Abstract
Apolipoprotein (apo) A-IV overexpression enhances chylomicron (CM) assembly and secretion in newborn swine intestinal epithelial cells by producing larger particles (Lu S, Yao Y, Cheng X, Mitchell S, Leng S, Meng S, Gallagher JW, Shelness GS, Morris GS, Mahan J, Frase S, Mansbach CM, Weinberg RB, Black DD. J Biol Chem 281: 3473–3483, 2006). To determine the impact of apo A-IV on microsomal triglyceride transfer protein (MTTP), IPEC-1 cell lines containing a tetracycline-regulatable expression system were used to overexpress native swine apo A-IV and “piglike” human apo A-IV, a mutant human apo A-IV with deletion of the EQQQ-rich COOH-terminus, previously shown to upregulate basolateral triglyceride (TG) secretion 5-fold and 25-fold, respectively. Cells were incubated 24 h with and without doxycycline and oleic acid (OA, 0.8 mM). Overexpression of the native swine apo A-IV and piglike human apo A-IV increased MTTP lipid transfer activity by 39.7% (P = 0.006) and 53.6% (P = 0.0001), respectively, compared with controls. Changes in mRNA and protein levels generally paralleled changes in activity. Interestingly, native swine apo A-IV overexpression also increased MTTP large subunit mRNA, protein levels, and lipid transfer activity in the absence of OA, suggesting a mechanism not mediated by lipid absorption. Overexpression of piglike human apo A-IV significantly increased partitioning of radiolabeled OA from endoplasmic reticulum (ER) membrane to lumen, suggesting increased net transfer of membrane TG to luminal particles. These results suggest that the increased packaging of TG into nascent CMs in the ER lumen, induced by apo A-IV, is associated with upregulation of MTTP activity at the pretranslational level. Thus MTTP is regulated by apo A-IV in a manner to promote increased packaging of TG into the CM core, which may be important in neonatal fat absorption.
Keywords: chylomicron, enterocyte, IPEC-1 cells, MTTP large subunit, oleic acid
apolipoprotein A-IV (apo A-IV) is a protein abundantly expressed in the small intestine, and expression is highly regulated by dietary lipid, especially in the newborn and sucking proximal small intestine (1, 6, 19). Over the years, myriad functions have been ascribed to apo A-IV, including roles as a postprandial satiety factor, participant in reverse cholesterol transport, lipoprotein antioxidant, anti-inflammatory agent, and antiatherosclerotic factor (11, 12, 14, 29, 32).
We have recently demonstrated a possible role for apo A-IV in enhancing enterocyte lipid secretion by facilitating the packaging of additional lipid into larger chylomicron particles using a tetracycline-regulatable expression system to overexpress apo A-IV in a newborn swine enterocyte cell line, IPEC-1 (23, 24). On the basis of these results, we have proposed that apo A-IV-mediated enhancement of chylomicron secretion may be important in the newborn pig, and probably the human infant, given the striking homology in intestinal maturation and function between the two species (3). Both likely utilize the chylomicron/intestinal lymphatic pathway for lipid absorption during the suckling period, in contrast to the neonatal rodent that appears to absorb significant amounts of fatty acids directly into the portal vein and may be less dependent on apo A-IV (5, 13). Both the human infant and newborn pig must absorb a large lipid load from breast milk during the suckling period, and apo A-IV is highly regulated by dietary lipid during the suckling period with decreased responsiveness after weaning (6, 7).
The mechanism by which apo A-IV enhances triglyceride (TG) transport may be related to structural features. The amphipathic α-helices in apo A-IV are more hydrophilic compared with those of other apolipoproteins and have a radial charge distribution that precludes deep penetration into lipid monolayers (34). These helical domains in swine apo A-IV are somewhat less hydrophilic compared with those of human apo A-IV (27). Weinberg et al. (34) have proposed that the presence of these hydrophilic helices enables apo A-IV to adopt an elastic, pressure-sensitive conformation at lipid interfaces. During chylomicron assembly, these hydrophilic α-helices would be recruited into the expanding nascent particle surface and by maintaining constant interfacial elasticity would stabilize particle growth and enable the packaging of more core lipid molecules per particle. Another potential mechanism may involve interaction of apo A-IV with apolipoprotein B (apo B) to retard prechylomicron trafficking through the endoplasmic reticulum (ER) to allow more time for core lipidation, as suggested by the work of Gallagher et al. (15).
Microsomal triglyceride transfer protein (MTTP), a heterodimeric protein complex possessing lipid transfer activity, functions in the small intestine and liver to transport ER membrane-bound lipid, primarily newly synthesized TG, to newly translated apo B in the ER lumen as the first step in TG-rich lipoprotein biogenesis (17, 18). This initial lipidation of apo B prevents proteosome-mediated degradation. In the small intestine, MTTP may also facilitate the further lipidation of nascent chylomicrons beyond the first apo B rescue step (30, 31). The MTTP large subunit (97 kDa) possesses the lipid transfer activity, and the smaller subunit, identical to protein disulfide isomerase (55 kDa), may maintain the large subunit in a soluble form and serve as a chaperone to target MTTP to the ER lumen. Mutations in the MTTP large subunit are responsible for the disease abetalipoproteinemia, characterized by inability to secrete intestinal chylomicrons or hepatic VLDL (35).
