Abstract
Specific inhibitors for individual cathepsins have been developed based on their tertiary structures of X-ray crystallography. Cathepsin B-specific inhibitors, CA-074 and CA-030, and cathepsin L specific inhibitors, CLIK-148 and CLIK-195, were designed as the epoxysuccinate derivatives. Cathepsin S inhibitor, CLIK-060, and cathepsin K inhibitor, CLIK-166, were synthesized. These inhibitors can use in vitro and also in vivo, and show no toxicity for experimental animals by the amounts used as the cathepsin inhibitor.
Various cathepsins are used in the processing of antigenic proteins. The CLIK-060 treatment to the autoimmune disease, Sjögren model mice, led to strongly suppress the expression of the pathological symptoms. Cathepsins L or K participates to the degradation of bone collagen. The CLIK-148 protects osteoporosis in animals and also protects the bone metastasis of cancer cells. Cathepsin L also enhances insulin-induced glucose uptake into 3T3-L1 adipocytes, suggesting cathepsin L plays the roles in adipogenesis and glucose tolerance in type 2 diabetes.
Keywords: cathepsin, inhibitor, cystatin, autoimmune diseases, osteoporosis, Sjögren syndrome
Introduction
Cysteine cathepsins make up an essential protease family located in lysozomes of all living cells. Eleven kinds of cysteine cathepsins have been registered in the human genome since 2001.1) Each cathepsin has different cleavage bond-specificity for substrate proteins, allowing induction of different physiological or pathological conditions. In general, the inhibitors for all cathepsins have been published and used, such as E-64,2-4) leupeptin and antipain,5,6) but no research has been published about specific inhibitors for individual cathepsins. We have developed specific inhibitors for individual cathepsins in cooperation with Asao (Taiho Pharmaceutical Co.),7-10) by a structure-based new drug design method using X-ray crystallography of individual cathepsin structures (substrate binding pocket), with epoxysuccinate (E-64) as the frame compound. We have shown specific binding mechanisms between individual cathepsins and their corresponding synthesized inhibitors.8,11) Only CA-074 is now commercially available from Sigma-Aldrich Co. and is used as the cathepsin B-specific inhibitor worldwide. Cathepsin L inhibitors,7,9) CLIK-148 and CLIK-195, and cathepsin S inhibitor, CLIK-060,7) are also being used in many laboratories in the world and the effectiveness have been already published in various journals.
Contents
Part I Structure-based development of specific inhibitors for individual cathepsins.
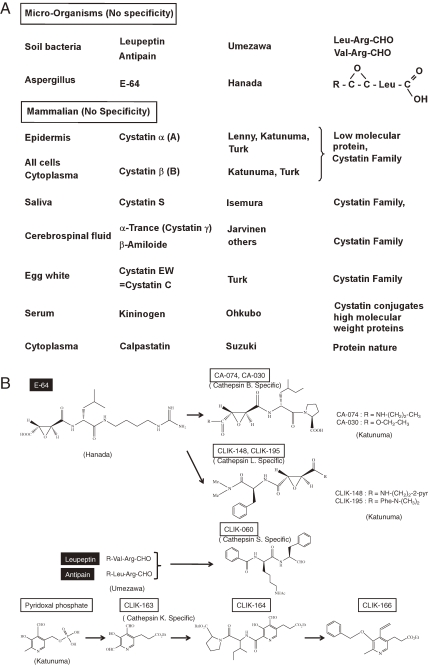
The classical cysteine protease inhibitors from micro-organisms origin and natural inhibitors located in mammalian organs, and cystatin families, were introduced in Fig. 1 .
Figure 1.
