Abstract
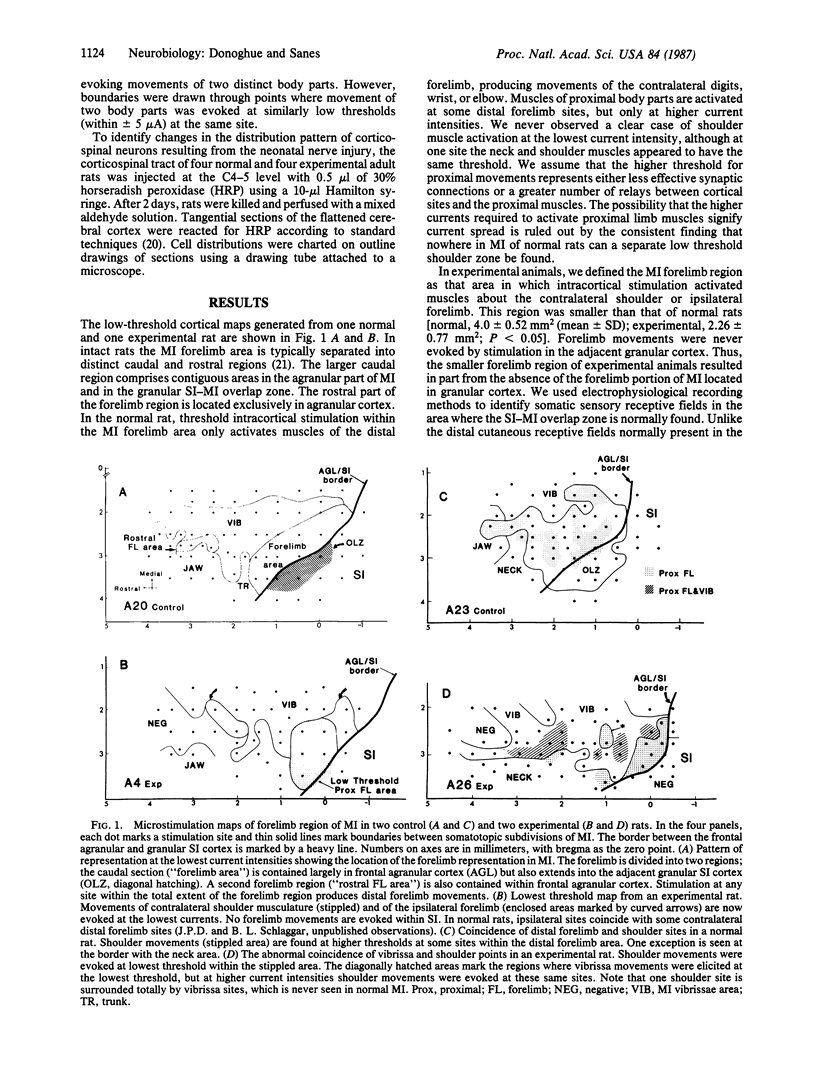
We investigated the effect of neonatal nerve lesions on cerebral motor cortex organization by comparing the cortical motor representation of normal adult rats with adult rats that had one forelimb removed on the day of birth. Mapping of cerebral neocortex with electrical stimulation revealed an altered relationship between the motor cortex and the remaining muscles. Whereas distal forelimb movements are normally elicited at the lowest threshold in the motor cortex forelimb area, the same stimuli activated shoulder and trunk muscles in experimental animals. In addition, an expanded cortical representation of intact body parts was present and there was an absence of a distinct portion of motor cortex. These data demonstrate that representation patterns in motor cortex can be altered by peripheral nerve injury during development.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cabana T., Martin G. F. Corticospinal development in the North-American opossum: evidence for a sequence in the growth of cortical axons in the spinal cord and for transient projections. Brain Res. 1985 Nov;355(1):69–80. doi: 10.1016/0165-3806(85)90007-0. [DOI] [PubMed] [Google Scholar]
- Devor M., Wall P. D. Effect of peripheral nerve injury on receptive fields of cells in the cat spinal cord. J Comp Neurol. 1981 Jun 20;199(2):277–291. doi: 10.1002/cne.901990209. [DOI] [PubMed] [Google Scholar]
- Devor M., Wall P. D. Reorganisation of spinal cord sensory map after peripheral nerve injury. Nature. 1978 Nov 2;276(5683):75–76. doi: 10.1038/276075a0. [DOI] [PubMed] [Google Scholar]
- Donoghue J. P. Contrasting properties of neurons in two parts of the primary motor cortex of the awake rat. Brain Res. 1985 Apr 29;333(1):173–177. doi: 10.1016/0006-8993(85)90141-6. [DOI] [PubMed] [Google Scholar]
- Donoghue J. P., Kerman K. L., Ebner F. F. Evidence for two organizational plans within the somatic sensory-motor cortex of the rat. J Comp Neurol. 1979 Feb 1;183(3):647–663. doi: 10.1002/cne.901830312. [DOI] [PubMed] [Google Scholar]
- Donoghue J. P., Parham C. Afferent connections of the lateral agranular field of the rat motor cortex. J Comp Neurol. 1983 Jul 10;217(4):390–404. doi: 10.1002/cne.902170404. [DOI] [PubMed] [Google Scholar]
- Donoghue J. P., Wise S. P. The motor cortex of the rat: cytoarchitecture and microstimulation mapping. J Comp Neurol. 1982 Nov 20;212(1):76–88. doi: 10.1002/cne.902120106. [DOI] [PubMed] [Google Scholar]
- Easter S. S., Jr, Purves D., Rakic P., Spitzer N. C. The changing view of neural specificity. Science. 1985 Nov 1;230(4725):507–511. doi: 10.1126/science.4048944. [DOI] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N., LeVay S. Plasticity of ocular dominance columns in monkey striate cortex. Philos Trans R Soc Lond B Biol Sci. 1977 Apr 26;278(961):377–409. doi: 10.1098/rstb.1977.0050. [DOI] [PubMed] [Google Scholar]
- Humphrey D. R., Reed D. J. Separate cortical systems for control of joint movement and joint stiffness: reciprocal activation and coactivation of antagonist muscles. Adv Neurol. 1983;39:347–372. [PubMed] [Google Scholar]
- Innocenti G. M., Frost D. O. Effects of visual experience on the maturation of the efferent system to the corpus callosum. Nature. 1979 Jul 19;280(5719):231–234. doi: 10.1038/280231a0. [DOI] [PubMed] [Google Scholar]