We have previously demonstrated that lipid absorption physiologically upregulates MTTP at the pretranslational level in IPEC-1 cells and in newborn swine small intestine (22). In more recent studies, we have demonstrated that overexpression of native swine apo A-IV using a tetracycline-regulatable expression system enhances basolateral TG secretion by approximately fivefold in IPEC-1 cells by producing larger chylomicron particles (23). When a “piglike” human apo A-IV mutant lacking the native EQQQ-rich carboxy terminus, which is absent in swine A-IV, is overexpressed, basolateral TG secretion increases 25-fold in the form of very large lipid particles (23). The reason for this striking enhancement is unclear at present, but once the carboxy terminus is removed, human apo A-IV is very similar structurally to swine apo A-IV, except that the α-helical repeats are in aggregate more hydrophilic in human apo A-IV compared with those of swine apo A-IV. This may result in differences in lipid binding and elasticity that allow more optimal surface stabilization of the nascent prechylomicron for assembly of a larger particle in the absence of the carboxy terminus. Thus the EQQQ-rich carboxy terminus of human apo A-IV appears to be inhibitory to TG secretory enhancement.
We hypothesized that in the face of the production of larger chylomicron particles and increased TG secretion induced by apo A-IV overexpression, there would be a need for increased MTTP for the lipidation of these particles in the ER. Furthermore, we would expect that this increased demand for MTTP would be greater with overexpression of the piglike human apo A-IV mutant than with the native swine apo A-IV protein. Therefore, we conducted a series of experiments in IPEC-1 cells to address the issue of possible regulation of MTTP expression and activity by apo A-IV overexpression.
MATERIALS AND METHODS
Materials.
The [9,10-3H]oleic acid (26.3 Ci/mmol) was purchased from Amersham Biosciences (Piscataway, NJ). Oleic acid (C18:1n-9; OA) and essentially fatty acid-free bovine serum albumin were purchased from Sigma Chemical (St. Louis, MO).
Cell culture.
The derivation of the IPEC-1 cell line has been described previously (16). Cells from passages 25 to 80 were used in these studies, and all cell culture was carried out at 37°C in an atmosphere containing 5% CO2. Undifferentiated IPEC-1 cells were maintained in serial passage in plastic culture flasks (75 cm2, Corning Glassworks, Corning, NY) in growth medium (GM): DMEM/F12 medium (GIBCO-BRL, Grand Island, NY) supplemented with 5% FBS (GIBCO-BRL), insulin (5 μg/ml), transferrin (5 μg/ml), selenium (5 ng/ml) (ITS Premix, BD Biosciences, Bedford, MA), epidermal growth factor (5 μg/l) (BD Biosciences), penicillin (50 μg/ml), and streptomycin (4 μg/ml) (GIBCO-BRL). To induce differentiation, undifferentiated cells were harvested by trypsinization, and 2 × 106 cells/well were plated on 75-mm-diameter collagen-coated filters (3.0-μm pore size) in Transwell culture plates (Costar, Corning, Corning, NY). Cells were maintained in serum-containing GM for 48 h and then switched to the same medium containing 10−7 M dexamethasone (Sigma), but without FBS. Medium was then changed every 2 days. We have previously shown that, after 10 days, IPEC-1 cells exhibit enterocytic features, including polarization with well-defined microvilli facing the apical medium (16). Cellular membrane integrity was assessed by measurement of apical medium lactate dehydrogenase activity (Sigma).
IPEC-1 cell lines with tetracycline-regulatable Apo A-IV expression.
The development and use of IPEC-1 cell lines stably transfected with a tetracycline-regulatable expression system (Tet-On) for native swine apo A-IV and piglike human apo A-IV overexpression have been previously described (23).
Incubation of cells with OA.
Differentiated IPEC-1 cells (10 days postplating Transwell filters in serum-free medium) were prepared, and fresh medium was added to both the apical and basolateral Transwell compartments. The apical medium contained 0.8 mM OA complexed with albumin (4:1 molar ratio) (26). This fatty acid concentration is in the physiological range, and above this concentration the basolateral secretion of triacylglycerol begins to plateau in IPEC-1 cells (16). For Tet-On induction, doxycycline (DOX) was added to the culture medium at the same time that the experimental incubation was started. Cells were incubated for 24 h followed by harvest of culture medium and cells.
Lipid radiolabeling with [3H]OA and isolation of ER fractions.
