Abstract
This paper proposes a novel framework for joint orientation distribution function (ODF) estimation and tractography based on a new class of tensor-kernels. Existing techniques estimate the local fiber orientation at each voxel independently so there is no running knowledge of confidence in the measured signal or estimated fiber orientation. In this work, fiber tracking is formulated as recursive estimation: at each step of tracing the fiber, the current estimate of the ODF is guided by the previous. To do this, second and higher order tensor based kernels are employed. A weighted mixture of these tensor-kernels is used for representing crossing and branching fiber structures. While tracing a fiber, the parameters of the mixture model are estimated based on the ODF at that location and a smoothness term that penalizes deviation from the previous estimate along the fiber direction. This ensures smooth estimation along the direction of propagation of the fiber.
In synthetic experiments, using a mixture of two and three components it is shown that this approach improves the angular resolution at crossings. In vivo experiments using two and three components examine the corpus callosum and corticospinal tract and confirm the ability to trace through regions known to contain such crossing and branching.
Keywords and phrases: Diffusion-weighted MRI, tractography, diffusion tensor estimation
1. Introduction
The advent of diffusion weighted magnetic resonance imaging has provided the opportunity for non-invasive investigation of neural architecture. Using this imaging technique, clinicians and neuroscientists want to ask how neurons originating from one region connect to other regions, or how well-defined those connections may be. For such studies, the quality of the results relies heavily on the chosen fiber representation and the method of reconstructing pathways.
To begin studying the microstructure of fibers, we need a model to interpret the diffusion weighted signal. Such models fall broadly into two categories: parametric and nonparametric. One of the simplest parametric models is the diffusion tensor which describes a Gaussian estimate of the diffusion orientation and strength at each voxel [1, 2]. While robust, this model can be inadequate in cases of mixed fiber presence or more complex orientations [3, 4]. To handle more complex diffusion patterns, various parametric models have been introduced: weighted mixtures [5–8], higher order tensors [9,10] and directional functions [11–13].
Nonparametric models often provide more information about the diffusion pattern. Instead of estimating a discrete number of fibers as in parametric models, nonparametric techniques estimate an orientation distribution function (ODF) describing an arbitrary configuration of fibers. For this estimation, Tuch et al. [14] introduced Q-ball imaging to numerically compute the ODF (also refered to as diffusion-ODF) using the Funk-Radon transform. The use of spherical harmonics simplified the computation with an analytic form [15–17]. A good review of both parametric and nonparametric models can be found in [18,19].
Deterministic tractography involves directly following the diffusion pathways. In the single tensor model, this means simply following the principal diffusion direction [20], while multi-fiber models often include techniques for determining the number of fibers present or when pathways branch [7, 21]. Since the estimated model at each location is inherently noisy, some methods perform path regularization using a filtering technique [22].
In order to perform deterministic tractography using nonparametric methods, one has to use a separate algorithm to find the diffusion-ODF (dODF) maxima. Since the dODF peaks are very smooth, the principal diffusion direction (PDD) obtained from it is very noisy [19]. Recent work has focused on estimating the fiber-ODF (fODF) either directly from the signal or from the dODF [23–25]. For example, Bloy et.al. [25] find them as maxima on the surface of a high-order tensor and [26] Schultz et.al. decompose a high-order tensor into a mixture of rank-1 tensors. Ramirez et.al. [27] provide a quantitative comparison of several such techniques.
Another popular technique to estimate the fODF has been to use spherical deconvolution [12, 28–32]. In this approach, a model for the signal response of a single-fiber is assumed and the observed signal is deconvolved to obtain the true fODF. This technique, sharpens the peak of the ODF and it becomes much easier to extract the maxima. The authors in [19] have shown improvement in tractography results with fODF computed using spherical deconvolution.
An alternative method is to use a mixture model of directional kernels, each representing a single fiber response. The advantages of the mixture model are that a separate algorithm is not needed to find the fODF maxima, since the kernel parameters includes the principal diffusion direction. Further, the scaling parameter can adjust the shape of the ODF, i.e., it can adjust the single fiber response based on the observed data (white matter, gray matter, CSF etc) and hence allows for better model fit for white matter and gray matter regions. The disadvantage, however, is that the number of fibers (components of mixture model) at each location have to be kept fixed or determined online during estimation.
2. Our contribution
In this work, we first propose a novel kernel based on second and higher order rank-1 tensors. The 2 parameters of the kernel determine the orientation and shape (scaling) of the kernel. We will use this tensor-kernel to represent fiber-ODF.
Second, while most of the approaches listed above perform dODF (or fODF) estimation independently at each voxel, we describe in this paper a method to estimate the model parameters and perform tractography simultaneously as we trace a fiber from its seed to termination. In this way, the estimation at each position builds upon the previous estimates along the fiber.
We use a mixture of two/three components and formulate our cost function in such a way that if there is one fiber, the components of the mixture align in the same general direction and disperse if there is more than one fiber at a given location. Further, we constrain the solution to be consistent with the model estimated at the previous location.
Using causal estimation in this way yields inherent path regularization and accurate fiber resolution at crossing angles not found with independent optimization.
3. Approach
In this section we propose a function using 2nd and higher-order tensors capable of representing ODF’s and examine some of its properties. We also define two different cost functions which are minimized in order to estimate the parameters of the mixture model.
3.1. Tensor-kernel
One of the goals of this paper is to extract the fiber-ODF from the diffusion-ODF. To do this, we need kernels (functions) which can accurately represent a fiber-ODF. 2nd and higher-order tensors provide a way to represent functions on the sphere (the fODF is a function on the sphere). In this work, we treat order-l tensors 𝕋 in a given orthonormal Cartesian coordinate system as quantities whose elements are addressed by l indices. The employed tensors are supersymmetric, i.e., their elements 𝕋i1i2…il are invariant under arbitrary permutations of indices i1i2…il. A function D(g) on the unit sphere is given by its induced homogeneous form. For an order-l tensor 𝕋 in three dimensions, it reads:
The above equation can be compactly written using the tensor contraction operator “:”
| (1) |
where ⊗ is the outer product operation and 𝔾l is a rank-1 tensor of order-l. The tensor contraction operator : is an inner product between matrices analogous to an inner product between vectors.
Let g1g2…gn ∈ 𝒮3 be the directions that represent a uniform sampling of the sphere. Then, we propose to use the following tensor-kernel to represent an ODF with principal diffusion direction along t:
| (2) |
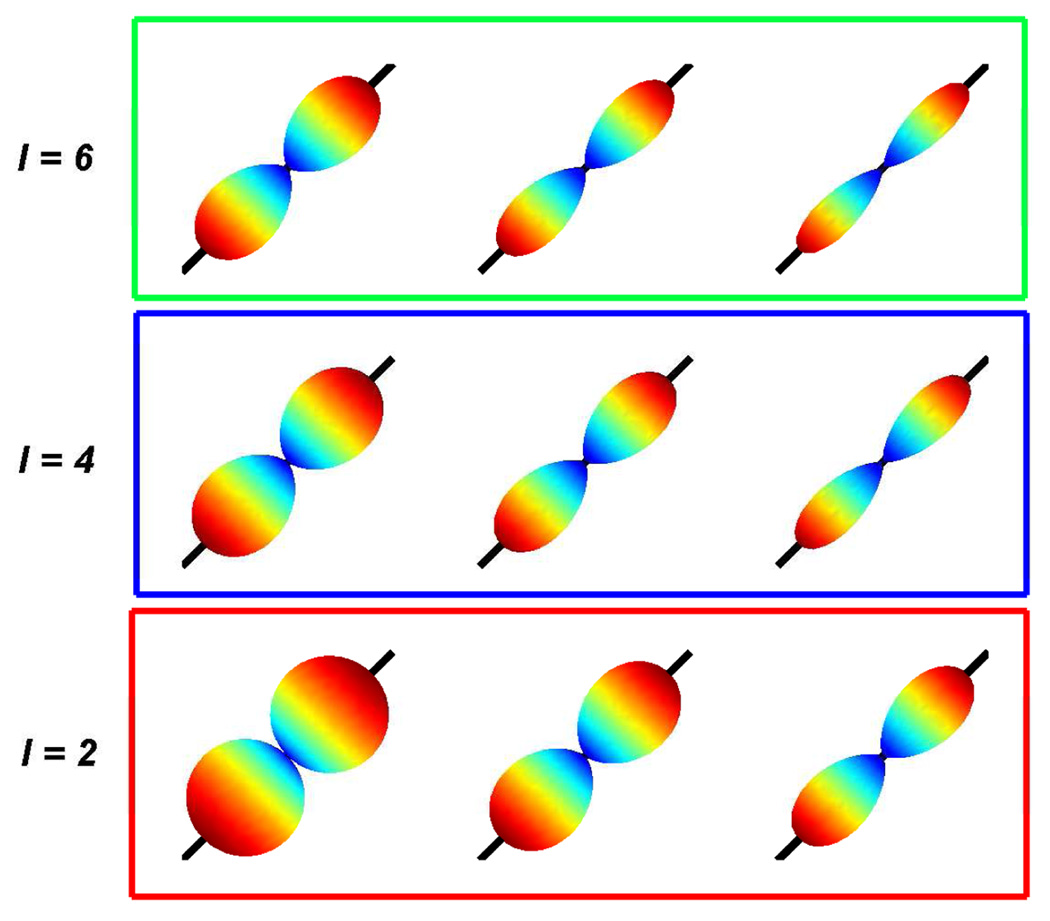
where 𝕋l is the order-l tensor of rank-1 in direction t, is order-l tensor in direction gi and p is a scaling parameter. Note that, a particular case of this function has been used in the literature with p = 1 [26, 33]. This is however, the first time we have modified it to have a scaling parameter, giving more flexibility to represent various kinds of shapes. Figure 1 shows the effect of the scaling parameter p on the shape of the function. Top row shows the function for order-6, middle for order-4 and bottom for order-2. Thus, we have a family of functions, given by their order, that can represent single fiber response. The higher order kernels differ from their lower order counterparts in terms of the scaling. Note that, we only require three parameters that can uniquely define the tensor-kernel: the principal diffusion direction t requires two parameters in spherical co-ordinates, and one scaling parameter p.