A. Development of cysteine cathepsin group specific inhibitors. a. Microorganism origin. A group of inhibitors comprise aliphatic aldehyde and dipeptide derivatives, which was discovered by Umezawa’s group. The other inhibitor comprise epoxysuccinyl amino acid derivatives, which was discovered by Hanada’s group2) from Aspergillus and named E-64. Main cysteine cathepsins and these inhibitors were discovered at the same time, between 1980 and 2000.2,3) b. Mammalian origin (cystatin family). Most cystatin families have a molecular weight of about 10,000; the high molecular weight inhibitors, kininogen group that participates in blood coagulation is characterized with a high molecular weight of the cystatin conjugates. B. Development of specific inhibitors for individual cathepsins by structure-based designs from tertiary structures of the binding pocket of cathepsins using their X-ray crystal structures, which were developed by Katunuma’s group. Cathepsin B-specific inhibitors (CA-074 and CA-030) and cathepsin L-specific inhibitors (CLIK 148 and CLIK-195) were designed E-64 as a frame. Cathepsin S-specific inhibitor (CLIK-060) was designed based on the structure of leupeptin and antipain. Cathepsin K-specific inhibitors (CLIK-163) was designed from pyridoxal phosphate.
Specific inhibitors for individual cathepsins have been developed in our group. Cathepsin B specific inhibitors, CA-074 and CA-030,12,13) and cathepsin L specific inhibitors, CLIK-148 and CLIK-195,7) were developed from the structure of epoxysuccinate derivatives as the frame compound. These derivatives were designed as individual cathepsin inhibitors, using X-ray crystallography of the tertiary structure of their substrate binding pocket.8,11,14)
As summarized in Table 1 , CA-030 and CA-074 specifically inhibit cathepsin B at 10−7 M, and CLIK-148 and CLIK-195 inhibit cathepsin L7) at 10−6 M, CLIK-060 inhibits cathepsin S7) at 10−6 M, and CLIK-164 inhibits cathepsin K9) at 10−5 M. Cathepsin K inhibitor CLIK-164 is a pyridoxal derivatives;9) and cathepsin K inhibitor CLIK-166 is an aromatic vinyl-derivative of pyridine ring. Their chemical structures are illustrated in Fig. 1B.
Table 1.
Inhibition specificities for various cathepsins
| Inhibition concentration |
Cathepsins | ||||
|---|---|---|---|---|---|
| 10−X M | B | L | S | K | |
| CA-030 | −7 | 100 | 0 | 0 | 0 |
| CA-074 | −7 | 100 | 0 | 0 | 0 |
| CLIK-148 | −6 | 0 | 100 | 30 | 0 |
| −7 | 0 | 63 | 0 | 0 | |
| CLIK-195 | −6 | 0 | 100 | 25 | 0 |
| −7 | 0 | 85 | 0 | 0 | |
| CLIK-060 | −6 | 25 | 30 | 100 | 10 |
| −7 | 0 | 0 | 86 | 0 | |
| CLIK-164 | −5 | 0 | 20 | 60 | 100 |
| −6 | 0 | 0 | 20 | 60 | |
| CLIK-166 | −4 | 0 | 0 | 17 | 100 |
| −5 | 0 | 0 | 0 | 100 | |
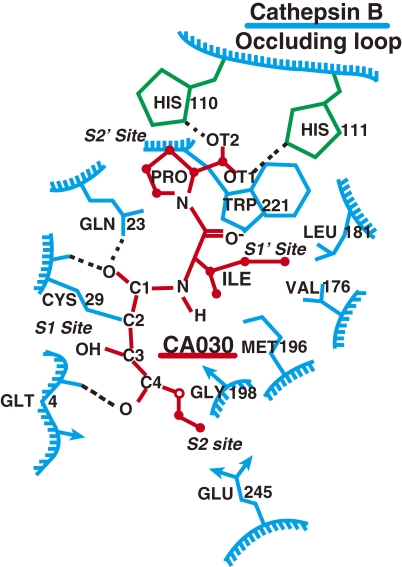
Cathepsin B-specific inhibitor, CA-030, is explained here as a typical example of structure-based inhibitor design11,14) that uses X-ray crystallograpical data of cathepsin B. The binding structure of cathepsin B with CA-030 is shown in Fig. 2 . The substrate or inhibitor is inserted into the substrate binding pocket in the vertical way. The upper part of the substrate binding pocket of cathepsin B is closed by a large occluding loop, and two moles of histidines are located side by side at the positions 110 and 111 to form an area of strong positive charge. Therefore, a proline was designed in the C-terminus of CA-074 and CA-030 to bind with the strong positive charges of these histidines in the occluding loop. When an epoxysuccinyl isoleucine is bound next to the proline, the epoxysuccinyl group of the inhibitor can easily approach Gln-23 located on the surface of the substrate binding pocket of cathepsin B to make an oxyanion hole. As a result, the active site of the Cys-29 of cathepsin B easily binds with that of epoxysuccinate; while other cathepsins do not possess such an occluding loop in the substrate binding pocket.
