Abstract
During hibernation in the 13-lined ground squirrel, Ictidomys tridecemlineatus, the cerebral cortex is electrically silent, yet the brainstem continues to regulate cardiorespiratory function. Previous work showed that neurons in slices through the medullary ventral respiratory column (VRC) but not the cortex are insensitive to high doses of pentobarbital during hibernation, leading to the hypothesis that GABAA receptors (GABAAR) in the VRC undergo a seasonal modification in subunit composition. To test whether alteration of GABAAR subunits are responsible for hibernation-associated pentobarbital insensitivity, we examined an array of subunits using RT-PCR and Western blots and identified changes in ε- and δ-subunits in the medulla but not the cortex. Using immunohistochemistry, we confirmed that during hibernation, the expression of ε-subunit-containing GABAARs nearly doubles in the VRC. We also identified a population of δ-subunit-containing GABAARs adjacent to the VRC that were differentially expressed during hibernation. As δ-subunit-containing GABAARs are particularly sensitive to ethanol (EtOH), multichannel electrodes were inserted in slices of medulla and cortex from hibernating squirrels and EtOH was applied. EtOH, which normally inhibits neuronal activity, excited VRC but not cortical neurons during hibernation. This excitation was prevented by bicuculline pretreatment, indicating the involvement of GABAARs. We propose that neuronal activity in the VRC during hibernation is unaffected by pentobarbital due to upregulation of ε-subunit-containing GABAARs on VRC neurons. Synaptic input from adjacent inhibitory interneurons that express δ-subunit-containing GABAARs is responsible for the excitatory effects of EtOH on VRC neurons during hibernation.
Keywords: GABAA receptor subunit, ventrolateral medulla, ethanol
hibernating animals provide a natural model with which to investigate mechanisms of circuit-specific neuronal plasticity. During torpor [body temperature (Tb) = 4°C], the forebrain is isoelectric (54), and the dendritic processes of neurons in the cortex, hippocampus, and thalamus retract (51, 52). In contrast, neuronal control of respiratory function is maintained during torpor (34). Throughout hibernation, between torpor bouts (3–21 days), squirrels spontaneously rewarm (Tb = 37°C) and resume activity for <24 h. Previous work in our laboratory (22) has shown that during these arousals (interbout arousal) neurons in the ventral respiratory column (VRC) are insensitive to high doses of barbiturates. In contrast, cortical neurons are highly sensitive to the inhibitory effects of pentobarbital throughout the year. Similarly, VRC neurons in squirrels are sensitive to pentobarbital during the summer active months. Sensitivity to muscimol, a GABA receptor agonist, is unchanged in both VRC and cortex throughout the year, indicating the presence of fully functional GABA receptors. As barbiturates are potent allosteric positive modulators of GABAA receptors, circuit-specific alterations in GABAARs are likely to be responsible for the pentobarbital insensitivity in this medullary brain region during hibernation.
The VRC is a column of neurons extending through the ventrolateral medulla that encompasses the essential rhythm and pattern generating circuitry of the respiratory control system (2). The VRC consists of the Bötzinger region in the rostral medulla, the pre-Bötzinger complex (pre-BötC), and the rostral ventral respiratory group (rVRG) to the caudal ventral respiratory group (cVRG) in the caudal medulla. Identification of the VRC in squirrels is based on anatomical landmarks with reference to rats (37). Findings in squirrels suggest that medullary GABAA receptors are involved in preserving respiratory function during the extreme conditions that define mammalian hibernation (22). However, the specific molecular changes that take place in GABAARs in respiratory brain regions during hibernation are unknown.
GABAARs are pentameric and typically composed of α1(2)-, β2(2)-, and γ2-subunits. Two molecules of GABA bind the receptor at junctions of the α- and β-subunits, while the γ2-subunit facilitates postsynaptic localization (4). Thus far 19 GABAAR subunits have been found in nature (12). While the predominant GABAAR found throughout the rodent brain is the α1β2γ2-subtype, there are regionally localized subtypes that incorporate other subunits (39). GABAAR subtypes can help to define distinct neuronal circuits, subcellular localization, and pharmacokinetics, thereby contributing to unique circuit characteristics (41, 44). Subunit substitutions can radically alter cellular and network responses to GABAAR modulators. For example, when the δ-subunit is inserted in place of γ2, GABAARs are predominantly extrasynaptic and display increased sensitivity to allosteric modulators, especially ethanol (EtOH; Ref. 21), so much that they have been termed “a target for alcohol” (47). Less well understood is the ε-subunit, the function and natural expression patterns of which are largely unknown. Transfection of the ε-subunit results in GABAARs that are insensitive to pentobarbital, propofol, and benzodiazepines (13, 14, 23), yet a natural role for the ε-subunit has not yet been established.
There are three potential hypotheses to explain the previously reported pentobarbital insensitivity in VRC neurons during hibernation (22). First, there is insertion of ε-subunit-containing GABAARs into VRC neurons during hibernation. Second, there is a decrease in δ-subunit-containing GABAARs during hibernation. A third explanation as to how VRC neurons could become seasonally insensitive to pentobarbital is by changes in GABAA receptor subtypes on neurons that project to VRC neurons. Each of these hypotheses can be tested with molecular, electrophysiological, and pharmacological approaches. For example, insertion of δ-subunit-containing GABAARs on inhibitory interneurons projecting to VRC neurons would result in an excitatory response to EtOH. We used several different techniques to test these hypotheses, including quantitative RT-PCR, Western blot, immunohistochemistry, and multichannel recording from brain slices, recognizing the challenges associated with such studies in squirrel. Based on our findings, we propose a model of GABAAR distribution (involving receptors expressing ε- and δ-subunits) that explains how VRC neurons may become seasonally insensitive to lethal doses of barbiturate. These findings hint at a solution for the problem of how medullary networks involved in cardiorespiratory control remain active during hibernation while most other (higher) brain regions are functionally switched off for energy conservation.
MATERIALS AND METHODS
Ethical approval.
All experimental procedures were in accordance with National Institutes of Health guidelines and were approved by the University of Wisconsin-Madison Institutional Animal Care and Use Committee.
Experimental animals.
Thirteen-lined ground squirrels (Ictidomys tridecemlineatus) were trapped in and around Madison, WI, between May and September. Animals were housed individually with access to food and water ad libitum. From May through September, animals were maintained at an ambient temperature of 22°C with a 12:12-h light-dark cycle [summer active (SA)]. In September-February, animals were housed in a dark room maintained at 4°C to facilitate hibernation. Seasonal hibernation is characterized by extended periods of time (3–21 days) spent in the torpid, hypometabolic state [torpor (T)], punctuated by a rapid return (∼2 h) to normothermia lasting for ∼12 h [interbout arousal (IBA)] before reentering torpor (10, 27). Food and water were removed after ∼2 wk of torpor/arousal cycles. During IBA, animals were active but did not eat or drink. All hibernating animals completed at least four full torpor bouts before being used in experiments. Torpor duration was monitored daily by the sawdust method (9). Briefly, animals were covered in sawdust upon entrance into torpor and checked every 24 h for either arousal (IBA) or a disruption of sawdust, indicating an arousal and reentrance into torpor since the last observation. Tb was measured with a rectal thermometer upon decapitation. Tb of torpid hibernators was 3–9°C, 35–38°C for IBA hibernators and 36–38°C for summer active animals. A total of 104 squirrels was used in the following experiments.
Tissue collection.
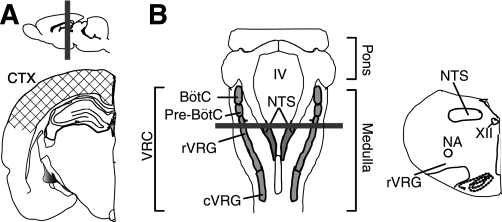
Brain tissue was collected from SA, T, and IBA animals. Animals (SA and IBA) were deeply anesthetized with isoflurane and killed by decapitation; T animals were placed in a cooled chamber and exposed to 5% isoflurane for 5 min before decapitation. The medulla (between C1 and the pons) was harvested. Cortical samples (CTX), including primary motor and somatosensory areas centered on a region equivalent to bregma −3.24 mm in rat (37), were harvested simultaneously (Fig. 1A). In a separate group of animals, tissues punches (diameter = 0.75 mm, depth = 0.75 mm; Stoelting, Wood Dale, IL) were taken from CTX, and two medullary regions previously reported to be seasonally insensitive to sodium pentobarbital, the VRC and the nucleus of the solitary tract (NTS; Ref. 22). Punches were taken from three slices (thickness = 750 μm) through the medulla inclusive of the BötC and extending through the cVRG (Fig. 1B). Tissue samples for Western blot were homogenized in buffer [65 mM Tris·HCl, pH 7.4, 0.108 M NaCl, and 0.05 M NaF, protease inhibitor (Roche, Indianapolis, IN), 2.57 mM EDTA, and 0.5% Triton X-100] and stored at −80°C. Total protein concentration of each extract was measured using a Nanodrop (Fisher Scientific, Waltham, MA).
Fig. 1.
