fusion proteins, or monoclonal anti-RANKL antibody (denosumab) have not yet been tested in IBD patients.
Bone Formation vs. Resorption in Animal Models of IBD
HLA-B27 transgenic rats that spontaneously develop inflammatory bowel disease, peripheral arthritis, ankylosing spondylitis, and skin lesions were also reported to have decreased bone strength (3). Papet et al. (76) showed that they have normal levels of osteocalcin (marker of bone formation), normal fractional protein synthesis in the bone, but significantly increased urinary excretion of deoxypyridinoline (marker of bone resorption). In another recent study, Rauner et al. (80) demonstrated increased RANKL-OPG ratio and increased osteocalcin mRNA in the bone of HLA-B27 rats. However, serum levels of type I collagen C-telopeptides (CTx), NH2-terminal propeptide of type I procollagen (P1NP), RANKL (all markers of bone resorption), as well as OPG, and osteocalcin did not differ significantly in this study. These data suggest that in HLA-B27 transgenic rats inflammation-associated bone loss is driven mainly by increased bone resorption, perhaps because of skewed RANKL-OPG ratio and that serum markers of bone metabolism may not always adequately reflect the mechanism of bone loss.
In murine studies, varying contributions of bone formation and resorption defects have been reported. Using IL-10−/− mice as a model of Crohn's-like colitis, Drezner-Pollak et al. (32) showed that the effect of intestinal inflammation on the development of osteopenia and osteoporosis was primarily mediated by deficient bone formation. This was evidenced by decreased cancellous double-labeled surface, mineralizing surface, serum osteocalcin level, and mineralized nodule number in bone marrow stromal cell cultures. The same group demonstrated later in dextran sodium sulfate (DSS) model of colitis that both the impaired bone formation and increased bone resorption contribute to the significant lowering of bone mass in this model, with decreased trabecular double-labeled surface, mineralizing surface, bone formation rate, and with increased bone resorption as indicated by elevated osteoclast number (41). Another recent study with DSS colitis also suggested a decrease in osteoblast maturation and function as the primary causes in the observed changes in bone density (42). Our group's findings related to osteoblast/osteocyte-specific Phex gene also support at least partial contribution of defective bone formation in colitis. Phex is a critical regulator of systemic Pi homeostasis (52), with an intrinsic role in osteoblast mineralization. Immortalized osteoblasts from Phex-deficient Hyp mice fail to mineralize under permissive conditions in vitro (109). We demonstrated that Phex expression is significantly depressed in various models of colitis (TNBS, adoptive T cell transfer, and IL-10−/− mice) (64, 104). This suppression can be reversed with anti-TNF antibody, and the effect of TNF on osteoblast mineralization correlates with decreased Phex protein and mRNA in vitro (104). Moreover, consistent with the inhibitory role of NF-κB on osteoblastic bone formation (22), TNF repressed Phex gene transcription via a concerted action of NF-κB and the enzymatic activity of poly(ADP-ribose)polymerase 1 (PARP-1). TNF failed to downregulate Phex expression in PARP-1−/− mice (64). This finding opens a potential avenue for reducing IBD-associated bone loss by modulating NF-κB activity via PARP-1 inhibition, a concept already being explored in acute inflammation and cancer (53, 86).
Adoptive transfer of naive CD4+CD45RBHi T cells to immunodeficient Rag or SCID mice results in colitis with similarities to human Crohn's disease. Using this model, Byrne et al. (21) demonstrated osteopenia which could be treated with recombinant osteoprotegerin (OPG-Fc) in mice with established colitis. Mice transferred with CD4+CD45RBHi T cells had a significant decrease in the number of osteoblasts and an increase in the number of osteoclasts in their tibias compared with mice transferred with CD4+CD45RBLo T cells. Colitis mice had elevated serum tartrate-resistant acid phosphatase (TRAP; osteoclast marker) and decreased serum alkaline phosphatase (ALP; osteoblast marker), suggesting both increased resorption and decreased bone formation. Surprisingly, OPG-Fc treatment resulted in a loss of detectable osteoclasts as well as osteoblasts. Although the authors concluded that “osteopenia was induced by inflammatory cell infiltration and not by malabsorption of calcium,” the presented data show that OPG-Fc did not alter TNF-positive mononuclear infiltrate in the bone while offering only measurement of serum Ca2+ and Pi concentration, which are not reliable markers of moderate changes in mineral (re)absorption (21).
Bone Formation vs. Resorption in IBD Patients
Biochemical markers that reflect the processes of bone resorption and bone formation, and thus bone turnover, can be measured in blood and urine (25). However, utilization of these markers in IBD patients has not delivered consistent results. Several studies have reported increased levels of markers of bone resorption without a compensatory increase in markers of formation (18, 19, 33, 84, 92, 94). Contrary data have also been published demonstrating reduced levels of bone formation markers and no change in resorptive markers in patients with long-standing, quiescent CD (90), elevated levels of both types of markers (9, 70), or no change between IBD patients and healthy controls (67).
