Abstract
Although elevation of shear stress increases production of vascular reactive oxygen species (ROS), the role of ROS in chronic flow overload (CFO) has not been well investigated. We hypothesize that CFO increases ROS production mediated in part by NADPH oxidase, which leads to endothelial dysfunction. In six swine, CFO in carotid arteries was induced by contralateral ligation for 1 wk. In an additional group, six swine received apocynin (NADPH oxidase blocker and anti-oxidant) treatment in conjunction with CFO for 1 wk. The blood flow in carotid arteries increased from 189.2 ± 25.3 ml/min (control) to 369.6 ± 61.9 ml/min (CFO), and the arterial diameter increased by 8.6%. The expressions of endothelial nitric oxide synthase (eNOS), p22/p47phox, and NOX2/NOX4 were upregulated. ROS production increased threefold in response to CFO. The endothelium-dependent vasorelaxation was compromised in the CFO group. Treatment with apocynin significantly reduced ROS production in the vessel wall, preserved endothelial function, and inhibited expressions of p22/p47phox and NOX2/NOX4. Although the process of CFO remodeling to restore the wall shear stress has been thought of as a physiological response, the present data implicate NADPH oxidase-produced ROS and eNOS uncoupling in endothelial dysfunction at 1 wk of CFO.
Keywords: reactive oxygen species, NADPH oxidase, chronic flow overload, endothelial dysfunction
flow overload (FO) occurs when the blood flow rate in blood vessels is elevated over its basal physiological level. FO may occur physiologically during exercise or pregnancy and pathophysiologically in arteriovenous fistula, contralateral stenosis, or cardiac hypertrophy (1, 6, 8, 15, 19, 48). Since flow-induced shear stress on the endothelium regulates vascular function and dictates structural homeostasis of the blood vessel wall, the blood flow rate is closely regulated. FO in an artery elicits an acute endothelium-derived vasodilatation mediated by endothelial nitric oxide (NO), prostacyclin (PGI2), endothelium-derived hyperpolarizing factor (EDHF) (3, 9, 37, 42), and subsequent vascular remodeling (8, 18, 29, 48). In addition to NO, reactive oxygen species (ROS) have been reported in FO. For instance, flow-mediated superoxide (O2−) generation was observed in arteries of normal conduit artery of rabbits (22), FO carotid arteries of mice (6), and coronary resistance arteries of humans (23).
ROS are involved in physiological and pathological processes. Recently, ROS (particularly O2−) have been recognized as signaling molecules to mediate specific cellular responses in the vasculature, including activating matrix metalloproteinases (MMPs), vascular remodeling, vascular smooth muscle cell (VSMC) hypertrophy, and cellular apoptosis (6, 28, 34, 43, 47, 51,). Furthermore, O2− production may lead to hydrogen peroxide (H2O2) formation, which is thought to regulate potassium channels (23). In the vasculature, there may be several sources of O2− such as NADPH oxidase, xanthine oxidase, mitochondria, and endothelial NO synthase (eNOS) when uncoupled (30).
The increase in ROS generation in hypertension, diabetes, hypercholesterolemia, atherosclerosis, and ischemia is considered deleterious to the vasculature (2, 5, 11, 12, 20, 25, 27, 33, 44). NADPH oxidase has been identified as a major source of ROS in blood vessels in response to chemical (e.g., hypercholesterolemia and diabetes) or physical (e.g., hypertension and oscillatory shear stress) stimuli (5, 7, 17, 20, 28, 44). The increase in ROS production from NADPH oxidase, as occurs in hypertension, atherosclerosis, and diabetes, attenuates endothelium-dependent vasodilatation and causes endothelial dysfunction (5, 20, 32, 41, 49, 53).
Although the increase in ROS production in CFO has been established in both conduit artery and various small arteries (6, 22, 23, 47) and endothelial dysfunction in CFO has also been found in mesenteric artery of obese rats (4), the effect of CFO on endothelial function of elastic (carotid) artery of large animal is unknown. Hence, we hypothesize that 1) the ROS increase compromises endothelial function in CFO and 2) the source of ROS is, in part, due to NADPH oxidase. To test these hypotheses, an intact porcine carotid artery was exposed to CFO for 1 wk by a contralateral ligation. ROS was detected with the spin trap N-tert-butyl-α-phenylnitrone (PBN) by electron paramagnetic resonance (EPR) spectroscopy. The production of ROS was also assayed with ethidium fluorescence analysis and luminol-enhanced chemiluminescence. The expressions of eNOS and NADPH oxidase were measured. Endothelial function was examined through endothelium-dependent relaxation in response to acetylcholine (ACh). Uncoupled eNOS in CFO was found with acute administration of eNOS cofactor tetrahydrobiopterin (BH4). In one additional group of swine, the animals with CFO carotid arteries were orally fed apocynin (4-hydroxy-3-methoxy-acetophenone) to determine the effect of NADPH oxidase in the vessel wall. The results support our hypotheses and demonstrate that CFO causes endothelial dysfunction in conduit vessels.
MATERIALS AND METHODS
Twelve male Duroc swine weighing 34 ± 4 kg (range 30–39 kg) were randomly divided into two groups. In group I, the right carotid artery was exposed to CFO for 1 wk by ligation of the contralateral carotid artery. In group II, the right carotid artery was exposed to CFO for 1 wk while animals were orally fed apocynin at the dose of 60 mg·kg−1·day−1 from the postoperative first day to the termination (CFO+A). The left carotid artery served as a control for each group and was harvested at the time of ligation. All experiments were performed in accordance with national and local ethical guidelines, including the Institute of Laboratory Animal Research (ILAR) Guide, Public Health Service policies, Animal Welfare Act, and an approved Indiana University School of Medicine IACUC protocol.
Animal Preparation
Surgical anesthesia was induced with ketamine (20 mg/kg im) and atropine (0.04 mg/kg im) and maintained with isofluorane (1–2%). Blood gas values were measured, and ventilation was adjusted to maintain normal values of Po2 and Pco2. In preliminary experiments, we measured the flow rates and external diameters to confirm that the left and right common carotid arteries are equivalent to ensure that one can serve as control for the other. Furthermore, the flow rates and external diameter of the right carotid artery were immediately measured after the left carotid artery was excised to quantify the immediate FO.
