Abstract
Aging is associated with insulin resistance and decreased insulin-stimulated glucose uptake into skeletal muscle. Although the mechanisms underlying age-related insulin resistance are not clearly defined, impaired defense against inflammation and tissue oxidative stress are likely causes. Heat shock proteins (HSPs) have been shown to protect tissue from oxidative stress and inhibit the activation of stress kinases such as JNK, known to interfere with the insulin signaling pathway. While the induction of HSPs via chronic heat treatment has been shown to protect skeletal muscle from obesity-related insulin resistance, the ability of heat treatment to improve insulin action in aged skeletal muscle is not known. In the present study, one bout of in vivo heat treatment applied to 24-mo-old Fischer 344 rats improved insulin-stimulated glucose uptake after 24 h in slow-twitch soleus muscles. In vitro heat treatment applied to young (3-mo-old) and aged (24-mo-old) soleus muscles increased expression of HSP72 and inhibited anisomycin-induced activation of JNK. In contrast, heat treatment had no effect on p38 MAPK, a MAPK strongly activated with anisomycin. Prior inhibition of HSP72 transcription with the pharmacological inhibitor KNK437 eliminated the ability of heat treatment to blunt JNK activation. This suggests that the ability of heat treatment to inhibit JNK activation in skeletal muscle is dependent on increased HSP72 expression. In conclusion, an acute bout of heat treatment can increase insulin-stimulated glucose uptake in aged skeletal muscle, with the underlying mechanism likely to be HSP72-mediated JNK inhibition.
Keywords: heat shock proteins, heat shock protein 72, JNK, insulin resistance
a decline in insulin action, or development of insulin resistance, is strongly associated with advancing age (6, 10, 13, 51). The significant decline in insulin action with age contributes to the prevalence of impaired glucose tolerance and type 2 diabetes in the elderly population. Aging is associated with chronic accumulation of reactive oxygen species, and several age-related pathologies, including diabetes, are thought to be due to oxidative stress (29). Oxidative stress can increase activation of inflammatory mediators such as JNK and inhibitor of κB kinase-β (30, 47), two stress kinases strongly implicated in the development of age-related insulin resistance (19). Prolonged activation of these kinases results in serine phosphorylation of the insulin receptor substrate 1, ultimately leading to impaired insulin signaling and insulin resistance. Insulin resistance in skeletal muscle, the tissue responsible for 75% of glucose utilization in the body, is an important risk factor for the development of type 2 diabetes (3).
A loss in heat shock protein (HSP) expression and function could contribute to oxidative stress and insulin resistance in skeletal muscle. A significant role for HSPs in diabetes and oxidative stress has been previously identified (26, 35, 54). Kurucz et al. (35) demonstrated that decreased expression of HSPs in patients with type 2 diabetes correlates with reduced insulin sensitivity. In addition, a small study demonstrated that heat therapy can modestly improve clinical parameters in patients with type 2 diabetes (27). More recent data from our laboratory (20) and others (7, 19, 32, 40) demonstrated that induction of HSPs with heat treatment can protect against obesity-related insulin resistance. Expression of HSPs and their upregulation in response to stress are significantly reduced with aging and diabetes (2, 33, 53). We previously showed that HSP expression is reduced in aging rat muscle and that this is associated with, and may contribute to, increased stress kinase activity and reduced insulin sensitivity (19). Lifelong overexpression of HSP72 in skeletal muscle protected mice from age-associated accumulation of oxidative damage and preserved muscle function (4, 49); however, the effects of increased HSP expression on insulin action in aging muscle are unknown.
The purpose of the current study was to determine if acute HSP upregulation via heat treatment could improve insulin action in aged skeletal muscle. We hypothesize that specific upregulation of HSP72 with heat treatment results in improved insulin action, most likely via inhibition of JNK activation in skeletal muscle.
MATERIALS AND METHODS
Materials.
[14C]mannitol and 2-deoxy-[1,2-3H]glucose were purchased from American Radiolabeled Chemicals (St. Louis, MO). Antibodies against phosphorylated (T183/Y185) SAPK/JNK, total SAPK/JNK, phosphorylated (T180/Y182) p38 MAPK, and total p38 MAPK were purchased from Cell Signaling (Beverly, MA). Anti-HSP72 antibody was obtained from Stressgen (Victoria, BC, Canada), and anti-tubulin was obtained from Sigma (St. Louis, MO). Goat anti-rabbit horseradish peroxidase (HRP)-conjugated secondary antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA), and goat anti-mouse HRP-conjugated secondary antibodies were obtained from Bio-Rad (Hercules, CA). KNK437 and anisomycin were obtained from Calbiochem (San Diego, CA). Insulin was purchased from Humalog (Elli Lilly, Indianapolis, IN), and enhanced chemiluminescence reagents were purchased from Amersham (Little Chalfont, Buckinghamshire, UK). All other reagents were obtained from Sigma.
Experimental animals.
The Fischer 344 (F344) rat strain provides a suitable aging model because of its relatively short life span (58), extensive characterization of age-associated changes, and the availability of barrier-reared aging colonies of F344 rats, maintained under the supervision of the National Institute on Aging. Male F344 rats were purchased at 3 and 24 mo of age (238.50 ± 5.11 and 393.0 ± 17.72 g body wt, respectively) from the National Institutes of Health. Rats were housed in pairs in large cages in a temperature-controlled facility (24 ± 1°C) with a 12:12-h light-dark cycle. All animals were given free access to Purina Rat Chow and water ad libitum. All protocols were approved by the Animal Care and Use Committee of the University of Kansas Medical Center.
In vivo heat treatment.