These differences highlight the difficulties in designing such clinical studies. In most cases, the discrepancies can be explained by small population sample and many confounding factors related to the heterogeneity of IBD: ethnicity, geographic region, CD vs. UC, duration of disease, disease activity index, active disease vs. remission, gender, steroid use, small intestinal involvement and/or resection, and age. These do not even account for factors like seasonality of vitamin D status. In a more uniform study, bone formation and bone resorption in quiescent Irish IBD patients (CD and UC, separately) were compared with age- and sex-matched healthy controls (39). Both CD and UC patients had significantly higher serum under carboxylated osteocalcin and bone-specific alkaline phosphatase concentrations, as well as significantly higher urinary type I collagen cross-linked N-telopeptides (NTx). Total serum osteocalcin was, however, significantly lower in CD patients compared with healthy control subjects (39). Simultaneously elevated markers of bone formation and resorption are a hallmark of increased rate of bone turnover, which in older adults contributes to faster bone loss and is recognized as a risk factor for fracture. However, lower total osteocalcin level may also suggest that the processes of bone resorption and bone formation are not coupled in IBD patients and that the uncoupled bone metabolism can be a significant risk factor for progressive loss of BMD in CD and UC patients, even during remission.
Several studies investigated bone turnover markers in IBD patients in the context of the RANK/RANKL/OPG pathway. Turk et al. (103) recently demonstrated elevated serum levels of free soluble RANKL, OPG, TNF, and IL-6 in both long-standing CD patients with low bone mass and in naive newly diagnosed patients with osteopenia compared with healthy controls. In general, osteocalcin levels were not different between CD and healthy patients, whereas CD patients had elevated NTx, thus suggesting increased bone resorption, which correlated well with serum cytokine levels. In a subset of patients, 81% of which were on corticosteroid therapy, low total osteocalcin level was accompanied by high NTx, thus suggesting that steroids trigger the uncoupling mechanism and result in simultaneous decrease in bone formation and increased bone resorption (103).
Analysis of bone loss in pediatric IBD patients presents with its unique difficulties. Some of the more critical differences in bone metabolism and bone mass assessment in pediatric and adult IBD patients have been reviewed by Sylvester (97) and Hill et al. (44). These difficulties begin with limitations of the use of dual-energy X-ray absorptiometry (DXA). Clinical reports generated from DXA measurements compare patient areal BMD (aBMD) with those of chronological age- and sex-matched healthy control subjects, returning a calculated aBMD Z-score. However, stunted growth seen in children with IBD, delayed puberty, decreased muscle strength, and delayed skeletal maturation makes BMD more a function of bone mineral accrual than of the child's chronological age. Therefore, low aBMD may not be an indication of a metabolic bone disease, but rather a reflection of delayed bone development, which makes clinical study design and interpretation so much more difficult. Moreover, DXA-derived aBMD is influenced by bone size and does not distinguish between osteomalacia (i.e., insufficient mineralization of bone matrix) and osteoporosis (insufficient amount of normally mineralized bone matrix). New more quantitative bone assessment methods relying on objective and not relative measures of bone health such as peripheral quantitative computed tomography provide a superior alternative.
Bone turnover marker data are also not easy to interpret in children. Similar to DXA results, they can be influenced by several confounding factors, such as growth rate, maturational delay, low bone mass, and nutritional deficits. After adjusting for several such confounders, Tuchman et al. (102) found that young IBD patients had low bone formation and elevated bone resorption markers. However, these were not newly diagnosed patients and they had received treatment for IBD at the time of study. A recent study with naive pediatric CD and UC patients found that biochemical markers of both bone formation and resorption were low compared with age- and sex-specific reference ranges (107). Histomorphometric data showed that bone formation activity was low on trabecular bone surfaces. Osteoclast-covered surface was low, whereas eroded surface was elevated, which might suggest increased bone resorption. The authors reasoned, however, that eroded surface would also be elevated when osteoblast activity is acutely suppressed. Given the low bone formation parameters and low osteoclast surface, it seems more likely that in children newly diagnosed with IBD suppressed bone formation is the primary mechanism leading to impaired bone accrual and low BMD.
SUMMARY
The simplistic view that skeletal abnormalities seen in IBD patients are related to vitamin D3 and calcium deficiency is challenged by the recent observations of the complex interplay between inflammatory mediators and key players in mineral homeostasis and their effect on the delicate balance of osteoblast-osteoclast activities. To date, neither patient nor the preclinical data with animal models of IBD provide a uniform explanation of the pathogenesis of bone loss in acute and chronic intestinal inflammation. Although no single animal model is a valid reflection of the complex and nonhomogenous human IBD, more well-designed animal studies are needed to understand the multifactorial nature of IBD-associated bone loss. A systematic review of these preclinical studies and studies with human IBD patients may help identify selected molecular targets and responsive subsets of patients to guide future therapies.
