Abstract
Desire to defecate is associated with a unique anal contractile response, the sensorimotor response (SMR). However, the precise muscle(s) involved is not known. We aimed to examine the role of external and internal anal sphincter and the puborectalis muscle in the genesis of SMR. Anorectal 3-D pressure topography was performed in 10 healthy subjects during graded rectal balloon distention using a novel high-definition manometry system consisting of a probe with 256 pressure sensors arranged circumferentially. The anal pressure changes before, during, and after the onset of SMR were measured at every millimeter along the length of anal canal and in 3-D by dividing the anal canal into 4 × 2.1-mm grids. Pressures were assessed in the longitudinal and anterior-posterior axis. Anal ultrasound was performed to assess puborectalis morphology. 3-D topography demonstrated that rectal distention produced an SMR coinciding with desire to defecate and predominantly induced by contraction of puborectalis. Anal ultrasound showed that the puborectalis was located at mean distance of 3.5 cm from anal verge, which corresponded with peak pressure difference between the anterior and posterior vectors observed at 3.4 cm with 3-D topography (r = 0.77). The highest absolute and percentage increases in pressure during SMR were seen in the superior-posterior portion of anal canal, reaffirming the role of puborectalis. The SMR anal pressure profile showed a peak pressure at 1.6 cm from anal verge in the anterior and posterior vectors and distinct increase in pressure only posteriorly at 3.2 cm corresponding to puborectalis. We concluded that SMR is primarily induced by the activation and contraction of the puborectalis muscle in response to a sensation of a desire to defecate.
Keywords: anal manometry, puborectalis
in humans, balloon distention of the rectum normally induces the recto-anal inhibitory reflex (RAIR) (6, 11) as well as the recto-anal contractile reflex (RACR) (9, 10, 21). RAIR is an enteric neuronal reflex that is mediated by nitric oxide, vasoactive intestinal peptide, and adenosine triphosphate and causes relaxation of internal anal sphincter (IAS) (18). The rate and method of rectal distention (intermittent vs. ramp) affect the properties of the RAIR, and this reflex response is classically absent in Hirschsprung's disease and other conditions such as after circular rectal myotomy and after lower anterior resection (4, 12, 22).
In addition to the aforementioned reflexes, recently, we have described the sensorimotor response (SMR) (5) that is also seen during balloon distention of the rectum. The SMR is a transient anal contraction that is usually seen overlying the initial relaxation phase of the RAIR (5). In healthy subjects, it coincides with the onset of a sensation for defecation (5). A recent study showed that the SMR was abnormal in patients with rectal hyposensitivity, suggesting a pathophysiological role for this response in anorectal disorders (19). However, the precise origin of the anal contractile response, in particular the anal or pelvic floor muscle(s) that is involved in the genesis of the SMR, is not known.
We hypothesized that the motor response that is seen during SMR is predominantly induced by a contraction of the puborectalis muscle. Therefore, our aims were 1) to perform detailed characterization of the pressure profiles in the anal high-pressure zone before, during, and after the onset of SMR and 2) to examine whether the anal contractile response during the SMR originates from the puborectalis muscle, the anal sphincters, or both by performing detailed contour mapping and vector analysis of the anal canal using a novel 3-D high-definition anorectal manometry (HDM) system.
MATERIALS AND METHODS
Subjects.
Ten healthy subjects (6 males, mean age 40 yr, range 18–55 yr) were recruited. All subjects filled out a Bowel Symptom Questionnaire that assessed their general and gastrointestinal health and anorectal function. All of them were asymptomatic and were not using laxatives or any other medications except multivitamins or oral contraceptives and had no history of previous gastrointestinal surgery other than appendectomy. All subjects had a normal physical examination. The study was approved by the Institutional Review Board of the University of Iowa, and all participants provided written informed consent.
Study protocol.