Twenty-four-month-old F344 rats (n = 7) were anesthetized with an intraperitoneal injection of pentobarbital sodium (5 mg/100 g body wt). While the animals were in the supine position, a thermal blanket (Chattanooga Group, Hixson, TN) was wrapped around the lower body, such that only the hindlimbs were completely covered. As previously described (52), the blanket temperature was set to 112°F and core body temperature was monitored using a rectal probe from ThermoWorks (Braintree Scientific, Braintree, MA). An average time of 40–55 min was needed to reach a body temperature of 41°C, after which the core temperature was maintained at 41–41.5°C for 20 min by opening and closing the blanket. For the sham treatment, rats were anesthetized and their core temperature was maintained at 35°C. After the heat or sham treatments, the rats were given 5 ml of 0.9% saline intraperitoneally to prevent dehydration and allowed free access to food and water.
Rats were then fasted overnight (12 h). At 24 h after heat or sham treatment, 3- and 24-mo-old F344 rats were anesthetized with pentobarbital sodium (5 mg/100 g body wt ip), and the soleus muscle, which consists predominantly of slow-twitch red fibers (84% type I, 16% type II) (11), was removed. Soleus muscles were split longitudinally prior to incubation to allow adequate diffusion of oxygen and substrates (19, 24).
Measurement of glucose transport activity.
After dissection, isolated soleus muscle strips were placed in small vials in a shaking incubator (35°C) for 60 min in Krebs-Henseleit bicarbonate (KHB) buffer with 8 mM glucose and 32 mM mannitol. In this and all subsequent incubation steps, the muscle strips were exposed to a gas phase of 95% O2-5% CO2. The soleus muscle strips were transferred to new vials containing 2 ml of KHB and 40 mM mannitol, without or with insulin (1 mU/ml), for 30 min at 29°C. Muscle strips were then incubated for 20 min at 29°C in 2 ml of KHB with 4 mM 2-[1,2-3H]deoxyglucose (1.5 μCi/ml) and 36 mM [14C]mannitol (0.2 μCi/ml), without or with insulin (1 mU/ml), as in the prior step. Finally, the muscle strips were blotted, clamp-frozen, and processed for determination of intracellular 2-deoxyglucose accumulation and extracellular space, as described previously (16, 19, 57).
In vitro heat treatment and muscle incubations.
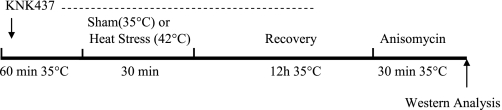
Soleus muscles were dissected from control (non-heat- or sham-treated) 3- and 24-mo-old rats (n = 10–12 rats per group) for in vitro muscle incubations. Muscles were split longitudinally, with care taken to maintain equal muscle size (<30 mg muscle wet wt) across age groups. After dissection, isolated soleus muscle strips were incubated in flasks containing 2 ml of MEM (Invitrogen, Carlsbad, CA) supplemented with 0.01% BSA, 2.2 g/l NaHCO3, 5 mM mannitol, 2.54 mM CaCl2, 10% FBS, and 1 ml/l Pen-Strep (all from Sigma) and 50 μU/ml insulin and exposed to a gas phase of 95% O2-5% CO2 at 35°C (17, 23). The MEM buffer is well suited for maintaining muscle strips during longer incubation protocols (1) and was used for all in vitro muscle incubations. The flasks were incubated in a temperature-controlled shaking water bath, as described above. For the in vitro heat treatment, muscles were incubated at 42°C for 30 min, while non-heat-treated muscles remained in recovery buffer at 35°C. After 30 min at 42°C or 35°C, muscles were allowed to recover for 12 h in the MEM recovery buffer at 35°C. After 6 h, the MEM buffer was replaced with fresh buffer to ensure adequate substrate availability (1, 18). For subsequent treatments, the muscles were transferred to fresh flasks without or with anisomycin (10 μg/ml) for 30 min in MEM buffer. When KNK437 (100 μM) was used, muscles were preincubated with KNK437 for 1 h and then subjected to heat or sham treatment in the presence of KNK437 for 30 min. After this step, muscles were incubated in fresh medium for the 12-h recovery period. KNK437 was maintained during most of the recovery time (8 h) to ensure that new protein synthesis of HSP72 did not occur. In preliminary experiments, we determined that the full 12-h recovery period with KNK437 resulted in nonspecific inhibition of MAPK signaling pathways. This inhibition did not occur if KNK437 was removed during the final 4 h of recovery. After the final incubation period with or without anisomycin, the muscles were rapidly frozen in liquid nitrogen and processed for Western blot analysis. Figure 1 depicts the in vitro incubation protocol.
Fig. 1.
In vitro muscle incubation protocol. Soleus muscle strips were dissected and incubated in recovery buffer at 35°C for 60 min. After 30 min of heat or sham treatment, soleus muscles were transferred to fresh vials containing recovery buffer at 35°C. Muscles were allowed to recover for 12 h and then incubated with or without anisomycin (10 μg/ml) for 30 min. A subset of soleus muscles was preincubated in KNK437 for 60 min at 35°C prior to heat treatment. The pharmacological inhibitor KNK437 was also present during 8 h of the recovery period in soleus muscles pretreated with KNK437 and removed for the last 4 h of recovery. After the final incubation step, muscles were clamp-frozen for future Western blot analysis.
Western blotting.
Clamp-frozen soleus muscles were homogenized in a 12:1 (vol/wt) ratio of ice-cold homogenization buffer (Biosource, Invitrogen). The buffer contains 10 mM Tris·HCl (pH 7.4), 100 mM NaCl, 1 mM each EDTA, EGTA, NaF, and PMSF, 2 mM Na3VO4, 20 mM Na4P2O7, 1% Triton X-100, 10% glycerol, 0.1% SDS, 0.5% deoxycholate, and 250 μl/5 ml protease inhibitor cocktail. Homogenized samples were rotated for 30 min and then centrifuged for 20 min at 3,000 rpm at 4°C. The protein concentration of the supernatant was determined by the Bradford method (Bio-Rad). Samples were prepared in 1× Laemmli buffer (final) containing 100 mM dithiothreitol and denatured in a boiling water bath for 5 min.
