Abstract
Parkin, whose mutations cause Parkinson disease (PD), controls oxidative stress by limiting the expression of monoamine oxidases (MAO)—mitochondrial enzymes responsible for the oxidative de-amination of dopamine. Here, we show that parkin performed this function by increasing the ubiquitination and degradation of estrogen-related receptors (ERR), orphan nuclear receptors that play critical roles in the transcription regulation of many nuclear-encoded mitochondrial proteins. All three ERRs (α, β and γ) increased the transcription of MAOs A and B; the effects were abolished by parkin, but not by its PD-linked mutants. Parkin bound to ERRs and increased their ubiquitination and degradation. In fibroblasts from PD patients with parkin mutations or brain slices from parkin knockout mice, degradation of ERRs was significantly attenuated. The results reveal the molecular mechanism by which parkin suppresses the transcription of MAOs to control oxidative stress induced by dopamine oxidation.
INTRODUCTION
Parkinson disease (PD) is a neurodegenerative movement disorder characterized by a selective loss of nigral dopaminergic neurons (1). Mutations of several genes, including parkin, are linked to the degeneration of these neurons and locomotor symptoms of PD (2). Thus, a key goal in PD research is to understand how mutations of genes such as parkin, which generally show wide expression patterns, lead to the selective degeneration of nigral DA neurons and ensuing PD. Many studies have shown that dopamine-induced oxidative stress plays a key role in the selective death of dopaminergic neurons (3). The enzymatic catabolism of dopamine, catalyzed by monoamine oxidase (MAO), produces a large amount of H2O2, which is converted to other reactive oxygen species (ROS). In addition, dopamine aldehyde generated in the oxidative de-amination reaction is 1000-fold more toxic in vivo than dopamine (4). Studies both in vivo (5) and in cell lines (6) have demonstrated that dopamine induces cell death through the generation of ROS. Our previous study has shown that parkin suppresses dopamine toxicity in SH-SY5Y cells by decreasing the level of ROS and oxidative stress (7). Parkin performs this function by reducing the mRNA levels of MAO (8). Overexpression of parkin in different cell lines such as SH-SY5Y and NIH3T3 greatly suppresses the message levels of MAO. On the other hand, the amounts of MAO mRNA are significantly increased in lymphocytes from a PD patient with homozygous deletion of parkin exon 4, compared with unaffected sibling heterozygous carriers or unrelated normal subjects (8). Consistent with these, parkin knockout (KO) mice have an increased level of the dopamine oxidation product DOPAC (9) and elevated MAO-B activity (10). Thus, previous studies have provided strong evidence that parkin regulates the transcription of MAOs to control dopamine-induced oxidative stress and ensuing selective death of dopaminergic neurons.
MAO has two isoforms, MAO-A and MAO-B, which are encoded by two distinct genes (11). Both MAOs are located on the cytoplasmic side of the mitochondrial outer membrane through a C-terminal tail anchored in the membrane (12,13). It has been found that PD patients have elevated MAO-B activity in the substantia nigra (14). MAO-B inhibitors have been widely used to delay the progression of PD symptoms and appear to have some neuroprotective effects (15). MAO-B KO mice are resistant to the PD toxin MPTP (16), as MAO-B is responsible for the oxidation of the protoxin MPTP to the active metabolite MPP+ (17), which specifically kills DA neurons through the selective uptake by the dopamine transporter (18). A large-scale screen has identified the nuclear orphan receptor ERRα (estrogen-related receptor α) and its co-activator PGC-1α as important inducers of both MAO-A and MAO-B (19). ERRα is a transcription factor of the nuclear receptor superfamily (20). The three members of the ERR family (α, β and γ) have no known endogenous ligand and seem to be constitutively active in most cells. The function of ERRα is highly regulated by the transcription co-activator PGC-1α (21). Together, they play a critical role in inducing genes involved in mitochondrial oxidative metabolism (22) and mitochondrial biogenesis (23).
The present study found that all three members of the ERR family significantly activated the transcription of MAO. These effects were abolished by parkin because parkin increased the ubiquitination and degradation of ERRs. PD-linked mutations of parkin disrupted this critical function, which results in uncontrolled expression of MAO. Thus, our study revealed the molecular mechanism by which parkin regulates MAO transcription to limit the intrinsic level of oxidative stress in dopaminergic neurons.
RESULTS
Parkin abrogates ERRα-induced activation of MAO-A and MAO-B promoters
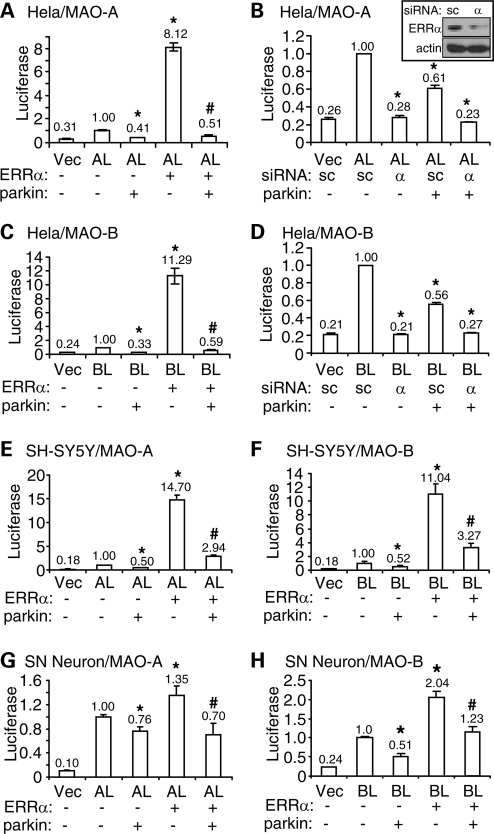
Our previous study has shown that parkin suppresses the transcription of MAO-A and MAO-B (8). Independent studies show that ERRα significantly induces the expression of both MAOs (19). To test whether parkin regulates the transcription of MAO through ERRα, we cloned the human MAO-A promoter (−1 to −2968 bp) or the human MAO-B promoter (−1 to −2779 bp) into the luciferase reporter construct pGL3. Co-transfection of ERRα with the MAO-A luciferase construct (AL) in HeLa cells significantly increased the activity of the promoter (Fig. 1A). The effect was almost completely abolished by parkin (Fig. 1A). In fact, parkin alone significantly reduced the activity of MAO-A promoter (Fig. 1A), presumably by suppressing the endogenous ERRα in HeLa cells. To confirm these, we used siRNA to knock down the expression of endogenous ERRα in HeLa cells. As shown in Figure 1B, ERRα knockdown significantly decreased the activity of MAO-A promoter. When endogenous ERRα was knocked down by its siRNA, parkin could not significantly suppress MAO-A promoter further (0.23 ± 0.01 versus 0.28 ± 0.02, P > 0.05, n = 9, Fig. 1B). It suggests that the effect of parkin on MAO-A promoter is mediated by ERRα.
Figure 1.
Parkin suppresses ERRα-induced activation of MAO-A and MAO-B promoters. (A–D) HeLa cells were transfected with a luciferase reporter of MAO-A promoter (AL) (A and B) or MAO-B promoter (BL) (C and D), together with parkin and ERRα (A and C) or parkin and siRNA against ERRα (α) or scrambled control (sc) sequence (B and D) as indicated. Luciferase activities were measured in cleared total cell lysates from these samples. Vec, pGL3 empty vector. Inset, ERRα or actin immunoblots of total cell lysates from HeLa cells transfected with siRNA against ERRα (α) or scrambled control sequence (sc). (E–H) The experiments on MAO-A promoter (A) and MAO-B promoter (C) were performed in SH-SY5Y cells (E and F) and rat embryonic midbrain neuronal cultures (G and H). *P < 0.05, versus AL or BL alone. #P < 0.05, versus the preceding bar, n = 9 for all experiments (three independent experiments, each with triplicate samples).
