Abstract
Bronchial vascular angiogenesis takes place in a variety of lung inflammatory conditions such as asthma, cystic fibrosis, lung cancer, and chronic pulmonary thromboembolic disease. However, it is unclear whether neovascularization is predominantly appropriate and preserves lung tissue or whether it contributes further to lung pathology through edema formation and inflammation. In the present study we examined airway and lung parenchymal function 14 days after left pulmonary artery ligation. In rats as well as higher mammals, severe pulmonary ischemia results in bronchial vascular proliferation. Using labeled microspheres, we demonstrated an 18-fold increase in systemic blood flow to the ischemic left lung. Additionally, vascular remodeling extended to the tracheal venules, which showed an average 28% increase in venular diameter. Despite this increase in vascularity, airways resistance was not altered nor was methacholine responsiveness. Since these measurements include the entire lung, we suggest that the normal right lung, which represented 78% of the total lung, obscured the ability to detect a change. When functional indexes such as diffusing capacity, in situ lung volume, and vascular permeability of the left lung could be separated from right lung, significant changes were observed. Thus when comparing average left lung values of rats 14 days after left pulmonary artery ligation to left lungs of rats undergoing sham surgery, diffusing capacity of the left lung decreased by 72%, left lung volume decreased by 38%, and the vascular permeability to protein increased by 58%. No significant differences in inflammatory cell recruitment were observed, suggesting that acute ischemic inflammation had resolved. We conclude that despite the preservation of lung tissue, the proliferating bronchial neovasculature may contribute to a sustained decrement in pulmonary function.
Keywords: angiogenesis, bronchial arteries
the prominent pathological feature of systemic angiogenesis in the lung takes place during conditions of chronic inflammation such as cystic fibrosis (2), asthma (12), pulmonary fibrosis (26), lung cancer (21), chronic obstructive pulmonary disease (COPD) (1), and chronic pulmonary thromboembolic disease (23). However, the regional extent of neovascularization appears to be somewhat differentiated based on whether the underlying lung pathology is predominantly an airway disease (asthma, chronic bronchitis of COPD, cystic fibrosis) vs. a lung parenchymal disorder (chronic thromboembolism). It is interesting that the pulmonary circulation appears not to participate in angiogenesis except under conditions of pneumonectomy (7) and chronic hypoxia (8). Numerous studies have shown that systemic angiogenic beds are functionally abnormal with a proinflammatory phenotype, demonstrating increased protein exudation, fluid flux, leukocyte recruitment, and vasodilation (17, 18). Little is known regarding the angiogenic bronchial vasculature, although clinically, hemoptysis remains a life-threatening consequence of the bronchial neovasculature (2, 10). Furthermore, the increased vascularity and microvascular permeability documented in asthmatic and COPD patients have been suggested to contribute to airflow obstruction and airways hyperreactivity (30). Despite this conjecture, few studies have addressed directly the contribution of bronchial vascularity to airway mechanics. Within the lung parenchyma, the bronchial vasculature anastomoses with pulmonary capillaries and it is assumed that the expanded angiogenic bronchial vasculature is essential to preserve lung tissue when pulmonary perfusion is compromised (4). However, little information is available regarding the functional characteristics of this vasculature in conditions such as chronic thromboembolic disease (22). Whether the neovasculature serves to preserve lung tissue or whether it contributes further to the lung pathology through edema formation and inflammation has not been determined. Our previous work in the mouse, a mammal with a limited subcarinal bronchial circulation, demonstrated increased inflammation and protein leak 21 days after complete left pulmonary artery obstruction (27). However, in the mouse model, neovascularization involves intercostal arteries bridging the pleural space to perfuse pulmonary capillaries (19). The ischemic condition in the mouse may be somewhat different from larger mammals with a patent and more extensive bronchial circulation able to respond immediately to the complete pulmonary ischemia. The growth of the bronchial vasculature in the rat after left pulmonary artery ligation (LPAL) has been described by Weibel (28) using a histologic approach and demonstrates proliferation of the bronchial vasculature in a manner similar to human neovascularization (9). Furthermore, preliminary work casting this neovasculature after LPAL in the rat shows changes in vessels perfusing the large airways and the trachea (25). Given the paucity of information concerning the consequences of bronchial neovascularization on lung function, the present study was designed to evaluate several physiological indexes of airway and parenchymal mechanics. We hypothesize that increased bronchial vascularity will cause decrements in lung function including increased airways resistance and decreased lung compliance. Our results confirmed increased airway and parenchymal vascularity 14 days after LPAL, yet this response did not alter airway resistance or reactivity. However, there were substantial changes in the lung parenchyma, which showed a markedly reduced left lung volume, significant permeability edema, and loss of capillary blood volume. These results suggest that in addition to the injurious effects of chronic ischemia, the bronchial circulation contributes to changes in overall lung function.
METHODS
LPAL.
