Abstract
Acute leg exercise increases brachial artery retrograde shear rate (SR), while chronic exercise improves vasomotor function. These combined observations are perplexing given the proatherogenic impacts of retrograde shear stress on the vascular endothelium and may be the result of brief protocols used to study acute exercise responses. Therefore, we hypothesized that brachial artery retrograde SR increases initially but subsequently decreases in magnitude during prolonged leg cycling. Additionally, we tested the role of cutaneous vasodilation in the elimination of increased retrograde SR during prolonged exercise. Brachial artery diameter and velocity profiles and forearm skin blood flow and temperature were measured at rest and during 50 min of steady-state, semirecumbent leg cycling (120 W) in 14 males. Exercise decreased forearm vascular conductance (FVC) and increased retrograde SR at 5 min (both P < 0.05), but subsequently forearm and cutaneous vascular conductance (CVC) rose while retrograde SR returned toward baseline values. The elimination of increased retrograde SR was related to the increase in FVC (r2 = 0.58; P < 0.05) and CVC (r2 = 0.32; P < 0.05). Moreover, when the forearm was cooled via a water-perfused suit between minutes 30 and 40 to blunt cutaneous vasodilation attending exercise, FVC was reduced and the magnitude of retrograde SR was increased from −49.7 ± 13.6 to −78.4 ± 16.5 s−1 (P < 0.05). Importantly, these responses resolved with removal of cooling during the final 10 min of exercise (retrograde SR: −46.9 ± 12.5 s−1). We conclude that increased brachial artery retrograde SR at the onset of leg cycling subsequently returns toward baseline values due in part to thermoregulatory cutaneous vasodilation during prolonged exercise.
Keywords: inactive limb, retrograde blood flow, forearm blood flow, skin blood flow, dynamic exercise
in peripheral conduit arteries, the blood flow pattern within a cardiac cycle is characterized by a large antegrade component during systole and a retrograde flow component in early diastole, followed by a subsequent phase of antegrade flow in mid and late diastole (3, 27). Considerable evidence suggests that retrograde shear stress associated with reversal of blood flow presents a detrimental stimulus to the endothelium. For example, cell and organ culture experiments indicate that oscillatory flow profiles with the inherent presence of retrograde shear forces stimulate a proinflammatory, proatherogenic endothelial cell phenotype (6, 8, 10, 17, 29, 49). Human experiments suggest that acutely increasing retrograde shear in the brachial artery (for 30 min) is sufficient to decrease the magnitude of endothelium-dependent flow-mediated vasodilation (40). Furthermore, post-mortem studies indicate that atherosclerotic lesions preferentially develop at bifurcations and other regions of the vasculature distinguished by oscillatory flow patterns (5, 23, 43, 46, 48). Thus retrograde blood flow and the associated oscillatory shear forces are thought to exert proatherogenic effects on the endothelium.
Given the negative influence of retrograde shear on the endothelium, it is surprising that acute dynamic exercise increases retrograde shear in conduit arteries of the inactive limb, which exhibit favorable adaptations to chronic exercise training (7, 24, 26, 32, 41). For example, acute lower limb exercise increases retrograde shear rate in the brachial artery (13, 15, 39), while 2–4 wk of lower limb exercise training increases brachial artery flow-mediated vasodilation, indicating beneficial adaptations within the endothelium (14, 32, 41). A possible explanation for this conundrum is that, to date, no studies have characterized retrograde shear rate during prolonged, steady-state dynamic exercise. Indeed, previous studies (13, 15, 39) demonstrating increased retrograde shear in the brachial artery during lower limb exercise have used short duration, incremental exercise protocols. These protocols lasted 9–15 min, with only 3 min at each workload, and only a single workload was >100 W. In contrast, exercise training studies demonstrating improvements in endothelial vasomotor function in the inactive limb have typically used longer duration steady-state exercise (e.g., 30 min) at a workload sufficient to induce thermoregulatory responses (32, 41). These two exercise protocols (short vs. prolonged duration) are characterized by dramatically different forearm vascular responses, with cutaneous and limb vasodilation prevalent in the latter (1, 2, 4, 20, 21, 34, 44). Therefore, blood flow and shear patterns assessed during a brief exercise bout (in the inactive limb) may not be representative of responses occurring with more sustained, steady-state exercise.
Our group (31) has recently demonstrated a positive association between sympathetic vasoconstrictor outflow and conduit artery retrograde shear rate. Accordingly, it is possible that increased retrograde shear in the inactive limb occurs early in a period of exercise owing to sympathetic vasoconstriction (1, 2, 4, 38) but is subsequently reduced or eliminated when thermoregulatory vasodilation ensues (20, 21, 44). To address this possibility, we studied brachial artery shear patterns and forearm skin blood flow during prolonged leg exercise at an intensity previously shown to produce thermoregulatory vasodilation in the forearm skin (20). We tested the hypothesis that brachial artery retrograde shear rate is increased initially but subsequently is decreased in magnitude or abolished during prolonged cycling exercise. Furthermore, we hypothesized that retrograde shear is inversely related to skin blood flow during prolonged exercise and can be influenced by altering the magnitude of cutaneous vasodilation.
METHODS
This study was approved by the Health Sciences Institutional Review Board of the University of Missouri, and each subject gave written, informed consent before participation.
Subjects.
Fourteen healthy, nonsmoking, normotensive male subjects completed this study (age: 28 ± 4 yr). Subject anthropometric characteristics were as follows: height 181 ± 4 (SD) cm, weight 76.3 ± 7.7 kg, and body mass index 23.3 ± 2.4 kg/m2.
Experimental measurements.
Experiments were performed in a temperature-controlled laboratory with the ambient temperature maintained at 23–24°C (average: 23.8 ± 0.8°C). Subjects were first seated in the semirecumbent position on a medical exam table equipped with an electrically braked cycle ergometer (Angio V2, Lode, Groningen, The Netherlands) and were then instrumented for measurement of heart rate via electrocardiogram (ECG; Quinton Q710, Bothell, WA) and arterial blood pressure on the left arm using an automated sphygmomanometer (Tango+, Suntech Medical Instruments, Raleigh, NC). The right forearm was passed through the sleeve of a water-perfused suit, although this sleeve remained unzipped and therefore did not cover the forearm for the majority of the protocol (see below). To obtain an index of skin blood flow, cutaneous red blood cell flux was measured on the right ventral forearm by laser-Doppler flowmetry (Periflux 5010, Perimed; Jarfalla, Sweden) with integrated laser-Doppler probes housed in local skin heaters (PF5020 local heating units and PeriFlux 5020 Temperature Unit; Perimed; Jarfalla, Sweden). Forearm skin temperature was measured with a copper-constantan thermocouple (Physitemp Instruments, Clifton, NJ) placed adjacent to the site of skin blood flow measurement. Core body temperature was measured via an ingestible pill telemetry system (HQ, Palmetto, FL).
