Abstract
The autonomic nervous system plays a central role in regulation of host defense and in physiological responses to sepsis, including changes in heart rate and heart rate variability. The cholinergic anti-inflammatory response, whereby infection triggers vagal efferent signals that dampen production of proinflammatory cytokines, would be predicted to result in increased vagal signaling to the heart and increased heart rate variability. In fact, decreased heart rate variability is widely described in humans with sepsis. Our studies elucidate this apparent paradox by showing that mice injected with pathogens demonstrate transient bradyarrhythmias of vagal origin in a background of decreased heart rate variability (HRV). Intraperitoneal injection of a large inoculum of Gram-positive or Gram-negative bacteria or Candida albicans rapidly induced bradyarrhythmias of sinus and AV nodal block, characteristic of cardiac vagal firing and dramatically increased short-term HRV. These pathogen-induced bradycardias were immediately terminated by atropine, an antagonist of muscarinic cholinergic receptors, demonstrating the role of vagal efferent signaling in this response. Vagal afferent signaling following pathogen injection was demonstrated by intense nuclear c-Fos activity in neurons of the vagal sensory ganglia and brain stem. Surprisingly, pathogen-induced bradycardia demonstrated rapid and prolonged desensitization and did not recur on repeat injection of the same organism 3 h or 3 days after the initial exposure. After recovery from the initial bradycardia, depressed heart rate variability developed in some mice and was correlated with elevated plasma cytokine levels and mortality. Our findings of decreased HRV and transient heart rate decelerations in infected mice are similar to heart rate changes described by our group in preterm neonates with sepsis. Pathogen sensing and signaling via the vagus nerve, and the desensitization of this response, may account for periods of both increased and decreased heart rate variability in sepsis.
Keywords: sepsis, heart rate variability, bradycardia, vagus nerve, autonomic nervous system
the autonomic nervous system plays a major role in sensing and responding to threats, including infection. Peripheral infectious or inflammatory signals are relayed via sympathetic and parasympathetic nerves to regulatory centers in the brain stem. Our group has previously shown that intraperitoneal administration of LPS or IL-1β or enteral administration of Campylobacter provoke signaling via the abdominal branches of the vagus nerve to activate the central autonomic network (10–14). Efferent autonomic signals then trigger physiological responses, including sickness behaviors and cardiorespiratory changes. Efferent cholinergic signaling via the vagus nerve has also been shown to limit the potentially damaging systemic inflammatory response and improve outcomes in animal models of sepsis and other acute inflammatory conditions. This critical host defense mechanism, discovered by Tracey and colleagues (4, 31) and termed the cholinergic anti-inflammatory response, involves decreased cytokine production by tissue macrophages via vagally released ACh binding to alpha7 nicotinic cholinergic receptors. If vagal efferent signaling to macrophages and to the heart are coupled, the cholinergic anti-inflammatory response would be associated with slowing of the heart rate via ACh binding to muscarinic cholinergic receptors on cardiac pacemaker cells (30). This would, in turn, increase heart rate variability (HRV), or the variations in intervals between each heart beat. In fact, however, we and others have shown that HRV is depressed in sepsis and sepsis-like illness in both animal models (7) and in humans (5, 7, 15, 17, 19–22, 24, 26, 29).
Effects of sepsis on heart rate and its variability are complex and not well understood. Decreased HRV in sepsis and other acute inflammatory conditions has been linked to the systemic inflammatory response (7, 29). Limited evidence suggests that in the course of sepsis, downregulation of heart rate responses to adrenergic and cholinergic signals occurs, limiting the normal heart rate accelerations and decelerations present in the healthy state. Interestingly, we have shown that some premature newborns with sepsis have recurrent brief heart rate decelerations superimposed on decreased HRV. Our prior studies in mice also showed that administration of LPS resulted in an early transient bradycardia followed by depressed HRV. Here, we demonstrate that live fungal and bacterial pathogens induce the same patterns, and provide evidence that pathogen-induced bradycardia represents vagus nerve activation.
METHODS
Mouse ECG telemetry.
Male C57BL/6 mice (Charles River) 8–12 wk of age were used for all experiments. Animals had free access to food and water throughout the experiments and were housed in a telemetry facility maintained at ambient temperature 20–22°C with a 12:12-h light-dark cycle. Experimental protocols were approved by the University of Virginia Animal Care and Use Committee and conform to the guidelines established in the American Physiology Society's “Guiding Principles for Research Involving Animals and Human Beings.”(1)
Telemetry probes (ETA-F20, Data Sciences International) were surgically implanted for continuous measurement of the ECG. Under isoflurane anesthesia, the telemetry battery was placed in the peritoneal cavity, and the ECG leads were tunneled under the skin to the right and left axillary regions. Experiments were conducted at least 6 days after surgery to allow recovery of baseline HRV. Mice were placed in their standard cages on a telemetry receiver connected via a data exchange matrix to a computer. Animals were allowed to acclimate to the telemetry facility overnight prior to commencing experiments.
The ECG signal was sampled at 2,000 Hz using Data Sciences International hardware and analyzed with Dataquest ART Gold 4.2 software. Our previous studies showed that frequency domain analysis does not provide additional information beyond that obtained from time series analysis in mouse sepsis experiments; therefore, we only performed time series analysis in the current studies (7). Time domain measures were calculated for each 5-min segment of R-R interval data. The normalized standard deviation of normal R-R intervals (SDNN) for each mouse was calculated by dividing the SDNN by the mean of all 5-min SDNN measurements in the hours immediately prior to pathogen or sham injection.