Figure 2.
Schematic presentation of CA-030 binding with cathepsin B. Proline on the C-terminus of CA-030 has strong affinity for histidines (2 mol) located in the occluding loop of cathepsin B. CA-030 forms an oxyanion hole with Gln-23 in the binding pocket of cathepsin B.
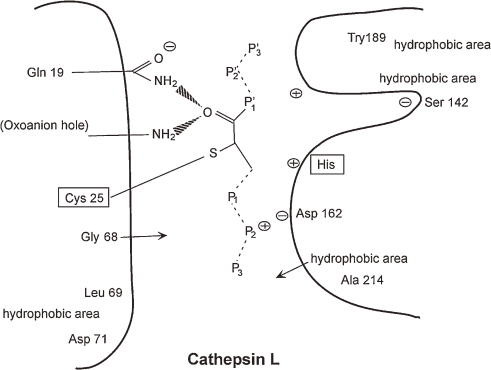
The mechanisms of specific binding of CLIK-148 with cathepsin L can be shown by X-ray co-crystallography (Fig. 3 ).8) The Try-189 is located at the end (bottom) of the substrate binding area which forms a hydrophobic area. The epoxysuccinate of CLIK-148 can easily bind with the active site of Cys-25 of cathepsin L, and the epoxysuccinate of CLIK-148 binds to Gln-19 to make an oxyanion hole. Thus, CLIK-148 binds very tightly and specifically with cathepsin L.
Figure 3.
Schematic illustration of specific binding of CLIK-148 with cathepsin L by X-ray co-crystallography.
The aromatic aldehyde attached to pyridoxal-3 propionate derivatives shows a specific affinity to cathepsin K. Cathepsin K-specific inhibitor, CLIK-164,9) an aromatic aldehyde derivatives. The cathepsin S-specific inhibitor, CLIK-060,7) have been synthesized as an aldehyde derivatives. These inhibitors show strong and specific inhibition with their individual cathepsins both in vitro and in vivo at 10−6–10−7 M (Table 1). They do not show any considerable toxicity at their inhibition concentrations used in rats and mice.
Part II Medical applications of specific inhibitors for individual cathepsins.
These specific inhibitors of individual cathepsins are useful for analyzing biological roles of cathepsins, such as the clarification of the pathogenesis of various diseases mediated by abnormal expression of specific cathepsins, and for development of powerful therapeutic drugs that originated from abnormal expression of special cathepsins.
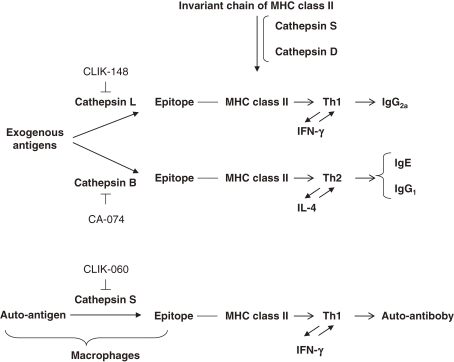
(a) Regulation of antigen processing by various cathepsins and the class switch of Th-cell responses by corresponding cathepsin inhibitors.15–19)
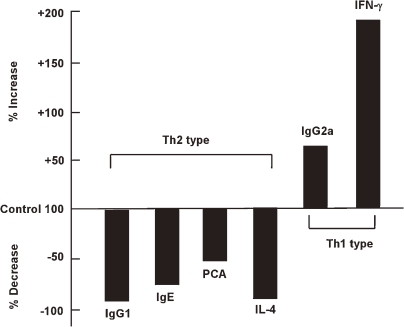
Antigen processing by cathepsins and the epitope presentation to major histocompatibility complex (MHC) class II are shown in Fig. 4 . The invariant chain binding to MHC class II molecules was removed by cathepsins S and D, and exogenous antigens are processed by various cathepsins. Therefore, different cathepsins produce different epitopes, even if processed from the same antigen, as shown in Fig. 5 . For instance, when cathepsin B participates in antigen processing to stimulate Th2 responses, IgG2a and γ-IFN production were promoted and IgE, IgG1 and IL-4 production were suppressed. Thus, classes of immune responses are switched between Th1 cells and Th2 cells by different cathepsin inhibitors, as shown in Figs. 4 and 5.