Brain regions harvested for molecular experiments and location of slices for electrophysiological recordings. Cortical samples (A), medullary samples (B), and punches containing the ventral respiratory column (VRC; B) were used for PCR and Western blot. Slices taken through the forebrain (A; hatched area) and through the medulla (B) were used for electrophysiological recordings. Solid gray bars indicate a sample location of coronal sections used for immunohistochemistry. IV, fourth ventricle; NTS, nucleus of the solitary tract; BötC, Bötzinger complex; Pre-BötC, pre-Bötzinger complex; rVRG, rostral ventral respiratory group; cVRG, caudal ventral respiratory group; NA, nucleus ambiguus; XII, hypoglossal nucleus.
Western blot.
Cortical and medullary lysates were subjected to SDS PAGE by separating 30 μg protein per lane on 12% gels. Proteins were transferred to 0.2 μm nitrocellulose membrane (Sigma-Aldrich, St. Louis, MO), blocked in Tris-buffered saline with Tween-20 (TBST; 50 mM Tris-HCl, pH 7.5, 15 mM NaCl, and 0.5% Tween-20) containing 5% Blotto (Santa Cruz Biotechnology, Santa Cruz, CA), washed in TBST, and incubated at 4°C overnight in TBST containing 1% Blotto and primary antibody [anti-GABAA δ 1:1,000, AB9752 (Millipore, Billerica, MA); anti-GABAA ε 1:500, 1:1,000, 1:5,000, ab35971 (Abcam, Cambridge, MA); anti-GABAA α1 1:1,000, PRB-564P (Covance, Emeryville, CA); and anti-GABAA γ2 1:1,000, PRB-569C (Covance)]. Subsequently, membranes were washed in TBST and incubated for 1.0 h in TBST containing 1% Blotto and secondary antibody (goat anti-rabbit or rabbit anti-mouse IgG 1:3,000; New England Biolabs, Ipswich, MA). Membranes were washed in TBST and incubated in enhanced chemiluminescent Western blotting substrate (ECL; Pierce, Rockford, IL) for 5 min. Chemiluminescence was detected by exposure to film (ISC Bioexpress, Kaysville, UT). Protein expression from crude homogenates was normalized to GAPDH (1:1,000 mms-580s; Covance) or histone 2B (1:1,000, ab45695; Abcam). A total of 51 animals were used for Western blot (24 for punches and 27 for whole medulla and CTX). A minimum of three samples from each behavioral state (SA, T, and IBA) was run on each gel, and all gels were replicated a minimum of 3 times with samples from different animals run on each replication.
Several antibodies to the GABAAR δ-subunit displayed nonspecific banding in Western blots of rat tissues (Santa Cruz sc-25705, Santa Cruz sc-31438, and Millipore AB9752). As there were no obvious differences in antigen affinity in squirrel tissues, we selected an antibody (Millipore AB9752) that has been used successfully in Western blots of rat hippocampus (28). For immunohistochemistry, we selected an antibody to the GABAAR δ-subunit raised in goat (Santa Cruz sc-31438) to colabel sections with antibodies made in rabbit and mouse.
Quantitative RT-PCR.
Cortex and whole medulla samples were collected as described above. Samples were stored at −80°C until homogenization in Trizol (Invitrogen, Carlsbad, CA). cDNA was synthesized from the extracted RNA with Moloney murine leukemia virus reverse transcriptase (Invitrogen). mRNA expression was normalized to GAPDH, the amplification of which did not vary significantly by behavioral state. Negative controls showed no detectable signal with any of the primer sets used, and each set displayed a single predominant peak in the dissociation curve at the expected melting temperature for each amplicon. The primer sequences were designed to span introns whenever possible to discount any product from genomic DNA. Primer specificity was assessed through NCBI BLAST analysis before use. Primer efficiency was tested through the use of serial dilutions. CT values from duplicate measurements were averaged, and relative expression levels were determined by the comparative CT method (26).
In the absence of sequence data from squirrels, PCR primer sequences were derived from rat and mouse sequences and tested for their ability to amplify cDNA from squirrels. DNA oligos were synthesized by Integrated DNA Technologies (Coralville, IA). The following DNA oligos recognized highly conserved sequences and amplified appropriately: GAPDH forward primer: 5′-ATGCCGCCTGGAGAAACC-3′ and reverse primer: 5′-GTAGCCCAGGATGCCCTTTAG-3′; GABAA α1 forward primer: 5′-ATCACAGAGGATGGCACCTTGC-3′ and reverse primer: 5′-TGGGCATCCATAGGGAAGTCC-3′; GABAA α2 forward primer: 5′-AGAACAACGCTTATGCAGTGGC-3′ and reverse primer: 5′-GTGGTTGCACTCTTGGAGATGG-3′; GABAA α3 forward primer: 5′-AACCGGGAGTCAGCTATCAAGG-3′ and reverse primer: 5′-TTGGGTGCCTGTATGCTTC-3′; GABAA α4 forward primer: 5′-GGTTTCTGCCAAGAAGGTACCC-3′ and reverse primer: 5′-TTTAAACAAACCGCCAGGCAC-3′; GABAA δ forward: 5′-GTCTGCCTGGTTCCATGATGT-3′ and reverse primer: 5′-GGAGGTGATGCGGATGCT-3′; GABAA ε forward: 5′-ACCTGAGCCTCAGCTGGA-3′ and reverse primer: 5′-GGT CCG AGG CTG TTG ACA-3′; and synaptophysin forward primer: 5′-CAGACAGGGAACACATGCAAGG-3′ and reverse primer: 5′-GGCCCAGCCTGTCTCCTTAAAC-3′.
Quantitative RT-PCR was performed with a Prism Sequence Detection System (model ABI 7000; Applied Biosystems, Foster City, CA). A total of 24 animals (8 each in SA, T, and IBA) was used for RT-PCR. All samples were run in duplicate at an annealing temperature of 60°C. After the final amplification cycle, a dissociation curve was generated to ensure that a single gene product was amplified. Samples were excluded on a well-by-well basis if contamination was evident when examining amplification plots. If samples were contaminated in control gene wells, the behavioral state mean for the control gene was used. By these criteria, only 2.2% of wells were excluded from the analysis.
To confirm that the PCR-amplified DNA was the correct molecular weight, gel electrophoresis was used to analyze the PCR product for a highly conserved subunit (GABAAR α1; Ref. 49). Samples from each behavioral state (SA, T, and IBA) and a DNA ladder (Promega, Madison, WI) were loaded on a 1% agarose gel (Bio-Rad, Hercules, CA) and run at 60 V for 2 h in a 1× Loenings buffer (4.36 mM Tris base, 15.44 mM NaH2PO4, and 902.6 μM Na EDTA). The gel was subsequently stained with ethidium bromide (3 μg/ml; Bio-Rad, Hercules, CA) for 10 min and destained for 30 min in double distilled H2O. Gels were photographed on an ultraviolet box (Fotodyne, Hartland, WI).
Immunohistochemistry.
Animals from each behavioral state (4 each in SA, T, and IBA) were perfused with cold saline followed by 4% paraformaldehyde in PBS (pH 7.4). Fixed brains were cryoprotected with 30% sucrose and sectioned coronally (30 μm) on a freezing microtome. Eight matched sections were selected from the medulla of each squirrel at 60-μm intervals (equivalent to bregma −13.9 to −13.1 mm in rat; 36). This is the location of the rVRG and cVRG in rat. Three matched sections were selected from the primary motor and somatosensory cortex (centered on the equivalent of bregma −3.24 mm in rat). All medullary and cortical sections were reacted simultaneously. Sections were washed extensively in 0.01M PBS. After 1.0 h in blocking solution (10% normal donkey serum in 0.01 M PBS), primary antibodies were applied for 48 h at 4°C in blocking solution and 0.3% Triton X-100. Primary antibodies were used at 1:1,000: anti-GABAA ε (ab35971; Abcam), anti-GABAA δ (sc-31438; Santa Cruz Biotechnology), and anti-GAD67 (MAB5406; Millipore). Alexa-Fluor-conjugated secondary antibodies (568 donkey anti-goat IgG, 488 donkey anti-rabbit IgG, 647 donkey anti-mouse IgG; Invitrogen) were used at 1:300 in 1% normal donkey serum and 0.75% Triton X-100. Negative controls (2 medullary and 1 cortical section from each behavioral state) were reacted simultaneously with the omission of the primary antibody. Sections were mounted and coverslipped with Vectashield Hard Set mounting medium for fluorescence (Vector Laboratories, Burlingame, CA). There were no labeled cells in negative control slices from all behavioral states.
Image acquisition and analysis.
All images were acquired during the same session using an Olympus Fluoview 500 laser-scanning confocal system (Tokyo, Japan) mounted on an AX-70 upright microscope. Images were analyzed using ImageJ software (W. Rasband, National Institutes of Health, Bethesda, MD). Images were scanned with different wavelengths sequentially to prevent bleed through. User-defined thresholds were applied uniformly to all images to measure the average pixel intensity, the number, and the area of particles. For quantitation, background fluorescence measured in negative control sections was subtracted from measurement of positive label. Data were normalized to the mean expression of label across all summer active sections. Images (see Figs. 1–9) were uniformly processed in Adobe Photoshop (Adobe Systems Incorporated, San Jose, CA) as follows: brightness levels adjusted, unsharp mask routine to improve edge detection, converted to 8-bit depth, and cropped.
