Abstract
Sympathetic nerve activity influences cerebral blood flow, but it is unknown whether augmented sympathetic nerve activity resets cerebral vasoreactivity to hypercapnia. This study tested the hypothesis that cerebral vasodilation during hypercapnia is restrained by lower-body negative pressure (LBNP)-stimulated sympathoexcitation. Cerebral hemodynamic responses were assessed in nine healthy volunteers [age 25 yr (SD 3)] during rebreathing-induced increases in partial pressure of end-tidal CO2 (PetCO2) at rest and during LBNP. Cerebral hemodynamic responses were determined by changes in flow velocity of middle cerebral artery (MCAV) using transcranial Doppler sonography and in regional cerebral tissue oxygenation (ScO2) using near-infrared spectroscopy. PetCO2 values during rebreathing were similarly increased from 41.9 to 56.5 mmHg at rest and from 40.7 to 56.0 mmHg during LBNP of −15 Torr. However, the rates of increases in MCAV and in ScO2 per unit increase in PetCO2 (i.e., the slopes of MCAV/PetCO2 and ScO2/PetCO2) were significantly (P ≤0.05) decreased from 2.62 ± 0.16 cm·s−1·mmHg−1 and 0.89 ± 0.10%/mmHg at rest to 1.68 ± 0.18 cm·s−1·mmHg−1 and 0.63 ± 0.07%/mmHg during LBNP. In conclusion, the sensitivity of cerebral vasoreactivity to hypercapnia, in terms of the rate of increases in MCAV and in ScO2, is diminished by LBNP-stimulated sympathoexcitation.
Keywords: cerebral blood flow velocity, cerebral tissue oxygenation, cerebral vascular conductance, lower-body negative pressure, partial pressure of end-tidal carbon dioxide
cerebral blood flow (CBF), as indicated by middle cerebral arterial blood flow velocity (MCAV), decreases during orthostatic challenges, e.g., head-up tilt (HUT) (3, 12, 13, 19, 23) or lower-body negative pressure (LBNP) (6, 8, 21, 33, 40). This cerebral hypoperfusion results from baroreflex-mediated sympathoexcitation (12) and/or hyperventilation-induced hypocapnia (6, 24) during orthostasis. Jordan et al. (12) reported that increase in MCAV was enhanced after autonomic ganglionic blockade, presumably due to release of sympathetic nerve influence. However, Zhang et al. (40) found that LBNP-induced decrease in MCAV persisted after trimethaphan was administered to block the baroreflex-mediated sympathoexcitation, suggesting that sympathetic nerve activity is not the major mechanism for reduction of CBF during orthostasis. The question remained as to whether cerebral vasoreactivity to hypercapnia could be reset or the sensitivity of cerebral vasodilation is acutely altered by the baroreflex-stimulated sympathoexcitation during orthostasis.
Cerebral vasoreactivity is an indicator of the functional reserve of the cerebral circulation (2, 14, 36), which appears to be impaired in patients with diffuse atherosclerosis and subclinical endothelial dysfunction (37) and with lacunar infarctions (28). The purpose of the present study was to examine the impact of steady-state LBNP-elicited sympathoexcitation or hyperadrenergic state on sensitivity of cerebral hemodynamic responses to CO2 stimulation. LBNP is extensively applied to stimulate sympathetic nerve activity. Mild LBNP without significant tachycardiac response and systemic hypotension appreciably increases muscle sympathetic nerve activity assessed by microneurography (31, 32) and by norepinephrine measurements in plasma and tissue (7, 16). Our hypothesis was that the sensitivity of cerebral vasoreactivity to hypercapnia would be diminished if baroreflex-stimulated sympathetic nerve activity restrained cerebral vasodilation or modulated cerebral vasomotor tone.
METHODS
Subjects.
Healthy young subjects [6 men and 3 women, age 25.0 yr (SD 3.1) and body mass index 23.8 kg/m2 (SD 3.8)] gave a written consent to participate in the study. The study protocol was approved by the Institutional Review Board for the Protection of Human Subjects at University of North Texas Health Science Center at Fort Worth. All volunteer participants were clinically confirmed to be free of cardiovascular, metabolic, renal, and pulmonary diseases. Before the experiment, the participants underwent laboratory orientation to familiarize them with the experimental procedure and measurements applied in the study.
Measurements.