Figure 4.
Class-switch Th-cell responses of antigen processing by different cathepsins.
Figure 5.
Changes in ovalbumin dependent production of anti-bodies and cytokines by administration of cathepsin B inhibitor (CA-074). The ovalbumin dependent production of antibodies and cytokines was class-switched by the administration of CA-074. Type 2 responses were suppressed, while type 1 responses were enhanced.
(b) Autoimmune diseases.20,21)
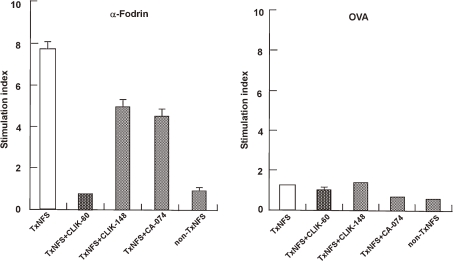
Autoimmune diseases are expressed by the presence of a special epitope derived from an auto-antigen processed by a particular cathepsin. Therefore, inhibition of autoantigen processing by a special cathepsin can inhibit auto-antibody production, suppressing the expression of the autoimmune disease. A typical autoimmune disease, Sjögren syndrome, was expressed by the processing of auto-antigen “α-fodrin” by cathepsin S, producing the responsible auto-antibody in a Sjögren mouse model. Cathepsin S inhibitor CLIK-060 specifically suppressed the expression of Sjögren syndrome (Fig. 6 ). Cell proliferation responses by α-fodrin in Sjögren model mice could be completely suppressed by cathepsin S inhibitor, but not by other cathepsin inhibitors and Sjögren syndrome could be almost completely subsided by CLIK-060 treatment (Table 2 ). No other therapy than glucocorticoid hormone administration is currently available for autoimmune diseases, but long term treatment of glucocorticoid hormone is not recommended, cathepsin S-specific inhibitor may provide a potential effective treatment.20,21)
Figure 6.
Immune response (stimulation index) of Sjögren syndrome through auto-antigen α-fodrin processing by cathepsin S and its suppression by CLIK-060. Using Sjögren model mice, the stimulation index of thymidine incorporation was determined. α-Fodrin (but not ovalbumin) specifically stimulated the index and cathepsin S inhibitor CLIK-060 specifically suppressed it.
Table 2.
Pathological symptoms of Sjögren syndrome model mice and their suppression by CLIK-060
| Pathological legion grade | ||
|---|---|---|
| Grade of legions | ||
| Lacrimal G | Submandibular G | |
| non-Tx SS | 0.3 | 0.3 |
| Tx | 3.8 | 3.0 |
| SS + CA-074 | 2.8 | 2.5 |
| SS + CLIK-148 | 3.5 | 2.8 |
| SS + CLIK-060 | 0.9 | 1.0 |
| Secretion volume of saliva and tears | ||
|---|---|---|
| Saliva | Tears | |
| non-Tx SS | 9.0 | 3.0 |
| Tx | 2.0 | 1.0 |
| SS + CLIK-060 | 7.0 | 2.9 |
Non-Tx-SS: Non-thymectomized Sjögren model mice.
Tx-SS: Thymectomized Sjögren model mice.
Secretion volume given in µl/20 min.
The morphological pathological legion in lacrimal gland and submandibular glands were prevented by cathepsin S inhibitor, CLIK-060, but not by the other inhibitors tested. Secretion volume of saliva and tears were decreased dramatically in the disease model animals, but the treatment of CLIK-060 prevented these Sjögren syndromes to the normal levels, as shown in Table 2.
