Abstract
Malnutrition during pregnancy adversely affects postnatal forebrain development; its effect upon brain stem development is less certain. To evaluate the role of tryptophan [critical for serotonin (5-HT) synthesis] on brain stem 5-HT and the development of cardiorespiratory function, we fed dams a diet ∼45% deficient in tryptophan during gestation and early postnatal life and studied cardiorespiratory variables in the developing pups. Deficient pups were of normal weight at postnatal day (P)5 but weighed less than control pups at P15 and P25 (P < 0.001) and had lower body temperatures at P15 (P < 0.001) and P25 (P < 0.05; females only). Oxygen consumption (V̇o2) was unaffected. At P15, deficient pups had an altered breathing pattern and slower heart rates. At P25, they had significantly lower ventilation (V̇e) and V̇e-to-V̇o2 ratios in both air and 7% CO2. The ventilatory response to CO2 (% increase in V̇e/V̇o2) was significantly increased at P5 (males) and reduced at P15 and P25 (males and females). Deficient pups had 41–56% less medullary 5-HT (P < 0.01) compared with control pups, without a difference in 5-HT neuronal number. These data indicate important interactions between nutrition, brain stem physiology, and age that are potentially relevant to understanding 5-HT deficiency in the sudden infant death syndrome.
Keywords: chemoreception, carbon dioxide, control of breathing, serotonin, sudden infant death syndrome
malnutrition during pregnancy and early postnatal life is well recognized to seriously impair forebrain development in the offspring (13, 17, 18, 31, 33). Studies in humans and animal models indicate widespread deficits in neuronal proliferation, differentiation, survival, synaptogenesis, and myelination in the forebrain (16–18, 33, 48). Yet the effects of early malnutrition upon the development of the brain stem and homeostatic functions mediated by it are poorly understood and incompletely characterized (43). Of special interest is the relationship of dietary deficiencies during gestation to the sudden infant death syndrome (SIDS), given that the underlying pathophysiological process of SIDS is thought to originate during gestation (24–26) and SIDS predominantly affects infants living in poverty, in whom poor nutrition during pregnancy is known to be a major problem (1, 25, 30).
SIDS is the sudden death of an infant at ≤12 postnatal months that remains unexplained by a complete autopsy, death scene investigation, and review of the clinical history (49). Typically, a seemingly healthy infant is found dead after a sleep period, with the unwitnessed death occurring during sleep itself or during one of the many arousals that occur throughout the night (25, 26). Studies of cardiorespiratory variables in infants who subsequently die of SIDS demonstrate multiple abnormalities in homeostasis, including episodic apnea and bradycardia (22, 23, 32, 41, 47), arousal deficits (21), and impaired gasping (41). Recently, we reported (12) that SIDS is associated with a deficiency of serotonin (5-HT) in regions of the medulla oblongata that are comprised of 5-HT cell bodies and their projection sites and are critical for respiratory and autonomic control. The 5-HT deficiency in the SIDS cases is characterized by a 26% decrease in levels of 5-HT measured by high-performance liquid chromatography (HPLC) and a 22% decrease in levels of tryptophan hydroxylase (TPH), the key biosynthetic enzyme for 5-HT, measured by Western blot (12). This partial deficiency appears to be associated with a constellation of secondary abnormalities in 5-HT receptor and transporter binding and 5-HT cell density and maturation that have likewise been reported in the medulla in SIDS infants in several independent data sets (12, 24, 29, 38, 39).
The possibility that SIDS is a disorder produced by medullary 5-HT deficiency led us to ask in an animal model whether a 5-HT-related dietary deficiency in gestation and early postnatal life can cause a deficiency of medullary 5-HT as well as associated abnormalities in homeostatic control. 5-HT is synthesized in the brain from tryptophan, an essential amino acid, with TPH as the rate-limiting enzyme (14, 15). Systemic tryptophan is taken up at the blood-brain barrier by neutral amino acid transporters (14, 46), and medullary 5-HT levels are known to be quite labile and dependent on tryptophan intake (14, 15). In the present study, we tested the hypothesis that maternal tryptophan deficiency produced by a diet deficient in tryptophan fed to rat dams just before and during pregnancy and extending into early postnatal life [to postnatal day (P)25] results in medullary 5-HT deficiency in the pups and is associated with alterations in cardiorespiratory regulation in early postnatal life. We focused on analysis of ventilation, metabolic rate, and heart rate (HR) in air and in 7% carbon dioxide (CO2) in male and female pups at P5, P15, and P25, i.e., the time frame possibly homologous to SIDS risk in the human infant (see discussion). We also measured the levels of 5-HT with HPLC, the number of medullary 5-HT neurons, and the intensity of 5-HT and TPH immunostaining/5-HT neuron at P25, i.e., the end point of the delivery of the tryptophan-deficient diet. The cellular analysis was performed in the same pups that underwent physiological assessment, thereby allowing for the potential demonstration of homeostatic deficits in pups with a documented medullary 5-HT deficiency.
MATERIALS AND METHODS
All experimental protocols were within the guidelines of the National Institutes of Health for animal use and care and were approved by the Dartmouth College Institutional Animal Use and Care Committee.
Experimental groups and diets.
Sprague-Dawley adult female rats were fed isocaloric, normal-protein, amino acid-based diets in which tryptophan was normal or reduced to values ∼55% of required (Harlan Teklad). The TD.99366 control diet was composed (g/kg) of the following ingredients: 3.5 l-alanine, 12.1 l-arginine HCl, 6.0 l-asparagine, 3.5 l-aspartic acid, 3.5 l-cystine, 40.0 l-glutamic acid, 23.3 glycine, 4.5 l-histidine HCl, monohydrate, 8.2 l-isoleucine, 11.1 l-leucine, 18.0 l-lysine HCl, 8.2 l-methionine, 7.5 l-phenylalanine, 3.5 l-proline, 3.5 l-serine, 8.2 l-threonine, 1.8 l-tryptophan, 5.0 l-tyrosine, 8.2 l-valine, 351.68 sucrose, 150.0 corn starch, 150.0 maltodextrin, 80.0 soybean oil, 30.0 cellulose, 35.0 mineral mix, 8.2 calcium phosphate, monobasic, monohydrate, 13.0 vitamin mix, 2.5 choline bitartrate, and 0.02 tertiary butylhydroquinone, an antioxidant. The TD.08125 tryptophan-deficient diet was equivalent to the TD.99366 diet except that it was reduced in tryptophan to 1 g/kg (∼55% of the estimated nutritional requirement). To keep an isonitrogenous/isocaloric modification of TD.99366, l-glutamic acid was increased to 41.18 g/kg. The animals were acclimated with the new food for at least 2 wk before mating and continued receiving the special diet throughout the pregnancy up to P25. There were four groups of litters: two from dams who received the amino acid control diet (control) and two from dams who received the tryptophan-restricted diet (tryptophan deficient). One set of diet and control litters was used for physiology at different ages: P5, P15, and P25. After evaluation at P25 the brains were used for immunohistochemistry. Separate groups of control and diet-treated litters were used for medullary tissue analysis of monoamine levels at P25. These were controls for another experiment in the laboratory at that time, and the pregnant dams had subcutaneous saline-releasing minipumps implanted at 4–5 days of gestational age.