During the experiment, the subject's heart rate (HR) was monitored from a standard electrocardiogram lead. Radial arterial pressure (ABP) was continuously measured by tonometry (Colin model 7000 Tonometry, San Antonio, TX). We (39) and others (15, 41) have shown this noninvasive ABP measurement to be highly correlated with ABP measured from intra-arterial catheter. Arterial O2 saturation (SaO2) was estimated by pulse oximetry (BIOPAC Oximeter, Santa Barbara, CA). MCAV was determined by transcranial Doppler (TCD) sonography using a 2-MHz probe (EZ-Dop DWL System, Singen, Germany) positioned over the left temporal window. Regional cerebral tissue oxygenation (ScO2) of the prefrontal cortex and regional forearm muscle oxygenation (SmO2) were monitored by near-infrared spectroscopy (NIRS, Somanetics, 4100 INVOS Cerebral Oximeter, Troy, MI) using sensors placed on the right side of the forehead and on the right brachioradialis, respectively. This NIRS technique has been validated, and the measured ScO2 is highly correlated with jugular venous oxygenation (9, 17). These measurements and the techniques of TCD sonography and NIRS have been applied in our studies (6, 8). Breath-by-breath inspired and expired fractions of O2 and CO2 were measured using a mass spectrometer (Perkin-Elmer, 1100 Medical Gas Analyzer, St. Louis, MO), which was calibrated against room air and gas containing 10% O2 and 10% CO2 balanced with N2. Continuous partial pressure of transcutaneous CO2 (PtcCO2) was determined by a Radiometer sensor placed on the subject's ear lobe (TOSCA 500, Copenhagen). The sensor's temperature was set and maintained at 42°C, which arterialized the local dermal capillary blood flow. This noninvasive continuous PtcCO2 is highly correlated with partial pressure of arterial CO2 (PaCO2) (4, 22, 26). Before each test, the PtcCO2 sensor was calibrated with TOSCA commercial gas of a known CO2 concentration. Analog data were continuously digitized on-line at 250 Hz by a computer interfaced with a data-acquisition system (BIOPAC MP150, Santa Barbara, CA). Partial pressure of end-tidal CO2 (PetCO2) was calculated off-line from the product of ambient barometric pressure and the fraction of end-tidal CO2. Cerebral vascular conductance (CVC) was estimated from the ratio of MCAV to mean arterial pressure (MAP).
Protocol.
The experiment was conducted with the subject in the supine position with the lower body sealed in an airtight LBNP box at a room temperature of 24 ± 1°C. The subject wore a face mask throughout the experiment. The subject rested for ≥15 min after instrumentation, and then baseline HR, ABP, MCAV, SaO2, ScO2, SmO2, PetCO2, and PtcCO2 were recorded for ∼3 min, followed by a rebreathing procedure (Fig. 1). During rebreathing the subject breathed into and out of a prefilled bag that contained ∼10 liters of the mixed gas with ∼3% CO2 and <40% O2 balanced with N2. This air mixture ensured that the fraction of inspired O2 remained above 21% at the end of the rebreathing procedure, which excluded hypoxic influence (Fig. 1). All subjects completed ≥6 min rebreathing except one who completed only 5 min rebreathing due to an equipment malfunction. After recovery from the rebreathing procedure, −15 Torr of LBNP was applied. While LBNP was maintained at −15 Torr, the cardiovascular, respiratory, and oxygenation data were collected for ≥2 min followed by the rebreathing-hypercapnia procedure superimposed on the mild, steady-state LBNP.
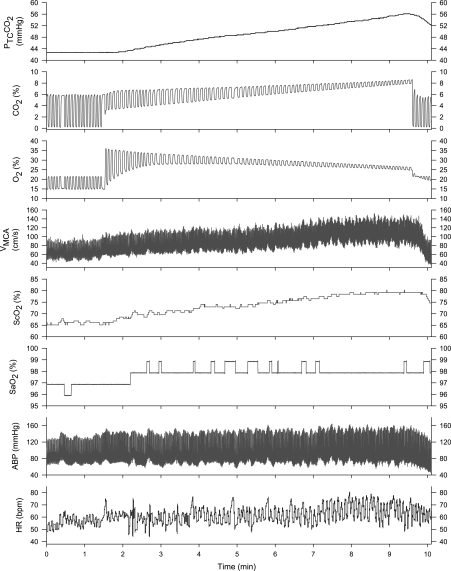
Fig. 1.
Rebreathing-induced hypercapnia at rest. Top to bottom: partial pressure of transcutaneous CO2 (PtcCO2), fractional CO2, fractional O2, blood flow velocity of middle cerebral artery (MCAV), cerebral tissue O2 saturation (ScO2), arterial O2 saturation (SaO2), arterial blood pressure (ABP), and heart rate (HR). There is a time delay in PtcCO2 response to the rebreathing procedure, although PtcCO2 parallels end-tidal CO2 during a ramp hypercapnia. At the end of the rebreathing procedure, the inspired O2 remained ≥21%.
Data analyses.
A section of 1-min continuous data before the rebreathing procedure at rest and during LBNP was averaged to provide control data. Data collected during 30-s interval of the rebreathing-elicited hypercapnia were averaged and plotted against the gradually increasing PetCO2 and PtcCO2. Control data at rest and during LBNP were compared by paired t-tests. Relationships of cerebral vascular conductance, MCAV, and cerebral tissue oxygenation with PetCO2 and PtcCO2 were analyzed by linear regression. All group data were reported as group mean ± SD. Values of P ≤0.05 were taken to indicate statistical significance. Statistic Analysis System software (SAS, Cary, NC) was used for the data analyses.