(c) Protection of osteoporosis and bone metastasis of cancer.10,22–24)
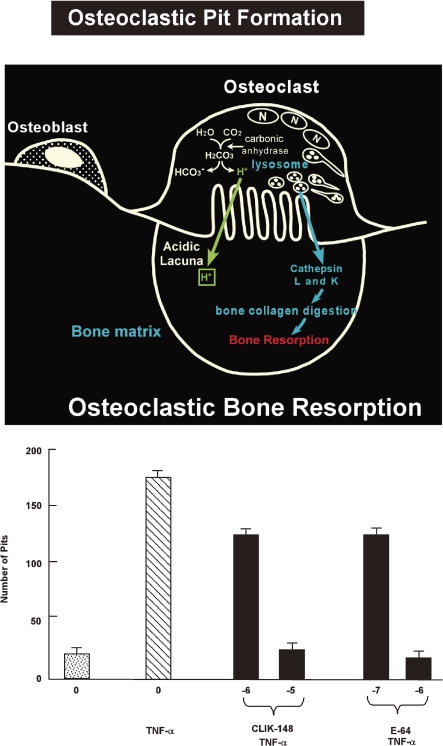
Bone and teeth mainly consist of type I collagen, which is mainly degraded by cathepsins L or K. Both cathepsins are therefore referred to as “collagenolytic cathepsins”.25) Some cancer cells secrete large amounts of collagenolytic cathepsins playing an important role in cancer metastasis and invasion in bone. Administration of inhibitor for cathepsin L or K has been shown significant protection against cancer metastasis and invasion in bone. An example of the pit formation mechanisms is shown in Fig. 7 .26)
Figure 7.
Mechanisms of osteoclastic bone resorption and pit formation by the cultured pit formation test (upper). Pit formation was stimulated by TNF-α addition and inhibited by cathepsin L inhibitor CLIK-148 addition (under).
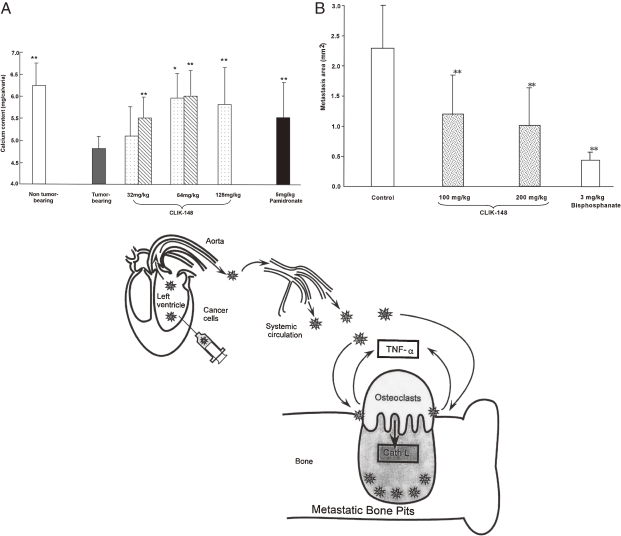
Inoculation of colon tumor 26PMF-15 cells into mouse calvaria resulted in 60–70% calcium released from the calvaria. This calcium release can be protected by CLIK-148 administration, as shown in Fig. 8A . Distant bone metastasis of melanoma cells A375, is illustrated in Fig. 8B. Upon injection into the left ventricles of rat hearts and the cancer cells were transported through the systemic circulation, the melanoma cells implant on the distant bone surface and make bone lacunue via osteoclasts. The CLIK-148 treatment protected half (50%) of these metastatic focuses formation (Fig. 8B). Bone degradation was mainly the result of osteoclasts which are located on the surface of bone, secreting large amounts of cathepsins L and K. On the contrary, parathyroid hormone can stimulate the metastasis. These cathepsins are secreted into bone acidic lacuna by the help of ATP proton pump by carbonic anhydrase. Therefore, administration of CLIK-148 and/or carbonic anhydrase inhibitors could suppress bone pit formation.
Figure 8.
Bone metastasis of cancer cells by secreted cathepsin L and its suppression by CLIK-148. A. Ca release from mouse calvaria by invasion of colon tumor-26 PMF-15 cells and its protection by CLIK-148 treatment. B. Melanoma cells (A357) were injected into the left ventricle of the heart causing distant bone metastasis via systemic circulation.