Pathogen administration.
Pathogens used in this study include Klebsiella pneumoniae (KP 43816 serotype 2), methicillin-resistant Staphylococcus aureus (MRSA strain COL), and Candida albicans (CA, SC5314 serotype A). Pathogens were grown overnight on a rotary shaker, bacteria in trypticase soy broth at 37°C and Candida in yeast-peptone-dextrose broth at 28°C (yielding spores and no hyphae). Subcultures were grown from a 1:1,000 dilution of the overnight culture, and organisms were then rinsed in sterile saline and diluted to the appropriate concentration. After pathogen injection in each experiment, inoculum size was confirmed by plating serial dilutions on agar plates, incubating overnight, and performing colony counts.
Following 4 h of baseline telemetry recording, mice were given an intraperitoneal injection of KP, MRSA, or CA in a volume of 0.1 ml. Sham-treated mice were given an intraperitoneal injection of vehicle (sterile endotoxin-free physiological saline). ECG recording was continued until death or 42 h.
Blood sampling for culture and cytokine assay.
In some experiments, at 18 h and 42 h after pathogen inoculation, blood was collected from the retroorbital venous plexus in a heparinized capillary tube. Mice were given light inhalational anesthesia with methoxyflurane for 1 min for the procedure. Blood was centrifuged, and plasma was stored at −80°C. Seven cytokines (IL-6, IL-10, TNF-α, IFN-γ, KC, MIP-1β, and G-CSF) were measured using Luminex multiplex analyte profiling technology. Plasma samples diluted 1:4 with mouse serum diluent and recombinant cytokine standards were incubated with fluorescent antibody-tagged microspheres (Bio-Rad), then with a biotin-labeled detection antibody followed by streptavidin-phycoerythrin. Microspheres were analyzed in a Bio-Plex 200 dual laser fluorometer (Bio-Rad), and cytokine concentrations were calculated from the standard curve with Bio-Plex Manager 4.0 software. Samples and standards were analyzed in duplicate. Lower limits of detection ranged from 1 to 17 pg/ml.
Effects of atropine or repeat pathogen exposure.
Mice undergoing radiotelemetric electrocardiogram recording were given intraperitoneal injections of KP, MRSA, and CA to elicit a bradyarrhythmia (107-108 CFU/mouse). Once the bradyarrhythmia developed, some mice were given an intraperitoneal injection of atropine 1 mg/kg. Other mice were not given atropine but were injected with the same inoculum size of the same pathogen 3 h and 3 days after the initial injection.
Brain preparation and c-Fos staining.
Because the three pathogens investigated in these studies had not previously been shown to activate vagal sensory neurons, or the vagal preganglionic neurons in the brain stem dorsal motor nucleus and nucleus ambiguus that innervate the heart, we assessed these neuronal populations using immunohistochemical detection of the activation marker c-Fos. In separate experiments, brains were perfusion fixed with a picric acid solution 90 min after intraperitoneal injection of KP, MRSA, CA, or sham. This time point was chosen to allow for the induction of c-Fos protein in response to the pathogen administration, which takes ∼60–90 min. Thus, c-Fos expression described in this study represents activation in vagal sensory ganglia and brain stem cells up to ∼30 min after pathogen administration. At the appropriate times following pathogen/saline injections, mice were perfused with saline followed by 4% paraformaldehyde in 0.1 M phosphate buffer with 15% saturated picric acid (pH 7.4). Brains were dissected, blocked, and cut into coronal sections on a Vibratome at 40-μm thickness. Sections were collected in one-in-six sets in six-well plates, and stored at 4°C in 0.1 M PB containing 0.1% sodium azide. One set from each brain stem was stained for c-Fos immunoreactivity, as previously described (14). Sections were incubated in anti-Fos (Ab5, Oncogene, Cambridge, MA; 1:50,000) for 72 h followed by overnight incubation in biotinylated goat anti-rabbit IgG (Jackson Immunoresearch; 1:1,000) and avidin-biotin-peroxidase complex (Vector ABC Elite kit, 1:500) for 4 h. Staining was completed using nickel-enhanced 3,3′-diaminobenzidine (DAB; 0.02%, nickelous ammonium sulfate 0.15%) in Tris-HCl (0.05 M, pH 7.6), yielding a black reaction product. An additional set of sections through the brain stem was double-stained for c-Fos immunoreactivity, as described above, followed by staining choline acetyltransferase (ChAT) immunoreactivity. For the second staining, sections were incubated in rabbit anti-ChAT polyclonal antibodies (Chemicon AB143; 1:2,000), followed by biotinylated goat anti-rabbit IgG (1:1,000), and ABC (Vector Laboratories; 1:750), and finally reacted with DAB (0.04%) yielding a reddish-brown cytoplasmic staining. Sections were then mounted on microscope slides, dehydrated, cleared, and coverslipped.
The entire vagal sensory ganglia (nodose and jugular, with petrosal) were dissected bilaterally from each animal, cryoprotected in 20% sucrose in 0.1 M PB, embedded in pairs in OCT compound (Tissue Tek), rapidly frozen, cut on a Cryostat into 12-μm sections, and thaw-mounted on sets of four microscope slides, such that every fourth section is present on each slide. The slide-mounted sections were subjected to the c-Fos immunohistochemical staining protocol, as described above.
Quantification of c-Fos expression.