Overexpression of ERRα also markedly increased the activity of MAO-B promoter; the effect was also abolished by parkin (Fig. 1C). Accordingly, ERRα siRNA significantly reduced the activity of MAO-B promoter and the effect could not be decreased further by parkin (Fig. 1D). To substantiate these results, we performed the same experiments in the dopaminergic neuroblastoma cell line SH-SY5Y (Fig. 1E and F) and primary rat embryonic midbrain neuronal cultures (Fig. 1G and H). Similar results were obtained in all these preparations, suggesting that the effects are independent of cell types.
Parkin suppresses ERRα-mediated induction of endogenous MAO-A and MAO-B
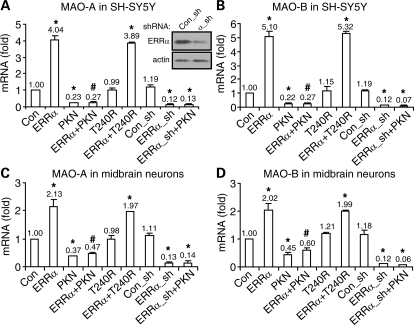
To substantiate our findings, we examined the impact of parkin and ERRα on the mRNA level of endogenous MAO-A and MAO-B. As shown in Figure 2A, the MAO-A mRNA level was markedly increased in SH-SY5Y cells infected with ERRα lentivirus, compared with the control GFP lentivirus. The effect was abolished by co-infection of the cells with sindbis virus overexpressing parkin, but not its PD-linked T240R mutant. Consistent with these, the wild-type (WT) parkin virus alone significantly reduced MAO-A level, whereas the T240R mutant did not have any significant effect. These results suggest that the action of parkin is dependent on its ubiquitin-protein ligase activity, which is lost in the T240R mutant (24). To strengthen our evidence, we used lentivirus to effectively deliver shRNA against ERRα or a scrambled control sequence. The ERRα shRNA lentivirus significantly knocked down endogenous ERRα in SH-SY5Y cells (Fig. 2A inset) or rat midbrain neuronal cultures (data not shown; the cognizant sequences are the same for both species). ERRα knockdown significantly decreased the message level of MAO-A; the effect was not significantly changed by co-infection of the cells with the parkin sindbis virus (Fig. 2A). Similar results were found for MAO-B (Fig. 2B). We also confirmed the findings in rat midbrain neuronal cultures (Fig. 2C and D). Thus, results from a variety of cell lines and primary neurons suggest that parkin suppresses MAO transcription by abrogating ERRα-induced transactivation of the MAO genes.
Figure 2.
Parkin decreases ERRα-induced expression of endogenous MAO-A and MAO-B. (A and B) Real-time quantitative RT–PCR measurements of the endogenous levels of MAO-A (A) or MAO-B (B) in SY-SY5Y cells infected with the indicated combinations of lentivirus expressing ERRα, ERRα shRNA (ERRα_sh), control shRNA (Con_sh) and sindbis virus expressing parkin (PKN) or its PD-linked T240R mutant. Inset: ERRα or actin immunoblots of total cell lysates from SH-SY5Y cells infected with lentivirus delivering shRNA of ERRα (α_sh) or control sequence (Con_sh). (C and D) The same experiments on MAO-A (C) and MAO-B (D) were performed in rat embryonic midbrain neuronal cultures. *P < 0.05, versus control (Con, GFP sindbis virus) or control shRNA (Con_sh); #P < 0.05, versus ERRα alone; n = 9 for all experiments (three independent experiments, each with triplicate samples).
Parkin binds to and ubiquitinates ERRα
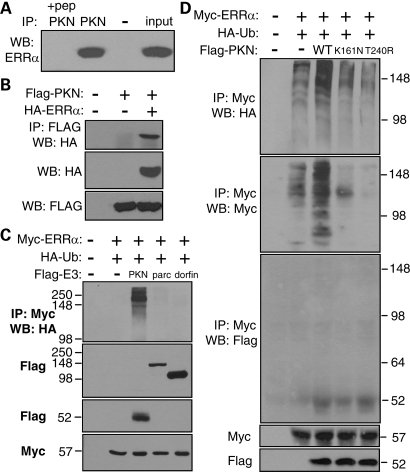
Since parkin is a ubiquitin-protein ligase, we tested whether ERRα is a substrate of parkin. Rat brain homogenates containing 4 mg of total proteins were immunoprecipitated with a specific antibody against parkin (4 µg) or the antibody plus its antigenic peptide (24). Anti-ERRα blot of the immunoprecipitates showed that ERRα was strongly co-immunoprecipitated with parkin. Co-immunoprecipitation (co-IP) was absent when the parkin antigenic peptide was included (Fig. 3A). To confirm the binding, we co-expressed parkin and ERRα in HEK293T cells and found that transfected parkin and ERRα indeed bound to each other in co-IP assays (Fig. 3B). To test whether parkin increases the ubiquitination of ERRα in a specific manner, we co-transfected Myc-ERRα, HA-ubiquitin (HA-Ub) and FLAG-tagged parkin, parc (25) or dorfin (26). All these ubiquitin-protein ligases share the RING-IBR-RING motif. As shown in Figure 3C, only parkin increased the ubiquitination of ERRα. Overexpression of parc or dorfin did not have any effect on the ubiquitination of ERRα. We then tested whether the ability of parkin to ubiquitinate ERRα is related to PD by transfecting HEK293T cells with Myc-ERRα, HA-Ub and FLAG-tagged WT parkin or its PD-linked K161N or T240R mutant. As shown in the first panel of Figure 3D, only WT parkin, but not its PD-linked mutants, increased the ubiquitination of Myc-ERRα. To rule out the possibility that the high molecular weigh smears might be due to auto-ubiquitinated parkin, we blotted the Myc immunoprecipitates with anti-Myc and found that Myc-ERRα was indeed modified in size and became high molecular weight smears only when WT parkin was co-transfected (Fig. 3D, panel 2). The PD-linked mutants did not have a significant effect over the basal ubiquitination of ERRα. Furthermore, Myc immunoprecipitates contained small amount of FLAG-parkin, but no significant amount of ubiquitinated FLAG-parkin (Fig. 3D, panel 3). Together, these data showed that parkin specifically increased the ubiquitination of ERRα.
Figure 3.
ERRα binds to parkin and increases its ubiquitination. (A) Cleared rat brain homogenates after ultracentrifugation were immunoprecipitated with parkin antibody (PKN) in the presence or absence of its antigenic peptide (pep). The immunoprecipitates and 1% of input cell lysates were western-blotted with antibody against ERRα. (B) HEK293T cells were transfected with FLAG-parkin and HA-ERRα as indicated. Anti-FLAG immunoprecipitates from cleared total cell lysates were blotted with anti-HA. (C) HEK293T cells were transfected with Myc-ERRα, HA-ubiquitin (HA-Ub) and FLAG-tagged ubiquitin-protein ligases such as parkin (PKN), parc or dorfin. Anti-Myc immunoprecipitates were blotted with anti-HA to examine the ubiquitination of Myc-ERRα. Total cell lysates were blotted with anti-FLAG or anti-Myc to show the expression levels of transfected proteins. (D) HEK293T cells were transfected with Myc-ERRα, HA-Ub and FLAG-tagged wild-type parkin (WT) or its PD-linked K161N or T240R mutant. Anti-Myc immunoprecipitates were blotted with anti-HA (first panel) or anti-Myc (second panel) to show the ubiquitination of Myc-ERRα, or with anti-FLAG (third panel) to show whether auto-ubiquitinated parkin was present in the Myc immunoprecipitates. Total cell lysates were blotted with anti-FLAG or anti-Myc to show the expression levels of transfected proteins. All experiments were repeated at least four different times with similar results.