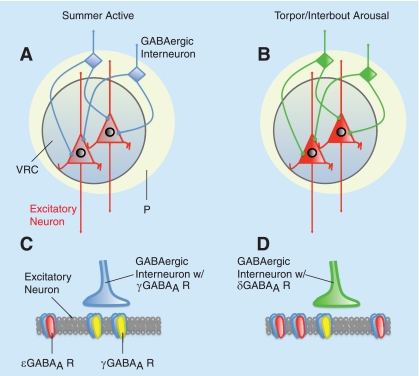
Fig. 9.
Proposed model of seasonal regulation of GABAAR expression in the VRC in 13-lined ground squirrels. A: excitatory neurons in the VRC express GABAAR ε-subunits (red) at low levels during the summer active period. In summer, inhibitory interneurons (blue) lacking δ-subunit GABAARs (presumably expressing γ-subunit-containing GABAARs) modulate VRC neuron excitability from nearby peripheral locations (P, yellow). B: during hibernation (T and IBA), excitatory VRC neurons upregulate GABAAR ε-expression (bright red). These neurons are modulated by inhibitory interneurons expressing δ-subunit-containing GABAARs (green) that are sensitive to allosteric positive modulation by EtOH. This season-specific plasticity that allows survival of normally lethal doses of barbiturate and EtOH may represent a shift from synaptic inhibition (C) to extrasynaptic inhibition (D).
To quantify the number of neurons immunopositive for both GABAAR δ and glutamic acid decarboxylase 67 (GAD67, a marker of GABAergic neurons) in the VRC and its periphery, two images (635 × 635μm) were taken in anatomical center of the VRC bilaterally, based on anatomical landmarks with reference to the rat (37). Three additional images were taken on one side dorsolateral to the VRC where we previously determined that the majority of GABAAR δ-expressing neurons were localized (see Supplemental Material, P1, P2, and P3 in Supplemental Fig. S1; Supplemental Material for this article is available online at the Am J Physiol Regul Integr Comp Physiol website). Two investigators, blinded to the identity of sections, counted double-labeled cells in each image (4 images/section, 8 sections/animal, and 4 animals/behavioral state). There was >95% concordance between the two investigators.
When appropriate, a two-way mixed ANOVA was used with a Tukey's honestly significant difference post hoc test to determine differences between regions and behavioral states. For nonparametric analyses, a Kruskal-Wallis rank sum test was used with subsequent post hoc pair-wise tests if the chi-square P value was <0.05.
Electrophysiology.
A total of 17 animals (7 SA and 10 IBA) was used in electrophysiological studies. Electrophysiological experiments were conducted during the summer active months (May to July) and during naturally occurring IBAs (November to February), which ruled out temperature-dependent effects. Detailed methods can be found in Hengen et al. (22). Briefly, brains were removed and medullary and cortical slices (350 μm thick) were cut with a vibrating microtome (Campden Instruments, Layfayette, IN). Cortical slices contained primary motor and primary somatosensory areas while medullary slices contained the NTS and VRC (Fig. 1, A and B). Slices were placed into an interface recording chamber (Warner Instruments, Hamden, CT) and subfused with warm artificial cerebrospinal fluid (aCSF, 37°C) at a rate of 8 ml/min. Slices were maintained at 37°C by an automated temperature controller (Harvard Apparatus, Holliston, MA). The composition of the aCSF was as follows (in mM): 120 NaCl, 26 NaHCO3, 20 glucose, 2 MgSO4, 1.0 CaCl2, and 1.25 Na2HPO4. To increase the yield of spontaneously active neurons, aCSF containing 9 mM KCl was used in all experiments. Previously, we determined that the GABAergic contributions to spontaneous activity were unaffected by the increased KCl concentration (22).
Experimental protocol.
Spontaneous activity was recorded simultaneously from medullary and cortical slices from the same animal. Two 16-channel extracellular electrodes arrays (model a4 × 4–3 mm100–177; Neuronexus, Ann Arbor, MI) were placed in the VRC, slightly ventrolateral to the nucleus ambiguus (Fig. 1, A and B). One array was placed in the NTS, and one array was inserted perpendicular to the cortical layers, centered on layer 3. Slices were allowed to equilibrate in 9 mM KCl aCSF at 37°C with electrodes inserted for 2 h. Baseline activity was then measured for 1 h, followed by sequential application of increasing doses of EtOH (0.001, 0.01, and 0.1 M EtOH in aCSF) for 40 min each. To examine the contribution of GABAARs to the neuronal response to EtOH, a subset of slices were treated with bicuculline, a selective GABAAR antagonist, before EtOH application. Spontaneous activity was recorded from two medullary slices from each of three IBA animals. After equilibration, a baseline was established, followed by 60 min of 100 μM bicuculline application (Tocris Bioscience, Ellisville, MO). Slices were then treated with 100 μM bicuculline together with 0.1M EtOH.
Data analysis.
Raw data were processed as described in Hengen et al. (22). Individual neurons were identified using principal component analysis (1). Neuronal activity was averaged in 5-min bins and normalized to the mean firing rate during the 1-h baseline recording before drug application. Waveforms that were recorded on multiple, adjacent channels were counted only once. Neurons were discarded from analysis if their mean baseline firing rate was ≤0.01 Hz, if they were silent for >10 consecutive min during the 1 h baseline, or if their firing rate was consistently decreasing during the 1-h baseline. Individual bins were discarded if the absolute firing rate was >500 Hz or if traces exhibited evidence of mechanical disturbances (i.e., normalized firing rate increased and then decreased >100 SD from the baseline mean in <3 min). Based on these criteria, 12.7% of neurons and <1% of data bins were discarded.
To examine possible artifacts due to drift across the duration of recordings, time control experiments were run for 340 min with no EtOH application. Time control experiments were analyzed with Wald t-tests to test for an individual effect of time. For statistical comparison, a Wald t-test for individual effects, or a likelihood ratio test to examine overall significance of interactions was used considering time, dose, animal, neuron, and behavioral state (R Foundation for Statistical Computing, Vienna, Austria). Differences were considered significant if P < 0.05. The total number of neurons per behavioral state was used as the number of independent samples for relevant statistical tests and calculation of SE. All data are reported as means ± SE.
RESULTS
Since GABAA receptor subunit composition in squirrel has not been described previously, we initially confirmed that the commonly reported synaptic GABAAR subunits were present in CTX, NTS,and VRC of 13-lined ground squirrels across the year (SA, T, and IBA). Western blot analysis revealed that GABAAR α1- and γ2-subunits, which participate in ligand binding and synaptic localization, respectively, were expressed abundantly in all regions in SA, T, and IBA (n = 9 animals/behavioral state; data not shown). Identified bands migrated at 51 kDa (α1) and 46 kD (γ2), which agrees with previously described subunit expression in mouse, rat, and human (17, 24, 42).
Expression of GABAAR ε-subunit.
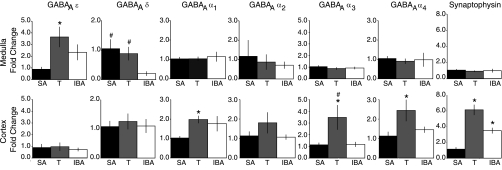
To determine if the seasonal expression of the GABAAR ε-subunit was consistent with pentobarbital insensitivity of VRC neurons during hibernation (22), we first examined transcriptional regulation of GABAAR subunits in the whole medulla and CTX. Cortical levels of mRNA for the ε-subunit were unchanged across the year (Fig. 2). In contrast, medullary mRNA for the ε-subunit was >3.5-fold greater in T than SA animals (Fig. 2). mRNA levels in the medulla during IBA showed a nonsignificant trend towards increase by comparison with SA animals (P = 0.16).
Fig. 2.
mRNA for GABAAR subunits and synaptophysin is differentially regulated in cortex and medulla across the circannual cycle. Quantitative analysis of mRNA for GABAAR subunits ε, δ, and α1–4 and synaptophysin in the medulla and cortex of the 13-lined ground squirrel during summer active (SA), torpor (T), and interbout arousal (IBA). In the medulla, GABAA ε-mRNA was >3.5-fold greater in T than SA, whereas cortical mRNA did not change. During IBA, medullary GABAAR δ mRNA was 20% of SA. Cortical δ-subunit mRNA did not change with behavioral state. GABAAR α-subunit mRNA changed with behavioral state only in cortex. Synaptophysin mRNA changed in cortical but not medullary samples. Data are normalized to SA. *P < 0.05, different from SA. #P < 0.05, different from IBA. Error bars indicate SE.