Vagal sensory neurons showing nuclear staining significantly darker than the surrounding cytoplasm were counted in one series of regularly spaced sections that were 48 μm apart, as previously described (12). Briefly, neuronal nuclei were considered immunopositive for c-Fos when the staining of the nucleus was visibly darker than the surrounding cytoplasm. In the caudal medulla, the number of double-labeled neurons (that contain both nuclear c-Fos stain and cytoplasmic ChAT stain) were assessed bilaterally in the nucleus ambiguus (NA), as well as in the dorsal motor nucleus of the vagus (DMNX). Every sixth section throughout one set was included, and counts were summed to yield the total number of double-labeled cells.
Statistical analysis.
For heart rate analysis, means were compared prepathogen and postpathogen injection using paired t-tests. For HRV analysis, the mean standard deviation of R-R intervals for each 5-min interval over the time course of the experiment was calculated for each mouse. SDNN was normalized to the 4-h baseline designated as “1,” and statistical significance of differences in SDNN at different time periods relative to the injection event was assessed using paired t-tests. Spearman correlation coefficient was calculated for plasma cytokines vs. HRV. c-Fos counts in the vagal sensory ganglia and in the brain stem vagal motor nuclei were analyzed using ANOVA with post hoc pairwise comparisons between each pathogen treatment and saline using Fisher's protected least significant difference test. Statistical testing was performed in GraphPad Prism version 4 with significance assessed at P < 0.05.
RESULTS
Intraperitoneal large inoculum pathogen injection rapidly induces bradyarrhythmias that are terminated by atropine.
Preliminary studies using a range of inoculum sizes of CA revealed that all mice injected with 107 CFU of CA developed an early transient bradyarrhythmia, whereas only 20% of mice had this response to 106 CFU (data not shown).
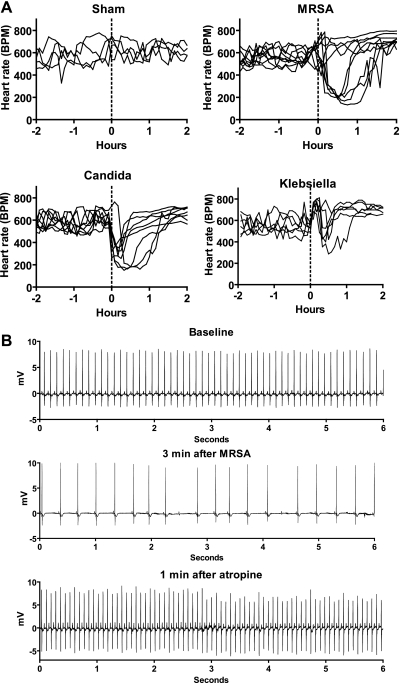
As shown in Fig. 1A, at baseline and following sham injection, mouse heart rate generally ranged from 500 to 700 beats per min (bpm) (n = 4). Intraperitoneal injection of a large inoculum of KP, MRSA, or CA (range 107-108 CFU/mouse; n = 6–9) led to early transient bradyarrhythmias in the majority of mice. A significant drop in HR, with rhythms similar to those shown in Fig. 2, B–D, occurred in all 8 mice injected with CA, 7 of 9 mice injected with MRSA, and 4 of 6 mice injected with KP (P < 0.001 comparing HR at maximum postinjection effect to baseline HR for each responder).
Fig. 1.
Pathogens induce bradycardia, which is terminated by atropine. A: heart rate (BPM, beats per min) of each mouse from 2 h before to 2 h after intraperitoneal injection of saline (sham n = 4) or high-inoculum pathogens at time 0, indicated by the dotted line (n = 6–9). B: atropine terminates pathogen-induced bradycardia. Six-second ECG tracings from a representative mouse at baseline, 3 min after intraperitoneal injection of methicillin-resistant Staphylococcus aureus (MRSA), and 1 min after injection of atropine. Similar results were obtained from mice injected with Klebsiella pneumoniae (KP), and Candida albicans (CA).
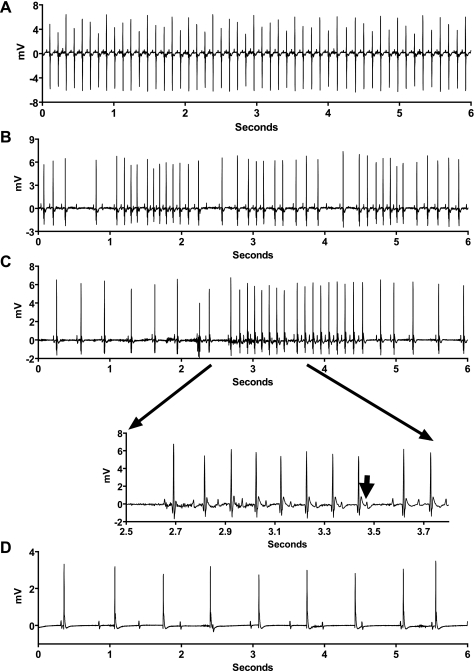
Fig. 2.
Electrocardiographic characteristics of pathogen-induced bradycardias. Representative ECG tracings within several minutes of injection of saline (A) or high-inoculum pathogens (B–D). Second-degree sinus exit block (B) and type 1 second-degree AV node block (Wenckebach) (C) show sinus bradycardia or exit block and AV node Wenckebach (inset, lengthening PR intervals followed by nonconducted P wave indicated by the arrow). D: short epoch of complete AV node block. These arrhythmias occurred in all mice injected with CA, 7 of 9 mice injected with MRSA, and 4 of 6 mice injected with KP.