The protocol was approved by the Johns Hopkins Animal Care and Use Committee. Sprague-Dawley male rats (75–100 g; Harlan, Indianapolis, IN), age-matched, were studied without surgery (naive), after recovery from left lateral thoracotomy (sham operated), or 14 days after LPAL. Rats undergoing surgery were anesthetized, intubated, and ventilated (90 breaths/min, 8 ml/kg) with an anesthetic/gas mixture (2% isoflurane in O2). As previously described, left lateral thoracotomy was performed at the fourth intercostal space to expose the left lung (25). The left pulmonary artery was isolated from the left bronchus, ligated, and the thoracotomy was closed with a suture while the animal was placed on positive end-expiratory pressure (2 cmH2O). Bupivicaine (2 mg/kg) was injected at the incision site for analgesia and the skin incision was closed with methyl acrylamide adhesive. Buprenorphine (0.05 mg/kg ip) was given for additional analgesia. Rats were removed from the ventilator, extubated, and allowed to recover. Sham-operated rats were treated similarly except without left pulmonary artery ligation (LPAL). No surgical procedures were performed on naive rats. After specified times following LPAL and unless otherwise described, anesthetized rats were euthanized by KCl injection into the left ventricle.
Neovascularization assessed by blood flow.
To determine the extent of neovascularization, systemic blood flow to the left lung was measured in rats 14 days after LPAL or after sham surgery (thoracotomy), using fluorescent microspheres (15-μm diameter; Invitrogen, Eugene, OR). Rats were anesthetized, ventilated as described above, the carotid artery was cannulated (PE 20), and 500,000 microspheres were infused (0.5 ml at 0.5 ml/min; Harvard Apparatus, Holliston, MA) followed by 1.0-ml heparinized saline flush. To prevent potential recirculation of microspheres in sham-operated rats, the left pulmonary artery was occluded immediately before microsphere injection. Subsequently, all rats were euthanized by exsanguination, and the left lung was excised. Microspheres lodged in the left lung were quantified after tissue digestion and fluorescent dye extraction, and the number calculated from the standard curve. Data are presented as the percent total microspheres delivered.
Tracheal vessel numbers by intravital microscopy.
Intravital microscopy of tracheal vessels was performed using procedures previously described (13). Briefly, in anesthetized, ventilated rats, the trachea was exposed and continuously superfused with warmed Krebs buffer. Still images were acquired over the length of the trachea (20× objective; 2–3 images/trachea). Quantification of the number of tracheal vessels was performed by 1) counting all vessels with sizes consistent with postcapillary venules (5–50 μm) within a visual field, and 2) identifying the main venule within a visual field and counting all vessels and daughter branches that fed into that venule. Additionally, a single postcapillary venule was visualized for 30 min and the images recorded on videotape. Leukocyte rolling velocity and the number of adherent cells (stationary for ≥ 30 s) in a 200-μm length of vessel (200 μm) were evaluated offline as previously described (13).
Lung carbon monoxide diffusing factor.
To quantify the ability of the lung to exchange gas, we used an index of carbon monoxide (CO) diffusion that is analogous to the CO-diffusing capacity used in humans. Since it is not precisely the same, we termed this the diffusion factor for carbon monoxide (DFCO). It is a measurement that compares the uptake of CO to that of the inert insoluble gas, Ne. This CO uptake is a direct measure of the capillary blood volume; with no red blood cells in the capillaries, the CO uptake would be similar to that of Ne. The DFCO was performed by rapidly inflating the lung at functional residual capacity with 2 ml of a gas mixture of 0.5% Ne and 0.5% CO. After 9 s, 2 ml was rapidly withdrawn, and the concentrations of Ne and CO were measured with a gas chromatograph (Agilent Technologies Micro GC model 3000A, Willmington DE). The DFCO is defined as 1 − (CO9/COc)/(Ne9/Nec), where the c and 9 subscripts refer to the calibration gas and the 9-s measurement time. DFCO thus varies between 0 and 1, with 0 reflecting negligible uptake of CO. Measurements were made in the whole lung, and then in left and right lungs separately.
Lung resistance, dynamic elastance, and airway smooth muscle responsiveness measurements.
In a separate group of anesthetized, intubated, and paralyzed (0.2 mg pancuronium bromide) rats, airway pressure and flow were continuously monitored (ADI Power Lab, Colorado Springs, CO). Three minutes after a standardized deep inspiration maneuver, brief inspiratory occlusions were applied and the resultant elastic and resistive pressures recorded. With the known tidal volume and inspiratory flow, respiratory system elastance and resistance were calculated, respectively, according to the methods described by Ewart and colleagues (5). Subsequently, a cumulative dose-response relationship to methacholine chloride aerosol was performed (0.1 to 10 mg/ml; Aerogen nebulizer, Galway, Ireland; 10-s delivery). Doses were increased until the peak airway pressure reached 25 cmH2O.
Pressure-volume relationships.
Quasi-static pressure-volume (P-V) relationships were determined according to procedures previously described (6). Briefly, rats were anesthetized with pentobarbital, the trachea cannulated, and the animal ventilated with 100% oxygen for 5 min. The tracheal cannula was then occluded, which led to complete degassing of the lungs before in situ testing. The carina was exposed and a vascular clip was placed on the right mainstem bronchus to allow measurement of the P-V relationship of the left lung. Subsequently, the left lung was clamped for right lung measurements. Airway pressure and volume were recorded (PowerLab, ADInstruments, Castle Hill, Australia). The limits of inflation and deflation were 35 and −10 cmH2O, respectively. Total lung capacity (volume at 35 cmH2O on the third inflation) and quasi-static compliance (slope of the deflation limb from 3 to 8 cmH2O) were determined for each lung and summed for total volume.