(d) Cathepsin L activity controls adipogenesis and glucose tolerance.23)
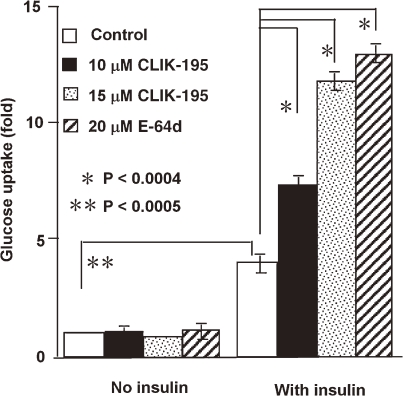
In vitro studies demonstrate the role of cathepsin L in the degradation of the matrix protein fibronectin, insulin receptor (IR) and insulin-like growth factor-1 receptor (IGF-IR), essential molecules for adipogenesis and glucose metabolism. Cathepsin L inhibition leads to the reduction of human and murine pre-adipocyte adipogenesis or lipid accumulation, protection of fibronectin from degradation, accumulation of IRβ and IGF-IRβ subunits, and an increase in glucose uptake (Fig. 9 ).23) Cathepsin L-deficient mice are lean and have reduced levels of serum glucose and insulin, but increased levels of muscle IRβ subunit, fibronectin and glucose transporter (Glut-4). Inhibition of cathepsin L by administration of CLIK-148 also demonstrated reduced body weight gain and serum insulin levels, and increased glucose tolerance due to increased levels of muscle IRβ subunit, fibronectin, and Glut-4 in both diet-induced obese mice and ob/ob mice. Increased levels of cathepsin L in obese and diabetic patients suggest that cathepsin L is a novel target for these metabolic disorders.
Figure 9.
Cathepsin L inhibition with CLIK-195 enhanced insulin-induced glucose uptake into 3T3-L1 adipocytes.23) Data represent glucose uptake (cpm) fold-increases relative to the cpm counts for cells treated with no insulin and no inhibitors.
Perspective and anticipation in the future (Discussion)
-
(1)
These chemically synthesized inhibitors show no particular toxicity in experimental animals, although long term toxicity needs further study. The effect on humans is also a major topic of future studies.
-
(2)
Inhibition specificities of some individual cathepsin inhibitors need to be improved. CA-074 and CA-030 (cathepsin B inhibitors) show absolute specificity at 10−7 M, while CLIK-148 and CLIK-195 (cathepsin L inhibitors) show high specificity at 10−7 M. However, CLIK-060 (cathepsin S inhibitor) show 86% inhibition at 10−7 M, while CLIK-060 is unstable. CLIK-164 and CLIK-166 (cathepsin K inhibitors) requires a concentration of 10−5 M. Cathepsin S inhibitor and cathepsin K inhibitor need further improvement in sensitivity and stability.
-
(3)
Immunity or suppression of IgE allergy expression needs to be reinforced. Cathepsin B inhibitor (CA-074 or CA-030) administration can be used for suppression of IgE allergy.
-
(4)
No effective therapy other than glucocorticoid hormone administration is available for autoimmune diseases at present in general. Incidence mechanisms have been clarified for autoimmune diseases, i.e., Sjögren syndrome. Therefore, cathepsin S inhibitors (CLIK-060) should be used in a clinical setting. This principal can use for other autoimmune diseases in general.
-
(5)
Cathepsin inhibitors have been synthesized by Taiho Pharmaceutical Co. in cooperation with Katunuma. Only CA-074, as a cathepsin B-specific inhibitor, is now commercially available from Sigma-Aldrich Co. Other cathepsin inhibitors (CLIKs) should be synthesized as commercially available products.
Profile
Nobuhiko Katunuma, male, was born in Nagasaki city, Japan, 1926. He graduated from School of Medicine, Nagoya University in 1953 and received the Ph.D. in Medical Sciences in 1958. He was appointed as an Associate Professor of Institute for Protein Research in Osaka University in Japan, 1959–1963. In 1963, He was appointed as a Professor of School of Medicine, Tokushima University. He was appointed as Dean of School of Medicine from 1980 to 1982, Tokushima University. He was appointed as a Professor of Tokushima Bunri University, Director of Institute for Health Sciences in 1992.