Subsequent to a left cervicotomy, the left common carotid artery was exposed gently to avoid vasomotion by dissection, and the in vivo external diameter was measured with the aid of a stereo microscope. The vessel was further dissected to place a flow probe (TS420 Transonic System). After data collection, the artery was ligated by suture and excised (length of ∼3 cm); the right carotid artery was not exposed to any surgical trauma. This measurement was taken on day 0 to avoid intervention on the experimental vessel. The incision was closed, and the animal was recovered. The animals in group II were fed apocynin (60 mg·kg−1·day−1) orally mixed in food. After 1 wk and following a right cervicotomy, the in vivo external diameter and flow rate of right carotid artery were measured (data points for week 1), and the vessel segment was excised. The excised vessels from control and experimental groups were immediately stored in 4°C HEPES physiological salt solution (HEPES-PSS, pH 7.4, mM: 142 NaCl, 4.7 KCl, 2.7 sodium HEPES, 3 HEPES acid, 1.17 MgSO4, 2.79 CaCl, 5.5 glucose) and divided into segments for various measurements after dissection of adjacent tissue. HEPES and HEPES salt were purchased from Sigma, whereas other chemicals were purchased from Fisher Scientific.
Vasoactivity
An isovolumic myograph, which retains the physiological loading of a pressure myograph with sensitivity of a wire myograph, was used to evaluate the vasoactivity of the artery (24). Briefly, the carotid vessel was cannulated on the connectors, which were fixed in a bath containing HEPES-PSS. The vessel was incubated in the bath at 37°C for 40 min. The vessel was stretched to in situ length with the aid of a digital caliper (resolution of 0.1 mm) and preloaded at a physiological pressure of 80 mmHg. Contraction or relaxation was pharmacologically induced with both ends closed. The contraction or relaxation of the vessel wall induces an increase or decrease in pressure, respectively. The diameter of the carotid artery remained approximately constant during vasoreactivity. The pressure and the external diameter were measured with a pressure transducer (Mikro-Tip SPR-524, Millar Instruments) and with a dimensional tracer (DiamTrak 3+, Australia; 10-μm resolution), respectively.
All arterial segments were precontracted (increase in pressure) to an approximate transluminal pressure (170 ± 20 mmHg) with phenylephrine (10−8 to 10−6 M), and thereafter endothelium-dependent relaxation (decrease in pressure) was assessed with a series of doses of acetylcholine (Ach, 10−9 to 10−6 M). The effect of eNOS uncoupling on endothelium-dependent relaxation was detected by incubation of the vessel segments in CFO with l-arginine (10−5 M) and eNOS cofactor BH4 (10−5 M) for 40 min. The circumferential tension of the vessel at every dose was calculated by Laplace's law, tension = pressure × radius. The % decrease in tension was calculated by the equation: %Tension = (Td − Ti)/(Tmax − Ti) × 100. where, Td, Ti, and Tmax are the tension at every dose (Td), physiological level (Ti), and maximum tension (Tmax) at submaximal concentration of phenylephrine, respectively. Non-receptor-dependent contraction to potassium (KCl, 60 mM) was used to verify identical contractility of VSMC in CFO. Endothelium-independent vasorelaxation to sodium nitroprusside (SNP, 10−5 M) was measured to verify the sensitivity of VSMC in response to NO.
ROS Detection
We detected ROS with paramagnetic resonance spectroscopy, ethidium fluorescence assay, and luminol derivative (L-012) enhanced chemiluminescence analysis as described below.
Electron paramagnetic resonance spectroscopy.
Immediately after the vessel was divided, the vessel ring for electron paramagnetic resonance (EPR) was videotaped from the side (5 mm in length) and cross-views under stereo microscope. The volume of the segment was calculated based on the product of cross-sectional area and axial length. After measurement by EPR as described below, ROS generation was expressed as mole per unit of volume.
ROS concentration in tissue samples was determined from the EPR spectra obtained by incubating the tissue samples with the spin trapping agent N-tert-butyl-α-phenylnitrone (PBN; Sigma) at 190 mM in HEPES-PSS for 30 min at 37°C in the dark. A ring incubated with 4-Hydroxy-TEMPO (a superoxide dismutase mimic) served as the control for ROS measurement. The tissue was subsequently inserted into a syringe along with the supernatant, immediately frozen in liquid nitrogen, and stored at −80°C until EPR analysis was performed. To avoid ROS produced during freezing and thawing of samples, the sample was quickly removed while in its frozen state from the syringe and placed in a Dewar containing liquid nitrogen. The Dewar was then inserted into the microwave cavity of the EPR spectrometer. The sample remained at liquid nitrogen temperature throughout the EPR analysis (1, 21).
The EPR equipment and settings were as follows. A Bruker ESP X-band spectrometer equipped with a TE102 cavity was utilized to detect signals. Parameters for the spectra were 9.4-GHz microwave frequency, 25.2-mW microwave power, 4.0-G modulation amplitude, 1×105 receiver gain, 5.24-s time constant, 3,330-G center magnetic field, and 100-G magnetic field sweep width. All experiments were completed at liquid nitrogen temperature.
Four EPR scans were taken per tissue sample and analyzed with Bruker WINEPR software (version 2.11) based on the spectral intensity and line width. ROS concentrations were determined with 2,2,6,6-tetramethylpiperidine 1-oxyl, TEMPO, solution (0.1 μM, Sigma) used as a concentration standard. All EPR parameters and conditions were applied to both standard and experimental samples.
Chemiluminescence.
A highly sensitive chemiluminescence probe, luminol derivative L-012 (Wako Chemicals) was used to detect ROS in the vessel tissue. Three arterial segments, 2–3 mm in length, were incubated in 96-well plates with HEPES-PSS at 37°C for 1 h. NADPH (0.3 M) and L-012 (500 μM) were administered in wells (36). Some of the segments were incubated with 1 mM oxyopurinol (a xanthine oxidase inhibitor), 50 μM rotenone (a mitochondria inhibitor), or 1 μM l-NAME (Nω-nitro-l-arginine methyl ester; an eNOS inhibitor) to verify the involvement of xanthine oxidase, mitochondria, and eNOS. Light emission was detected by ultra-sensitive photon counter (Wallac EnVision, 2104 Multilabel Reader, PerkinElmer). Counts were obtained at 1-min intervals for 40 min. ROS levels were reported as relative light units after subtracting background luminescence and were normalized to dry tissue weight.
