Abstract
The aim of this study was to assess the utility of 3He MRI to noninvasively probe the effects of positive end-expiratory pressure (PEEP) maneuvers on alveolar recruitment and atelectasis buildup in mechanically ventilated animals. Sprague-Dawley rats (n = 13) were anesthetized, intubated, and ventilated in the supine position (4He-to-O2 ratio: 4:1; tidal volume: 10 ml/kg, 60 breaths/min, and inspiration-to-expiration ratio: 1:2). Recruitment maneuvers consisted of either a stepwise increase of PEEP to 9 cmH2O and back to zero end-expiratory pressure or alternating between these two PEEP levels. Diffusion MRI was performed to image 3He apparent diffusion coefficient (ADC) maps in the middle coronal slices of lungs (n = 10). ADC was measured immediately before and after two recruitment maneuvers, which were separated from each other with a wait period (8–44 min). We detected a statistically significant decrease in mean ADC after each recruitment maneuver. The relative ADC change was −21.2 ± 4.1 % after the first maneuver and −9.7 ± 5.8 % after the second maneuver. A significant relative increase in mean ADC was observed over the wait period between the two recruitment maneuvers. The extent of this ADC buildup was time dependent, as it was significantly related to the duration of the wait period. The two postrecruitment ADC measurements were similar, suggesting that the lungs returned to the same state after the recruitment maneuvers were applied. No significant intrasubject differences in ADC were observed between the corresponding PEEP levels in two rats that underwent three repeat maneuvers. Airway pressure tracings were recorded in separate rats undergoing one PEEP maneuver (n = 3) and showed a significant relative difference in peak inspiratory pressure between pre- and poststates. These observations support the hypothesis of redistribution of alveolar gas due to recruitment of collapsed alveoli in presence of atelectasis, which was also supported by the decrease in peak inspiratory pressure after recruitment maneuvers.
Keywords: atelectasis, ventilator-induced lung injury, respiratory gas distribution, 3He diffusion magnetic resonance imaging, apparent diffusion coefficient
in recent years, the goals of mechanical ventilation have shifted from normalizing gas exchange to protecting the lungs from ventilator-associated lung injury (VALI) (33). One common and significant cause of VALI is the application of excessive mechanical stress to the alveolar walls (9, 43). Such mechanical stress is a result of lung overdistension (10, 56) and the development of atelectasis (40, 47, 60). VALI manifests itself through alveolar cell death (16), inflammatory mediator activation (54), microvascular injury (25), and interstitial matrix remodeling (38). Terminal bronchioles and alveolar ducts also may be injured by mechanical ventilation (5, 55).
Various strategies, including low tidal volume (VT) ventilation, positive end-expiratory pressure (PEEP), and alveolar recruitment maneuvers have been used to attenuate VALI in acute lung injury. In particular, recruitment maneuvers attempt to treat atelectasis by increasing the proportion of aerated alveoli while preventing the overdistension of already ventilated lung regions. While low VT improved the outcomes of acute lung injury patients (1, 2), studies on PEEP and alveolar recruitment maneuvers have yielded contradictory results (2, 6, 36, 37). These discrepancies might reflect the fact that lung protection can be achieved only if ventilated alveolar sizes are preserved and if harmful overdistention is avoided (33). Unfortunately, the response to mechanical ventilation at the alveolar and distal airway levels is difficult to investigate. The only direct demonstration of alveolar behavior during ventilatory maneuvers is provided by in vivo microscopy (7, 21, 47, 48). This technique is limited to the visualization of subpleural tissue and is too invasive to be used in humans. In the absence of direct measurements, it is presumed that alveolar behavior is reflected in whole lung measurements of physiological variables (29, 31) or in radiographic changes most often assessed with computed tomography (CT) (8, 17, 18, 32, 44, 45). However, CT provides only limited, indirect information about airway size at the highly vulnerable acinar level (3, 28, 55).
Hyperpolarized 3He MRI has the potential to fill this gap in knowledge. This technique provides information about regional restriction in airway gas diffusivity that is consistent with the size of ventilated airspaces at the acinar level, including the alveoli, respiratoy bronchioles, and alveolar sacs (63). This is accomplished via measurements of the apparent diffusion coefficient (ADC) of 3He gas atoms (57). Hyperpolarized 3He MRI is a noninvasive, nonionizing, and sensitive technique that rivals current modalities of evaluating functional lung markers and facilitates quantitative measurements of changes over time (15). Hyperpolarized 3He MRI provides structural information at the scale of alveoli and airways distal to the terminal bronchiole (62) despite the fact that spatial resolution does not permit the visualization of individual airspace units. This is based on the intrinsic ability of hyperpolarized 3He MRI to deliver statistical information about the large numbers of airspaces within each imaging voxel (63). Therefore, further investigation of ADC values can yield a better understanding of the behavior of ventilated acinar microstructures during mechanical ventilation, complementing the data provided by lung CT.
Our global aim was twofold: 1) to develop the use of regional ADC measurements as a noninvasive probe to longitudinally assess the effects of atelectasis and alveolar recruitment maneuvers and 2) to determine outcomes when different ventilatory maneuvers are used. This approach relies on the assumption that, during mechanical ventilation, changes in ADC are related to shifts in the dimensions of ventilated acini caused by variable alveolar inflation. In this study, we used hyperpolarized 3He diffusion MRI to assess the effects of superimposed alveolar recruitment maneuvers on ADC distribution, which were obtained through the intermittent application of PEEP trials in a group of anesthetized rats undergoing mechanical ventilation. Previous studies (11, 12) have shown that healthy rats ventilated under similar conditions are prone to the formation of atelectasis. Through this study, we tested the following specific hypotheses: 1) ADC measurements at a fixed VT can detect alterations in the size of ventilated airspaces due to changes in the proportion of collapsed alveoli and 2) the restoration of collapsed alveoli through recruitment maneuvers can be quantified by measurements of ADC.
MATERIALS AND METHODS
Preparation of animals for imaging.
All animal experiments complied with National Institutes of Health guidelines and were performed in accordance with protocols set forth by the Institutional Animal Care and Use Committee of the University of Pennsylvania. Experiments were performed on healthy, male Sprague-Dawley rats (n = 13, 300 ± 50 g body wt). Animals were acclimated for 1 wk before procedures and were maintained under identical environmental conditions with ad libitum access to food and water. For all procedures, animals were sedated with intraperitoneal pentobarbital (40–60 mg/kg) and were maintained anesthetized with one-third of the original dose (15–20 mg/kg) every 60–90 min. Animals were intubated with a 2-in.-long, 14-gauge angiocatheter (BD, Franklin Lakes, NJ) with a sealant (UHU Tac adhesive putty, Saunders Manufacturing, Readfield, ME) placed around the glottis to prevent gas from leaking out of the lungs around the tube. This placement compensated for our use of uncuffed endotracheal access, and, in almost all cases, no significant leakage was observed over a 5-s breath hold at an inflation level of 20 cmH2O measured with a pressure transducer connected to the endotracheal tube. The position of the tube was readjusted whenever a non-negligible leak was detected. Once the airway was secured, animals were temporarily paralyzed with 1 mg/kg intravenous pancuronium bromide (Abbot Labs, North Chicago, IL) and connected to a custom-built MRI-compatible small animal ventilator capable of delivering gas volumes accurate within ±100 μl. An MRI-compatible pressure sensor connected to the endotracheal tube allowed for real-time monitoring of airway pressure during the imaging study. The animals' body temperature was maintained at 37°C by a flow of warm air through the bore of the magnet, and temperature was continuously monitored using a rectal probe. A veterinary pulse oximeter (Nonin Medical, Plymouth, MN) with an optical probe was attached to the hindfoot to monitor heart rate and O2 saturation levels.
ADC imaging protocol.