After an overnight fast, subjects attended the motility lab. No routine bowel preparation was used. With the subject lying down in the left lateral position, an HDM probe (Sierra Scientific, Los Angeles, CA) was placed into the anorectum. This rigid probe measures 6.4 cm in length and has an outer diameter of 10.75 mm. It has 256 pressure sensors that are arranged in 16 rows, and each row has 16 circumferentially oriented sensors. Each sensor has a 4-mm center linear spacing and 2.1-mm circumferential spacing. Although pressure is sensed at 4-mm intervals, the software linearly interpolates the space between sensors to provide measurements at 1-mm spacing with negligible error. The probe has a central lumen for inflation and a Luer lock at one end through which a balloon is attached. The probe is attached to an amplifier and recorder system, and the manometric and topographic images are displayed on a computer monitor using specialized software (Motility Visualization System v.2.2, Sierra Scientific). The HDM system operates at a frequency response of >20 Hz, a scan rate of 10 Hz, and an output resolution of 0.1 mmHg. The probe was calibrated immediately before the procedure by placement in a calibration chamber, where it was zeroed to atmospheric pressure and set to a range of pressure up to 300 mmHg. The sensor calibration residual is ± 2 mmHg in the 0–100-mmHg range and 2% of reading in the 100–300-mmHg range. The lubricated probe was inserted into the rectum and placed such that the outermost panel of pressure sensors was located at the anal verge. Because the probe has circumferential pressure sensors, it was oriented such that the posterior portion corresponded to the dorsal aspect of the subject. This allowed for standardization of the vector measurements in the rectum and the anal canal.
The probe was held in place manually by one of the investigators throughout the study. After a rest period of 7 min, we assessed the resting and squeeze pressure profiles. Next, the rectal balloon was inflated in a stepwise, graded fashion using an intermittent balloon distention technique (15, 16). The subjects were given a Likert-like sensation chart and asked to describe their sensations. Rectal balloon distentions were performed by using a hand-held syringe in 10-ml increments until the subject reported a first sensation and thereafter in 30-ml increments until maximum tolerable volume or a total volume of 320 ml was reached. Each distention was held for at least 30 s and after deflation and rest period of 2 min was reinflated to the next volume. The subject was unaware of the timing or the volume of balloon distention. The threshold balloon volumes that induced a first sensation, desire to defecate, urge to defecate, and the maximum tolerable volume were recorded.
In seven subjects, an anal ultrasound was performed by placing a 8-mm ultrasound probe (Hitachi, San Jose, CA) into the rectum. The distance from the anal verge where the puborectalis muscle was best visualized and its thicknesses at the three o'clock and nine o'clock positions were examined.
Data analysis.
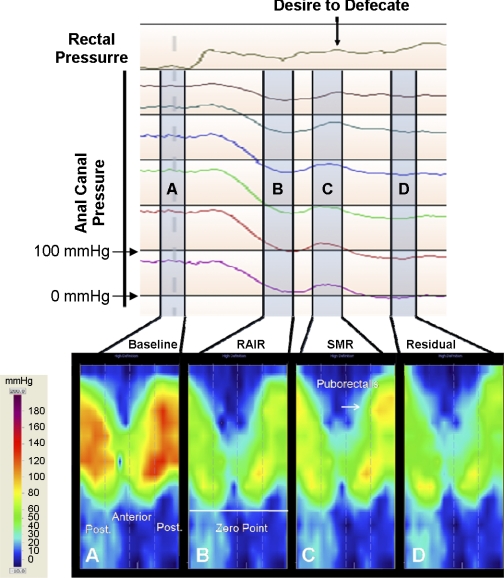
The manometric and topographic HDM data were examined to assess the presence of SMR and to define its motor characteristics. An example of the SMR response as seen with manometry and 3-D topography is shown in Fig. 1. The top line of the manometric image corresponds to the rectal balloon pressure, and the lower lines represent the anal pressure changes at different distances from the anal verge. Rectal balloon distention induces a reflex relaxation of the anal canal or RAIR. Immediately after the RAIR reaches its nadir, the SMR is visualized manometrically as a transient contraction with an increase in anal canal pressure. This anal contractile response coincided with the subject signaling the onset for a desire to defecate.
Fig. 1.
Top: continuous recording of the rectal and anal manometric pressure sequences. Bottom: 4 separate snapshots or zones of the high-resolution topographic pressure profiles taken before, during, and after a sensorimotor response (SMR) in a healthy subject. A: anal and rectal resting pressure profile before rectal balloon distention. Following this, the rectal balloon distention can be seen as a rise in the pressure in the rectal pressure channel. The rectal balloon distention induces a recto-anal inhibitory reflex (RAIR) that is seen as a relaxation of the anal resting pressure throughout all anal channels. B: pressure changes just before the onset of the SMR and predominately consists of anal relaxation. C: subject signals a sensation of desire to defecate, and simultaneously there is a unique anal contractile response that is seen throughout the anal high-pressure zone, which represents the SMR. The topographic image further shows a transient contraction of the puborectalis muscle (Arrow). Following the SMR, the RAIR continues to inhibit anal pressures throughout the anal canal, and this residual pressure is represented D. All measurements were taken at a specific time point within the A, B, C, and D ranges.