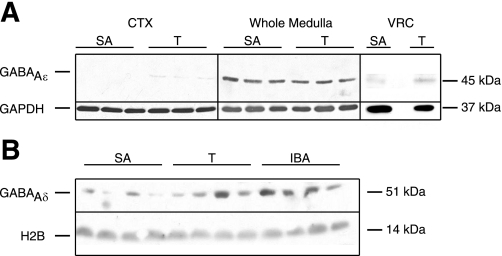
To confirm that regional and seasonal regulation of the GABAAR ε-subunit mRNA was reflected in protein expression, we examined cortical and medullary expression of the ε-subunit with Western blot (n = 9 animals/behavioral state examined, 3 animals/behavioral state shown). GABAAR ε-subunit protein was expressed robustly in the whole medulla yet was near the threshold for detection in CTX (SA, T, and IBA; Fig. 3A). Western blots of punches of the VRC (as opposed to whole medulla; n = 8 animals/behavioral state examined, 1 animal/behavioral state shown) showed GABAAR ε-protein in all behavioral states (Fig. 3A). Protein expression in the VRC was visibly increased during T by comparison with SA, consistent with the RT-PCR data (above) and the previously reported (22) pentobarbital insensitivity of VRC neurons during hibernation (Fig. 3A). The identified band migrated at 45 kDa, which is consistent with a synthetic peptide corresponding to human GABAAR ε. In the NTS, a cardiorespiratory brain region that shows partial pentobarbital insensitivity (22), GABAAR ε-protein expression was similar to that of VRC samples (data not shown).
Fig. 3.
GABAAR ε- and δ-subunits are differentially regulated in cortex and medulla across the circannual cycle. A: GABAAR ε-subunit protein expression is increased in punches of VRC during hibernation. Cortical samples (CTX), whole medulla samples, and punches centered on the VRC were analyzed for GABAAR ε-subunit protein expression. GABAAR ε-subunit protein is enriched in the medulla by comparison with the cortex in SA and T animals. GABAAR ε-subunit expression is increased in VRC punches in T compared with SA. An empty lane separates SA and T in the Western blot of VRC punches. B: during hibernation (T and IBA), GABAAR δ-subunit protein is increased in whole medulla lysates by comparison with SA. GAPDH or H2B was used as a loading control.
Total protein concentration in equal sized punches from the VRC in T and IBA animals was greater than in SA animals (P < 0.001 and P < 0.005, respectively; see Supplemental Fig. S2). In contrast, protein concentration in equal sized punches from CTX and NTS did not vary across the year. In light of this inconsistent relationship in the VRC, normalizing to total protein for quantification of Western blots would introduce an error. Consequently, we turned to immunohistochemistry for protein quantification.
Expression of GABAAR δ-subunit.
An alternative explanation for seasonal pentobarbital insensitivity in VRC neurons due to changes in GABAAR ε-subunit expression is a decrease in δ-subunit-containing GABAARs, which, unlike ε-subunit-containing GABAARs, have increased sensitivity to allosteric modulators including barbiturates (33). Thus we examined mRNA for the GABAAR δ-subunit in the CTX and medulla to more critically evaluate transcriptional control of extrasynaptic GABAAR expression across the circannual cycle. In cortical samples, mRNA for the GABAAR δ-subunit was unchanged across the circannual cycle and, indeed, was the most stable of all six subunits examined (Fig. 2). In contrast, GABAAR δ-mRNA levels decreased significantly during IBA by comparison with SA (P < 0.05; Fig. 2). While this finding would be consistent with our second hypothesis, rates of transcription and translation can be affected by temperature changes during hibernation. Therefore, we used Western blot to examine protein expression in whole medulla as well as in punches of the VRC. While there was considerable variability among animals, δ-subunit expression increased robustly in the whole medulla during T and IBA by comparison with SA (n = 9 animals per behavioral state; Fig. 3B). Surprisingly, we were unable to detect δ-subunit protein in punches of the VRC in SA, T, or IBA (n = 8 per behavioral state; data not shown), suggesting that δ-subunit-containing GABAARs are expressed in the medulla but not specifically within the VRC. To reconcile the mismatch between medullary δ-subunit mRNA and protein expression during hibernation, we used immunohistochemistry for the δ-subunit using a different antibody (see below).
Expression of GABAAR α-subunits.
To confirm that the increases in GABAAR ε- and δ-subunit protein in the medulla were not the result of a general upregulation of GABAAR-subunits, we examined the expression of mRNA for GABAAR α1–4-subunits. Medullary GABAA α1–4-subunits did not change across the circannual cycle (n = 8 per behavioral state; Fig. 2). Taken together, the RT-PCR and Western blot data indicate that regulation of the GABAAR ε- and δ-subunits in the medulla and/or the VRC during hibernation differs greatly from the CTX and is not due to generalized changes in GABAAR subunit expression.
Synaptic alterations during hibernation.
Cortical, thalamic, and hippocampal neurons undergo dendritic retraction and synaptic degradation during each bout of torpor, followed by regrowth on arousal (51, 52). As many GABAARs are localized at synapses and as synaptic GABAARs contain α-subunits, we predicted that cortical GABAAR α-subunit mRNA would show evidence of this degradation and regrowth. Consistent with this, levels of mRNA for the α1-, α3-, and α4-subunits (Fig. 2; n = 8 per behavioral state) were significantly increased in cortical samples during T by comparison with SA animals, and mRNA for the α2-subunit similarly exhibited a nonsignificant trend towards increased expression (Fig. 2). In IBA animals, the increase in α1-mRNA compared with SA approached significance (176 ± 39% of summer active; P = 0.06; Fig. 2). No equivalent evidence for dendritic retraction and regrowth was detected in medullary samples. To address the issue of primer specificity, we confirmed the molecular weight of the PCR product recognized by our oligonucleotides against GABAA α1-subunit with gel electrophoresis (data not shown).
To further confirm that the transcriptional regulation of the α1-, α3-, and α4-subunits seen in CTX might be at least partially explained by state-dependent synaptic plasticity, we examined mRNA for synaptophysin (a presynaptic vesicle glycoprotein) in cortical tissues from SA, IBA, and T animals. Cortical synaptophysin mRNA increased more than sixfold in T compared with SA animals (P < 0.001), consistent with synaptic remodeling previously described (51, 52) in hibernating squirrels (Fig. 2). During IBA, cortical synaptophysin mRNA increased nearly 3.5-fold compared with SA animals (P < 0.005), and synaptophysin mRNA in CTX was significantly greater in T than IBA (P < 0.005). These data further underscore the absence of any change in cortical GABAAR ε- and δ-subunit mRNA during hibernation. We found no detectable changes in medullary synaptophysin mRNA across the circannual cycle (Fig. 2). Combined with the lack of change in GABAAR α-subunits, these data suggest that synapses in the medulla may be protected from hibernation-related retraction of dendrites and synaptic remodeling.
GABAA ε-immunoreactivity in VRC neurons changes with behavioral state.
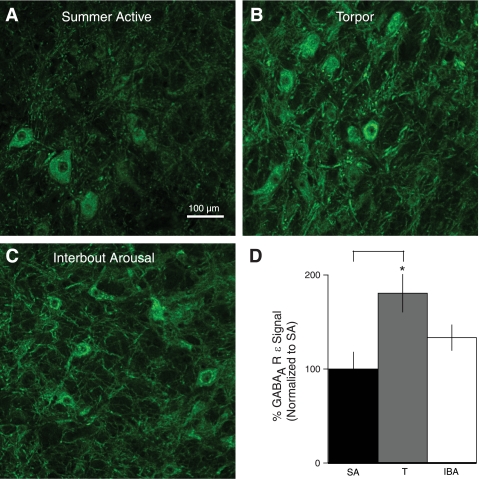
Since state-dependent differences in total protein concentration in a fixed volume punch of the VRC made accurate quantification of Western blots difficult (Supplemental Fig. S1), we used immunohistochemistry to assess GABAAR ε-subunit expression in the VRC across the year. The predominant pattern of staining in the VRC was in the cytoplasm of neurons and their processes (Fig. 4). GABAAR ε-immunofluorescent signal intensity in the VRC of T animals was nearly double that of SA animals (P < 0.05; Fig. 4). Signal intensity in the VRC in IBA was intermediate between T and SA but not significantly different from either behavioral state (Fig. 4D). These immunocytochemical data confirm our observations in Western blots of punches through the VRC, that levels of GABAAR ε protein are greater in T than in SA.
Fig. 4.
GABAAR ε-subunit expression in the VRC is seasonally regulated. A: immunofluorescent signal intensity for GABAAR ε-subunit is low in the VRC during the SA period. B: GABAAR ε-immunofluorescence in the VRC is increased during T (181% SA; P < 0.05). C: GABAAR ε-immunofluorescence in the VRC during IBA is intermediate between T and SA (133% of SA; NS). D: quantitative analysis of GABAAR ε-immunofluorescence during SA, T, and IBA shows a significant increase in GABAAR ε-subunit expression during T by comparison with SA (*P < 0.05). Error bars indicate SE.
The overall increase in GABAAR ε-immunoreactivity in sections through the VRC in T by comparison with SA animals was due to both an increase in the number of stained neurons (169%; P = 0.09) and an increase in the level of fluorescence in individual cells. There was a nonsignificant increase in the number of labeled neurons in IBA by comparison with SA animals (129.4%; P > 0.05). Mean signal intensity expressed as a percentage of SA intensity was as follows: IBA > T > SA (IBA = 104.2 ± 0.27% of SA intensity, n = 1,502 neurons; T = 102.3 ± 0.26% of SA intensity, n = 1,695; and SA = 100 ± 0.27%, n = 1,161; P < 0.001 for all comparisons). The size of GABAAR ε-immunoreactive neurons did not differ between behavioral states (P = 0.23).