To determine the role of vagal efferent signaling in pathogen-induced bradycardia, mice were administered each pathogen followed by intraperitoneal administration of atropine or saline once the bradyarrhythmias developed. All mice showed similar responses, and ECG data from a representative mouse are shown in Fig. 1B. The bradyarrhythmias began within 1–3 min of pathogen injection. Atropine was then given and terminated the bradyarrhythmias in 0.5–2 min. Sham treatment did not result in any significant changes in heart rate or rhythm.
Characteristics of the pathogen-induced bradyarrhythmias are shown in Fig. 2 and include sinus bradycardia, type 1 second degree sinus exit block (sinus Wenckebach), type 1 second degree AV node block (AV Wenckebach), and complete AV block. The bradycardia began within 1–3 min of intraperitoneal injection and resolved spontaneously within 30–90 min.
All three pathogens activate vagal sensory and brain stem autonomic neurons.
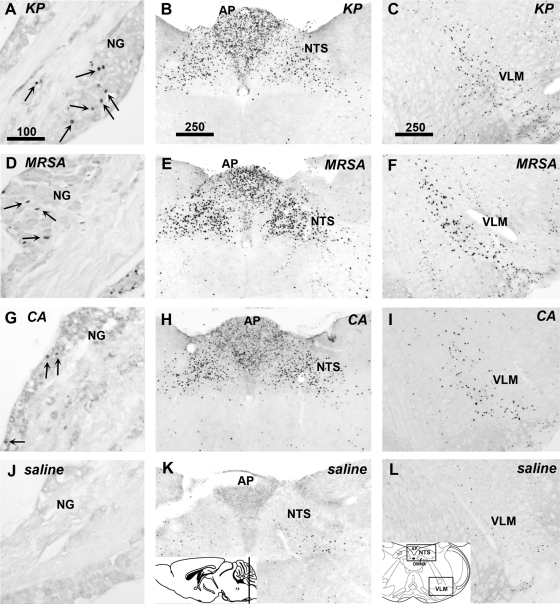
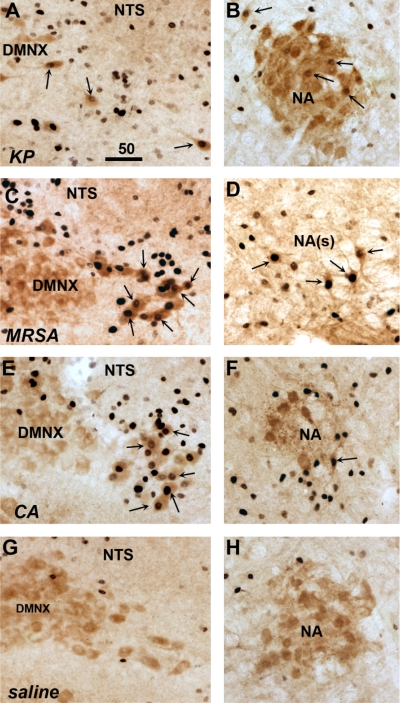
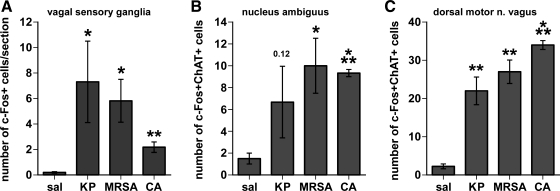
To determine whether vagal sensory and motor neurons are activated in response to the live pathogen administration, sections of vagal sensory ganglia, and caudal brain stem sections containing the targets of the vagal sensory fibers, as well as the motor neurons in the DMNX and NA, were examined for evidence of induction of the activation marker c-Fos protein. A 90-min timepoint was chosen for these studies to allow time for c-Fos protein expression to manifest after pathogen injection (typically, 60 min after vagus nerve stimulation). In saline-treated mice, no or very few vagal sensory neurons were positively labeled for c-Fos protein. However, many vagal sensory neurons from animals treated with the live pathogens expressed c-Fos protein (Fig. 3, left). In the brain stem, all three pathogens dramatically induced c-Fos protein in central autonomic regions, notably the nucleus of the solitary tract and area postrema (NTS and AP, Fig. 3, middle panels), as well as the ventrolateral medulla (VLM, Fig. 3, right). All three pathogens also induced c-Fos protein in cholinergic neurons in lateral portions of the DMNX and in the NA (Fig. 4). Quantitative assessment, summarized in Fig. 5, demonstrated significant increases in c-Fos expression by pathogen injections in the vagal sensory neurons [ANOVA, F (3,9) = 4.12, P = 0.04], as well as in the ChAT-immunoreactive neurons in the NA [F (3,9) = 4.54, P = 0.03] and the DMNX [F (3,9) = 41.0, P < 0.0001]. In mice treated with KP and MRSA, vagal sensory ganglia show particularly large numbers of c-Fos-labeled neurons. ChAT-c-Fos double-labeled neurons occurred most prominently in the lateral portions of the DMNX in mice injected with MRSA and CA, and their presence was less abundant in KP-injected mice.
Fig. 3.
Pathogens rapidly activate vagal sensory neurons in the nodose ganglia and viscerosensory neurons in the caudal brain stem. Immunohistochemical staining for c-Fos was performed 90 min after inoculation with KP (A–C), MRSA (D–F), CA (G–I) or saline (J–L). c-Fos protein is seen as black staining in cell nuclei. All three pathogens induced c-Fos in vagal sensory neurons of the nodose ganglia (NG; A, D, G, J). Strong increases in c-Fos expression were also seen in caudal brain stem viscerosensory regions, including the area postrema (AP) and nucleus of the solitary tract (NTS) (B, E, H, K). The ventrolateral medulla also exhibited c-Fos staining following inoculation with each pathogen compared with sham (ventrolateral medulla, VLM; C, F, I, L). Sections shown are representative of findings in 3 mice in each group. Insets: level of brain stem section (K) and the regions of interest (L). Scale bars in A–C indicate micrometers.