RESULTS
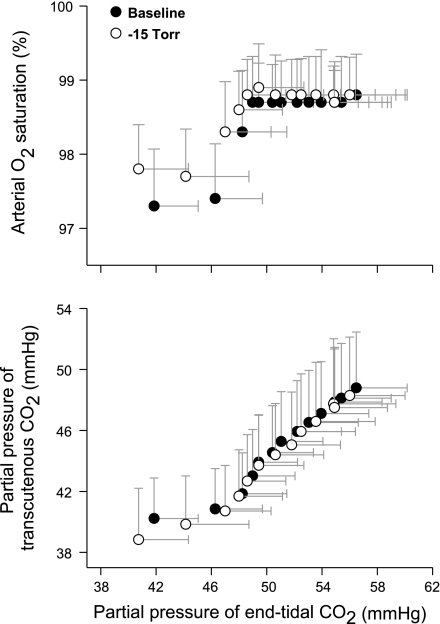
Table 1 summarizes the data at rest and during LBNP before the rebreathing-induced hypercapnia. LBNP at −15 Torr did not significantly change HR or blood pressure values. These similar cardiovascular data suggested a complete recovery from the prior rebreathing protocol. Mild steady-state LBNP induced modest hypocapnia prior to rebreathing, as indicated by significantly lower PetCO2 and PtcCO2 values, which was associated with a higher SaO2. LBNP effectively augmented sympathetic nerve activity, as indicated by lower cerebral vascular conductance and muscle O2 saturation. During the rebreathing procedure, PetCO2 gradually increased to 56.5 ± 3.7 and 56.0 ± 4.0 mmHg at rest and during LBNP, respectively. This hypercapnia during rebreathing was not confounded by hypoxia because inspired O2 was maintained ≥21% (Fig. 1) and SaO2 remained elevated throughout rebreathing at rest and with LBNP (Fig. 2). Although PtcCO2 was highly correlated with PetCO2, a delay of approximately 30–60 s in PtcCO2-assessed hypercapnia was observed during the rebreathing procedure, accompanied by a smaller magnitude of PtcCO2 response vs. that of PetCO2 (Fig. 2, bottom) during the rebreathing procedure at rest or during LBNP. Following the changes in PetCO2, PtcCO2 values similarly increased to 48.8 ± 3.7 and 48.3 ± 3.8 mmHg at rest and during LBNP, respectively.
Table 1.
Control data before the rebreathing-hypercapnia procedure
| HR, beats/min | SBP, mmHg | DBP, mmHg | MAP, mmHg | MCAV, cm/s | CVC, U | SaO2, % | ScO2, % | PetCO2, mmHg | PtcCO2, mmHg | SmO2, % | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | 60 ± 9 | 116 ± 14 | 60 ± 8 | 79 ± 9 | 61.0 ± 6.8 | 0.78 ± 0.10 | 97.2 ± 0.8 | 68.2 ± 6.8 | 41.9 ± 3.2 | 40.2 ± 2.7 | 67.4 ± 3.6 |
| LBNP | 63 ± 8 | 119 ± 10 | 65 ± 5 | 83 ± 6 | 57.3 ± 9.3 | 0.70 ± 0.11 | 97.8 ± 0.6* | 66.4 ± 6.7 | 40.7 ± 3.6* | 38.8 ± 3.4* | 64.4 ± 6.3* |
| P | 0.149 | 0.548 | 0.139 | 0.197 | 0.224 | 0.057 | 0.008 | 0.055 | 0.016 | 0.014 | 0.037 |
Data are means ± SD of the mean. P indicates P values from paired t-tests. LBNP, lower-body negative pressure; HR, heart rate; SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure; MCAV, mean blood flow velocity of middle cerebral artery; CVC, estimated cerebral vascular conductance; SaO2, systemic arterial O2 saturation; ScO2, cerebral tissue O2 saturation; PetCO2, partial pressure of end-tidal CO2; PtcCO2, partial pressure of transcutaneous CO2; SmO2: muscle tissue O2 saturation; n = 9 in all group data except SmO2 (n = 7).
Significant difference based on the paired t-test (P ≤ 0.05).
Fig. 2.
Associations of PetCO2 with arterial O2 saturation and with PtcCO2 at rest and during lower-body negative pressure (LBNP). Top: SaO2 increases within a minute of rebreathing, which reaches plateau throughout the end of the rebreathing procedure at rest or during LBNP. Bottom: compared with PetCO2, responses of PtcCO2 to rebreathing are less robust and delayed. Data are group mean ± SD from 9 subjects.
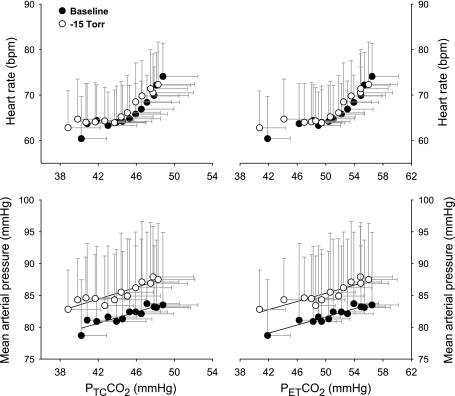
The rebreathing-elicited hypercapnia stimulated tachycardiac and hypertensive responses (Fig. 3). Neither the tachycardiac response nor the hypertensive response in terms of unit increase in PtcCO2 or PetCO2 was statistically different at rest and during LBNP −15 Torr, suggesting a constant sensitivity of the chemoreflex-mediated systemic hemodynamic response.
Fig. 3.