GRANTS
This research was support by National Institute of Diabetes and Digestive and Kidney Diseases Grants 5R37DK033209 and 3R37DK033209-27S1 (to F. K. Ghishan).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Abreu MT, Kantorovich V, Vasiliauskas EA, Gruntmanis U, Matuk R, Daigle K, Chen S, Zehnder D, Lin YC, Yang H, Hewison M, Adams JS. Measurement of vitamin D levels in inflammatory bowel disease patients reveals a subset of Crohn's disease patients with elevated 1,25-dihydroxyvitamin D and low bone mineral density. Gut 53: 1129–1136, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Airaksinen MS, Eilers J, Garaschuk O, Thoenen H, Konnerth A, Meyer M. Ataxia and altered dendritic calcium signaling in mice carrying a targeted null mutation of the calbindin D28k gene. Proc Natl Acad Sci USA 94: 1488–1493, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Akhter MP, Jung LK. Decreased bone strength in HLA-B27 transgenic rat model of spondyloarthropathy. Rheumatology (Oxford) 46: 1258–1262, 2007 [DOI] [PubMed] [Google Scholar]
- 4. Akhter S, Kutuzova GD, Christakos S, DeLuca HF. Calbindin D9k is not required for 1,25-dihydroxyvitamin D3-mediated Ca2+ absorption in small intestine. Arch Biochem Biophys 460: 227–232, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Alexander RT, Woudenberg-Vrenken TE, Buurman J, Dijkman H, van der Eerden BC, van Leeuwen JP, Bindels RJ, Hoenderop JG. Klotho prevents renal calcium loss. J Am Soc Nephrol 20: 2371–2379, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ali T, Lam D, Bronze MS, Humphrey MB. Osteoporosis in inflammatory bowel disease. Am J Med 122: 599–604, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. American Gastroenterological Association medical position statement: guidelines on osteoporosis in gastrointestinal diseases. Gastroenterology 124: 791–794, 2003 [DOI] [PubMed] [Google Scholar]
- 9. Ardizzone S, Bollani S, Bettica P, Bevilacqua M, Molteni P, Bianchi Porro G. Altered bone metabolism in inflammatory bowel disease: there is a difference between Crohn's disease and ulcerative colitis. J Intern Med 247: 63–70, 2000 [DOI] [PubMed] [Google Scholar]
- 10. Ashcroft AJ, Cruickshank SM, Croucher PI, Perry MJ, Rollinson S, Lippitt JM, Child JA, Dunstan C, Felsburg PJ, Morgan GJ, Carding SR. Colonic dendritic cells, intestinal inflammation, and T cell-mediated bone destruction are modulated by recombinant osteoprotegerin. Immunity 19: 849–861, 2003 [DOI] [PubMed] [Google Scholar]
- 11. Atkins GJ, Welldon KJ, Wijenayaka AR, Bonewald LF, Findlay DM. Vitamin K promotes mineralization, osteoblast-to-osteocyte transition, and an anticatabolic phenotype by γ-carboxylation-dependent and -independent mechanisms. Am J Physiol Cell Physiol 297: C1358–C1367, 2009 [DOI] [PubMed] [Google Scholar]
- 12. Benchimol EI, Ward LM, Gallagher JC, Rauch F, Barrowman N, Warren J, Beedle S, Mack DR. Effect of calcium and vitamin D supplementation on bone mineral density in children with inflammatory bowel disease. J Pediatr Gastroenterol Nutr 45: 538–545, 2007 [DOI] [PubMed] [Google Scholar]
- 13. Bernstein CN, Sargent M, Leslie WD. Serum osteoprotegerin is increased in Crohn's disease: a population-based case control study. Inflamm Bowel Dis 11: 325–330, 2005 [DOI] [PubMed] [Google Scholar]
- 14. Bernstein CN, Seeger LL, Anton PA, Artinian L, Geffrey S, Goodman W, Belin TR, Shanahan F. A randomized, placebo-controlled trial of calcium supplementation for decreased bone density in corticosteroid-using patients with inflammatory bowel disease: a pilot study. Aliment Pharmacol Ther 10: 777–786, 1996 [DOI] [PubMed] [Google Scholar]
- 15. Bianco SD, Peng JB, Takanaga H, Suzuki Y, Crescenzi A, Kos CH, Zhuang L, Freeman MR, Gouveia CH, Wu J, Luo H, Mauro T, Brown EM, Hediger MA. Marked disturbance of calcium homeostasis in mice with targeted disruption of the Trpv6 calcium channel gene. J Bone Miner Res 22: 274–285, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bindels RJ, Ramakers PL, Dempster JA, Hartog A, van Os CH. Role of Na+/Ca2+ exchange in transcellular Ca2+ transport across primary cultures of rabbit kidney collecting system. Pflügers Arch 420: 566–572, 1992 [DOI] [PubMed] [Google Scholar]
- 17. Binkley N. Is vitamin D the fountain of youth? Endocr Pract 15: 590–596, 2009 [DOI] [PubMed] [Google Scholar]