Fig. 4.
Pathogens activate cholinergic visceromotor neurons of the dorsal motor nucleus of the vagus (DMNX) and nucleus ambiguus (NA). Dual immunohistochemical staining was performed for choline acetyltransferase (ChAT, reddish-brown cytoplasm staining) and c-Fos protein (black nuclear staining) 90 min after inoculation of mice with KP (A and B), methicillin-resistant SA (MRSA; C and D), CA (E and F), or saline (G and H). Double-labeled neurons indicated by arrows are found in the dorsal motor nucleus of the vagus (DMNX; A, C, E, G) and the nucleus ambiguus (NA; B, D, F, H), both within compact and more caudal semicompact portions [NA(s)]. Saline injection resulted in few or no double-labeled neurons (G and H). Sections shown are representative of findings in 3 mice in each group. Scale bar in A indicates micrometers.
Fig. 5.
Quantitation of pathogen-induced c-Fos expression in vagal sensory and motor neurons. Graphs summarize the immunohistochemistry results from mice 90 min after intraperitoneal injection of saline (sal; n = 4) or pathogens (KP, MRSA, or CA, n = 3 each). c-Fos-positive cells were counted in vagal sensory neurons (A) and dual c-Fos and choline acetyltransferase (ChAT)-positive cells in cholinergic neurons in the nucleus ambiguus and the dorsal motor nucleus of the vagus (B, C). (*P < 0.05; **P < 0.005, ***P < 0.0005 vs. saline control).
Pathogen effects on heart rate variability and cytokines.
For HRV experiments, KP and CA were administered at a dose that resulted in 30% mortality (mean CFU/mouse 2.8 × 105 KP, n = 10, and 2.6 × 107 CA, n = 8). For MRSA, the mean CFU/mouse was 4.3 × 107 (n = 9), which resulted in transient bradycardia and decreased HRV but no mortality. A 10-fold higher inoculum of MRSA resulted in 100% mortality, and therefore, the lower inoculum was chosen for these experiments. Preliminary studies showed that bacteria-injected mice that appeared well 2 days later survived long-term, while some of those injected with CA appeared well for several weeks and then developed signs of illness and died.
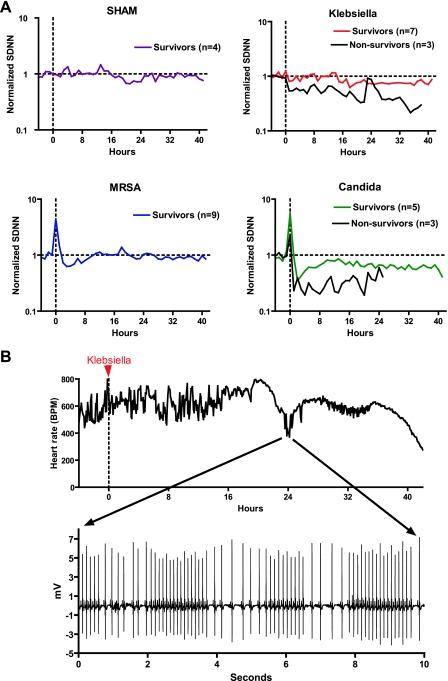
A shown in Fig. 6A, sham injection did not alter heart rate variability. With these inoculum sizes, initial pathogen-induced bradycardias occurred in all mice injected with CA and 5 of 9 mice injected with MRSA, resulting in increased heart rate variability in the 1st h after injection, seen as an upward spike in the SDNN at time 0 (Fig. 6A, bottom). In contrast, only 3 of 10 mice administered an LD30 of KP had initial pathogen-induced bradycardias, and the arrhythmias were milder and of shorter duration than those associated with the other two organisms, resulting in no significant increase in mean normalized SDNN at time 0 (Fig. 6A, top).
Fig. 6.
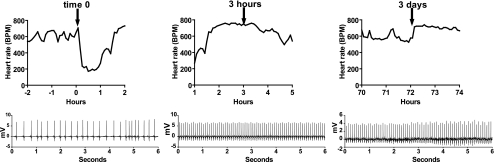
Pathogens decrease and increase heart rate variability. A: decreased heart rate variability (HRV) is associated with mortality in sepsis. At time 0 (vertical dashed line), mice were injected with saline (SHAM), KP, MRSA, or CA. HRV (SDNN, standard deviation of normal R-R intervals) was normalized to the 4-h baseline prior to injection, denoted as “1” (horizontal dashed line). Average normalized SDNN from −4 h to +42 h relative to injection for surviving (colored lines) and nonsurviving mice (black lines) is shown (n = 8–10 per pathogen). B: sepsis induces both decreased HRV and transient HR decelerations. Heart rate of a mouse injected with Klebsiella at time 0 (red arrow) is shown. Note absence of the early bradycardic response with 106 colony-forming units of inoculum. At 18 h after injection, HRV was depressed, and at 24 h, there was a transient episode of recurrent heart rate decelerations (ECG tracing shown in inset) resulting in increased HRV. Subsequently, HR returned to normal but variability was decreased, and 40 h after injection, there was sinus bradycardia followed by death.