Ethidium fluorescent assay.
A 5-mm ring was cut from the segment of carotid artery and incubated with nitrogen-bubbled HEPES-PSS (to remove possible ROS) containing dihydroethidium (DHE, Sigma) at optimal concentration (determined in pilot experiments) of 0.2 μM for 30 min at 37°C in the dark (47). Another ring that served as a control for ROS measurement was first incubated with 4-hydroxy-TEMPO (Tempol, Sigma), an O2− scavenger, for 30 min at 37°C, and then with DHE for 30 min at 37°C. An additional ring without DHE incubation was used as the control for autofluorescence of the vessel tissue. A 4′-6-diamidino-2-phenylindole (DAPI) staining was performed to determine nuclear area, which was a reference for analysis of ethidium fluorescence. After being washed five times with saline, the rings were sliced at 20-μm-thick transverse sections by a cryostat (Leica CM 1850). Confocal microscopy (LSM 510 META, Zeiss) was used to visualize the fluorescence (excitation/emission: 518/605 nm, 63 × oil objective). A gray scale analysis was carried out to determine the fluorescence area fraction. The autofluorescence of vessel tissue was subtracted to remove the background.
Protein Expression
The segment for Western blotting was homogenized in a lysis buffer and then incubated on ice for 1 h. The sample was centrifuged at 1,000 g for 15 min at 1°C, and the supernatant was drawn off. The total value of protein was measured by a BCA kit (Bio-Rad). Equal amounts of protein (25 μg) were loaded, electrophoresed in 10% SDS-PAGE gel, and transferred onto a ployvinylidene difluoride membrane. After blocking the sample for 2 h in 8% dried milk in TBS-Tween buffer, the membrane was incubated overnight at 4°C with specific primary antibody with either anti-eNOS (1:1,000 dilution in blocking buffer, BD transduction laboratory), anti-p22phox (1:1,000, Santa Cruz Biotech) anti-p47phox (1:500, Santa Cruz Biotech), anti-NOX2 (1:250, Santa Cruz Biotech), or anti-NOX4 (1:250, Santa Cruz Biotech). The membrane was then rinsed and incubated with horseradish peroxidase-conjugated secondary antibody (Santa Cruz Biotech) for 2 h (eNOS: goat anti-mouse 1:3,000 dilution in blocking buffer; p22phox and p47phox: goat anti-rabbit 1:5,000, NOX2 and NOX4: bovine anti-goat 1:5,000). The specific protein was detected by enhanced chemiluminescence (ECL; Amersham) and evaluated by densitometry (Sigma Scan). All samples from each group were simultaneously probed with anti-β-actin, a mouse monoclonal antibody (primary antibody 1:1,000 dilution in blocking buffer, Santa Cruz Biotech), to correct for sample loading.
Statistical Analysis
All data given in the text and figures are expressed as means ± SD. Student's t-test (two-tailed distribution, two-sample unequal variance) and Duncan's test following ANOVA were used to detect differences between groups. For all analyses, P < 0.05 was used to indicate statistical significance.
RESULTS
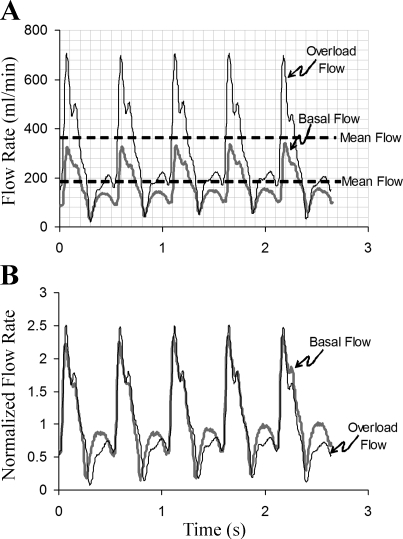
The blood flow in carotid arteries was significantly increased in CFO and CFO+A after contralateral ligation (189.2 ± 25.3, 369.6 ± 61.9, and 382.3 ± 62.5 ml/min in the control, CFO, and CFO+A groups, respectively; P < 0.001). The flow rate in the apocynin treatment group was not significantly different than the untreated group. Figure 1 represents a typical tracing of a control and a carotid artery exposed to CFO. The mean and peak of the blood flow doubled in CFO (Fig. 1A). After normalization of pulsatile blood flow with mean flow rate, the two curves with respect to their mean values largely overlapped (Fig. 1B), which suggests that the oscillatory components of pulsatile blood flow were not significantly changed in the CFO model.
Fig. 1.
A typical waveform of blood flow in the carotid artery at baseline and flow overload (CFO). A: the real time recordings. B: the normalized tracing by the mean flow rate. The normalized flow tracing at chronic flow overload (CFO) approximately overlaps the normalized baseline.
The outer diameter of the vessel significantly increased by 8.6% (4.60 ± 0.41 to 4.98 ± 0.46 mm) after 1 wk of exposure to CFO (P < 0.05) and did not change significantly after treatment with apocynin (4.98 ± 0.46 to 5.06 ± 0.48 mm). We did not observe a significant change of arterial wall thickness in either the CFO or the CFO+A group compared with the control group (data not shown). The systemic blood pressure measured at the femoral artery did not change after either surgical ligation of the carotid artery or after treatment with apocynin compared with the pressure before ligation (88 ± 12 vs. 86 ± 11 mmHg). The wall shear stress (WSS; WSS ∼ QD−3, where Q and D represent flow rate and inner diameter, respectively) was found to remain elevated by ∼50% after 1 wk of the CFO and CFO+A groups.