To determine whether the increased number of GABAAR ε-immunopositive neurons during T and IBA by comparison with SA were concentrated in any part of the VRC, we examined eight matched sections through the VRC (4 animals/behavioral state). When data from the two VRC sample areas were combined, there was significant effect of region: the number of GABAAR ε-immunopositive neurons in one section through the rVRG (section 3, corresponding to bregma −13.3 in rat; Supplemental Fig. S2C) more than doubled in T by comparison with SA animals (T, 51.5 ± 7.6 neurons/section; SA 23.3 ± 5.9; P < 0.05).
We also quantified GABAAR ε-subunit immunoreactivity in the NTS of the same sections used for quantification of GABAAR ε-subunit in the VRC. Label in the NTS was punctate with very few immunoreactive neurons, and signal intensity did not change across the year (P = 0.52). Immunocytochemical data from sections through the CTX stained for GABAAR ε-subunit confirmed Western blot observations, as ε-immunoreactivity was nearly undetectable in cortical sections.
GABAA δ-immunoreactivity changes with behavioral state in the VRC.
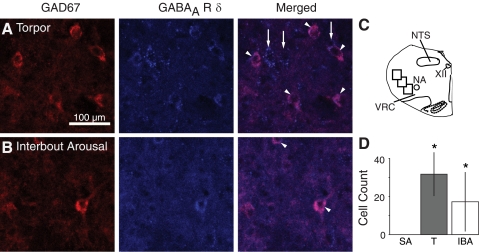
Although Western blots indicated that δ-subunit expression increased robustly during hibernation in the whole medulla (T and IBA; Fig. 3B), we could not detect δ-subunit protein in punches of the VRC in any behavioral state, suggesting that δ-subunit-containing GABAARs are expressed in the medulla but not specifically within the VRC. Virtually no GABAAR δ-immunoreactive neurons were found in the VRC in any behavioral state, and no neurons were found double labeled for GABAAR ε- and δ-subunits. However, δ-immunoreactive neurons were consistently observed peripheral to the VRC, in particular dorsolaterally (Fig. 5). To determine whether the number of δ-immunopositive neurons dorsolateral to the VRC increased during hibernation, we examined eight matched sections through the rostrocaudal extent of the VRC (4 animals/behavioral state). Sections were also stained for GAD67 to identify GABAergic neurons. Very few GAD67-immunopositive neurons and no double-labeled neurons were found in the VRC in any behavioral state. No double-labeled neurons were found in any of the three sample areas dorsolateral to the VRC in SA animals. In contrast, in IBA and T animals, many double-labeled neurons were found dorsolateral to the VRG (Fig. 5). In IBA, a total of 17.3 ± 15.6 double-labeled neurons was found in images through three sample areas in eight sections per animal (P < 0.05, compared with SA) and in T, 31.8 ± 11.5 double-labeled neurons were found (P < 0.05, compared with SA).
Fig. 5.
GAD67 and GABAAR δ-immunoreactivity in the VRC during hibernation. A and B: colocalization of GAD67 and GABAAR δ-immunoreactivity in neurons dorsolateral to the VRC in T and IBA. Some, but not all, GABAAR δ-immunoreactive neurons were immunopositive for GAD67. Double-labeled neurons are indicated by arrowheads in the merged image. Arrows indicate cells positive for GABAAR δ-subunit but not GAD67. C: 3 regions dorsolateral to the VRC were analyzed in each of 8 coronal sections through the VRC (P1, P2, and P3, see Supplemental Fig. S2). D: no double-labeled neurons were found in SA animals. Number of double-labeled neurons in T and IBA animals was significantly greater than SA. *P < 0.05, different than SA.
Regional and seasonal effects of EtOH on spontaneous neuronal activity in VRC neurons.
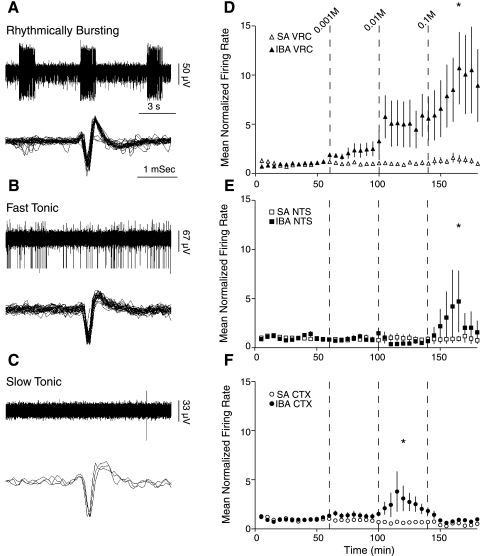
As δ-subunit-containing GABAARs are highly sensitive to EtOH (20, 31), we recorded neuronal activity in brain slices containing the VRC in response to increasing doses of EtOH in hibernating (IBA) and SA animals. Neuronal activity from the multichannel electrode array placed in the region of the VRC was initially categorized as follows: rhythmically bursting (<10%; Fig. 6A), fast tonic (∼60%; Fig. 6B), and slow tonic (∼40%; Fig. 6C). During the equilibration period, all rhythmically bursting cells converted into tonically active cells.
Fig. 6.
Neuronal responses to ethanol (EtOH) are seasonally and regionally regulated in the cortex and medulla of the 13-lined ground squirrel. Spontaneous activity of neurons in the VRC in a region anatomically consistent with the Pre-BötC demonstrated 3 distinct patterns of activity. A–C: examples of initial firing patterns seen in VRC neurons, rhythmically bursting (A), fast tonic (B), and slow tonic (C). D: mean normalized firing rate of VRC neurons in SA animals (▵) and IBA (▴) in response to 0.001, 0.01, and 0.1 M EtOH (applied at vertical bars, t = 60, 100, and 140 min). E: mean normalized firing rate of NTS neurons in SA (□) and IBA (■) in response to increasing doses of EtOH. F: mean normalized firing rate of CTX neurons in SA (○) and IBA (●) in response to the 3 doses of EtOH. Single unit activity was normalized to mean baseline activity for that neuron. *P < 0.05, significant difference between IBA and SA.
In slices from SA animals (n = 4 animals), increasing doses of EtOH (0.001, 0.01, and 0.1 M) failed to alter the spontaneous firing rate of neurons in the VRC (n = 33 neurons) (Fig. 6D), consistent with the absence of δ-subunit protein expression in Western blots and the absence of δ-immunoreactive neurons. All three doses of EtOH increased the firing rate of VRC neurons (n = 70 neurons) in slices from IBA animals (n = 4 animals) three- to eightfold (by comparison with SA animals, P < 0.05; Fig. 6D), consistent with the expression of δ-subunit-containing GABAARs on inhibitory neurons that modulate VRC activity.
In the NTS (n = 28 neurons), the two lower doses of EtOH failed to alter the activity of neurons in IBA animals by comparison with SA animals (Fig. 6D). There was, however, a significantly different response to 0.1 M EtOH (n = 28 neurons; Fig. 6D). In CTX, all three doses of EtOH reduced the firing rate of neurons in SA animals (n = 57 neurons). Cortical neurons in IBA animals showed variable responses to EtOH (n = 57 neurons; Fig. 6). In response to 0.01 M EtOH, cortical neurons in IBA animals increased activity by 83 ± 37%, whereas cortical neurons in SA animals showed reduced activity (30 ± 16%; Fig. 6F). There was no significant effect of time on spontaneous neuronal activity in the VRC (n = 49 neurons) or the NTS (n = 36 neurons) in time-control experiments (3 IBA and 3 SA). Cortical neurons (n = 48 neurons) displayed higher variability in spontaneous activity, yet bursts in activity (<2 min) during time control experiments were transient and considered to be of no biological significance.
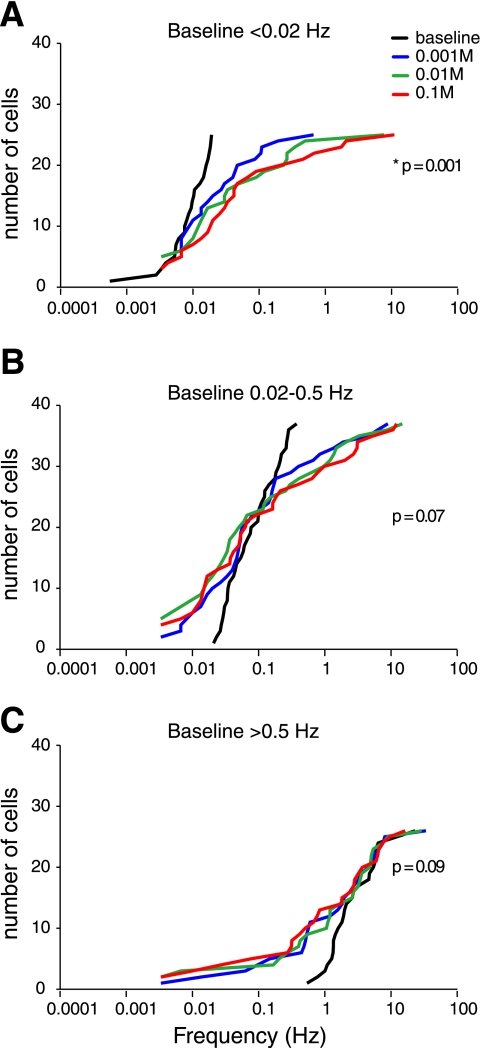
The excitatory response of VRC neurons in IBA animals to increasing doses of EtOH (Fig. 6D) was due to the activity of a subset of neurons (25 of 88 neurons). The majority of VRC neurons were nearly silent in baseline conditions (60 min). Neurons with baseline activity of <0.02 Hz were excited by the application of increasing doses of EtOH (Fig. 7A; P < 0.005, baseline compared with 0.1 M EtOH). Neurons with baseline activity between 0.02 and 0.5 Hz were variably affected by the increasing doses of EtOH (Fig. 7B; P = 0.07), while nearly all neurons with baseline activity >0.5 Hz showed a trend towards inhibition at increasing doses of EtOH (Fig. 7C; P = 0.09). These data indicate that mean network activity in the VRC is driven by a subset of neurons but that the majority of VRC neurons are either unchanged or inhibited by EtOH.