Responses of heart rate and mean arterial pressure during the rebreathing elicited hypercapnia at rest and during LBNP. Top: rate of tachycardiac response stimulated by CO2 is not significantly different at rest and during LBNP, i.e., 1.21 vs. 1.02 beats·min−1·mmHg−1 (P = 0.46) in terms of unit increase in partial pressure of transcutaneous CO2 (top left); 0.87 vs 0.79 beats·min−1·mmHg−1 (P = 0.69) in terms of unit increase in partial pressure of end-tidal CO2 (top right). Bottom: the magnitude of increase in mean arterial pressure during hypercapnia also is not statistically different at rest and during LBNP: i.e., 0.36 vs. 0.45 mmHg/mmHg (P = 0.37) in terms of unit increase in partial pressure of transcutaneous CO2 (bottom left); 0.25 vs. 0.35 mmHg/mmHg (r = 0.20) in terms of unit increase in partial pressure of end-tidal CO2 (bottom right). Data are group mean ± SD from 9 subjects.
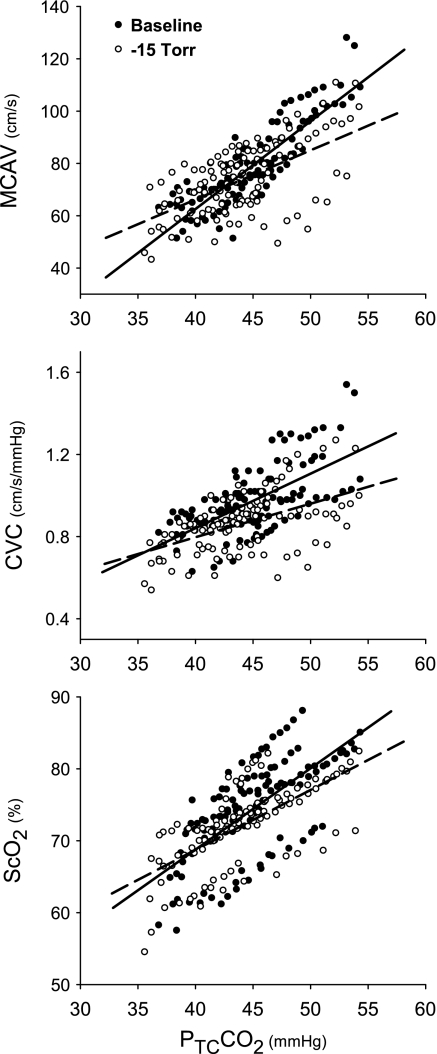
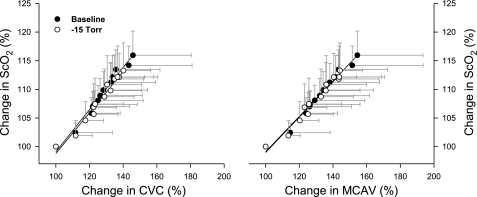
However, the rates of increase in MCAV, CVC, and ScO2 per unit increase in CO2 were greater at rest than during LBNP (Fig. 4). Table 2 summarizes the slope data of MCAV/PtcCO2, CVC/ PtcCO2, ScO2/PtcCO2, MCAV/PetCO2, CVC/PetCO2, and ScO2/PetCO2. Nevertheless, the group slopes of ΔScO2/ΔCVC or ΔScO2/ΔMCAV were essentially identical at rest and during LBNP (Fig. 5).
Fig. 4.
Cerebral hemodynamic responses to hypercapnia. All cerebral hemodynamic responses are linearly (P < 0.001) associated with increase in PtcCO2 during rebreathing. The rates of cerebral hemodynamic responses to hypercapnia are significantly greater at rest than during LBNP (−15 Torr). The slopes of MCAV/PtcCO2 (cm·s−1·mmHg−1) are 3.38 vs. 1.90 (P = 0.0001); cerebral vascular conductance (CVC)/PtcCO2 (unit/mmHg) are 0.026 vs. 0.017 (P = 0.0162); and ScO2/PtcCO2 (%/mmHg) are 1.13 vs. 0.83 (P = 0.0434). Solid line, regression line at rest; dash line, regression line during LBNP.
Table 2.
Slope data of cerebral hemodynamic response during hypercapnia
| Baseline |
−15 Torr |
||||||
|---|---|---|---|---|---|---|---|
| Slope | P | r | Slope | P | r | Baseline vs. −15 Torr, P Value* | |
| MCAV/PtcCO2, cm · s−1 · mmHg−1 | 3.38 ± 0.18 | 0.0001 | 0.88 | 1.90 ± 0.24 | 0.0001 | 0.61 | 0.0001 |
| CVC/PtcCO2, U/mmHg | 0.026 ± .003 | 0.0001 | 0.64 | 0.017 ± .003 | 0.0001 | 0.53 | 0.0162 |
| ScO2/PtcCO2, %/mmHg | 1.13 ± 0.12 | 0.0001 | 0.67 | 0.83 ± 0.09 | 0.0001 | 0.66 | 0.0434 |
| MCAV/PetCO2, cm · s−1 · mmHg−1 | 2.62 ± 0.16 | 0.0001 | 0.83 | 1.68 ± 0.18 | 0.0001 | 0.67 | 0.0002 |
| CVC/PetCO2, U/mmHg | 0.020 ± .003 | 0.0001 | 0.61 | 0.013 ± .002 | 0.0001 | 0.52 | 0.0350 |
| ScO2/PetCO2, %/mmHg | 0.89 ± 0.10 | 0.0001 | 0.66 | 0.63 ± 0.07 | 0.0001 | 0.64 | 0.0342 |
Slope data include the estimate of the SD of the slopes.