IPEC-1 cells were incubated for 24 h with 12 μCi [3H]OA/well, and cellular ER vesicles were isolated as described (20, 36). Radiolabeled differentiated cells from four Transwell plates were pooled for each experimental condition, and undifferentiated, untreated cells from five flasks were added to the preparation to serve as a carrier. After harvest, cells were suspended in buffer D [0.25 M sucrose, 5 mM EDTA, 10 mM HEPES, and protease inhibitors (Complete, Roche Diagnostics, Indianapolis, IN) at pH 7.3]. Cells were then disrupted by N2 cavitation by using a Parr bomb (Parr Instruments, Moline, IL) set at 1,500 psi for 20 min at 4°C followed by rapid venting with return to atmospheric pressure. The cell homogenate was then transferred to a tight-fitting Dounce homogenizer and given five strokes. The preparation was then centrifuged for 1.4 × 104 g·min at 4°C in a Sorvall RC5C centrifuge (Dupont Instruments, Wilmington, DE). The supernatant was transferred to a polyallomar tube and centrifuged for 9 × 106 g·min at 4°C in a TH-641 rotor in a Sorvall Ultra 80 ultracentrifuge (Dupont Instruments). The pellet containing the ER and Golgi fractions was suspended in buffer D supplemented with 2% bovine serum albumin. The suspension was then centrifuged again for 3 × 106 g·min at 4°C, followed by harvest of the pellet. The pellet was resuspended in buffer E [0.25 M sucrose, 30 mM HEPES, 2.5 mM magnesium acetate, 30 mM KCl, and protease inhibitors (Complete, Roche Diagnostics, Indianapolis, IN)] at pH 7.2 in a loose-fitting Dounce homogenizer with five strokes. The suspension was transferred to a 12-ml polyallomar tube and brought to 3 ml with buffer E, adjusted to 1.22 M sucrose, and successively overplayed with 2.6 ml each of 1.15 M, 0.86 M, and 0.25 M sucrose. The tubes were centrifuged for 14.4 × 106 g·min at 4°C in a Sorvall Ultra 80 ultracentrifuge (Dupont Instruments). The microsomal membranes were collected as the pellet plus membranes remaining in the 1.22 M sucrose layer and combined into one sample. Samples that were not subjected to carbonate treatment were immediately frozen to −80°C. Aliquots of the whole cell homogenate and ER fractions were used for protein determination and measurement of galactosyltransferase activity (8) as a marker for Golgi contamination and glucose-6-phosphatase activity (9) as an ER marker. Compared with the whole cell homogenate, there was a sixfold enrichment in glucose-6-phosphatase specific activity in the ER fraction with no appreciable galactosyltransferase activity. Thus there was satisfactory marker enzyme enrichment and minimal cross contamination of the subcellular fractions.
Isolation of ER vesicle contents and membranes.
ER membranes and contents were isolated as described by Banerjee (2). One volume of ice-cold 200 mM Na2CO3, pH 11.3, was added to freshly collected ER vesicles to achieve a final concentration of 100 mM Na2CO3 and incubated on ice for 30 min. Samples were centrifuged for 6.4 × 106 g·min at 4°C in a Sorvall Ultra 80 ultracentrifuge (Dupont Instruments). The supernatant (containing organelle contents) and pellet (containing organelle membranes) were frozen at −80°C for later lipid extraction.
Radiolabeled lipid analysis.
The total lipid in the ER vesicles, as well as the ER membrane and lumen preparations, was extracted (33); the extracts were applied to silica gel G plates and subjected to thin-layer chromatography using petroleum ether-diethyl ether-acetic acid 80:20:1 (vol/vol/vol). The band corresponding to TG was identified by comparison to a cochromatographed standard by exposure to iodine vapor and scraped off the plate for liquid scintillation counting. Content of radiolabeled lipid was expressed as disintegrations per minute per milligram protein.
Analysis of MTTP and Apo A-IV mRNA by quantitative RT-PCR.
Total RNA was extracted from cells (10), and aliquots (2–10 μg) were treated with 0.5 units of DNAse RQ1 (Promega, Madison, WI) at 37°C for 60 min in 50 μl 40 mM Tris·HCl, pH 7.5, 6 mM MgCl2, 10 mM NaCl, 10 mM DTT, 20 U RNase inhibitor (RNAsin, Promega). The RNA was then sequentially extracted with phenol-chloroform and chloroform, precipitated with ethanol, washed once (with 70% ethanol), and resuspended in 20–40 μl H2O. For cDNA synthesis, 2 μg of total RNA was used with the Omniscript RT kit (Qiagen, Valencia, CA) with oligo(dT) nucleotides at 37°C for 1 h.
The QuantiTect SYBR Green PCR kit (Qiagen) was used for real-time PCR using the following primers: β-actin, forward 5′-CTCCTTCCTGGGCATGGA-3′ (sense, nt 426–443), reverse 5′-CGCACTTCATGATCGAGTTGA-3′ (antisense nt 470–490); swine MTTP large subunit, forward 5′-GCAAAACGCCTCTTCATGCT-3′ (sense, nt 1964–1983), reverse 5′-CAATTAAGGCCTCCAGTCTTTGG-3′ (antisense, nt 2004–2026).
Following reverse transcription, real-time quantitative PCR was performed by using the Applied Biosystems Prism 7900HT sequence detection system (Applied Biosystems, Foster, CA). The following PCR profile was used: 2 min at 50°C, 10 min at 90°C, and 40 cycles of 15 s at 95°C and 60 s at 60°C. The amount of SYBR Green was measured at the end of each cycle. Analyses were performed in duplicate. To generate the standard curves for MTTP large subunit and β-actin, serially diluted cDNA was prepared, and real-time PCR was performed as above.
Western blot analysis of apolipoproteins and MTTP large subunit.
Rabbit anti-swine apo A-IV and MTTP large subunit and anti-human apo A-IV antibodies were generated as described previously (4, 6). The antisera were subjected to ammonium sulfate precipitation and used directly as a 1:1,000 dilution for Western blot analysis.
One milliliter of basolateral culture medium was collected with added protease inhibitors (Complete, Roche Diagnostics, Indianapolis, IN) on ice, and medium was concentrated 4:1 (Centriplus YM-10, Millipore, Austin, TX). Samples were electrophoresed on an 8% (apo A-IV and MTTP large subunit) SDS-PAGE gel followed by transfer to a nitrocellulose filter. Western blotting was conducted using the ECL Western blot kit according to the manufacturer's protocol (Amersham Pharmacia Biotech, Piscataway, NJ). The blots were scanned, and digital densitometry was carried out using NIH ImageJ software.
MTTP lipid transfer activity assay.