Permeability by Evans blue dye extravasation.
Evans blue dye (30 mg/kg; 0.5–0.7 ml) was infused via tail vein in anesthetized and ventilated rats and allowed to circulate for 30 min. After death by exsanguination, the intravascular Evans blue dye was flushed from the systemic vasculature (20 ml heparinized saline) by retrograde infusion through the descending aorta. Large airways were dissected from parenchyma at the level of the mainstem bronchi. With this approach, strictly large airway responses were separated from parenchymal responses. Tissues (left lung, right lung, trachea) were removed, weighed, and placed in formamide (5 ml lung, 1 ml trachea) for dye extraction (60°C for 24 h). Dye content was measured (200-μl aliquot) in a plate reader (absorbance at 595 nm). Tissues were then placed in an oven to dry and subsequently were weighed.
Bronchoalveolar lavage.
In separate groups of animals 14 days after surgery and immediately after death, the right lung was ligated and the left lung lavaged (3 × 1.0 ml PBS). Bronchoalveolar lavage fluid was gently aspirated, total recovered volume recorded, and total cell count (Bright Line Hemacytometer, Horsham, PA) and differential cell counts (Cytospin 4; Shandon, Pittsburgh, PA and Diff-quick staining; Dade Bering, Newark, DE) were determined where ∼1,000 cells/rat were evaluated.
Statistics.
All data are presented as means ± SE. Blood flow, vessel numbers, and DFCO of sham and LPAL rats, were compared by unpaired t-test. ANOVA was performed when comparing more than two groups followed by Newman-Keuls post-hoc analysis.
Wilcoxon signed rank test was used to evaluate adherent leukocyte cell numbers relative to zero. A P value < 0.05 was accepted as significant.
RESULTS
Neovascularization.
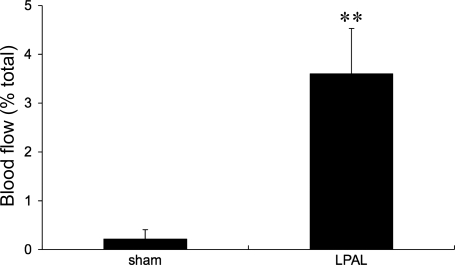
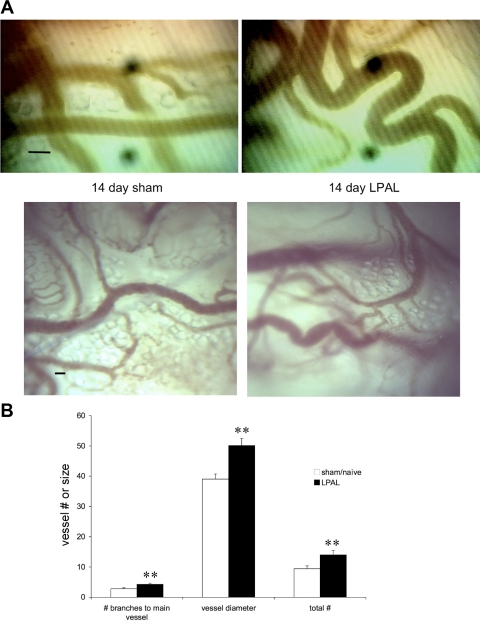
New functional systemic vessel growth to the left lung after LPAL was quantified using fluorescent microspheres injected into the systemic circulation. Fourteen days after LPAL, blood flow to the left lung of rats averaged 3.6 ± 0.9% of the cardiac output whereas systemic blood flow to the left lung of sham-operated rats was 0.2 ± 0.2% of cardiac output (P = 0.002, n = 8 rats/group; Fig. 1). These results confirmed our previous observations demonstrating a significant increase in systemic blood flow to the left lung 14 days after LPAL (25). To further confirm that vessels of the trachea also proliferate after LPAL, we examined the trachea using intravital microscopy. Predominantly capillaries and venules are observed on the ventral surface of the trachea, and increased numbers of these vessels were quantified. Figure 2A demonstrates both bright-field (bottom images) and still images taken from video recordings (top images) of tracheal vessels of sham-operated and LPAL rats. Note the dilated and tortuous vessels of the LPAL images relative to the sham-operated rats. Since the number of vessels or vessel size did not differ in naive and sham-operated rats, they were grouped together (n = 11 rats) and compared with LPAL rats (n = 8 rats). As seen in Fig. 2B, the total number of vessels seen, vessel diameters, and the number of capillaries feeding into a main venule were all significantly greater in LPAL rats than in the sham-operated group (P < 0.009 for each).
Fig. 1.
Blood flow (as a % of total spheres injected) to the left lung 14 days after thoracotomy (sham) and left pulmonary artery ligation (LPAL). There was a significant increase in systemic perfusion of the left lung, confirming bronchial neovascularization. **P = 0.002; n = 8 rats/group.
Fig. 2.
A: tracheal vascular remodeling 14 days after LPAL. Top images are still frames taken from video recordings, and bottom images are bright-field images of tracheal vessels of rats after sham surgery and LPAL. Note the dilated and tortuous vessels of the LPAL relative to the sham-operated rats. Images provide confirmation that vascular remodeling occurs in the trachea after LPAL (representative images from individual rats; bar = 20 μm). B: tracheal vessel numbers and diameter in naive/sham rats (n = 11) and LPAL rats (n = 8). The total number of vessels seen, vessel diameters, and the number of capillaries feeding into a main venule were all significantly greater in LPAL rats than in the sham-operated group. **P < 0.009.