Protein samples (30–100 μg) were subjected to SDS-PAGE (6.25–10% gel) followed by a wet transfer to a nitrocellulose membrane for 90 min (200 mA per tank). Membranes were blocked for 1 h at room temperature in Tris-buffered saline-0.1% Tween 20 (TBST)-5% nonfat dry milk and then incubated overnight with the appropriate primary antibodies at a concentration of 1:1,000. Antibodies were diluted in TBST and 5% BSA. After three brief rinses in TBST, blots were incubated in TBST-1% nonfat dry milk supplemented with a HRP-conjugated goat anti-rabbit secondary antibody at a concentration of 1:10,000 for 1 h at room temperature. Bands were visualized by enhanced chemiluminescence and quantified using densitometry (ImageJ, National Institutes of Health). Blots were stripped and normalized for total protein or tubulin as loading controls.
Citrate synthase assay.
Citrate synthase activity was assessed in muscle lysates using a modification of the protocol (48) of Srere (50) used previously in our laboratory (20). The absorbance was recorded at 405 nm every 20 s for 3 min at 30°C with use of a MRXII microplate reader and kinetic software package (Dynex Technologies, Chantilly, VA). The linear portion of the reaction curve was used to calculate activity levels of citrate synthase (in μmol·g−1·min−1).
Statistical analysis.
Two-way ANOVA was used when both age and treatment differences were studied. This was followed by a post hoc comparison using the Student-Newman-Keuls test when necessary. Values are means ± SE. Statistical significance was set at P < 0.05.
RESULTS
Increased insulin-stimulated glucose uptake with heat treatment in aged rats.
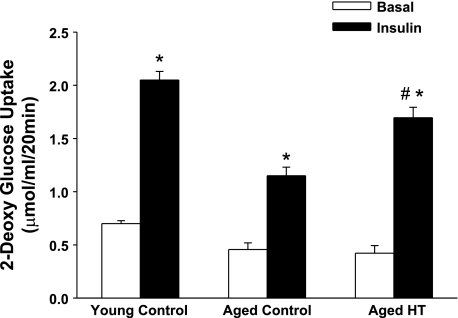
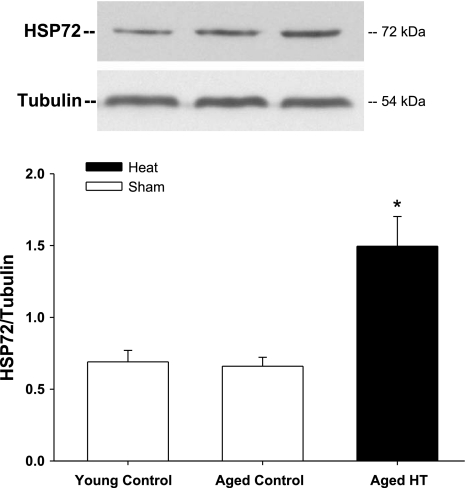
Our previous findings indicate decreased glucose uptake in soleus muscles from 24-mo-old F344 rats and decreased activation of insulin signaling intermediates compared with soleus muscles from 3-mo-old F344 rats (19). In the present study, we subjected 24-mo-old F344 rats to lower body heat treatment with a thermal blanket (core temperature 41°C for 20 min) under low-dose anesthesia, while control rats (3 and 24 mo old) were subjected to a sham treatment (core temperature 35°C for 20 min). At 24 h after sham or heat treatment, soleus muscles were dissected and basal- and insulin-stimulated glucose uptake was measured. Values for insulin-stimulated glucose uptake in soleus muscles from sham-treated animals were similar to our previous findings in young and aged rats (19), with a decrease in glucose uptake of 44% in muscles from aged rats compared with young rats (Fig. 2). However, soleus muscle insulin-stimulated glucose uptake in 24-mo-old rats was significantly improved following one bout of heat treatment. Basal glucose uptake was not altered by prior heat treatment. As shown in Fig. 3, HSP72 protein expression was increased 24 h after heat treatment, coinciding with increased insulin-stimulated glucose uptake in these muscles. Analysis of HSP72 and JNK activation in response to heat was next performed on isolated muscles in vitro. The advantage of this in vitro model is the ability to assess the effects of heat treatment on skeletal muscle independent of whole body physiological changes or heat effects on other organ systems.
Fig. 2.
Acute heat treatment (HT) improves glucose uptake in soleus muscles from aged rats. Three- and 24-mo-old Fischer 344 (F344) rats were subjected to sham treatment (control, 35°C for 20 min), and 24-mo-old rats were subjected to heat treatment (41°C for 20 min) using a thermal blanket. The rats were allowed to recover, and soleus muscles were removed 24 h after treatment. Basal (without insulin) and insulin-stimulated (1 mU/ml) glucose transport was assessed using 2-[1,2-3H]deoxyglucose, [14C]mannitol, and 2-deoxyglucose. *P < 0.001 vs. basal. #P < 0.001, insulin heat treatment vs. insulin aged control. Values are means of ± SE for 4–7 muscles per group.
Fig. 3.
Acute heat treatment increases heat shock protein (HSP) 72 expression in soleus muscles from aged rats. Three- and 24-mo-old F344 rats were subjected to sham treatment (control, 35°C for 20 min), and 24-mo-old rats were subjected to heat treatment (41°C for 20 min) using a thermal blanket. The rats were allowed to recover, and soleus muscles were removed 24 h after treatment, frozen, and analyzed for HSP72 expression by Western blotting. *P < 0.01 vs. sham. Values are means ± SE for 4–7 muscles per group.
Heat treatment protocol and muscle viability following heat stress.