The right brachial artery was imaged longitudinally 2–5 cm above the antecubital fossa by a 2-D high resolution ultrasound system (Logiq 7; GE Medical Systems, Milwaukee, WI), using a 12-MHz multifrequency linear-array transducer (31). Continuous Doppler velocity was simultaneously obtained using the same probe in pulsed-wave mode, operating at a linear frequency of 5 MHz. Doppler velocity signals were corrected at an insonation angle of 60°, and measurements were performed with a large sample volume to encompass the vessel lumen without extending outside of it. Once a satisfactory image was acquired, the right arm was secured and the transducer was stabilized using a custom designed clamp. The location of the transducer was marked on the skin to ensure placement was kept constant throughout the study.
Experimental protocol.
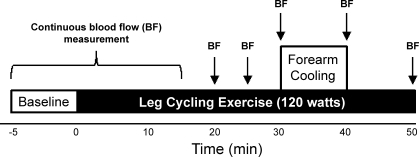
After instrumentation for the measurements described above, subjects rested quietly for 10 min. A schematic of the experimental protocol is illustrated in Fig. 1. The protocol began with a 5-min baseline period during which all variables were measured continuously with the exception of arterial blood pressure (every minute). After baseline data collection was completed, a 50-min bout of cycling exercise was initiated at a workload of 120 W. This protocol (fixed workload) was chosen because it has been shown to increase core temperature sufficiently to stimulate thermoregulatory vasodilation during prolonged leg cycling, producing marked forearm hyperemia directed to the cutaneous circulation (20). We chose not to scale the workload to subject V̇o2max because insufficient data are available regarding how relative effort impacts the distribution of blood flow within the forearm vasculature during prolonged exercise. Continuous data collection was extended through the initial 15 min of exercise to document the temporal profile of retrograde shear during the initial stages of work. Thereafter, 1-min data samples were collected every 5 min through 30 min of exercise, with additional data samples collected at minute 40 (forearm cooling) and minute 50 (see Fig. 1). Blood pressure was measured every 5 min throughout the experiment.
Fig. 1.
Schematic of the experimental protocol. Five minutes of baseline data were recorded before the subject began cycling at a cadence of 60 rpm in the semirecumbent position. This level of exercise was maintained for 50 min while brachial artery blood flow patterns, blood pressure, core temperature, and forearm skin blood flow and temperature were recorded. The forearm was cooled from minutes 30 to 40 by circulating 10°C water through the sleeve of a water-perfused suit covering the arm. Arrows indicate when blood flow measurements were recorded.
Between minutes 30 and 40, the forearm was cooled by circulating 10°C water through the water-perfused suit. The goal of this intervention was to provide a localized cooling stimulus to the skin of the forearm and thereby superimpose a vasoconstrictor signal that would oppose thermoregulatory vasodilation during exercise. This required the suit to be zipped up to cover the surface of the forearm, while plastic bags of 10°C water were used to cool the exposed hand. Because the cooling capacity of the water-perfused suit relies on uniform contact between the skin and water-perfused tubes, it was not possible to obtain skin blood flow measurements and cool the forearm skin simultaneously. Therefore, these measurements were only taken during the initial 30 min of exercise. Since compression of the forearm can alter shear patterns in the upstream conduit artery (40), caution was exercised when zipping up the sleeve of the water perfused suit. In addition, we completed post hoc analysis of the relationship between the decrease in forearm temperature induced by local cooling and the magnitude of forearm vasoconstriction to test whether cooling per se was the driving stimulus for reductions in forearm blood flow. This relationship was statistically significant (P < 0.001), yielding r2 = 0.63, indicating that cooling, and not compression, likely explains the decrease in forearm vascular conductance observed while cooling the forearm during exercise.
After 10 min of forearm cooling, the water-perfused suit was unzipped and peeled away from the forearm. The remaining 10 min of exercise were used as a control to ensure that changes observed during forearm cooling were not due to factors other than the local cooling stimulus. At the end of the exercise period, the site of skin blood flow measurement was locally heated to 43°C to achieve maximal skin blood flow (22, 28).
Data analysis.
Images of the brachial artery and associated velocity waveform were acquired at 30 Hz using a custom Labview program interfaced to the video output (640 × 480 pixels) of the Doppler ultrasound machine through a PCI-1411 video card (National Instruments, Austin, TX). Skin blood flow and heart rate data were digitized at 1,000 Hz with signal-processing software (Chart v5.2 and Powerlab; ADInstruments, Bella Vista, NSW, Australia). The ECG signal and a trigger pulse synchronized to the R-wave of the ECG were also embedded as data streams (33 pts/image) into an AVI file containing the video images from the ultrasound machine.
Off-line analyses of brachial artery diameters and velocities were performed using a custom-designed edge-detection and wall tracking software (Labview; National Instruments). The blood velocity measurements obtained using this software were validated against those obtained directly from the Logiq 7 ultrasound machine analysis program. In brief, the user identified a region of interest on the portion of the image where the brachial artery walls were clearest. The internal edges of the artery were then detected by thresholding and iterative regression procedures similar to a previously described method (11). For each video frame, a thresholding algorithm searched for two appropriate edges, starting from the vessel centerline and proceeding outward in both directions. The algorithm detected 10–100 points along each edge, depending on the number of horizontal pixels in the region of interest. It then searched iteratively, according to preset criteria, for a subset of points along each edge that were best fit by a first-order equation, discarding points that substantially lowered the correlation coefficient. When a best-fit solution converged for each edge, the difference between the mid-points of the two lines was taken as the average internal diameter of the artery. Pixels were converted to units of mm according to the calibration scale generated by the Doppler machine. To decode the Doppler waveform, a method similar to that described by Green (13) was used. Each video frame was subtracted from the previous frame so that the difference image was a narrow, vertical subimage containing the updated Doppler information for each video frame. The width of the subimage was determined by the front-panel display settings of the ultrasound monitor (2–32 pixels wide); for the settings used throughout the present protocols, the subimage was uniformly 4 pixels wide. Another thresholding algorithm was then used to detect both the upper and lower edges of the Doppler signal in the subimage, and the data were concatenated to those of the previous scan line. The resulting reconstruction of the Doppler signal resulted in an effective data rate of 120 points/s (4 pixels × 30 Hz), compared with the single diameter measurement obtained per video frame (30 points/s). Velocity and diameter data were synchronized to the other channels of embedded AVI data, which were downsampled from 1,000 to 120 Hz, and all parameters were then output to a text file. The file, containing mean blood velocity, antegrade mean velocity, and retrograde mean velocity data, was subsequently processed by a second LabView program that used the R-wave trigger pulse to analyze each parameter on a beat-by-beat basis. Antegrade and retrograde mean blood velocities per cardiac cycle were calculated using the average of only positive or negative data points, respectively, yielding within-cardiac cycle values for these parameters.