Lung diffusing factor.
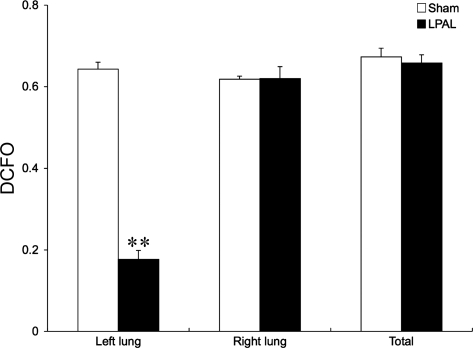
To estimate changes in pulmonary capillary blood volume, we measured the DFCO. Figure 3 shows a comparison of the left, right, and total lung in sham-operated rats (4–6 rats) and in rats 14 days after LPAL (n = 6 rats). A large, significant decrease in the diffusing factor was observed only in the left lung after LPAL (P < 0.001).
Fig. 3.
Lung diffusing factor (DFCO) of the left, right, and total lung in sham-operated rats (n = 4–6) and in rats 14 days after LPAL (n = 6). Note the large, significant decrease in the diffusing factor only in the left lung after LPAL. **P < 0.001.
Having confirmed that the newly expanded systemic vasculature to the ischemic left lung did not fill the pulmonary vascular compartment of the lung, a series of tests were performed to evaluate the overall pulmonary function of the lung.
Pressure-volume relationships.
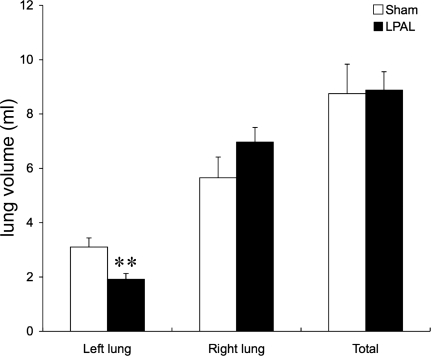
In situ pressure-volume relationships demonstrated that total lung volume was not altered by LPAL compared with sham-operated rats (Fig. 4). However, a significant reduction in left lung volume was seen (P = 0.007) after LPAL. A trend toward increased right lung volume was observed, but this did not reach statistical significance (P = 0.14). The left lung fraction in sham-operated rats was 36 ± 1% of total lung volume, and this value decreased to 22 ± 2% in the LPAL rats (P < 0.001; n = 6 rats/group). Static lung compliance decreased in the left lung after LPAL (0.23 ± 0.02 ml/cmH2O to 0.14 ± 0.03 ml/cmH2O; P = 0.026). In the right lung, static lung compliance increased from 0.46 ± 0.04 ml/cmH2O in sham-operated rats to 0.63 ± 0.03 ml/cmH2O after LPAL. However, when studied in vivo in a separate group of rats where the contributions of left and right lungs could not be separated, total lung elastance (1/compliance) did not differ between sham-operated and LPAL rats before or 14 days or even 21 days after surgery.
Fig. 4.
In situ lung volumes of left, right, and the total lung in sham-operated and LPAL rats (n = 6/group). Left lung volume was seen after LPAL. The left lung fraction in sham-operated rats was 36% of total lung volume, and this value decreased to 22% in the LPAL rats. **P = 0.007.
Airways reactivity.
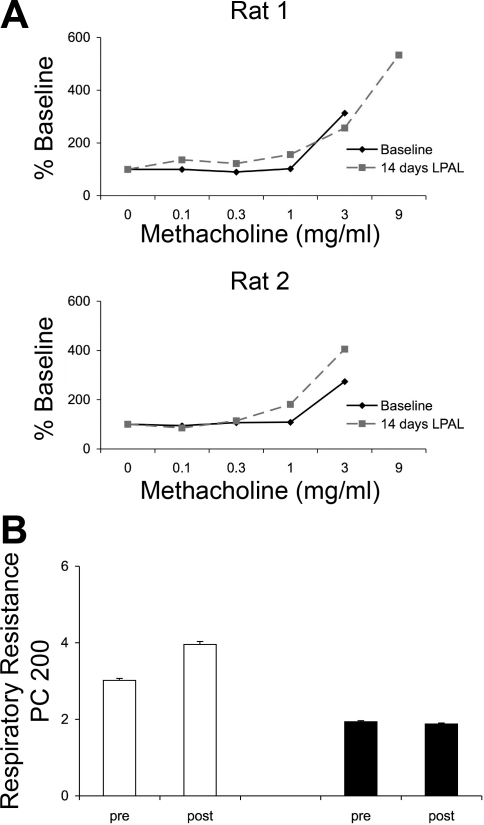
Baseline resistance was not altered by surgery in either sham-operated or LPAL rats and averaged 0.22 ± 0.03 cmH2O·ml−1·s for the entire group. Representative methacholine concentration-response curves are shown in Fig. 5A for two representative rats. As can be seen, no differences in responsiveness were observed 14 days after LPAL. Since no changes were observed, two additional rats for both sham-operated and LPAL groups were studied 21 days after surgery, and these showed no difference from 14-day results. The provocative methacholine concentration that caused a doubling of resistance (PC 200) was calculated from each concentration-response relationship, and average responses for sham-operated and LPAL groups are shown in Fig. 5B. No significant changes in responsiveness were observed between either group, sham operated vs. LPAL, or pre- vs. postsurgery for either group (n = 5/group; P > 0.05). Methacholine-induced changes in elastance were similar in both groups at both time points of evaluation (sham operated vs. LPAL PC 200: 4.2 ± 1.8 vs. 3.3 ± 0.6 mg/ml).