- 18. Bischoff SC, Herrmann A, Goke M, Manns MP, von zur Muhlen A, Brabant G. Altered bone metabolism in inflammatory bowel disease. Am J Gastroenterol 92: 1157–1163, 1997 [PubMed] [Google Scholar]
- 19. Bjarnason I, Macpherson A, Mackintosh C, Buxton-Thomas M, Forgacs I, Moniz C. Reduced bone density in patients with inflammatory bowel disease. Gut 40: 228–233, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bronner F. Recent developments in intestinal calcium absorption. Nutr Rev 67: 109–113, 2009 [DOI] [PubMed] [Google Scholar]
- 21. Byrne FR, Morony S, Warmington K, Geng Z, Brown HL, Flores SA, Fiorino M, Yin SL, Hill D, Porkess V, Duryea D, Pretorius JK, Adamu S, Manoukian R, Danilenko DM, Sarosi I, Lacey DL, Kostenuik PJ, Senaldi G. CD4+CD45RBHi T cell transfer induced colitis in mice is accompanied by osteopenia which is treatable with recombinant human osteoprotegerin. Gut 54: 78–86, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chang J, Wang Z, Tang E, Fan Z, McCauley L, Franceschi R, Guan K, Krebsbach PH, Wang CY. Inhibition of osteoblastic bone formation by nuclear factor-kappaB. Nat Med 15: 682–689, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen H, Xu H, Dong J, Li J, Ghishan FK. Tumor necrosis factor-α impairs intestinal phosphate absorption in colitis. Am J Physiol Gastrointest Liver Physiol 296: G775–G781, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Christakos S, Ajibade DV, Dhawan P, Fechner AJ, Mady LJ. Vitamin D: metabolism. Endocrinol Metab Clin North Am 39: 243–253, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Civitelli R, Armamento-Villareal R, Napoli N. Bone turnover markers: understanding their value in clinical trials and clinical practice. Osteoporos Int 20: 843–851, 2009 [DOI] [PubMed] [Google Scholar]
- 26. Collins JF, Bai L, Ghishan FK. The SLC20 family of proteins: dual functions as sodium-phosphate cotransporters and viral receptors. Pflügers Arch 447: 647–652, 2004 [DOI] [PubMed] [Google Scholar]
- 27. Corut A, Senyigit A, Ugur SA, Altin S, Ozcelik U, Calisir H, Yildirim Z, Gocmen A, Tolun A. Mutations in SLC34A2 cause pulmonary alveolar microlithiasis and are possibly associated with testicular microlithiasis. Am J Hum Genet 79: 650–656, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Coutu DL, Wu JH, Monette A, Rivard GE, Blostein MD, Galipeau J. Periostin, a member of a novel family of vitamin K-dependent proteins, is expressed by mesenchymal stromal cells. J Biol Chem 283: 17991–18001, 2008 [DOI] [PubMed] [Google Scholar]
- 29. Cranenburg EC, Schurgers LJ, Vermeer C. Vitamin K: the coagulation vitamin that became omnipotent. Thromb Haemost 98: 120–125, 2007 [PubMed] [Google Scholar]
- 30. De Groot T, Bindels RJ, Hoenderop JG. TRPV5: an ingeniously controlled calcium channel. Kidney Int 74: 1241–1246, 2008 [DOI] [PubMed] [Google Scholar]
- 31. Di Stefano M, Veneto G, Malservisi S, Cecchetti L, Minguzzi L, Strocchi A, Corazza GR. Lactose malabsorption and intolerance and peak bone mass. Gastroenterology 122: 1793–1799, 2002 [DOI] [PubMed] [Google Scholar]
- 32. Dresner-Pollak R, Gelb N, Rachmilewitz D, Karmeli F, Weinreb M. Interleukin 10-deficient mice develop osteopenia, decreased bone formation, and mechanical fragility of long bones. Gastroenterology 127: 792–801, 2004 [DOI] [PubMed] [Google Scholar]
- 33. Duggan P, O'Brien M, Kiely M, McCarthy J, Shanahan F, Cashman KD. Vitamin K status in patients with Crohn's disease and relationship to bone turnover. Am J Gastroenterol 99: 2178–2185, 2004 [DOI] [PubMed] [Google Scholar]
- 34. El-Matary W, Sikora S, Spady D. Bone mineral density, vitamin D, and disease activity in children newly diagnosed with inflammatory bowel disease. Dig Dis Sci 2010. Aug 20. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 35. Eto N, Tomita M, Hayashi M. NaPi-mediated transcellular permeation is the dominant route in intestinal inorganic phosphate absorption in rats. Drug Metab Pharmacokinet 21: 217–221, 2006 [DOI] [PubMed] [Google Scholar]
- 36. Fries W, Giacomin D, Plebani M, Martin A. Effect of experimental colitis on bone metabolism in the rat. Digestion 55: 229–233, 1994 [DOI] [PubMed] [Google Scholar]