ADC maps of 3He were acquired using a double-acquisition ADC imaging technique as previously described (14). This technique has been shown to minimize the effect of cross terms between diffusion gradients and imaging gradients (42) as well as that of background field gradients (including susceptibility gradients induced by the air-tissue interface in the lungs), which, if ignored, could potentially result in an overestimated value of the diffusion coefficient. For this purpose, a series of five hyperpolarized gas breaths (3He-to-O2 ratio: 4:1) were delivered to the rat at the designated VT. The sixth breath was then held at the end-inspiratory level for 3 s to acquire the first series of diffusion-weighted images corresponding to the given set of five diffusion gradient factors (b values) and using the positive-negative ordering of bipolar diffusion-sensitizing gradients, as shown in Fig. 1A. This procedure was then immediately repeated in an identical manner with the reverse (negative-positive) order of bipolar diffusion gradients in a second breath hold. With this configuration, each ADC acquisition was composed of 10 diffusion-weighted images and took 18 s to complete. The b value was determined by the following equation:
where the gyromagnetic ratio of 3He (γ−) = 32.43 MHz/T. G(j) is the diffusion-sensitizing gradient amplitude corresponding to the jth image in the series at time t(j), where δ is the diffusion gradient duration (from the beginning of the ramp to the end of gradient flat top), Δ is the diffusion time (defined as the time between the beginning of the first gradient lobe to the beginning of the second gradient lobe), and τ is the gradient ramp time. The images for corresponding b values from each breath were pairwise multiplied, raised to the power of 0.5 to yield the geometric mean of the two sets of diffusion-weighted images, and then fit to the following equation to yield ADC values on a pixel-by-pixel basis:
| (1) |
where S0 is the signal of the first image, S(j) is that of the jth image, and NPE is the number of pulses triggered per image (i.e., the number of phase-encoded lines) α and T1,O2 represent the radiofrequency (RF) pulse flip angle and O2-induced relaxation time constant, respectively. Considering the negligible effect of the O2-induced depolarization effect over the relatively short timescale of 3 s, an overall value of T1,O2 = 19 s, corresponding to an alveolar Po2 of 103 mmHg, was assumed throughout the lung (46).
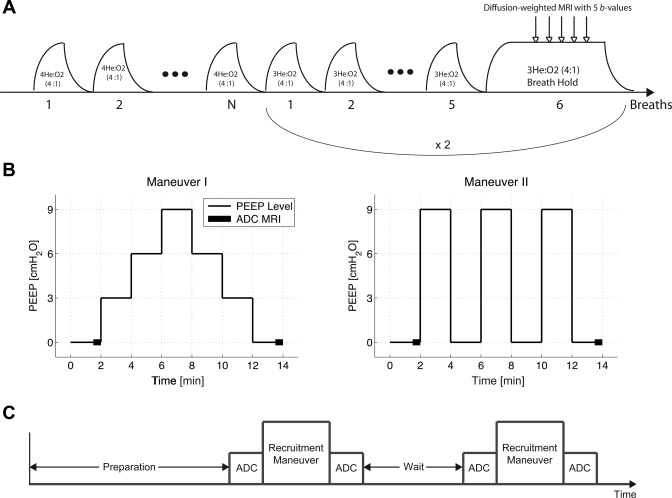
Fig. 1.
A: schematic diagram of the ventilation protocol for end-inspiratory apparent diffusion coefficient (ADC) imaging in rat lungs with hyperpolarized 3He MRI. b values, diffusion gradient factors. B: schematic diagram of the two positive end-expiratory pressure (PEEP) recruitment maneuvers. The thick line segments represent ADC acquisitions. C: overall experimental protocol comprised of two recruitment maneuvers sandwiched between two pairs of ADC measurements and separated by a wait time period, as shown in Table 1.
3He gas was hyperpolarized through spin exchange collisions with optically pumped rubidium atoms, as previously described (58), using a commercial prototype (IGI.9600.He, GE Healthcare, Durham, NC) to a level of ∼30% over 14 h. During imaging, hyperpolarized 3He was stored in a 400-ml Tedlar bag (Jensen Inert Products, Coral Springs, FA) positioned inside the magnet bore, which provided a relaxation time constant in the order of 30–45 min and was refilled during the imaging session as needed. Imaging was performed on a 50-cm bore 4.7-T MRI scanner (Varian, Palo Alto, CA) equipped with 12-cm 25-G/cm gradients and a quadrature 8-leg birdcage RF coil with internal diameter of 7 cm (Stark Contrast, Erlangen, Germany) tuned to the 3He resonance frequency of 152.95 MHz. The animal was placed supine in the RF coil inside the MRI scanner, and overall RF pulse width calibration was performed to estimate the applied imaging flip angle to the rat. This task was performed during a 2-s single breath hold of hyperpolarized 3He. ADC imaging was performed on the middle coronal slice using a diffusion-weighted gradient echo pulse sequence with the following parameters: field of view = 6 × 6 cm2, slice thickness = 5 mm, α = 4−5°, matrix size = 64 × 64 pixels, repetition time = 8.1 ms, and echo time = 4.8 ms. Diffusion weighting was performed along the phase-encode direction, corresponding to the left-right direction with reference to the rat body and using a diffusion time of Δ = 1 ms, a gradient duration of δ = 200 μs, and b values = 0, 5.27, 3.09, 1.41, and 0 s/cm2, all with a fixed ramp time of τ = 180 μs. The zero b value was repeated at the beginning and end of the sequence to allow for a more accurate estimation of α for every single voxel.
Mechanical ventilation protocols.
Ventilation protocols were designed to assess the effects of atelectasis buildup in mechanically ventilated anesthetized rats as well as the effectiveness of PEEP recruitment maneuvers in reopening collapsed alveoli. To promote the formation of atelectasis, intubated rats were ventilated at zero end-expiratory pressure (ZEEP) in the supine position for at least 1 h before the beginning of imaging. The ventilator was set to a respiratory rate of 60 breaths/min, an inspiration-to-expiration ratio (I:E) of 1:2, and a VT of 10 ml/kg. These settings were maintained unchanged throughout the entire study. Room air was used as the inspired gas during the initial phase. Approximately 10 min before the start of the imaging procedures, the inspired gas was switched to a normoxic 4He-O2 mixture. This was done to prevent diffusion measurement errors induced by different gas mixtures in the inspired and residual capacity at different PEEP levels. The source gas was then accordingly switched to 3He-O2 for any planned ADC measurements. The O2 source was moisturized by passing through a water column before reaching the animal. PEEP was applied by submerging the expired gas line into a graduated water column filled to the desired liquid level.
The ADC imaging protocol was performed on n = 10 rats. The remaining animals (n = 3) were only observed for respiratory pressure signals as they underwent the recruitment maneuvers. Two different recruitment maneuvers were used in this study. The first maneuver consisted of an incremental increase of the PEEP level from 0 to 9 cmH2O (at 3-cmH2O steps) followed by an incremental decrease back to the ZEEP level. The second maneuver consisted of an alternating series of PEEP levels between 0 and 9 cmH2O, eventually landing on the ZEEP level. During all recruitment maneuvers, each PEEP level was maintained for 2 min before switching to the following step. Imaging animals were divided into two groups (n = 5), and each group underwent either of the recruitment maneuvers. Figure 1B shows a schematic diagram of each recruitment strategy.
Upon the completion of preparation procedures, a baseline ADC measurement was performed on the rat at the ZEEP level (corresponding to premaneuver I), as described in the imaging protocol. The animal, depending on the group that it belonged to, then underwent either of the recruitment maneuvers. Upon returning to the ZEEP level, a second ADC measurement was performed (corresponding to postmaneuver I). At this point, the rat entered a wait period ranging anywhere from 8 to 44 min while still inside the magnet bore, during which it was kept ventilated on 4He-O2 with no change in breathing rate and posture (one exception to the wait time is discussed below). The rat then underwent another set of recruitment maneuver, and an identical pair of ADC measurements were performed (corresponding to pre- and postmaneuver II), similar to the first half of the protocol. This entire procedure is schematically shown in Fig. 1C.
There were a few exceptions, as described below. To characterize the ADC response to intermediate changes in PEEP level as well as to assess the intrasubject repeatability of ADC measurements during PEEP maneuvers, two of the animals in the stepwise PEEP maneuver group (Fig. 1B, left) underwent two additional cycles of wait and recruitment identical to their first cycles. In these animals, ADC was measured at each PEEP level. One of the animals in the alternating PEEP maneuver group underwent an extended wait period of up to 104 min while maintained under anesthesia under mechanical ventilation. Several intermittent ADC measurements were performed during this time period to demonstrate the atelectasis buildup over an extended time period. In both of these exceptional situations, ADC was measured at all intermittent PEEP levels in addition to pre- and postmaneuver points.
Data analysis.