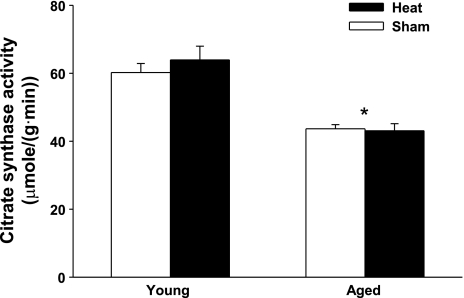
Preliminary studies were performed to determine optimal conditions for assessing the effects of heat treatment on skeletal muscle in vitro. In vitro heat treatment (42°C) for 30 min results in a significant increase in HSP72 protein expression without any nonspecific effects of heat stress on MAPK activation (data not shown) and was therefore used in the current study. The recovery interval was chosen on the basis of a need to see increased HSP protein expression while also maintaining muscle viability in vitro. Previous studies examining the in vivo heat stress response in muscle found maximal HSP72 expression at 4–48 h after heat treatment, depending on the muscle type (43). We found the maximal response to in vitro heat treatment in soleus muscles at 12 h after heat treatment (3–24 h recovery times tested, data not shown). At the 12-h recovery time point, we observed no significant changes in muscle quality (tissue coloration and resting length) in heat- or sham-treated muscles. In addition, total protein concentration as assessed by the Bradford assay was not different between heat- and sham-treated muscles (6.01 ± 0.18 and 5.61 ± 0.27 mg/ml in sham and heat-treated muscles, respectively, n = 6 per group). To verify that muscle metabolic function is not substantially altered by heat stress, activity levels of citrate synthase were measured in young and aged soleus muscles. Citrate synthase activity levels were greater in the young than the aged soleus muscles (Fig. 4), as has been reported previously (12). However, citrate synthase activity levels did not differ between muscles subjected to sham or heat treatment and allowed to recover for 12 h. On the basis of these combined measurements, we can conclude that the in vitro heat stress protocol developed here is relatively mild and results in minimal nonspecific effects in skeletal muscle at the 12-h time point.
Fig. 4.
Heat treatment does not alter citrate synthase activity in soleus muscles. Soleus muscles from young (3-mo-old) and aged (24-mo-old) F344 rats were subjected to sham (35°C for 30 min) or heat (42°C for 30 min) treatment. Soleus muscles were allowed to recover for 12 h and then frozen and analyzed for citrate synthase activity. *P < 0.001, aged vs. young. Values are means ± SE for 3 muscles per group.
In vitro heat treatment induces HSP72 expression in soleus muscles from young and aged rats.
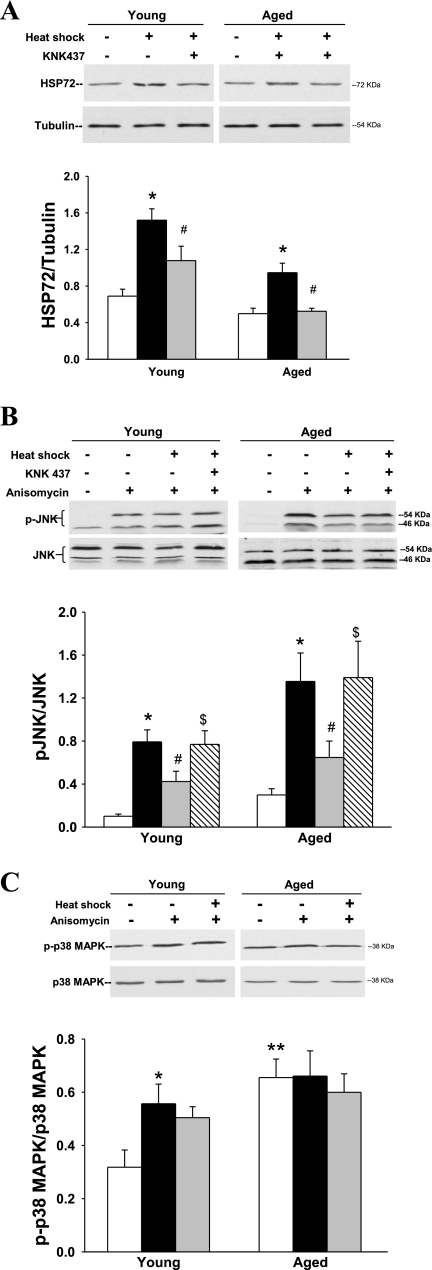
Young and aged soleus muscles demonstrate a robust induction of HSP72 protein expression 12 h after heat treatment (120% and 90% increase above basal, respectively; Fig. 5A). However, the heat stress response is considerably less in the aged than the young soleus.
Fig. 5.
Effects of in vitro heat treatment on HSP72 expression and MAPK activation. Soleus muscles from young (3-mo-old) and aged (24-mo-old) F344 rats were subjected to sham (35°C for 30 min) or heat (42°C for 30 min) treatment. A reversible pharmacological inhibitor of HSPs, KNK437 (100 μM), was included in the incubation buffer 1 h prior to, during, and 8 h after heat treatment for a subgroup of muscles. A: muscles were allowed to recover for 12 h and then frozen and analyzed for HSP72 expression by Western blotting. Blots were stripped and probed for tubulin as a loading control. B and C: for assessment of MAPK activation, a set of muscles was stimulated with anisomycin (10 μg/ml) for 30 min after the 12-h recovery period and then snap-frozen. KNK437 was also included in a subset of muscles prior to and during heat treatment (hatched bars). Muscle lysates were analyzed for phosphorylated (p)-JNK/total JNK and for phosphorylated p38 MAPK/total p38 MAPK with and without heat treatment and anisomycin stimulation. *P < 0.001 vs. sham. #P < 0.001, heat + KNK437 vs. heat and heat + anisomycin vs. sham + anisomycin. $P < 0.001, heat + KNK437 + anisomycin vs. heat + anisomycin. P < 0.001, all aged vs. all young groups in A and B; **P < 0.01, young vs. aged sham in C. Values are means of ± SE for 4–7 aged muscles and 7–20 young muscles per group.
In vitro heat treatment prevents JNK activation.