Endothelial Function
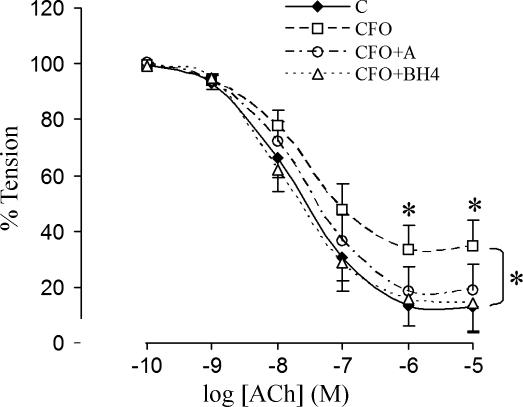
The endothelial function was quantified by the % decrease in tension in response to ACh (Fig. 2). The dose curves indicated that endothelial function was compromised in the CFO group (P < 0.05) but was preserved with apocynin treatment. This result implied that endothelial dysfunction in CFO was related to ROS upregulation. Furthermore, we found that the endothelial dysfunction of the vessels in CFO were completely reserved after acute incubation with BH4 and l-arginine, which suggests that eNOS uncoupling may play a role in CFO. The endothelium-independent vasorelaxation in response to SNP did not show differences in groups, which implied that the VSMC did not develop resistance to nitric oxide (103.5 ± 15.5, 102.6 ± 17.2, and 103.6 ± 21.1% for control, CFO, and CFO+A, respectively). The potassium-induced contraction, which is not receptor-dependent, did not reveal differences of VSMC contractility (tension) in the three groups (49.6 ± 6.98, 51.3 ± 7.66, and 51.1 ± 7.96 mN/mm for control, CFO, and CFO+A, respectively).
Fig. 2.
Endothelium-dependent vasorelaxation of carotid arteries. The arteries were precontracted to approximate tension with phenylephrine (PE), and dose-responsive vasorelaxation was induced with acetylcholine (ACh). C, control group; CFO, chronic flow overload group; CFO+A, chronic flow overload group treated with apocynin; CFO+BH4, vessel segment in CFO acute incubation with tetrahydrobiopterin (BH4). *Significant difference between groups (P < 0.05; ANOVA followed by Duncan's test for multiple groups).
ROS Generation
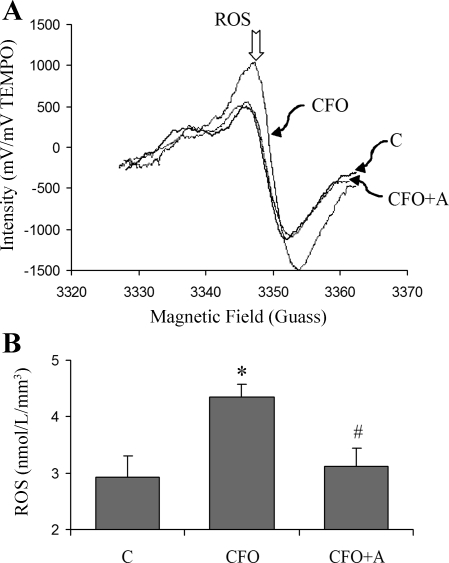
The EPR measurements are presented in Fig. 3. ROS generation significantly increased in the CFO group. Apocynin treatment restored ROS generation to the control value. Since PBN is lipophilic and forms stable lipid-derived, spin adducts (radical bound to spin trap), it provides reliable overall concentration measurements of ROS in biological tissue under physiological conditions without lipid radical adduct extraction that may involve artifacts such as failure to retrieve all of the adducts from the biological sample and additional ROS generation during centrifugation. Since PBN is indeed able to trap NO in vascular tissue, we verified that NO was not significantly produced in our experimental preparation with DAF-2DA (NO probe) and l-NAME (an eNOS inhibitor).
Fig. 3.
A: representative samples of the EPR spectra. The concentration of ROS is proportional to the signal intensity. B: ROS concentration by EPR spectroscopy using the PBN as a spin trap in HEPE-PSS. *Significant difference compared with control (P < 0.05). #Significant difference compared with CFO (P < 0.05).
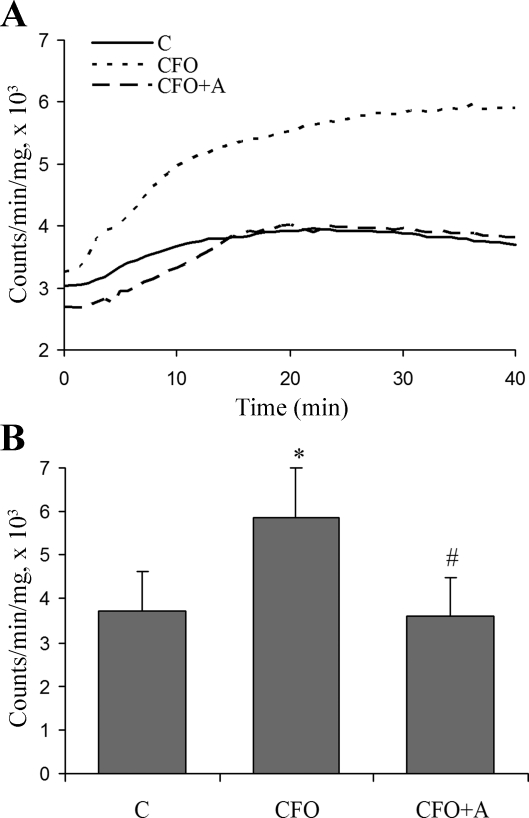
Luminol derivative L-012 is highly sensitive at physiological range of pH (7.5) and reacts with various types of ROS generated from various biological tissues. L-012 enhanced chemiluminescence increased in the vessel tissue in CFO after addition of NADPH (Fig. 4). A 1-wk treatment of apocynin decreased the level of ROS. The inhibitors of xanthine oxidase, mitochondria, and eNOS did not cause any change of chemiluminescence (data not shown), which indicates that those ROS sources were not involved in ROS production in CFO.
Fig. 4.
ROS production determined by chemiluminescence analysis in arterial segments. A luminol derivative L-012 was used to enhance chemiluminescence by ROS in arterial segments. A: representative samples of L-012 enhanced-chemiluminescence. B: ROS production measured by chemiluminescence analysis. *Significant difference compared with control (P < 0.05). #Significant difference compared with CFO (P < 0.05).