The dams and pups were kept in the Animal Research Facility at Dartmouth in controlled temperature and light conditions with lights on from 7 AM to 7 PM and lights off from 7 PM to 7 AM. All experiments were performed between 9 AM and 5 PM. Food and water were available ad libitum to the dam. The rat pups were separated from the dam for the physiology experiments and were immediately brought back to the litter upon their completion, usually within 20 min. A total of 36 pups from both sexes were studied physiologically from two litters under the control diet (8 males and 10 females) and three litters under the tryptophan-deficient diet (9 males and 9 females).
Head-out and whole body plethysmography under normoxia and hypercapnia.
Physiological studies were conducted by using either a head-out (7, 8, 35) or a whole body (4, 28, 40) plethysmograph to measure ventilation (V̇e), tidal volume (Vt), respiratory frequency (f), and oxygen consumption (V̇o2). The rectal temperature was measured before and after each experiment with a small thermistor probe. The volume of the head-out plethysmograph used to measure ventilation in P5 rat pups was ∼39 ml (2.5-cm diameter, 8-cm-long cylinder). For the P15 and P25 pups, we measured ventilation with a whole body plethysmograph with a volume of ∼685 ml (7.5-cm diameter, 14.5-cm-long cylinder) connected to a reference chamber of the same size. The inflow gas for the plethysmograph was humidified and matched to the outflow connected to a vacuum system. The flow rate was ∼1 l/min for the whole body plethysmograph; ∼100 ml/min of outflow gas served O2 and CO2 analyzers (S3-A from Applied Electrochemistry and Capstar-100 from CWE, respectively). In the head-out box, the chamber with the pup's head was not sealed and was constantly flushed by either room air or the 7% CO2 challenge gas at a flow rate of ∼0.5 l/min. A port very close to the pup's head was connected to the O2 and CO2 analyzers, which, because of the flow rate of the gas analyzer sampling, obtained expired gas from the pup's nose. We measured chamber pressure by transducer and calibrated the plethysmograph with 0.1-ml injections for the head-out plethysmograph and with 0.2-ml injections for the whole body plethysmograph. The chamber temperature for both the head-out and whole body plethysmographs was measured by a thermometer continuously, and it was kept at ∼30°C by heat lamp. The measurement of breathing for the whole body plethysmograph during air or 7% CO2 was recorded for ∼3 min.
The same pups were studied at all three ages. HR and V̇e were measured during room air and hypercapnia (7% CO2 challenge) periods. The measurement of HR was performed with a telemetric device (CTA-F40 from Data Systems International) with two ECG leads that were placed on the pup's chest skin and kept in place with an elastic bandage. After the ECG leads were placed the pup was placed inside the box, and measurements of breathing and HR were recorded when the animal was settled and calm. For the head-out box, Vt was directly proportional to the pressure deflection caused by the pup's thorax movements, and it was calculated based on the calibration of the box with a known volume of air. For the whole body box, Vt was calculated with the plethysmograph temperature at that time and the pup's averaged temperature from measurements before it was placed in the box and after it was removed from the box. Breathing frequency (f) was counted and used to estimate V̇e per breath.
Medullary tissue 5-HT and metabolite levels.
For analysis of medullary tissue 5-HT, as well as norepinephrine, epinephrine, 3,4-dihydroxyphenylacetic acid (DOPAC, a dopamine metabolite), dopamine, 5-hydroxyindoleacetic acid (5-HIAA, a 5-HT metabolite), and homovanillic acid (HVA, a catecholamine metabolite), pups were killed by pentobarbital overdose and the medullae were removed and frozen en bloc. Ten control (5 male, 5 female) and 10 diet-exposed (5 male, 5 female) P26 pups were used for this analysis. In an effort to measure 5-HT in regions heavily concentrated with 5-HT neurons, we dissected a triangular wedge of tissue from the midline with the base at the ventral medullary surface. This tissue wedge included the majority of medullary 5-HT neurons, which are heavily concentrated in the raphé obscurus, raphé magnus (RM), and raphé pallidus. The tissue was shipped in a frozen state and with medullary levels of 5-HT, and its metabolites were measured by HPLC at the Neurochemistry Core, Vanderbilt University School of Medicine (Nashville, TN) according to published protocols (https://medschool.mc.vanderbilt.edu/cmn/node/12).
TPH and 5-HT immunohistochemistry and data analysis.
On completion of the physiological experiments, the pups were deeply anesthetized with ketamine and xylazine (100 mg/kg and 15 mg/kg, respectively) for perfusion fixation of the brain. After perfusion of 4% paraformaldehyde via the right heart ventricle, brains were removed and equilibrated in 30% sucrose for at least 24 h before being frozen on crushed dry ice. Brains were sectioned frozen at 40 μm thick with a rotary microtome with a chuck cooled with a Peltier device. Sections were collected in 0.1 M phosphate-buffered saline (PBS) and processed floating. For immunohistochemistry, primary antisera were diluted together in buffer containing 0.1% bovine serum albumin (BSA), 0.3% Triton X-100, and PBS. Primary antisera included anti-TPH raised in sheep, which recognizes both isoforms of TPH, i.e., TPH1 and TPH2 (Chemicon/Millipore, Billerica, MA; 1:1,000), and anti-5-HT raised in rabbit (Chemicon/Millipore; 1:10,000). Tissue sections were incubated with both of these primary antisera together for 24 h at room temperature or for 2–3 days in the cold room (4°C) with mild agitation. After rinsing several times with PBS, the tissue was incubated with fluorophore-conjugated secondary antisera diluted to 1:100 in 0.1% BSA, 0.3% Triton X-100, and PBS. Secondary antisera included Alexa 488-conjugated donkey anti-sheep and Alexa 647-conjugated donkey anti-rabbit (Invitrogen, Carlsbad, CA). After rinsing, immunolabeled tissue sections were mounted on glass slides, dried, and coverslipped with mounting medium containing 90% glycerol and 10% PBS pH 8.5–9.