Cells were homogenized in Cellytic-M mammalian lysis/extraction reagent (Sigma-Aldrich, St. Louis, MO) and dialyzed against 200 ml of 20 mM Tris·HCl, 0.2 mM EDTA, and 100 mM KCl and 20% glycerol, pH 7.4. MTTP lipid transfer activity was assayed by using a commercially available kit per the manufacturer's instructions (Chylos, Woodbury, NY).
Statistical analysis.
Data in experimental groups were analyzed by one-way ANOVA, followed by the Fisher's least significant difference test to compare specific groups using the StatPlus software package. Two-group comparisons were made by Student's t-test. Statistical significance was set at a two-tailed P value of <0.05.
RESULTS
Regulation of expression of native swine apo A-IV and piglike human apo A-IV in IPEC-1 cells by use of a tetracycline-regulatable expression system.
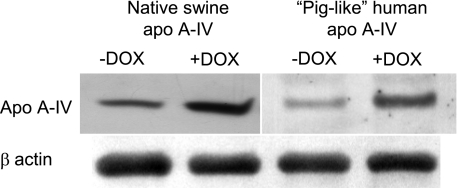
As shown previously (23), in the present studies treatment of differentiated IPEC-1 Tet-On cell lines with DOX resulted in increased basolateral secretion of native swine apo A-IV and piglike human apo A-IV (Fig. 1).
Fig. 1.
Native swine (left) and “piglike” human mutant apolipoprotein (apo) A-IV (right) proteins secreted by IPEC-1 cells incubated for 24 h with (+) and without (−) 400 ng/ml doxycycline (DOX) as assessed by Western blot analysis.
Influence of apo A-IV overexpression on MTTP lipid transfer activity, protein, and mRNA.
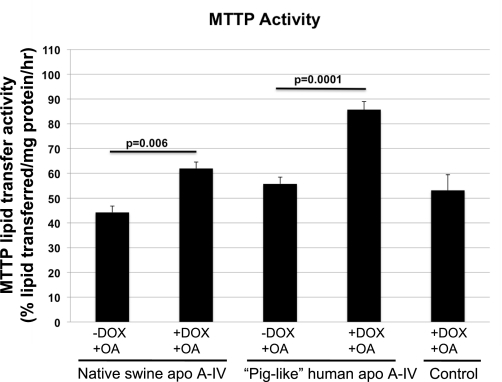
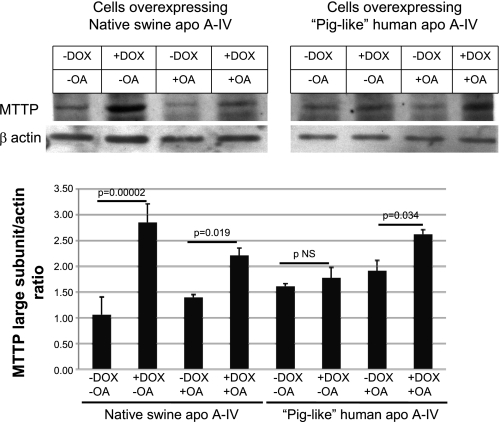
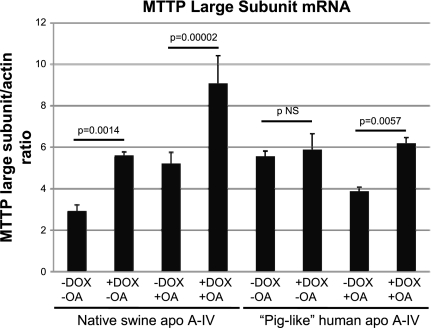
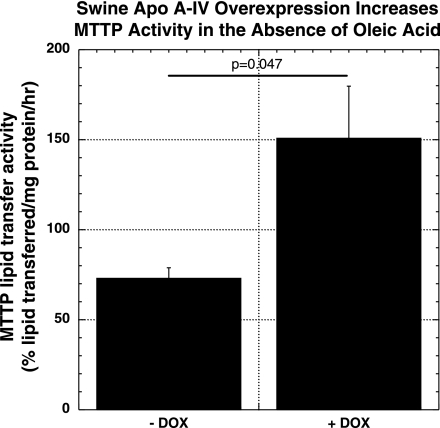
As shown in Fig. 2, overexpression of native swine apo A-IV and piglike human apo A-IV in the setting of treatment with 0.8 mM OA increased MTTP lipid transfer activity by 39.7 and 53.6%, respectively. Figure 3 shows results of Western blot analysis of MTTP large subunit protein in these two cell lines with and without DOX treatment and with and without OA incubation. In the case of native swine apo A-IV overexpression, MTTP large subunit protein levels increased significantly with DOX treatment with or without OA, suggesting that induction of MTTP by native swine apo A-IV overexpression is not solely dependent on exogenous lipid absorption and that there may be a direct regulatory effect of apo A-IV on MTTP. Figure 4 shows that MTTP large subunit mRNA levels parallel changes in protein levels for native swine apo A-IV, suggesting that the regulation of MTTP by apo A-IV occurs at the pretranslational level. As shown in Fig. 5, MTTP lipid transfer activity parallels the increases in MTTP large subunit mRNA and protein with native swine apo A-IV overexpression in the absence of OA.
Fig. 2.