To assess the effects of heat treatment on activation of JNK, sham- and heat-treated soleus muscles were acutely stimulated with anisomycin for 30 min. Anisomycin is a potent activator of the stress-activated protein kinases JNK (21) and p38 MAPK (36, 45). In non-heat-treated muscles, anisomycin induced a robust increase in JNK phosphorylation, a measure of JNK activation (Fig. 5B). Phosphorylation of JNK was much greater in the aged than in the young soleus muscles, as previously shown (19, 41). In muscles subjected to prior heat treatment, the same concentration of anisomycin resulted in significantly less JNK phosphorylation in young and aged animals (Fig. 5B). Anisomycin can also stimulate p38 MAPK activation (Fig. 5C). However, contrary to the effect on JNK activation, heat treatment had no effect on the phosphorylation of p38 MAPK in the young or aged soleus muscles (Fig. 5C). Basal phosphorylation of p38 MAPK was much greater in the aged soleus and did not further increase with anisomycin treatment.
KNK437 inhibits heat induced-HSP72 expression.
Previous studies demonstrated a strong relationship between HSP72 expression and JNK inactivation (7, 20). To assess the effects of HSP72 activation with heat treatment on JNK in the current study, we utilized a pharmacological approach. KNK437 is a specific inhibitor of inducible HSPs (31, 56) and effectively inhibits HSP72 at the mRNA level and prevents transcription of new HSP72 (56). Muscles were preincubated with KNK437 (100 μM) for 1 h and then subjected to an in vitro heat or sham treatment. Since KNK437 is a reversible inhibitor, it was included during most of the recovery period as well (8 h of the total 12 h of recovery). HSP72 induction with heat treatment was significantly decreased in muscles from young and aged rats incubated in the presence of KNK437 (Fig. 5A). KNK437 had no significant effect on the muscles without heat treatment (data not shown).
Inhibition of HSP72 with KNK437 reverses heat shock-mediated JNK inhibition.
To determine the impact of HSP72 upregulation on JNK activation, we measured anisomycin-induced JNK phosphorylation in the presence of the HSP72 inhibitor KNK437. Anisomycin-induced JNK phosphorylation was reduced with heat treatment as previously described, but in the muscles incubated with KNK437, heat treatment did not decrease JNK activation (Fig. 5B). Thus, pharmacological inhibition of the HSP72 response reversed the heat-mediated inhibition of JNK. These data suggest that JNK inactivation via heat treatment is dependent on increased HSP72 expression.
DISCUSSION
New findings from the current study demonstrate that one bout of heat treatment, much like one bout of exercise training, can significantly increase insulin-stimulated glucose uptake in aged skeletal muscle. Heat treatment eliminated the ∼40% reduction in insulin-stimulated glucose uptake that occurs in skeletal muscle from aged rats (19). Our in vitro data demonstrate that acute heat treatment results in decreased JNK phosphorylation in skeletal muscle and that this decrease appears dependent on induction of HSP72.
Decreased HSP expression could play an important role in the development of skeletal muscle insulin resistance. Aging and type 2 diabetes are characterized by a diminished heat stress response, specifically, decreased expression of HSP72 (7, 19, 20, 46). Previous studies demonstrate an inverse correlation between HSP72 mRNA expression and the severity of insulin resistance (5, 35). More recently, Chung et al. (7) showed that HSP72 protein levels were decreased, and JNK phosphorylation increased, in skeletal muscle from obese, insulin-resistant patients. Our findings from the current study support these data and demonstrate a decrease in HSP72 activation and an increase in JNK phosphorylation in aged, insulin-resistant skeletal muscle. Decreased HSP expression in skeletal muscle could be due to increased stress kinase activation characteristic of insulin-resistant tissue. Glycogen synthase kinase 3 and the MAPKs, ERK and JNK, are known to negatively regulate the primary HSP transcription factor heat shock factor-1 (HSF-1). Glycogen synthase kinase 3, ERK, and JNK phosphorylate HSF-1 on serine residues 303, 307, and 363, respectively, which maintains HSF-1 in an inactive state in normal physiological conditions (8, 22). However, overactivation of these stress kinases as occurs in conditions of insulin resistance, could result in repression of HSF-1 activation and decreased expression of HSP72. In this manner, aging and insulin resistance are associated with an interdependent cycle of increased stress kinase activity, decreased HSP expression, and reduced insulin sensitivity (28). Therapeutic upregulation of HSPs could potentially interfere with this adverse cycle by decreasing stress kinase activation.
Increased HSP72 expression via heat treatment, genetic overexpression, or pharmacological means resulted in the prevention of insulin resistance in a manner that was tightly associated with decreased JNK phosphorylation (7). Previous studies demonstrated that the absence of JNK results in protection from obesity-induced insulin resistance (25) and free fatty acid-induced defects in insulin action (15, 42). It has been suggested that HSP72 functions as a natural inhibitor of JNK. Although the exact mechanism of JNK inhibition by HSP72 is unknown, it is independent of HSP72 chaperone function, since removal of the ATPase domain of HSP72 still inhibits JNK activation (55). In NIH 3T3 cells, HSP72 has been shown to bind to JNK and prevent its activation by the upstream kinases SEK1 and MKK7 (44). Other investigators suggest that HSP72 prevents stress-mediated inactivation of a phosphatase that targets JNK (14, 39) or results in inhibition of DLK1, a kinase upstream of JNK (9). Future studies are necessary to identify the exact mechanisms by which HSP72 results in JNK inactivation.
In conclusion, our findings demonstrate that a single bout of heat treatment can increase insulin-stimulated glucose uptake in aged skeletal muscle. Our findings in vitro suggest that decreased phosphorylation of JNK via heat treatment could, at least in part, be the underlying mechanism by which heat treatment increases insulin-stimulated glucose uptake. Induction of HSP72 appears to be crucial to these beneficial effects of heat treatment, but future studies are needed to demonstrate the specific role of HSP72 in mediating skeletal muscle insulin action. Several HSP72-inducing compounds have been explored for treating diabetic neuropathy (34) and myocardial infarction (38). In addition, a recent clinical trial demonstrated, for the first time, the insulin-sensitizing effect of BGP-15, a coinducer of HSPs (37). The direct effects of HSP-inducing treatments, such as the hydroximic acid derivative BGP-15, heat, and exercise training, on insulin action in skeletal muscle warrant future study.