Fig 1.
Tensor-kernels of different orders and scales. Top row - order-6, middle - order-4 and bottom - order-2. Left to right: p = 0.5, 1, 2.
3.2. Mixture Model
In diffusion weighted imaging, image contrast is related to the strength of water diffusion, and our goal is to accurately relate these signals to an underlying model of fiber orientation. At each image voxel, diffusion is measured along a set of distinct gradients, g1, …, gn ∈ 𝒮3 (on the unit sphere), producing the corresponding signal, S = [ s1, …, sn ]T ∈ ℛn. Thus, the signal S is a function defined on the sphere.
A popular method to represent functions on the sphere is using spherical harmonics (SH) [15–17]. Given any bandlimited signal S defined on the sphere, one can write it as an expansion in terms of the SH basis as: , for any direction (θ, ϕ), where Yl,m are the basis functions given by:
and Pl,m is the associated Legendre polynomial. The above equations can be written as a linear system of equations and cl,m can be computed using the Moore-Penrose pseudo inverse. To make the method robust to noise, the authors in [17] proposed a regularized version by adding a smoothness constraint.
Once the signal representation is obtained in the SH basis, the corresponding dODF can be analytically computed using the Funk-Radon transform [17]. This method is very fast and robust to noise. In the remainder of this work, we will assume that the dODF has been pre-computed using spherical harmonics (see [17] for details). There has been a lot of work recently to extract the fODF from dODF using spherical deconvolution [12, 28–30, 32]. This method assumes a fixed single-fiber response while allowing for variable number of fibers to be estimated.
In this work, we seek to estimate the fODF using a different strategy by allowing for variation in single-fiber response but keeping the number of fibers fixed. This can be done by fitting the dODF using a mixture of tensor-kernels. While one can assume a mixture of any number of components, we choose to start with a mixture involving two and three weighted tensor kernels. While the work of Behrens et al. [34] showed that at a b-value of 1000 the maximum number of detectable fibers is two, several other studies restricted their analysis to two-fiber models due to problems with estimating more than two components. [6, 7, 23]. In this work, we perform experiments on synthetic data using two components, and later show an extension to three components. Similar results are shown for in-vivo data.
The model we intend to use in this study is given by:
| (3) |
where D(gi) is the dODF in direction gi, wj are the respective weights of the components, is the l-th order rank-1 tensor obtained from the gradient direction gi, while is l-th order rank-1 tensors with principal diffusion direction tj. N is the total number of components used in the mixture, which in our case could be two or three. Thus, a two-component dODF can be uniquely represented using 10 parameters (if tj ∈ ℛ3) or 8 parameters if the directions are represented in spherical coordinates. We should also clarify that, D is the dODF but the individual components of the mixture model give the fODF in direction tj. In the remainder of this section will focus on estimating the free parameters of this mixture model, i.e., .
3.3. Independent Estimation
Given the scanner signal S ∈ ℛn, we compute the corresponding dODF F = [F1, F2, …Fn] ∈ ℛn using spherical harmonics [17] and min-max normalize it [14]. One can now minimize the following cost function to estimate the parameters:
| (4) |
where we have used the short-hand notation D(gi) = Di and F (gi) = Fi. Equation (4) can be minimized using the Levenberg-Marquardt (LM) nonlinear optimizer. However, the minimum could be a degenrate solution with negative ŵj and/or p̂j. To overcome this problem, we use the exponential map to guarantee positivity of the weight and scaling parameters [10], i.e., we use wj = exp(−ŵj) and pj = exp(p̂j) in equation (4):
| (5) |
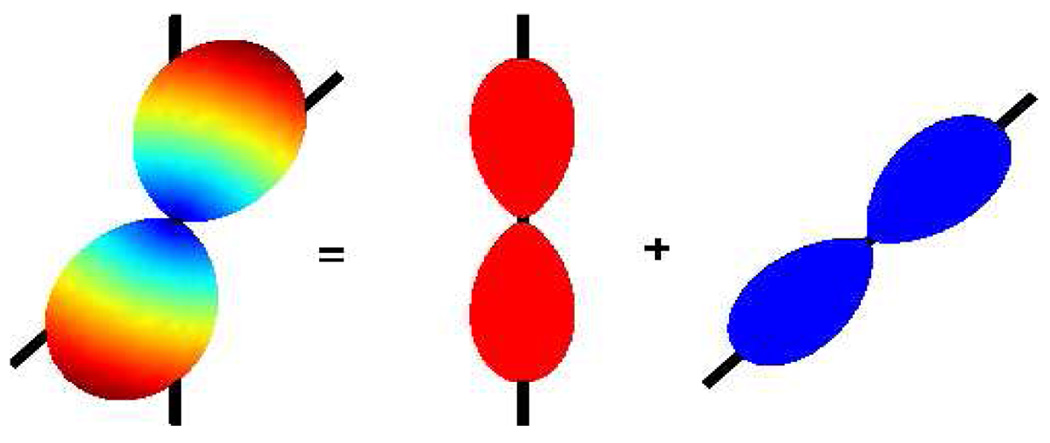
To obtain volume fraction contribution for each of component, one can normalize the weights so that they sum to 1. Figure 2 shows how the individual fiber-ODF’s combine to give the observed dODF. Note that, maxima of F would not have revealed the individual fiber composition of the dODF.
Fig 2.
Mixture components of dODF at an angle of 45°
Given the signal at each location in the brain, one could independently estimate the dODF and the mixture model parameters as is done in most of the techniques presented in the literature. However, in this paper, we make the observation that the diffusion of water molecules is not independent and is highly correlated along the direction of the fiber bundle. We wil demonstrate that taking this into account significantly improves tractography results.
3.4. Smooth Estimation and Tractography
In this work, we take this spatial correlation into account and propose to do simultaneous model estimation and tractography. This means, we recursively estimate the model parameters at location x based on the observed dODF at x and the model parameters estimated at x − 1 (previous location along the fiber tract). Once the model parameters are estimated, we propagate a unit step along the most consistent direction. Causal estimation and tracking in this way allows for smooth and accurate estimation of the fiber.
In order to take into account the estimated model parameters at the previous location x − 1, we modify the cost function in equation (5):
| (6) |
where, the first is the data consistency term at location x, the 2nd, 3rd and 4th terms ensure that the weights, scale and direction are consistent with the previous estimation at x − 1. The constants λ1, λ2, λ3 are user defined constants that determine the influence of the previous estimate on the current. In our experiments, we empirically obtained these constants by choosing the ones that gave the best tractography results on synthetic data. One could however use a strategy that either does a brute force search or minimizes the error to obtain these constants. We however noticed that the estimation works well for a braod range of values. Equation (6) can be minimized using an LM optimizer.
To perform tractography, we repeatedly minimize equation (6) at each voxel location x and then propagate a unit step in the most consistent direction. The principal diffusion directions are given by t1 and t2 and hence there is no need for a separate peak finding algorithm. Smooth parameter estimation along the fiber in this way gives significantly better results than independent estimation, as is clear from synthetic and in-vivo results given in the next section. A summary of the entire algorithm is given below:
|
Before we conclude this section, we note some existing techniques that do spatial regularization during estimation [35,36]. In [35], the authors estimate a single tensor using the log-Euclidean metric along with a spatial smoothness term. In [36], the authors extend it for smooth estimation of the spherical harmonic coefficients. Both of these methods assume a spatial neighborhood (3 × 3 × 3) within which the regularisation is done. However, none of these methods perform simultaneous tractography and estimation in their framework (as is proposed in this work).
We also note some techniques that have used mixture model to represent ODFs. In the recently proposed work of [26], the authors use a tensor decomposition approach to find a mixture of rank-1 tensors of order-l that best fit the given dODF. Some of the important differences with this method are: a) The authors estimate the standard high-order tensor of rank-1 with a single fixed scale (p = 1). In order to compensate for the scaling, they deconvolve the dODF using a fixed kernel and map it to an appropriate space so that a rank-1 order-l tensor can be fit to it. b). Once again the authors perform an independent estimation at each voxel location and then do tractography on it. In the work of [11], the authors use a mixture model of von-Mises Fisher function to estimate an ODF. They also perform independent estimation at each voxel and thus ignore the correlation in fiber geometry. In [37] the authors estimate the parameters of a two-tensor Gaussian mixture model using relaxation labeling as their regularizer.