Fig. 5.
Methacholine reactivity. A: the methacholine concentration-response relationships in 2 representative rats before (baseline) and 14 days after LPAL. Baseline resistance averaged 0.22 ± 0.03 cmH2O·ml−1·s for the entire group. No obvious differences in responsiveness to methacholine were apparent. B: the provocative methacholine concentration that caused a doubling of respiratory resistance (PC 200) was calculated from each concentration-response relationship and average responses for sham-operated and LPAL groups are shown. No significant changes in responsiveness were observed between either group, sham vs. LPAL or pre- vs. postsurgery for either group (n = 5 rats/group).
Inflammation.
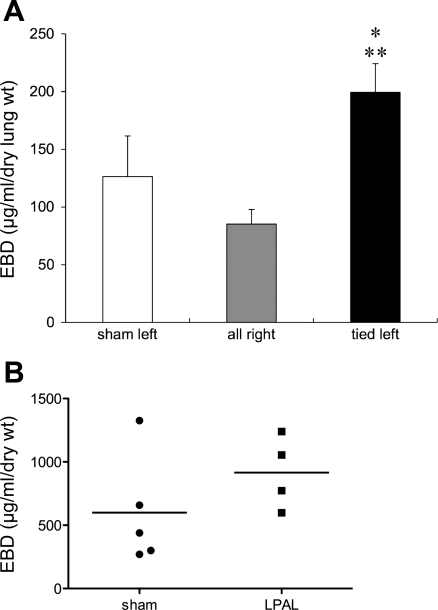
To assess inflammatory aspects of the neovasculature, we studied protein leak in sham-operated rats (n = 5) and rats 14 days after LPAL (n = 5) using Evans blue dye measurements. Since the right lungs from sham operated and tied rats did not differ, we combined these and present group average values of Evans blue dye (μg·ml−1·g dry lung weight−1) in Fig. 6A. Overall, a significant difference in protein extravasation was observed (P = 0.006). Sham left lungs did not differ from right lungs, yet left lungs from LPAL rats showed significantly greater extravasation than sham left lungs (P < 0.05) and right lungs (P < 0.01). Results of tracheal Evans blue dye are shown in Fig. 6B. Although differences between sham-operated and LPAL rats did not reach statistical significance (P = 0.079), a trend toward increased tracheal vascular permeability was present.
Fig. 6.
A: lung vascular permeability measured as Evans blue dye (EBD; μg·ml−1·g dry lung wt−1) concentration in left lungs from sham-operated rats, LPAL-tied left lungs, and right lungs of sham-operated and LPAL rats (n = 5 rats/group). Sham left lungs did not differ from right lungs, yet LPAL-tied left lungs showed significantly greater extravasation than sham left lungs (*P < 0.05) and right lungs (**P < 0.01). B: Evans blue dye extravasation in the trachea of sham-operated and LPAL rats. Each point represents an individual rat. Differences between sham-operated and LPAL rats did not reach statistical significance (P = 0.079).
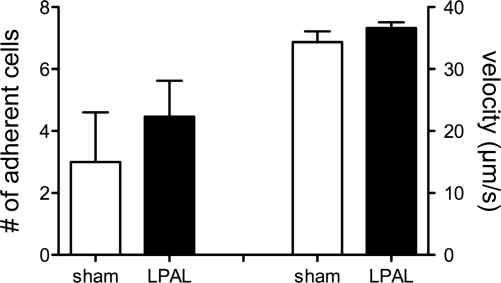
Recruitment of inflammatory cells to the left lung was evaluated in bronchoalveolar lavage fluid 14 days after sham or LPAL surgery. As seen in Table 1, neither total cell counts nor differentials showed differences between the two groups of rats. Additionally, we evaluated inflammatory cell recruitment to the tracheal postcapillary venules using intravital microscopy (Fig. 7). Leukocyte velocity did not differ between sham-operated and LPAL rats, averaging 36 ± 1 μm/s for the entire group (n = 20 rats). The baseline number of adherent leukocytes was variable in sham-operated rats and did not differ from zero, whereas after LPAL, there was a significant increase in the number of adherent cells (P < 0.004). However, when comparing the absolute number of adherent cells in postcapillary venules of sham-operated and LPAL rats, there were no significant differences between the two groups.
Table 1.
Recruitment of inflammatory cells to left lung of sham-operated or LPAL rats
| Total Cell Count | Neutrophils, % | Monocytes/Macrophages, % | Lymphocytes, % | |
|---|---|---|---|---|
| Sham | 21,438 ± 5,411 | 0.3 ± 0.3 | 89.5 ± 3.1 | 9.8 ± 2.7 |
| LPAL | 26,563 ± 2,525 | 0.5 ± 0.3 | 86.5 ± 4.4 | 13 ± 4.3 |
Values are means ± SE; n = 4/group. No significant differences, sham vs. left pulmonary artery ligation (LPAL).