Data analysis was performed using custom MATLAB (Mathworks, Natick, MA) programs developed in the authors' laboratory. ADC analysis was performed on a pixel-by-pixel basis at a submillimeter planar resolution (∼0.94 × 0.94 mm2). The signal in the acquired images was bias corrected for the background noise (24) according to the following equation: , where Ŝ is the bias-corrected signal and σ is the inherent noise in the MR image. σ calculated from the following equation: , where Ḃ is the average background signal intensity corresponding to a 10 × 10-pixel region far away from the lungs in the acquired image. To distinguish lung tissue from background, pixels with a signal-to-noise ratio (SNR) below a specific threshold were excluded from analysis. The SNR threshold was iteratively varied between 5 and 20 for each image, and the highest threshold value was selected such that the entire lung parenchyma was retained after the low SNR pixels were masked. After background pixels were excluded from the image, the time evolution of the signal intensity of valid pixels was fit to Eq. 1 to yield maps of regional ADC values. To enhance the visualization of ADC distribution in the imaged slice, the computed ADC maps were smoothed using a two-dimensional moving average filter by replacing the ADC value of each voxel with the average value of its surrounding 3 × 3 grid.
To assess the effectiveness of PEEP maneuvers in recruiting collapsed alveoli, statistical analysis was performed to test for any significant decline in ADC distribution from the premaneuver (baseline) to the postmaneuver state. Data points from the two different recruitment maneuver strategies were combined into one pool, and the statistical analysis specifically focused on pre-/post-recruitment ADC values acquired at the ZEEP level. Additionally, to assess the atelectasis buildup during the wait period between the two consecutive recruitment maneuvers, statistical analysis was performed to test for any significant increase in ADC distribution from the initial point to the final point of the wait period. ADC measurements from the repeat maneuvers in the two rats of the first group were treated as independent pre-/postmaneuver trials. In addition to mean and SD, skewness was computed for the ADC distribution in each imaged slice. The group mean and SD of these three distribution parameters were subsequently calculated and compared between each pair of time points. To normalize the effect of absolute difference in ADC values among the animals, the relative changes in mean and SD of ADC distributions before and after the maneuvers as well as between the initial and final points of the wait period were calculated. A β probability density function was fit to each ADC map to assist in the visualization and qualitative assessment of the associated frequency distribution histogram.
The respiratory pressure trace captured on the three additional rats (which did not undergo MRI) was analyzed to test for any significant decline in the peak inspiratory pressure (PIP) from the premaneuver (baseline) state to the postmaneuver state. The mean value of PIP at the ZEEP level before and after the recruitment maneuver was used to test for statistically significant differences in the relative change of PIP.
A generalized two-way ANOVA test was used with an unequal sample size for each pair of time points, where each animal was used as a control for itself. Post hoc pairwise statistical comparisons of mean, SD, and skewness quantities were performed using Tukey's honestly significant difference criterion. The significance level of 0.05, corresponding to a confidence level of 95%, was considered for all the statistical analyses.
RESULTS
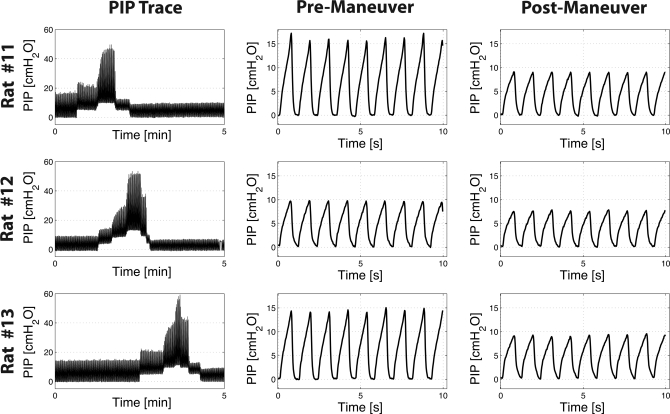
Figure 2 shows respiratory pressure traces from the three separate rats that underwent one stepwise PEEP recruitment cycle, similar to what is shown in Fig. 1B, left. Also shown in Fig. 2 is the representative respiratory trend from pre- and postrecruitment phases of each rat demonstrating the relative change in PIP levels. A significant relative difference of ΔPIP = −32.7 ± 8.5 % (P < 0.022) in pre- and postrecruitment PIP values was measured in all three animals. These changes support the occurrence of alveolar recruitment by PEEP manuvers.
Fig. 2.
Time history of peak inspiratory pressure (PIP) for three rats that were not imaged with hyperpolarized 3He MRI. These animals underwent the stepwise recruitment maneuver, shown in Fig. 1B, left. All three rats showed a significant change in PIP before and after application of the PEEP maneuver; a similar trend was also observed with the 3He ADC results.
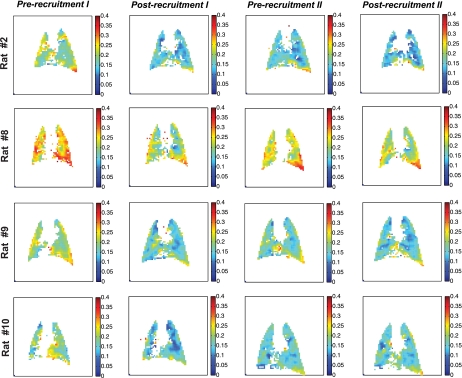
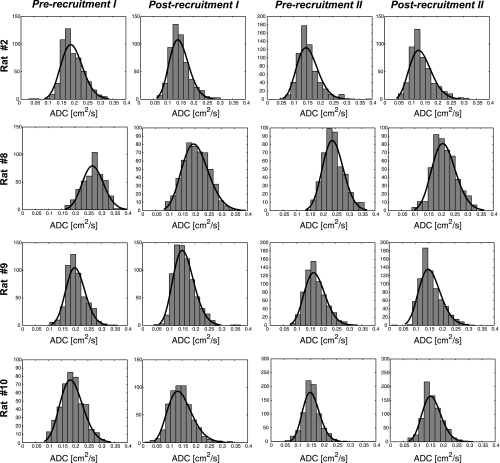
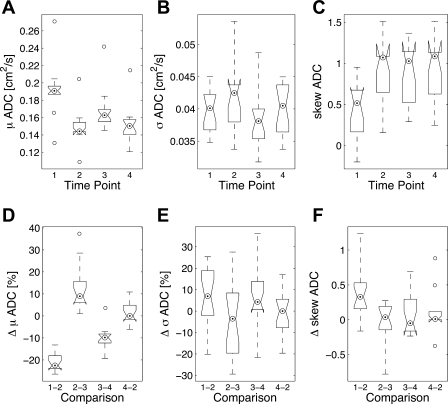
Figure 3 shows ADC maps for a select number of animals at the following four study time points: ADC = 0.19 ± 0.03 cm2/s (baseline, prerecruitment I), ADC = 0.15 ± 0.02 cm2/s (beginning of the wait period, postrecruitment I), ADC = 0.17 ± 0.02 cm2/s (after the wait period, prerecruitment II), and finally ADC = 0.15 ± 0.02 cm2/s (postrecruitment II). The corresponding frequency distribution histograms for these ADC maps are shown in Fig. 4, along with the best fit to a β probability density function. Table 1 shows the distribution parameters for all the ADC maps. The relative changes in mean and skewness of ADC distributions between different time points are shown in Table 2. Specifically, comparisons were performed to assess the changes in the above ADC distribution parameters between pre- and postrecruitment points of maneuver I, between the initial and final points of the wait period, between pre- and postrecruitment points of maneuver II, and finally between the two postrecruitment points of the separate maneuvers, as shown in Fig. 5, A–C.
Fig. 3.
Maps of ADC distribution (cm2/s) in the middle coronal slice of four representation rats that underwent the study protocol shown in Fig. 1C. The ADC map in each column corresponds to each of the four time points in the recruitment maneuver study.
Fig. 4.
Frequency distribution histograms corresponding to the ADC maps shown in Fig. 3 for the four representative rats. Also overlaid on the histograms are the best β fits for qualitative assessment of the distribution functions.
Table 1.