Fig. 7.
Cumulative frequency histogram of VRC neurons from animals during interbout arousal. EtOH increases spontaneous neuronal activity in the VRC by activating a subpopulation of neurons that are silent in control conditions. A: cumulative frequency histogram of all VRC neurons with a baseline-firing rate <0.02 Hz. Spontaneous activity of neurons at baseline (black line) is nearly absent and increases progressively in response to 0.001 M EtOH (blue line), 0.01 M EtOH (green line), and 0.1 M EtOH (red line). B: EtOH differentially affects VRC neurons firing between 0.02 Hz and 0.5 Hz at baseline (black). Each of 3 doses of EtOH increases the activity of approximately one-half of these neurons and decreases the activity of the other one-half. C: VRC neurons firing >0.5 Hz at baseline (black) are progressively inhibited by increasing doses of EtOH. *Significant difference between baseline and 0.1 M EtOH.
GABAAR contribution to excitation by EtOH.
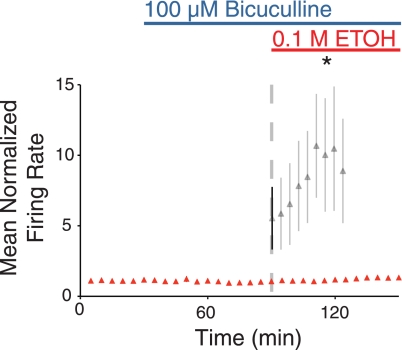
To confirm that GABAARs are integral to the excitation of VRC neurons by EtOH, slices from IBA animals (n = 3) were pretreated with 100 μM bicuculline before the application of 0.1 M EtOH. Following 1 h of bicuculline, 0.1 M EtOH and 100 μM bicuculline were coapplied for 1 h. There was no difference between spontaneous activity at steady state in bicuculline and spontaneous activity in the presence of 0.1 M EtOH and bicuculline (133 ± 13% of bicuculline steady state; Fig. 8). Thus the GABAAR antagonist bicuculline prevented the excitation of VRC neurons elicited by 0.1 M EtOH alone (P < 0.05).
Fig. 8.
The GABAAR antagonist bicuculline inhibits neuronal responses to EtOH. Spontaneous activity of VRC neurons before bicuculline and during bicuculline (blue line) and EtOH coapplication (blue and red lines). Individual units are normalized to the last 30 min of bicuculline treatment (before the application of EtOH). Corresponding VRC neuronal activity during 0.1 M EtOH application (from Fig. 6) is overlaid for comparison (gray trace). *P < 0.05, different than EtOH treatment.
DISCUSSION
We have identified hibernation-associated changes in GABAAR subunits in neurons in the medulla that are not evident in the cortex of the 13-lined ground squirrel. These changes are consistent with a previous description of seasonal pentobarbital insensitivity localized to the VRC (22). Specifically, seasonal expression of the GABAAR ε-subunit in VRC neurons prevents inhibition by pentobarbital and EtOH during hibernation states (T and IBA). Synaptic input from adjacent inhibitory interneurons that express δ-subunit-containing GABAARs further prevents inhibition of VRC neurons by pentobarbital and EtOH. A proposed model of hibernation-associated regulation of GABAAR subunit expression in the ventrolateral medulla in the 13-lined ground squirrel is shown in Fig. 9. In light of these data demonstrating a critical role for GABAARs in hibernation, it is difficult to ignore the possibility that hibernation states (IBA and T) may be ligand driven. If so, this model illustrates a solution to the problem of how medullary networks involved in cardiorespiratory control remain active during hibernation while most other (higher) brain regions are functionally silenced for energy conservation.
A key feature of the proposed model (Fig. 9) is the inhibition of GABAergic inhibitory interneurons due to δ-subunit-containing GABAARs on their surface. Extrasynaptic GABAARs, including those containing δ-subunits, are highly sensitive to ambient levels of GABA from adjacent synaptic transmission, as well as being the primary targets for allosteric modulators such as pentobarbital (19). The proposed model makes very specific predictions with respect to recording from VRC neurons following application of EtOH to a slice preparation: neurons should show a net excitation. Had the expression of GABAAR-expressing δ-subunits been on noninhibitory neurons in this network, the VRC would have been inhibited by EtOH. Likewise, had the expression of GABAAR-expressing δ-subunits merely decreased during hibernation, there would have been no GABAAR-dependent effect of EtOH.
Experimental limitations.
Hibernation provides a unique opportunity to study naturally occurring mechanisms underlying regionally and seasonally regulated neuronal activity. However, there are significant challenges and limitations associated with studies in the wild-caught, 13-lined ground squirrel. First, specific activity states can only be studied at certain times of year. Second, genome-specific primers and species-specific antibodies are not currently available. Consequently, in this study we used a range of molecular and immunohistological techniques to identify the mechanism(s) responsible for pentobarbital insensitivity in the VRC during hibernation that was described previously (22). Each method builds on and supports the original observation that cardiorespiratory neurons are insensitive to the effects of lethal doses of barbiturate via plasticity of expressed GABAAR subtypes. Predictions of the hypothetical model (Fig. 9) were tested with electrophysiological and pharmacological approaches that avoid species-related issues (GABAA receptors: bicuculline and muscimol; ε- and δ-subunits: pentobarbital and EtOH). The most disparate respiratory states are SA and T, and we find a significant difference between GABAAR ε-subunit immunoreactivity in the VRC in these two behavioral states. We also found a trend towards increased GABAAR ε-subunit expression in the VRC in IBA by comparison with SA, and the electrophysiological data enabled us to unambiguously confirm the functional presence of barbiturate- and EtOH-insensitive GABAARs during IBA compared with SA. Taken together, there is compelling evidence for the seasonal insertion of GABAAR with ε- and δ-subunits in the VRC.
Temperature-related confounds (40) were avoided in this study by the inclusion of IBA animals (Tb = 37°C). We found greater total protein concentration in Western blots of equal sized punches of the VRC in T and IBA animals by comparison with SA animals, which was not seen in punches of CTX and NTS. We speculate that this hibernation-associated difference in total protein per unit volume of VRC tissue may be related to the maintenance of synaptic connectivity necessary for respiratory function at low temperatures in this brain region.
Identity of neuronal networks and recorded neurons.
We cannot say with certainty that the neurons we recorded in the VRC are definitively respiratory. The anatomy of the squirrel medulla is very similar to the rat, and placement of microelectrode arrays was restricted to a region comparable to the rat VRC. Furthermore, neurons with properties characteristic of previously described respiratory neurons (rhythmic bursting; Fig. 6A) were identified at this site (2, 3, 8, 45, 18). Previous studies (15) of brainstem networks controlling phrenic motoneurons show third-order neurons that project to premotor neurons in the VRC. The location of these neurons overlaps with the region dorsolateral to the VRC in which we found neurons immunopositive for the δ-subunit during hibernation (P1, P2; Supplemental Fig. 2; Fig. 9), many of which were colabeled with GAD67, a marker of GABAergic neurons. This region in the rat is also rich in calbindin and parvalbumin-immunopositive neurons, which are frequently associated with GABAergic function (36, 2, 3). GABAergic transmission plays a key role in respiratory neuron excitability (57, 43). As there are few GABAergic neurons within the VRC (55, 36), one of the sources of GABAergic input is likely to be this population of third-order neurons dorsolateral to the VRC.
While we cannot identify the specific role of any of the neurons recorded (i.e., rhythm generating, premotor, etc.), a majority of neurons in the VRC appear to be involved in the control of breathing (2, 3). Our finding that in IBA 25/88 of VRC neurons are excited by EtOH suggests that GABAergic/δ-subunit-expressing neurons normally inhibit the activity of a subset of VRC neurons and that this inhibition is relieved by the application of EtOH (Fig. 9). Thirty-seven neurons were unaffected by EtOH, suggesting that they did not receive input from GABAAR δ-subunit-expressing neurons or that these inputs were severed in the slice preparation. We also found that 0.1 M EtOH application to medullary slices from IBA squirrels produced a transient yet significant excitation of the NTS, a medullary region that is also critical to cardiorespiratory function. The NTS lies adjacent to a region containing GAD67/δ-immunoreactive neurons and may also receive input from δ-subunit-expressing GABAergic neurons similar to the VRC (see P3, Supplemental Fig. S2).