Fig. 7.
Leukocyte adherence (no. of cells; left panel) and velocity (μm/s; right panel) in tracheal postcapillary venules of sham-operated (n = 7) and LPAL rats (n = 13). Leukocyte velocity did not differ between sham-operated and LPAL rats, averaging 36 ± 1 μm/s for the entire group (n = 20 rats). When comparing the absolute number of adherent cells in postcapillary venules of sham-operated and LPAL rats, there were no significant differences between the two groups.
DISCUSSION
Systemic angiogenesis of the airways and lung parenchyma occurs in a variety of lung pathologies; however, the impact of this neovascularization is not known. Although it is generally assumed the bronchial vascular bed sustains the lung when pulmonary perfusion is limited (4), speculation exists that hyperpermeable, dilated vessels in the airways during inflammatory states such as asthma contribute to airflow obstruction and airways hyperreactivity (30). Parenchymal function has not been assessed after chronic thromboembolism when bronchial angiogenesis is likely to occur. In this study we evaluated several indexes of airway and parenchymal function 14 days after occluding the left pulmonary artery. We and others have shown a vigorous proliferation of the bronchial vasculature in the rat after ligating the left pulmonary artery (25, 28). Our initial results confirmed the establishment of a patent systemic vasculature to the left lung with perfusion averaging 3.6% of cardiac output (Fig. 1). This value was somewhat greater than our previous result using radiolabeled microspheres (25) and approximately equivalent to that measured using single-photon emission-computed tomography (SPECT) and micro-CT (4.0% 10 days after LPAL) (29). We believe that the sensitivity of our blood flow measurement currently is greater with fluorescent than radiolabeled spheres due to the potential loss of radioactive ligand from the microspheres used previously. In rats undergoing sham surgery, perfusion to the left lung averaged 0.2% and reflects the very small contribution of bronchial blood flow to the left lung normally.
Because of previous casting of the systemic circulation after LPAL and noticing a profusion of small arteries directed toward the trachea (25), we sought to further evaluate changes in tracheal vascularity. Using the experimental set-up previously established to monitor leukocyte recruitment to tracheal postcapillary venules (13), we imaged the tracheal vasculature and quantified differences in vessel numbers and size offline. These in vivo data add to the substantial histologic data available for the rat tracheal microcirculation (16). Our approach allowed us to measure predominantly venules since arterioles rarely appear on the ventral perspective of the trachea. Since the perturbation to the animal was LPAL, we were surprised to see the small but significantly increased numbers of venules, vessel size, and numbers of capillaries/venules draining into larger vessels (Fig. 2B). Although this observation might be explained by sustained vasodilation, we think this unlikely given the corresponding increase in vessel tortuosity. At this time, we have no explanation of the mechanism responsible for this upstream remodeling of tracheal venules during pulmonary ischemia. It is interesting to note that McDonald and colleagues have shown similar vessel remodeling in the trachea of mice after Mycoplasma pulmonis infection (15). However, in their model, airways are directly exposed to the angiogenic stimulus with its inflammatory sequelae. In the current model, tracheal venules are not intimately connected to the downstream vascular changes in the pulmonary vasculature. At this point, we can only speculate that endothelial cell growth factors are delivered from ischemic parenchyma to tracheal vessels via lymphatics or mucociliary movement but clearly this issue requires greater study.
However, the morphometry demonstrated increased vessel number and size, which is compatible with the hypothesis that increased vascular area in the airway wall contributes to airways hyperreactivity (30). A geometric hypothesis has long been suggested to contribute to airways narrowing either through direct encroachment on the airway lumen or reducing airway-parenchymal interdependence (20). However, we saw no changes in airways resistance or methacholine reactivity. This may reflect an inability to detect small changes in just the left lung, since the right lung should remain normal. The resistance measurement was of the total lung, and since the right lung is ≈2/3 of total lung volume (Fig. 4), a change in left lung resistance alone would have to be substantial enough to rise above the normal variation in total lung resistance level. For this reason, we explored several aspects of pulmonary function in which left lung/right lung comparisons could be made directly and independently.
We measured changes in diffusing capacity as an index of the changes in lung vascular volume during conditions of no pulmonary perfusion but with a confirmed increase in systemic perfusion of the left lung by 14 days. Although there was no pulmonary arterial perfusion to the left lung, the pulmonary capillaries should still be connected to the left atrium, and the bronchial venous drainage flows through these pulmonary veins (24). We thus expected that capillaries would still be filled with blood, although at a lower volume compared with the right lung, proportional to the reduction in mean capillary pressure. We were, therefore, very surprised to find a dramatically attenuated diffusing capacity (Fig. 3), consistent with almost no capillary filling. Neither right lung nor total lung diffusing capacity were altered in LPAL rats and demonstrated values similar to those of sham-operated rats. These results suggest that there is a substantial loss of functional capillaries in the left lung. However, in mice, the alveolar walls are maintained after LPAL (6), so this result suggests that at least at 14 days, the septal walls do not require much of a vasculature to maintain this structure.