Summary of ADC measurement results at all time points in 10 rats expressed as the mean, SD, and skewness of the ADC distribution in the imaged slice
|
Prerecruitment I |
Postrecruitment I |
Prerecruitment II |
Postrecruitment II |
||||||
|---|---|---|---|---|---|---|---|---|---|
| ADC, cm2/s | ADC skewness | ADC, cm2/s | ADC skewness | Wait Time, min | ADC, cm2/s | ADC skewness | ADC, cm2/s | ADC skewness | |
| Rat 1 | 0.20 ± 0.04 | 0.17 | 0.14 ± 0.04 | 1.40 | |||||
| Rat 2 | 0.20 ± 0.04 | 0.44 | 0.15 ± 0.04 | 0.97 | 8 | 0.16 ± 0.04 | 1.24 | 0.14 ± 0.04 | 1.08 |
| 0.14 ± 0.04 | 1.08 | 15 | 0.16 ± 0.03 | 1.03 | 0.14 ± 0.04 | 1.08 | |||
| 0.14 ± 0.04 | 1.08 | 22 | 0.16 ± 0.04 | 1.32 | 0.14 ± 0.04 | 1.08 | |||
| Rat 3 | 0.19 ± 0.05 | 0.67 | 0.15 ± 0.05 | 0.83 | |||||
| Rat 4 | 0.19 ± 0.04 | 0.79 | 0.16 ± 0.03 | 0.63 | 10 | 0.17 ± 0.04 | 0.79 | 0.16 ± 0.03 | 0.63 |
| 0.16 ± 0.03 | 0.63 | 8 | 0.17 ± 0.04 | 0.83 | 0.15 ± 0.04 | 1.51 | |||
| 0.15 ± 0.04 | 1.51 | 18 | 0.16 ± 0.04 | 1.37 | 0.15 ± 0.04 | 1.13 | |||
| Rat 5 | 0.19 ± 0.03 | 0.61 | 0.14 ± 0.04 | 1.25 | 10 | 0.16 ± 0.04 | 1.10 | ||
| Rat 6 | 0.17 ± 0.04 | 0.95 | 0.14 ± 0.04 | 1.07 | 15 | 0.15 ± 0.03 | 1.11 | ||
| 0.14 ± 0.04 | 1.07 | 45 | 0.15 ± 0.03 | 1.15 | |||||
| 0.14 ± 0.04 | 1.07 | 85 | 0.16 ± 0.04 | 1.11 | |||||
| 0.14 ± 0.04 | 1.07 | 105 | 0.18 ± 0.05 | 0.29 | |||||
| Rat 7 | 0.13 ± 0.04 | 0.35 | 0.11 ± 0.04 | 0.65 | 30 | 0.15 ± 0.04 | 0.46 | 0.12 ± 0.04 | 1.15 |
| Rat 8 | 0.27 ± 0.04 | −0.20 | 0.20 ± 0.05 | 0.16 | 32 | 0.24 ± 0.04 | 0.41 | 0.21 ± 0.05 | 0.25 |
| Rat 9 | 0.20 ± 0.04 | 0.59 | 0.16 ± 0.04 | 0.81 | 30 | 0.17 ± 0.04 | 0.73 | 0.16 ± 0.04 | 0.83 |
| Rat 10 | 0.19 ± 0.04 | 0.16 | 0.15 ± 0.05 | 0.62 | 44 | 0.16 ± 0.03 | 0.33 | 0.16 ± 0.04 | 0.62 |
| Group means ± SD | 0.19 ± 0.03 | 0.45 ± 0.34 | 0.15 ± 0.02 | 0.94 ± 0.33 | 0.17 ± 0.02 | 0.88 ± 0.37 | 0.15 ± 0.02 | 0.94 ± 0.36 | |
Values for apparent diffusion coefficients (ADCs) are means ± SD. Blank fields indicate missing data points. Multiple ADC measurements in rats 2, 4, and 6 are shown on separate lines with reference to their baseline values.
Table 2.
Relative changes in mean ADC and ADC skewness for each pair of time points in the study
| Change in Mean ADC, % |
Change in ADC Skewness, % |
|||||||
|---|---|---|---|---|---|---|---|---|
| From prerecruitment I to postrecruitment I | Atelectasis buildup during the wait period between the manuevers | From prerecuitment II to postrecruitment II | Comparision between postrecruitment I and postrecruitment II | From prerecruitment I to postrecruitment I | Atelectasis buildup during the wait period between the manuevers | From prerecuitment II to postrecruitment II | Comparision between postrecruitment I and postrecruitment II | |
| Rat 1 | −26.4 | 1.23 | ||||||
| Rat 2 | −23.8 | 4.3 | −10.0 | −6.1 | 0.53 | 0.28 | −0.16 | 0.12 |
| 14.1 | −12.3 | 0.0 | −0.06 | 0.06 | 0.00 | |||
| 16.2 | −13.9 | 0.0 | 0.23 | −0.23 | 0.00 | |||
| Rat 3 | −20.5 | 0.16 | ||||||
| Rat 4 | −18.1 | 9.0 | −8.2 | 0.0 | −0.16 | 0.16 | −0.16 | 0.00 |
| 5.8 | −7.2 | −1.7 | 0.20 | 0.68 | 0.88 | |||
| 5.8 | −9.7 | −4.4 | −0.14 | −0.23 | −0.38 | |||
| Rat 5 | −23.0 | 11.2 | 0.63 | −0.15 | ||||
| Rat 6 | −13.2 | 1.0 | 0.12 | 0.04 | ||||
| 6.8 | 0.08 | |||||||
| 14.3 | 0.04 | |||||||
| 28.5 | −0.78 | |||||||
| Rat 7 | −16.7 | 37.4 | −19.3 | 10.9 | 0.30 | −0.20 | 0.69 | 0.49 |
| Rat 8 | −24.5 | 18.2 | −11.3 | 4.9 | 0.36 | 0.25 | −0.16 | 0.09 |
| Rat 9 | −22.0 | 8.6 | −9.0 | −1.2 | 0.22 | −0.08 | 0.10 | 0.02 |
| Rat 10 | −23.5 | 6.3 | 3.6 | 10.1 | 0.46 | −0.29 | 0.30 | 0.00 |
| Group means ± SD | −21.2 ± 4.1 | 12.5 ± 9.7 | −9.7 ± 5.8 | 1.2 ± 5.5 | 0.38 ± 0.36 | −0.03 ± 0.27 | 0.09 ± 0.35 | 0.12 ± 0.33 |
| P value | <5 × 10−8 | <2 × 10−4 | <5.1 × 10−4 | 0.507 | 0.010 | 0.700 | 0.461 | 0.282 |
Fig. 5.
Summary of ADC measurement results described in terms of the mean (A), SD (B), and skewness (C) of the ADC distribution in each imaged slices reported for each of the four time points according to Table 1. D–F: relative changes in these three parameters corresponding to the four pairwise comparisons shown in Table 2. The edges of each box correspond to the lower and upper quartile values, and the center-dotted circle represents the median ADC of each group. The whiskers extend from each end of the box to the most extreme values within 1.5 times the interquartile range. Data points in each group with values beyond the ends of the whiskers are considered outliers and are displayed as open circles.
Figure 5, D and E, on the other hand, shows the pairwise relative change in distribution parameters between the four time points. The relative change in the mean ADC value was significant before and after both recruitment maneuvers, with a 21.2 ± 4.1% decline (P < 5 × 10−8) in the first maneuver and a 9.7 ± 5.8% decline (P < 5.1 × 10−4) in the second maneuver, as shown in Fig. 5D. Pairwise comparisons revealed that the relative decline in mean ADC was significantly smaller in the second recruitment compared with the first recruitment. A similarly significant relative change in mean ADC was observed over the wait period between the two recruitment maneuvers, with a 12.5 ± 9.7% buildup (P < 2 × 10−4) over the designated wait time window. No statistically significant difference in mean ADC was observed between the two postrecruitment states.
SDs of ADC distributions in the imaged slices were somewhat larger in both postrecruitment states compared with their corresponding prerecruitment states, even though this change was not statistically significant, corresponding to 6.1 ± 13.6% and 6.8 ± 15.1% for the first and second maneuvers, respectively, as shown in Fig. 5E. The ADC distribution was significantly more skewed to the right at baseline, 0.45 ± 0.34% (P < 0.011), compared with all other maneuver points. However, the three subsequent time points did not show any significant change in this regard. The ADC skewness in the second prerecruitment was slightly lower than the corresponding postrecruitment distribution, although not significantly.