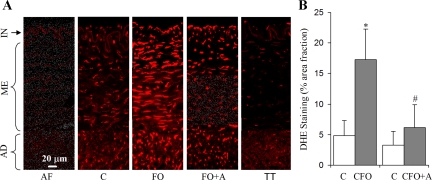
Confocal microscopic images of ethidium fluorescence showed that ROS increased in all three wall layers in CFO group (Fig. 5A). The fraction area of fluorescent assay in the carotid vessels exposed to CFO was more than three times higher than that of control segments (Fig. 5B). With the treatment of apocynin, the production of ROS was significantly lower than that in the CFO group (Fig. 5A). When the tissue from either the control or the CFO group was first incubated with Tempol, the DHE assay was almost completely eliminated by Tempol (Fig. 5A).
Fig. 5.
The fluorescent detection of ROS by dihydroethidium (DHE). A: photographs from the confocal microscopy, where the bright red spots indicate ROS production. The autofluorescence from ECM (AF) of tissue was evaluated by visualizing the tissue without DHE incubation (blank DHE) using the confocal microscopy. To mimimize autofluorescence, the parameters of confocal microscopy were maintained the same as the autofluorescent data but at lower laser power. There were significant spots indicating basal production of ROS in all three layers (intima, media, and adventitia). Treated with apocynin, the spots were smaller and less intense than those of CFO but still larger and brighter than those of control (C). TT, vessel segment treated with Tempol (Tempol is a cell-permeable SOD and catalase mimetic). With pre-incubation with Tempol, the confocal microscopic spots were drastically decreased in the tissue segment. AD, adventitial layer; ME, medial layer; IN, intimal layer. B: the fractional area of fluorescent assay of the arterial sections based on the measurement of the confocal microscopic images. *Significant difference compared with control (P < 0.05). #Significant difference compared with CFO (P < 0.05).
Expression of eNOS and NADPH Oxidase
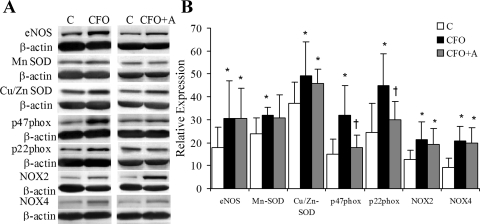
The expression of eNOS was significantly upregulated in the CFO group and unaffected by apocynin treatment (Fig. 6). The subunits p22phox and p47phox are well known to be expressed ubiquitously in endothelial and VSMC. The protein expression of cytosolic assembling subunits of NADPH oxidase (p22phox and p47phox) were significantly elevated in carotid segments exposed to CFO but not in CFO+A (Fig. 6). NOX2 and NOX4 (NADPH oxidase 2 and 4) were found to be upregulated in CFO and CFO+A.
Fig. 6.
The proteins expression evaluated with Western blot. A: Western blotting bands. The molecular weight was confirmed as eNOS, 120; p22phox, 22; p47phox, 47; NOX2, 91; and NOX4: 55. B: ratios of total pixels of the bands were measured by use of imaging software. *Significant difference in comparison between control and CFO or CFO+A (P < 0.05). †Significant difference in comparison between CFO and CFO+A (P < 0.05).
DISCUSSION
The major findings are that chronic shear stress elevation of ∼50% increases ROS production mediated by NADPH oxidase and induces endothelial dysfunction in swine carotid artery. Recent observations suggest that NADPH oxidase is directly involved in O2− production in mouse carotid arteries where the flow was increased by a factor of three to five by construction of an arteriovenous fistula (6). The present study demonstrates a similar finding in conduit artery of the porcine model in response to a more modest increase in WSS. Moreover, we show for the first time endothelial dysfunction as a consequence of increased oxidative stress in conduit artery where eNOS uncoupling may play a role during CFO.
The porcine carotid artery was exposed to approximately twice the physiological flow rate for 1 wk in this study. Although the change of flow rate was relatively small compared with a typical arteriovenous fistula (over fivefold increase in flow rate) (18), the objective was to study a model with minimal disturbance since surgery elicits inflammatory response and consequent oxidative stress. The diameter enlargement of the artery was accordingly small (8.6%). Such a change in flow and diameter in a conduit artery may occur under physiological conditions, such as in exercise (15, 50). Interestingly, ROS generation increased by onefold in response to the relatively small changes in flow or WSS (56% increase) as shown in Fig. 3. Although mechanical stimulations, including oscillatory WSS and cyclic stretch, have been shown to increase ROS production in the vasculature (7, 14, 17, 28), this is the first report of increased ROS generation in response to CFO in a conduit vessel of a large animal.
The diameter increase during CFO is attributed to a physiological response to restore the homeostatic shear stress on the endothelium. It is well known that acute vasodilatation is stimulated by WSS elevation (16). Following acute vasodilatation, chronic remodeling occurs and further increases the diameter (6, 8). Interestingly, the complete restoration of WSS requires a relatively long period in an arteriovenous fistula; e.g., over 4 wk in carotid arteries of rabbits (47), over 3 wk in the carotid arteries of mice (6), and 6 mo in the carotid arteries of dogs (18). In the present study, the WSS was not restored and remained elevated after 1 wk. In fact, a conduit artery possesses the capacity to acutely increase its diameter up to 15% in response to a 10- to 20-fold increase in flow (8, 40). Even in the 1-wk study, the diameter may enlarge by 15–20% in response to a three- to fivefold increase in flow rate (6, 18). Hence, acute diameter enlargement in response to CFO depends on the magnitude of WSS and the subsequent remodeling duration since the restoration of WSS may be very long. Since vasodilatation increases vascular stretch, which can activate NADPH oxidase in blood vessel wall (31), the stretch may play a role in CFO. The elevated stretch results from diameter increase (flow-induced vasodilatation) and vascular wall softening due to NO-induced vascular tone reduction. Both effects increase the magnitude of stretch at pulsatile blood pressure.
The expansive remodeling of the blood vessel wall in response to an increase in flow rate has been previously viewed as a physiological adaptation to restore the WSS (18, 52). Here, we show that the remodeling response leads to compromised endothelial function (over a 1-wk period). The endothelium-dependent vasodilatation in the present study (Fig. 2) is significantly decreased in response to a modest flow increase in a relatively short period. In resistance arteries, Pourageaud et al. observed that CFO increased the endothelium-dependent vasodilatation to ACh, and flow-induced vasodilatation was slightly (35) but not statistically attenuated. In diabetic rats, CFO results in significant endothelial dysfunction in mesenteric arteries (4). The mechanism involved in the different endothelial responses in large conduit arteries and small resistance arteries to CFO is unclear. It may be that resistance arteries are exposed to more significant variations of blood flow under physiological conditions due to lateral-contralateral adjustment of microcirculation and adapt to larger variation of blood flow compared with large conduit arteries.