4. Experimental Evaluation
We first use experiments with synthetic data to validate our technique against ground truth. We confirm that our approach reliably recognizes crossing fibers over a broad range of angles. Comparing against two alternative multi-fiber optimization techniques, we find that the constrained tractography approach gives consistently superior results. Next, we perform tractography through crossing fiber fields and qualitatively examine the underlying orientations and branchings (see 4.2). Lastly, we examine a real dataset to demonstrate how constrained estimation is able to pick up fibers and branchings known to exist in vivo yet undetectable using deconvolved spherical harmonics and single tensor models (see 4.3).
Following the experimental method of generating synthetic data found in [19, 26, 30], we pull from our real data set the 300 voxels with highest fractional anisotropy (FA) and compute the average eigenvalues among these voxels: {1200, 100, 100} µm2/msec (FA=0.91). We generated synthetic MR signals according to a Gaussian mixture model as done in [14], using these eigenvalues to form an anisotropic tensor at both b = 1000 and b = 3000, with 81 gradient directions uniformly spread on the hemisphere. We generate two separate data sets, each with a different level of Rician noise along the fiber direction: low-noise (SNR ≈ 10 dB) and high-noise (SNR ≈ 5 dB).
Throughout the experiments, we draw comparison to two other independent optimization techniques. In what follows, we use the 2nd and 4th order tensor kernels. First, we use the proposed tensor-kernel within the mixture model framework, but do an independent optimization using equation (5). Second, we use spherical harmonics for modeling [30] and fiber-ODF (fODF) sharpening with peak detection as described in [19] (order l = 6, regularization L = 0.006). This provides a comparison with an independently estimated, model-free representation. Last, when performing tractography on real data, we use single-tensor streamline tractography as a baseline1.
4.1. Angular resolution
While the independent optimization techniques can be run on individually generated voxels, care must be taken in constructing reasonable scenarios to test the causal filter. For this purpose, we constructed an actual 2D field through which to navigate (see Figure 5 ). In the middle is one long fiber pathway where the tracker begins estimating a single tensor but then runs into a field of voxels with two crossed fibers at a fixed angle. We computed the angular error over this region using both sharpened spherical harmonics and independent optimization (5). We generated several similar fields, each at a different fixed angle. By varying the size of the crossing region or the number of fibers run, we ensured that each technique performed estimation on at least 500 voxels.
Fig 5.
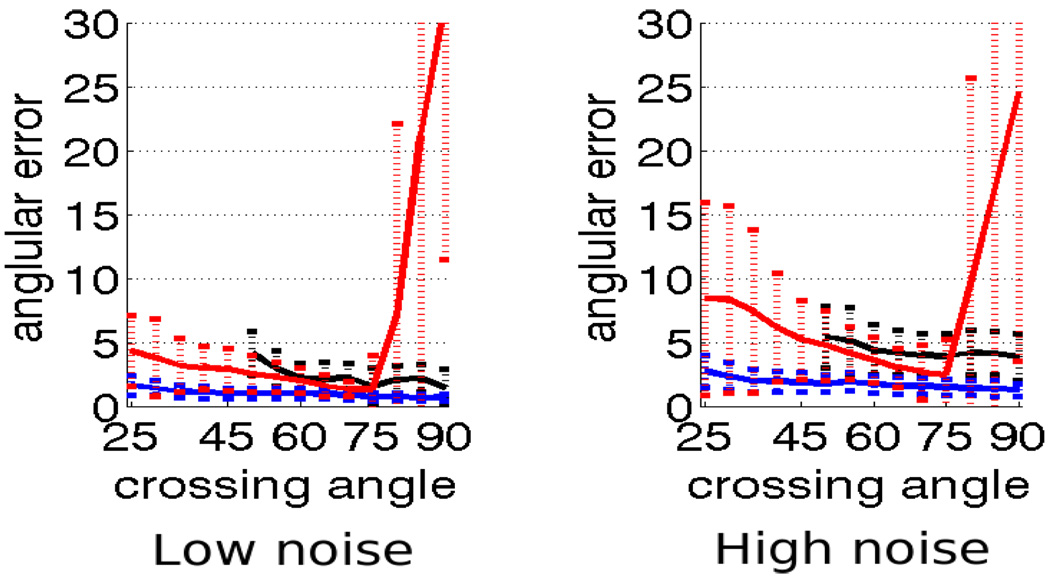
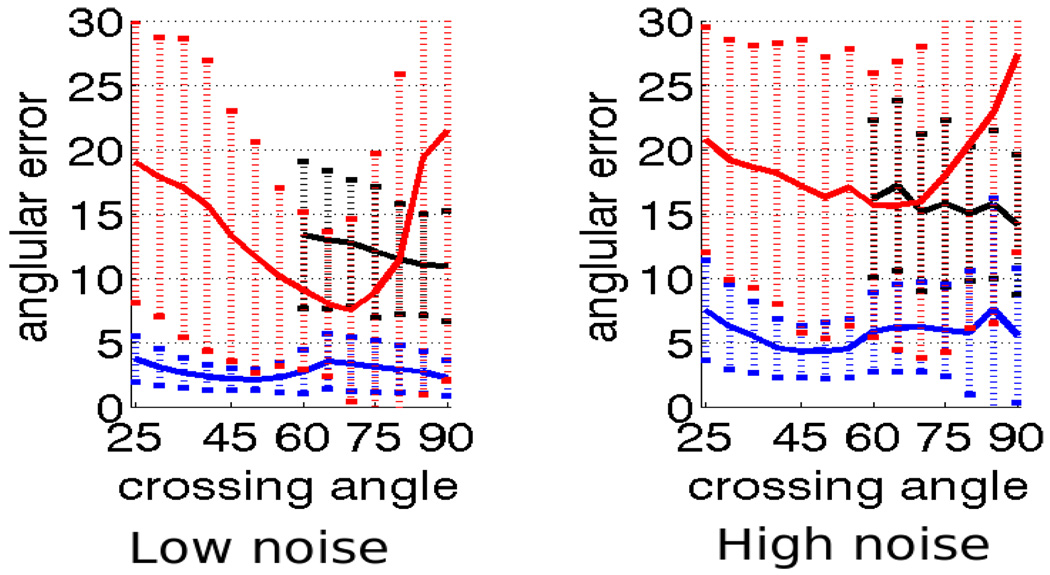
Angular error for 2nd-order kernel using constrained optimization (blue), independent optimization (red) and sharpened spherical harmonics (black) for b = 3000. The dotted vertical bars show range of one standard deviation. Left: low-noise, right: high-noise.
We looked at the error in angular resolution by comparing the constrained approach to independent optimization and sharpened spherical harmonics. For the tensor kernels (two components), we show comparison using both, 2nd and 4th order tensors. Consistent with the results reported in [17, 19], sharpened spherical harmonics are generally unable to detect and resolve angles below 50° for b = 1000. Figures 3 and 4 confirm this.
Fig 3.
Angular error for 2nd-order kernel using constrained optimization (blue), independent optimization (red) and sharpened spherical harmonics (black) for b = 1000. The dotted vertical bars show range of one standard deviation. Left: low-noise, right: high-noise.
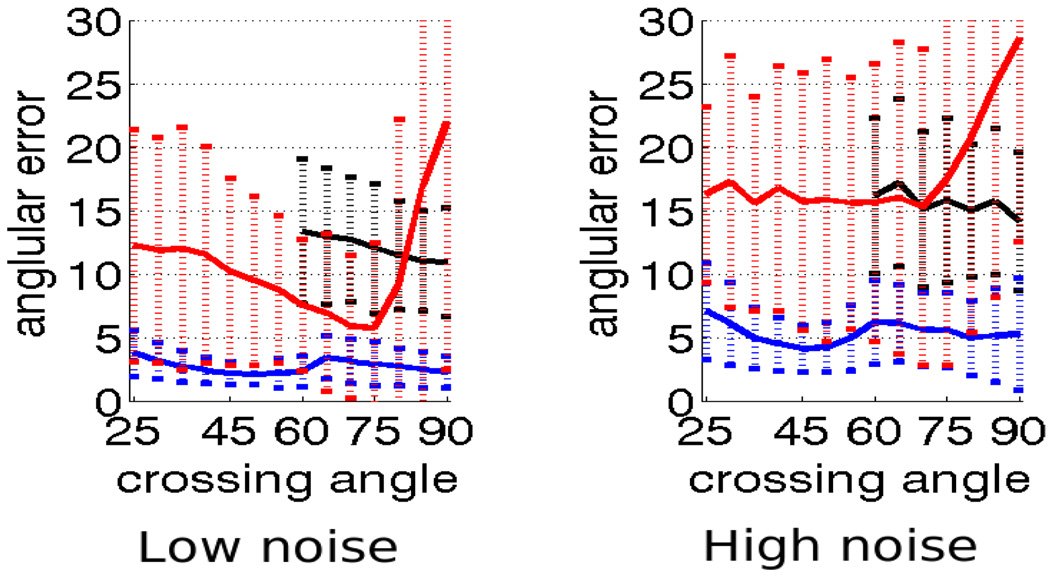
Fig 4.
Angular error for 4th-order kernel using constrained optimization (blue), independent optimization (red) and sharpened spherical harmonics (black) for b = 1000. The dotted vertical bars show range of one standard deviation. Left: low-noise, right: high-noise.
As expected, the independent optimization technique also reports large errors since the LM optimizer gets stuck in local minimum. For example, any dODF can be represented using a single component with significant weight and appropriate scaling parameter, while rendering the contribution from the other component to zero by setting the weight to be zero. Such solutions contribute to large errors when using independent optimization.