The manometric anal pressure changes were measured at four time points during the course of rectal distention and are depicted in Fig. 1. This figure displays simultaneous topographic and manometric images. The four time points designated as Fig. 1, A, B, C, and D, correspond to the following components of the recording: Fig. 1A, the baseline anal pressure; Fig. 1B, the anal relaxation pressure immediately before the onset of the SMR; Fig. 1C, the highest amplitude of the anal contractile response; and Fig. 1D, the residual pressure of the anal relaxation response. The topographic images shown in Fig. 1 provide a 3-D pressure profile image, with the outer edges of the map showing the pressure changes in the posterior portion of the anal canal and the middle or the inner portion of the image showing the pressure changes in the anterior portion of the anal canal. The topographic view also allowed us to define the zero point or the anal verge, which was the point where we observed a sharp drop in anal pressure that equaled atmospheric pressure (Fig. 1B). After the anal canal length was set using the topographic pressure map and the four time points were identified using the manometric recording, a detailed pressure analysis could be performed.
We analyzed the anal pressures at each of the four time points and at every millimeter distance along the length of the anal canal from the anal verge. From these individual pressure values, we calculated an average circumferential anal pressure at a resolution of one millimeter. The topographic pressure map was divided into 4-mm × 2.1-mm grids, and an average anal pressure per grid was calculated. This allowed us to provide a multidimensional analysis of the anal canal pressures both in the longitudinal and in the circumferential axes. Using this vector mapping method, we compared the pressures in the anterior half with the posterior half of the anal canal at a resolution of 4 mm.
The SMR anal contractile response was also analyzed for the changes in pressure. We measured anal pressures immediately before the onset of SMR as well as the peak SMR amplitude. The absolute SMR pressure increase was calculated at every millimeter distance along the length of the anal canal. Additionally, the anal canal was subdivided into 1-cm segments, and the percentage increase in pressure during the SMR and in each segment was calculated.
Anal ultrasound data were analyzed for the distance from the anal verge, where the puborectalis muscle was best visualized in each subject.
Statistical analysis.
The anterior and posterior anal canal pressures during the baseline period were compared at every 4-mm distance along the anal canal using Student's t-test. Also, the anterior and posterior and circumferential anal pressures before, during, and after the SMR were compared at every millimeter distance using a two-tailed student's t-test. The percent change in anal pressure for the four segments of the anal canal (Fig. 1, A, B, C, and D) were compared using ANOVA and a Bonferroni's multiple-comparison posttest analysis. Statistical significance was considered when P < 0.05. The distance from the anal verge at which the puborectalis muscle was identified with anal ultrasound was compared and correlated with the distance from the anal verge, where the pressure difference between the anterior and posterior half of the anal canal was the highest per subject with Pearson correlation coefficient. This most likely represented the level at which the puborectalis contributed the greatest fraction of the total anal pressure.
RESULTS
An SMR was present in all of our subjects and was demonstrated both manometrically and topographically (Fig. 1). The SMR coincided with the onset of a desire to defecate in all of our subjects. The average duration of the SMR was 6.4 ± 0.5 s, and the mean rectal distention volume to induce SMR was 107 ± 6 ml. The rectal distention volume that induced a first sensation was 17 ± 3.4 ml, desire to defecate was 100.0 ± 14.8 ml, and urge to defecate was 160.0 ± 15.1 ml. All subjects tolerated the HDM and ultrasound recording satisfactorily.
SMR pressure profiles and contribution of puborectalis muscle.
The topographic pressure image during each of the four time points shown in Fig. 1 facilitates a better spatiotemporal visualization of the anal pressure changes. Here, one can visualize the activation or relaxation of the individual muscle components that surround the anal canal. For example, the puborectalis muscle can be identified in the superior-posterior (Fig. 1, top) section of the image. Through these sequential topographic pressure images, it is also possible to visualize the origin and progression of the anal contractile response that occurs during SMR and how the activation and contraction of the puborectalis muscle appears to be the predominant muscle contributing to this response.