Diameter and velocity measurements were used to calculate brachial artery blood flow and shear rate. Blood flow was calculated as Vm·Π·(D2/4)·60, where Vm is mean blood velocity (cm/s) and D is arterial diameter (cm), and shear rate (s−1) was defined as 4 × Vm/D (31). For calculations of antegrade and retrograde shear rate, antegrade and retrograde mean blood velocities were used, respectively. Oscillatory shear index, an indicator of the magnitude of shear oscillation, was defined as follows: (∣retrograde shear∣)/(∣antegrade shear∣) + (∣retrograde shear∣) (31, 47). Forearm vascular conductance (ml·min−1·mmHg−1) was calculated as the ratio of forearm blood flow to mean arterial pressure.
Statistical analysis.
Statistical analysis was performed using SigmaStat (Systat Software, Chicago, IL). Relationships between changes in retrograde shear rate and forearm/skin vascular conductance during exercise were assessed by linear regression. For each variable, a one-way repeated measures ANOVA was performed to assess the effect of time. When a significant time effect was detected, Dunnet's post hoc procedure was used to determine where differences occurred. This post hoc procedure facilitates comparison of sequentially determined data samples against a baseline or reference condition/time point, and therefore data during the first 30 min of exercise (i.e., prolonged exercise with no intervention) were compared with baseline, whereas data collected at the end of forearm cooling and subsequently at the end of exercise were compared to precooling values (i.e., at 30 min). Differences were considered statistically significant when P < 0.05. All values are presented as means ± SE unless otherwise indicated.
RESULTS
Biphasic shear pattern and forearm hemodynamic response during prolonged leg cycling.
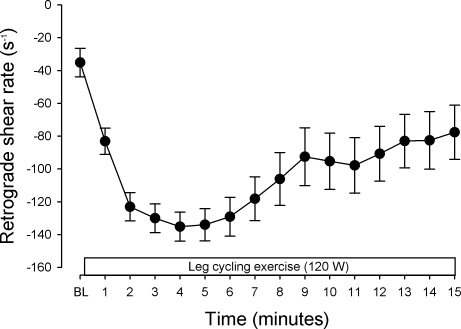
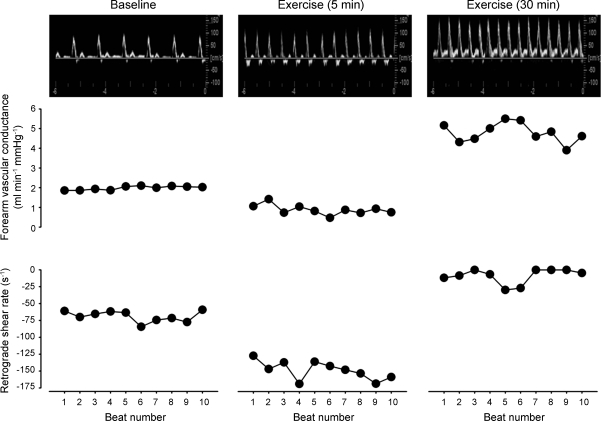
Figure 2 displays minute by minute average retrograde shear rate for baseline and the initial 15 min of exercise. The magnitude of retrograde shear rate increased (i.e., became more negative) through the first 5 min of exercise and then decreased toward baseline values from minutes 5 to 15. Figure 3 displays original Doppler flow profiles, beat-to-beat forearm vascular conductance, and retrograde shear rate responses from a representative subject at baseline, 5 min, and 30 min of exercise. Retrograde shear rate was increased when forearm vascular conductance was decreased at 5 min, while decreases in retrograde shear rate were associated with increases in forearm vascular conductance at 30 min of exercise.
Fig. 2.
Brachial artery retrograde shear rate averaged over 1-min intervals at baseline and through 15 min of leg cycling exercise. The magnitude of retrograde shear rate was transiently increased (i.e., became more negative) through the first 5 min of exercise and then decreased toward baseline values from minutes 5 to 15. Data are means ± SE. BL, baseline.
Fig. 3.
Original Doppler ultrasound flow profiles, beat-to-beat forearm vascular conductance, and retrograde shear rate responses from a single subject at baseline, 5 min, and 30 min of exercise. Forearm vascular conductance and retrograde shear rate data are presented for 10 cardiac cycles at each time point. Note the corresponding changes in retrograde shear rate and forearm vascular conductance at 5 vs. 30 min.
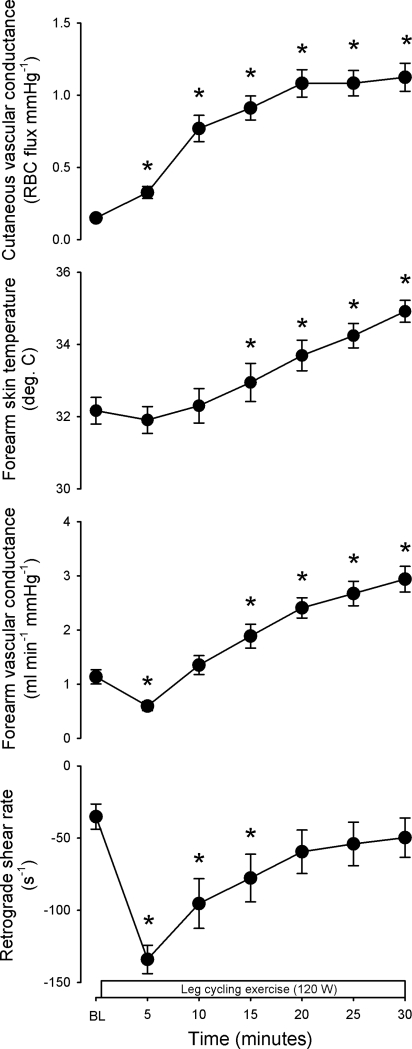
Figure 4 displays group responses at baseline and through 30 min of exercise for cutaneous vascular conductance, forearm skin temperature, forearm vascular conductance, and retrograde shear rate. The magnitude of retrograde shear rate increased while forearm vascular conductance decreased from baseline to 5 min of exercise (P < 0.05 vs. baseline), and these events appeared to be correlated, although this relationship was not statistically significant (r2 = 0.26, p = 0.061). After 5 min of exercise, the direction of changes in retrograde shear rate and forearm vascular conductance was subsequently reversed, and retrograde shear rate was no longer statistically different from baseline at 20 min of exercise (P > 0.05), while forearm vascular conductance was elevated from baseline by 15 min of exercise (P < 0.05). Cutaneous vascular conductance was increased from 5 through 30 min of exercise (P < 0.05 vs. baseline). Similar responses were seen when cutaneous vascular conductance was expressed as a percentage of maximal values obtained during local heating [7.7 ± 0.8, 16.7 ± 2.5, 38.1 ± 4.2, 45.5 ± 4.2, 53.7 ± 4.2, 54.0 ± 4.2, and 55.5 ± 4.2%CVCmax for baseline and 5*, 10*, 15*, 20*, 25*, and 30* minutes of exercise, respectively (*P < 0.05 vs. baseline)]. Table 1 displays responses in core temperature and cardiovascular measurements through 30 min of exercise. Expected responses to prolonged, mild-to-moderate intensity leg cycling were observed in core temperature, heart rate, blood pressure, and forearm hemodynamics. In addition, the oscillatory shear index was increased initially (P < 0.05 vs. baseline), indicating enhanced bidirectional flow, but subsequently normalized through 30 min of exercise. Brachial artery diameter decreased at 5 min but was then elevated from baseline by 15 min of exercise.