Parkin enhances the degradation of ERRα
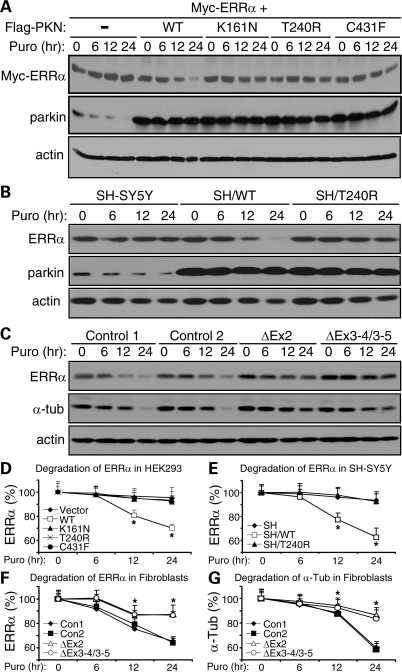
After HEK293T cells were co-transfected with Myc-ERRα and WT parkin or its PD-linked mutants (K161N, T240R or C431F), we treated the cells with puromycin (100 µm) for various durations to block new protein synthesis. Western blotting of the total cell lysate with anti-Myc showed that the degradation of transfected ERRα was significantly sped up by WT parkin, but not by any of its PD-linked mutants (Fig. 4A and D). To confirm this, we examined the degradation rates of endogenous ERRα in SH-SY5Y cells and SH-SY5Y cells overexpressing WT parkin or its T240R mutant. Indeed, the degradation of endogenous ERRα in SH-SY5Y cells was much faster when WT, but not mutant, parkin was overexpressed (Fig. 4B and E). We also used primary human skin fibroblasts from two normal control subjects and two PD patients with parkin mutations (ΔEx2: homozygous deletion of exon 2; ΔEx3-4/3-5: compound heterozygous deletions of exons 3–4 and 3–5). Degradation of endogenous ERRα was significantly slowed down when parkin was mutated (Fig. 4C and F). The degradation of α-tubulin, a known substrate of parkin (24), was shown as a positive control (Fig. 4C and G), whereas the degradation of actin, which is not a substrate of parkin, was shown as a negative control (Fig. 4C). These data conclusively showed that parkin was a ubiquitin-protein ligase for ERRα.
Figure 4.
Parkin enhances the degradation of ERRα. (A and D) HEK293T cells were co-transfected with Myc-ERRα and empty vector (−) or FLAG-tagged wild-type parkin (WT) or its PD-linked K161N, T240R or C431F mutant. Cells were treated with puromycin (100 µm) for the indicated durations to block new protein synthesis. Cleared total cell lysates were immunoblotted with anti-Myc to examine the degradation of transfected Myc-ERRα, parkin and endogenous actin (A). Quantification of Myc-ERRα levels is shown in (D). *P < 0.05, WT versus mutants or empty vector at the same time point, n = 3. (B and E) SH-SY5Y cells or SH-SY5Y cells stably expressing WT parkin (SH/WT) or the T240R mutant (SH/T240R) were treated with puromycin (100 µm) as indicated. Cleared total cell lysates were immunoblotted with antibodies against ERRα, parkin and actin to monitor their degradation (B). Quantification of the degradation of endogenous ERRα is shown in (E). *P < 0.05, SH/WT versus SH/T240R or SH at the same time point, n = 3. (C, F and G) Skin fibroblasts from normal control subjects and PD patients with parkin mutations (ΔEx2 for homozygous exon 2 deletion; ΔEx3-4/3-5 for compound heterozygous deletions of exons 3–4 and 3–5) were treated with puromycin (100 µm) as indicated. Cleared total cell lysates were immunoblotted with antibodies against ERRα, α-tubulin (α-tub) and actin to monitor their degradation (C). Quantification of the degradation of endogenous ERRα is shown in (F) and that of α-tubulin in (G). *P < 0.05, control cells versus parkin-deficient cells at the same time point, n = 3.
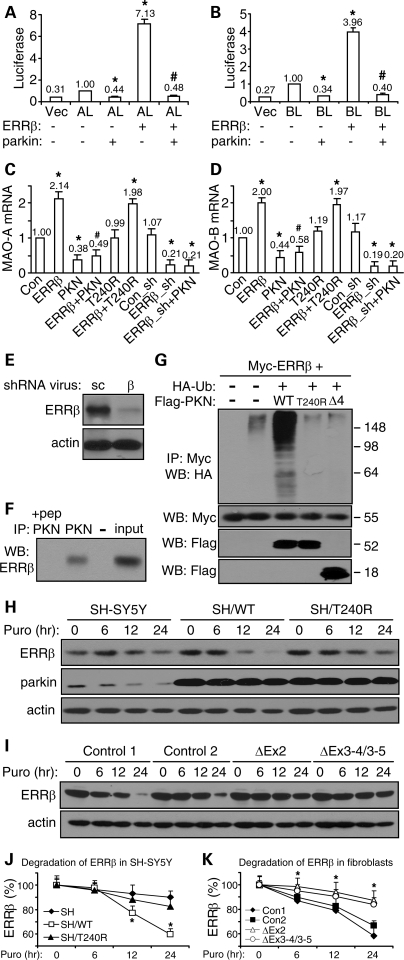
Parkin ubiquitinates and degrades ERRβ to suppress the induction of MAOs by ERRβ
The three members of the ERR family of orphan nuclear receptors (ERRα, ERRβ and ERRγ) share extensive sequence homology and certain functions (27). Thus, we tested whether ERRβ acts in the same manner as ERRα in the transcription suppression of MAOs by parkin. Overexpression of ERRβ in HeLa cells significantly increased the activity of MAO-A promoter (Fig. 5A) and MAO-B promoter (Fig. 5B). Co-expression of parkin abrogated these effects of ERRβ (Fig. 5A and B). When rat embryonic midbrain neuronal cultures were infected with ERRβ lentivirus, the mRNA levels of endogenous MAO-A (Fig. 5C) and MAO-B (Fig. 5D) were significantly increased. The effects were totally abolished by co-infection of sindbis virus expressing WT parkin but not its T240R mutant (Fig. 5C and D). Consistent with these, lentiviral delivery of ERRβ shRNA significantly reduced the message levels of endogenous MAO-A (Fig. 5C) and MAO-B (Fig. 5D). The effects were not decreased further by parkin sindbis virus (Fig. 5C and D). Western blotting of rat embryonic midbrain neuronal cultures infected with ERRβ shRNA lentivirus showed marked reduction of ERRβ expression level, compared with cultures infected with lentivirus of scrambled control shRNA (Fig. 5E). Thus, ERRβ strongly induced MAO-A and MAO-B, and the effects were abolished by parkin.
Figure 5.