We first tested the hypothesis that TPH and 5-HT immunostaining is decreased in the RM in the tryptophan-deficient pups compared with age-matched control pups. We used 18 control (8 male, 10 female) and 18 diet-exposed (9 male, 9 female) P26 pups for this analysis. The tissue sections from control and tryptophan-deficient pups were processed in parallel as matched pairs or matched quadruplets with aliquots of the same reagents. The tissue sections dually immunolabeled for both TPH and 5-HT were digitally photographed with convention fluorescence illumination and a ×10 objective. For each immunohistochemical run, tissue was photographed at the same time with the same illumination and exposure. For each rat, every fourth section was processed. Of these, at least three sections containing RM were photographed. In each photograph of the RM four to seven cells were randomly selected. For each of these cells a small circle, ∼10 μm2 in area, was placed over the soma in a location that did not overlap the nucleus. Within this circle, the intensity of TPH and 5-HT immunolabeling was measured with Slidebook image acquisition software. In addition, areas of the tissue section where there was little detectable TPH or 5-HT immunolabeling were sampled to determine background fluorescence intensities. For these sampled areas, the mean intensity of each fluorescent channel was determined. Background fluorescence intensity values were subtracted from somatic intensity values for each cell for both Alexa 488 and Alexa 647. The intensity of somatic staining per cell was then averaged for each section of RM sampled. Subsequently, the intensity of each section was averaged together, yielding an individual animal average. Individual averages of immunolabeling intensity were then normalized to their pair-matched control(s) sampled identically, and the average percent deviation from control values was determined.
We next tested the hypothesis that the number of 5-HT neurons, identified by TPH immunostaining, is decreased in the RM in the tryptophan-deficient pups compared with age-matched control pups. To quantify the number of cells in the RM, the same sections were analyzed, and the average number of cells per section was determined for each rat. For this analysis, the same photographs of RM were analyzed by visualizing TPH immunolabeling. All immunolabeled cells that were dispersed in a triangular pattern above the pyramids were counted from each section. Individual averages were used to calculate a group average.
Statistical analysis.
An unpaired t-test was used to analyze the data, comparing V̇e, Vt, respiratory rate, V̇o2, HR, brain tissue amine levels, and number of TPH-immunolabeled cells between control and tryptophan-deficient diet groups in males and in females at each postnatal age. Intensity of immunolabeling between groups was compared with a pair-matched t-test. For the CO2 response data (% increase in V̇e/V̇o2; see Fig. 5) a two-way repeated-measures ANOVA was applied with age and diet as factors and a Bonferroni post hoc test.
Fig. 5.
Postnatal development of the ventilatory response to CO2 expressed as % increase in V̇e/V̇o2 as a function of postnatal age and effect on this CO2 response of exposure to a tryptophan-deficient diet. Dark bars, control diet group; light bars, tryptophan-deficient diet group. These normalized data were analyzed with a 2-way repeated-measures ANOVA with diet and age as factors. There was an overall effect of diet (P < 0.001) and age (P < 0.001). The interactive effect (age × diet) was significant in males (P < 0.001) and of borderline significance in females (P < 0.06). *Post hoc testing (Bonferroni) showed significance in males at P5, P15, and P25 (P < 0.01) and in females at P15 (P < 0.03) and P25 (P < 0.01).
RESULTS
Effect of tryptophan-deficient diet on body weight, body temperature, and oxygen consumption.
The body weights of rat pups from dams fed the tryptophan-deficient diet were the same as those of pups from dams fed the control diet at P5 but were significantly less at P15 and P25 in both males and females (P < 0.001; Fig. 1, top). Basal body temperature was significantly lower in the tryptophan-deficient pups (Fig. 1, middle) at P15 in males and females (P < 0.001) and at P25 in females (P < 0.05). V̇o2 was not significantly different between the two groups at any of the three ages studied (Fig. 1, bottom).
Fig. 1.
Body weight, body temperature, and oxygen consumption (V̇o2) during early postnatal life in rat pups from dams fed a normal diet or a tryptophan-deficient diet. ●, Data from control males (n = 8) and females (n = 10); ○, data from tryptophan-deficient diet males (n = 9) and females (n = 9). Statistical significance (t-test): *P < 0.05, ***P < 0.001. P5, P15, P25, postnatal days 5, 15, and 25.
Effects of tryptophan-deficient diet on ventilation and heart rate while breathing air or 7% CO2 at P5.
Exposure to the tryptophan-deficient diet had no significant effects on V̇e, the ratio of V̇e to V̇o2, Vt, f, or HR while breathing air or 7% CO2 in male or female pups at P5 (Fig. 2).
Fig. 2.
Ventilation (V̇e), V̇e-to-V̇o2 ratio, tidal volume (Vt), respiratory frequency (RR), and heart rate (HR) at postnatal age P5 in rat pups from dams fed a normal diet or a tryptophan-deficient diet. There were no significant differences between values in pups exposed to the normal or the tryptophan-deficient diet (t-test); n in each case is as in Fig. 1. RA, room air.
Effects of tryptophan-deficient diet on ventilation and heart rate while breathing air or 7% CO2 at P15.
At P15, tryptophan-deficient diet-exposed pups had increased V̇e breathing air (males, P < 0.01; females, P < 0.01) and 7% CO2 (females, P < 0.05) compared with age-matched control pups (Fig. 3). This reflected a combination of a greater Vt breathing air (males, P < 0.01; females, P < 0.05) and 7% CO2 (males and females, P < 0.01) together with a tendency for a slower f, which reached significance during the breathing of 7% CO2 (males, P < 0.01; females, P < 0.01). Ventilation normalized to metabolic rate (V̇e/V̇o2) was significantly increased during air breathing in diet-exposed male pups (P < 0.03), an effect that was of borderline significance in females (P < 0.07). There was no significant effect during 7% CO2 exposure. HR was significantly lower at P15 while breathing air in males (P < 0.01) and females (P < 0.05).
Fig. 3.