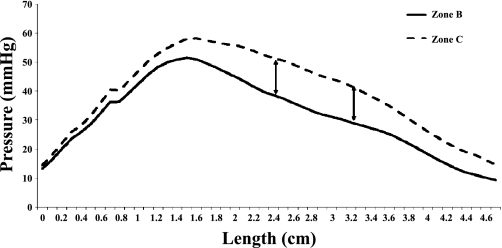
This role of the puborectalis muscle was further confirmed by the measurements of the mean circumferential pressures immediately before and during the SMR and is shown in Fig. 2. We found a statistically significant (P ≤ 0.05) increase in anal canal pressure during the SMR from a distance of 2.4 cm to 3.2 cm from the anal verge. This location is represented with arrows in Fig. 2 and corresponds to the puborectalis muscle. (Fig. 4).
Fig. 2.
The circumferential pressure changes along the length of the anal canal before and during the SMR, zones B and C, respectively. The arrows outline the location where there was a significant (P ≤ 0.05) rise in pressure during the SMR. This location (2.4–3.2 cm from the anal verge) is the same location at which the puborectalis muscle was identified to generate anal canal pressure in Fig. 3.
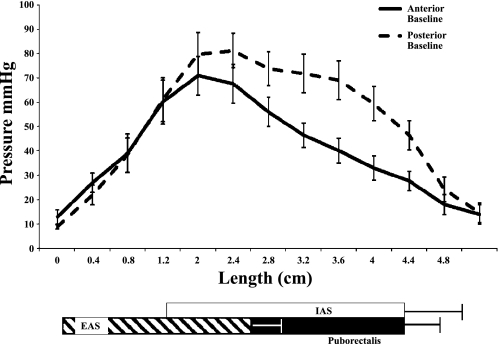
Fig. 4.
Mean pressure changes along the length of the anal canal from all subjects in the anterior and posterior vectors during baseline, i.e., resting. The locations of the 3 anal canal muscular structures that contribute to the anal high-pressure zone as described by Mittal and colleagues (11a, 14). IAS, internal anal sphincter; EAS, external anal sphincter.
Anal pressure changes during SMR.
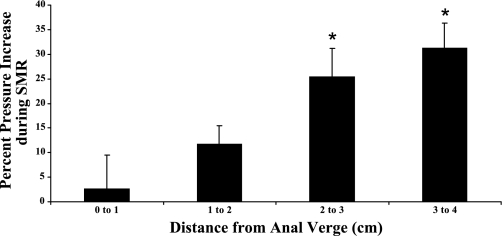
The peak increase in anal pressure during SMR was seen at about 2.8 cm from the anal verge (Fig. 2) and spanned a distance of 0.8 cm from 2.4 cm to 3.2 cm. Because this region corresponds to the locale of the puborectalis muscle within the anal canal, it further suggests that the anal contractile response during SMR is most likely induced by the puborectalis. The percent increase in anal pressure at each centimeter distance from the anal verge during the SMR is shown in Fig. 3. The superior portion of the anal canal shows the greatest percent increase, with the superior 2 cm of the anal canal having a significant percentage pressure increase compared with the most distal section of the anal canal. This again reflects the contraction of the puborectalis muscle.
Fig. 3.
Mean percent increase in anal pressure during the SMR from all subjects at every centimeter along the anal canal. The superior portion shows the largest percentage increase in pressure, with the superior 2 cm of the anal canal showing a more significant (*P < 0.05) percentage pressure increase compared with the more distal sections of the anal canal. The means ± SE are shown per segment. Data are the means from all subjects.
Vector and longitudinal changes in basal anal sphincter pressure.
When the baseline anal pressure was analyzed along the length of the anal canal, the peak pressure was seen at 1.6 cm from the anal verge, both in the anterior and posterior vectors (Fig. 4). A second smaller peak or hump was also seen consistently in all subjects at ∼3.2 cm. However, this hump was only seen in the posterior region and was distinctively absent in the anterior pressure profile (Fig. 4). The posterior-specific hump spanned a distance of 1.6 cm and was seen between 2.4 cm to 4.0 cm from the anal verge. When we compared the mean anterior vs. mean posterior basal sphincter pressures at 4-mm increments from the anal verge, there was no difference up to a distance of 2.4 cm but a significant pressure difference between 2.4 cm and 4.0 cm (Table 1). This pressure difference and its location most likely correspond to the posterior sling fibers of the puborectalis muscle. Thus the puborectalis offers a distinct and important contribution to the resting anal sphincter profile, over and above the peak pressure offered by the IAS in both the anterior and posterior vectors that begin at 1.6 cm.