Compared with the 4-h baseline period, 3–4 h after pathogen injection, mean HRV was significantly decreased in mice in all three pathogen groups (decrease in mean SDNN 20%, 31%, and 53% for KP, MRSA, and CA, respectively, P < 0.0005). MRSA-treated mice had return of normal HRV by 10 h after injection and maintained normal HRV thereafter. Six mice (3 each in KP and CA groups) died 19–42 h after injection, and all showed markedly decreased HRV. Near the time of death, heart rate was very slow, and HRV was increased. Interestingly, as shown in Fig. 6B, one mouse injected with KP at time 0 (indicated by red arrow) did not have an initial bradycardic response, but at 18 h, it had decreased HRV and at 24 h, it had developed bradyarrhythmias (Fig. 6B, inset). This response resembled the early pathogen-induced bradycardia seen with high-inoculum pathogen injection (compare Figs. 6B and 2B) and resulted in a dramatic increase in HRV. This was followed by a prolonged period of depressed HRV and death at 42 h.
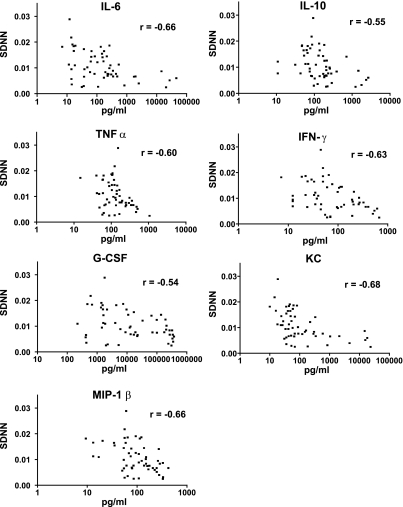
To demonstrate the association between abnormal HRV and the systemic inflammatory response to infection, plasma levels of seven cytokines were measured in all surviving mice at 18 h and 42 h after injection of pathogen or saline control. Seven cytokines were analyzed: IL-6, IL-10, TNFα, IFN-γ, G-CSF, KC, and MIP-1β. As expected, administration of each pathogen caused a significant rise in plasma levels of all cytokines. Cytokine levels were highest in mice that died, were lower at 42 h than at 18 h in mice that survived, and were similar among the three pathogens studied (data not shown). HRV was calculated in the 2-h window prior to blood sampling for cytokine analysis (average SDNN from each 5-min interval from 16–18 h and 40–42 h after pathogen or saline injection). As shown in Fig. 7, average SDNN was significantly inversely correlated with plasma levels of each cytokine (Spearman correlation coefficient ranging from −0.68 to −0.54).
Fig. 7.
Cytokines are inversely correlated with HRV. Seven cytokines were assayed in plasma 18 h and 42 h after injection of saline (n = 4) or KP, MRSA, or CA (n = 8–10 each). Cytokine level (pg/ml, note logarithmic scale) is plotted against HRV (SDNN, ms). Spearman correlation coefficients “r” ranged from −0.54 to −0.68, as indicated.
Desensitization to pathogen-induced bradycardia.
Following the initial pathogen-induced bradycardia, repeated injections of the same size inoculum of the same pathogen 3 h and 3 days later did not result in a bradyarrhythmia. These experiments were done with MRSA and CA only, as repeat injection of high-inoculum KP resulted in rapid deterioration and death. As shown in Fig. 8, the initial injection of MRSA or CA resulted in a bradyarrhythmia in most mice, which resolved by 90 min. HR was then in the high-normal range with depressed variability. Three hours after the first injection, the same injection was given and there were no bradyarrhythmias. Two high-inoculum injections of MRSA or CA were lethal in a small number of mice, while others survived and appeared well several days later. Seventy-two hours after the initial injection, surviving mice were administered the same size inoculum of freshly prepared MRSA or CA, and there was again no significant decrease in heart rate or change in rhythm. Mean heart rate at baseline and lowest heart rate within 30 min of the first pathogen injection were 587 ± 72 and 221 ± 74 (mean ± SD, n = 6, P < 0.001). In contrast, for 30 min after the second (3 h later), and third (3 days later) pathogen injections in the same mice, HR did not drop (659 ± 112 after 3 h injection and 568 ± 222 after 3 day injection; means ± SD, n = 3–6). Of note, occasionally a mouse did not develop bradycardia with the first injection but did respond to the second, whereas all mice that responded to the first injection failed to respond to the second injection 3 h later.
Fig. 8.
Desensitization to pathogen-induced bradycardia. Heart rate responses to identical pathogen injections (arrows) at time 0, 3 h, and 3 days. Representative 6-s ECG tracings within 15 min of each injection are shown. Data are from a single mouse injected with MRSA and are representative of results from 6 mice injected with MRSA or CA (n = 3 each).
DISCUSSION
The key role of the autonomic nervous system in sensing and responding to infection is only beginning to be appreciated. We provide novel evidence that pathogens can rapidly activate vagal afferent and efferent signaling and cause pronounced bradyarrhythmias. Surprisingly, this response undergoes rapid and prolonged desensitization, perhaps contributing to decreased HRV described in sepsis.