V̇e, V̇e/V̇o2, Vt, RR, and HR at postnatal age P15 in rat pups from dams fed a normal diet (dark bars) or a tryptophan-deficient diet (light bars). Significant differences between control and tryptophan-deficient diet treatment (t-test): *P < 0.05, **P < 0.01; n in each case is as in Fig. 1.
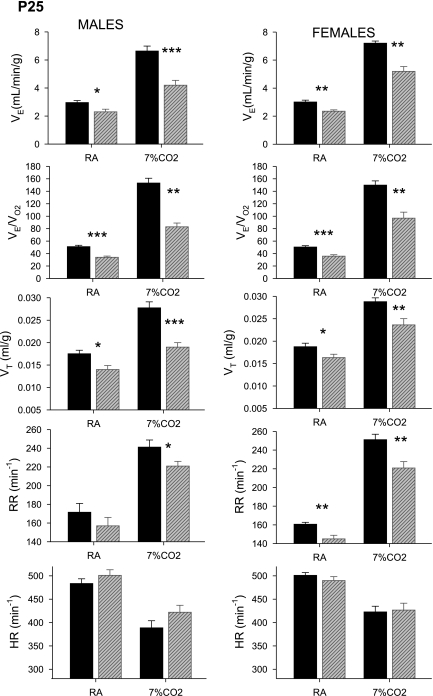
Effects of tryptophan-deficient diet on ventilation and heart rate while breathing air or 7% CO2 at P25.
At P25, diet-exposed pups had significantly decreased V̇e breathing air (male, P < 0.05; female, P < 0.01) and 7% CO2 (male, P < 0.001; female, P < 0.01) (Fig. 4). This lower V̇e reflected a combination of a smaller Vt breathing air (males, P < 0.05; females, P < 0.05) and 7% CO2 (males, P < 0.001; females, P < 0.01) together with a slower f, which reached significance during the breathing of air in females (P < 0.01) and 7% CO2 in males (P < 0.05) and females (P < 0.01). When ventilation was normalized to each pup's metabolic rate, V̇e/V̇o2 was also significantly lower in the diet-exposed pups breathing air (males and females, P < 0.001) and 7% CO2 (males and females, P < 0.01). At P25 there was no difference in HR between treated and control pups.
Fig. 4.
V̇e, V̇e/V̇o2, Vt, RR, and HR at postnatal age P25 in rat pups from dams fed a normal diet (dark bars) or a tryptophan-deficient diet (light bars). Significant differences between control and tryptophan-deficient diet treatment (t-test): *P < 0.05, **P < 0.01, ***P < 0.001; n in each case is as in Fig. 1.
Effects of tryptophan-deficient diet on CO2 response at P5, P15, and P25.
In the control and diet-exposed pups, there was a significant effect on the CO2 response (represented as % increase in V̇e/V̇o2) of both age and diet in both sexes (P < 0.001; 2-way repeated-measures ANOVA with age and diet as factors) and there was a significant interactive effect in males (P < 0.001) and a borderline significant interactive effect in females (P < 0.06) (Fig. 5). Post hoc testing (Bonferroni) indicated significant effects in males at P5 (increased response: P < 0.001) and in both sexes at P15 (decreased response: males, P < 0.001; females, P < 0.03) and P25 (decreased response: males, P < 0.001; females, P < 0.01).
Effect of tryptophan-deficient diet on 5-HT and 5-HIAA content in medulla.
Analysis by HPLC of medullary tissue from wedge-shaped samples of the medullary raphé of P25 pups showed significantly decreased levels of 5-HT and its metabolite, 5-HIAA, in diet-exposed males (P < 0.01 and P < 0.001, respectively) and females (P < 0.001 and P < 0.001, respectively) compared with control pups (Fig. 6). The average decrease in 5-HT was 41% in males and 56% in females; that of 5-HIAA was 56% in males and 74% in females. There were no significant differences in norepinephrine, epinephrine, DOPAC, dopamine, or HVA.
Fig. 6.
Medullary tissue monoamine levels detected by high-performance liquid chromatography (HPLC) in P15 rat pups from dams fed a control or a tryptophan-deficient diet; n = 5 males and females in each group. Significance tested by t-test: **P < 0.01, ***P < 0.001. DOPAC, 3,4-dihydroxyphenylacetic acid, a dopamine (DA) metabolite; 5-HIAA, 5-hydroxyindoleacetic acid, a serotonin (5-HT) metabolite; HVA, homovanillic acid, a catecholamine metabolite.
Effect of tryptophan-deficient diet on tryptophan hydroxylase, 5-HT content, and number of 5-HT neurons in raphé magnus.
Medullary tissue sections from RM of P25 diet-exposed and control pups were analyzed for the intensity of immunolabeling for TPH and 5-HT per cell (Fig. 7). For this, the intensity of immunolabeling was measured within individual cell soma, but not in axon terminal fields where additional tissue 5-HT is located. In addition, we counted the number of TPH-labeled cells per section within RM. The intensity of somatic TPH immunolabeling per cell in diet-exposed pups was the same as in control pups for both males and females (Fig. 7). For males, the intensity of 5-HT immunolabeling measured in the soma was decreased 30% or more in eight of nine diet-exposed pups compared with their controls (P < 0.05). For females, four of nine female rats also showed a 27% or greater reduction in 5-HT intensity relative to pair-matched control pups, but as a group 5-HT immunolabeling intensity was more variable and not significantly different from that in control pups. Counts of the number of TPH-labeled cells per section in RM showed no difference between tryptophan-deficient diet-exposed and control pups in both males and females. For males, the mean ± SE number of TPH-labeled cells in RM of control pups (n = 8) was 23.7 ± 1.7 vs. 24.8 ± 0.7 in diet-exposed pups (n = 9). For females, the cell number in control pups (n = 9) was 29.3 ± 1.9 vs. 28.4 ± 1.8 in diet-exposed pups (n = 9).
Fig. 7.