Baseline vs. −15 Torr P value indicates significant difference in the slopes between baseline and LBNP.
Fig. 5.
Correlations of ScO2 vs. CVC responses and ScO2 vs. MCAV responses during the rebreathing-hypercapnia at rest and with LBNP. Change in cerebral tissue oxygenation is significantly explained by change in cerebral perfusion, in terms of changes in CVC (left) or MCAV (right). There is no significant difference between the baseline (at rest) and −15 Torr (during LBNP) in either the slope of ΔSaO2/ΔCVC 0.37 vs. 0.36 (P = 0.67) or the slope of ΔSaO2/ΔMCAV 0.31 vs. 0.31 (P = 0.98). Data are group mean ± SD from 9 subjects.
DISCUSSION
This study suggests that the sensitivity of cerebral vasomotor reactivity to hypercapnia is diminished during LBNP −15 Torr compared with the baseline response. Specifically, the data show that LBNP-augmented sympathetic nerve activity decreases the functional reserve of the cerebral circulation in response to CO2 stimulus.
Evidence of sympathoexcitation during LBNP.
Orthostatic challenges stimulate adrenergic activity as indicated by increases in muscle sympathetic nerve activity (31, 32) and plasma norepinephrine (7, 16). Our previous study (5) confirmed that arterial plasma renin activity and norepinephrine concentrations were elevated by LBNP. In the present study, we observed that muscle tissue O2 saturation fell slightly but significantly during LBNP (Table 1). Since O2 demand (or metabolic rate during LBNP) in resting skeletal muscle was considered to remain constant, a decrease in the control SmO2 was most likely associated with a decrease in O2 delivery and/or blood flow, caused by the sympathoexcitation-stimulated peripheral vasoconstriction during LBNP. However, mild LBNP-stimulated sympathoexcitation did not significantly change either heart rate or systemic arterial pressure, indicating a limited disturbance in the systemic homeostasis.
During orthostatic challenge, cerebral hypoperfusion may result from baroreflex-mediated sympathoexcitation (12) and/or hyperventilation-induced hypocapnia (6, 24). This study revealed modest declines in PetCO2 and PtcCO2 during LBNP before the rebreathing procedure. Furthermore, the LBNP-induced reduction of venous return or central hypovolemia could diminish cardiac output, which might influence the cerebral hemodynamic response. However, this central hypovolemic effect on the shift of cerebral perfusion was insignificant based on current (Table 1) and previous observations (8). Furthermore, cerebral autoregulation in response to systemic hypotension was maintained during the LBNP-induced central hypovolemia (8). Shoemaker et al. (35) reported an increase in cardiac output (∼1.1 l/min) during a rebreathing protocol with a change in PetCO2 from 40 to 58 mmHg. Collectively, these studies suggested that central hypovolemia during mild LBNP would not be a significant factor on the sensitivity of cerebral vasoreactivity.
Effect of sympathoexcitation on cerebral vasoreactivity.
This study demonstrates that the cerebral hemodynamic responses, in terms of increases in CVC, MCAV, or ScO2 during the rebreathing-elicited hypercapnia, were less sensitive during LBNP than at rest. These results confirm the hypothesis that sympathoexcitation significantly diminishes the cerebrovascular sensitivity in response to hypercapnia during LBNP. This finding is complementary to a previous report (12) of augmented MCAV response to CO2 stimulus during ganglionic blockade. Collectively, these results show that the LBNP-activated sympathoexcitation modulated the sensitivity of the cerebral vasoreactivity to hypercapnia. Nonetheless, a previous study (20) did not find a change in the cerebral vasoreactivity during LBNP. This difference might be related to a different hypercapnic protocol applied in the study (20). However, exercise-augmented sympathetic nerve activity did not alter (25) or even enhanced (30) cerebral CO2 reactivity. This discrepancy in the cerebral hemodynamic response is probably related to the exercise-elicited central command, as evident with significant tachycardia during mild to moderate exercise such as handgrip (1, 29) or leg cycling (25). This feedforward mechanism overrides the brain stem, which interferes with the chemoreflex and baroreflex. Furthermore, the exercise-induced increases in metabolic rate, blood plasma potassium, osmolality, and temperature may counteract the sympathetic influence on the systemic and cerebral vasomotor response (30).
The rate of increase in MCAV, CVC, and ScO2 per unit hypercapnia (i.e., mmHg increase in CO2) was smaller during LBNP; the relationship of the changes in ScO2 with MCAV or with CVC during the rebreathing procedure was unaltered by LBNP (Fig. 5). These results demonstrate that the response of cerebral tissue O2 saturation to hypercapnia is related to the change in cerebral O2 delivery (8, 36), and that an increase in ScO2 stimulated by hypercapnia reflects an increase in cerebral perfusion indicated by the changes in MCAV and CVC (Fig. 1). This observation demonstrates that cerebral tissue oxygenation depends on cerebral perfusion and their relationship was not affected by sympathetic nerve activity.