Fig. 4.
Forearm cutaneous vascular conductance, forearm skin temperature, forearm vascular conductance, and retrograde shear rate as a function of time at baseline and throughout 30 min of exercise. Data are means ± SE. *P < 0.05 vs. baseline.
Table 1.
Core temperature and cardiovascular measurements at baseline and through 30 min of exercise
| Leg Cycling Exercise, 120 W |
|||||||
|---|---|---|---|---|---|---|---|
| Baseline | 5 min | 10 min | 15 min | 20 min | 25 min | 30 min | |
| Core temperature, °C | 37.08 ± 0.07 | 37.14 ± 0.08 | 37.30 ± 0.07* | 37.42 ± 0.08* | 37.53 ± 0.08* | 37.61 ± 0.08* | 37.63 ± 0.07* |
| Heart rate, beats/min | 61 ± 3 | 121 ± 5* | 130 ± 7* | 133 ± 7* | 136 ± 7* | 139 ± 7* | 140 ± 7* |
| Systolic blood pressure, mmHg | 115 ± 3 | 160 ± 3* | 173 ± 4* | 170 ± 4* | 170 ± 5* | 173 ± 5* | 168 ± 4* |
| Diastolic blood pressure, mmHg | 65 ± 1 | 72 ± 3* | 65 ± 3 | 64 ± 2 | 58 ± 2* | 60 ± 1 | 59 ± 2 |
| Mean arterial pressure, mmHg | 81 ± 1 | 101 ± 2* | 101 ± 2* | 99 ± 2* | 96 ± 2* | 97 ± 2* | 95 ± 2* |
| Forearm blood flow, ml/min | 92.1 ± 10.7 | 59.5 ± 8.3 | 136.8 ± 18.5* | 187.9 ± 22.8* | 230.1 ± 18.8* | 262.2 ± 24.1* | 281.8 ± 25.0* |
| Antegrade blood velocity, cm/s | 12.5 ± 0.7 | 23.1 ± 1.3* | 25.1 ± 1.7* | 27.9 ± 1.9* | 30.0 ± 1.6* | 31.4 ± 2.0* | 32.3 ± 2.0* |
| Retrograde blood velocity, cm/s | −3.6 ± 0.9 | −13.3 ± 0.9* | −9.8 ± 1.7* | −8.3 ± 1.7* | −6.6 ± 1.6* | −6.2 ± 1.7 | −5.7 ± 1.6 |
| Mean blood velocity, cm/s | 10.6 ± 0.9 | 7.7 ± 1.0 | 16.3 ± 2.2* | 20.4 ± 2.2* | 24.0 ± 1.7* | 25.9 ± 2.5* | 27.1 ± 2.4* |
| Antegrade shear rate, s−1 | 118.8 ± 6.8 | 232.1 ± 15.4* | 242.4 ± 18.6* | 257.4 ± 20.3* | 269.5 ± 16.8* | 273.6 ± 19.6* | 277.7 ± 19.4* |
| Mean shear rate, s−1 | 99.7 ± 8.4 | 76.6 ± 10.4 | 155.9 ± 22.5* | 187.4 ± 21.1* | 215.1 ± 16.8* | 225.8 ± 23.8* | 233.0 ± 22.2* |
| Oscillatory shear index | 0.19 ± 0.04 | 0.37 ± 0.01* | 0.26 ± 0.04 | 0.21 ± 0.04 | 0.15 ± 0.03 | 0.14 ± 0.04 | 0.13 ± 0.03 |
| Brachial artery diameter, mm | 4.22 ± 0.10 | 4.02 ± 0.09* | 4.19 ± 0.10 | 4.39 ± 0.11* | 4.50 ± 0.10* | 4.63 ± 0.09* | 4.69 ± 0.10* |
Values are means ± SE; n = 14.
P < 0.05 vs. baseline.
Elimination of increased brachial artery retrograde shear rate during prolonged leg cycling: role of forearm cutaneous vasodilation.
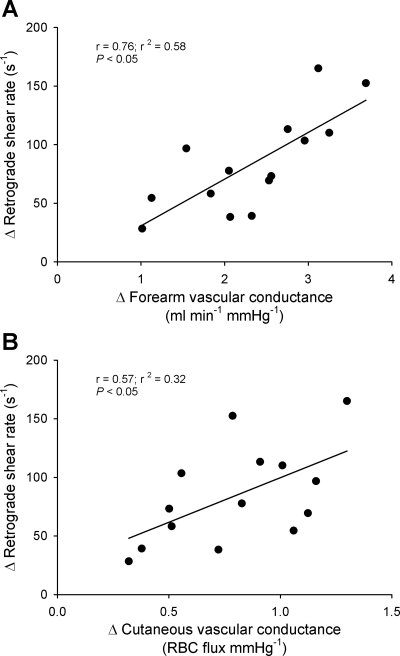
Figure 5 displays the relationship between changes in cutaneous and forearm vascular conductance and retrograde shear rate from 5 to 30 min of exercise. Significant positive relationships between changes in both cutaneous (r2 = 0.32; P = 0.034) and forearm (r2 = 0.58; P = 0.002) vascular conductance and retrograde shear rate were observed.