Tryptophan-deficient diet had no detectable effect on tryptophan hydroxylase (TPH) immunolabeling but decreased the intensity of perisomatic 5-HT immunolabeling in males. A: average intensity of TPH immunolabeling per cell of individual tryptophan-deficient pups expressed as % relative to pair-matched control pups. Each symbol represents the values for 1 animal, with the horizontal bars marking the group average. There was no detectable effect of the diet exposure on TPH immunolabeling in males (M) or females (F), and groups averaged 96% and 100% of controls, respectively. A′ and A″ show representative TPH immunolabeling in the raphé magnus from pair-matched control male (cM, A′) and tryptophan-deficient diet-exposed male (dM, A″). In both cases, TPH-labeled cells (arrows) are clearly visible. B: intensity of perisomatic 5-HT immunolabeling following tryptophan-deficient diet exposure decreases on average to 65% of control values in males (raw intensity values compared with control by t-test: *P < 0.05). Intensity of 5-HT immunolabeling in females was more variable and averaged 96% control values. B′ and B″ show immunolabeling for 5-HT (arrows) in the same cells as A′ and A″. Cell bodies are clearly visible in male control tissue (cM, B′) but less distinct in tissue from tryptophan-deficient diet-exposed male tissue (dM, B″). Scale bar, 30 μm.
DISCUSSION
While the detrimental consequences on cognitive development due to malnutrition in early life are well recognized, little is known of the effects of malnutrition upon homeostatic control regulated by the brain stem. Of special interest are the effects of deficiencies in nutrients that are critical to the synthesis of neurotransmitters involved in brain stem control of homeostasis, such as the amino acid tryptophan required for the synthesis of 5-HT. 5-HT is used by groups of medullary neurons that influence respiratory patterning, gasping, thermal stability, chemosensitivity, HR, and blood pressure (25). In this study, we framed the issue of the effects of nutritional deficiency upon homeostatic control in early life in the context of SIDS, given that SIDS is associated with medullary 5-HT deficiency, likely originates in gestation, and is related to poverty (12, 24–26, 29, 30, 38, 39). Our major findings are that 1) dietary deficiency of tryptophan during gestation and early postnatal life (P0–P25) results in decreased tissue levels of 5-HT measured at P25 in the midline medulla, the key site of the medullary 5-HT system and a region relevant to homeostatic control; 2) decreased medullary 5-HT levels in tryptophan-deficient pups are associated with homeostatic deficits, confirming that optimal homeostasis depends on proper diet; and 3) several homeostatic deficits are age specific, suggesting that different vulnerable periods may exist for different functions. Indeed, these key findings related to diet and brain stem neurochemistry and the interaction of diet with vulnerable periods in the regulation of homeostasis are relevant to the issue of malnutrition and homeostasis in early life beyond the SIDS problem.
Technical limitations of physiological studies.
The major technical limitation in physiological studies of developing rodents involves the measurement of Vt. We employed a head-out plethysmograph with a neck seal for the P5 pups, the smallest tested. This technique accurately and reliably measures Vt by the pressure changes created in the closed body chamber by lung expansion and contraction (7, 8, 35). As the pups grow and become more active, however, they are more difficult to study in the head-out chamber, and we then employ a whole body plethysmograph in which we place the entire pup at P15 and P25. This technique measures Vt via the gas laws applied to the change in chamber pressure that occurs as the inspired gas within the sealed chamber is warmed and further wetted when the inspirate moves from the cooler ambient temperature to the warmer pup temperature (4). However, in smaller animals (all ages studied here) with rapid breathing frequencies, the change in chamber pressure can also reflect a second physical event, the rarefaction and compression of the gas as it flows through the tiny airways (35, 36). Thus, at P15, and less likely at P25, the “tidal volume” that we estimate can be in part due to a pressure change related to airway caliber and resistance (35, 36). This limitation must be put into the context of the value of obtaining ventilatory data from relatively unstressed, unanesthetized pups.
The ideal experimental design in studies using rodent neonates would be to assign one pup from each litter to an experimental group. To minimize costs, both in terms of financial and animal welfare issues, we have chosen to accept the limitation of using all pups from each litter.
An additional limitation of our study design is the necessary stress of the brief maternal separation along with handling of the pup. This may contribute to the slightly higher V̇o2 and V̇e/V̇o2 observed in our results compared with previously published data (45).
Tryptophan depletion-induced effects on pup medullary 5-HT levels.
Tryptophan is critical for the generation of several compounds in the brain, including 5-HT, products of the kynurenine pathway, and the pineal hormone melatonin (44). In this study, we focused on the consequences of tryptophan depletion for 5-HT synthesis and function, while recognizing that deficiencies in related compounds may contribute to the brain stem-mediated outcomes. We ascertained the effect of the maternal dietary tryptophan deficiency on the pups by measuring the level of medullary raphé 5-HT from tissue samples obtained at P25. The 5-HT levels were reduced by 41% and 56% in males and females, respectively. The major 5-HT metabolite, 5-HIAA, was similarly reduced, indicating that the decrease in the 5-HT levels was due to decreased synthesis rather than rapid degradation and turnover. The dietary effect of tryptophan depletion was specific to 5-HT and did not affect other biogenic amines in the medullary tissue samples. Maternal tryptophan depletion did not change the number of medullary 5-HT neurons, as measured by cell counts of TPH-immunoreactive cells, nor did it affect the amount (intensity) of TPH immunolabeling per cell. Yet the amount of 5-HT immunolabeling per cell was decreased in the tryptophan-depleted pups, most consistently in males. These observations are consistent with a previous study reporting no change in TPH cell number in the RM in dietary-depleted pups at the same age (37). Gonzalez et al. (16) reported, however, that a tryptophan-depletion diet results in a decrease in 5-HT cell number. Our results could explain this finding, since these investigators counted cells with 5-HT immunolabeling (16) and decreased immunolabeling of 5-HT per cell as we found may cause some cells to fall below the level of detection, resulting in a lower cell count. In our study, the decreased 5-HT labeling per cell body provides additional support for the HPLC data showing decreases in 5-HT and its metabolites in total tissue. Interestingly, the effect on 5-HT immunolabeling was not as consistent in females as it was in males, suggesting that males and females may adapt to tryptophan depletion differently. The reduction in the medullary 5-HT levels in the pups was only partial as expected because the maternal dietary depletion was also partial. When we applied a diet with 100% depletion of tryptophan, the dams lost weight quickly and did not conceive. The partial 5-HT reduction observed in our study stands in contrast to the genetically engineered Lmx1b conditional knockout mice with a complete reduction in brain stem 5-HT neurons (19, 20). Given that the depletion of medullary 5-HT levels is partial in SIDS infants (12), the dietary-depleted pups appear to represent a closer animal model of the 5-HT defect in SIDS infants.
Tryptophan depletion-induced effects on pup growth.