Role of GABAA receptors in respiratory control.
GABAergic neurotransmission plays an important role in the control of breathing (30, 43, 38, 25, 57). Glycinergic neurotransmission is responsible for reciprocal inhibition of expiratory neurons in the pre-Bötzinger complex, which contains the respiratory rhythm-generating network, and the excitability of expiratory neurons is modulated by both GABAA and glycine receptors (43). Additionally, inspiratory neurons in the VRC are tonically inhibited during the expiratory phase of breathing primarily by activation of GABAARs (25). Zuperku and McCrimmon (57) presented compelling evidence for a dynamic, nonlinear mechanism of gain modulation in the respiratory control system mediated by GABAergic neurons and GABAA receptors. This gain modulation allows the respiratory rhythm to be maintained while increasing or decreasing the output of the system when conditions change, such as during exercise, development, weight gain, hypoxia, or aging (57). Hibernation imposes a significant challenge to the respiratory control system for gain modulation to maintain normal blood gas levels. The location and balance of GABAARs expressing ε- and δ-subunits are thus potentially very important to the functioning of respiratory networks, as both subunits are capable of forming extrasynaptic ligand channels that can provide tonic mediation of cell excitability, independent of synaptic transmission (48, 53, 5). Recent preliminary data in rats indicate that there are GABAARs expressing ε- and δ-subunits in the ventrolateral medulla and that the responses of VRC neurons to pentobarbital are similar to those of SA squirrels (K. Hengen, unpublished observations). It will be interesting to determine whether there are changes in the expression of these subunits in response to demands for enhanced gain modulation in respiratory control.
GABAAR stoichometry.
To our knowledge, these are the first data to support the existence of naturally expressed, pentobarbital-resistant GABAARs, initially described in transfected HEK-293 cells and formed by α(2)β(2)ε-subunits (13). Because normal ligand binding requires two α and two β-subunits, these data and our previous work suggest α(2)β(2)ε receptors lack a γ2-subunit. Evidence suggests that GABAARs receptors lacking a γ-subunit are primarily extrasynaptic (4); thus the ε-subunit-containing GABAARs described here in the VRC may be extrasynaptic. This will need to be confirmed in future studies, as a recent report of ε-subunit-containing GABAARs in the locus coeruleus of rats suggested that these receptors were pentobarbital sensitive and located perisynaptically (7). In light of this finding, knowing the identity of the other four subunits will be important, as they may contribute to the affinity of allosteric modulators.
Biological significance.
The hypothetical configuration of GABAAR subtypes in the VRC (Fig. 9) could explain the insensitivity of hibernating squirrels to a wide variety of anesthetics that act on GABAARs (13, 14, 23) including lethal doses of pentobarbital (35). Pentobarbital, propofol, and other anesthetics interact with VRC neurons, at high doses abolish neuronal activity in medullary slices (11), and inhibit neuronal control of respiration in vivo (16, 56). EtOH, pentobarbital, and other anesthetics have limited natural or evolutionary relevance, but these compounds act on GABAARs in a manner similar to that of neurosteroids (53, 46, 6, 19). Furthermore, during pregnancy when neurosteroid levels are greatly elevated, GABAARs containing the δ-subunit are upregulated (28). We speculate that circannual changes in the levels of neurosteroids in hibernators underlie the reorganization of GABAARs such that forebrain networks are silenced while respiratory function is maintained. On entry into torpor, this pentobarbital-like ligand, in combination with temperature-dependent cessation of neuronal excitability below 15°C (54, 50), would silence all neurons capable of binding this ligand. Only by means of a regionally restricted ligand binding capacity could neurons in the forebrain be silenced and those in the medulla remain functional. Nonetheless, ligand insensitivity in the medulla would not prevent the temperature-dependent retraction of neuronal processes described previously in cortex (51, 52, 40). While there is compelling evidence that the forebrain undergoes morphological remodeling in torpor, our data suggest that the medulla does not. Our analyses of cortical synaptophysin mRNA are consistent with the report by von der Ohe et al. (51, 52) that up to 65% of synapses are degraded and reformed in a temperature-dependent manner. However, synaptophysin mRNA did not change in the medulla, which appears to be spared from synaptic degradation during torpor. We hypothesize that the increased total protein concentration in the VRC during hibernation states (IBA, T; Fig. S1) may represent the upregulation of a factor responsible for preventing the synaptic retraction observed elsewhere in the brain. Determining whether this change in total protein concentration is due to alterations in intracellular scaffolding proteins, extracellular matrix proteins, or glia will provide further insight into mammalian survival at low Tb.
In conclusion, changes that the nervous system undergoes during hibernation may differ greatly by region. Our data suggest that, during hibernation, medullary regions controlling breathing are protected from the temperature-dependent synaptic remodeling that occurs in the cortex. Alterations in GABAA receptor ε- and δ-subunits on distinct populations of neurons in the VRC may provide a network that permits breathing and survival at Tb < 4°C. These findings in hibernators advance our understanding of the impact of barbiturates and EtOH on the respiratory control system.
GRANTS
This work was supported by the National Institutes on Aging Grant AG-18760 (to M. Behan). K. Hengen was supported by T32 GM007507.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge input from Nathan Nelson and Drs. Hannah Carey, Adrianne Huxtable, Mathew Jones, Clark Nelson, and Thomas Tubon. We thank Dr. Cecile Ané for assistance with statistical modeling.
REFERENCES
- 1. Adamos DA, Kosmidis EK, Theophilidis G. Performance evaluation of PCA-based spike sorting algorithms. Comput Methods Programs Biomed 91:232–244, 2008 [DOI] [PubMed] [Google Scholar]
- 2. Alheid GF, Gray PA, Jiang MC, Feldman JL, McCrimmon DR. Parvalbumin in respiratory neurons of the ventrolateral medulla of the adult rat. J Neurocytol 31:693–717, 2002 [DOI] [PubMed] [Google Scholar]
- 3. Alheid GH, Milsom WK, McCrimmon DR. Pontine influences on breathing: an overview. Respir Physiol Neurobiol 143:105–114, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Alldred MJ, Mulder-Rosi J, Lingenfelter SE, Chen G, Lüscher B. Dintinct γ2 subunit domains mediate clustering and synaptic function of postsynaptic GABAA receptors and gephryn. J Neurosci 25:594–603, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Baur R, Kaur KH, Sigel E. Diversity of structure and function of alpha1alpha6beta3delta GABAA receptors: comparison with alpha1beta3delta and alpha6beta3delta receptors. J Biol Chem 285:17398–17405, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Belelli D, Lambert JJ. Neurosteroids: endogenous regulators of the GABA(A) receptors. Nat Rev Neurosci 6:567–575, 2005 [DOI] [PubMed] [Google Scholar]
- 7. Belujon P, Baufreton J, Grandoso L, Boué-Grabot E, Batten TFC, Ugedo L, Garret M, Taupignon AI. Inhibitory transmission in locus coeruleus neurons expressing GABAA receptor epsilon subunit has a number of unique properties. J Neurophysiol 102:2312–2325, 2009 [DOI] [PubMed] [Google Scholar]
- 8. Bianchi AL, Denavit-Saubie M, Champagnat J. Central control of breathing in mammals: neuronal circuitry, membrane properties, and neurotransmitters. Physiol Rev 75:1–45, 1995 [DOI] [PubMed] [Google Scholar]
- 9. Carey HV, Martin SL. Preservation of intestinal gene expression during hibernation. Am J Physiol Gastrointest Liver Physiol 271:G805–G813, 1996 [DOI] [PubMed] [Google Scholar]
- 10. Carey HV, Andrews MT, Martin SL. Mammalian hibernation: cellular and molecular responses to depressed metabolism and low temperature. Physiol Rev 83:1153–1181, 2003 [DOI] [PubMed] [Google Scholar]
- 11. Chen K, Godfrey DA. Sodium pentobarbital abolishes bursting spontaneous activity of dorsal cochlear nucleus in rat brain slices. Hear Res 149:216–222, 2000 [DOI] [PubMed] [Google Scholar]
- 12. Collingridge GL, Olsen RW, Peters J, Spedding M. A nomenclature for ligand-gated ion channels. Neuropharmacology 56:2–5, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Davies PA, Hanna MC, Hales TG, Kirkness EF. Insensitivity to anaesthetic agents conferred by a class of GABAA receptor subunit. Nature 385:820–823, 1997 [DOI] [PubMed] [Google Scholar]