We provide three lines of evidence that pathogen-induced bradycardia is vagally mediated: the arrhythmias have characteristics typically associated with vagal activation, are rapidly terminated by the muscarinic cholinergic antagonist atropine, and are associated with neuronal activation in the autonomic network. The finding that live pathogens activate vagal sensory neurons, the solitary tract nucleus, and preganglionic vagal motor neurons in the DMNX and NA suggests a reflex loop whereby the detection of pathogens by visceral sensory neurons, such as those of the vagus nerve, rapidly leads to a vagal efferent cardiovascular response. Our finding of c-Fos in cholinergic neurons in lateral portions of the DMNX and in the NA shown to innervate the heart (6, 28) is supportive of such an assertion. Pathogen-induced bradycardia may be an extension of the cholinergic anti-inflammatory reflex identified by Gallowitsch-Puerta and Tracey, whereby efferent vagal signaling to tissue macrophages dampens inflammation (8), although it is not yet known whether efferent vagal signaling to the heart and to other organs and tissue macrophages are coupled.
The rapid onset of the bradycardic response suggests that pathogens exert a direct effect on the nervous system, leading to vagal efferent firing, rather than an indirect effect via cytokines or other secondary mediators. Quorum-sensing signaling molecules produced by Gram-negative bacteria (acylhomoserine lactones) have been shown to induce a rapid and marked bradycardic response in rats through vagal efferent activation (9), but the mechanisms by which the nervous system detects pathogens, such as bacteria or Candida, or their products (such as LPS or acylhomoserine lactones) are not well established. Pathogens may activate vagal sensory neurons either peripherally or within the ganglia (via the circulation) by acting on pattern recognition receptors such as Toll-like receptors (TLRs) on the neuronal membrane. To date, TRLs have not been identified in vagal sensory nerves in the peritoneal cavity, but TLR mRNA and protein have been demonstrated in the vagal sensory (nodose) ganglion (18), identifying one possible signaling route for pathogens to activate the autonomic nervous system.
In our studies, pathogen-induced bradycardia underwent rapid and prolonged desensitization, which could reflect changes in receptor sensitivity or in activation of downstream signaling pathways, as seen in LPS tolerance. We also found, as expected, that infected mice had decreased HRV. HRV depression in sepsis has been widely reported in humans and, although the mechanisms are poorly understood, decreased adrenergic and/or cholinergic activity or sensitivity are likely to be involved. Viral infection can transiently desensitize neuronal M2 muscarinic receptors in the lung (25), and it is plausible that other pathogens could alter muscarinic receptor number or sensitivity in cardiac pacemaker cells or in cholinergic neurons. Whether pathogen programming of autonomic responses occurs through direct pathogen effects on cells or via secondary mediators released in the systemic inflammatory response to sepsis is unknown. Our current studies show that high levels of circulating cytokines in bacterial and fungal sepsis are correlated with depressed heart rate variability, and our prior studies showed that administration of TNF-α is sufficient to depress HRV. Cytokines have been shown to decrease expression of muscarinic cholinergic receptors in the intestine (2) and lung (3), and cytokine effects on cholinergic receptors on cardiac pacemaker cells remain to be determined.
Perspectives and Significance
Pathogen-exposed mice have transient bradyarrhythmias and depressed HRV, which may represent vagal hyperactivation and desensitization, respectively. These changes also occur in premature neonates with sepsis (16–19, 21, 24) and in fetuses in the setting of asphyxia (23) or intrauterine infection (27). In these conditions, the vagus nerve may be serving a key role in sensing a threat to survival, and the resulting physiological response (abnormal heart rate characteristics) could serve as a sentinel for impending clinical deterioration.
GRANTS
This work was supported by the following funding sources: NICHD 051609 (to K. D. Fairchild), NIGMS 64640 (to J. R. Moorman), NIMH 068834 (to L. Goehler), The Wallace H. Coulter Foundation Translational Research Award, and University of Virginia Children's Hospital.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
We would like to acknowledge the expert technical assistance of Bobi Thornhill.
REFERENCES
- 1. American Physiological Society Guiding principles for research involving animals, and human beings. Am J Physiol Regul Integr Comp Physiol 283: R281–R283, 2002 [DOI] [PubMed] [Google Scholar]
- 2. Akiho H, Khan WI, Al-Kaabi A, Blennerhassett P, Deng Y, Collins SM. Cytokine modulation of muscarinic receptors in the murine intestine. Am J Physiol Gastrointest Liver Physiol 293: G250–G255, 2007 [DOI] [PubMed] [Google Scholar]
- 3. Barnes PJ, Haddad EB, Rousell J. Regulation of muscarinic M2 receptors. Life Sci 60: 1015–1021, 1997 [DOI] [PubMed] [Google Scholar]
- 4. Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW, Tracey KJ. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 405: 458–462, 2000 [DOI] [PubMed] [Google Scholar]