Implication of the study.
Cerebral vasoreactivity is considered an indicator of the functional reserve of the cerebral circulation (2, 14, 36). An important clinical implication of the present study is that some physiological and pathological conditions, such as aging, hypertension, congestive heart failure, and obstructive sleep apnea, which are commonly associated with a heightened sympathetic nerve activity or hyperadrenergic activity, may diminish the functional reserve of cerebral hemodynamic responses to metabolic demand. This diminished cerebral vasoreactivity places these individuals at an increased risk for cerebral hypoperfusion and potentially compromises their physiological or cognitive function when cerebral tissue O2 demand increases. Our results suggest that prevention or treatment of a hyperadrenergic state may help improve the functional reserve of the cerebral circulation or the cerebral hemodynamic response.
Methodological considerations.
Transcranial Doppler sonography measures blood flow velocity, which is affected by both blood flow and diameter of the vessel. As the blood vessel diameter was not measured, we could not compare CBF under the different conditions. On the other hand, diameters of conduit vessels, such as the middle cerebral artery, are reported to be relatively constant in response to changes in CO2 and LBNP (34), so the measured responses of MCAV should provide a reliable index of changes in CBF. Although NIRS is a common technique applied to monitor regional tissue oxygenation (9, 18, 27), it cannot differentiate the oxyhemoglobin contents within the various vessels of the region. However, the hemoglobin volume in the venous bed or capacitance vessels is predominant compared with that within the arterial bed or resistance vessels (38). Thus the tissue oxygenation is more related to a change in the venous oxyhemoglobin, and ScO2 has been validated to be significantly correlated with jugular venous oxygenation (9, 17). We believe that NIRS is able to measure the response of ScO2. Breath-by-breath PetCO2 and continuous PtcCO2 were applied to monitor the rebreathing-elicited hypercapnia. However, these indirect changes in Pco2 could differ from PaCO2 or pH in the cerebral tissue. Nonetheless, in healthy subjects without circulatory or respiratory disease, a breath-by-breath measured continuous response in PetCO2 or PtcCO2 should faithfully reflect the change in PaCO2 (or tissue pH) during a ramp hypercapnia at rest or during steady-state orthostatic challenge (10, 11). Furthermore, the study outcome was the same whether the cerebral vasoreactivity data were plotted against the changes in PetCO2 or PtcCO2 during the rebreathing-elicited hypercapnia (Table 2). The order of the rebreathing tests at rest and during LBNP was not randomized. However, neither the control cardiovascular data (Table 1) nor the response or the change in arterial O2 saturation and CO2 (Fig. 2) were different with and without LBNP. Furthermore, the sensitivity of the chemoreflex-stimulated tachycardia and hypertensive responses remained constant at rest and during LBNP (Fig. 3). We postulated that the residual effect of the prior breathing protocol at rest on the chemoreceptor and the systemic hemodynamic and respiratory functions during LBNP should be insignificant. Therefore, the difference in cerebral vasoreactivity to hypercapnia at rest and during LBNP was not due to the order of the rebreathing tests.
Conclusion.
The present study suggests that the orthostasis-stimulated sympathoexcitation diminishes the sensitivity of cerebral vasoreactivity, in terms of the magnitude of the responses in MCAV, CVC, and ScO2 during hypercapnia. Our results provide evidence that pathological and physiological conditions associated with a heightened sympathetic nerve activity diminish the functional reserve of the cerebral circulation or the cerebral hemodynamic response during CO2 stimulation.
GRANTS
This study was supported in part by National Heart, Lung, and Blood Institute Grant R01-HL-65613 and a Government Scholarship from the People's Republic of China to P. Zhang.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We sincerely thank our volunteer subjects for their cheerful cooperation and Dr. Robert Mallet for his comments and suggestions.