Parkin also degrades ERRβ to limit its induction of MAOs. (A and B) HeLa cells were transfected with a luciferase reporter of MAO-A promoter (AL) (A) or MAO-B promoter (BL) (B), together with parkin and ERRβ as indicated. Luciferase activities were measured in cleared total cell lysates from these samples. *P < 0.05, versus AL or BL alone. #P < 0.05, versus the preceding bar, n = 9 for all experiments (three independent experiments, each with triplicate samples). (C and D) Real-time quantitative RT–PCR measurements of the endogenous levels of MAO-A (C) or MAO-B (D) in rat embryonic midbrain neuronal cultures infected with the indicated combinations of lentivirus expressing ERRβ, ERRβ shRNA (ERRβ_sh), control shRNA (Con_sh) and sindbis virus expressing parkin (PKN) or its PD-linked T240R mutant. *P < 0.05, versus control (Con, GFP sindbis virus) or control shRNA (Con_sh). #P < 0.05, versus ERRβ alone, n = 9 for all experiments (three independent experiments, each with triplicate samples). (E) ERRβ or actin immunoblots of total cell lysates from rat midbrain neuronal cultures infected with lentivirus delivering shRNA against ERRβ (β) or scrambled control sequence (sc). (F) Cleared rat brain homogenates after ultracentrifugation were immunoprecipitated with parkin antibody (PKN) in the presence or absence of its antigenic peptide (pep). The immunoprecipitates and 1% of input cell lysates were western-blotted with anti-ERRβ. (G) HEK293T cells were transfected with Myc-ERRβ, HA-ubiquitin (HA-Ub) and FLAG-tagged wild-type parkin (WT) or its PD-linked T240R or Δ4 (exon 4 deletion) mutant. Anti-myc immunoprecipitates were blotted with anti-HA to show the ubiquitination levels of myc-ERRβ. All experiments in (E–G) were repeated at least three different times with similar results. (H and J) SH-SY5Y cells or SH-SY5Y cells stably expressing WT parkin (SH/WT) or the T240R mutant (SH/T240R) were treated with puromycin (100 µm) as indicated. Cleared total cell lysates were immunoblotted with antibodies against ERRβ, parkin and actin to monitor their degradation (H). Quantification of the degradation of endogenous ERRβ is shown in (J). *P < 0.05, SH/WT versus SH/T240R or SH at the same time point, n = 3. (I and K) Skin fibroblasts from normal control subjects and PD patients with parkin mutations were treated with puromycin (100 µm) as indicated. Cleared total cell lysates were immunoblotted with antibodies against ERRβ and actin to monitor their degradation (I). Quantification of the degradation of endogenous ERRβ is shown in (K) *P < 0.05, control cells versus parkin-deficient cells at the same time point, n = 3.
We then tested whether parkin bound to ERRβ and increased its ubiquitination and degradation. In the rat brain homogenate, the co-IP of endogenous parkin and ERRβ was robustly observed; the effect was completely disrupted by the parkin antigenic peptide (Fig. 5F). When HEK293T cells were co-transfected with ERRβ and parkin or its PD-linked mutants (T240R or Δ4, exon 4 deletion), the ubiquitination of ERRβ was significantly increased by parkin, but not by its mutants (Fig. 5G). Using the puromycin chase assay, we found that the degradation of endogenous ERRβ was significantly enhanced in SH-SY5Y cells stably expressing WT parkin, but not its T240R mutant (Fig. 5H and J). Consistent with this, degradation of endogenous ERRβ was significantly slowed down in skin fibroblasts from PD patients with parkin mutations, compared with those from normal controls (Fig. 5I and K).
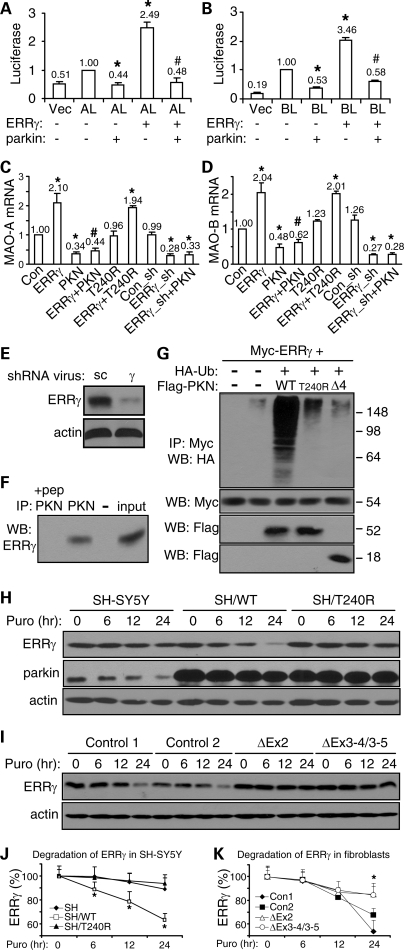
Parkin also suppresses ERRγ-induced expression of MAOs
Previous report of the induction of MAO-B by ERRγ (27) led us to examine whether parkin might suppress the action of ERRγ on MAO expression. Overexpression of ERRγ in HeLa cells significantly increased the activity of MAO-A promoter (Fig. 6A) and MAO-B promoter (Fig. 6B). The increases were totally abolished by co-expression of parkin (Fig. 6A and B). When rat embryonic midbrain neuronal cultures were infected with ERRγ lentivirus, the mRNA levels of endogenous MAO-A (Fig. 6C) and MAO-B (Fig. 6D) were significantly increased. Co-infection with sindbis virus expressing parkin, but not its T240R mutant, abolished these effects (Fig. 6C and D). Consistent with these, ERRγ knockdown by lentiviral delivery of shRNA significantly reduced the message levels of both MAOs. The effects were not decreased further by co-infection with parkin sindbis virus (Fig. 6C and D). Western blotting of rat embryonic midbrain neuronal cultures infected with ERRγ shRNA lentivirus showed marked knockdown of endogenous ERRγ (Fig. 6E).
Figure 6.
ERRγ-induced expression of MAOs is suppressed by parkin through the ubiquitination and degradation of ERRγ. (A and B) Luciferase activities in HeLa cells transfected with a luciferase reporter of MAO-A promoter (AL) (A) or MAO-B promoter (BL) (B), together with parkin and ERRγ as indicated. *P < 0.05, versus AL or BL alone. #P < 0.05, versus the preceding bar, n = 9 for all experiments (three independent experiments, each with triplicate samples). (C and D) Real-time quantitative RT–PCR measurements of the endogenous levels of MAO-A (C) or MAO-B (D) in rat embryonic midbrain neuronal cultures infected with the indicated combinations of lentivirus expressing ERRγ, ERRγ shRNA (ERRγ_sh), control shRNA (Con_sh) and sindbis virus expressing parkin (PKN) or its PD-linked T240R mutant. *P < 0.05, versus control (Con, GFP sindbis virus) or control shRNA (Con_sh). #P < 0.05, versus ERRγ alone, n = 9 for all experiments (three independent experiments, each with triplicate samples). (E) ERRγ or actin immunoblots of total cell lysates from rat midbrain neuronal cultures infected with lentivirus delivering shRNA against ERRγ (γ) or scrambled control sequence (sc). (F) Cleared rat brain homogenates after ultracentrifugation were immunoprecipitated with parkin antibody (PKN) in the presence or absence of its antigenic peptide (pep). The immunoprecipitates and 1% of input cell lysates were western-blotted with anti-ERRγ. (G) HEK293T cells were transfected with Myc-ERRγ, HA-ubiquitin (HA-Ub) and FLAG-tagged wild-type parkin (WT) or its PD-linked T240R or Δ4 (exon 4 deletion) mutant. Anti-myc immunoprecipitates were blotted with anti-HA to show the ubiquitination levels of myc-ERRγ. All experiments in (E–G) were repeated at least three different times with similar results. (H and J) SH-SY5Y cells or SH-SY5Y cells stably expressing WT parkin (SH/WT) or the T240R mutant (SH/T240R) were treated with puromycin (100 µm) as indicated. Cleared total cell lysates were immunoblotted with antibodies against ERRγ, parkin and actin to monitor their degradation (H). Quantification of the degradation of endogenous ERRγ is shown in (J). *P < 0.05, SH/WT versus SH/T240R or SH at the same time point, n = 3. (I and K) Skin fibroblasts from normal control subjects and PD patients with parkin mutations were treated with puromycin (100 µm) for the indicated durations to block new protein synthesis. Cleared total cell lysates were immunoblotted with antibodies against ERRγ and actin to monitor their degradation (I). Quantification of the degradation of endogenous ERRγ is shown in (K) *P < 0.05, control cells versus parkin-deficient cells at the same time point, n = 3.