Pups from dams fed the tryptophan-deficient diet had body weights similar to control pups at P5 but had significantly lower body weights at P15 (−30%) and P25 (−40%) in both sexes. Other rodent models with reduced brain 5-HT levels also exhibit growth retardation during the postnatal period. Lmx1b conditional knockout mice with complete absence of 5-HT neurons exhibit growth retardation from ∼P4 until ∼P55. At ∼P20 these mice have ∼40% lower body weights, an effect that begins to lessen by P25. These mice have severely abnormal breathing and excess mortality in early postnatal life (20). Transgenic mice lacking TPH2, the brain-specific form of TPH, have almost absent brain tissue 5-HT levels, a 50% mortality in the first 4 wk of life, and growth retardation that begins at ∼P6 and ends at ∼P64, i.e., after weaning they “catch up” (2). At ∼P25, these mice have ∼50% lower body weight than control pups (2). Thus normal amounts of brain 5-HT are necessary for optimal growth during early postnatal life but not during gestation or after a certain postnatal age; with severe or near-total absence of 5-HT there is early life mortality and growth retardation (2, 20). In our tryptophan-depletion diet model, we see no early life mortality but do detect growth retardation. The mechanism for this growth retardation is unknown. Mice lacking TPH2 drink milk and have normal digestion, are able to compete with littermates for milk, and can vocalize normally to alert mothers. They have, however, a deficiency in serum levels of insulin-like growth factor (IGF) until ∼2 mo of age (2). It is not clear how the 5-HT deficiency is linked to IGF.
Age-specific respiratory and/or autonomic effects in tryptophan-depleted rat pups.
In this study, the maternal dietary depletion of tryptophan extended from conception through pregnancy into the postnatal period to P25. Nevertheless, the effects were characterized by different patterns at specific ages, that is, the effects were neither uniform nor present at all the postnatal ages analyzed. Despite maternal pre- and postnatal tryptophan deficiency, there were no respiratory, HR, or temperature effects at P5. There was, however, in males a small but significant increase in the CO2 response measured as percent increase in V̇e/V̇o2. At P15, a distinct complex of abnormalities was detected, including 1) decreased body temperature; 2) decreased HR at rest while breathing air; 3) larger Vt and slower breathing frequencies while breathing air or 7% CO2; 4) greater V̇e values in air for males and in air and 7% CO2 for females; and 5) a decreased CO2 response expressed as percent increase in V̇e/V̇o2 in both sexes. The level of V̇e relative to the metabolic rate while breathing air was unaffected. At P25 a different constellation of physiological abnormalities was detected, with 1) decreased body temperature in females; 2) no changes in HR; 3) decreased baseline V̇e breathing room air in males and in females; 4) ∼40% decreased V̇e/V̇o2 while breathing air in males and females, indicating hypoventilation; 5) 40–50% decreased absolute value of V̇e and absolute value of V̇e/V̇o2 while breathing 7% CO2 in males and females; and 6) ∼17% decreased CO2 response expressed as percent increase in V̇e/V̇o2 in males and females due to decreases in Vt and f. Thus the pups at P25 have a decreased drive to breathe in air and a lessened response to CO2.
The development of the brain occurs in successive stages, with different structures and functions maturing in orderly and predictable sequences according to different rates and timing. Human and animal studies indicate that malnutrition imposed at a critical period of brain development is likely to cause permanent impairments in brain structure and function. Dobbing and Sands (10, 11) have emphasized that in humans this critical period extends from the later half of pregnancy to about 2 postnatal years, i.e., the age range in which malnutrition is most common worldwide. Although the precise developmental sequences of the respiratory, HR, CO2 response, and temperature parameters mediated by the medullary 5-HT system in rat pups is unknown, our control data indicate dramatic changes in them between P5, P15, and P25. The absence of an effect of tryptophan depletion (environmental factor) at P5 suggests that the medullary 5-HT system is not a prerequisite for homeostatic function at this age. The demonstration of different profiles of physiological abnormalities at P15 and P25 suggests that the medullary 5-HT system is at different stages of development, the requirement for 5-HT varies according to the stage, and the effect of the deficiency of tryptophan is consequently different. The observation that HR is affected at P15 but not P5 or P25, for example, suggests that the influence of 5-HT on cardiac regulation is most pronounced at this age and P15 is the vulnerable period for 5-HT deficiency upon cardiac patterning. Rats pups with 5-HT lesions induced at P2 demonstrate age-dependent changes in HR and spontaneous bradycardias (7), as do pet-1 null transgenic mice with reduced numbers of medullary 5-HT neurons (8). The observation that an abnormality in CO2 sensitivity is present at P15 and P25 is consistent with cell culture data showing that 5-HT neurons become CO2 sensitive at P12 or later and that the function of 5-HT neurons in central chemoreception becomes more manifest at ∼P12 (6).
The question arises of what age in the rodent is homologous to the peak window of risk for SIDS in the human infant, i.e., 2–4 postnatal months, and whether the vulnerable period of P15–P25 to tryptophan deficiency in rat pups corresponds to the human vulnerable period of SIDS. We cannot pinpoint an exact postnatal age in the rat that coincides with a given postnatal age in the human infant, but we can examine age dependence over a compressed developmental period. For example, others have outlined key rodent postnatal development periods (9): phase 1: early development, from P0 to P10, with eyes closed, no hearing, Hering-Breuer and laryngeal inhibitory reflexes present (34), and baroreflex present (42); phase 2: transitional period, from P11 to P14, with eyes open and hearing present (3); and phase 3: late development, from P15 to P21, when sleep EEG becomes adultlike (5). Within this entire developmental window of P5–P25 we hope to capture age-dependent changes that could be relevant for the age-dependent vulnerability to SIDS. A review of rat postnatal brain stem development indicates that between P11 and P14 there is a major neurochemical reorganization of neurons involved in the control of breathing as well as in other vital functions such as vision, hearing, temperature control, and sleep state (27).
In conclusion, we report abnormal profiles of homeostatic function in rat pups exposed to maternal tryptophan deficiency during pregnancy and the early postnatal period that are age specific. These data indicate a tight link between diet, vulnerable developmental periods, and physiological dysfunction that is relevant to the conceptionalization of all dietary deficiencies of the central nervous system in early life. Moreover, our data indicate that maternal dietary depletions may have adverse effects on the development of the brain stem and brain stem-mediated function in offspring that are just as detrimental as their effects on the forebrain and cognition, with dietary tryptophan depletion potentially contributing to SIDS and explaining in part the prevalence of SIDS among impoverished populations. The age-dependent effects of tryptophan depletion on respiratory function provide an interesting correlate to the age dependence of SIDS and suggest a plausible mechanism by which malnutrition may increase the risk of SIDS. These dietary data suggest that dietary deficiencies in amino acids that form neurotransmitters important for homeostasis may contribute to the high mortality of malnourished neonates and infants living in poverty (30).