NADPH oxidase (NOX) has been recognized as a major source of O2− in the vasculature in response to mechanical stimulation (17, 28). It is known that p22phox is an important trans-membrane protein that combines with NOX to assemble NADPH oxidase complex and is known to be upregulated by WSS (39). The p47phox, as cytosolic subunit of NADPH oxidase, may also affect the activity of NOX. NOX4 is a p22phox-dependent enzyme (2) and does not require cytosolic proteins (p47phox, p67phox) for its activity (10, 26, 38). Apocynin is an inhibitor of NADPH oxidase under in vivo condition where H2O2 and myeloperoxidase is present and is suggested to inhibit the translocation of cytoplasmic subunits (45). It is also possible that treatment of apocynin in this study shifted the balance of oxidative stress in vascular tissue through nonspecific antioxidant effects (13, 36). Since the expression of NOX isoforms may be located in endothelial and VSMC, we cannot separate the effect of WSS on endothelium and circumferential stretch acting throughout the vessel wall on NOX isoforms. The elucidation of the role of NOX in endothelial and VSMC during CFO requires further study.
Ethidium fluorescence visualized by confocal microscopy indicates that ROS increases not only in endothelium but also in the media and in the adventitia. This is interesting because WSS acts directly on the luminal surface of endothelium; i.e., WSS is not likely to directly stimulate the media and the adventitia. A fundamental question involves the mechanism by which elevated WSS sensed by the endothelium transmits its effect on more remote regions of the wall. A possible mechanism may be similar to hypertension via the NADPH oxidase pathway (44, 45, 49). The common factor in CFO and hypertension is the increase in circumferential stretch and stress. Hypertension increases circumferential stress and strain by an increase in blood pressure, whereas flow-induced vasodilatation increases vessel stretch similarly through an increase in diameter. The circumferential stress or strain, mediated by mechanotransduction in CFO, may activate NADPH oxidase and elicit ROS generation.
In summary, ROS production increases in porcine carotid arteries in response to a onefold increase in the blood flow rate, which leads to endothelial dysfunction. NOX2 and NOX4 oxidase and p22phox and p47phox are upregulated in CFO, and NADPH oxidase is likely involved in the increase in oxidative stress. The chronic use of apocynin prevents the elevation of ROS levels, even though NOX2 and NOX4 are upregulated, and preserves endothelial function. The mechanisms by which apocynin prevents the upregulation of p22phox and p47phox but not NOX2 and 4 remain unclear. Although the process of CFO-induced remodeling to restore WSS has previously been thought of as a physiological response, the present data suggest that CFO mediated by ROS causes endothelial dysfunction, which may result from eNOS uncoupling in the first week of outward vascular remodeling.
GRANTS
This research was supported in part by National Heart, Lung, and Blood Institute Grants R01 HL055554-11 and R01-HL-084529.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
The authors acknowledge Hans Mooij, Charles Dang, and Carlos Linares for excellent technical assistance.
REFERENCES
- 1. Bailey DM, Davies B, Young IS, Jackson MJ, Davison GW, Isaacson R, Richardson RS. EPR spectroscopic detection of free radical outflow from an isolated muscle bed in exercising humans. J Appl Physiol 94: 1714–1718, 2003 [DOI] [PubMed] [Google Scholar]
- 2. Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 87: 245–313, 2007 [DOI] [PubMed] [Google Scholar]
- 3. Brandes RP, Schmitz-Winnenthal FH, Feletou M, Godecke A, Huang Vanhoutte PM PL, Fleming I, Busse R. An endothelium-derived hyperpolarizing factor distinct from NO and prostacyclin is a major endothelium-dependent vasodilator in resistance vessels of wild-type and endothelial NO synthase knockout mice. Proc Natl Acad Sci USA 97: 9747–9752, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bouvet C, Belin de Chantemèle E, Guihot AL, Vessières E, Bocquet A, Dumont O, Jardel A, Loufrani L, Moreau P, Henrion D. Flow-induced remodeling in resistance arteries from obese Zucker rats is associated with endothelial dysfunction. Hypertension 50: 248–254, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res 87: 840–844, 2000 [DOI] [PubMed] [Google Scholar]
- 6. Castier Y, Brandes RP, Leseche G, Tedgui A, Lehoux S. p47phox-Dependent NADPH oxidase regulates flow-induced vascular remodeling. Circ Res 97: 533–540, 2005 [DOI] [PubMed] [Google Scholar]
- 7. De Keulenaer GW, Chappell DC, Ishizaka N, Nerem RM, Alexander RW, Griendling KK. Oscillatory and steady laminar shear stress differentially affect human endothelial redox state. Circ Res 82: 1094–1101, 1998 [DOI] [PubMed] [Google Scholar]
- 8. Di Stefano I, Koopmans DR, Langille BL. Modulation of arterial growth of the rabbit carotid artery associated with experimental elevation of blood flow. J Vasc Res 35: 1–7, 1998 [DOI] [PubMed] [Google Scholar]
- 9. Garland CJ, Plane F, Kemp Cocks TM BK. Endothelium-dependent hyperpolarization: a role in the control of vascular tone. Trends Pharmacol Sci 16: 23–30, 1995 [DOI] [PubMed] [Google Scholar]