- 37. Fugate SE, Ramsey AM. Resistance to oral vitamin K for reversal of overanticoagulation during Crohn's disease relapse. J Thromb Thrombolysis 17: 219–223, 2004 [DOI] [PubMed] [Google Scholar]
- 38. Gattineni J, Bates C, Twombley K, Dwarakanath V, Robinson ML, Goetz R, Mohammadi M, Baum M. FGF23 decreases renal NaPi-2a and NaPi-2c expression and induces hypophosphatemia in vivo predominantly via FGF receptor 1. Am J Physiol Renal Physiol 297: F282–F291, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gilman J, Shanahan F, Cashman KD. Altered levels of biochemical indices of bone turnover and bone-related vitamins in patients with Crohn's disease and ulcerative colitis. Aliment Pharmacol Ther 23: 1007–1016, 2006 [DOI] [PubMed] [Google Scholar]
- 40. Giral H, Caldas Y, Sutherland E, Wilson P, Breusegem S, Barry N, Blaine J, Jiang T, Wang XX, Levi M. Regulation of rat intestinal Na-dependent phosphate transporters by dietary phosphate. Am J Physiol Renal Physiol 297: F1466–F1475, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hamdani G, Gabet Y, Rachmilewitz D, Karmeli F, Bab I, Dresner-Pollak R. Dextran sodium sulfate-induced colitis causes rapid bone loss in mice. Bone 43: 945–950, 2008 [DOI] [PubMed] [Google Scholar]
- 42. Harris L, Senagore P, Young VB, McCabe LR. Inflammatory bowel disease causes reversible suppression of osteoblast and chondrocyte function in mice. Am J Physiol Gastrointest Liver Physiol 296: G1020–G1029, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hewison M. Vitamin D and the immune system: new perspectives on an old theme. Endocrinol Metab Clin North Am 39: 365–379, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hill RJ, Brookes DS, Davies PS. Bones in pediatric Crohn's disease: a review of fracture risk in children and adults. Inflamm Bowel Dis 2010. Sep 7. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 45. Hoenderop JG, Nilius B, Bindels RJ. Calcium absorption across epithelia. Physiol Rev 85: 373–422, 2005 [DOI] [PubMed] [Google Scholar]
- 46. Hu MC, Shi M, Zhang J, Pastor J, Nakatani T, Lanske B, Razzaque MS, Rosenblatt KP, Baum MG, Kuro-o M, Moe OW. Klotho: a novel phosphaturic substance acting as an autocrine enzyme in the renal proximal tubule. FASEB J 24: 3438–3450, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Huybers S, Apostolaki M, van der Eerden BC, Kollias G, Naber TH, Bindels RJ, Hoenderop JG. Murine TNFΔARE Crohn's disease model displays diminished expression of intestinal Ca2+ transporters. Inflamm Bowel Dis 14: 803–811, 2008 [DOI] [PubMed] [Google Scholar]
- 48. Huybers S, Naber TH, Bindels RJ, Hoenderop JG. Prednisolone-induced Ca2+ malabsorption is caused by diminished expression of the epithelial Ca2+ channel TRPV6. Am J Physiol Gastrointest Liver Physiol 292: G92–G97, 2007 [DOI] [PubMed] [Google Scholar]
- 49. Ichikawa T, Horie-Inoue K, Ikeda K, Blumberg B, Inoue S. Steroid and xenobiotic receptor SXR mediates vitamin K2-activated transcription of extracellular matrix-related genes and collagen accumulation in osteoblastic cells. J Biol Chem 281: 16927–16934, 2006 [DOI] [PubMed] [Google Scholar]
- 50. Kanis JA, Melton LJ, 3rd, Christiansen C, Johnston CC, Khaltaev N. The diagnosis of osteoporosis. J Bone Miner Res 9: 1137–1141, 1994 [DOI] [PubMed] [Google Scholar]
- 51. Khanal RC, Nemere I. Regulation of intestinal calcium transport. Annu Rev Nutr 28: 179–196, 2008 [DOI] [PubMed] [Google Scholar]
- 52. Kiela PR, Ghishan FK. Recent advances in the renal-skeletal-gut axis that controls phosphate homeostasis. Lab Invest 89: 7–14, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kirkland JB. Poly ADP-ribose polymerase-1 and health. Exp Biol Med (Maywood) 235: 561–568, 2010 [DOI] [PubMed] [Google Scholar]
- 54. Kolek OI, Hines ER, Jones MD, LeSueur LK, Lipko MA, Kiela PR, Collins JF, Haussler MR, Ghishan FK. 1α,25-Dihydroxyvitamin D3 upregulates FGF23 gene expression in bone: the final link in a renal-gastrointestinal-skeletal axis that controls phosphate transport. Am J Physiol Gastrointest Liver Physiol 289: G1036–G1042, 2005 [DOI] [PubMed] [Google Scholar]
- 55. Koushik SV, Wang J, Rogers R, Moskophidis D, Lambert NA, Creazzo TL, Conway SJ. Targeted inactivation of the sodium-calcium exchanger (Ncx1) results in the lack of a heartbeat and abnormal myofibrillar organization. FASEB J 15: 1209–1211, 2001 [DOI] [PubMed] [Google Scholar]