Table 1.
Changes in the baseline anal pressure at each 4-mm level from the anal verge and at the anterior and posterior vectors
| Length From Anal Verge, cm | Anterior, mmHg | Posterior, mmHg | P |
|---|---|---|---|
| 0 | 12.77 | 8.8 | 0.165 |
| 0.4 | 26.92 | 21.88 | 0.381 |
| 0.8 | 38.98 | 38.24 | 0.946 |
| 1.2 | 60.15 | 61.01 | 0.947 |
| 1.6 | 70.86 | 79.69 | 0.482 |
| 2 | 67.5 | 81.16 | 0.221 |
| 2.4 | 56.02 | 73.75 | 0.067 |
| 2.8 | 46.42 | 71.77 | 0.014 |
| 3.2 | 40.02 | 68.96 | 0.01 |
| 3.6 | 32.84 | 59.31 | 0.007 |
| 4 | 27.59 | 46.44 | 0.016 |
| 4.4 | 17.92 | 24.19 | 0.319 |
| 4.8 | 13.86 | 14.53 | 0.904 |
Correlation between anal ultrasound and HDM pressure profile.
Anal ultrasound data were obtained in seven out of ten subjects. We found that the distance from the anal verge, where the puborectalis was best identified using anal ultrasound, was located at a mean distance of 3.5 cm. This corresponded nicely with the location from the anal verge, where the pressure difference was highest between the anterior and posterior half of anal canal, mean 3.4 cm, which most likely corresponded to the puborectalis (Table 2), P = 0.6. The Pearson correlation coefficient was 0.77. Owing to technical reasons, the ultrasound study could not be performed in three subjects.
Table 2.
Individual values for the distance from the anal verge where the puborectalis was best identified with anal ultrasound and with HDM
| HDM, cm | Ultrasound, cm |
|---|---|
| 3.2 | 3.75 |
| 3.2 | 3.5 |
| 3.2 | 3 |
| 3.2 | 3.5 |
| 4.0 | 4.75 |
| 3.2 | 2.5 |
| 3.6 | 3.5 |
| Mean =3.37 | Mean =3.5 |
HDM, high-definition 3-D topography
DISCUSSION
Our main objective was to examine the anal sphincter spatiotemporal changes during SMR and to delineate the predominant anal muscle component for this response using 3-D high-definition anorectal pressure topography. The SMR was consistently seen in all of our subjects overlying the RAIR. Its onset coincided with a desire to defecate in all of our subjects, as previously described (5). Detailed topographic and vector characterization of the anal contractile response during the SMR revealed that the rise in anal pressure was largely attributable to the contraction of the puborectalis muscle. The absolute pressure change showed that the highest increase in the anal region corresponded to the location of the puborectalis muscle. Additionally, the greatest percentage increase in pressure during the SMR was also seen at the superior portion of the anal canal, further supporting our hypothesis that the anal contractile response seen during the SMR is primarily attributable to the contraction of the puborectalis muscle. Furthermore, anal ultrasound studies provided independent validation that the pressure profiles observed in the superior-posterior portion of the anal canal with 3-D topography corresponded to the location where the puborectalis muscle provided the greatest fraction of the total anal pressure, reaffirming the role of this muscle in the genesis of this response.
The neurohumoral mediators for this anal excitatory muscle response are not known and remain to be explored. It is unclear whether this is a locally mediated response similar to RAIR (20) or whether it is modulated through a spinal or even central process. Because the response is invariably triggered with the onset of a need to defecate, we suspect that this must be centrally modulated. Additionally, much like the graded changes in RAIR (12, 22), the SMR also exhibits a larger response at higher volumes of rectal distention (5). Supra spinal and parasympathetic pathways are implicated in the modulation of RAIR. We suspect that the sacral parasympathics may be involved in the SMR as evidenced by modulation of puborectalis through stimulation of sacral plexus (2, 13). Future studies of the SMR in patients with neurological conditions and lower bowel dysfunction may demonstrate pathophysiological changes, much like the alterations in RAIR seen in patients with Hirschsprung's disease (20) and spinal cord injury (1).