Microsomal triglyceride transfer protein (MTTP) lipid transfer activity after incubation for 24 h with 0.8 mM oleic acid (OA) and with and without 400 ng/ml DOX. Nontransfected IPEC-1 cells were used as a control. Bars represent mean activity expressed as percent lipid transferred per mg protein per hour ± SE from 3 Transwells as described under methods. Statistical significance is indicated by 2-tailed P values.
Fig. 3.
MTTP large subunit protein in IPEC-1 cells overexpressing native swine apo A-IV (top, left) and piglike human mutant apo A-IV (top, right) incubated for 24 h with and without 0.8 mM OA and with and without 400 ng/ml DOX as assessed by Western blot analysis using β-actin to control for protein loading. Graph at bottom depicts results of densitometric analysis of the Western blots. Bars represent mean values for the MTTP large subunit/β-actin ratio ± SE from 3 Transwells as described under methods. Statistical significance is indicated by 2-tailed P values. NS, not significant.
Fig. 4.
MTTP large subunit mRNA in IPEC-1 cells overexpressing native swine apo A-IV and piglike human mutant apo A-IV incubated for 24 h with and without 0.8 mM OA and with and without 400 ng/ml DOX as assessed by quantitative RT-PCR analysis using β-actin to control for sample loading. Bars represent mean values for the MTTP large subunit/β-actin ratio ± SE from 3 Transwells as described under methods. Statistical significance is indicated by 2-tailed P values.
Fig. 5.
MTTP lipid transfer activity in IPEC-1 cells overexpressing native swine apo A-IV after incubation for 24 h without OA and with and without 400 ng/ml DOX. Bars represent mean activity expressed as percent lipid transferred per milligram protein per hour ± SE from 4 Transwells as described under methods. Statistical significance is indicated by the 2-tailed P value.
The regulation of MTTP by the piglike human apo A-IV is more complex. Although there is a trend toward an increase in MTTP large subunit protein (Fig. 3) and mRNA (Fig. 4) with DOX treatment without OA, the changes do not reach statistical significance. However, in the presence of OA, MTTP large subunit protein (Fig. 3) and mRNA (Fig. 4) levels both increase significantly with DOX treatment. Therefore, MTTP regulation by the piglike human apo A-IV appears to be more dependent on the presence of absorbed fatty acid.
Influence of apo A-IV overexpression on ER lipid content and membrane/lumen partitioning.
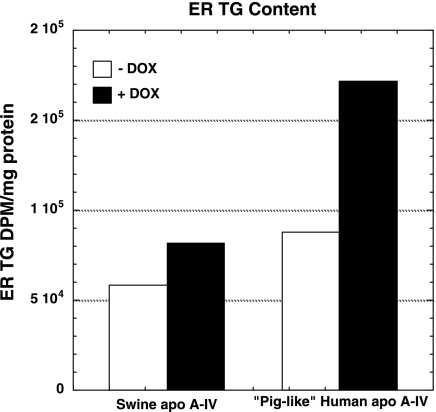
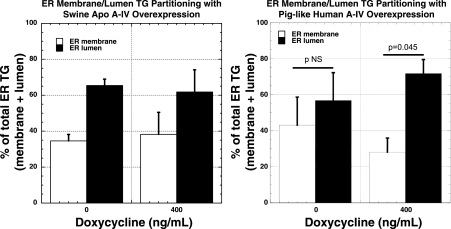
The function of MTTP in transferring TG from the ER membrane to luminal nascent lipoprotein particles was assessed by isolation of ER vesicles, which were further subjected to carbonate treatment to liberate ER lumen contents for analysis. Differentiated IPEC-1 cells overexpressing native swine apo A-IV and piglike human apo A-IV were incubated with radiolabeled OA (0.8 mM) with and without DOX for 24 h prior to cell fractionation and lipid analysis. With overexpression of native swine apo A-IV, total ER (membrane plus lumen) content of labeled TG increased by 1.4-fold (Fig. 6), but the proportion of labeled TG in ER membrane and lumen did not change (Fig. 7, left). However, overexpression of piglike human apo A-IV increased ER total labeled TG content 2.0-fold (Fig. 6) and decreased membrane-associated TG from 43.2 to 28.2% and increased luminal TG from 56.8 to 71.8% of total ER TG radioactivity (Fig. 7, right), suggesting significantly increased net transfer of membrane TG to nascent luminal particles.
Fig. 6.
Endoplasmic reticulum (ER) total radiolabeled TG content in IPEC-1 cells overexpressing native swine (A) and piglike human mutant (B) apo A-IV as assessed by incubation for 24 h with radiolabeled OA with and without 400 ng/ml DOX followed by cell fractionation, lipid extraction, thin-layer chromatography, and scintillation counting as described under methods. Bars represent mean radiolabeled lipid content as dpm/cell protein from pooled samples from 4 Transwells as described under methods.
Fig. 7.
Percentage of total ER radiolabeled TG in ER membranes and ER lumen isolated after carbonate treatment in IPEC-1 cells overexpressing native swine (left) and piglike human mutant (right) apo A-IV as assessed by incubation for 24 h with radiolabeled OA with and without 400 ng/ml DOX. Bars represent mean percentage of total ER radiolabeled lipid content ± SE from 3 experiments as described under methods. Statistical significance is indicated by 2-tailed P values.