However, in the constrained estimation technique, we constrain the weight and the scaling parameter to be close to its previous estimate, which results in a better estimation of the parameters. For in-vivo as well as synthetic data, the user defined values of λ1, λ2, λ3 in equation (6) were emprirically found to be 2.5, 1, 0.15 respectively for a second order tensor kernel. Thus, the deviation of the weights from the previous estimate had the largest penalty, the scaling parameter had a little more flixibility while the penalty on direction parameter was still lower. Intuitively, for the case of a single fiber, this means that equally-weighted, similarly aligned components are more preferable than a single component with the correct direction but zero contribution from the second component. Thus, upon encountering a region of dispersion, the second component is poised and ready to begin branching. Overall, the relative weighting of the penalty terms ensures that there is no abrupt change in either the weight or the scale of the estimated fODF along the fiber, but still allowing flexibility to capture fiber crossings at high angles.
This experiment demonstrates that for b = 1000, the constrained approach consistently resolves angles down to 25–30° with 5° error compared to independent optimization which reports very high error for certain angles and sharpened spherical harmonics, which fails to reliably resolve below 60° with as much as 15° error. For b = 3000, the constrained approach consistently resolves down to 20–30° with 1–2° error compared to sharpened spherical harmonics which cannot resolve below 50° with 5° error (Figure 5). Further, the angular errors reported using the constrained optimization with 2nd and 4th order tensor kernels are very close and hence we will only show in-vivo results using the 2nd-order tensor kernel.
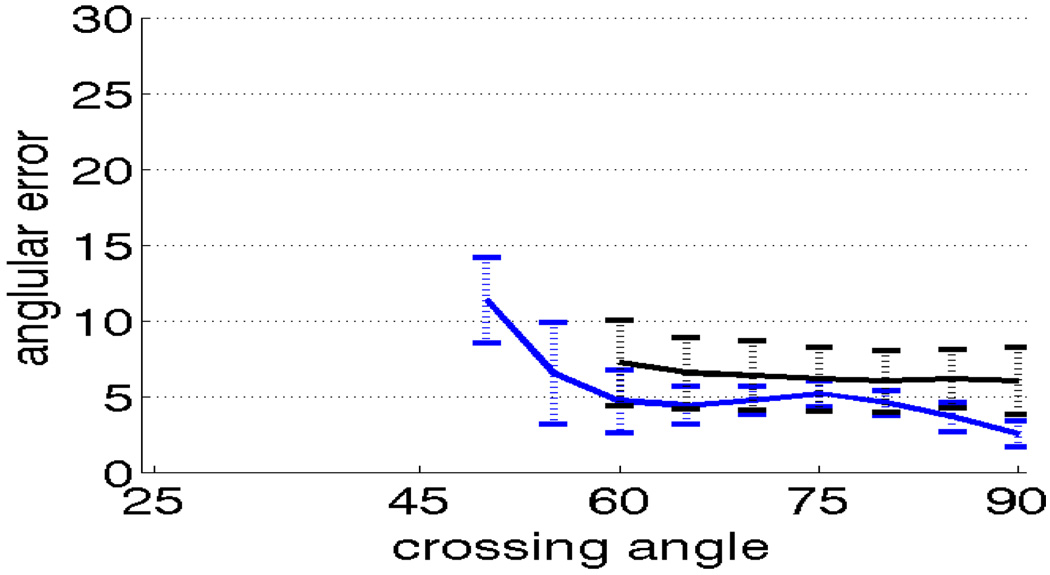
We also performed a similar experiment to test how well our method performs in case of three crossing fibers. In this case we used a mixture of three components with different crossing angles as described earlier. Figure 6 demonstrates that the proposed method has lower angular error than sharpened spherical harmonics. Figure 7 shows orientation and weights of three components as they trace a fiber that passes through a fiber field with two crossing bundles. Notice that, the weights are zero when there is only one component, while the weight for two components is non-zero in the region with two-fibers and then fall back to single component being dominant once again, in accordance with the data. This demonstrates that the proposed method can adaptively change the weights depending on the number of fibers present at each voxel.
Fig 6.
Angular error for 2nd-order kernel using constrained optimization (blue), and sharpened spherical harmonics (black) for b = 3000 and three component model with three fiber crossings (high noise). The dotted vertical bars show range of one standard deviation.
Fig 7.
Figure shows tracing a three-component model through a field of single and two-tensors (crossing at 60°). The weights of each of the components are also shown, indicating that two of them are zero in case a single fiber is present and only one of them is zero in case two fibers are present. Note that, since the weight of the third component is zero, its orientation is inconsequential.
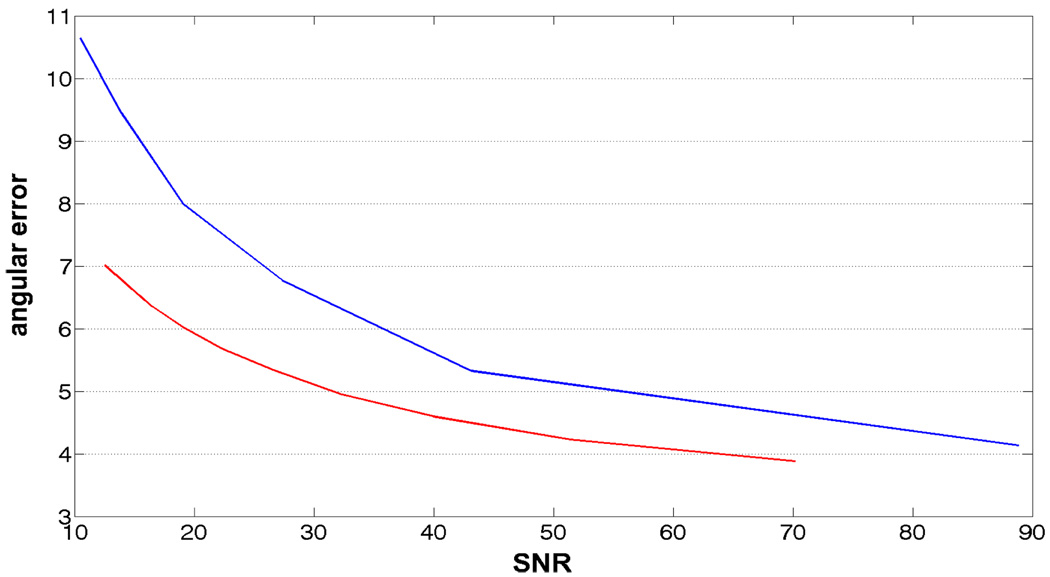
Finally, Figure 8 shows the precision of the proposed method in the presence of varying levels of noise. On the x-axis is the SNR , where σs and σn are standard deviation of the signal and noise respectively. We should note that the SNR reported is for the mean baseline image, and that the actual SNR varies quite significantly in each gradient direction. Thus, an SNR of 20 for the baseline image could mean an SNR of 0.25 in the fiber direction while 35 in the direction orthogonal to it. This important fact should be kept in mind while inferring the results for Figure 8.
Fig 8.
Figure shows the precision of the proposed method in the presence of varying levels of noise. X-axis is the SNR, while y-axis is the angular error. Blue is for b = 1000, while red is for b = 3000.
4.2. Synthetic tractography
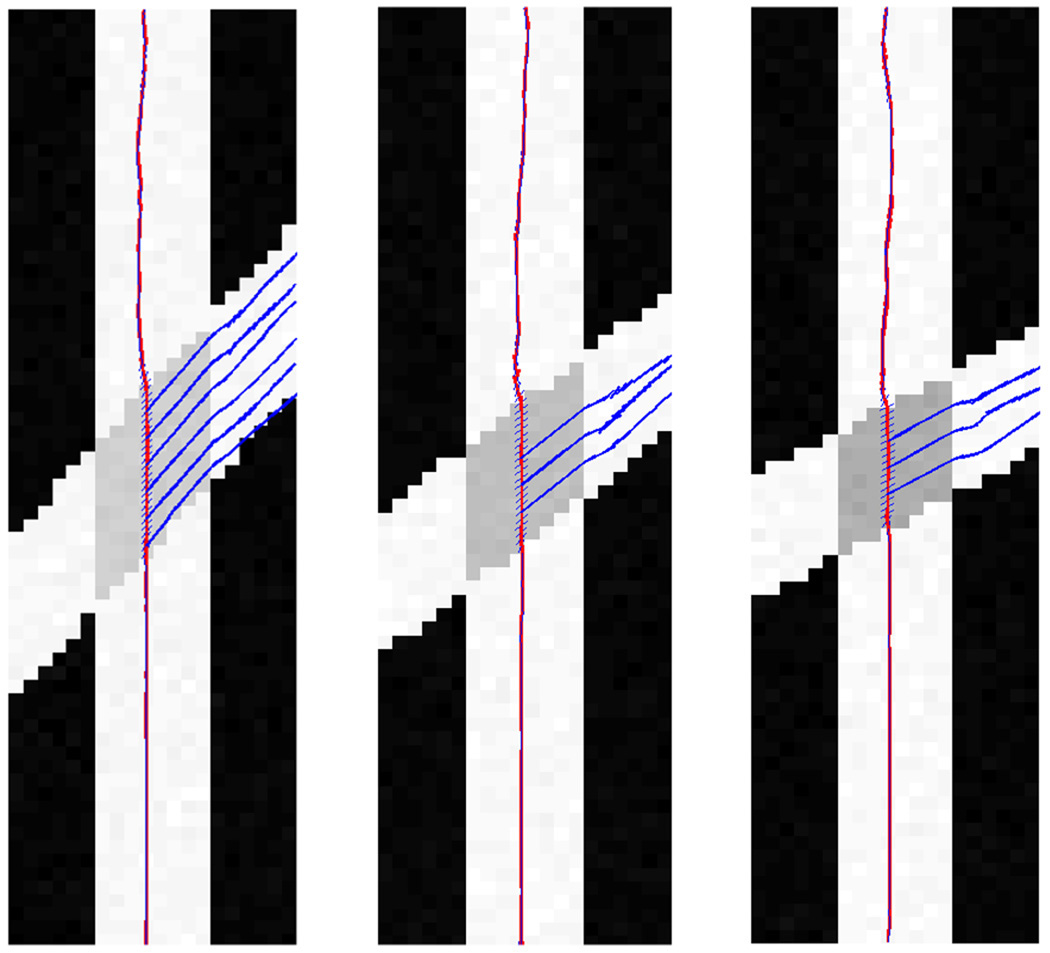
Having verified the technique’s accuracy, we now turn to the resulting tractography. Figure 9 provides examples of synthetic crossing fiber fields each at different fixed angles: 40°, 50°, 60° (b = 3000, high-noise). In our experiments, we start fibers from the bottom and propagate upward where they gradually encounter the crossing region. Here we show one such fiber and use blue glyphs to indicate the second component detected as it passes through the crossing region. In general, we found that in regions with only one true fiber present (those outside the crossing), the second component roughly aligned with the first.