The functional significance of the SMR is also unknown. One possible explanation is that the SMR acts as an involuntary mechanism to signal onset of imminent defecation or help to maintain continence during the abrupt changes in rectal pressure from distention or movements of gas or stool in the distal rectum. This hypothesis may explain why the SMR is associated with the onset of a desire to defecate and larger rectal distention volumes produced a stronger SMR response (5). A second possibility is that a transient contraction of the puborectalis would allow the individual to sense whether the contents are solid, which would have a weight and mass to press against during the SMR, rather than gas. The consistency of rectal contents has previously been shown to influence defecation (17). This concept also explains why the SMR is associated with the desire to defecate. It therefore follows that the arousal of a desire to defecate induces an anal canal contraction that is mediated by voluntary, striated muscle, such as the puborectalis.
In this study, we also performed detailed analysis of the spatiotemporal pressure changes along the anal canal. We found that in the resting stage there are two peaks in the anal canal profile; the first peak occurred at 1.6 cm, and this largely corresponds to the IAS (11a; 14). The second less prominent inflection of the pressure profile, spanning a distance of ∼1.6–1.8 cm occurred at a distance of 2.4 cm to 4.0 cm from the anal verge. This most likely corresponded to the location of the posterior sling fibers of the puborectalis muscle. Furthermore, we found that there was a significant difference in the pressure profile between the anterior and posterior region of the anal canal at rest for the same 1.6-cm distance, spanning between 2.4 cm to 4.0 cm from the verge. Liu et al. (11a) found the puborectalis to be located 2.5 cm to 4.0 cm from the anal verge using luminal ultrasound pull-though technique, which matches exactly with this study's observation that the posterior vector pressure from 2.4 cm to 4.0 cm is largely attributable to the puborectalis muscle (11a).
The detailed pressure and vector analysis was largely possible because of the superior and 3-D resolution of the HDM system. This imaging modality has the advantage of simultaneously measuring pressure recordings over the entire length of the anal canal and also circumferentially. This degree of fine detail allowed for concurrent assessment and correlation of anatomy with pressure profiles, rather than performing correlations using two different techniques such as manometry and ultrasound (8) or MRI (3, 7). Additionally, the closely spaced sensors oriented circumferentially and longitudinally along the length of the anal canal provided a continuous display of pressure without loss of signals, unlike a water-perfused, air-perfused, or solid-state pressure transducers that can only provide pressure measurements at predetermined points or levels. HDM not only allowed for a higher resolution and more clear understanding of the anal canal pressures, but also facilitated the ability to distinguish each component of the high-pressure zone. Thus in this regard our measurements and anatomical delineation correspond to those described by Mittal and colleagues (14) and confirm and extend their findings. HDM has implications for improving our understanding and diagnosis of anorectal disorders.
In conclusion, this study reaffirms the presence of SMR in healthy subjects. Furthermore, through detailed topographic and manometric characterization using HDM, our study demonstrates that the anal contractile response seen during the SMR is primarily induced by the activation and contraction of the puborectalis muscle.
GRANTS
This research was supported in part by NIH grant R01DK 57100-03. G. Cheeney was supported through a Clinical Research Fellowship from the Doris Duke Charitable Foundation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
This work was presented at Digestive Disease Week, New Orleans, LA, 2010 and published as an abstract in Gastroenterology 138: S544, 2010. Current affiliation for J. M. Remes-Troche: Digestive Physiology and Motility Department, Medical-Biological Research Institute, University of Veracruz, Veracruz, Mexico.