He is nominated to an Honorary Member of Japanese Society of Biochemistry and Molecular Biology, an Honorary Member of Japanese Society of Vitamin and BioFactors, an Honorary Member of American Society of Biochemistry and Molecular Biology, an Honorary Member of American Society of Cancer Research.
He awarded the National Violet Ribbon Decoration for excellent scientists and artists from Japanese Emperor in 1991.
References
- 1).Turk V., Turk B., Turk D. (2001) Lysosomal cysteine proteases facts and opportunities. EMBO J. 20, 4629–4633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2).Hanada K., Tamai M., Yamagishi M., Ohmura S., Sawada J., Tanaka I. (1978) Isolation and characterization of E-64, a new thiol protease Inhibitor. Agric. Biol. Chem. Tokyo 42, 523–528 [Google Scholar]
- 3).Hashida S., Towatari T., Kominami E., Katunuma N. (1980) Inhibition by E-64 derivatives of rat liver cathepsin B and cathepsin L in vitro and in vivo. J. Biochem. 88, 1805–1811 [DOI] [PubMed] [Google Scholar]
- 4).Hara K., Kominami E., Ii K., Katunuma N. (1988) Effect of proteinase inhibitors on intracellular processing of cathepsin B, H and L in rat macrophages. FEBS Lett. 231, 229–231 [DOI] [PubMed] [Google Scholar]
- 5).Hori M., Hemmi H., Suzukake K., Hayashi H., Uehara Y., Takeuti T., Umezawa H. (1978) Biosynthesis of leupeptin. J. Antibiot. (Tokyo) 31, 95–98 [DOI] [PubMed] [Google Scholar]
- 6).Umezawa H., Aoyagi T., Morishita T., Kunimoto S., Matsuzaki M., Hamada M., Takeuchi T. (1970) Chymostatin, a new chymotrypsin inhibitor produced by actinomycetes. J. Antibiot. (Tokyo) 23, 425–427 [DOI] [PubMed] [Google Scholar]
- 7).Katunuma N., Murata E., Kakegawa H., Matsui A., Tsuzuki H., Tsuge H., Turk D., Turk V., Fukushima M., Tada Y., Asao T. (1999) Structure based development of novel specific inhibitors for cathepsin L and cathepsin S in vitro and in vivo. FEBS Lett. 458, 6–10 [DOI] [PubMed] [Google Scholar]
- 8).Tsuge H., Nishimura T., Tada Y., Asao T., Turk D., Turk V., Katunuma N. (1996) Inhibition mechanism of cathepsin L-specific inhibitors based on the crystal structure of papain-CLIK148 complex. Biochem. Biophys. Res. Commun. 266, 411–416 [DOI] [PubMed] [Google Scholar]
- 9).Katunuma N., Matsui A., Inubushi T., Murata E., Kakegawa H., Ohba Y., Turk D., Turk V., Tada Y., Asao T. (2000) Structure-based development of pyridoxal propionate derivatives as specific inhibitors of cathepsin K in vitro and in vivo. Biochem. Biophys. Res. Commun. 267, 850–854 [DOI] [PubMed] [Google Scholar]
- 10).Katunuma N., Tsuge H., Nukatsuka M, Asao T., Fukushima M. (2002) Structure-based design of specific cathepsin inhibitors and their application to protection of bone metastases of cancer cells. Arch. Biochem. Biophys. 397 (2), 305– 311 [DOI] [PubMed] [Google Scholar]
- 11).Turk D., Podobnik M., Popovic T., Katunuma N., Bode W., Huber R., Turk V. (1995) Crystal structure of cathepsin B inhibited with CA030 at 2.0-Å resolution: A basis for the design of specific epoxysuccinyl inhibitors. Biochemistry 34, 4791–4797 [DOI] [PubMed] [Google Scholar]
- 12).Murata M., Miyashita S., Yokoo C., Tamai M., Hanada K., Katunuma N. (1991) Novel epoxysuccinyl peptides. Selective inhibitors of cathepsin B, in vitro. FEBS Lett. 280, 307–310 [DOI] [PubMed] [Google Scholar]
- 13).Katunuma N., Kominami E. (1995) Structure, properties, mechanisms, and assays of cysteine protease inhibitors: cystatins and E-64 derivatives. Methods Enzymol. 251, 382–397 [DOI] [PubMed] [Google Scholar]