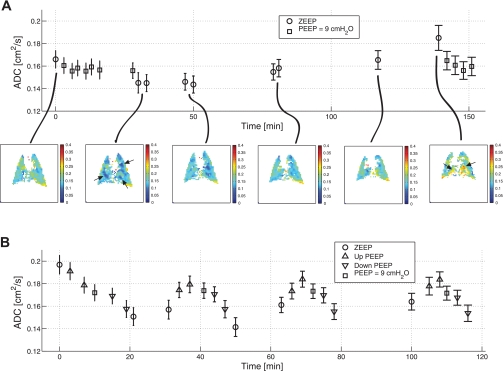
Figure 6A shows ADC measurements in rat 6, which underwent the extended wait period to allow atelectasis buildup. The error bars shown on this diagram represent the SD of ADC distribution in the imaged slice. An ADC value of 0.17 ± 0.04 cm2/s was observed at baseline, after the animal was mechanically ventilated at ZEEP for ∼1 h. After an initial recruitment maneuver with alternating PEEP levels between 0 and 9 cmH2O and a return to ZEEP, the ADC value decreased to 0.14 ± 0.04 cm2/s (intermittent ADC measurements were performed at PEEP = 9 cmH2O points, as shown in Fig. 6). The animal then remained ventilated at ZEEP for over 100 min, and intermittent ADC measurements revealed a slow and steady increase in mean ADC to a value slightly higher than the beginning of the study, 0.18 ± 0.05 cm2/s. Subsequent application of high PEEP resulted in a decline in ADC to a value approximating that observed at PEEP = 9 cmH2O near the beginning of the experiment. No postrecruitment ADC at the ZEEP level was measured at the end due to the depleted hyperpolarized 3He in the reservoir.
Fig. 6.
A: maintenance of rat 6 ventilated at zero end-expiratory pressure (ZEEP) in the supine position for >100 min after a recruitment maneuver resulted in a slowly increasing ADC trend as evidenced by intermediate measurements. ADC maps corresponding to some of the ZEEP states are shown for reference. The error bars represent the SD of the ADC distribution in the imaged slice. The areas shown by arrows depict localized changes in the ADC value. B: end-inspiratory ADC measurements during the stepwise PEEP maneuvers in rat 2 showed good repeatability after the initial recruitment maneuver. The ADC measurements at the final ZEEP levels of third and fourth cycle are missing due to depleted hyperpolarized 3He gas in the reservoir.
Figure 6B shows rat 2, which underwent a total of three consecutive stepwise PEEP maneuvers after the baseline recruitment trial. The first PEEP maneuver progressively decreased end-inspiratory ADC from a starting value at ZEEP of 0.20 ± 0.04 cm2/s. ADC also continued to decrease during the descending limb of the ramp, reaching a value at ZEEP of 0.15 ± 0.04 cm2/s. Then, each subsequent repetition resulted in a cyclic change in the ADC value as a function of the PEEP level, a pattern consistent in all three ramp cycles. No significant difference in the mean ADC value was observed between the corresponding PEEP levels of the three maneuver cycles after the initial recruitment. This indicated that the variation of the PEEP level as a function of time had no significant effect on intrasubject variability of ADC measurements through multiple cycles over the timescale of the experiment.
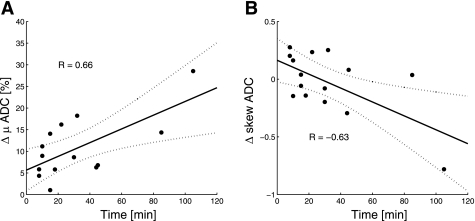
The time course of ADC buildup measured for all rats as a function of time over the wait period between the two recruitment maneuvers is shown in Fig. 7A. The buildup slope was calculated as 9.5 (2.8, 16.3)%h−1, with an overall correlation coefficient of R = 0.66, after one outlier was excluded, as judged by the 1.5 interquartile range. Figure 7B shows the trend of change in ADC distribution skewness as a function of wait time between the two recruitment maneuvers and was calculated as −0.36 (−0.63,−0.09) h−1, with an overall inverse correlation coefficient of R = −0.63.
Fig. 7.
A: linear correlation of the relative change in mean ADC as a function of wait time showed a steady increase in the ADC value in all the imaged rats. One data point was determined as an outlier based on the 1.5 quartile and was excluded from the correlation analysis. B: linear correlation of the change in ADC skewness as a function of wait time showed a steady shift of the distribution toward the lower values in all the imaged rats. The dotted lines represent the 95% confidence intervals in both A and B.
DISCUSSION
Measurements of 3He ADC allowed for the detection and quantification of time-dependent increases in acinar airway size induced by atelectasis buildup and increased inflation of ventilated alveoli in lungs of anesthetized supine rats under mechanical ventilation. The subsequent application of PEEP maneuvers caused ADC to significantly decrease. The latter observation is linked to intrapulmonary gas redistribution due to the recruitment of collapsed alveoli. Therefore, imaging of regional diffusivity of respiratory gas using hyperpolarized 3He MRI may allow for the quantitative assessment of the effects of atelectasis on ventilated alveoli under anesthesia as well as an analysis of the effect of ventilatory maneuvers on alveolar recruitment.
Effects of atelectasis on alveolar inflation.
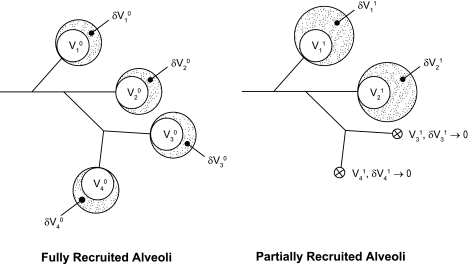
Ventilation without PEEP promotes hypoxemia, a progressive rise in inspiratory pressures, and decay of lung compliance (4, 13, 22, 34, 49). Chest CT studies (22, 30, 49) have documented that these phenomena are associated with regional atelectasis, causing a decrease in the number of ventilated alveoli. Functional residual capacity (FRC) and thoracic dimensions are lower than normal (11, 12, 23), but it is thought that the smaller number of ventilated alveoli, rather than a decrease in their sizes, accounts for most of this volume loss (19). Ventilated alveoli are supported by chest wall recoil and increased interalveolar traction forces (35). As a consequence of their smaller number, these alveoli should then reach abnormally large end-inspiratory dimensions and higher recoil pressures for a constant applied VT (19, 52), as schematically demonstrated in Fig. 8. According to this model, decreases in inspiratory alveolar inflation and PIP are the logical consequence of a recruitment-induced increase in the pool of aerated alveoli (26). This event would lead to the redistribution of inspired gas to a larger number of alveoli that have smaller sizes for a constant VT.
Fig. 8.
A simple model of alveolar collapse and recruitment showing residual Vi0 and fractional δVi0 volumes for the ith representative alveolar unit. Postatelectasis states are shown as Vi1 and δVi1, respectively. A collapsed airway unit is described by both Vi1 → 0 and δVi1 → 0, with nominal boundary conditions: FRCs ≈ Σ Vis and VTs ≈ Σδ Vis, where FRC is functional residual capacity, VT is tidal volume, and s is the state of interest. The underlying assumptions are VT0 = VT1 and Vi0 = Vi1 for aerated alveoli. When a fraction of alveoli is collapsed, for a fixed VT the patent airspaces undergo a proportional increase in size due to the redistribution of inspired gas. Traction forces due to alveolar interdependence may also cause airspace enlargement in the presence of atelectasis.
Hyperinflation of ventilated alveoli is accepted as the main mechanism of compliance decrease in atelectasis and acute lung injury (20) and as one of the pathways through which atelectasis promotes VALI (39). In fact, surfactant-deficient rats ventilated with high VT showed evidence of alveolar damage only in the areas of the lungs that were not atelectatic (55). However, the direct demonstration of alveolar hyperinflation during atelectasis is limited to in vivo microscopy studies (21, 47), which also showed that recruitment maneuvers restored normal alveolar size and dynamics (21). In the absence of strong evidence, it can be argued that changes in lung compliance and PIP during atelectasis may also be explained by other mechanisms. Progressive decreases in lung compliance during constant VT mechanical ventilation have been ascribed to surfactant aggregation causing increased surface tensions (53) and to stress recovery in elastic lung tissue elements (27). Both mechanisms should cause a decrease rather than an increase in alveolar sizes. The observed changes in respiratory gas ADC as a function of the recruitment maneuvers and ventilation on ADC provide support to the concept that atelectasis increases the propensity of ventilated alveolar units to hyperinflation.
Response of ADC to recruitment maneuvers.