When rat brain lysates were immunoprecipitated with parkin antibody in the presence or absence of its antigenic peptide, ERRγ was strongly present in parkin immunoprecipitates, but not when the antigenic peptide was included (Fig. 6F). In HEK293T cells co-transfected with ERRγ and parkin or its mutants (T240R or Δ4), ubiquitination of ERRγ was significantly increased by parkin, but not by its PD-linked mutants (Fig. 6G). Degradation of endogenous ERRγ was significantly enhanced in SH-SY5Y cells stably overexpressing WT, but not T240R mutant, parkin, compared with the situation in parental SH-SY5Y cells (Fig. 6H and J). Accordingly, degradation of endogenous ERRγ was significantly attenuated in dermal fibroblasts from PD patients with parkin mutations, compared with normal controls (Fig. 6I and K).
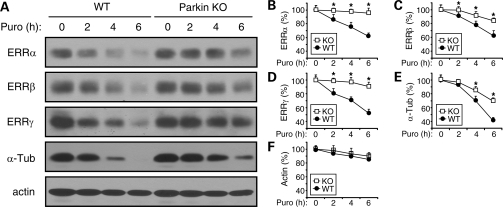
Parkin accelerates the degradation of ERRα, β and γ in vivo
To confirm that parkin was indeed a ubiquitin-protein ligase for ERRα, β and γ, we examined the degradation of the three ERRs in parkin KO mice and WT control. Midbrain slices from WT mice and parkin KO mice were treated with 100 µm puromycin for various durations to block new protein synthesis. Western blotting of total cell lysates with antibodies against ERRα, ERRβ or ERRγ showed that the degradation of endogenous ERRα, β and γ was significantly attenuated in parkin KO mice, compared with that in WT controls (Fig. 7A–D). As expected, the degradation of α-tubulin, a known substrate of parkin, was also significantly decreased in parkin KO mice (Fig. 7A and E). In contrast, the degradation of actin was very similar between the two genotypes (Fig. 7A and F).
Figure 7.
Parkin facilitates the degradation of ERRα, β and γ in vivo. (A) Midbrain brain slices from wild-type (WT) or parkin knockout (KO) mice were treated with puromycin (100 µm) for the indicated durations to block new protein synthesis. Cleared total cell lysates were immunoblotted with antibodies against ERRα, ERRβ, ERRγ, α-tubulin (α-tub) or actin to examine their degradation. (B–F) Quantification for the degradation of endogenous ERRα (B), ERRβ (C), ERRγ (D), α-tubulin (E) and actin (F). *P < 0.05, parkin KO mice versus WT mice at the same time point, n = 3.
DISCUSSION
A critical challenge for PD research is to understand the selective loss of nigral dopaminergic neurons, which leads to the hallmark motor symptoms of the disease. Loss-of-function mutations of parkin cause a selective degeneration of nigral DA neurons and PD, despite the wide expression pattern of parkin. It suggests that parkin may protect certain cellular processes that render dopaminergic neurons uniquely vulnerable. One of these vulnerabilities is oxidative stress induced by dopamine oxidation. The oxidative de-amination of dopamine, which is catalyzed by MAO, produces large amounts of ROS. Thus, a key mechanism to control oxidative stress is to set the appropriate level of MAO expression. Our previous studies have shown that parkin reduces dopamine toxicity and oxidative stress (7) by suppressing the mRNA level of MAO (8). The present study revealed the molecular mechanism by which parkin suppresses MAO transcription.
We found that ERRα was the key link between parkin and MAO transcription. Consistent with previous independent studies (19,27), our results showed that ERRα significantly increased the activity of MAO-A and MAO-B promoters in a variety of cells such as HeLa, SH-SY5Y and rat midbrain neuronal cultures (Fig. 1). Parkin abolished ERRα-induced transactivation of MAO promoters (Fig. 1). ERRα knockdown experiments confirmed that parkin was indeed acting on ERRα to regulate MAO promoters (Fig. 1). Consistent with these, mRNA levels of endogenous MAO-A and MAO-B in the human dopaminergic neuroblastoma cell line SH-SY5Y and primary rat midbrain neurons were also significantly increased by ERRα, and the upregulation was abrogated by WT, but not PD-linked mutant, parkin (Fig. 2). Since parkin mutants such as T240R do not have ubiquitin-protein ligase activity, we tested whether ERRα was a substrate of parkin. Our experiments showed that parkin bound to ERRα and increased its ubiquitination (Fig. 3) and degradation (Fig. 4). Transient expression of parkin in HEK293T cells or stable expression of parkin in SH-SY5Y markedly accelerated the degradation of transfected or endogenous ERRα in an E3-dependent manner (Fig. 4A and B). The data were corroborated by the finding that the degradation of endogenous ERRα was greatly attenuated in human primary skin fibroblasts from PD patients with parkin mutations, compared with normal subjects (Fig. 4C and F). Furthermore, the degradation of endogenous ERRα was significantly slowed down in brain slices from parkin KO mice, compared with WT controls (Fig. 7A and B). The results unequivocally demonstrated that ERRα was a substrate of parkin. When we tested ERRβ and ERRγ, we found that both of them act in the same way as ERRα in activating the transcription of MAO-A and MAO-B (Figs 5 and 6). The effects of all three ERRs were abolished by parkin because parkin increased their ubiquitination and degradation (Figs 5–7).
Thus, the study showed that all three members of the ERR family of orphan nuclear receptors mediated the transcription suppression effect of parkin on MAO-A and MAO-B. The revelation of parkin–ERR–MAO pathway has many implications for PD research. Parkin is expressed widely in many different types of cells including neurons and glia, whereas MAO-A is primarily expressed in catecholaminergic neurons and MAO-B in serotonergic neurons and glial cells (11). This allows parkin to regulate MAO-A expression in DA neurons and MAO-B expression in glial cells to lower the oxidation of cytosolic dopamine and released dopamine in a concerted manner. The modest neuroprotective effect of MAO-B inhibitors points to the need to identify a better way to control MAO-B (15). XCT790, a specific reverse agonist of ERRα, has been found to inhibit MAO transcription in a large-scale screen (19). Since parkin exerts similar action on MAO transcription, it seems an attractive idea to test whether selective ERR inhibitors might have neuroprotective effects on nigral DA neurons in PD. In light of the encouraging clinical trials of selective MAO-B inhibitors, which appear to be neuroprotective against PD (15), it may be useful to explore ways to selectively control MAO expression, instead of using irreversible MAO inhibitors to inactive the enzyme in an uncontrolled manner. Because MAO-B is responsible for oxidizing the PD protoxin MPTP to its active metabolite MPP+, PD patients with parkin mutations, who have increased level of MAO (8), may have higher vulnerability to MPTP-like PD toxins.