Fig 9.
Single fiber producing branches at angles of 40°, 50° and 60° (left to right) in the crossing region. Notice that, the directions of the 2 components align when there is only one fiber.
The tractography was initialized, by estimating the parameters in the seed region using a dictionary-based matching pursuit algorithm [38]. In our implementation, we simply project against a dictionary populated with two tensor-kernel components at various p-values and equal weights. While our signal is produced at 81 gradient directions, we use 341 directions to construct the dictionary, thus any error is due to the method’s sensitivity to noise and discretization. This provides a very good starting point for the proposed constrained tractography algorithm.
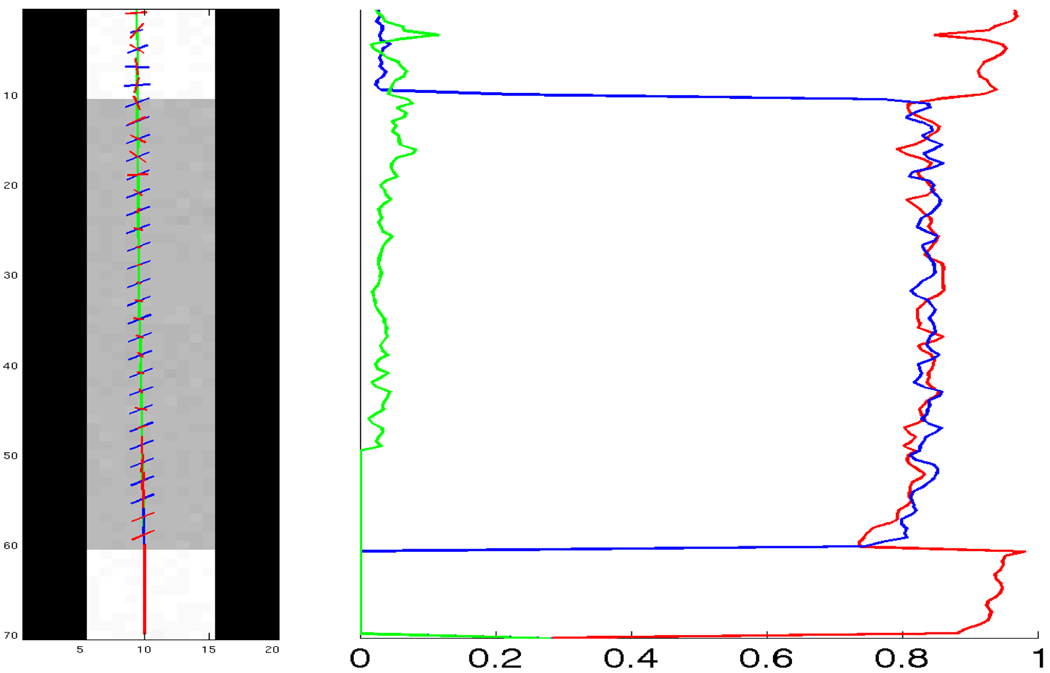
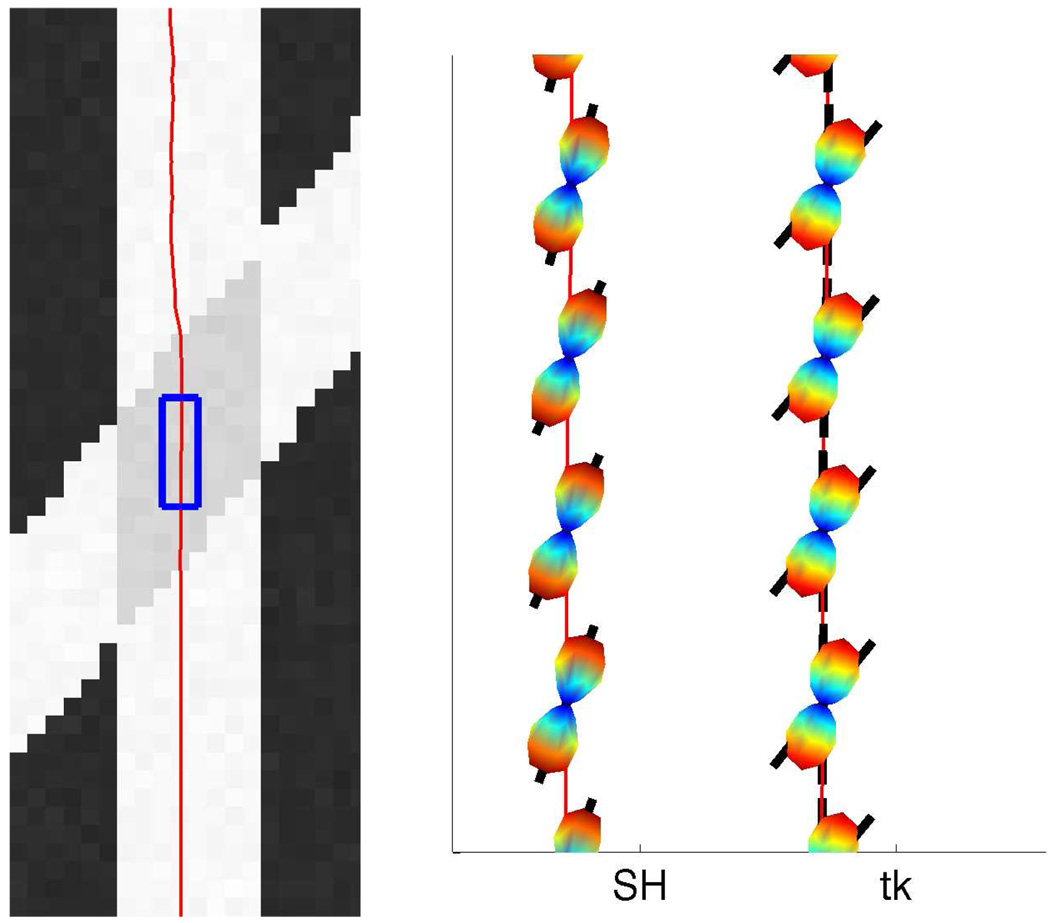
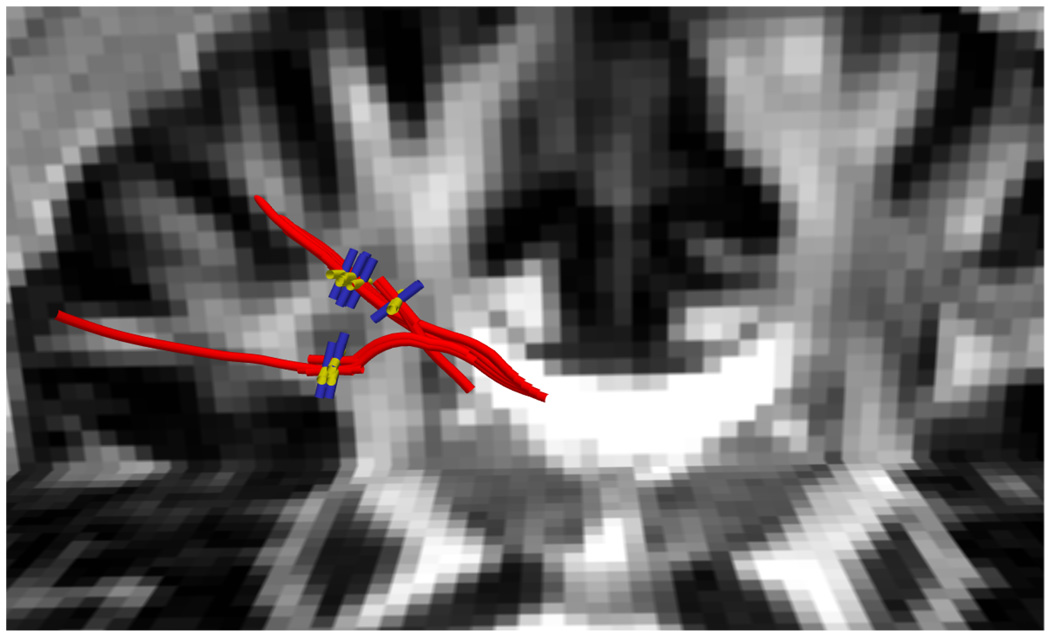
In Figure 10 we show another 40° field (b = 3000, noisy) but take a closer look at several points along a single fiber as it passes through the crossing region. We also examine the corresponding ODFs reconstructed using sharpened spherical harmonics and the constrained approach. As expected, the sharpened spherical harmonics do not detect the crossing but result in a single angle. A close examination of the reported axes shows the bias toward a single averaged axis as reported in [23, 26, 39]. In contrast, the proposed approach results are consistent and accurate.