As discussed below, ADC is directly related to the size of aerated acinar airspaces (57), which are mostly represented by alveoli and alveolar sacs. We believe that the effect of recruitment maneuvers on ADC values and distribution is related to the reopening of a small but significant portion of the atelectatic lung, whereas the ADC increase at ZEEP may have been caused by the slow accumulation of collapsed groups of alveoli during the mechanical ventilation of the anesthetized animals in the supine position. The presence of atelectasis in rats after ventilation at ZEEP is supported by the decrease in PIP observed after recruitment maneuvers in three animals that underwent the same maneuvers as in the imaging animals (Fig. 2). As discussed above, the decrease in PIP suggests the occurrence of alveolar recruitment. In addition, recent studies (11, 12) have also documented atelectasis in healthy rats after the rats were ventilated at low PEEP and constant VT for up to 150 min during general anesthesia and paralysis. On the other hand, we note that a higher observed PIP can be a result of an increase in alveolar surface tension causing a decrease in lung compliance, in which case alveolar size is expected to decrease rather than increase with atelectasis. Therefore, the observed increase of ADC was not necessarily trivial and could not have been predicted solely on the basis of PIP measurements.
After the induction of anesthesia and an initial preparatory period under mechanical ventilation at ZEEP in the supine position (for a minimum of 1 h), application of the first recruitment maneuver led to a significant decrease in the mean ADC value at the ZEEP level, due to reopening of previously collapsed alveoli. This pattern was universally detected in all studied animals, as shown in Fig. 5A, and was also observed in the second maneuver, although with a smaller relative decline in mean ADC (Fig. 5D). The two maneuvers were separated from each other by a wait period during which ADC increased toward its prerecruitment value. This phenomenon was most likely due to the slow and progressive accumulation of atelectasis, as suggested by the incremental ADC measurements shown in the representative case in Fig. 6A. Even though the duration of the wait period was substantially different among the study animals, the change in ADC from the initial to final points of the wait period was significant in all the rats that underwent MRI (n = 10). The magnitude of the relative change in ADC was shown to be moderately correlated to the wait time, as shown in Fig. 7A, thereby explaining the difference between the two maneuvers. The fact that the two postrecruitment ADC measurements were statistically identical suggests that the lungs returned to the same alveolar configuration after the recruitment maneuvers were applied independent of their initial conditions and history of PEEP trials.
Postrecruitment ADC distributions were almost invariably skewed toward the higher values, whereas their peaks moved in the opposite direction, as shown in Fig. 4. This change was statistically significant in the first maneuver and suggests that the majority of the newly recruited alveoli exhibit a smaller ADC value. However, the change in skewness was not significant in the second maneuver, as shown in Fig. 5C. The large variability of ADC skewness in the second maneuver is believed to be primarily a function of the different buildup time among the different rats. In fact, the trends shown in Fig. 7B demonstrate that the shift in ADC skewness is moderately correlated to the time of the wait period between the two maneuvers.
The behavior of ADC measurements in the two animals that underwent multiple repeats of stepwise PEEP maneuvers showed a fairly good intrasubject repeatability of mean ADC as a function of PEEP level, as shown in Fig. 6B, with the obvious exception of ZEEP states. This substudy, even though not performed on all the animals, provided confidence on how repeatable the recruitment maneuvers and their associated ADC responses were in a given animal as a basis for the entire study. The specific mean ADC variation as a function of PEEP level, however, turned out to be partly unexpected. When considering the end-inspiratory state of the lung, PEEP can be simply interpreted as an elevated inflation level. Therefore, at end inspiration, higher PEEP levels were expected to result in an overall higher ADC value driven by an increase in airway and alveolar size. However, in the PEEP up ramp, as shown in Fig. 6B, the ADC value corresponding to PEEP = 9 cmH2O was invariably lower than that of PEEP = 6 cmH2O. This pattern was consistent across the three repeats. We hypothesize that this behavior is governed by an incremental reopening of the atelectatic portions of the lung, presumably at different opening pressure levels. This hypothesis is compatible with recent observations made with confocal microscopy of alveoli in mice lungs by Namati et al. (41). On the PEEP down ramp, the observed ADC values were consistently lower than their upward counterparts, which may be a cumulative effect of lung tissue hysteresis and an additionally recruited group of alveoli inaccessible before reaching the PEEP = 9 cmH2O value. These phenomena present great potential in extracting additional information about airways dynamics, and they are the subject of further investigation in ongoing research.
Anatomic correlates and limitations of ADC.
ADC provides a measurement of the restricted diffusion of hyperpolarized 3He atoms within the lungs and is therefore directly related to average airspace dimensions within each imaged voxel (15). Once the proximal conductive airways are excluded from analysis, ADC should be especially accurate to probe airway geometry at the acinar level, where diffusion is the primary mechanism of gas transport (62) and where alveoli and alveolar sacs are the most represented airspaces. Use of hyperpolarized gas MRI for ADC measurement has been evaluated extensively in vitro (15, 57) and ex vivo (61). Validation of ADC measurements as an estimate of alveolar and small airway size remains a challenge and the subject of ongoing research (51). This endeavor reflects the lack of a precise morphometric gold standard for regional in vivo measurements and a persistent uncertainty about subtle changes to alveolar structure during freezing or fixation for histology. However, ADC is known to correlate well with certain disease processes, adverse environmental exposures, and therapeutic agents that modify alveolar dimensions (51, 59, 63).
Several sophisticated models have been developed and extensively studied using numerical simulations and compared with in vitro lung specimens as well as artificial lung models. Recent work in this area has demonstrated the possibility to exploit gas diffusion measurements to extract longitudinal and transverse airway dimensions and alveolar diameter along multiple directions (50). The general implication of these models, however, is that diffusion components monotonically increase with larger airway sizes. For example, as shown by Yablonskiy et al. (63), standard stereology parameters of lung microstructure, including the surface-to-volume ratio and number of alveoli per unit lung volume, which are the most commonly used measurements to characterize lung morphology, are closely related to gas diffusion MRI measurements in acinar airways.
Responses of ADC during mechanical ventilation should therefore primarily reflect dimensional changes at the alveolar level. The contribution of small airways, such as respiratory bronchioles, on the measured ADC values cannot be ruled out, especially at the typical imaging MRI resolution in the order of millimeters, where each voxel effectively contains a group of small airways, alveolar ducts, and terminal alveoli. However, since ADC measurements at the voxel level correspond to gas diffusion effects averaged over hundreds of small airway units enclosed in each voxel, the contribution of gas diffusivity in acinar airways bears a high statistical power over a multitude of orientations.
Relevance and possible uses of ADC measurements.
The key factors that link mechanical ventilation on anesthetized subjects to outcomes relate to the effects of mechanical ventilation on the size and mechanics of the alveoli and distal airspaces (33). Therefore, the goal of modern mechanical ventilation is to prevent VALI by avoiding the excessive stretching of airspaces while maintaining a reasonable level of ventilation gas exchange. The observed changes in ADC were relatively small yet significant for the modest sample size of this study and may have physiological and biological relevance (38). Hyperpolarized 3He diffusion MRI is likely to demonstrate more striking ADC changes in animal models of lung injury in which atelectasis is more prominent than in healthy animals. More accurate analysis of alveolar mechanics will require the study of regional ADC heterogeneity and quantification of distribution patterns (instead of only mean values and distribution parameters) and preferably over the entire lung using a three-dimensional imaging sequence. Furthermore, the modeling challenge associated with the coupled dynamics of alveolar expansion (i.e., variable volume) and alveolar collapse (i.e., binary closure) is a major task requiring dedicated research. In future, the combination of ADC measurements with markers of lung injury and inflammation may allow us to investigate the topographical correlations between alterations of alveolar mechanics and the biological effects of mechanical ventilation.
Conclusions.
Measurement of regional respiratory gas diffusivity using hyperpolarized 3He ADC MRI has the potential to become an important investigational tool to evaluate the effects of lung pathology and mechanical ventilation settings on the mechanics of alveoli and behavior of acinar airways. Through the use of this technology with various lung injury models and during different ventilatory strategies, the applicability of undemonstrated hypotheses about alveolar behavior may be disproven or confirmed. A human investigational use of hyperpolarized 3He MRI using the proposed technology is possible and can help to study similar phenomena in human subjects.
GRANTS
This work was supported by National Institutes of Health Grants R01-HL-064741, R01-HL-077241 and R21-EB-008173.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
The authors acknowledge the support of the Small Animal Imaging Facility of the Department of Radiology, University of Pennsylvania.