- 56. Kumari M, Khazai NB, Ziegler TR, Nanes MS, Abrams SA, Tangpricha V. Vitamin D-mediated calcium absorption in patients with clinically stable Crohn's disease: A pilot study. Mol Nutr Food Res 54: 1085–1091, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R, Nabeshima YI. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 390: 45–51, 1997 [DOI] [PubMed] [Google Scholar]
- 58. Kutuzova GD, Akhter S, Christakos S, Vanhooke J, Kimmel-Jehan C, Deluca HF. Calbindin D (9k) knockout mice are indistinguishable from wild-type mice in phenotype and serum calcium level. Proc Natl Acad Sci USA 103: 12377–12381, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kuwabara A, Tanaka K, Tsugawa N, Nakase H, Tsuji H, Shide K, Kamao M, Chiba T, Inagaki N, Okano T, Kido S. High prevalence of vitamin K and D deficiency and decreased BMD in inflammatory bowel disease. Osteoporos Int 20: 935–942, 2009 [DOI] [PubMed] [Google Scholar]
- 60. Larsen S, Bendtzen K, Nielsen OH. Extraintestinal manifestations of inflammatory bowel disease: epidemiology, diagnosis, and management. Ann Med 42: 97–114, 2010 [DOI] [PubMed] [Google Scholar]
- 61. Leslie WD, Miller N, Rogala L, Bernstein CN. Vitamin D status and bone density in recently diagnosed inflammatory bowel disease: the Manitoba IBD Cohort Study. Am J Gastroenterol 103: 1451–1459, 2008 [DOI] [PubMed] [Google Scholar]
- 62. Lichtenstein GR, Sands BE, Pazianas M. Prevention and treatment of osteoporosis in inflammatory bowel disease. Inflamm Bowel Dis 12: 797–813, 2006 [DOI] [PubMed] [Google Scholar]
- 63. Lu P, Boros S, Chang Q, Bindels RJ, Hoenderop JG. The beta-glucuronidase klotho exclusively activates the epithelial Ca2+ channels TRPV5 and TRPV6. Nephrol Dial Transplant 23: 3397–3402, 2008 [DOI] [PubMed] [Google Scholar]
- 64. Majewski PM, Thurston RD, Ramalingam R, Kiela PR, Ghishan FK. Cooperative role of NF-κB and poly (ADP-ribose) polymerase 1 (PARP-1) in the TNF-induced inhibition of PHEX expression in osteoblasts. J Biol Chem 285: 34828–34838, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Margolis DS, Kim D, Szivek JA, Lai LW, Lien YH. Functionally improved bone in calbindin-D28k knockout mice. Bone 39: 477–484, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Marks J, Debnam ES, Unwin RJ. Phosphate homeostasis and the renal-gastrointestinal axis. Am J Physiol Renal Physiol 299: F285–F296, 2010 [DOI] [PubMed] [Google Scholar]
- 67. Martin A, Fries W, Luisetto G, Mussolin L, Plebani M, Giacomin D, Naccarato R. Bone-density and calcium-metabolism in patients with long-standing, quiescent Crohns-disease. Eur J Gastroenterol Hepatol 6: 611–616, 1994 [Google Scholar]
- 68. Mascarenhas MR, Thayu M. Pediatric inflammatory bowel disease and bone health. Nutr Clin Pract 25: 347–352, 2010 [DOI] [PubMed] [Google Scholar]
- 69. Miheller P, Muzes G, Racz K, Blazovits A, Lakatos P, Herszenyi L, Tulassay Z. Changes of OPG and RANKL concentrations in Crohn's disease after infliximab therapy. Inflamm Bowel Dis 13: 1379–1384, 2007 [DOI] [PubMed] [Google Scholar]
- 70. Miheller P, Toth M, Molnar E, Zagoni T, Racz K, Tulassay Z. [Serum bone marker measurements in bone metabolism disorders associated with inflammatory bowel diseases]. Orv Hetil 142: 1557–1560, 2001 [PubMed] [Google Scholar]
- 71. Moe SM. Disorders involving calcium, phosphorus, and magnesium. Prim Care 35: 215–237, v–vi, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Moschen AR, Kaser A, Enrich B, Ludwiczek O, Gabriel M, Obrist P, Wolf AM, Tilg H. The RANKL/OPG system is activated in inflammatory bowel disease and relates to the state of bone loss. Gut 54: 479–487, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Nakashima T, Takayanagi H. Osteoimmunology: crosstalk between the immune and bone systems. J Clin Immunol 29: 555–567, 2009 [DOI] [PubMed] [Google Scholar]
- 74. Ohnishi M, Nakatani T, Lanske B, Razzaque MS. Reversal of mineral ion homeostasis and soft-tissue calcification of klotho knockout mice by deletion of vitamin D 1alpha-hydroxylase. Kidney Int 75: 1166–1172, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Okunade GW, Miller ML, Pyne GJ, Sutliff RL, O'Connor KT, Neumann JC, Andringa A, Miller DA, Prasad V, Doetschman T, Paul RJ, Shull GE. Targeted ablation of plasma membrane Ca2+-ATPase (PMCA) 1 and 4 indicates a major housekeeping function for PMCA1 and a critical role in hyperactivated sperm motility and male fertility for PMCA4. J Biol Chem 279: 33742–33750, 2004 [DOI] [PubMed] [Google Scholar]