Fig 10.
Single fiber tractography with the second component at 40°. Right: Estimated fODF using sharpened spherical harmonics (SH) and constrained optimization using 2nd-order tensor kernel (tk). Notice that, SH is able to pick up only 1 component, in the crossing region.
4.3. In vivo tractography
We tested our approach on a real human brain scan acquired on a 3-Tesla GE system using an echo planar imaging (EPI) diffusion weighted image sequence. A double echo option was used to reduce eddy-current related distortions. To reduce impact of EPI spatial distortion, an eight channel coil was used to perform parallel imaging using Array Spatial Sensitivity Encoding Techniques (GE) with a SENSE-factor (speed-up) of 2. Acquisitions have 51 gradient directions with b = 900 and eight baseline scans with b = 0. The original GE sequence was modified to increase spatial resolution, and to further minimize image artifacts. The following scan parameters were used: TR 17000 ms, TE 78 ms, FOV 24 cm, 144x144 encoding steps, 1.7 mm slice thickness. The scan had 85 axial slices parallel to the AC-PC line covering the whole brain.
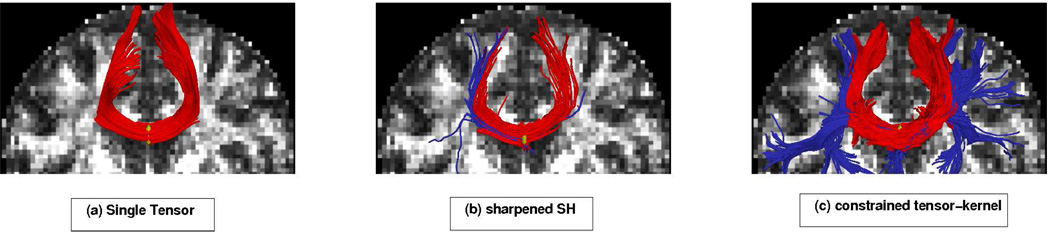
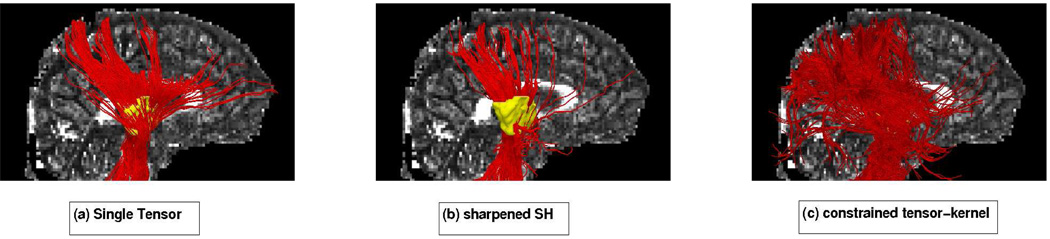
We first focused on fibers originating in the corpus callosum. Specifically, we sought to trace out the lateral transcallosal fibers that run through the corpus callosum out to the lateral gyri. It is known that single-tensor streamline tractography only traces out the dominant pathways forming the U-shaped callosal radiation (Figures 11a and 12a). Several studies document this phenomena, among them the works of [19] and [26] have side-by-side comparisons. These fibers have been reported using diffusion spectrum imaging [21], probabilistic tractography [12, 19, 40], and more recently with tensor decomposition [26].
Fig 11.
Constrained tractography picks up many fiber paths consistent with the underlying structures. Both single-tensor streamline and sharpened spherical harmonics (SH) are unable to find the majority of these pathways. Blue colored fibers indicate lateral and transcallosal fibers.
Fig 12.
Closeup of upper right in Figure 10c.
We start with two basic experiments: first examining the tracts surrounding a single coronal slice and second looking at all tracts passing through the corpus callosum. We seed each algorithm multiple times in voxels at the intersection of the mid-sagital plane and the corpus callosum. To explore branchings found using the proposed technique, we considered a component to be branching if it was separated from the primary component by less than 40°. Similarly, with sharpened spherical harmonics, we considered it a branch if we found additional maxima over the same range. We terminated fibers when the generalized fractional anisotropy (std/rms) [14] of the estimated signal fell below 0.05.
For the first experiment, Figure 12 shows tracts originating from within a few voxels intersecting a particular coronal slice. For a reference backdrop, we use a coronal slice showing the intensity of fractional anisotropy (FA) placed a few voxels behind the seeded coronal position. Keeping in mind that these fibers are intersecting or are in front of the image plane, this roughly shows how the fibers navigate the areas of high anisotropy (bright regions). Similar to the results in [19, 26], figure 11b shows that sharpened spherical harmonics only pick up a few fibers intersecting the U-shaped callosal radiata. In contrast, our proposed method traces out many pathways consistent with the apparent anatomy. To emphasize transcallosal tracts, we color as blue those fibers exiting a corridor of ±22 mm around the mid-sagittal plane. Figure 8 provides a closer inspection of Figure 7c where, to emphasize the underlying anatomy influencing the fibers, we use as a backdrop the actual coronal slice passing through the voxels used to seed this run.
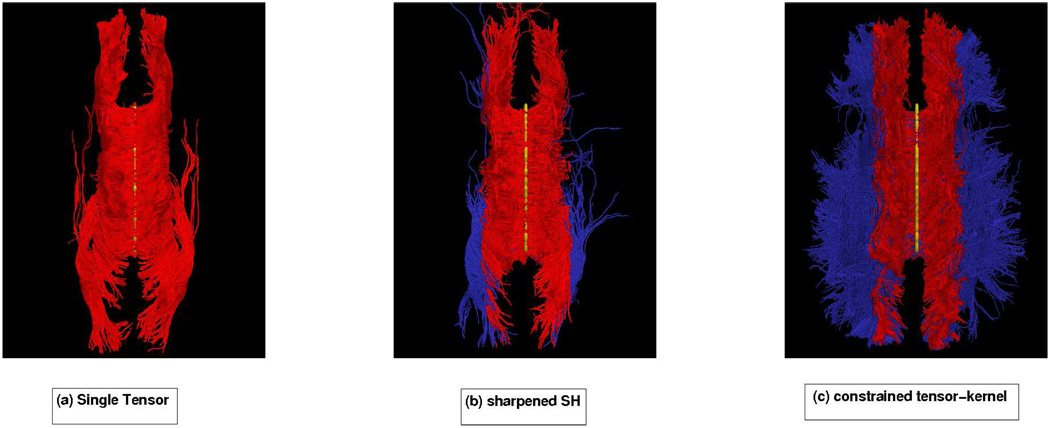
For the second experiment, we seeded in all voxels of the corpus callosum lying in the mid-sagittal plane. Figure 9 shows the top view of the entire corpus callosum to see the overall difference between the different methods. Here again we emphasize with blue the transcallosal fibers found using all of the methods.