Fig. 5.
Linear regression analysis of the relationship between changes in forearm (A) and cutaneous (B) vascular conductance and changes in retrograde shear rate from minutes 5 to 30 of exercise in all 14 subjects.
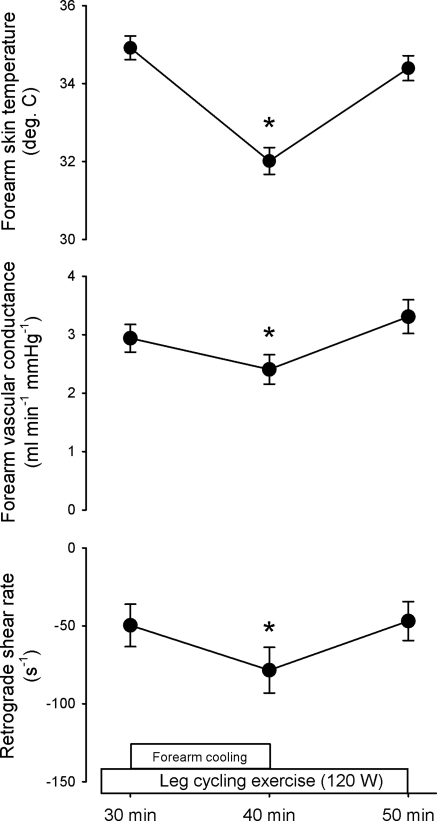
The contribution of cutaneous and forearm vasodilation to the removal of increased retrograde shear rate after its peak at 5 min was assessed by cooling the forearm during prolonged exercise. Figure 6 displays the effect of forearm cooling from 30 to 40 min of exercise on forearm skin temperature, forearm vascular conductance, and retrograde shear rate. Forearm cooling increased the magnitude of retrograde shear rate while decreasing forearm vascular conductance and skin temperature (all P < 0.05 vs. 30 min). The removal of forearm cooling resolved these responses so that retrograde shear rate, forearm vascular conductance, and forearm skin temperature were not different between 30 and 50 min of exercise (all P > 0.05). Table 2 displays exercise core temperature and cardiovascular responses before, during, and after forearm cooling. Forearm cooling during exercise had no impact on core temperature or systemic hemodynamics. In contrast, forearm hemodynamics were altered in a manner consistent with downstream vasoconstriction. Oscillatory shear index was increased by forearm cooling (P < 0.05), while this and other forearm hemodynamic responses resolved when cooling was removed.
Fig. 6.
Effect of forearm cooling and subsequent removal of cooling on forearm skin temperature, forearm vascular conductance, and retrograde shear rate during steady-state exercise. The forearm was cooled from minutes 30 to 40 of exercise by circulating 10°C water through the sleeve of a water-perfused suit covering the arm. Data are means ± SE. *P < 0.05 vs. 30 min.
Table 2.
Exercise core temperature and cardiovascular measurements before, during, and after forearm cooling
| Leg Cycling Exercise, 120 W |
|||
|---|---|---|---|
| 30 min | 40 min (forearm cooling) | 50 min | |
| Core temperature, °C | 37.63 ± 0.07 | 37.76 ± 0.09* | 37.86 ± 0.09* |
| Heart rate, beats/min | 140 ± 7 | 138 ± 7 | 140 ± 7 |
| Systolic blood pressure, mmHg | 168 ± 4 | 166 ± 4 | 165 ± 4 |
| Diastolic blood pressure, mmHg | 59 ± 12 | 56 ± 2 | 53 ± 2* |
| Mean arterial pressure, mmHg | 95 ± 2 | 92 ± 2 | 91 ± 2* |
| Forearm blood flow, ml/min | 281.8 ± 25.0 | 222.2 ± 23.2* | 300.9 ± 26.5 |
| Antegrade blood velocity, cm/s | 32.3 ± 2.0 | 29.5 ± 1.9* | 32.5 ± 2.2 |
| Retrograde blood velocity, cm/s | −5.7 ± 1.6 | −9.2 ± 1.7* | −5.6 ± 1.5 |
| Mean blood velocity, cm/s | 27.1 ± 2.4 | 21.4 ± 2.1* | 27.4 ± 2.4 |
| Antegrade shear rate, s−1 | 277.7 ± 19.4 | 252.8 ± 16.3* | 270.6 ± 18.9 |
| Mean shear rate, s−1 | 233.0 ± 22.2 | 183.4 ± 19.0* | 228.2 ± 20.8 |
| Oscillatory shear index | 0.13 ± 0.03 | 0.22 ± 0.04* | 0.13 ± 0.03 |
| Brachial artery diameter, mm | 4.69 ± 0.10 | 4.69 ± 0.09 | 4.83 ± 0.09* |
Values are means ± SE; n = 14.
P < 0.05 vs. 30 min.
DISCUSSION
The major novel finding of this study is that initial increases in brachial artery retrograde shear rate at the onset of leg cycling exercise are removed as steady-state exercise progresses. The normalization of retrograde shear as exercise continues is due in part to cutaneous vasodilation, as cooling the forearm skin and evoking local vasoconstriction increase the magnitude of retrograde shear rate during prolonged exercise. Collectively, these data indicate that brachial artery retrograde shear rate is significantly increased at the onset of leg exercise when downstream vasoconstriction is observed. However, as exercise progresses, subsequent thermoregulatory cutaneous vasodilation increases limb vascular conductance, contributing to the return of retrograde shear rate back toward baseline values.
In humans, the onset of large muscle mass, dynamic leg exercise elicits a robust vasoconstrictor response in the forearm vasculature (1, 2, 4, 38). Blair et al. (4) showed that this response could be abolished by proximal blockade of sympathetic neural transmission using local anesthetic or by intra-arterial infusion of bretylium tosylate, which prevents the release of neurotransmitters from adrenergic nerve terminals. These results suggest the initial vasoconstriction in the forearm during leg exercise is mediated by increased sympathetic vasoconstrictor outflow. When leg exercise is prolonged, however, the initial vasoconstrictor response in the forearm is reversed, and limb vasodilation is observed (20, 44). Forearm vasodilation in this setting is linearly related to the increase in core temperature (44), is confined to the cutaneous vascular bed (20, 21), and therefore represents a cardiovascular adjustment to the thermoregulatory demands of sustained exercise (19, 34).