GRANTS
This work was supported by National Institute of Child Health and Human Development Grant PO1-HD-036379.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Adams WR, Kiefer SW, Badia-Elder N. Tryptophan deficiency and alcohol consumption in rats as a model for disadvantaged human populations: a preliminary study. Med Anthropol 16: 175–191, 1995 [DOI] [PubMed] [Google Scholar]
- 2. Alenina N, Kikic D, Todiras M, Mosienko V, Qadri F, Plehm R, Boye P, Vilianovitch L, Sohr R, Tenner K, Hortnagl H, Bader M. Growth retardation and altered autonomic control in mice lacking brain serotonin. Proc Natl Acad Sci USA 106: 10332–10337, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Balakrishnan V, Becker M, Lohrke S, Nothwang HG, Guresir E, Friauf E. Expression and function of chloride transporters during development of inhibitory neurotransmission in the auditory brainstem. J Neurosci 23: 4134–4145, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bartlett D, Jr, Tenney SM. Control of breathing in experimental anemia. Respir Physiol 10: 384–395, 1970 [DOI] [PubMed] [Google Scholar]
- 5. Blumberg MS, Karlsson KA, Seelke AM, Mohns EJ. The ontogeny of mammalian sleep: a response to Frank and Heller (2003). J Sleep Res 14: 91–98, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Corcoran AE, Hodges MR, Wu Y, Wang W, Wylie CJ, Deneris ES, Richerson GB. Medullary serotonin neurons and central CO2 chemoreception. Respir Physiol Neurobiol 168: 49–58, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cummings KJ, Commons KG, Fan KC, Li A, Nattie EE. Severe spontaneous bradycardia associated with respiratory disruptions in rat pups with fewer brain stem 5-HT neurons. Am J Physiol Regul Integr Comp Physiol 296: R1783–R1796, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cummings KJ, Li A, Deneris ES, Nattie EE. Bradycardia in serotonin-deficient Pet-1-/- mice: influence of respiratory dysfunction and hyperthermia over the first 2 postnatal weeks. Am J Physiol Regul Integr Comp Physiol 298: R1333–R1342, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Davis SE, Solhied G, Castillo M, Dwinell M, Brozoski D, Forster HV. Postnatal developmental changes in CO2 sensitivity in rats. J Appl Physiol 101: 1097, 2006 [DOI] [PubMed] [Google Scholar]
- 10. Dobbing J, Sands J. Quantitative growth and development of human brain. Arch Dis Child 48: 757–767, 1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dobbing J, Sands J. Vulnerability of developing brain. IX. The effect of nutritional growth retardation on the timing of the brain growth-spurt. Biol Neonate 19: 363–378, 1971 [DOI] [PubMed] [Google Scholar]
- 12. Duncan JR, Paterson DS, Hoffman JM, Mokler DJ, Borenstein NS, Belliveau RA, Krous HF, Haas EA, Stanley C, Nattie EE, Trachtenberg FL, Kinney HC. Brainstem serotonergic deficiency in sudden infant death syndrome. JAMA 303: 430–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Eleftheriades M, Creatsas G, Nicolaides K. Fetal growth restriction and postnatal development. Ann NY Acad Sci 1092: 319–330, 2006 [DOI] [PubMed] [Google Scholar]
- 14. Fadda F. Tryptophan-free diets: a physiological tool to study brain serotonin function. News Physiol Sci 15: 260–264, 2000 [DOI] [PubMed] [Google Scholar]
- 15. Gessa GL, Biggio G, Fadda F, Corsini GU, Tagliamonte A. Tryptophan-free diet: a new means for rapidly decreasing brain tryptophan content and serotonin synthesis. Acta Vitaminol Enzymol 29: 72–78, 1975 [PubMed] [Google Scholar]
- 16. Gonzalez EM, Penedo LA, Oliveira-Silva P, Campello-Costa P, Guedes RC, Serfaty CA. Neonatal tryptophan dietary restriction alters development of retinotectal projections in rats. Exp Neurol 211: 441–448, 2008 [DOI] [PubMed] [Google Scholar]
- 17. Gressens P, Muaku SM, Besse L, Nsegbe E, Gallego J, Delpech B, Gaultier C, Evrard P, Ketelslegers JM, Maiter D. Maternal protein restriction early in rat pregnancy alters brain development in the progeny. Brain Res Dev Brain Res 103: 21–35, 1997 [DOI] [PubMed] [Google Scholar]
- 18. Guesry P. The role of nutrition in brain development. Prevent Med 27: 189–194, 1998 [DOI] [PubMed] [Google Scholar]
- 19. Hodges MR, Tattersall GJ, Harris MB, McEvoy SD, Richerson DN, Deneris ES, Johnson RL, Chen ZF, Richerson GB. Defects in breathing and thermoregulation in mice with near-complete absence of central serotonin neurons. J Neurosci 28: 2495–2505, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hodges MR, Wehner M, Aungst J, Smith JC, Richerson GB. Transgenic mice lacking serotonin neurons have severe apnea and high mortality during development. J Neurosci 29: 10341–10349, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Horne RS, Parslow PM, Ferens D, Bandopadhayay P, Osborne A, Watts AM, Cranage SM, Adamson TM. Arousal responses and risk factors for sudden infant death syndrome. Sleep Med 3, Suppl 2: S61–S65, 2002 [DOI] [PubMed] [Google Scholar]
- 22. Kahn A, Blum D. Home monitoring of infants considered at risk for the sudden infant death syndrome. Four years' experience (1977–1981). Eur J Pediatr 139: 94–100, 1982 [DOI] [PubMed] [Google Scholar]
- 23. Kelly DH, Golub H, Carley D, Shannon DC. Pneumograms in infants who subsequently died of sudden infant death syndrome. J Pediatr 109: 249–254, 1986 [DOI] [PubMed] [Google Scholar]
- 24. Kinney HC, Randall LL, Sleeper LA, Willinger M, Belliveau RA, Zec N, Rava LA, Dominici L, Iyasu S, Randall B, Habbe D, Wilson H, Mandell F, McClain M, Welty TK. Serotonergic brainstem abnormalities in Northern Plains Indians with the sudden infant death syndrome. J Neuropathol Exp Neurol 62: 1178–1191, 2003 [DOI] [PubMed] [Google Scholar]