- 14).Musil D., Zucic D., Turk D., Engh R.A., Mayr I., Huber R., Popovic T., Turk V., Towatari T., Katunuma N., Bode W. (1991) The refined 2.15 Å X-ray crystal structure of human liver cathepsin B: the structural basis for its specificity. EMBO J. 10, 2321–2330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15).Katunuma N., Matsui A., Endo K., Hanba J., Sato A., Nakano M., Yuto Y., Tada Y., Asao T., Himeno K., Maekawa Y., Inubushi T. (2000) Inhibition of intracellular cathepsin activities and suppression of immune responses mediated by helper T lymphocyte type-2 by peroral or intraperitoneal administration of vitamin B6. Biochem. Biophys. Res. Commun. 271, 151–155 [DOI] [PubMed] [Google Scholar]
- 16).Maekawa Y., Himeno K., Ishikawa H., Hisaeda H., Sakai T., Dainichi T., Asao T., Good R.A., Katunuma N. (1998) Switch of CD4+ T cell differentiation from Th2 to Th1 by treatment with cathepsin B inhibitor in experimental leishmaniasis. J. Immunol. 161, 2120–2127 [PubMed] [Google Scholar]
- 17).Katunuma, N. (1997) New aspects on antigen presentation mechanism for immune-responses and allergy expression. Medical Aspects of Proteases and Protease Inhibitors. IOS Press, Amsterdam, pp. 153–172. [Google Scholar]
- 18).Kitamura H., Kamon H., Sawa S., Park S.J., Katunuma N., Ishihara K., Murakami M., Hirano T. (2005) IL-6-STAT3 controls intracellular MHC class II αβ dimer level through cathepsin S activity in dendritic cells. Immunity 23, 491–502 [DOI] [PubMed] [Google Scholar]
- 19).Zhang T., Maekawa Y., Yasutomo K., Ishikawa H., Fawzy Nashed B., Dainichi T., Hisaeda H., Sakai T., Kasai M., Mizuochi T., Asao T., Katunuma N., Himeno K. (2000) Pepstatin-A sensitive aspartic proteases in lysosome are involved in degradation of the invariant chain and antigen-processing in antigen presenting cells of mice infected with Leishmania major. Biochem. Biophys. Res. Commun. 276, 693–701 [DOI] [PubMed] [Google Scholar]
- 20).Saegusa K., Ishimura N., Yanagi K., Arakaki R., Ogawa K., Saito I., Katunuma N., Hayashi Y. (2002) Cathepsin S inhibitor prevents autoantigen presentation and autoimmunity. J. Clin. Invest. 110 (3), 361–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21).Ishimaru N., Arakaki R., Katunuma N., Hayashi Y. (2004) Critical role of cathepsin inhibitors for autoantigen processing and autoimmunity. Adv. Enzyme Regul. 44, 309–320 [DOI] [PubMed] [Google Scholar]
- 22).Katunuma N. (1997) Molecular mechanisms of bone collagen degradation in bone resorption. J. Bone Miner. Metab. 15, 1–8 [Google Scholar]
- 23).Yang M., Zhang Y., Pan J., Sun J., Liu J., Libby P., Sukhova G.K., Doria A., Katunuma N., Peroni O.D., Guerre-Millo M., Kahn B.B., Clement K., Shi G.P. (2007) Cathepsin L activity controls adipogenesis and glucose tolerance. Nat. Cell Biol. 9, 970–977 [DOI] [PubMed] [Google Scholar]
- 24).Delaissé J.M., Eeckhout Y., Vaes G. (1984) In vivo and in vitro evidence for the involvement of cysteine proteinases in bone resorption. Biochem. Biophys. Res. Commun. 125 (2), 441–447 [DOI] [PubMed] [Google Scholar]
- 25).Kirschke H., Kembhavi A.A., Bohley P., Barrett A.J. (1982) Action of rat liver cathepsin L on collagen and other substrates. Biochem. J. 201 (2), 367–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26).Katunuma, N. (1992) Intracellular Protein Degradation Mechanisms. Tokyo Kagaku Dojin, Tokyo. [Google Scholar]