- 10. Geiszt M, Kopp JB, Varnai P, Leto TL. Identification of renox, an NAD(P)H oxidase in kidney. Proc Natl Acad Sci USA 97: 8010–8014, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Guzik TJ, Mussa S, Gastaldi D, Sadowski J, Ratnatunga C, Pillai R, Channon KM. Mechanisms of increased vascular superoxide production in human diabetes mellitus: role of NAD(P)H oxidase and endothelial nitric oxide synthase. Circulation 105: 1656–1662, 2002 [DOI] [PubMed] [Google Scholar]
- 12. Harrison D, Griendling KK, Landmesser U, Hornig B, Drexler H. Role of oxidative stress in atherosclerosis. Am J Cardiol 91: 7A–11A, 2003 [DOI] [PubMed] [Google Scholar]
- 13. Heumüller S, Wind S, Barbosa-Sicard E, Schmidt HH, Busse R, Schröder K, Brandes RP. Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension 51: 211–217, 2008 [DOI] [PubMed] [Google Scholar]
- 14. Howard AB, Alexander RW, Nerem RM, Griendling KK, Taylor WR. Cyclic strain induces an oxidative stress in endothelial cells. Am J Physiol Cell Physiol 272: C421–C427, 1997 [DOI] [PubMed] [Google Scholar]
- 15. Huang SY, Tawney KW, Bender PR, Groves BM, McCullough RG, McCullough RE, Micco AJ, Macno-Johnson A, Cymerman A, Greene Reeves JT ER. Internal carotid arterial flow velocity with exercise before and after acclimatization to 4,300 m. J Appl Physiol 71: 1469–1476, 1991 [DOI] [PubMed] [Google Scholar]
- 16. Hull SS, Kaiser L, Jaffe MD, Sparks HV. Endothelium-dependent flow-induced dilation of canine femoral and saphenous arteries. Blood Vessels 23: 183–198, 1986 [DOI] [PubMed] [Google Scholar]
- 17. Hwang J, Saha A, Boo YC, Sorescu GP, McNally JS, Holland SM, Dikalov S, Giddens DP, Griendling KK, Harrison DG, Jo H. Oscillatory shear stress stimulates endothelial production of O2− from p47phox-dependent NAD(P)H oxidases, leading to monocyte adhesion. J Biol Chem 278: 47291–47298, 2003 [DOI] [PubMed] [Google Scholar]
- 18. Kamiya A, Togawa T. Adaptive regulation of wall shear stress to flow change in the canine carotid artery. Am J Physiol Heart Circ Physiol 239: H14–H21, 1980 [DOI] [PubMed] [Google Scholar]
- 19. Kassab GS, Imoto K, White FC, Rider CA, Fung YC, Bloor CM. Coronary arterial tree remodeling in right ventricular hypertrophy. Am J Physiol Heart Circ Physiol 265: H366–H375, 1993 [DOI] [PubMed] [Google Scholar]
- 20. Griendling KK, Sorescu D, Ushio-Fukai M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ Res 86: 494–501, 2000 [DOI] [PubMed] [Google Scholar]
- 21. Kozlov AV, Szalay L, Umar F, Fink B, Kropik K, Nhl H, Redl H, Bahrami S. EPR analysis reveals three tissues responding to endotoxin by increased formation of reactive oxygen and nitrogen species. Free Radic Biol Med 34: 1555–1562, 2003 [DOI] [PubMed] [Google Scholar]
- 22. Laurindo FR, Pedro Mde A, Barbeiro HV, Pileggi F, Carvalho MH, Augusto O, da Luz PL. Vascular free radical release. Ex vivo and in vivo evidence for a flow-dependent endothelial mechanism. Circ Res 74: 700–709, 1994 [DOI] [PubMed] [Google Scholar]
- 23. Liu Y, Zhao H, Li H, Kalyanaraman B, Nicolosi AC, Gutterman DD. Mitochondrial sources of H2O2 generation play a key role in flow-mediated dilation in human coronary resistance arteries. Circ Res 93: 573–580, 2003 [DOI] [PubMed] [Google Scholar]
- 24. Lu X, Kassab GS. Vasoactivity of blood vessels using a novel isovolumic myograph. Ann Biomed Eng 35: 356–366, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Manevich Y, Al-Mehdi A, Muzykantov V, Fisher AB. Oxidative burst and NO generation as initial response to ischemia in flow-adapted endothelial cells. Am J Physiol Heart Circ Physiol 280: H2126–H2135, 2001 [DOI] [PubMed] [Google Scholar]
- 26. Martyn KD, Frederick LM, von Loehneysen K, Dinauer MC, Knaus UG. Functional analysis of Nox4 reveals unique characteristics compared to other NADPH oxidases. Cell Signal 28: 28, 2005 [DOI] [PubMed] [Google Scholar]
- 27. Matsumoto T, Miyamori K, Kobayashi T, Kamata K. Apocynin normalizes hyperreactivity to phenylephrine in mesenteric arteries from cholesterol-fed mice by improving endothelium-derived hyperpolarizing factor response. Free Radic Biol Med 41: 1289–1303, 2006 [DOI] [PubMed] [Google Scholar]
- 28. McNally JS, Davis ME, Giddens DP, Saha A, Hwang J, Dikalov S, Jo H, Harrison DG. Role of xanthine oxidoreductase and NAD(P)H oxidase in endothelial superoxide production in response to oscillatory shear stress. Am J Physiol Heart Circ Physiol 285: H2290–H2297, 2003 [DOI] [PubMed] [Google Scholar]
- 29. Miller VM, Burnett JCJ. Modulation of NO and endothelin by chronic increases in blood flow in canine femoral arteries. Am J Physiol Heart Circ Physiol 263: H103–H108, 1992 [DOI] [PubMed] [Google Scholar]
- 30. Mueller CF, Laude K, McNally JS, Harrison DG. ATVB in focus: redox mechanisms in blood vessels. Arterioscler Thromb Vasc Biol 25: 274–278, 2005 [DOI] [PubMed] [Google Scholar]
- 31. Oeckler RA, Kaminski PM, Wolin MS. Stretch enhances contraction of bovine coronary arteries via an NAD(P)H oxidase-mediated activation of the extracellular signal-regulated kinase mitogen-activated protein kinase cascade. Circ Res 92: 23–31, 2003 [DOI] [PubMed] [Google Scholar]