Many independent lines of studies have suggested that parkin plays a key role in the function of mitochondria (28). In parkin-deficient Drosophila, abnormal mitochondrial morphology is seen in indirect flight muscle and spermatids (29,30). Increased expression of some mitochondrial proteins (31) and elevated oxidative stress (30) are observed in these flies. Consistent with these, parkin-deficient mice also exhibit alterations in the levels of mitochondrial proteins and increased peroxidation of proteins and lipids (32). Parkin KO mice have an increased level of the dopamine oxidation product DOPAC (9) and elevated MAO-B activity (10). In lymphocytes from PD patient with exon 4 deletion of parkin, MAO expression is significantly increased (8). One mechanism for parkin to regulate mitochondrial functions in a coordinated manner is through the ERR family of nuclear receptors. ERRα is a key metabolic regulator that controls many nuclear-encoded mitochondrial enzymes involved in energy metabolism and mitochondrial biogenesis (33). ERRα and its co-activator PGC-1α act in a concerted manner to increase mitochondrial transcription factor A (TFAM), which regulates transcription of mitochondria-encoded genes and participates in the replication of mitochondrial DNA. Although ERRα is largely in the nucleus, there is always a small fraction of any transcription factor in the cytosol as it is synthesized there. By degrading the cytosolic pool of ERRα through ubiquitin-dependent proteolysis, parkin limits the amount of ERRα that can be imported into the nucleus and may thus control the expression levels of many nuclear-encoded mitochondrial proteins in the same manner as it regulates MAO expression. The involvement of parkin in mitochondrial biology is undoubtedly more complex, as recent findings show that parkin is recruited to depolarized mitochondria to trigger their autophagy (34). Thus, parkin may impact on mitochondria from its biogenesis to degradation. Superimposed on the ROS produced by mitochondrial dysfunction, the large amounts of oxyradicals generated in the MAO-catalyzed oxidative de-amination of dopamine may render dopaminergic neurons particularly vulnerable to oxidative stress. Therefore, the parkin/ERR pathway appears to control oxidative stress stemming from both mitochondrial dysfunction and dopamine oxidation, which are critically important for the survival of nigral dopaminergic neurons.
MATERIALS AND METHODS
Reagents, constructs and viruses
Monoclonal anti-ERRα, ERRβ, ERRγ were purchased from R & D (Perseus Proteomics, Inc., Tokyo). Polyclonal antibody against actin and monoclonal antibody against myc were from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Polyclonal antibody against parkin was generated as reported previously (24). Monoclonal anti-flag (M2) was from Sigma (St Louis, MO, USA); monoclonal anti-hemagglutinin was purchased from Roche (Indianapolis, IN, USA). Human MAO-A promoter (−1 to −2968 bp) and human MAO-B promoter (−1 to −2779 bp) were cloned by PCR from human genomic DNA BAC clone RP5-879N19 and RP1-27K14, respectively. They were fully verified by sequencing and subcloned into pGL3-enhancer (Promega). Complementary DNAs (cDNAs) for ERRα, β and γ were provided by Dr Christina Teng at the National Institute of Environmental Health Sciences, Dr Vincent Giguere at McGill University and Dr Uwe Borgmeyer at the University of Hamburg, respectively. Myc-tagged ERRα, ERRβ and ERRγ were generated by subcloning the original cDNA into pCMV-Tag3 (Agilent), which adds an N-terminal Myc tag. The cDNAs were also subcloned into pLenti6 (Invitrogen, Carlsbad, CA, USA) for the lentiviral expression of the three ERRs. FLAG-tagged human parkin and its mutants were generated previously (24). These parkin cDNAs were also subcloned into pSinRep5 (Invitrogen) for the generation of sindbis virus in BHK cells. We found that parkin or its mutants could not be expressed very strongly by lentivirus. Thus, we expressed them with sindbis virus. Both lentivirus and sindbis virus are very efficient in infecting neurons and cell lines that are hard to transfect (e.g. SH-SY5Y). FLAG-parc was provided by Dr Wei Gu at Columbia University. FLAG-dorfin was provided by Dr Gen Sobue at Nagoya University. The shRNA sequences of rat and human ERRα, ERRβ and ERRγ were obtained from Openbiosystems (clone ID TRCN0000026166, TRCN0000022205, TRCN0000027078, respectively). The sequence for scrambled control shRNA is GTGGACTCTTGAAAGTACTAT. Double-stranded DNA oligonucleotides of these sequences were cloned into pLKO.1 vector (Addgene) for lentivirus-mediated knockdown of endogenous ERRα, β or γ in rat midbrain neuronal cultures. The following siRNAs (Ambion) were used in luciferase reporter assays: GAGCAUCCCAGGCUUCUCAtt (human ERRα), GCUUCGGUCAUUUGGAAGAtt (human ERRβ), GGAAACUGUAUGAUGACUGtt (human ERRγ).
Cell lines, neuronal cultures and parkin KO mice
HeLa cells, HEK 293 cells, SH-SY5Y and BHK cells were purchased from ATCC (Manassas, VA, USA) and maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum, 2 mm/ml l-glutamine, 100 μg/ml penicillin and 100 μg/ml streptomycin (Invitrogen). SH-SY5Y cells stably expressing parkin or its K161N or T240R mutant were generated previously (7). Primary skin fibroblasts from two PD patients with parkin mutations and two normal subjects are described previously (35). Primary rat embryonic midbrain neuronal cultures were prepared from rat embryos at E17 (36). Cultures on cover slips were maintained in 24-well plates in neurobasal media supplemented with 2% B27 (Invitrogen) and AraC (5 µmol/l, Sigma) for 12 days before they were infected with various viruses or transfected with various plasmids. Parkin KO mice were generated by deleting exon 2 of the murine parkin gene in mouse embryonic stem cells through homologous recombination. Mice with the same deletion have been described before (37).
Luciferase reporter assay
HeLa cells, SH-SY5Y cells or neuronal cultures were co-transfected with various reporter constructs and the renilla luciferase construct using FuGENE HD (Roche). We kept the total amount of plasmids constant by using empty vector to make up any differences. Luciferase activity was quantified in a luminometer (BioTek) using the Dual-Luciferase Reporter Assay System according to the manufacturer's protocol and data analysis methods (Promega). All samples were assayed in triplicate. The raw count on firefly luciferase activity of a sample was divided by the count on renilla luciferase to normalize transfection efficiency. This ratio for a given sample was then normalized against that of the reporter construct AL (MAO-A luciferase reporter) or BL (MAO-B luciferase reporter), which was shown as 1.00 ± standard error of measurement.
Quantitative real-time RT–PCR
SH-SY5Y cells or neuronal cultures (12 DIV) were infected with various recombinant sindbis viruses and lentiviruses. Total RNA was extracted using TRIzol Reagent (Invitrogen). First-strand cDNA was synthesized with oligo (dT) or random hexamers as primers, using the SuperScript First-strand Synthesis System according to the manufacturer's protocol (Invitrogen). An equal volume mixture of the products was used as the template for quantitative real-time PCR amplification as described previously (8). The primers for human MAO-A were CTGATCGACTTGCTAAGCTAC and ATGCACTGGATGTAAAGCTTC (102 bp), and those for rat MAO-A were CTGATCGACTTGCTAAACTAC and ATGGACTGGATATAATGCTTC (137 bp). The primers for human MAO-B were GCTCTCTGGTTCCTGTGGTATGTG and TCCGCTCACTCACTTGACCAGATC (118 bp), and those for rat MAO-B were GCACTGTGGTTCCTGTGGTATGTG and TCCGCTCACTCACTTGACCAGATC (118 bp). The primers for human GAPDH were GACAACAGCCTCAAGATCATCAG and ATGGCATGGACTGTGGTCATGAG (122 bp), and those for rat GAPDH were GACAACTCCCTCAAGATTGTCAG and ATGGCATGGACTGTGGTCATGAG (122 bp). Each sample was run in triplicate.