REFERENCES
- 1. The Acute Respiratory Distress Syndrome Network Ventilation with lower tidal volumes compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med 342: 1301–1308, 2000 [DOI] [PubMed] [Google Scholar]
- 2. Amato MB, Barbas CS, Medeiros DM, Magaldi RB, Schettino GP, Lorenzi-Filho G, Kairalla RA, Deheinzelin D, Munoz C, Oliveira R, Takagaki TY, Carvalho CR. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med 338: 347–354, 1998 [DOI] [PubMed] [Google Scholar]
- 3. Bellani G, Messa C, Guerra L, Spagnolli E, Foti G, Patroniti N, Fumagalli R, Musch G, Fazio F, Pesenti A. Lungs of patients with acute respiratory distress syndrome show diffuse inflammation in normally aerated regions: a [18F]-fluoro-2-deoxy-d-glucose PET/CT study. Crit Care Med 37: 2216–2222, 2009 [DOI] [PubMed] [Google Scholar]
- 4. Bendixen HH, Hedley-Whyte J, Laver MB. Impaired oxygenation in surgical patients during general anesthesia with controlled ventilation. a concept of atelectasis. N Engl J Med 269: 991–996, 1963 [DOI] [PubMed] [Google Scholar]
- 5. Bilek AM, Dee KC, Gaver DP., 3rd Mechanisms of surface-tension-induced epithelial cell damage in a model of pulmonary airway reopening. J Appl Physiol 94: 770–783, 2003 [DOI] [PubMed] [Google Scholar]
- 6. Brower RG, Lanken PN, MacIntyre N, Matthay MA, Morris A, Ancukiewicz M, Schoenfeld D, Thompson BT; National Lung, and Heart, Blood Institute ARDS Clinical Trials Network Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med 351: 327–336, 2004 [DOI] [PubMed] [Google Scholar]
- 7. Carney DE, Bredenberg CE, Schiller HJ, Picone AL, McCann UG, Gatto LA, Bailey G, Fillinger M, Nieman GF. The mechanism of lung volume change during mechanical ventilation. Am J Respir Crit Care Med 160: 1697–1702, 1999 [PubMed] [Google Scholar]
- 8. Crotti S, Mascheroni D, Caironi P, Pelosi P, Ronzoni G, Mondino M, Marini JJ, Gattinoni L. Recruitment and derecruitment during acute respiratory failure: a clinical study. Am J Respir Crit Care Med 164: 131–140, 2001 [DOI] [PubMed] [Google Scholar]
- 9. Dreyfuss D, Saumon G. Ventilator-induced lung injury: lessons from experimental studies. Am J Respir Crit Care Med 157: 294–323, 1998 [DOI] [PubMed] [Google Scholar]
- 10. Dreyfuss D, Soler P, Basset G, Saumon G. High inflation pressure pulmonary edema. Respective effects of high airway pressure, high tidal volume, and positive end-expiratory pressure. Am Rev Respir Dis 137: 1159–1164, 1988 [DOI] [PubMed] [Google Scholar]
- 11. Duggan M, McCaul CL, McNamara PJ, Engelberts D, Ackerley C, Kavanagh BP. Atelectasis causes vascular leak and lethal right ventricular failure in uninjured rat lungs. Am J Respir Crit Care Med 167: 1633–1640, 2003 [DOI] [PubMed] [Google Scholar]
- 12. Duggan M, McNamara PJ, Engelberts D, Pace-Asciak C, Babyn P, Post M, Kavanagh BP. Oxygen attenuates atelectasis-induced injury in the in vivo rat lung. Anesthesiology 103: 522–531, 2005 [DOI] [PubMed] [Google Scholar]
- 13. Egbert LD, Laver MB, Bendixen HH. Intermittent deep breaths and compliance during anesthesia in man. Anesthesiology 24: 57–66, 1963 [Google Scholar]
- 14. Emami K, Kadlecek S, Pickup s Woodburn JM, Zhu J, Yu J, Vahdat V, Ishii M, Cadman R, Rajaei S, Nakayama T, Cox C, Guyer R, Law M, Stephen M, Shrager J, Lipson DA, Gefter W, Rizi RR. An improved technique for measurement of gas diffusion anisotropy in lung. In: Proceedings of the International Society of Magnetic Resonance Medicine Berlin: 2007 [Google Scholar]
- 15. Fain SB, Korosec FR, Holmes JH, O'Halloran R, Sorkness RL, Grist TM. Functional lung imaging using hyperpolarized gas MRI. J Magn Reson Imaging 25: 910–923, 2007 [DOI] [PubMed] [Google Scholar]
- 16. Frank JA, Matthay MA. Science review: mechanisms of ventilator-induced injury. Crit Care 7: 233–241, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gattinoni L, Caironi P, Cressoni M, Chiumello D, Ranieri VM, Quintel M, Russo S, Patroniti N, Cornejo R, Bugedo G. Lung recruitment in patients with the acute respiratory distress syndrome. N Engl J Med 354: 1775–1786, 2006 [DOI] [PubMed] [Google Scholar]
- 18. Gattinoni L, Caironi P, Pelosi P, Goodman LR. What has computed tomography taught us about the acute respiratory distress syndrome? Am J Respir Crit Care Med 164: 1701–1711, 2001 [DOI] [PubMed] [Google Scholar]
- 19. Gattinoni L, Pesenti A. The concept of “baby lung”. Intensive Care Med 31: 776–784, 2005 [DOI] [PubMed] [Google Scholar]
- 20. Gattinoni L, Pesenti A, Avalli L, Rossi F, Bombino M. Pressure-volume curve of total respiratory system in acute respiratory failure. Computed tomographic scan study. Am Rev Respir Dis 136: 730–736, 1987 [DOI] [PubMed] [Google Scholar]
- 21. Halter JM, Steinberg JM, Schiller HJ, DaSilva M, Gatto LA, Landas S, Nieman GF. Positive end-expiratory pressure after a recruitment maneuver prevents both alveolar collapse and recruitment/derecruitment. Am J Respir Crit Care Med 167: 1620–1626, 2003 [DOI] [PubMed] [Google Scholar]
- 22. Hedenstierna G. Alveolar collapse and closure of airways: regular effects of anaesthesia. Clin Physiol Funct Imaging 23: 123–129, 2003 [DOI] [PubMed] [Google Scholar]
- 23. Hedenstierna G, Strandberg A, Brismar B, Lundquist H, Svensson L, Tokics L. Functional residual capacity, thoracoabdominal dimensions, and central blood volume during general anesthesia with muscle paralysis and mechanical ventilation. Anesthesiology 62: 247–254, 1985 [DOI] [PubMed] [Google Scholar]
- 24. Henkelman RM. Measurement of signal intensities in the presence of noise in MR images. Med Phys 12: 232–233, 1985 [DOI] [PubMed] [Google Scholar]
- 25. Hernandez LA, Coker PJ, May S, Thompson AL, Parker JC. Mechanical ventilation increases microvascular permeability in oleic acid-injured lungs. J Appl Physiol 69: 2057–2061, 1990 [DOI] [PubMed] [Google Scholar]
- 26. Hickling KG. The pressure-volume curve is greatly modified by recruitment. A mathematical model of ARDS lungs. Am J Respir Crit Care Med 158: 194–202, 1998 [DOI] [PubMed] [Google Scholar]
- 27. Huang YC, Weinmann GG, Mitzner W. Effect of tidal volume and frequency on the temporal fall in lung compliance. J Appl Physiol 65: 2040–2045, 1988 [DOI] [PubMed] [Google Scholar]
- 28. Hubmayr RD. Perspective on lung injury and recruitment: a skeptical look at the opening and collapse story. Am J Respir Crit Care Med 165: 1647–1653, 2002 [DOI] [PubMed] [Google Scholar]
- 29. Jonson B, Richard JC, Straus C, Mancebo J, Lemaire F, Brochard L. Pressure-volume curves and compliance in acute lung injury: evidence of recruitment above the lower inflection point. Am J Respir Crit Care Med 159: 1172–1178, 1999 [DOI] [PubMed] [Google Scholar]
- 30. Lundquist H, Hedenstierna G, Strandberg A, Tokics L, Brismar B. CT-assessment of dependent lung densities in man during general anaesthesia. Acta Radiol 36: 626–632, 1995 [PubMed] [Google Scholar]
- 31. Lynch JP, Mhyre JG, Dantzker DR. Influence of cardiac output on intrapulmonary shunt. J Appl Physiol 46: 315–321, 1979 [DOI] [PubMed] [Google Scholar]
- 32. Malbouisson LM, Muller JC, Constantin JM, Lu Q, Puybasset L, Rouby JJ, Group CTSAS. Computed tomography assessment of positive end-expiratory pressure-induced alveolar recruitment in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med 163: 1444–1450, 2001 [DOI] [PubMed] [Google Scholar]