- 76. Papet I, El Yousfi M, Godin JP, Mermoud AF, Davicco MJ, Coxam V, Breuille D, Obled C. HLA-B27 rats develop osteopaenia through increased bone resorption without any change in bone formation. J Musculoskelet Neuronal Interact 8: 251–256, 2008 [PubMed] [Google Scholar]
- 77. Poser JW, Esch FS, Ling NC, Price PA. Isolation and sequence of the vitamin K-dependent protein from human bone. Undercarboxylation of the first glutamic acid residue. J Biol Chem 255: 8685–8691, 1980 [PubMed] [Google Scholar]
- 78. Price PA, Williamson MK. Primary structure of bovine matrix Gla protein, a new vitamin K-dependent bone protein. J Biol Chem 260: 14971–14975, 1985 [PubMed] [Google Scholar]
- 79. Raggatt LJ, Partridge NC. Cellular and molecular mechanisms of bone remodeling. J Biol Chem 285: 25103–25108, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Rauner M, Stupphann D, Haas M, Fert I, Glatigny S, Sipos W, Breban M, Pietschmann P. The HLA-B27 transgenic rat, a model of spondyloarthritis, has decreased bone mineral density and increased RANKL to osteoprotegerin mRNA ratio. J Rheumatol 36: 120–126, 2009 [DOI] [PubMed] [Google Scholar]
- 81. Renkema KY, Alexander RT, Bindels RJ, Hoenderop JG. Calcium and phosphate homeostasis: concerted interplay of new regulators. Ann Med 40: 82–91, 2008 [DOI] [PubMed] [Google Scholar]
- 82. Renkema KY, Nijenhuis T, van der Eerden BC, van der Kemp AW, Weinans H, van Leeuwen JP, Bindels RJ, Hoenderop JG. Hypervitaminosis D mediates compensatory Ca2+ hyperabsorption in TRPV5 knockout mice. J Am Soc Nephrol 16: 3188–3195, 2005 [DOI] [PubMed] [Google Scholar]
- 83. Riancho JA, Valero C, Hernandez JL, Ortiz F, Zarrabeitia A, Alonso MA, Pena N, Pascual MA, Gonzalez-Macias J, Zarrabeitia MT. Association of the F352V variant of the Klotho gene with bone mineral density. Biogerontology 8: 121–127, 2007 [DOI] [PubMed] [Google Scholar]
- 84. Robinson RJ, Iqbal SJ, Abrams K, Al-Azzawi F, Mayberry JF. Increased bone resorption in patients with Crohn's disease. Aliment Pharmacol Ther 12: 699–705, 1998 [DOI] [PubMed] [Google Scholar]
- 85. Romas E. Clinical applications of RANK-ligand inhibition. Intern Med J 39: 110–116, 2009 [DOI] [PubMed] [Google Scholar]
- 86. Rouleau M, Patel A, Hendzel MJ, Kaufmann SH, Poirier GG. PARP inhibition: PARP1 and beyond. Nat Rev Cancer 10: 293–301, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Rudnicki M, Frolich A, Transbol I. Inappropriate hypercalcitriolemia in ileum-resected patients with Crohn's disease. Miner Electrolyte Metab 18: 52–55, 1992 [PubMed] [Google Scholar]
- 88. Sabbagh Y, O'Brien SP, Song W, Boulanger JH, Stockmann A, Arbeeny C, Schiavi SC. Intestinal npt2b plays a major role in phosphate absorption and homeostasis. J Am Soc Nephrol 20: 2348–2358, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Schoeber JP, Hoenderop JG, Bindels RJ. Concerted action of associated proteins in the regulation of TRPV5 and TRPV6. Biochem Soc Trans 35: 115–119, 2007 [DOI] [PubMed] [Google Scholar]
- 90. Schoon EJ, Geerling BG, Van Dooren IM, Schurgers LJ, Vermeer C, Brummer RJ, Stockbrugger RW. Abnormal bone turnover in long-standing Crohn's disease in remission. Aliment Pharmacol Ther 15: 783–792, 2001 [DOI] [PubMed] [Google Scholar]
- 91. Schoon EJ, Muller MC, Vermeer C, Schurgers LJ, Brummer RJ, Stockbrugger RW. Low serum and bone vitamin K status in patients with longstanding Crohn's disease: another pathogenetic factor of osteoporosis in Crohn's disease? Gut 48: 473–477, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Schulte C, Dignass AU, Mann K, Goebell H. Reduced bone mineral density and unbalanced bone metabolism in patients with inflammatory bowel disease. Inflamm Bowel Dis 4: 268–275, 1998 [DOI] [PubMed] [Google Scholar]
- 93. Shikhare G, Kugathasan S. Inflammatory bowel disease in children: current trends. J Gastroenterol 45: 673–682, 2010 [DOI] [PubMed] [Google Scholar]