Changes in left lung volume were consistent with the changes in diffusing capacity suggesting a smaller left lung (Fig. 4). In sham-operated rats the left lung comprised approximately one-third of total lung volume. After LPAL, this value decreased significantly while total lung volume remained constant and right lung volume trended up. Although these values might represent structural changes in the left lung, our previous work in mice demonstrated no changes in alveolar size despite overall changes in left lung volume (6). The decreased left lung volume could result from loss of unneeded alveolar tissue, but increased fluid/protein leak could also account for the overall changes in left lung volume at this time point. Using the albumin-binding properties of Evans blue dye, we assessed protein exudation in the lung after LPAL. Results demonstrated that the perfusing vasculature of the left lung showed enhanced protein extravasation compared with the sham and right lungs where the pulmonary circulations were predominantly evaluated. Thus the increased bronchial vascular perfusion of the left lung (3.6% of cardiac output) contributed to protein leak beyond that of the normal pulmonary vasculature to the left lung (1/3 cardiac output) or right lung (2/3 cardiac output). Changes in lung volume observed could be related to this increased protein leak with likely concomitant fluid leak.
Additional measurements of inflammation showed that inflammatory cell recruitment to the tracheal neovasculature by intravital microscopy and left lung bronchoalveolar lavage did not differ between sham-operated and LPAL rats. These results are consistent with results published in the mouse model of LPAL demonstrating that although there is an early increase in lavaged inflammatory cells, by 14 days, sham-operated and LPAL show no differences (14). However, these results of inflammatory cell recruitment were obtained and compared in a sham lung with 1/3 of cardiac output compared with one with 3.6% of cardiac output and clear differences in vascular volume. Thus normalization to circulating volume might suggest a much increased inflammatory cell burden after LPAL.
In this study, we chose to evaluate indexes of lung function at a time when a new vasculature was established and functional. Additionally, the 14-day time point was well beyond the time of acute ischemic injury. We expect that had we selected a significantly later time point, the effects observed such as increased vascular permeability might be much greater and likely contribute more to decrements in lung function, particularly lung compliance. Kelly and colleagues studied the effects of LPAL on in vivo lung mechanics in a dog model 3 mo after surgery and used a low-resistance tracheal tube that divided left lung from right lung responses (11). They showed an increase in left lung elastance and an increase in left lung resistance that was predominantly accounted for by an increase in tissue resistance. These authors concluded that the mechanical changes were due to the increased bronchial vascularity which widened the interstitium and thickened the visceral pleura. Additionally, Charan and Carvalho (3) studied sheep 1 yr after left pulmonary artery ligation. Although they made no measurements of pulmonary mechanics, they demonstrated through casting the vasculature, complete filling of pulmonary capillaries by the proliferating bronchial vasculature (3). Thus our results in the rat at an early time point are compatible with observations made at later time points after LPAL in larger mammals (3, 11). Our results demonstrate the early effects of neovascularization on lung function in a mammal where the normal bronchial vasculature proliferates and expands within the lung.
To conclude, we have shown that 14 days after LPAL there is an expanded bronchial vasculature in the left lung as well as tracheal venular remodeling. No changes in airways resistance or airways reactivity to methacholine were observed perhaps due to the in vivo measurements where normal right lung properties predominate. However, LPAL was associated with a decrease in left lung volume and a decrease in diffusing capacity, consistent with a substantially reduced capillary volume. Increased airway vascular permeability was observed in the left lung, which may contribute to the loss of lung volume. Although the lung is sustained during severe pulmonary ischemia, the bronchial neovasculature may contribute to the loss of lung function.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant RO1-HL-088005.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Bergeron C, Boulet LP. Structural changes in airway diseases: characteristics, mechanisms, consequences, and pharmacologic modulation. Chest 129: 1068–1087, 2006 [DOI] [PubMed] [Google Scholar]
- 2. Brinson GM, Noone PG, Mauro MA, Knowles MR, Yankaskas JR, Sandhu JS, Jaques PF. Bronchial artery embolization for the treatment of hemoptysis in patients with cystic fibrosis. Am J Respir Crit Care Med 157: 1951–1958, 1998 [DOI] [PubMed] [Google Scholar]
- 3. Charan NB, Carvalho P. Angiogenesis in bronchial circulatory system after unilateral pulmonary artery obstruction. J Appl Physiol 82: 284–291, 1997 [DOI] [PubMed] [Google Scholar]