This biphasic forearm vascular response to leg cycling suggests that differences may exist between short and more prolonged duration exercise with respect to beat-to-beat blood flow and shear patterns. Indeed, previous studies (3, 31) have shown that retrograde velocity and shear patterns in peripheral conduit arteries are affected by acute changes in sympathetic nerve activity and vascular tone in the distal circulation. Therefore, we reasoned that brachial artery shear patterns would vary as a function of downstream forearm vascular conductance during prolonged, steady-state exercise. In support of our hypothesis, the brachial artery retrograde shear rate increased at the onset of leg exercise when forearm vascular conductance was reduced but subsequently decreased as forearm vascular conductance increased during prolonged exercise. The increase in the magnitude of retrograde shear rate at exercise onset appeared to be correlated with decreased forearm vascular conductance, while the subsequent reduction in retrograde shear rate as exercise progressed was significantly related to changes in forearm and skin vascular conductance. In addition, local cooling of the forearm, which decreased skin temperature and forearm vascular conductance, increased retrograde shear rate during prolonged exercise. Thus, after an initial increase in brachial artery retrograde shear rate at the onset of exercise, subsequent increases in cutaneous and therefore whole limb vascular conductance cause retrograde shear rate to return to baseline values as exercise progresses.
The return of brachial artery retrograde shear rate to baseline values during prolonged exercise was associated with concomitant increases in antegrade and mean shear rate (see Table 1). Overall, these responses indicate the conversion toward a more anti-atherogenic stimulus to the endothelium during sustained exercise. Indeed, similar shear patterns have been observed in the brachial artery during rhythmic handgrip exercise and appear to be responsible for the increases in endothelium-dependent flow-mediated vasodilation after chronic forearm exercise training (42). Therefore, the current observations provide a link between brachial artery shear patterns prevalent during acute leg exercise and improvements in endothelial vasomotor function associated with chronic leg exercise training (32, 41). These data support the proposal by Tanaka et al. (37) that increases in brachial artery endothelial vasomotor function caused by large muscle mass dynamic exercise training are the result of chronic elevations in mean shear stress and further suggest a role for thermoregulatory cutaneous vasodilation in these responses.
Interestingly, we noted a decrease in brachial artery diameter at the onset of leg exercise which was followed by progressive increases throughout prolonged exercise. To our knowledge, these are the first measurements of conduit artery diameter in the inactive limb during prolonged, steady-state exercise in a thermoneutral environment. Changes in brachial artery diameter and mean shear rate were directionally similar such that decreased shear rate at exercise onset was associated with decreased artery diameter, whereas increased shear during prolonged exercise was associated with increased diameter. This directionally similar relationship is predictable on the basis of shear-mediated changes in diameter (12, 25), as previously demonstrated in vessels perfusing the active muscle during rhythmic handgrip exercise (33). These observations support the possibility that mean shear rate is a primary determinant of conduit artery diameter in the inactive tissues during prolonged, steady-state exercise.
We have previously shown that the effect of increased sympathetic nerve activity on brachial artery retrograde shear rate is minimized if arterial pressure is elevated at rest (31). In contrast, the current results and those of others (39) suggest that this is not the case during dynamic exercise. That is, mean arterial pressure is elevated during dynamic exercise; yet retrograde shear rate is also increased at exercise onset. It is unclear why the regulatory role of arterial pressure in this context is altered during exercise, but systemic effects of exercise may be involved. For example, changes in arterial stiffness during dynamic exercise suggest that vascular compliance is increased in the peripheral circulation (35). It has been suggested that conduit artery retrograde blood flow (and shear stress) is tightly linked to the relation between systemic perfusion pressure and the “critical closing pressure” in the downstream resistance vessels (16). If this is true, then factors that affect resistance vessel caliber at a given pressure (e.g., compliance) may alter the integrated effects of systemic perfusion pressure and downstream pressure on conduit artery blood flow patterns, thereby increasing the arterial pressure necessary to maintain completely antegrade blood flow when the sympathetic nervous system is activated. These concepts warrant further investigation.
Cutaneous vascular conductance was modestly but significantly increased at the onset of exercise when brachial artery retrograde shear rate was at its highest (Fig. 4). This finding suggests that forearm cutaneous vascular conductance is not a primary determinant of brachial artery retrograde shear rate at the onset of exercise. Indeed, as discussed above, it appears that whole limb (i.e., downstream) vascular conductance determines the magnitude of conduit artery retrograde shear rate, and at the onset of exercise a clear reduction in forearm vascular conductance was observed. At rest (in thermoneutral conditions), ∼30% of forearm blood flow is directed to the skin (9), which indicates that only a small portion of forearm vascular conductance resides in the cutaneous circulation. Therefore, brachial artery retrograde shear rate is likely influenced more by downstream skeletal muscle vascular conductance (vs. skin) during resting conditions and at the onset of exercise. In contrast, prolonged mild to moderate intensity leg cycling represents a scenario in which 75–80% of forearm blood flow is directed to the skin circulation (20, 30), and therefore forearm vascular conductance is primarily determined by cutaneous vascular conductance in this setting. Thus, a potential explanation for why increased cutaneous vascular conductance caused a decrease in the magnitude of retrograde shear rate (and vice versa) during prolonged exercise, and not at the onset of exercise, is that only during prolonged exercise was the cutaneous circulation the major effector of forearm vascular conductance. Importantly, although an increase in retrograde shear rate occurring concomitant with a decrease in forearm vascular conductance at exercise onset fits with our overall hypothesis regarding the regulation of shear rate profiles during exercise and a modest trend was noted for these phenomena to be correlated (r2 = 0.26; P = 0.061), this study was not designed to assess the contributing mechanisms to the initial increase in retrograde shear rate at the onset of exercise. It is plausible that factors other than downstream vascular conductance alter shear patterns in the transition from rest to exercise.
Perspectives
Primary aging and type II diabetes are associated with a reduced capacity to increase skin blood flow during thermal stress (18, 36, 45). As such, the transition during exercise to more favorable shear patterns in the nonactive limb when thermoregulatory cutaneous vasodilation increases limb vascular conductance may be less complete in these populations. This would presumably lead to a greater maintenance of the initial elevation in brachial artery retrograde shear rate during prolonged exercise. Indeed, the potential implication for improvements and/or the preservation of systemic vascular health in these populations through regular aerobic exercise requires further investigation.
Conclusions
In summary, we have shown for the first time that the robust increase in the magnitude of brachial artery retrograde shear rate at exercise onset was removed during prolonged, steady-state leg cycling. In addition, a role for thermoregulatory cutaneous vasodilation in the removal of increased retrograde shear was demonstrated by manipulation of the forearm vasodilation attendant to exercise. We conclude that increased brachial artery retrograde shear rate at the onset of leg cycling subsequently returns toward resting values as exercise progresses, due in part to thermoregulatory cutaneous vasodilation and the associated increase in downstream vascular conductance.