Immunoprecipitation and western blot
Transient transfection of various constructs in HEK293 cells was performed using calcium phosphate for 48 h. Cells cultured in 10 cm dishes were washed three times in cold phosphate-buffered saline and lysed on ice-cold lysis buffer (1% Triton X-100, 10 mm Tris, pH 7.6, 50 mm NaCl, 30 mm sodium pyrophosphate, 50 mm NaF, 5 mm EDTA and 0.1 mm Na3VO4) for 20 min. Lysates were centrifuged at 16 000g at 4°C, and the supernatant fractions containing equal amounts of total proteins were immunoprecipitated with indicated antibodies. The samples were boiled in 2x SDS-loading buffer for 5 min followed by separation on SDS–PAGE and analysis by western blots with various antibodies using ECL. We used 4 mg of total proteins for immunoprecipitation in rat brain homogenates and 2 mg of total protein for immunoprecipitation in transfected cells. Generally, 4 µg of antibody was used and the incubation was overnight.
Puromycin chase assay
HEK293 cells transfected with various constructs, SH-SY5Y cells and their derivatives and primary human skin fibroblasts were treated with puromycin (100 μm) for different durations as indicated. Equal fractions (1%) of total cell lysates were western-blotted with the indicated antibodies to examine the degradation of cognizant proteins in the absence of new protein synthesis. For WT or parkin KO mice, 250 μm thick midbrain slices containing dopaminergic neurons were incubated with 100 μm puromycin in EBSS solution for different durations. Slices were homogenized in lysis buffer (1% Triton X-100, 10 mm Tris, pH 7.6, 50 mm NaCl, 30 mm sodium pyrophosphate, 50 mm NaF, 5 mm EDTA and 0.1 mm Na3VO4). Equal volumes (1% of total) of cleared supernatants were western-blotted with the indicated antibodies.
FUNDING
This work is supported by NIH grants NS061856, NYSTEM contract C024406, and NSFC grant 30928006 (J.F.).
ACKNOWLEDGEMENTS
We thank Dr Christina Teng at the National Institute of Environmental Health Sciences for ERRα cDNA, Dr Vincent Giguere at McGill University for ERRβ cDNA, Dr Uwe Borgmeyer at the University of Hamburg for ERRγ cDNA, Dr Wei Gu at Columbia University for parc cDNA and Dr Gen Sobue at Nagoya University for dorfin cDNA. We thank Dr Ted Dawson at Johns Hopkins University and Dr Flint Beal at Cornell University for critical review of this manuscript.
Conflict of Interest statement. None declared.
REFERENCES
- 1.Lang A.E., Lozano A.M. Parkinson's disease. First of two parts. N. Engl. J. Med. 1998;339:1044–1053. doi: 10.1056/NEJM199810083391506. doi:10.1056/NEJM199810083391506. [DOI] [PubMed] [Google Scholar]
- 2.Hardy J. Genetic analysis of pathways to Parkinson disease. Neuron. 2010;68:201–206. doi: 10.1016/j.neuron.2010.10.014. doi:10.1016/j.neuron.2010.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jenner P., Olanow C.W. Oxidative stress and the pathogenesis of Parkinson's disease. Neurology. 1996;47:S161–S170. doi: 10.1212/wnl.47.6_suppl_3.161s. [DOI] [PubMed] [Google Scholar]
- 4.Burke W.J. 3,4-dihydroxyphenylacetaldehyde: a potential target for neuroprotective therapy in Parkinson's disease. Curr. Drug Target CNS Neurol. Disord. 2003;2:143–148. doi: 10.2174/1568007033482913. doi:10.2174/1568007033482913. [DOI] [PubMed] [Google Scholar]
- 5.Hastings T.G., Lewis D.A., Zigmond M.J. Role of oxidation in the neurotoxic effects of intrastriatal dopamine injections. Proc. Natl Acad. Sci. USA. 1996;93:1956–1961. doi: 10.1073/pnas.93.5.1956. doi:10.1073/pnas.93.5.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luo Y.Q., Umegaki H., Wang X.T., Abe R., Roth G.S. Dopamine induces apoptosis through an oxidation-involved SAPK/JNK activation pathway. J. Biol. Chem. 1998;273:3756–3764. doi: 10.1074/jbc.273.6.3756. doi:10.1074/jbc.273.6.3756. [DOI] [PubMed] [Google Scholar]
- 7.Jiang H., Ren Y., Zhao J., Feng J. Parkin protects human dopaminergic neuroblastoma cells against dopamine-induced apoptosis. Hum. Mol. Genet. 2004;13:1745–1754. doi: 10.1093/hmg/ddh180. doi:10.1093/hmg/ddh180. [DOI] [PubMed] [Google Scholar]
- 8.Jiang H., Jiang Q., Liu W., Feng J. Parkin suppresses the expression of monoamine oxidases. J. Biol. Chem. 2006;281:8591–8599. doi: 10.1074/jbc.M510926200. doi:10.1074/jbc.M510926200. [DOI] [PubMed] [Google Scholar]
- 9.Itier J.M., Ibanez P., Mena M.A., Abbas N., Cohen-Salmon C., Bohme G.A., Laville M., Pratt J., Corti O., Pradier L., et al. Parkin gene inactivation alters behaviour and dopamine neurotransmission in the mouse. Hum. Mol. Genet. 2003;12:2277–2291. doi: 10.1093/hmg/ddg239. doi:10.1093/hmg/ddg239. [DOI] [PubMed] [Google Scholar]
- 10.Casarejos M.J., Solano R.M., Menendez J., Rodriguez-Navarro J.A., Correa C., Garcia D.Y., Mena M.A. Differential effects of l-DOPA on monoamine metabolism, cell survival and glutathione production in midbrain neuronal-enriched cultures from parkin knockout and wild-type mice. J. Neurochem. 2005;94:1005–1014. doi: 10.1111/j.1471-4159.2005.03249.x. doi:10.1111/j.1471-4159.2005.03249.x. [DOI] [PubMed] [Google Scholar]
- 11.Shih J.C., Chen K., Ridd M.J. Monoamine oxidase: from genes to behavior. Annu. Rev. Neurosci. 1999;22:197–217. doi: 10.1146/annurev.neuro.22.1.197. doi:10.1146/annurev.neuro.22.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Binda C., Newton-Vinson P., Hubalek F., Edmondson D.E., Mattevi A. Structure of human monoamine oxidase B, a drug target for the treatment of neurological disorders. Nat. Struct. Biol. 2002;9:22–26. doi: 10.1038/nsb732. doi:10.1038/nsb732. [DOI] [PubMed] [Google Scholar]
- 13.Rebrin I., Geha R.M., Chen K., Shih J.C. Effects of carboxyl-terminal truncations on the activity and solubility of human monoamine oxidase B. J. Biol. Chem. 2001;276:29499–29506. doi: 10.1074/jbc.M100431200. doi:10.1074/jbc.M100431200. [DOI] [PubMed] [Google Scholar]
- 14.Riederer P., Jellinger K. Neurochemical insights into monoamine oxidase inhibitors, with special reference to deprenyl (selegiline) Acta Neurol. Scand. Suppl. 1983;95:43–55. doi: 10.1111/j.1600-0404.1983.tb01516.x. doi:10.1111/j.1600-0404.1983.tb01516.x. [DOI] [PubMed] [Google Scholar]
- 15.Olanow C.W., Rascol O., Hauser R., Feigin P.D., Jankovic J., Lang A., Langston W., Melamed E., Poewe W., Stocchi F., et al. A double-blind, delayed-start trial of rasagiline in Parkinson's disease. N. Engl. J. Med. 2009;361:1268–1278. doi: 10.1056/NEJMoa0809335. doi:10.1056/NEJMoa0809335. [DOI] [PubMed] [Google Scholar]