GRANTS
The project was supported by National Institutes of Health Grants AG-031575 and P20 RR-016475 from the IDeA Networks of Biomedical Research Excellence Program of the National Center for Research Resources. A. A. Gupte was supported by a Kansas University Medical Center Biomedical Research Training Program award, and core support was provided by National Institute of Child Health and Human Development Grant HD-002528.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
The authors thank Jill Morris and Brittany Gorres for assistance with this research.
Present address of A. A. Gupte: The Methodist Hospital Research Institute, 6565 Fannin, F8-060, Houston, TX 77030 (e-mail: aagupte@tmhs.org).
REFERENCES
- 1. Alkhateeb H, Chabowski A, Bonen A. Viability of the isolated soleus muscle during long-term incubation. Appl Physiol Nutr Metab 31: 467–476, 2006 [DOI] [PubMed] [Google Scholar]
- 2. Atalay M, Oksala NK, Laaksonen DE, Khanna S, Nakao C, Lappalainen J, Roy S, Hanninen O, Sen CK. Exercise training modulates heat shock protein response in diabetic rats. J Appl Physiol 97: 605–611, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Bjornholm M, Zierath JR. Insulin signal transduction in human skeletal muscle: identifying the defects in type II diabetes. Biochem Soc Trans 33: 354–357, 2005 [DOI] [PubMed] [Google Scholar]
- 4. Broome CS, Kayani AC, Palomero J, Dillmann WH, Mestril R, Jackson MJ, McArdle A. Effect of lifelong overexpression of HSP70 in skeletal muscle on age-related oxidative stress and adaptation after nondamaging contractile activity. FASEB J 20: 1549–1551, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Bruce CR, Carey AL, Hawley JA, Febbraio MA. Intramuscular heat shock protein 72 and heme oxygenase-1 mRNA are reduced in patients with type 2 diabetes: evidence that insulin resistance is associated with a disturbed antioxidant defense mechanism. Diabetes 52: 2338–2345, 2003 [DOI] [PubMed] [Google Scholar]
- 6. Chen M, Bergman RN, Porte D., Jr Insulin resistance and beta-cell dysfunction in aging: the importance of dietary carbohydrate. J Clin Endocrinol Metab 67: 951–957, 1988 [DOI] [PubMed] [Google Scholar]
- 7. Chung J, Nguyen AK, Henstridge DC, Holmes AG, Chan MH, Mesa JL, Lancaster GI, Southgate RJ, Bruce CR, Duffy SJ, Horvath I, Mestril R, Watt MJ, Hooper PL, Kingwell BA, Vigh L, Hevener A, Febbraio MA. HSP72 protects against obesity-induced insulin resistance. Proc Natl Acad Sci USA 105: 1739–1744, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dai R, Frejtag W, He B, Zhang Y, Mivechi NF. c-Jun NH2-terminal kinase targeting and phosphorylation of heat shock factor-1 suppress its transcriptional activity. J Biol Chem 275: 18210–18218, 2000 [DOI] [PubMed] [Google Scholar]
- 9. Daviau A, Proulx R, Robitaille K, Di Fruscio M, Tanguay RM, Landry J, Patterson C, Durocher Y, Blouin R. Down-regulation of the mixed-lineage dual leucine zipper-bearing kinase by heat shock protein 70 and its co-chaperone CHIP. J Biol Chem 281: 31467–31477, 2006 [DOI] [PubMed] [Google Scholar]
- 10. DeFronzo RA. Glucose intolerance and aging. Diabetes Care 4: 493–501, 1981 [DOI] [PubMed] [Google Scholar]
- 11. Delp MD, Duan C. Composition and size of type I, IIA, IID/X, and IIB fibers and citrate synthase activity of rat muscle. J Appl Physiol 80: 261–270, 1996 [DOI] [PubMed] [Google Scholar]
- 12. Figueiredo PA, Ferreira RM, Appell HJ, Duarte JA. Age-induced morphological, biochemical, and functional alterations in isolated mitochondria from murine skeletal muscle. J Gerontol A Biol Sci Med Sci 63: 350–359, 2008 [DOI] [PubMed] [Google Scholar]
- 13. Fink RI, Kolterman OG, Griffin J, Olefsky JM. Mechanisms of insulin resistance in aging. J Clin Invest 71: 1523–1535, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gabai VL, Meriin AB, Yaglom JA, Wei JY, Mosser DD, Sherman MY. Suppression of stress kinase JNK is involved in HSP72-mediated protection of myogenic cells from transient energy deprivation. HSP72 alleviates the stress-induced inhibition of JNK dephosphorylation. J Biol Chem 275: 38088–38094, 2000 [DOI] [PubMed] [Google Scholar]
- 15. Gao Z, Zhang X, Zuberi A, Hwang D, Quon MJ, Lefevre M, Ye J. Inhibition of insulin sensitivity by free fatty acids requires activation of multiple serine kinases in 3T3-L1 adipocytes. Mol Endocrinol 18: 2024–2034, 2004 [DOI] [PubMed] [Google Scholar]
- 16. Geiger PC, Han DH, Wright DC, Holloszy JO. How muscle insulin sensitivity is regulated: testing of a hypothesis. Am J Physiol Endocrinol Metab 291: E1258–E1263, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Geiger PC, Wright DC, Han DH, Holloszy JO. Activation of p38 MAP kinase enhances sensitivity of muscle glucose transport to insulin. Am J Physiol Endocrinol Metab 288: E782–E788, 2005 [DOI] [PubMed] [Google Scholar]
- 18. Gulve EA, Cartee GD, Youn JH, Holloszy JO. Prolonged incubation of skeletal muscle increases system A amino acid transport. Am J Physiol Cell Physiol 260: C88–C95, 1991 [DOI] [PubMed] [Google Scholar]