REFERENCES
- 1. Ainslie PN, Ashmead JC, Ide K, Morgan BJ, Poulin MJ. Differential responses to CO2 and sympathetic stimulation in the cerebral and femoral circulations in humans. J Physiol 566: 613–624, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bonoczk P, Panczel G, Nagy Z. Vasoreactivity in patients with periventricular white matter lucency. Acta Neurol Scand 110: 254–259, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Carey BJ, Manktelow BN, Panerai RB, Potter JF. Cerebral autoregulatory responses to head-up tilt in normal subjects and patients with recurrent vasovagal syncope. Circulation 104: 898–902, 2001 [DOI] [PubMed] [Google Scholar]
- 4. Casati A, Squicciarini G, Malagutti G, Baciarello M, Putzu M, Fanelli A. Transcutaneous monitoring of partial pressure of carbon dioxide in the elderly patient: a prospective, clinical comparison with end-tidal monitoring. J Clin Anesth 18: 436–440, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Formes K, Wray DW, OYurvati A, Weiss M, Shi X. Sympathetic cardiac influence and arterial blood pressure instability. Autonom Neurosci Basic Clin 118: 116–124, 2005 [DOI] [PubMed] [Google Scholar]
- 6. Formes K, Zhang P, Tierney N, Schaller F, Shi X. Chronic physical activity mitigates cerebral hypoperfusion during central hypovolemia in elderly humans. Am J Physiol Heart Circ Physiol 298: H1029–H1037, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Goldsmith SR, Francis GS, Cowley AW, Cohn JN. Response of vasopressin and norepinephrine to lower body negative pressure in humans. Am J Physiol Heart Circ Physiol 243: H970–H973, 1982 [DOI] [PubMed] [Google Scholar]
- 8. Guo H, Tierney N, Schaller F, Raven PB, Smith SA, Shi X. Cerebral autoregulation is preserved during orthostatic stress superimposed with systemic hypotension. J Appl Physiol 100: 1785–1792, 2006 [DOI] [PubMed] [Google Scholar]
- 9. Henson LC, Calalang C, Temp JA, Ward DS. Accuracy of a cerebral oximeter in healthy volunteers under conditions of isocapnic hypoxia. Anesthesiology 88: 58–65, 1998 [DOI] [PubMed] [Google Scholar]
- 10. Immink RV, Secher NH, Roos CM, Pott F, Madsen PL, van Lieshout JJ. The postural reduction in middle cerebral artery blood velocity is not explained by PaCO2. Eur J Appl Physiol 96: 609–614, 2006 [DOI] [PubMed] [Google Scholar]
- 11. Immink RV, Truijen J, Secher NH, Van Lieshout JJ. Transient influence of end-tidal carbon dioxide tension on the postural restraint in cerebral perfusion. J Appl Physiol 107: 816–823, 2009 [DOI] [PubMed] [Google Scholar]
- 12. Jordan J, Shannon JR, Diedrich A, Black B, Costa F, Robertson D, Biaggioni I. Interaction of carbon dioxide and sympathetic nervous system activity in the regulation of cerebral perfusion in humans. Hypertension 36: 383–388, 2000 [DOI] [PubMed] [Google Scholar]
- 13. Jorgensen LG, Perko M, Perko G, Secher NH. Middle cerebral artery velocity during head-up tilt induced hypovolaemic shock in humans. Clin Physiol 13: 323–336, 1993 [DOI] [PubMed] [Google Scholar]
- 14. Kadoi Y, Hinohara H, Kunimoto F, Saito S, Ide M, Hiraoka H, Kawahara F, Goto F. Diabetic patients have an impaired cerebral vasodilatory response to hypercapnia under propofol anesthesia. Stroke 34: 2399–2403, 2003 [DOI] [PubMed] [Google Scholar]
- 15. Kemmotsu O, Ueda M, Otsuka H, Yamamura T, Okamura A, Ishikawa T, Winter DC, Eckerle JS. Blood pressure measurement by arterial tonometry in controlled hypotension. Anesth Analg 73: 54–58, 1991 [DOI] [PubMed] [Google Scholar]
- 16. Khan MH, Sinoway LI, MacLean DA. Effects of graded LBNP on MSNA and interstitial norepinephrine. Am J Physiol Heart Circ Physiol 283: H2038–H2044, 2002 [DOI] [PubMed] [Google Scholar]
- 17. Kim MB, Ward DS, Cartwright CR, Kolano J, Chlebowski S, Henson LC. Estimation of jugular venous O2 saturation from cerebral oximetry or arterial O2 saturation during isocapnic hypoxia. J Clin Monit Comput 16: 191–199, 2000 [DOI] [PubMed] [Google Scholar]
- 18. Kolb JC, Ainslie PN, Ide K, Poulin MJ. Protocol to measure acute cerebrovascular and ventilatory responses to isocapnic hypoxia in humans. Respir Physiol Neurobiol 141: 191–199, 2004 [DOI] [PubMed] [Google Scholar]
- 19. Lagi A, Cencetti S, Corsoni V, Georgiadis D, Bacalli S. Cerebral vasoconstriction in vasovagal syncope: any link with symptoms? A transcranial Doppler study. Circulation 104: 2694–2698, 2001 [DOI] [PubMed] [Google Scholar]
- 20. LeMarbre G, Stauber S, Khayat RN, Puleo DS, Skatrud JB, Morgan BJ. Baroreflex-induced sympathetic activation does not alter cerebrovascular CO2 responsiveness in humans. J Physiol 551: 609–616, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Levine BD, Giller CA, Lane LD, Buckey JC, Blomqvist CG. Cerebral versus systemic hemodynamics during graded orthostatic stress in humans. Circulation 90: 298–306, 1994 [DOI] [PubMed] [Google Scholar]