- 25. Kinney HC, Richerson GB, Dymecki SM, Darnall RA, Nattie EE. The brainstem and serotonin in the sudden infant death syndrome. Annu Rev Pathol 4: 517–550, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kinney HC, Thach BT. The sudden infant death syndrome. N Engl J Med 361: 795–805, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kubin L, Volgin DV. Developmental profiles of neurotransmitter receptors in respiratory motor nuclei. Respir Physiol Neurobiol 164: 64–71, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li A, Zhou S, Nattie E. Simultaneous inhibition of caudal medullary raphe and retrotrapezoid nucleus decreases breathing and the CO2 response in conscious rats. J Physiol 577: 307–318, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Machaalani R, Say M, Waters KA. Serotoninergic receptor 1A in the sudden infant death syndrome brainstem medulla and associations with clinical risk factors. Acta Neuropathol 117: 257–265, 2009 [DOI] [PubMed] [Google Scholar]
- 30. Malloy MH, Eschbach K. Association of poverty with sudden infant death syndrome in metropolitan counties of the United States in the years 1990 and 2000. South Med J 100: 1107–1113, 2007 [DOI] [PubMed] [Google Scholar]
- 31. Marin MC, De Tomas ME, Serres C, Mercuri O. Protein-energy malnutrition during gestation and lactation in rats affects growth rate, brain development and essential fatty acid metabolism. J Nutr 125: 1017–1024, 1995 [DOI] [PubMed] [Google Scholar]
- 32. Meny RG, Carroll JL, Carbone MT, Kelly DH. Cardiorespiratory recordings from infants dying suddenly and unexpectedly at home. Pediatrics 93: 44–49, 1994 [PubMed] [Google Scholar]
- 33. Morgane PJ, Austin-LaFrance R, Bronzino J, Tonkiss J, Diaz-Cintra S, Cintra L, Kemper T, Galler JR. Prenatal malnutrition and development of the brain. Neurosci Biobehav Rev 17: 91–128, 1993 [DOI] [PubMed] [Google Scholar]
- 34. Mortola JP. Respiratory Physiology of Newborn Mammals: A Comparative Perspective. Baltimore, MD: Johns Hopkins Univ. Press, 2001 [Google Scholar]
- 35. Mortola JP, Fisher JT, Sant'Ambrogio G. Vagal control of the breathing pattern and respiratory mechanics in the adult and newborn rabbit. Pflügers Arch 401: 281–286, 1984 [DOI] [PubMed] [Google Scholar]
- 36. Mortola JP, Frappell PB. On the barometric method for measurements of ventilation, and its use in small animals. Can J Physiol Pharmacol 76: 937–944, 1998 [DOI] [PubMed] [Google Scholar]
- 37. Orozco-Suarez S, Del Angel AR, Beas-Zarate C, Manjarrrez G, Feria-Velasco A. Corn feeding during development induces changes in the number of serotonergic neurons in the raphe nuclei. Int J Dev Neurosci 21: 13–22, 2003 [DOI] [PubMed] [Google Scholar]
- 38. Panigrahy A, Filiano J, Sleeper LA, Mandell F, Valdes-Dapena M, Krous HF, Rava LA, Foley E, White WF, Kinney HC. Decreased serotonergic receptor binding in rhombic lip-derived regions of the medulla oblongata in the sudden infant death syndrome. J Neuropathol Exp Neurol 59: 377–384, 2000 [DOI] [PubMed] [Google Scholar]
- 39. Paterson DS, Trachtenberg FL, Thompson EG, Belliveau RA, Beggs AH, Darnall R, Chadwick AE, Krous HF, Kinney HC. Multiple serotonergic brainstem abnormalities in sudden infant death syndrome. JAMA 296: 2124–2132, 2006 [DOI] [PubMed] [Google Scholar]
- 40. Penatti EM, Berniker AV, Kereshi B, Cafaro C, Kelly ML, Niblock MM, Gao HG, Kinney HC, Li A, Nattie EE. Ventilatory response to hypercapnia and hypoxia after extensive lesion of medullary serotonergic neurons in newborn conscious piglets. J Appl Physiol 101: 1177–1188, 2006 [DOI] [PubMed] [Google Scholar]
- 41. Poets CF, Meny RG, Chobanian MR, Bonofiglo RE. Gasping and other cardiorespiratory patterns during sudden infant deaths. Pediatr Res 45: 350–354, 1999 [DOI] [PubMed] [Google Scholar]
- 42. Quigley KS, Myers MM, Shair HN. Development of the baroreflex in the young rat. Auton Neurosci 121: 26–32, 2005 [DOI] [PubMed] [Google Scholar]
- 43. Rubio L, Torrero C, Regalado M, Salas M. Alterations in the solitary tract nucleus of the rat following perinatal food restriction and subsequent nutritional rehabilitation. Nutr Neurosci 7: 291–300, 2004 [DOI] [PubMed] [Google Scholar]
- 44. Ruddick JP, Evans AK, Nutt DJ, Lightman SL, Rook GA, Lowry CA. Tryptophan metabolism in the central nervous system: medical implications. Expert Rev Mol Med 8: 1–27, 2006 [DOI] [PubMed] [Google Scholar]
- 45. Saiki C, Mortola JP. Effect of CO2 on the metabolic and ventilatory responses to ambient temperature in conscious adult and newborn rats. J Physiol 491: 261–269, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sodhi MS, Sanders-Bush E. Serotonin and brain development. Int Rev Neurobiol 59: 111–174, 2004 [DOI] [PubMed] [Google Scholar]
- 47. Southall DP, Richards J, Brown DJ, Johnston PG, de Swiet M, Shinebourne EA. 24-hour tape recordings of ECG and respiration in the newborn infant with findings related to sudden death and unexplained brain damage in infancy. Arch Dis Child 55: 7–16, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tonkiss J, Galler J, Morgane PJ, Bronzino JD, Austin-LaFrance RJ. Prenatal protein malnutrition and postnatal brain function. Ann NY Acad Sci 678: 215–227, 1993 [DOI] [PubMed] [Google Scholar]
- 49. Willinger M, James LS, Catz C. Defining the sudden infant death syndrome (SIDS): deliberations of an expert panel convened by the National Institute of Child Health and Human Development. Pediatr Pathol 11: 677–684, 1991 [DOI] [PubMed] [Google Scholar]