DISCUSSION
Our laboratory has previously demonstrated a role for apo A-IV in enhancing lipid absorption in the neonatal small intestine by promoting the assembly and secretion of larger chylomicrons transporting increased core TG (23, 24). Apo A-IV expression in suckling piglet small intestine is particularly sensitive to induction by lipid absorption, and these animals are dependent on a high-fat breast milk diet for rapid growth and development (3, 6, 7). Previous studies demonstrated that controlled overexpression of native swine apo A-IV into the physiological range in IPEC-1 cells using a tetracycline-responsive expression system enhanced basolateral TG secretion approximately fivefold (23). Similar experiments with native human apo A-IV increased TG secretion twofold (23). However, a piglike mutant human apo A-IV with deletion of the apparently inhibitory EQQQ-rich carboxy terminus upregulated TG secretion 25-fold (23). To enable such increases in chylomicron secretion, the molecular machinery for chylomicron assembly and lipidation must be adaptable to the rapid increase in fatty acid flux into the cell and reesterification into TG in the ER. In particular, MTTP, which is a key player in chylomicron assembly and lipidation in the ER, must be able to accommodate these changes.
In the present studies, we examined the response of MTTP to the need for enhanced chylomicron TG packaging with overexpression of native swine apo A-IV and piglike human A-IV. These two apo A-IV proteins were selected for these experiments because of the large magnitude of basolateral TG secretory enhancement induced by these two proteins documented in our previous overexpression studies (23). The results of the present studies show that the increased packaging of TG into nascent chylomicrons in the ER lumen in IPEC-1 cells, induced by both apo A-IV proteins, is generally associated with upregulation of MTTP activity, as well as MTTP large subunit protein and mRNA levels. Overall, our data support the concept that MTTP is regulated by apo A-IV in a manner to promote increased packaging of TG into the prechylomicron resulting in basolateral lipid secretory enhancement.
For native swine apo A-IV, in addition to upregulation of MTTP lipid transfer activity by apo A-IV overexpression in the presence of OA, MTTP large subunit protein and mRNA levels were coordinately increased as well, suggesting pretranslational regulation. Interestingly, native swine apo A-IV overexpression induced MTTP large subunit protein and mRNA, as well as lipid transfer activity, both with and without OA treatment, suggesting that the regulatory mechanism may not be wholly dependent on the absorption of exogenous fatty acid.
In the case of the piglike human apo A-IV mutant that greatly enhances chylomicron TG secretion, MTTP lipid transfer activity was also increased by apo A-IV overexpression to a greater degree than that noted for native swine apo A-IV, presumably reflecting the increased need for packaging of core TG into the nascent chylomicron mediated by overexpression of the piglike human apo A-IV mutant. However, the only statistically significant change in protein and mRNA levels occurred with both DOX and OA treatment, suggesting a more complex regulatory mechanism that may be more dependent on exogenous fatty acid absorption. This may, in part, be due to the fact that the piglike human apo A-IV mutant, although similar structurally to native swine apo A-IV after deletion of the carboxy terminus, still possesses amino acid and charge differences compared with the native swine protein, and these experiments were conducted in a swine intestinal cell system.
When ER radiolabeled TG content was measured with determination of ER membrane to lumen partitioning as an overall measure of transfer of TG to ER luminal particles by MTTP, overexpression of both apo A-IV proteins increased ER TG content, with the greatest magnitude increase observed with piglike human apo A-IV, as expected. However, a significant shift to luminal partitioning was noted only for piglike human apo A-IV overexpression. Possible explanations include the fact that this technique is a relatively gross assessment of MTTP function, and differences may be observable only with large-magnitude changes in chylomicron lipidation, such as that associated with overexpression of the mutant human protein. Also, significant increases in overall TG flux through the ER may likely occur in the case of native swine apo A-IV overexpression before there is an actual shift in the ratio of ER to luminal TG dpm, possibly reflecting prechylomicron budding from the ER membrane in the prechylomicron transport vesicle as the rate-limiting step in chylomicron trafficking and lipid absorption (25, 28).
The changes in MTTP expression and activity induced by apo A-IV overexpression may occur via at least two possible mechanisms. First, the increased TG content in the ER induced by apo A-IV overexpression may secondarily upregulate MTTP large subunit at the transcriptional level. We have previously shown that MTTP large subunit expression is regulated by dietary lipid absorption in newborn swine jejunum and by OA in IPEC-1 cells in a fashion similar to that of apo A-IV (22). In additional studies in IPEC-1 cells, we have documented that the MTTP large subunit gene is a specific target of the nuclear receptor, hepatocyte nuclear factor-4α (HNF-4α), as is also the case for apo A-IV (21). We have also demonstrated that expression of HNF-4α is induced by a high-fat diet in newborn swine jejunum, as well as by fatty acid treatment in IPEC-1 cells. This may in part explain the upregulation of MTTP large subunit mRNA with OA treatment in the present studies. However, an intriguing finding in the present studies is the observation that, in the absence of OA treatment and in serum-free medium devoid of lipid, overexpression of native swine apo A-IV resulted in the robust induction of MTTP large subunit protein and mRNA. This suggests that the observed regulation by apo A-IV is not dependent on increased exogenous fatty acid esterification and flux of TG into the ER. It is possible that apo A-IV overexpression may induce or activate a trans-acting factor or other regulatory element that increases MTTP large subunit transcription or may in some manner impact on mRNA stability. Additional studies are underway to elucidate this regulatory mechanism.