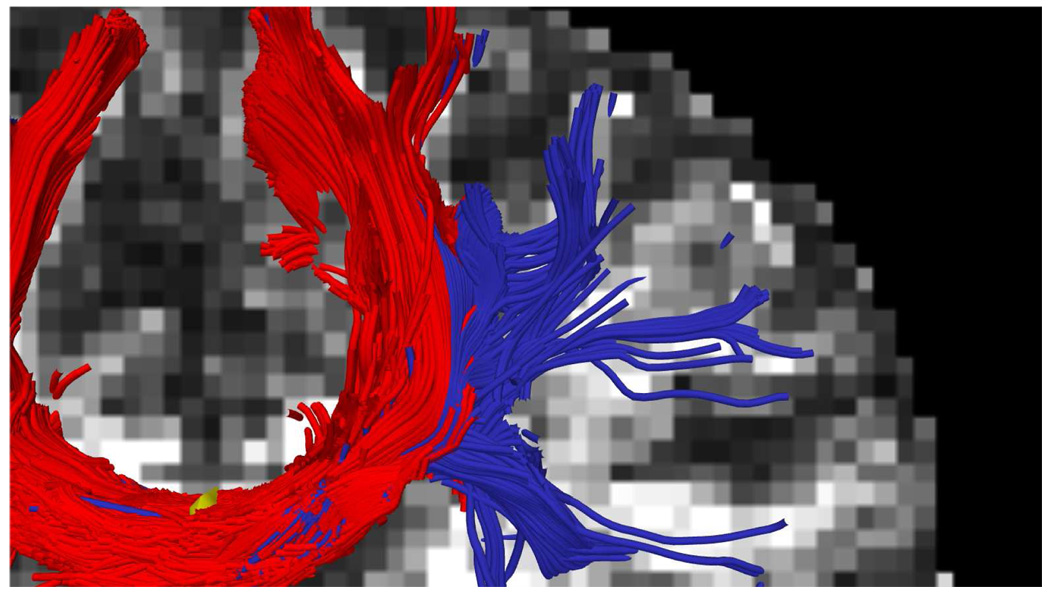
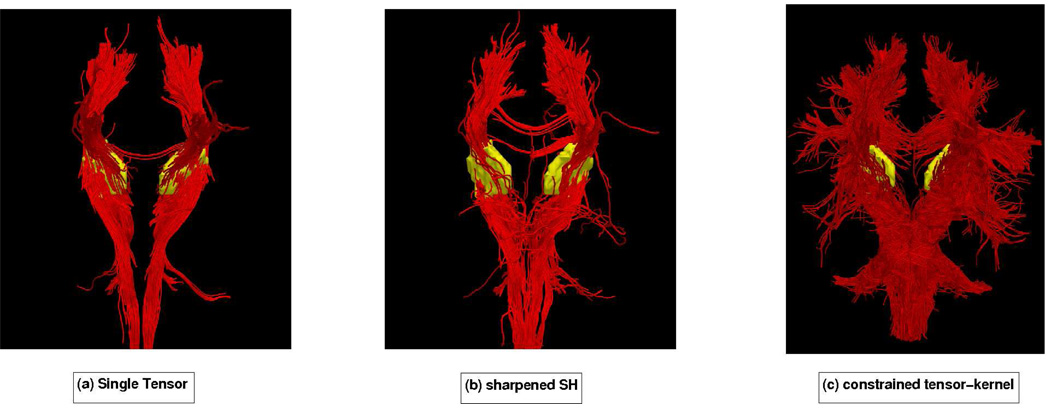
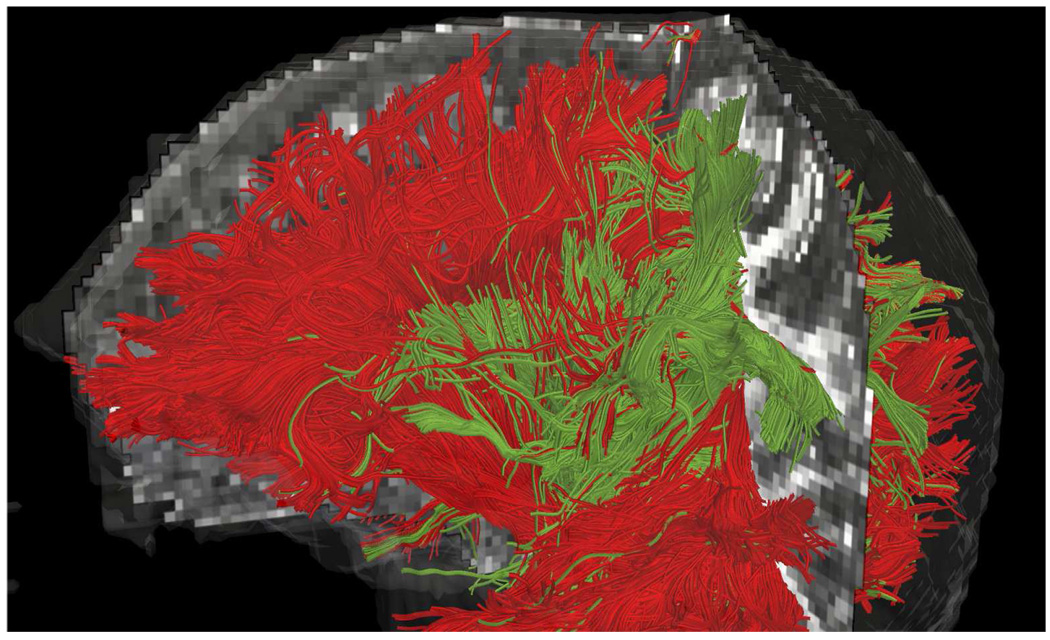
Next we examined cortico-spinal tract by seeding in the internal capsule to trace out the pathways reaching up into the primary motor cortex at the top of the brain as well as down into the hippocampal regions near the brain stem. Figure 11 shows frontal views for each technique with seeding (yellow). Figure 14 shows this same result from a side view where we can see that the constrained approach picks up many of the corticospinal pathways, consistent with the anatomy. Figure 12 shows a combined view of the corpus callosum and corticospinal tract. Notice that the tracts corresponding to each of these pathways terminates in different region (gyri) of the brain, consistent with the anatomy. As reported elsewhere [34], single-tensor tractography only follows the dominant corticospinal tract to the primary motor cortex. Similar pathways were also found with sharpened spherical harmonics.
Fig 14.
Sagittal view with seeding in the internal capsule (yellow). While both single-tensor and sharpened SH tend to follow the dominant corticospinal tract to the primary motor cortex, the constrained tractography method follows many more pathways.
Next, we report results using the three-component model (Figure 17). In this case, we seeded the mid-sagittal plane of the corpus callosum and traced the region known to contain three crossing bundles, namely the corpus callosum (CC) the superior longitudinal fasciculus (SLF) and cortico-spinal tract (CST). The proposed method picks up these three major pathways, with red showing the CC fibers, blue the direction of the CST and yellow the SLF. Thus, the three component model is able to pick up the three-crossings in the in-vivo data.
Fig 17.
Figure shows crossing of the three fiber bundles in the corona radiata. Red indicates the corpus callosum fibers, blue indicates direction of CST while yellow shows direction of SLF.
Note that in case we use the two-component model, the region of intersection between the transcallosal fibers, the CST, and the SLF, leads the tractography algorithm to report several end-to-end connections that are not necessarily present, e.g. fibers originating in the left internal capsule do not pass through the corpus callosum. Many of the lateral extensions are callosal fibers that are picked up while passing through this juncture. We believe that finding such pathways are inevitable (in the absence of any a-priori knowledge of the anatomy) due to porr model fitting and could be removed during post-processing as is typically done.
5. Conclusion
Studies involving deterministic tractography rely on the underlying model estimated at each voxel as well as the reconstructed pathways. In this work, we demonstrated that using a constrained estimation approach for tractography, robust estimates of much higher accuracy are obtained than independent estimation techniques. The proposed approach gives significantly lower angular error (5°) in regions with fiber crossings compared to using sharpened spherical harmonics (15–20°), and it is able to reliably resolve crossings down to 25–30° compared to spherical harmonics which reaches only down to 50–60°. In-vivo results on the corpus callosum and corticospinal tract demonstrate the ability of the tensor-kernel in a constrained mixture model framework to find fiber tracts which are not traced using either the single tensor model or the sharpened spherical harmonics approach, but are nevertheless present.
Future work involves incorporating prior knowledge during tractography to avoid tracts reaching in areas that are not anatomically connected but are picked-up due to partial voluming of the data. Further, we would extend this model to a mixture of three components to analyze in-vivo data at b = 3000 or higher b-values where fiber crossings are better detected. In eqn (6), we assumed the noise model to be Gaussian, and future work involves using a Rician noise model for better estimation.
Fig 13.
Tracing fibers originating from the center of the entire corpus callosum with view from the top. Constrained tractography is able to find many of the lateral projections (blue) while single-tensor is unable to find any and few are found with sharpened spherical harmonics. Seed region indicated in yellow.
Fig 15.
Frontal view of the cortico-spinal tract in each hemisphere with seeding done in the internal capsule (yellow).
Fig 16.
Combined view of corpus callosum (red) and corticospinal tract (green) as obtained using constrained second order tensor-kernel. Notice that different gyri are connected to the corpus callosum and corticospinal tract
Footnotes
Using the freely available Slicer 2.7 (http://www.slicer.org).