- 14. Davies PA, Kirkness EF, Hales TG. Evidence for the formation of functionally distinct αβγε GABAA receptors. J Physiol 537:101–113, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dobbins EG, Feldman JL. Brainstem network controlling descending drive to phrenic motoneurons in rat. J Comp Neurol 374:64–86, 1994 [DOI] [PubMed] [Google Scholar]
- 16. Eikermann M, Fassbender P, Zaremba S, Jordan AS, Rosow C, Malhotra A, Chamberlin NL. Pentobarbital dose-dependently increases respiratory genioglossus muscle activity while impairing diaphragmatic function in anesthetized rats. Anesthesiology 110:1327–1334, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fatemi SH, Reutiman TJ, Folsom TD, Thuras PD. GABAA receptor downregulation in brains of subjects with autism. J Autism Dev Disord 39:223–230, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Feldman JL, Mitchell GS, Nattie EE. Breathing: rhythmicity, plasticity, chemosensitivity. Annu Rev Neurosci 26:239–266, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Feng HJ, Macdonald RL. Barbiturates require the N terminus and first transmembrane domain of the delta subunit for enhancement of alpha1beta3delta GABAA receptor currents. J Biol Chem 285:23614–23621, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gatta E, Cupello A, Pellistri F, Robello M. GABA(A) receptors of cerebellar granule cells in culture: explanation of overall insensitivity to ethanol. Neuroscience 162:1187–1191, 2009 [DOI] [PubMed] [Google Scholar]
- 21. Glykys J, Peng Z, Chandra D, Homanics GE, Houser CR, Mody I. A new naturally occurring GABA(A) receptor subunit partnership with high sensitivity to ethanol. Nat Neurosci 10:40–48, 2007 [DOI] [PubMed] [Google Scholar]
- 22. Hengen KB, Behan M, Carey HV, Jones MV, Johnson SM. Hibernation induced pentobarbital insensitivity in medulla but not cortex. Am J Physiol Regul Integr Comp Physiol 297:R1028–R1036, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Irnaten M, Walwyn WM, Wang J, Venkatesan P, Evans C, Chang KSK, Andresen MC, Hales TG, Mendelowitz D. Pentobarbital enhances GABAergic neurotransmission to cardiac parasympathetic neurons, which is prevented by expression of a GABAA ε subunit. Anesthesiology 97:717–724, 2002 [DOI] [PubMed] [Google Scholar]
- 24. Kralic JE, Korpi ER, O'Buckley TK, Homanics GE, Morrow AL. Molecular and pharmacological characterization of GABAA receptor α1 subunit knockout mice. J Pharmacol Exp Ther 302:1037–1045, 2002 [DOI] [PubMed] [Google Scholar]
- 25. Krolo M, Stuth EA, Tonkovic-Capin M, Hopp FA, McCrimmon DR, Zuperku EJ. Relative magnitude of tonic and phasic synaptic excitation of medullary inspiratory neurons in dogs. Am J Physiol Regul Integr Comp Physiol 279:R639–R649, 2000 [DOI] [PubMed] [Google Scholar]
- 26. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2[-delta delta C(T)] method. Methods 25:402–408, 2001 [DOI] [PubMed] [Google Scholar]
- 27. Lyman CP, Willis JS, Malan A, Wang LCH. Hibernation in Mammals and Birds. New York: Academic Press, 1982 [Google Scholar]
- 28. Maguire K, Ferando I, Simonsen C, Mody I. Excitability changes related to GABAA receptor plasticity during pregnancy. J Neurosci 29:9592–9601, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Martyniuk CJ, Aris-Brosou S, Drouin G, Cahn J, Trudeau VL. Early evolution of ionotropic GABA receptors and selective regimes acting on the mammalian-specific theta and epsilon subunits. PloS One 2:e894, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. McCrimmon DR, Mitchell GS, Dekin MS. Glutamate, GABA and serotonin in ventilatory control. In: Regulation of Breathing, edited by Dempsey JA, Pack AI. New York: Marcel Dekker, 1995, p. 151–218 [Google Scholar]
- 31. McElroy B, Zakaria A, Glass JD, Prosser RA. Ethanol modulates mammalian circadian clock phase resetting through extrasynaptic GABA receptor activation. Neuroscience 164: 842–848, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. McKernan RM, Whiting PJ. Which GABAA-receptor subtypes really occur in the brain? Trends Neurosci 19:139–143, 1996 [DOI] [PubMed] [Google Scholar]
- 33. Meera P, Olsen RW, Otis TS, Wallner M. Etomidate, propofol and the neurosteroid THDOC increase GABA efficacy of recombinant alpha4beta4delta and alpha4beta3 GABA A receptos expressed in HEK cells. Neuropharmacology 56:155–160, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Milsom Wk, Harris MB, Reid SG. Do descending influences alternate to produce episodic breathing? Respir Physiol 110:307–317, 1997 [DOI] [PubMed] [Google Scholar]
- 35. Miyazawa S, Shiina T, Takewaki T, Shimizu Y. Extension of time until cardiac arrest after injection of a lethal dose of pentobarbital in the hibernating Syrian hamster. J Vet Med Sci 71:383–385, 2009 [DOI] [PubMed] [Google Scholar]
- 36. Paxinos G, Pascal C, Wang H, Wang PY. Chemoarchitectonic Atlas of the Rat Brainstem. San Diego, CA: Academic, 1999 [Google Scholar]
- 37. Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego, CA: Academic, 2004 [Google Scholar]
- 38. Pierrefiche O, Schwarzacher SW, Bischoff AM, Richter DW. Blockade of synaptic inhibition within the pre-Bötzinger complex in the cat suppresses respiratory rhythm generation in vivo. J Physiol 509:245–254, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G. GABA(A) receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience 101:815–850, 2000 [DOI] [PubMed] [Google Scholar]
- 40. Popov VI, Medvedev NI, Patrushev IV, Ignat'ev DA, Morenkov ED, Stewart MG. Reversible reduction in dendritic spines in CA1 of rat and ground squirrel subjected to hypothermia-normothermia in vivo: a three-dimensional electron microscope study. Neuroscience 149:549–560, 2007 [DOI] [PubMed] [Google Scholar]
- 41. Rudolph U, Möhler H. GABA-based therapeutic approaches: GABAA receptor subtype functions. Curr Opin Pharmacol 6:18–23, 2006 [DOI] [PubMed] [Google Scholar]
- 42. Sancar F, Czajkowski C. A GABAA receptor mutation linked to human epilepsy (γ2R43Q) impairs cell surface expression of αβγ receptors. J Biol Chem 279:47034–47039, 2004 [DOI] [PubMed] [Google Scholar]
- 43. Shao XM, Feldman JL. Respiratory rhythm generation and synaptic inhibition of expiratory neurons in the pre-Bötzinger complex: differential roles of glycinergic and GABAergic neural transmission. J Neurophysiol 77:1853–1860, 1997 [DOI] [PubMed] [Google Scholar]
- 44. Sieghart W, Sperk G. Subunit composition, distribution and function of GABA(A) receptor subtypes. Curr Top Med Chem 2:795–816, 2002 [DOI] [PubMed] [Google Scholar]
- 45. Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL. Pre-Bötzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science 254:726–729, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Stell BM, Brickley SG, Tang CY, Farrant M, Mody I. Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by delta subunit-containing GABAA receptors. Proc Natl Acad Sci USA 100:14439–14444, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sundstrom-Poromaa I, Smith DH, Gong QH, Sabado TN, Li X, Light A, Wiedmann M, Williams K, Smith SS. Hormonally regulated α4β2δ GABAA receptors are a target for alcohol. Nat Neurosci 5:721–722, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Takahashi A, Mashimo T, Uchida I. GABAergic tonic inhibition of substantia gelatinosa neurons in mouse spinal cord. Neuroreport 17:1331–1335, 2006 [DOI] [PubMed] [Google Scholar]
- 49. Tsang SY, Ng SK, Xu Z, Xue H. The evolution of GABAA receptor-like genes. Mol Biol Evol 24:599–610, 2006 [DOI] [PubMed] [Google Scholar]
- 50. Volgushev M, Vidyasagar TR, Chistiakova M, Yousef T, Eysel UT. Membrane properties in rat visual cortical cells during reversible cooling. J Physiol 522:59–76, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Von der Ohe CG, Darian-Smith C, Garner CG, Heller HC. Ubiquitous and temperature-dependent neural plasticity in hibernators. J Neurosci 26:10590–10598, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Von der Ohe CG, Garner CG, Darian-Smith C, Heller HC. Synaptic protein dynamics in hibernation. J Neurosci 27:84–92, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wagner DA, Goldschen-Ohm MP, Hales TG, Jones MV. Kinetics and spontaneous open probability conferred by the epsilon subunit of the GABAA receptor. J Neurosci 25:10462–10468, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Walker JM, Glotzbach SF, Berger RJ, Heller HC. Sleep and hibernation in ground squirrels (Citellus spp): electrophysiological observations. Am J Physiol Regul Integr Comp Physiol 233:R213–R221, 1977 [DOI] [PubMed] [Google Scholar]
- 55. Wang H, Stornetta RL, Rosin DL, Guyenet PG. Neurokinin-1 receptor-immunoreactive neurons of the ventral respiratory group in the rat. J Comp Neurol 434:128–146, 2001 [DOI] [PubMed] [Google Scholar]
- 56. Yang CY, Tan PC, We WC, Hsu JC, See LC, Chai CY. Inhibitory effects of propofol on neuron firing activities in the rostral ventrolateral medulla. Chin J Physiol 50:251–257, 2007 [PubMed] [Google Scholar]
- 57. Zuperku EJ, McCrimmon DR. Gain modulation of respiratory neurons. Respir Physiol Neurobiol 131:121–133, 2002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.