In summary, these results suggest that the increased packaging of TG into nascent chylomicrons in the ER lumen of newborn swine small intestinal epithelial cells, induced by apo A-IV, is associated with upregulation of MTTP lipid transfer activity, which is crucial for particle lipidation. In the case of native swine apo A-IV, overexpression also increases MTTP large subunit protein, mRNA, and lipid transfer activity in the absence of OA, suggesting the presence of a mechanism of regulation that is not dependent on the influx of exogenous lipid. Thus MTTP is regulated by apo A-IV in a manner to facilitate increased packaging of TG into the prechylomicron core, resulting in enterocyte lipid secretory enhancement. This regulation may be important in the neonatal small intestine where efficient absorption of large lipid loads from breast milk is crucial for normal growth and development.
GRANTS
This work was supported by National Institute of Child Health and Human Development Grant HD22551 (D. D. Black), National Institute of Diabetes and Digestive and Kidney Diseases Grant DK46900 (M. M. Hussain), the American Heart Association (X. Pan), and funding from the Children's Foundation Research Center of Memphis at Le Bonheur Children's Medical Center (D. D. Black).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Apfelbaum TF, Davidson NO, Glickman RM. Apolipoprotein A-IV synthesis in the rat small intestine: regulation by dietary triglyceride. Am J Physiol Gastrointest Liver Physiol 252: G662– G666, 1987 [DOI] [PubMed] [Google Scholar]
- 2. Banerjee D. Studies of nascent lipoproteins in isolated hepatic microsomes and Golgi cell fractions. Methods Enzymol 129: 283– 297, 1986 [DOI] [PubMed] [Google Scholar]
- 3. Black DD. Development and physiological regulation of intestinal lipid absorption. I. Development of intestinal lipid absorption: cellular events in chylomicron assembly and secretion. Am J Physiol Gastrointest Liver Physiol 293: G519– G524, 2007 [DOI] [PubMed] [Google Scholar]
- 4. Black DD, Davidson NO. Intestinal apolipoprotein synthesis and secretion in the suckling pig. J Lipid Res 30: 207– 218, 1989 [PubMed] [Google Scholar]
- 5. Black DD, Rohwer-Nutter PL. Intestinal apolipoprotein synthesis in the newborn piglet. Pediatr Res 29: 32– 38, 1991 [DOI] [PubMed] [Google Scholar]
- 6. Black DD, Rohwer-Nutter PL, Davidson NO. Intestinal apo A-IV gene expression in the piglet. J Lipid Res 31: 497– 505, 1990 [PubMed] [Google Scholar]
- 7. Black DD, Wang H, Hunter F, Zhan R. Intestinal expression of apolipoprotein A-IV and C-III is coordinately regulated by dietary lipid in newborn swine. Biochem Biophys Res Commun 221: 619– 624, 1996 [DOI] [PubMed] [Google Scholar]
- 8. Brew K, Shaper JH, Olsen KW, Trayer IP, Hill RL. Cross-linking of the components of lactose synthesis with dimethylpimelimidate. J Biol Chem 250: 1434– 1444, 1975 [PubMed] [Google Scholar]
- 9. Bublitz C. An assay for glucose-6-phosphatase based on the formation of glucose. Mol Cell Biochem 108: 141– 144, 1991 [DOI] [PubMed] [Google Scholar]
- 10. Chomcyznski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162: 156– 159, 1987 [DOI] [PubMed] [Google Scholar]
- 11. Duverger N, Tremp G, Gaillaud JM, Emmanuel F, Castro G, Fruchart JC, Steinmetz A, Denefle P. Protection against atherogenesis in mice mediated by human apolipoprotein A-IV. Science 273: 966– 968, 1996 [DOI] [PubMed] [Google Scholar]
- 12. Dvorin E, Gorder NL, Benson DM, Gotto AM. Apolipoprotein A-IV: a determinate for binding and uptake of high density lipoproteins by rat hepatocytes. J Biol Chem 261: 15714– 15718, 1986 [PubMed] [Google Scholar]
- 13. Ee LC, Zheng S, Yao L, Tso P. Lymphatic absorption of fatty acids and cholesterol in the neonatal rat. Am J Physiol Gastrointest Liver Physiol 279: G325– G331, 2000 [DOI] [PubMed] [Google Scholar]
- 14. Fujimoto K, Cardelli JA, Tso P. Increased apolipoprotein A-IV in rat mesenteric lymph after lipid meal acts as a physiological signal for satiation. Am J Physiol Gastrointest Liver Physiol 262: G1002– G1006, 1992 [DOI] [PubMed] [Google Scholar]
- 15. Gallagher JW, Weinberg RB, Shelness GS. ApoA-IV tagged with the ER retention signal KDEL perturbs the intracellular trafficking and secretion of apoB. J Lipid Res 45: 1826– 1834, 2004 [DOI] [PubMed] [Google Scholar]
- 16. Gonzalez-Vallina R, Wang H, Zhan R, Berschneider HM, Lee RM, Davidson NO, Black DD. Lipoprotein and apolipoprotein secretion by a newborn piglet intestinal cell line (IPEC-1). Am J Physiol Gastrointest Liver Physiol 271: G249– G259, 1996 [DOI] [PubMed] [Google Scholar]