REFERENCES
- 1. Beuret-Blanquart F, Weber J, Gouverneur JP, Demangeon S, Denis P. Colonic transit time and anorectal manometric anomalies in 19 patients with complete transection of the spinal cord. J Auton Nerv Syst 30: 199–207, 1990 [DOI] [PubMed] [Google Scholar]
- 2. Bharucha AE. Pelvic floor: anatomy and function. Neurogastroenterol Motil 18: 507–519, 2006 [DOI] [PubMed] [Google Scholar]
- 3. Cornella JL, Hibner M, Fenner DE, Kriegshauser JS, Hentz J, Magrina JF. Three-dimensional reconstruction of magnetic resonance images of the anal sphincter and correlation between sphincter volume and pressure. Am J Obstet Gynecol 189: 130–135, 2003 [DOI] [PubMed] [Google Scholar]
- 4. Cortesini C, Pucciani F, Carassale GL, Paparozzi C. Anorectal physiology after anterior resection and pull-through operation. Eur Surg Res 15: 176–183, 1983 [DOI] [PubMed] [Google Scholar]
- 5. De Ocampo S, Remes-Troche JM, Miller MJ, Rao SSC. Rectoanal sensorimotor response in humans during rectal distension. Dis Colon Rectum 50: 1639–1646, 2007 [DOI] [PubMed] [Google Scholar]
- 6. Denny-Brown D, Robertson EG. An investigation of the nervous control of defaecation. Brain 58: 256–310, 1935 [DOI] [PubMed] [Google Scholar]
- 7. Fenner DE, Kriegshauser JS, Lee HH, Beart RW, Weaver A, Cornella JL. Anatomic and physiologic measurements of the internal and external anal sphincters in normal females. Obstet Gynecol 91: 369–374, 1998 [DOI] [PubMed] [Google Scholar]
- 8. Gantke B, Schafer A, Enck P, Lubke HJ. Sonographic, manometric, and myographic evaluation of the anal sphincters morphology and function. Dis Colon Rectum 36: 1037–1041, 1993 [DOI] [PubMed] [Google Scholar]
- 9. Garry RC. The responses to stimulation of the caudal end of the large bowel in the cat. J Physiol 78: 208–224, 1933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Goligher JC, Hughes ES. Sensibility of the rectum and colon. Its role in the mechanism of anal continence. Lancet 1: 543–547, 1951 [DOI] [PubMed] [Google Scholar]
- 11. Gowers WR. The automatic action of the sphincter ani. Proc R Soc Lond B Biol Sci 26: 77–84, 1877 [Google Scholar]
- 11a. Liu J, Guaderrama N, Nager CW, Pretorius DH, Master S, Mittal RK. Functional correlates of anal canal anatomy: puborectalis muscle and anal canal pressure. Am J Gastroenterol 101: 1092–1097, 2006 [DOI] [PubMed] [Google Scholar]
- 12. Monteiro FJ, Regadas FS, Murad-Regadas SM, Rodrigues LV, Leal VM. Comparative evaluation of the effect of sustained inflation and rapid inflation/deflation of the intrarectal balloon upon rectoanal inhibitory reflex parameters in asymptomatic subjects. Tech Coloproctol 11: 323–326, 2007 [DOI] [PubMed] [Google Scholar]
- 13. Percy JP, Neill ME, Swash M, Parks AG. Electrophysiological study of motor nerve supply of pelvic floor. Lancet 1: 16–17, 1981 [DOI] [PubMed] [Google Scholar]
- 14. Raizada V, Mittal RK. Pelvic floor anatomy and applied physiology. Gastroenterol Clin North Am 37: 493–509, vii, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rao SS, Azpiroz F, Diamant N, Enck P, Tougas G, Wald A. Minimum standards of anorectal manometry. Neurogastroenterol Motil 14: 553–559, 2002 [DOI] [PubMed] [Google Scholar]
- 16. Rao SS, Hatfield R, Soffer E, Rao S, Beaty J, Conklin JL. Manometric tests of anorectal function in healthy adults. Am J Gastroenterol 94: 773–783, 1999 [DOI] [PubMed] [Google Scholar]
- 17. Rao SS, Kavlock R, Rao S. Influence of body position and stool characteristics on defecation in humans. Am J Gastroenterol 101: 2790–2796, 2006 [DOI] [PubMed] [Google Scholar]
- 18. Rattan S, Sarkar A, Chakder S. Nitric oxide pathway in rectoanal inhibitory reflex of opossum internal anal sphincter. Gastroenterology 103: 43–50, 1992 [DOI] [PubMed] [Google Scholar]
- 19. Remes-Troche JM, De Ocampo S, Paulson J, Rao S. Rectoanal reflexes and sensorimotor response in rectal hyposensitivity. Dis Colon Rectum 53: 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sangwan YP, Solla JA. Internal anal sphincter: advances and insights. Dis Colon Rectum 41: 1297–1311, 1998 [DOI] [PubMed] [Google Scholar]
- 21. Sun WM, Read NW, Donnelly TC. Anorectal function in incontinent patients with cerebrospinal disease. Gastroenterology 99: 1372–1379, 1990 [DOI] [PubMed] [Google Scholar]
- 22. Sun WM, Read NW, Prior A, Daly JA, Cheah SK, Grundy D. Sensory and motor responses to rectal distention vary according to rate and pattern of balloon inflation. Gastroenterology 99: 1008–1015, 1990 [DOI] [PubMed] [Google Scholar]