- 19. Gupte AA, Bomhoff GL, Geiger PC. Age-related differences in skeletal muscle insulin signaling: the role of stress kinases and heat shock proteins. J Appl Physiol 105: 839–848, 2008 [DOI] [PubMed] [Google Scholar]
- 20. Gupte AA, Bomhoff GL, Swerdlow RH, Geiger PC. Heat treatment improves glucose tolerance and prevents skeletal muscle insulin resistance in rats fed a high-fat diet. Diabetes 58: 567–578, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hazzalin CA, Le Panse R, Cano E, Mahadevan LC. Anisomycin selectively desensitizes signalling components involved in stress kinase activation and fos and jun induction. Mol Cell Biol 18: 1844–1854, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. He B, Meng YH, Mivechi NF. Glycogen synthase kinase 3β and extracellular signal-regulated kinase inactivate heat shock transcription factor 1 by facilitating the disappearance of transcriptionally active granules after heat shock. Mol Cell Biol 18: 6624–6633, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Henriksen EJ, Bourey RE, Rodnick KJ, Koranyi L, Permutt MA, Holloszy JO. Glucose transporter protein content and glucose transport capacity in rat skeletal muscles. Am J Physiol Endocrinol Metab 259: E593–E598, 1990 [DOI] [PubMed] [Google Scholar]
- 24. Henriksen EJ, Holloszy JO. Effect of diffusion distance on measurement of rat skeletal muscle glucose transport in vitro. Acta Physiol Scand 143: 381–386, 1991 [DOI] [PubMed] [Google Scholar]
- 25. Hirosumi J, Tuncman G, Chang L, Gorgun CZ, Uysal KT, Maeda K, Karin M, Hotamisligil GS. A central role for JNK in obesity and insulin resistance. Nature 420: 333–336, 2002 [DOI] [PubMed] [Google Scholar]
- 26. Hojlund K, Wrzesinski K, Larsen PM, Fey SJ, Roepstorff P, Handberg A, Dela F, Vinten J, McCormack JG, Reynet C, Beck-Nielsen H. Proteome analysis reveals phosphorylation of ATP synthase β-subunit in human skeletal muscle and proteins with potential roles in type 2 diabetes. J Biol Chem 278: 10436–10442, 2003 [DOI] [PubMed] [Google Scholar]
- 27. Hooper PL. Hot-tub therapy for type 2 diabetes mellitus. N Engl J Med 341: 924–925, 1999 [DOI] [PubMed] [Google Scholar]
- 28. Hooper PL, Hooper PL. Inflammation, heat shock proteins, and type 2 diabetes. Cell Stress Chaperones 15: 761–770, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature 440: 944–948, 2006 [DOI] [PubMed] [Google Scholar]
- 30. Kaneto H, Matsuoka TA, Katakami N, Kawamori D, Miyatsuka T, Yoshiuchi K, Yasuda T, Sakamoto K, Yamasaki Y, Matsuhisa M. Oxidative stress and the JNK pathway are involved in the development of type 1 and type 2 diabetes. Curr Mol Med 7: 674–686, 2007 [DOI] [PubMed] [Google Scholar]
- 31. Koishi M, Yokota S, Mae T, Nishimura Y, Kanamori S, Horii N, Shibuya K, Sasai K, Hiraoka M. The effects of KNK437, a novel inhibitor of heat shock protein synthesis, on the acquisition of thermotolerance in a murine transplantable tumor in vivo. Clin Cancer Res 7: 215–219, 2001 [PubMed] [Google Scholar]
- 32. Kokura S, Adachi S, Manabe E, Mizushima K, Hattori T, Okuda T, Nakabe N, Handa O, Takagi T, Naito Y, Yoshida N, Yoshikawa T. Whole body hyperthermia improves obesity-induced insulin resistance in diabetic mice. Int J Hyperthermia 23: 259–265, 2007 [DOI] [PubMed] [Google Scholar]
- 33. Kregel KC, Moseley PL, Skidmore R, Gutierrez JA, Guerriero V., Jr HSP70 accumulation in tissues of heat-stressed rats is blunted with advancing age. J Appl Physiol 79: 1673–1678, 1995 [DOI] [PubMed] [Google Scholar]
- 34. Kurthy M, Mogyorosi T, Nagy K, Kukorelli T, Jednakovits A, Talosi L, Biro K. Effect of BRX-220 against peripheral neuropathy and insulin resistance in diabetic rat models. Ann NY Acad Sci 967: 482–489, 2002 [DOI] [PubMed] [Google Scholar]
- 35. Kurucz I, Morva A, Vaag A, Eriksson KF, Huang X, Groop L, Koranyi L. Decreased expression of heat shock protein 72 in skeletal muscle of patients with type 2 diabetes correlates with insulin resistance. Diabetes 51: 1102–1109, 2002 [DOI] [PubMed] [Google Scholar]
- 36. Li Y, Inoki K, Vacratsis P, Guan KL. The p38 and MK2 kinase cascade phosphorylates tuberin, the tuberous sclerosis 2 gene product, and enhances its interaction with 14-3-3. J Biol Chem 278: 13663–13671, 2003 [DOI] [PubMed] [Google Scholar]
- 37. Literati-Nagy B, Kulcsar E, Literati-Nagy Z, Buday B, Peterfai E, Horvath T, Tory K, Kolonics A, Fleming A, Mandl J, Koranyi L. Improvement of insulin sensitivity by a novel drug, BGP-15, in insulin-resistant patients: a proof of concept randomized double-blind clinical trial. Horm Metab Res 41: 374–380, 2009 [DOI] [PubMed] [Google Scholar]
- 38. Lubbers NL, Polakowski JS, Wegner CD, Burke SE, Diaz GJ, Daniell KM, Cox BF. Oral bimoclomol elevates heat shock protein 70 and reduces myocardial infarct size in rats. Eur J Pharmacol 435: 79–83, 2002 [DOI] [PubMed] [Google Scholar]