- 17. Hussain MM, Bakillah A. New approaches to target microsomal triglyceride transfer protein. Curr Opin Lipidol 19: 572– 578, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Iqbal J, Hussain MM. Intestinal lipid absorption. Am J Physiol Endocrinol Metab 296: E1183– E1194, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kalogeris TJ, Rodriguez MD, Tso P. Control of synthesis and secretion of intestinal apolipoprotein A-IV by lipid. J Nutr 127: 537S– 543S, 1997 [DOI] [PubMed] [Google Scholar]
- 20. Kumar NS, Mansbach CM. Determinants of triacylglycerol transport from the endoplasmic reticulum to the Golgi in intestine. Am J Physiol Gastrointest Liver Physiol 273: G18– G30, 1997 [DOI] [PubMed] [Google Scholar]
- 21. Leng S, Lu S, Yao Y, Kan Z, Morris GS, Stair BR, Cherny MA, Black DD. Hepatocyte nuclear factor-4 mediates apolipoprotein A-IV transcriptional regulation by fatty acid in newborn swine enterocytes. Am J Physiol Gastrointest Liver Physiol 293: G475– G483, 2007 [DOI] [PubMed] [Google Scholar]
- 22. Lu S, Huffman M, Yao Y, Mansbach CM, Cheng X, Black DD. Regulation of microsomal triglyceride transfer protein expression in developing swine. J Lipid Res 43: 1303– 1311, 2002 [PubMed] [Google Scholar]
- 23. Lu S, Yao Y, Cheng X, Mitchell S, Leng S, Meng S, Gallagher JW, Shelness GS, Morris GS, Mahan J, Frase S, Mansbach CM, Weinberg RB, Black DD. Overexpression of apolipoprotein A-IV enhances lipid secretion in IPEC-1 cells by increasing chylomicron size. J Biol Chem 281: 3473– 3483, 2006 [DOI] [PubMed] [Google Scholar]
- 24. Lu S, Yao Y, Meng S, Cheng X, Black DD. Overexpression of apolipoprotein A-IV enhances lipid transport in newborn swine intestinal epithelial cells. J Biol Chem 277: 31929– 31937, 2002 [DOI] [PubMed] [Google Scholar]
- 25. Mansbach CM, 2nd, Gorelick F. Development and physiological regulation of intestinal lipid absorption. II. Dietary lipid absorption, complex lipid synthesis, and the intracellular packaging and secretion of chylomicrons. Am J Physiol Gastrointest Liver Physiol 293: G645– G650, 2007 [DOI] [PubMed] [Google Scholar]
- 26. Murthy S, Albright E, Mathur SN, Davidson NO, Field FJ. Apolipoprotein B mRNA abundance is decreased by eicosapentaenoic acid in CaCo-2 cells. Arterioscler Thromb 12: 691– 700, 1992 [DOI] [PubMed] [Google Scholar]
- 27. Navarro MA, Acin S, Iturralde M, Calleja L, Carnicer R, Guzman-Garcia MA, Gonzalez-Ramon N, Mata P, Isabel B, Lopez-Bote CJ, Lampreave F, Pineiro A, Osada J. Cloning, characterization and comparative analysis of pig plasma apolipoprotein A-IV. Gene 325: 157– 164, 2004 [DOI] [PubMed] [Google Scholar]
- 28. Neeli I, Siddiqi SA, Siddiqi S, Lagakos WS, Binas B, Gheyi T, Storch J, Mansbach CM., 2nd Liver fatty acid-binding protein initiates budding of pre-chylomicron transport vesicles from intestinal endoplasmic reticulum. J Biol Chem 282: 17974– 17984, 2007 [DOI] [PubMed] [Google Scholar]
- 29. Qin X, Swertfeger DK, Zheng S, Hui DY, Tso P. Apolipoprotein A-IV: a potent endogenous inhibitor of lipid oxidation. Am J Physiol Heart Circ Physiol 274: H1836– H1840, 1998 [DOI] [PubMed] [Google Scholar]
- 30. Sheena V, Hertz R, Nousbeck J, Berman I, Magenheim J, Bar-Tana J. Transcriptional regulation of human microsomal triglyceride transfer protein by hepatocyte nuclear factor-4alpha. J Lipid Res 46: 328– 341, 2005 [DOI] [PubMed] [Google Scholar]
- 31. Shelness GS, Ledford AS. Evolution and mechanism of apolipoprotein B-containing lipoprotein assembly. Curr Opin Lipidol 16: 325– 332, 2005 [DOI] [PubMed] [Google Scholar]
- 32. Vowinkel T, Mori M, Krieglstein CF, Russell J, Saijo F, Bharwani S, Turnage RH, Davidson WS, Tso P, Granger DN, Kalogeris TJ. Apolipoprotein A-IV inhibits experimental colitis. J Clin Invest 114: 260– 269, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang H, Berschneider HM, Du J, Black DD. Apolipoprotein secretion and lipid synthesis: regulation by fatty acids in newborn swine intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol 272: G935– G942, 1997 [DOI] [PubMed] [Google Scholar]
- 34. Weinberg RB, Cook VR, DeLozier JA, Shelness GS. Dynamic interfacial properties of human apolipoproteins A-IV and B-17 at the air/water and oil/water interface. J Lipid Res 41: 1419– 1427, 2000 [PubMed] [Google Scholar]
- 35. Wetterau JR, Aggerbeck LP, Bouma ME, Eisenberg C, Munck A, Hermier M, Schmitz J, Gay G, Rader DJ, Gregg RE. Absence of microsomal triglyceride transfer protein in individuals with abetalipoproteinemia. Science 258: 999– 1001, 1992 [DOI] [PubMed] [Google Scholar]
- 36. Yao Y, Eshun JK, Lu S, Berschneider HM, Black DD. Regulation of triacylglycerol and phospholipid trafficking by fatty acids in newborn swine intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol 282: G817– G824, 2002 [DOI] [PubMed] [Google Scholar]