- 4. Deffebach ME, Charan NB, Lakshminarayan S, Butler J. The bronchial circulation-small, but a vital attribute of the lung. Am Rev Respir Dis 135: 463–481, 1987 [DOI] [PubMed] [Google Scholar]
- 5. Ewart S, Levitt R, Mitzner W. Respiratory system mechanics in mice measured by end-inflation occlusion. J Appl Physiol 79: 560–566, 1995 [DOI] [PubMed] [Google Scholar]
- 6. Fields MJ, Bishai JM, Mitzner W, Wagner EM. Effects of pulmonary ischemia on lung morphology. Am J Physiol Lung Cell Mol Physiol 293: L254–L258, 2007 [DOI] [PubMed] [Google Scholar]
- 7. Hsia CC, Herazo LF, Fryder-Doffey F, Weibel ER. Compensatory lung growth occurs in adult dogs after right pneumonectomy. J Clin Invest 94: 405–412, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hyvelin JM, Howell K, Nichol A, Costello CM, Preston RJ, McLoughlin P. Inhibition of Rho-kinase attenuates hypoxia-induced angiogenesis in the pulmonary circulation. Circ Res 97: 185–191, 2005 [DOI] [PubMed] [Google Scholar]
- 9. Karsner H, Ghoreyeb A. Studies in infarction: the circulation in experimental pulmonary embolism. J Exp Med 18: 507–522, 1913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kaufman SL, Kan JS, Mitchell SE, Flaherty JT, White RI., Jr Embolization of systemic to pulmonary artery collaterals in the management of hemoptysis in pulmonary atresia. Am J Cardiol 58: 1130–1132, 1986 [DOI] [PubMed] [Google Scholar]
- 11. Kelly SM, Bates JH, Michel RP. Altered mechanical properties of lung parenchyma in postobstructive pulmonary vasculopathy. J Appl Physiol 77: 2543–2551, 1994 [DOI] [PubMed] [Google Scholar]
- 12. Li X, Wilson JW. Increased vascularity of the bronchial mucosa in mild asthma. Am J Respir Crit Care Med 156: 229–233, 1997 [DOI] [PubMed] [Google Scholar]
- 13. Lim LH, Bochner BS, Wagner EM. Leukocyte recruitment in the airways: an intravital microscopic study of rat tracheal microcirculation. Am J Physiol Lung Cell Mol Physiol 282: L959–L967, 2002 [DOI] [PubMed] [Google Scholar]
- 14. McClintock JY, Wagner EM. Role of IL-6 in systemic angiogenesis of the lung. J Appl Physiol 99: 861–866, 2005 [DOI] [PubMed] [Google Scholar]
- 15. McDonald DM. Angiogenesis and remodeling of airway vasculature in chronic inflammation. Am J Respir Crit Care Med 164: S39–S45, 2001 [DOI] [PubMed] [Google Scholar]
- 16. McDonald DM. Endothelial gaps and permeability of venules in rat tracheas exposed to inflammatory stimuli. Am J Physiol Lung Cell Mol Physiol 266: L61–L83, 1994 [DOI] [PubMed] [Google Scholar]
- 17. McDonald DM, Baluk P. Significance of blood vessel leakiness in cancer. Cancer Res 62: 5381–5385, 2002 [PubMed] [Google Scholar]
- 18. Middleton J, Americh L, Gayon R, Julien D, Aguilar L, Amalric F, Girard JP. Endothelial cell phenotypes in the rheumatoid synovium: activated, angiogenic, apoptotic and leaky. Arthritis Res Ther 6: 60–72, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mitzner W, Lee W, Georgakopoulos D, Wagner E. Angiogenesis in the mouse lung. Am J Pathol 157: 93–101, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mitzner W, Wagner EM, Brown RH. Is asthma a vascular disorder? Chest 107: 403–409, 1995 [DOI] [PubMed] [Google Scholar]
- 21. Muller KM, Meyer-Schwickerath M. Bronchial arteries in various stages of bronchogenic carcinoma. Pathol Res Pract 163: 34–46, 1978 [DOI] [PubMed] [Google Scholar]
- 22. Remy-Jardin M, Bouaziz N, Dumont P, Brillet PY, Bruzzi J, Remy J. Bronchial and nonbronchial systemic arteries at multi-detector row CT angiography: comparison with conventional angiography. Radiology 233: 741–749, 2004 [DOI] [PubMed] [Google Scholar]
- 23. Remy-Jardin M, Duhamel A, Deken V, Bouaziz N, Dumont P, Remy J. Systemic collateral supply in patients with chronic thromboembolic and primary pulmonary hypertension: assessment with multi-detector row helical CT angiography. Radiology: 274–281, 2005 [DOI] [PubMed] [Google Scholar]
- 24. Schraufnagel DE. Microvascular casting of the lung: bronchial versus pulmonary artery filling. Scanning Microsc 3: 575–578, 1989 [PubMed] [Google Scholar]
- 25. Sukkar A, Jenkins J, Sánchez J, Wagner E. Inhibition of CXCR2 attenuates bronchial angiogenesis in the ischemic rat lung. J Appl Physiol 104: 1470–1475, 2008 [DOI] [PubMed] [Google Scholar]
- 26. Turner-Warwick M. Precapillary systemic-pulmonary anastomoses. Thorax 18: 225–237, 1963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wagner EM, Karagulova G, Jenkins J, Bishai J, McClintock J. Changes in lung permeability after chronic pulmonary artery obstruction. J Appl Physiol 100: 1224–1229, 2006 [DOI] [PubMed] [Google Scholar]
- 28. Weibel ER. Early stages in the development of collateral circulation to the lung in the rat. Circ Res 8: 353–376, 1960 [DOI] [PubMed] [Google Scholar]
- 29. Wietholt C, Roerig DL, Gordon JB, Haworth ST, Molthen RC, Clough AV. Bronchial circulation angiogenesis in the rat quantified with SPECT and micro-CT. Eur J Nucl Med Mol Imaging 35: 1124–1132, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zanini A, Chetta A, Imperatori AS, Spanevello A, Olivieri D. The role of the bronchial microvasculature in the airway remodelling in asthma and COPD. Respir Res 11: 132, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]