- 94. Silvennoinen J, Risteli L, Karttunen T, Risteli J. Increased degradation of type I collagen in patients with inflammatory bowel disease. Gut 38: 223–228, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. St-Arnaud R, Arabian A, Travers R, Barletta F, Raval-Pandya M, Chapin K, Depovere J, Mathieu C, Christakos S, Demay MB, Glorieux FH. Deficient mineralization of intramembranous bone in vitamin D-24-hydroxylase-ablated mice is due to elevated 1,25-dihydroxyvitamin D and not to the absence of 24,25-dihydroxyvitamin D. Endocrinology 141: 2658–2666, 2000 [DOI] [PubMed] [Google Scholar]
- 96. Suzuki Y, Landowski CP, Hediger MA. Mechanisms and regulation of epithelial Ca2+ absorption in health and disease. Annu Rev Physiol 70: 257–271, 2008 [DOI] [PubMed] [Google Scholar]
- 97. Sylvester FA. IBD and skeletal health: children are not small adults! Inflamm Bowel Dis 11: 1020–1023, 2005 [DOI] [PubMed] [Google Scholar]
- 98. Sylvester FA, Wyzga N, Hyams JS, Davis PM, Lerer T, Vance K, Hawker G, Griffiths AM. Natural history of bone metabolism and bone mineral density in children with inflammatory bowel disease. Inflamm Bowel Dis 13: 42–50, 2007 [DOI] [PubMed] [Google Scholar]
- 99. Tabb MM, Sun A, Zhou C, Grun F, Errandi J, Romero K, Pham H, Inoue S, Mallick S, Lin M, Forman BM, Blumberg B. vitamin K2 regulation of bone homeostasis is mediated by the steroid and xenobiotic receptor SXR. J Biol Chem 278: 43919–43927, 2003 [DOI] [PubMed] [Google Scholar]
- 100. Takeuchi Y, Suzawa M, Fukumoto S, Fujita T. Vitamin K(2) inhibits adipogenesis, osteoclastogenesis, and ODF/RANK ligand expression in murine bone marrow cell cultures. Bone 27: 769–776, 2000 [DOI] [PubMed] [Google Scholar]
- 101. Thurston RD, Larmonier CB, Majewski PM, Ramalingam R, Midura-Kiela M, Laubitz D, Vandewalle A, Besselsen DG, Muhlbauer M, Jobin C, Kiela PR, Ghishan FK. Tumor necrosis factor and interferon-gamma down-regulate Klotho in mice with colitis. Gastroenterology 138: 1384–1394, 1394.e1–2, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Tuchman S, Thayu M, Shults J, Zemel BS, Burnham JM, Leonard MB. Interpretation of biomarkers of bone metabolism in children: impact of growth velocity and body size in healthy children and chronic disease. J Pediatr 153: 484–490, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Turk N, Cukovic-Cavka S, Korsic M, Turk Z, Vucelic B. Proinflammatory cytokines and receptor activator of nuclear factor kappaB-ligand/osteoprotegerin associated with bone deterioration in patients with Crohn's disease. Eur J Gastroenterol Hepatol 21: 159–166, 2009 [DOI] [PubMed] [Google Scholar]
- 104. Uno JK, Kolek OI, Hines ER, Xu H, Timmermann BN, Kiela PR, Ghishan FK. The role of tumor necrosis factor alpha in down-regulation of osteoblast Phex gene expression in experimental murine colitis. Gastroenterology 131: 497–509, 2006 [DOI] [PubMed] [Google Scholar]
- 105. Urakawa I, Yamazaki Y, Shimada T, Iijima K, Hasegawa H, Okawa K, Fujita T, Fukumoto S, Yamashita T. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature 444: 770–774, 2006 [DOI] [PubMed] [Google Scholar]
- 106. Villa-Bellosta R, Ravera S, Sorribas V, Stange G, Levi M, Murer H, Biber J, Forster IC. The Na+-Pi cotransporter PiT-2 (SLC20A2) is expressed in the apical membrane of rat renal proximal tubules and regulated by dietary Pi. Am J Physiol Renal Physiol 296: F691–F699, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106a. vitamin K2. Monograph. Altern Med Rev 14: 284–293, 2009 [PubMed] [Google Scholar]
- 107. Ward LM, Rauch F, Matzinger MA, Benchimol EI, Boland M, Mack DR. Iliac bone histomorphometry in children with newly diagnosed inflammatory bowel disease. Osteoporos Int 21: 331–337, 2010 [DOI] [PubMed] [Google Scholar]
- 108. Williams KB, DeLuca HF. Characterization of intestinal phosphate absorption using a novel in vivo method. Am J Physiol Endocrinol Metab 292: E1917–E1921, 2007 [DOI] [PubMed] [Google Scholar]
- 109. Xiao ZS, Crenshaw M, Guo R, Nesbitt T, Drezner MK, Quarles LD. Intrinsic mineralization defect in Hyp mouse osteoblasts. Am J Physiol Endocrinol Metab 275: E700–E708, 1998 [DOI] [PubMed] [Google Scholar]
- 110. Zarrabeitia MT, Hernandez JL, Valero C, Zarrabeitia AL, Ortiz F, Gonzalez-Macias J, Riancho JA. Klotho gene polymorphism and male bone mass. Calcif Tissue Int 80: 10–14, 2007 [DOI] [PubMed] [Google Scholar]