- 39. Meriin AB, Yaglom JA, Gabai VL, Zon L, Ganiatsas S, Mosser DD, Zon L, Sherman MY. Protein-damaging stresses activate c-Jun N-terminal kinase via inhibition of its dephosphorylation: a novel pathway controlled by HSP72. Mol Cell Biol 19: 2547–2555, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Morino S, Kondo T, Sasaki K, Adachi H, Suico MA, Sekimoto E, Matsuda T, Shuto T, Araki E, Kai H. Mild electrical stimulation with heat shock ameliorates insulin resistance via enhanced insulin signaling. PLoS ONE 3: e4068, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mylabathula DB, Rice KM, Wang Z, Uddemarri S, Kinnard RS, Blough ER. Age-associated changes in MAPK activation in fast- and slow-twitch skeletal muscle of the F344/NNiaHSD × Brown Norway/BiNia rat model. Exp Gerontol 41: 205–214, 2006 [DOI] [PubMed] [Google Scholar]
- 42. Nguyen MT, Satoh H, Favelyukis S, Babendure JL, Imamura T, Sbodio JI, Zalevsky J, Dahiyat BI, Chi NW, Olefsky JM. JNK and tumor necrosis factor-α mediate free fatty acid-induced insulin resistance in 3T3-L1 adipocytes. J Biol Chem 280: 35361–35371, 2005 [DOI] [PubMed] [Google Scholar]
- 43. Oishi Y, Taniguchi K, Matsumoto H, Ishihara A, Ohira Y, Roy RR. Muscle type-specific response of HSP60, HSP72, and HSC73 during recovery after elevation of muscle temperature. J Appl Physiol 92: 1097–1103, 2002 [DOI] [PubMed] [Google Scholar]
- 44. Park HS, Lee JS, Huh SH, Seo JS, Choi EJ. Hsp72 functions as a natural inhibitory protein of c-Jun N-terminal kinase. EMBO J 20: 446–456, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rolli M, Kotlyarov A, Sakamoto KM, Gaestel M, Neininger A. Stress-induced stimulation of early growth response gene-1 by p38/stress-activated protein kinase 2 is mediated by a cAMP-responsive promoter element in a MAPKAP kinase 2-independent manner. J Biol Chem 274: 19559–19564, 1999 [DOI] [PubMed] [Google Scholar]
- 46. Selsby JT, Judge AR, Yimlamai T, Leeuwenburgh C, Dodd SL. Life long calorie restriction increases heat shock proteins and proteasome activity in soleus muscles of Fischer 344 rats. Exp Gerontol 40: 37–42, 2005 [DOI] [PubMed] [Google Scholar]
- 47. Shoelson SE, Lee J, Yuan M. Inflammation and the IKKβ/IκB/NF-κB axis in obesity- and diet-induced insulin resistance. Int J Obes Relat Metab Disord 27 Suppl 3: S49–S52, 2003 [DOI] [PubMed] [Google Scholar]
- 48. Smirnova IV, Kibiryeva N, Vidoni E, Bunag R, Stehno-Bittel L. Abnormal EKG stress test in rats with type 1 diabetes is deterred with low-intensity exercise programme. Acta Diabetol 43: 66–74, 2006 [DOI] [PubMed] [Google Scholar]
- 49. Smolka MB, Zoppi CC, Alves AA, Silveira LR, Marangoni S, Pereira-Da-Silva L, Novello JC, Macedo DV. HSP72 as a complementary protection against oxidative stress induced by exercise in the soleus muscle of rats. Am J Physiol Regul Integr Comp Physiol 279: R1539–R1545, 2000 [DOI] [PubMed] [Google Scholar]
- 50. Srere PA. Citrate synthase. Methods Enzymol 13: 3–11, 1969 [Google Scholar]
- 51. Supiano MA, Hogikyan RV, Morrow LA, Ortiz-Alonso FJ, Herman WH, Galecki AT, Halter JB. Aging and insulin sensitivity: role of blood pressure and sympathetic nervous system activity. J Gerontol 48: M237–M243, 1993 [DOI] [PubMed] [Google Scholar]
- 52. Tolson JK, Roberts SM. Manipulating heat shock protein expression in laboratory animals. Methods 35: 149–157, 2005 [DOI] [PubMed] [Google Scholar]
- 53. Vasilaki A, Jackson MJ, McArdle A. Attenuated HSP70 response in skeletal muscle of aged rats following contractile activity. Muscle Nerve 25: 902–905, 2002 [DOI] [PubMed] [Google Scholar]
- 54. Welsh N, Margulis B, Borg LA, Wiklund HJ, Saldeen J, Flodstrom M, Mello MA, Andersson A, Pipeleers DG, Hellerstrom C, et al. Differences in the expression of heat-shock proteins and antioxidant enzymes between human and rodent pancreatic islets: implications for the pathogenesis of insulin-dependent diabetes mellitus. Mol Med 1: 806–820, 1995 [PMC free article] [PubMed] [Google Scholar]
- 55. Yaglom JA, Gabai VL, Meriin AB, Mosser DD, Sherman MY. The function of HSP72 in suppression of c-Jun N-terminal kinase activation can be dissociated from its role in prevention of protein damage. J Biol Chem 274: 20223–20228, 1999 [DOI] [PubMed] [Google Scholar]
- 56. Yokota S, Kitahara M, Nagata K. Benzylidene lactam compound, KNK437, a novel inhibitor of acquisition of thermotolerance and heat shock protein induction in human colon carcinoma cells. Cancer Res 60: 2942–2948, 2000 [PubMed] [Google Scholar]
- 57. Young DA, Uhl JJ, Cartee GD, Holloszy JO. Activation of glucose transport in muscle by prolonged exposure to insulin. Effects of glucose and insulin concentrations. J Biol Chem 261: 16049–16053, 1986 [PubMed] [Google Scholar]
- 58. Yu BP, Masoro EJ, McMahan CA. Nutritional influences on aging of Fischer 344 rats. I. Physical, metabolic, and longevity characteristics. J Gerontol 40: 657–670, 1985 [DOI] [PubMed] [Google Scholar]