- 32. Oelze M, Warnholtz A, Faulhaber J, Wenzel P, Kleschyov AL, Coldewey M, Hink U, Pongs O, Fleming I, Wassmann S, Meinertz T, Ehmke H, Daiber A, Munzel T. NADPH oxidase accounts for enhanced superoxide production and impaired endothelium-dependent smooth muscle relaxation in BKbeta1−/− mice. Arterioscler Thromb Vasc Biol 26:1753–1759, 2006 [DOI] [PubMed] [Google Scholar]
- 33. Ohara Y, Peterson TE, Harrison DG. Hypercholesterolemia increases endothelial superoxide anion production. J Clin Invest 91: 2546–2551, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pagano PJ, Ito Y, Tornheim K, Gallop PM, Tauber AI, Cohen RA. An NADPH oxidase superoxide-generating system in the rabbit aorta. Am J Physiol Heart Circ Physiol 268: H2274–H2280, 1995 [DOI] [PubMed] [Google Scholar]
- 35. Pourageaud F, De Mey JG. Vasomotor responses in chronically hyperperfused and hypoperfused rat mesenteric arteries. Am J Physiol Heart Circ Physiol 274: H1301–H1307, 1998 [DOI] [PubMed] [Google Scholar]
- 36. Schlüter T, Steinbach AC, Steffen A, Rettig R, Grisk O. Apocynin-induced vasodilation involves Rho kinase inhibition but not NADPH oxidase inhibition. Cardiovasc Res 80: 271–279, 2008 [DOI] [PubMed] [Google Scholar]
- 37. Scotland RS, Madhani M, Chauhan S, Moncada S, Andresen J, Nilsson H, Hobbs AJ, Ahluwalia A. Investigation of vascular responses in endothelial nitric oxide synthase/cyclooxygenase-1 double-knockout mice: key role for endothelium-derived hyperpolarizing factor in the regulation of blood pressure in vivo. Circulation 111: 796–803, 2005 [DOI] [PubMed] [Google Scholar]
- 38. Shiose A, Kuroda J, Tsuruya K, Hirai M, Hirakata H, Naito S, Hattori M, Sakaki Y, Sumimoto H. A novel superoxide-producing NAD(P)H oxidase in kidney. J Biol Chem 276: 1417–1423, 2001 [DOI] [PubMed] [Google Scholar]
- 39. Silacci P, Desgeorges A, Mazzolai L, Chambaz C, Hayoz D. Flow pulsatility is a critical determinant of oxidative stress in endothelial cells. Hypertension 38: 1162–1166, 2001 [DOI] [PubMed] [Google Scholar]
- 40. Smiesko V, Kozik J, Dolezel S. Role of endothelium in the control of arterial diameter by blood flow. Blood Vessels 22: 247–251, 1985 [PubMed] [Google Scholar]
- 41. Sonta T, Inoguchi T, Tsubouchi H, Sekiguchi N, Kobayashi K, Matsumoto S, Utsumi H, Nawata H. Evidence for contribution of vascular NAD(P)H oxidase to increased oxidative stress in animal models of diabetes and obesity. Free Radic Biol Med 37: 115–123, 2004 [DOI] [PubMed] [Google Scholar]
- 42. Sun D, Huang A, Smith CJ, Stackpole CJ, Connetta JA, Shesely EG, Koller A, Kaley G. Enhanced release of prostaglandins contributes to flow-induced arteriolar dilation in eNOS knockout mice. Circ Res 85: 288–293, 1999 [DOI] [PubMed] [Google Scholar]
- 43. Taniyama Y, Griendling KK. Reactive oxygen species in the vasculature: molecular and cellular mechanisms. Hypertension 426: 1075–1081, 2003 [DOI] [PubMed] [Google Scholar]
- 44. Fukui T, Ishizaka N, Rajagopalan S, Laursen JB, Capers Q, Taylor WR, Harrison DG, de Leon H, Wilcox JN, Griendling KK. p22phox mRNA expression and NADPH oxidase activity are increased in aortas from hypertensive rats. Circ Res 80: 45–51, 1997 [DOI] [PubMed] [Google Scholar]
- 45. Touyz RM, Schiffrin EL. Reactive oxygen species in vascular biology: implications in hypertension. Histochem Cell Biol 122: 339–352, 2004 [DOI] [PubMed] [Google Scholar]
- 46. Touyz RM. Apocynin, NADPH oxidase, and vascular cells: a complex matter. Hypertension 51: 172–174, 2008 [DOI] [PubMed] [Google Scholar]
- 47. Tronc F, Mallat Z, Lehoux S, Wassef M, Esposito B, Tedgui A. Role of matrix metalloproteinases in blood flow-induced arterial enlargement. Arterioscler Thromb Vasc Biol 20: e120–e126, 2000 [DOI] [PubMed] [Google Scholar]
- 48. Tuttle JL, Nachreiner RD, Bhuller AS, Condict KW, Connors BA, Herring BP, Dalsing MC, Unthank JL. Shear level influences resistance artery remodeling: wall dimensions, cell density, and eNOS expression. Am J Physiol Heart Circ Physiol 281: H1380–H1389, 2001 [DOI] [PubMed] [Google Scholar]
- 49. Ungvari Z, Csiszar A, Huang A, Kaminski PM, Wolin MS, Koller A. High pressure induces superoxide production in isolated arteries via protein kinase C-dependent activation of NAD(P)H oxidase. Circulation 108: 1253–1258, 2003 [DOI] [PubMed] [Google Scholar]
- 50. Wang J, Wolin MS, Hintze TH. Chronic exercise enhances endothelium-mediated dilation of epicardial coronary artery in conscious dogs. Circ Res 73: 829–838, 1993 [DOI] [PubMed] [Google Scholar]
- 51. Zafari AM, Ushio-Fukai M, Akers M, Yin Q, Shah A, Harrison DG, Taylor WR, Griendling KK. Novel role of NADH/NADPH oxidase derived hydrogen peroxide in angiotensin II-induced hypertrophy of rat vascular smooth muscle cells. Hypertension 32: 488–495, 1998 [DOI] [PubMed] [Google Scholar]
- 52. Zarins CK, Zatina MA, Giddens DP, Ku DN, Glagov S. Shear stress regulation of artery lumen diameter in experimental atherogenosis. J Vasc Surg 5: 413–420, 1987 [PubMed] [Google Scholar]
- 53. Zhu J, Mori T, Huang T, Lombard JH. Effect of high-salt diet on NO release and superoxide production in rat aorta. Am J Physiol Heart Circ Physiol 286: H575–H583, 2004 [DOI] [PubMed] [Google Scholar]