- 22. Nosovitch MA, Johnson JO, Tobias JD. Noninvasive intraoperative monitoring of carbon dioxide in children: end-tidal versus transcutaneous techniques. Paediatr Anaesth 12: 48–52, 2002 [DOI] [PubMed] [Google Scholar]
- 23. Novak V, Novak P, Spies JM, Low PA. Autoregulation of cerebral blood flow in orthostatic hypotension. Stroke 29: 104–111, 1998 [DOI] [PubMed] [Google Scholar]
- 24. Novak V, Spies JM, Novak P, McPhee BR, Rummans TA, Low PA. Hypocapnia and cerebral hypoperfusion in orthostatic intolerance. Stroke 29: 1876–1881, 1998 [DOI] [PubMed] [Google Scholar]
- 25. Ogoh S, Hayashi N, Inagaki M, Ainslie PN, Miyamoto T. Interaction between the ventilatory and cerebrovascular responses to hypo- and hypercapnia at rest and during exercise. J Physiol 586: 4327–4338, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Oshibuchi M, Cho S, Hara T, Tomiyasu S, Makita T, Sumikawa K. A comparative evaluation of transcutaneous and end-tidal measurements of CO2 in thoracic anesthesia. Anesth Analg 97: 776–779, 2003 [DOI] [PubMed] [Google Scholar]
- 27. Peltonen JE, Kowalchuk JM, Paterson DH, DeLorey DS, duManoir GR, Petrella RJ, Shoemaker JK. Cerebral and muscle tissue oxygenation in acute hypoxic ventilatory response test. Respir Physiol Neurobiol 155: 71–81, 2007 [DOI] [PubMed] [Google Scholar]
- 28. Pretnar-Oblak J, Sabovic M, Sebestjen M, Pogacnik T, Zaletel M. Influence of atorvastatin treatment on l-arginine cerebrovascular reactivity and flow-mediated dilatation in patients with lacunar infarctions. Stroke 37: 2540–2545, 2006 [DOI] [PubMed] [Google Scholar]
- 29. Rasmussen P, Plomgaard P, Krogh-Madsen R, Kim YS, van Lieshout JJ, Secher NH, Quistorff B. MCA Vmean and the arterial lactate-to-pyruvate ratio correlate during rhythmic handgrip. J Appl Physiol 101: 1406–1411, 2006 [DOI] [PubMed] [Google Scholar]
- 30. Rasmussen P, Stie H, Nielsen B, Nybo L. Enhanced cerebral CO2 reactivity during strenuous exercise in man. Eur J Appl Physiol 96: 299–304, 2006 [DOI] [PubMed] [Google Scholar]
- 31. Rea RF, Hamdan M, Clary MP, Randels MJ, Dayton PJ, Strauss RG. Comparison of muscle sympathetic responses to hemorrhage and lower body negative pressure in humans. J Appl Physiol 70: 1401–1405, 1991 [DOI] [PubMed] [Google Scholar]
- 32. Rea RF, Wallin BG. Sympathetic nerve activity in arm and leg muscles during lower body negative pressure in humans. J Appl Physiol 66: 2778–2781, 1989 [DOI] [PubMed] [Google Scholar]
- 33. Serrador JM, Picot PA, Rutt BK, Shoemaker JK, Bondar RL. MRI measures of middle cerebral artery diameter in conscious humans during simulated orthostasis. Stroke 31: 1672–1678, 2000 [DOI] [PubMed] [Google Scholar]
- 34. Serrador JM, Picot PA, Rutt BK, Shoemaker JK, Bondar RL. MRI measures of middle cerebral artery diameter in conscious humans during simulated orthostasis. Stroke 31: 1672–1678, 2000 [DOI] [PubMed] [Google Scholar]
- 35. Shoemaker JK, Vovk A, Cunningham DA. Peripheral chemoreceptor contributions to sympathetic and cardiovascular responses during hypercapnia. Can J Physiol Pharmacol 80: 1136–1144, 2002 [DOI] [PubMed] [Google Scholar]
- 36. Terborg C, Gora F, Weiller C, Rother J. Reduced vasomotor reactivity in cerebral microangiopathy : a study with near-infrared spectroscopy and transcranial Doppler sonography. Stroke 31: 924–929, 2000 [DOI] [PubMed] [Google Scholar]
- 37. Vicenzini E, Altieri M, Michetti PM, Ricciardi MC, Ciccariello M, Shahabadi H, Puccinelli F, Lenzi GL, Di Piero V. Cerebral vasomotor reactivity is reduced in patients with erectile dysfunction. Eur Neurol 60: 85–88, 2008 [DOI] [PubMed] [Google Scholar]
- 38. Watzman HM, Kurth CD, Montenegro LM, Rome J, Steven JM, Nicolson SC. Arterial and venous contributions to near-infrared cerebral oximetry. Anesthesiology 93: 947–953, 2000 [DOI] [PubMed] [Google Scholar]
- 39. Wray DW, Formes KJ, Weiss MS, OYurvati AH, Raven PB, Zhang R, Shi X. Vagal cardiac function and arterial blood pressure stability. Am J Physiol Heart Circ Physiol 281: H1870–H1880, 2001 [DOI] [PubMed] [Google Scholar]
- 40. Zhang R, Levine BD. Autonomic ganglionic blockade does not prevent reduction in cerebral blood flow velocity during orthostasis in humans. Stroke 38: 1238–1244, 2007 [DOI] [PubMed] [Google Scholar]
- 41. Zorn EA, Wilson MB, Angel JJ, Zanella J, Alpert BS. Validation of an automated arterial tonometry monitor using Association for the Advancement of Medical Instrumentation standards. Blood Press Monit 2: 185–188, 1997 [PubMed] [Google Scholar]