GRANTS
This study was supported by National Institutes of Health Grants R01-HL-093167 (to P. J. Fadel), P01-HL-052490 (to M. H. Laughlin), P01-HL-095486 (to M. J. Davis), and T32-AR-048523 (to G. H. Simmons).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Dr. David Edwards, University of Delaware, for providing an initial version of the LabView data acquisition program. In addition, we thank the subjects who participated in this study.
REFERENCES
- 1. Bevegard BS, Shepherd JT. Reaction in man of resistance and capacity vessels in forearm and hand to leg exercise. J Appl Physiol 21: 123–132, 1966 [DOI] [PubMed] [Google Scholar]
- 2. Bishop JM, Donald KW, Taylor SH, Wormald PN. The blood flow in the human arm during supine leg exercise. J Physiol 137: 294–308, 1957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Blackshear WM, Phillips DJ, Strandness DE. Pulsed Doppler assessment of normal human femoral artery velocity patterns. J Surg Res 27: 73–83, 1979 [DOI] [PubMed] [Google Scholar]
- 4. Blair DA, Glover WE, Roddie IC. Vasomotor responses in the human arm during leg exercise. Circ Res 9: 264–274, 1961 [Google Scholar]
- 5. Caro CG, Fitz-Gerald JM, Schroter RC. Arterial wall shear and distribution of early atheroma in man. Nature 223: 1159–1161, 1969 [DOI] [PubMed] [Google Scholar]
- 6. Chien S. Effects of disturbed flow on endothelial cells. Ann Biomed Eng 36: 554–562, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Clarkson P, Montgomery HE, Mullen MJ, Donald AE, Powe AJ, Bull T, Jubb M, World M, Deanfield JE. Exercise training enhances endothelial function in young men. J Am Coll Cardiol 33: 1379–1385, 1999 [DOI] [PubMed] [Google Scholar]
- 8. Conway DE, Williams MR, Eskin SG, McIntire LV. Endothelial cell responses to atheroprone flow are driven by two separate flow components: low time-average shear stress and fluid flow reversal. Am J Physiol Heart Circ Physiol 298: H367–H374, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cooper KE, Edholm OG, Mottram RF. The blood flow in skin and muscle of the human forearm. J Physiol 128: 258–267, 1955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dai G, Kaazempur-Mofrad MR, Natarajan S, Zhang Y, Vaughn S, Blackman BR, Kamm RD, Garcia-Cardena G, Gimbrone MA. Distinct endothelial phenotypes evoked by arterial waveforms derived from atherosclerosis-susceptible and -resistant regions of human vasculature. Proc Natl Acad Sci USA 101: 14871–14876, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Davis MJ. An improved, computer-based method to automatically track internal and external diameter of isolated microvessels. Microcirculation 12: 361–372, 2005 [DOI] [PubMed] [Google Scholar]
- 12. Gori T, Dragoni S, Lisi M, Di Stolfo G, Sonnati S, Fineschi M, Parker JD. Conduit artery constriction mediated by low flow: a novel noninvasive method for the assessment of vascular function. J Am Coll Cardiol 51: 1953–1958, 2008 [DOI] [PubMed] [Google Scholar]
- 13. Green D, Cheetham C, Reed C, Dembo L, O'Driscoll G. Assessment of brachial artery blood flow across the cardiac cycle: retrograde flows during cycle ergometry. J Appl Physiol 93: 361–368, 2002 [DOI] [PubMed] [Google Scholar]
- 14. Green DJ. Exercise training as vascular medicine: direct impacts on the vasculature in humans. Exerc Sport Sci Rev 37: 196–202, 2009 [DOI] [PubMed] [Google Scholar]
- 15. Green DJ, Bilsborough W, Naylor LH, Reed C, Wright J, O'Driscoll G, Walsh JH. Comparison of forearm blood flow responses to incremental handgrip and cycle ergometer exercise: relative contribution of nitric oxide. J Physiol 562: 617–628, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Halliwill JR, Minson CT. Retrograde shear: backwards into the future? Am J Physiol Heart Circ Physiol 298: H1126–H1127, 2010 [DOI] [PubMed] [Google Scholar]
- 17. Hastings NE, Simmers MB, McDonald OG, Wamhoff BR, Blackman BR. Atherosclerosis-prone hemodynamics differentially regulates endothelial and smooth muscle cell phenotypes and promotes pro-nflammatory priming. Am J Physiol Cell Physiol 293: C1824–C1833, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Holowatz LA, Thompson-Torgerson C, Kenney WL. Aging and the control of human skin blood flow. Front Biosci 15: 718–739, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Johnson JM, Proppe DW. Cardiovascular adjustments to heat stress. In: Handbook of Physiology, section 4: Environmental Physiology, edited by Fregly MJ, Blatteis CM. Bethesda, MD: Am. Physiol. Soc., 1996, sect. 4, vol. 1, chapt. 11, p. 215–243 [Google Scholar]
- 20. Johnson JM, Rowell LB. Forearm skin and muscle vascular responses to prolonged leg exercise in man. J Appl Physiol 39: 920–924, 1975 [DOI] [PubMed] [Google Scholar]
- 21. Kamon E, Belding HS. Dermal blood flow in the resting arm during prolonged leg exercise. J Appl Physiol 26: 317–320, 1969 [DOI] [PubMed] [Google Scholar]
- 22. Kellogg DL, Jr, Liu Y, Kosiba IF, O'Donnell D. Role of nitric oxide in the vascular effects of local warming of the skin in humans. J Appl Physiol 86: 1185–1190, 1999 [DOI] [PubMed] [Google Scholar]
- 23. Ku DN, Giddens DP, Zarins CK, Glagov S. Pulsatile flow and atherosclerosis in the human carotid bifurcation. Positive correlation between plaque location and low oscillating shear stress. Arteriosclerosis 5: 293–302, 1985 [DOI] [PubMed] [Google Scholar]
- 24. Lavrencic A, Salobir BG, Keber I. Physical training improves flow-mediated dilation in patients with the polymetabolic syndrome. Arterioscler Thromb Vasc Biol 20: 551–555, 2000 [DOI] [PubMed] [Google Scholar]
- 25. Lie M, Sejersted OM, Kiil F. Local regulation of vascular cross section during changes in femoral arterial blood flow in dogs. Circ Res 27: 727–737, 1970 [DOI] [PubMed] [Google Scholar]