- 16.Grimsby J., Toth M., Chen K., Kumazawa T., Klaidman L., Adams J.D., Karoum F., Gal J., Shih J.C. Increased stress response and beta-phenylethylamine in MAOB-deficient mice. Nat. Genet. 1997;17:206–210. doi: 10.1038/ng1097-206. doi:10.1038/ng1097-206. [DOI] [PubMed] [Google Scholar]
- 17.Langston J.W., Irwin I., Langston E.B., Forno L.S. Pargyline prevents MPTP-induced parkinsonism in primates. Science. 1984;225:1480–1482. doi: 10.1126/science.6332378. doi:10.1126/science.6332378. [DOI] [PubMed] [Google Scholar]
- 18.Javitch J.A., D'Amato R.J., Strittmatter S.M., Snyder S.H. Parkinsonism-inducing neurotoxin, N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine: uptake of the metabolite N-methyl-4-phenylpyridine by dopamine neurons explains selective toxicity. Proc. Natl Acad. Sci. USA. 1985;82:2173–2177. doi: 10.1073/pnas.82.7.2173. doi:10.1073/pnas.82.7.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Willy P.J., Murray I.R., Qian J., Busch B.B., Stevens W.C., Jr, Martin R., Mohan R., Zhou S., Ordentlich P., Wei P., et al. Regulation of PPARgamma coactivator 1alpha (PGC-1alpha) signaling by an estrogen-related receptor alpha (ERRalpha) ligand. Proc. Natl Acad. Sci. USA. 2004;101:8912–8917. doi: 10.1073/pnas.0401420101. doi:10.1073/pnas.0401420101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giguere V., Yang N., Segui P., Evans R.M. Identification of a new class of steroid hormone receptors. Nature. 1988;331:91–94. doi: 10.1038/331091a0. doi:10.1038/331091a0. [DOI] [PubMed] [Google Scholar]
- 21.Schreiber S.N., Knutti D., Brogli K., Uhlmann T., Kralli A. The transcriptional coactivator PGC-1 regulates the expression and activity of the orphan nuclear receptor estrogen-related receptor alpha (ERRalpha) J. Biol. Chem. 2003;278:9013–9018. doi: 10.1074/jbc.M212923200. doi:10.1074/jbc.M212923200. [DOI] [PubMed] [Google Scholar]
- 22.Puigserver P., Spiegelman B.M. Peroxisome proliferator-activated receptor-gamma coactivator 1 alpha (PGC-1 alpha): transcriptional coactivator and metabolic regulator. Endocr. Rev. 2003;24:78–90. doi: 10.1210/er.2002-0012. doi:10.1210/er.2002-0012. [DOI] [PubMed] [Google Scholar]
- 23.Schreiber S.N., Emter R., Hock M.B., Knutti D., Cardenas J., Podvinec M., Oakeley E.J., Kralli A. The estrogen-related receptor alpha (ERRalpha) functions in PPARgamma coactivator 1alpha (PGC-1alpha)-induced mitochondrial biogenesis. Proc. Natl Acad. Sci. USA. 2004;101:6472–6477. doi: 10.1073/pnas.0308686101. doi:10.1073/pnas.0308686101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ren Y., Zhao J.H., Feng J. Parkin binds to alpha/beta tubulin and increases their ubiquitination and degradation. J. Neurosci. 2003;23:3316–3324. doi: 10.1523/JNEUROSCI.23-08-03316.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nikolaev A.Y., Li M., Puskas N., Qin J., Gu W. Parc: a cytoplasmic anchor for p53. Cell. 2003;112:29–40. doi: 10.1016/s0092-8674(02)01255-2. doi:10.1016/S0092-8674(02)01255-2. [DOI] [PubMed] [Google Scholar]
- 26.Niwa J., Ishigaki S., Doyu M., Suzuki T., Tanaka K., Sobue G. A novel centrosomal ring-finger protein, dorfin, mediates ubiquitin ligase activity. Biochem. Biophys. Res. Commun. 2001;281:706–713. doi: 10.1006/bbrc.2001.4414. doi:10.1006/bbrc.2001.4414. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Z., Chen K., Shih J.C., Teng C.T. Estrogen-related receptors-stimulated monoamine oxidase B promoter activity is down-regulated by estrogen receptors. Mol. Endocrinol. 2006;20:1547–1561. doi: 10.1210/me.2005-0252. doi:10.1210/me.2005-0252. [DOI] [PubMed] [Google Scholar]
- 28.Lin M.T., Beal M.F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. doi:10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- 29.Greene J.C., Whitworth A.J., Kuo I., Andrews L.A., Feany M.B., Pallanck L.J. Mitochondrial pathology and apoptotic muscle degeneration in Drosophila parkin mutants. Proc. Natl Acad. Sci. USA. 2003;100:4078–4083. doi: 10.1073/pnas.0737556100. doi:10.1073/pnas.0737556100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pesah Y., Pham T., Burgess H., Middlebrooks B., Verstreken P., Zhou Y., Harding M., Bellen H., Mardon G. Drosophila parkin mutants have decreased mass and cell size and increased sensitivity to oxygen radical stress. Development. 2004;131:2183–2194. doi: 10.1242/dev.01095. doi:10.1242/dev.01095. [DOI] [PubMed] [Google Scholar]
- 31.Greene J.C., Whitworth A.J., Andrews L.A., Parker T.J., Pallanck L.J. Genetic and genomic studies of Drosophila parkin mutants implicate oxidative stress and innate immune responses in pathogenesis. Hum. Mol. Genet. 2005;14:799–811. doi: 10.1093/hmg/ddi074. doi:10.1093/hmg/ddi074. [DOI] [PubMed] [Google Scholar]
- 32.Palacino J.J., Sagi D., Goldberg M.S., Krauss S., Motz C., Wacker M., Klose J., Shen J. Mitochondrial dysfunction and oxidative damage in parkin-deficient mice. J. Biol. Chem. 2004;279:18614–18622. doi: 10.1074/jbc.M401135200. doi:10.1074/jbc.M401135200. [DOI] [PubMed] [Google Scholar]
- 33.Villena J.A., Kralli A. ERRalpha: a metabolic function for the oldest orphan. Trends Endocrinol. Metab. 2008;19:269–276. doi: 10.1016/j.tem.2008.07.005. doi:10.1016/j.tem.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Narendra D., Tanaka A., Suen D.F., Youle R.J. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J. Cell Biol. 2008;183:795–803. doi: 10.1083/jcb.200809125. doi:10.1083/jcb.200809125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ren Y., Jiang H., Yang F., Nakaso K., Feng J. Parkin protects dopaminergic neurons against microtubule-depolymerizing toxins by attenuating microtubule-associated protein kinase activation. J. Biol. Chem. 2009;284:4009–4017. doi: 10.1074/jbc.M806245200. doi:10.1074/jbc.M806245200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ren Y., Liu W., Jiang H., Jiang Q., Feng J. Selective vulnerability of dopaminergic neurons to microtubule depolymerization. J. Biol. Chem. 2005;280:34105–34112. doi: 10.1074/jbc.M503483200. doi:10.1074/jbc.M503483200. [DOI] [PubMed] [Google Scholar]
- 37.Perez F.A., Palmiter R.D. Parkin-deficient mice are not a robust model of parkinsonism. Proc. Natl Acad. Sci. USA. 2005;102:2174–2179. doi: 10.1073/pnas.0409598102. doi:10.1073/pnas.0409598102. [DOI] [PMC free article] [PubMed] [Google Scholar]