- 33. Marini JJ, Gattinoni L. Ventilatory management of acute respiratory distress syndrome: a consensus of two. Crit Care Med 32: 250–255, 2004 [DOI] [PubMed] [Google Scholar]
- 34. Mead J, Collier C. Relation of volume history of lungs to respiratory mechanics in anesthetized dogs. J Appl Physiol 14: 669–678, 1959 [Google Scholar]
- 35. Mead J, Takishima T, Leith D. Stress distribution in lungs: a model of pulmonary elasticity. J Appl Physiol 28: 596–608, 1970 [DOI] [PubMed] [Google Scholar]
- 36. Meade MO, Cook DJ, Guyatt GH, Slutsky AS, Arabi YM, Cooper DJ, Davies AR, Hand LE, Zhou Q, Thabane L, Austin P, Lapinsky S, Baxter A, Russell J, Skrobik Y, Ronco JJ, Stewart TE; Lung Open Ventilation Study Investigators Ventilation strategy using low tidal volumes, recruitment maneuvers, and high positive end-expiratory pressure for acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA 299: 637–645, 2008 [DOI] [PubMed] [Google Scholar]
- 37. Mercat A, Richard JC, Vielle B, Jaber S, Osman D, Diehl JL, Lefrant JY, Prat G, Richecoeur J, Nieszkowska A, Gervais C, Baudot J, Bouadma L, Brochard L; Expiratory Pressure Study Group Positive end-expiratory pressure setting in adults with acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA 299: 646–655, 2008 [DOI] [PubMed] [Google Scholar]
- 38. Moriondo A, Pelosi P, Passi A, Viola M, Marcozzi C, Severgnini P, Ottani V, Quaranta M, Negrini D. Proteoglycan fragmentation and respiratory mechanics in mechanically ventilated healthy rats. J Appl Physiol 103: 747–756, 2007 [DOI] [PubMed] [Google Scholar]
- 39. Muders T, Wrigge H. New insights into experimental evidence on atelectasis and causes of lung injury. Best Pract Res Clin Anaesthesiol 24: 171–182, 2010 [DOI] [PubMed] [Google Scholar]
- 40. Muscedere JG, Mullen JB, Gan K, Slutsky AS. Tidal ventilation at low airway pressures can augment lung injury. Am J Respir Crit Care Med 149: 1327–1334, 1994 [DOI] [PubMed] [Google Scholar]
- 41. Namati E, Thiesse J, de Ryk J, McLennan G. Alveolar dynamics during respiration: are the pores of Kohn a pathway to recruitment? Am J Respir Cell Mol Biol 38: 572–578, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Neeman M, Freyer JP, Sillerud LO. A simple method for obtaining cross-term-free images for diffusion anisotropy studies in NMR microimaging. Magn Reson Med 21: 138–143, 1991 [DOI] [PubMed] [Google Scholar]
- 43. Parker JC, Hernandez LA, Peevy KJ. Mechanisms of ventilator-induced lung injury. Crit Care Med 21: 131–143, 1993 [DOI] [PubMed] [Google Scholar]
- 44. Pelosi P, Goldner M, McKibben A, Adams A, Eccher G, Caironi P, Losappio S, Gattinoni L, Marini JJ. Recruitment and derecruitment during acute respiratory failure: an experimental study. Am J Respir Crit Care Med 164: 122–130, 2001 [DOI] [PubMed] [Google Scholar]
- 45. Puybasset L, Cluzel P, Gusman P, Grenier P, Preteux F, Rouby JJ. Regional distribution of gas and tissue in acute respiratory distress syndrome. I. Consequences for lung morphology. CT Scan ARDS Study Group. Intensive Care Med 26: 857–869, 2000 [DOI] [PubMed] [Google Scholar]
- 46. Saam B, Happer W, Middleton H. Nuclear relaxation of 3He in the presence of O2. Phys Rev A 52: 862–865, 1995 [DOI] [PubMed] [Google Scholar]
- 47. Schiller HJ, McCann UG, 2nd, Carney DE, Gatto LA, Steinberg JM, Nieman GF. Altered alveolar mechanics in the acutely injured lung. Crit Care Med 29: 1049–1055, 2001 [DOI] [PubMed] [Google Scholar]
- 48. Steinberg J, Schiller HJ, Halter JM, Gatto LA, Dasilva M, Amato M, McCann UG, Nieman GF. Tidal volume increases do not affect alveolar mechanics in normal lung but cause alveolar overdistension and exacerbate alveolar instability after surfactant deactivation. Crit Care Med 30: 2675–2683, 2002 [DOI] [PubMed] [Google Scholar]
- 49. Strandberg A, Tokics L, Brismar B, Lundquist H, Hedenstierna G. Atelectasis during anaesthesia and in the postoperative period. Acta Anaesthesiol Scand 30: 154–158, 1986 [DOI] [PubMed] [Google Scholar]
- 50. Sukstanskii AL, Yablonskiy DA. In vivo lung morphometry with hyperpolarized 3He diffusion MRI: theoretical background. J Magn Reson 190: 200–210, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tanoli TS, Woods JC, Conradi MS, Bae KT, Gierada DS, Hogg JC, Cooper JD, Yablonskiy DA. In vivo lung morphometry with hyperpolarized 3He diffusion MRI in canines with induced emphysema: disease progression and comparison with computed tomography. J Appl Physiol 102: 477–484, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Terragni PP, Rosboch G, Tealdi A, Corno E, Menaldo E, Davini O, Gandini G, Herrmann P, Mascia L, Quintel M, Slutsky AS, Gattinoni L, Ranieri VM. Tidal hyperinflation during low tidal volume ventilation in acute respiratory distress syndrome. Am J Respir Crit Care Med 175: 160–166, 2007 [DOI] [PubMed] [Google Scholar]
- 53. Thet LA, Clerch L, Massaro GD, Massaro D. Changes in sedimentation of surfactant in ventilated excised rat lungs. Physical alterations in surfactant associated with the development and reversal of atelectasis. J Clin Invest 64: 600–608, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tremblay L, Valenza F, Ribeiro SP, Li J, Slutsky AS. Injurious ventilatory strategies increase cytokines and c-fos m-RNA expression in an isolated rat lung model. J Clin Invest 99: 944–952, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Tsuchida S, Engelberts D, Peltekova V, Hopkins N, Frndova H, Babyn P, McKerlie C, Post M, McLoughlin P, Kavanagh BP. Atelectasis causes alveolar injury in nonatelectatic lung regions. Am J Respir Crit Care Med 174: 279–289, 2006 [DOI] [PubMed] [Google Scholar]
- 56. Tsuno K, Prato P, Kolobow T. Acute lung injury from mechanical ventilation at moderately high airway pressures. J Appl Physiol 69: 956–961, 1990 [DOI] [PubMed] [Google Scholar]
- 57. Verbanck S, Paiva M. Simulation of the apparent diffusion of helium-3 in the human acinus. J Appl Physiol 103: 249–254, 2007 [DOI] [PubMed] [Google Scholar]
- 58. Walker TG, Happer W. Spin-exchange optical pumping of noble-gas nuclei. Rev Mod Phys 69: 629–642, 1997 [Google Scholar]
- 59. Waters B, Owers-Bradley J, Silverman M. Acinar structure in symptom-free adults by helium-3 magnetic resonance. Am J Respir Crit Care Med 173: 847–851, 2006 [DOI] [PubMed] [Google Scholar]
- 60. Webb HH, Tierney DF. Experimental pulmonary edema due to intermittent positive pressure ventilation with high inflation pressures. Protection by positive end-expiratory pressure. Am Rev Respir Dis 110: 556–565, 1974 [DOI] [PubMed] [Google Scholar]
- 61. Woods J, Choong C, Yablonskiy D, Bentley J, Wong J, Pierce J, Cooper J, Macklem P, Conradi M, Hogg J. Hyperpolarized 3He diffusion MRI and histology in pulmonary emphysema. Magn Reson Med 56: 1293–1300, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Yablonskiy DA, Sukstanskii AL, Leawoods JC, Gierada DS, Bretthorst GL, Lefrak SS, Cooper JD, Conradi MS. Quantitative in vivo assessment of lung microstructure at the alveolar level with hyperpolarized 3He diffusion MRI. Proc Natl Acad Sci USA 99: 3111–3116, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Yablonskiy DA, Sukstanskii AL, Woods JC, Gierada DS, Quirk JD, Hogg JC, Cooper JD, Conradi MS. Quantification of lung microstructure with hyperpolarized 3He diffusion MRI. J Appl Physiol 107: 1258–1265, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]