- 26. Linke A, Schoene N, Gielen S, Hofer J, Erbs S, Schuler G, Hambrecht R. Endothelial dysfunction in patients with chronic heart failure: systemic effects of lower-limb exercise training. J Am Coll Cardiol 37: 392–397, 2001 [DOI] [PubMed] [Google Scholar]
- 27. McDonald DA. The relation of pulsatile pressure to flow in arteries. J Physiol 127: 533–552, 1955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Minson CT, Berry LT, Joyner MJ. Nitric oxide and neurally mediated regulation of skin blood flow during local heating. J Appl Physiol 91: 1619–1626, 2001 [DOI] [PubMed] [Google Scholar]
- 29. O'Keeffe LM, Muir G, Piterina AV, McGloughlin T. Vascular cell adhesion molecule-1 expression in endothelial cells exposed to physiological coronary wall shear stresses. J Biomech Eng 131: 081003, 2009 [DOI] [PubMed] [Google Scholar]
- 30. Ooue A, Ichinose T, Inoue Y, Nishiyasu T, Koga S, Kondo N. Changes in blood flow in conduit artery and veins of the upper arm during leg exercise in humans. Eur J Appl Physiol 103: 367–373, 2008 [DOI] [PubMed] [Google Scholar]
- 31. Padilla J, Young CN, Simmons GH, Deo SH, Newcomer SC, Sullivan JP, Laughlin MH, Fadel PJ. Increased muscle sympathetic nerve activity acutely alters conduit artery shear rate patterns. Am J Physiol Heart Circ Physiol 298: H1128–H1135, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pullin CH, Bellamy MF, D. B, Ashton M, Davies B, Williams S, Goodfellow J, Wilson JF, Lewis MJ. Time course of changes in endothelial function following exercise in habitually sedentary men. J Exerc Physiol Online 7: 14–22, 2004 [Google Scholar]
- 33. Pyke KE, Poitras V, Tschakovsky ME. Brachial artery flow-mediated dilation during handgrip exercise: evidence for endothelial transduction of the mean shear stimulus. Am J Physiol Heart Circ Physiol 294: H2669–H2679, 2008 [DOI] [PubMed] [Google Scholar]
- 34. Rowell LB, O'Leary DS, Kellogg DL. Integration of cardiovascular control system in dynamic exercise. In: Handbook of Physiology: Exercise: Regulation and Integration of Multiple Systems, edited by Rowell LB, Shepherd JT. Bethesda, MD: Am. Physiol. Soc., 1996, sect. 12, chapt. 17, p. 770–840 [Google Scholar]
- 35. Sharman JE, McEniery CM, Campbell R, Pusalkar P, Wilkinson IB, Coombes JS, Cockcroft JR. Nitric oxide does not significantly contribute to changes in pulse pressure amplification during light aerobic exercise. Hypertension 51: 856–861, 2008 [DOI] [PubMed] [Google Scholar]
- 36. Sokolnicki LA, Strom NA, Roberts SK, Kingsley-Berg SA, Basu A, Charkoudian N. Skin blood flow and nitric oxide during body heating in type 2 diabetes mellitus. J Appl Physiol 106: 566–570, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tanaka H, Shimizu S, Ohmori F, Muraoka Y, Kumagai M, Yoshizawa M, Kagaya A. Increases in blood flow and shear stress to nonworking limbs during incremental exercise. Med Sci Sports Exerc 38: 81–85, 2006 [DOI] [PubMed] [Google Scholar]
- 38. Taylor J, Hand G, Johnson D, Seals D. Augmented forearm vasoconstriction during dynamic exercise in healthy older men. Circulation 86: 1789–1799, 1992 [DOI] [PubMed] [Google Scholar]
- 39. Thijssen DH, Dawson EA, Black MA, Hopman MT, Cable NT, Green DJ. Brachial artery blood flow responses to different modalities of lower limb exercise. Med Sci Sports Exerc 41: 1072–1079, 2009 [DOI] [PubMed] [Google Scholar]
- 40. Thijssen DH, Dawson EA, Tinken TM, Cable NT, Green DJ. Retrograde flow and shear rate acutely impair endothelial function in humans. Hypertension 53: 986–992, 2009 [DOI] [PubMed] [Google Scholar]
- 41. Tinken TM, Thijssen DHJ, Black MA, Cable NT, Green DJ. Time course of change in vasodilator function and capacity in response to exercise training in humans. J Physiol 586: 5003–5012, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tinken TM, Thijssen DHJ, Hopkins N, Dawson EA, Cable NT, Green DJ. Shear stress mediates endothelial adaptations to exercise training in humans. Hypertension 55: 312–318, 2010 [DOI] [PubMed] [Google Scholar]
- 43. VanderLaan PA, Reardon CA, Getz GS. Site specificity of atherosclerosis: site-selective responses to atherosclerotic modulators. Arterioscler Thromb Vasc Biol 24: 12–22, 2004 [DOI] [PubMed] [Google Scholar]
- 44. Wenger CB, Roberts MF, Stolwijk JA, Nadel ER. Forearm blood flow during body temperature transients produced by leg exercise. J Appl Physiol 38: 58–63, 1975 [DOI] [PubMed] [Google Scholar]
- 45. Wick DE, Roberts SK, Basu A, Sandroni P, Fealey RD, Sletten D, Charkoudian N. Delayed threshold for active cutaneous vasodilation in patients with Type 2 diabetes mellitus. J Appl Physiol 100: 637–641, 2006 [DOI] [PubMed] [Google Scholar]
- 46. Wissler RW, Strong JP. Risk factors and progression of atherosclerosis in youth. Am J Pathol 153: 1023–1033, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wu SP, Ringgaard S, Oyre S, Hansen MS, Rasmus S, Pedersen EM. Wall shear rates differ between the normal carotid, femoral, and brachial arteries: an in vivo MRI study. J Magn Reson Imaging 19: 188–193, 2004 [DOI] [PubMed] [Google Scholar]
- 48. Zarins CK, Giddens DP, Bharadvaj BK, Sottiurai VS, Mabon RF, Glagov S. Carotid bifurcation atherosclerosis. Quantitative correlation of plaque localization with flow velocity profiles and wall shear stress. Circ Res 53: 502–514, 1983 [DOI] [PubMed] [Google Scholar]
- 49. Ziegler T, Bouzourene K, Harrison VJ, brunner HR, Hayoz D. Influence of oscillatory and unidirectional flow environments on the expression of endothelin and nitric oxide synthase in cultured endothelial cells. Arterioscler Thromb Vasc Biol 18: 686–692, 1998 [DOI] [PubMed] [Google Scholar]