- Innocenti G. M., Frost D. O. The postnatal development of visual callosal connections in the absence of visual experience or of the eyes. Exp Brain Res. 1980;39(4):365–375. doi: 10.1007/BF00239301. [DOI] [PubMed] [Google Scholar]
- Ivy G. O., Killackey H. P. Ontogenetic changes in the projections of neocortical neurons. J Neurosci. 1982 Jun;2(6):735–743. doi: 10.1523/JNEUROSCI.02-06-00735.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivy G. O., Killackey H. P. The ontogeny of the distribution of callosal projection neurons in the rat parietal cortex. J Comp Neurol. 1981 Jan 20;195(3):367–389. doi: 10.1002/cne.901950302. [DOI] [PubMed] [Google Scholar]
- Kaas J. H., Merzenich M. M., Killackey H. P. The reorganization of somatosensory cortex following peripheral nerve damage in adult and developing mammals. Annu Rev Neurosci. 1983;6:325–356. doi: 10.1146/annurev.ne.06.030183.001545. [DOI] [PubMed] [Google Scholar]
- Merzenich M. M., Kaas J. H., Wall J. T., Sur M., Nelson R. J., Felleman D. J. Progression of change following median nerve section in the cortical representation of the hand in areas 3b and 1 in adult owl and squirrel monkeys. Neuroscience. 1983 Nov;10(3):639–665. doi: 10.1016/0306-4522(83)90208-7. [DOI] [PubMed] [Google Scholar]
- Merzenich M. M., Kaas J. H., Wall J., Nelson R. J., Sur M., Felleman D. Topographic reorganization of somatosensory cortical areas 3b and 1 in adult monkeys following restricted deafferentation. Neuroscience. 1983 Jan;8(1):33–55. doi: 10.1016/0306-4522(83)90024-6. [DOI] [PubMed] [Google Scholar]
- Neafsey E. J., Sievert C. A second forelimb motor area exists in rat frontal cortex. Brain Res. 1982 Jan 28;232(1):151–156. doi: 10.1016/0006-8993(82)90617-5. [DOI] [PubMed] [Google Scholar]
- O'Leary D. D., Stanfield B. B., Cowan W. M. Evidence that the early postnatal restriction of the cells of origin of the callosal projection is due to the elimination of axonal collaterals rather than to the death of neurons. Brain Res. 1981 Jul;227(4):607–617. doi: 10.1016/0165-3806(81)90012-2. [DOI] [PubMed] [Google Scholar]
- Sanderson K. J., Welker W., Shambes G. M. Reevaluation of motor cortex and of sensorimotor overlap in cerebral cortex of albino rats. Brain Res. 1984 Feb 6;292(2):251–260. doi: 10.1016/0006-8993(84)90761-3. [DOI] [PubMed] [Google Scholar]
- Shatz C. J., Stryker M. P. Ocular dominance in layer IV of the cat's visual cortex and the effects of monocular deprivation. J Physiol. 1978 Aug;281:267–283. doi: 10.1113/jphysiol.1978.sp012421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sica R. E., Sanz O. P., Cohen L. G., Freyre J. D., Panizza M. Changes in the N1-P1 component of the somatosensory cortical evoked response in patients with partial limb amputation. Electromyogr Clin Neurophysiol. 1984 Jun-Jul;24(5):415–427. [PubMed] [Google Scholar]
- Stoney S. D., Jr, Thompson W. D., Asanuma H. Excitation of pyramidal tract cells by intracortical microstimulation: effective extent of stimulating current. J Neurophysiol. 1968 Sep;31(5):659–669. doi: 10.1152/jn.1968.31.5.659. [DOI] [PubMed] [Google Scholar]
- Wiesel T. N., Hubel D. H. Comparison of the effects of unilateral and bilateral eye closure on cortical unit responses in kittens. J Neurophysiol. 1965 Nov;28(6):1029–1040. doi: 10.1152/jn.1965.28.6.1029. [DOI] [PubMed] [Google Scholar]