References
- 1.Basser PJ, Mattiello J, LeBihan D. MR diffusion tensor spectroscopy and imaging. Biophysical Journal. 1994;vol. 66(no. 1):259–267. doi: 10.1016/S0006-3495(94)80775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Behrens T, Woolrich M, Jenkinson M, Johansen-Berg H, Nunes R, Clare S, Matthews P, Brady J, Smith S. Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magnetic Resonance in Medicine. 2003;vol. 50:1077–1088. doi: 10.1002/mrm.10609. [DOI] [PubMed] [Google Scholar]
- 3.Alexander D, Barker G, Arridge S. Detection and modeling of non-Gaussian apparent diffusion coefficient profiles in human brain data. Magnetic Resonance in Medicine. 2002;vol. 48:331–340. doi: 10.1002/mrm.10209. [DOI] [PubMed] [Google Scholar]
- 4.Frank L. Characterization of anisotropy in high angular resolution diffusion-weighted MRI. Magnetic Resonance in Medicine. 2002;vol. 47:1083–1099. doi: 10.1002/mrm.10156. [DOI] [PubMed] [Google Scholar]
- 5.Alexander A, Hasan K, Tsuruda J, Parker D. Analysis of partial volume effects in diffusion-tensor MRI. Magnetic Resonance in Medicine. 2001;vol. 45:770–780. doi: 10.1002/mrm.1105. [DOI] [PubMed] [Google Scholar]
- 6.Tuch D, Reese T, Wiegella M, Makris N, Belliveau J, Wedeen V. High angular resolution diffusion imaging reveals intravoxel white matter fiber heterogeneity. Magnetic Resonance in Medicine. 2002;vol. 48:577–582. doi: 10.1002/mrm.10268. [DOI] [PubMed] [Google Scholar]
- 7.Kreher B, Schneider J, Mader I, Martin E, Hennig J, Il’yasov K. Multitensor approach for analysis and tracking of complex fiber configurations. Magnetic Resonance in Medicine. 2005;vol. 54:1216–1225. doi: 10.1002/mrm.20670. [DOI] [PubMed] [Google Scholar]
- 8.Friman O, Farnebäck G, Westin C-F. A Bayesian approach for stochastic white matter tractography. Trans. on Medical Imaging. 2006;vol. 25(no. 8):965–978. doi: 10.1109/tmi.2006.877093. [DOI] [PubMed] [Google Scholar]
- 9.Basser PJ, Pajevic S. Spectral decomposition of a 4th-order covariance tensor: Applications to diffusion tensor MRI. Signal Processing. 2007;vol. 87:220–236. [Google Scholar]
- 10.Barmpoutis A, Hwang MS, Howland D, Forder J, Vemuri B. Regularized positive-definite fourth order tensor field estimation from DW-MRI. Neuroimage. 2009 doi: 10.1016/j.neuroimage.2008.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McGraw T, Vemuri B, Yezierski B, Mareci T. Von Mises-Fisher mixture model of the diffusion ODF. Int. Symp. on Biomedical Imaging. 2006:65–68. doi: 10.1109/ISBI.2006.1624853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaden E, Anwander A, Knsche T. Parametric spherical deconvolution: Inferring anatomical connectivity using diffusion MR imaging. Neuroimage. 2007;vol. 37:477–488. doi: 10.1016/j.neuroimage.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 13.Rathi Y, Michailovich O, Shenton ME, Bouix S. Directional functions for orientation distribution estimation. Medical Image Analysis. 2009 doi: 10.1016/j.media.2009.01.004. DOI: 10.1016/j.media.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tuch D. Q-ball imaging. Magnetic Resonance in Medicine. 2004;vol. 52:1358–1372. doi: 10.1002/mrm.20279. [DOI] [PubMed] [Google Scholar]
- 15.Anderson A. Measurement of fiber orientation distributions using high angular resolution diffusion imaging. Magnetic Resonance in Medicine. 2005;vol. 54(no. 5):1194–1206. doi: 10.1002/mrm.20667. [DOI] [PubMed] [Google Scholar]
- 16.Hess C, Mukherjee P, Han E, Xu D, Vigneron D. Q-ball reconstruction of multimodal fiber orientations using the spherical harmonic basis. Magnetic Resonance in Medicine. 2006;vol. 56:104–117. doi: 10.1002/mrm.20931. [DOI] [PubMed] [Google Scholar]
- 17.Descoteaux M, Angelino E, Fitzgibbons S, Deriche R. Regularized, fast, and robust analytical Q-ball imaging. Magnetic Resonance in Medicine. 2007;vol. 58:497–510. doi: 10.1002/mrm.21277. [DOI] [PubMed] [Google Scholar]
- 18.Alexander D. Multiple-fiber reconstruction algorithms for diffusion MRI. Annals of the New York Academy of Sciences. 2005;vol. 1064 doi: 10.1196/annals.1340.018. [DOI] [PubMed] [Google Scholar]
- 19.Descoteaux M, Deriche R, Anwander A. Deterministic and probabilistic q-ball tractography: from diffusion to sharp fiber distributions. Tech. Rep. 6273, INRIA Sophia Antipolis. 2007 August [Google Scholar]
- 20.Basser P, Pajevic S, Pierpaoli C, Duda J, Aldroubi A. In vivo fiber tractography using DT-MRI data. Magnetic Resonance in Medicine. 2000;vol. 44:625–632. doi: 10.1002/1522-2594(200010)44:4<625::aid-mrm17>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 21.Hagmann P, Reese T, Tseng W-Y, Meuli R, Thiran J-P, Wedeen VJ. Diffusion spectrum imaging tractography in complex cerebral white matter: An investigation of the centrum semiovale. Int. Symp. on Magnetic Resonance in Medicine. 2004;623 [Google Scholar]
- 22.Zhang F, Goodlett C, Hancock E, Gerig G. Probabilistic fiber tracking using particle filtering. MICCAI. 2007:144–152. doi: 10.1007/978-3-540-75759-7_18. [DOI] [PubMed] [Google Scholar]
- 23.Zhan W, Yang Yihong. How accurately can the diffusion profiles indicate multiple fiber orientations ? A study on general fiber crossings in diffusion MRI. Journal of Magnetic Resonance. 2006;vol. 183:193–202. doi: 10.1016/j.jmr.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 24.Seunarine K, Cook P, Hall M, Embleton K, Parker G, Alexander D. Exploiting peak anisotropy for tracking through complex structures. MMBIA. 2007:1–8. [Google Scholar]
- 25.Bloy L, Verma R. On computing the underlying fiber directions from the diffusion orientation distribution function. MICCAI. 2008:1–8. doi: 10.1007/978-3-540-85988-8_1. [DOI] [PubMed] [Google Scholar]
- 26.Schultz T, Seidel H. Estimating crossing fibers: A tensor decomposition approach. Trans. on Visualization and Computer Graphics. 2008;vol. 14(no. 6):1635–1642. doi: 10.1109/TVCG.2008.128. [DOI] [PubMed] [Google Scholar]
- 27.Ramirez-Manzanares A, Cook P, Gee J. A comparison of methods for recovering intra-voxel white matter fiber architecture from clinical diffusion imaging scans. MICCAI. 2008:305–312. doi: 10.1007/978-3-540-85988-8_37. [DOI] [PubMed] [Google Scholar]
- 28.Jian B, Vemuri B. A unified computational framework for deconvolution to reconstruct multiple fibers from diffusion weighted MRI. IEEE Trans. on Medical Imaging. 2007;vol. 26(no. 11):1464–1471. doi: 10.1109/TMI.2007.907552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jansons K, Alexander D. Persistent angular structure: New insights from diffusion MRI data. Inverse Problems. 2003;vol. 19:1031–1046. doi: 10.1007/978-3-540-45087-0_56. [DOI] [PubMed] [Google Scholar]
- 30.Tournier J-D, Calamante F, Gadian D, Connelly A. Direct estimation of the fiber orientation density function from diffusion-weighted MRI data using spherical deconvolution. NeuroImage. 2004;vol. 23:1176–1185. doi: 10.1016/j.neuroimage.2004.07.037. [DOI] [PubMed] [Google Scholar]
- 31.Kumar R, Barmpoutis A, Vemuri BC, Carney PR, Mareci TH. Multi-fiber reconstruction from DW-MRI using a continuous mixture of von Mises-Fisher distributions. MMBIA. 2008:1–8. doi: 10.1007/978-3-642-02498-6_12. [DOI] [PubMed] [Google Scholar]
- 32.Tournier J-D, Yeh C, Calamante F, Cho H, Connelly A, Lin P. Resolving crossing fibres using constrained spherical deconvolution: validation using diffusion-weighted imaging phantom data. Neuroimage. 2008;vol. 41:617–625. doi: 10.1016/j.neuroimage.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 33.Basser P, Pajevic S. A normal distribution for tensor-valued random variables: applications to diffusion tensor MRI. IEEE Trans. on Medical Imaging. 2003;vol. 22:785–794. doi: 10.1109/TMI.2003.815059. [DOI] [PubMed] [Google Scholar]
- 34.Behrens T, Johansen-Berg H, Jbabdi S, Rushworth M, Woolrich M. Probabilistic diffusion tractography with multiple fibre orientations: What can we gain? NeuroImage. 2007;vol. 34:144–155. doi: 10.1016/j.neuroimage.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arsigny Vincent, Fillard Pierre, Pennec Xavier, Ayache Nicholas. Clinical DT-MRI estimation, smoothing and fiber tracking with log-euclidean metrics. IEEE Trans. on Medical Imaging. 2007;vol. 26:1472–1482. doi: 10.1109/TMI.2007.899173. [DOI] [PubMed] [Google Scholar]
- 36.Assemlal H-E, Tschumperlé D, Brun L. A variational framework for the robust estimation of odfs from high angular resolution diffusion images. Tech. Rep., les cahiers du GREYC. 2007 April [Google Scholar]
- 37.Sotiropoulos S, Bai Li, Morgan P, Auer D, Constantinescu C, Tench C. A regularized two-tensor model fit to low angular resolution diffusion images using basis directions. Journal of Magnetic Resonance in Medicine. 2008;vol. 28:199–209. doi: 10.1002/jmri.21380. [DOI] [PubMed] [Google Scholar]
- 38.Mallat S, Zhang Z. Matching pursuits with time-frequency dictionaries. Trans. on Signal Processing. 1993;vol. 41:3397–2415. [Google Scholar]
- 39.Tournier J-D, Calamante F, Connelly A. Robust determination of the fibre orientation distribution in diffusion MRI: Non-negativity constrained super-resolved spherical deconvolution. NeuroImage. 2007;vol. 35:1459–1472. doi: 10.1016/j.neuroimage.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 40.Anwander A, Descoteaux M, Deriche R. Probabilistic Q-Ball tractography solves crossings of the callosal fibers. Human Brain Mapping. 2007:342. [Google Scholar]