- 5. Cao H, Lake DE, Griffin MP, Moorman JR. Increased nonstationarity of neonatal heart rate before the clinical diagnosis of sepsis. Ann Biomed Eng 32: 233–244, 2004 [DOI] [PubMed] [Google Scholar]
- 6. Corbett EK, Mary DA, McWilliam PN, Batten TF. Age-related loss of cardiac vagal preganglionic neurones in spontaneously hypertensive rats. Exp Physiol 92: 1005–1013, 2007 [DOI] [PubMed] [Google Scholar]
- 7. Fairchild KD, Saucerman JJ, Raynor LL, Sivak JA, Xiao Y, Lake DE, Moorman JR. Endotoxin depresses heart rate variability in mice: Cytokine and steroid effects. Am J Physiol Regul Integr Comp Physiol 297: R1019–R1027, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gallowitsch-Puerta M, Tracey KJ. Immunologic role of the cholinergic anti-inflammatory pathway and the nicotinic acetylcholine alpha 7 receptor. Ann NY Acad Sci 1062: 209–219, 2005 [DOI] [PubMed] [Google Scholar]
- 9. Gardiner SM, Chhabra SR, Harty C, Williams P, Pritchard DI, Bycroft BW, Bennett T. Haemodynamic effects of the bacterial quorum sensing signal molecule, N-(3-oxododecanoyl)-l-homoserine lactone, in conscious, normal and endotoxaemic rats. Br J Pharmacol 133: 1047–1054, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gaykema RP, Goehler LE, Lyte M. Brain response to cecal infection with Campylobacter jejuni: analysis with Fos immunohistochemistry. Brain Behav Immun 18: 238–245, 2004 [DOI] [PubMed] [Google Scholar]
- 11. Gaykema RP, Goehler LE, Tilders FJ, Bol JG, McGorry M, Fleshner M, Maier SF, Watkins LR. Bacterial endotoxin induces fos immunoreactivity in primary afferent neurons of the vagus nerve. Neuroimmunomodulation 5: 234–240, 1998 [DOI] [PubMed] [Google Scholar]
- 12. Goehler LE, Gaykema RP, Hammack SE, Maier SF, Watkins LR. Interleukin-1 induces c-Fos immunoreactivity in primary afferent neurons of the vagus nerve. Brain Res 804: 306–310, 1998 [DOI] [PubMed] [Google Scholar]
- 13. Goehler LE, Gaykema RP, Hansen MK, Anderson K, Maier SF, Watkins LR. Vagal immune-to-brain communication: a visceral chemosensory pathway. Auton Neurosci 85: 49–59, 2000 [DOI] [PubMed] [Google Scholar]
- 14. Goehler LE, Gaykema RP, Opitz N, Reddaway R, Badr N, Lyte M. Activation in vagal afferents and central autonomic pathways: early responses to intestinal infection with Campylobacter jejuni. Brain Behav Immunol 19: 334–344, 2005 [DOI] [PubMed] [Google Scholar]
- 15. Griffin MP, Lake DE, Bissonette EA, Harrell FE, Jr, O'Shea TM, Moorman JR. Heart rate characteristics: novel physiomarkers to predict neonatal infection and death. Pediatrics 116: 1070–1074, 2005 [DOI] [PubMed] [Google Scholar]
- 16. Griffin MP, Lake DE, Moorman JR. Heart rate characteristics and laboratory tests in neonatal sepsis. Pediatrics 115: 937–941, 2005 [DOI] [PubMed] [Google Scholar]
- 17. Griffin MP, Lake DE, O'Shea TM, Moorman JR. Heart rate characteristics and clinical signs in neonatal sepsis. Pediatr Res 61: 222–227, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Griffin MP, Moorman JR. Toward the early diagnosis of neonatal sepsis and sepsis-like illness using novel heart rate analysis. Pediatrics 107: 97–104, 2001 [DOI] [PubMed] [Google Scholar]
- 19. Griffin MP, O'Shea TM, Bissonette EA, Harrell FE, Jr, Lake DE, Moorman JR. Abnormal heart rate characteristics preceding neonatal sepsis and sepsis-like illness. Pediatr Res 53: 920–926, 2003 [DOI] [PubMed] [Google Scholar]
- 20. Griffin MP, Scollan DF, Moorman JR. The dynamic range of neonatal heart rate variability. J Cardiovasc Electrophysiol 5: 112–124, 1994 [DOI] [PubMed] [Google Scholar]
- 21. Kovatchev BP, Farhy LS, Cao H, Griffin MP, Lake DE, Moorman JR. Sample asymmetry analysis of heart rate characteristics with application to neonatal sepsis and systemic inflammatory response syndrome. Pediatr Res 54: 892–898, 2003 [DOI] [PubMed] [Google Scholar]
- 22. Lake DE, Richman JS, Griffin MP, Moorman JR. Sample entropy analysis of neonatal heart rate variability. Am J Physiol Regul Integr Comp Physiol 283: R789–R797, 2002 [DOI] [PubMed] [Google Scholar]
- 23. Low JA, Pancham SR, Worthington DN. Intrapartum fetal heart rate profiles with and without fetal asphyxia. Am J Obstet Gynecol 127: 729–737, 1977 [DOI] [PubMed] [Google Scholar]
- 24. Moorman JR, Lake DE, Griffin MP. Heart rate characteristics monitoring for neonatal sepsis. IEEE Trans Biomed Eng 53: 126–132, 2006 [DOI] [PubMed] [Google Scholar]
- 25. Moreno L, Jacoby DB, Fryer AD. Dexamethasone prevents virus-induced hyperresponsiveness via multiple mechanisms. Am J Physiol Lung Cell Mol Physiol 285: L451–L455, 2003 [DOI] [PubMed] [Google Scholar]
- 26. Nelson JC, Rizwan u Griffin MP, Moorman JR. Probing the order within neonatal heart rate variability. Pediatr Res 43: 823–831, 1998 [DOI] [PubMed] [Google Scholar]
- 27. Salafia CM, Ghidini A, Sherer DM, Pezzullo JC. Abnormalities of the fetal heart rate in preterm deliveries are associated with acute intra-amniotic infection. J Soc Gynecol Investig 5: 188–191, 1998 [DOI] [PubMed] [Google Scholar]
- 28. Takanaga A, Hayakawa T, Tanaka K, Kawabata K, Maeda S, Seki M. Immunohistochemical characterization of cardiac vagal preganglionic neurons in the rat. Auton Neurosci 106: 132–137, 2003 [DOI] [PubMed] [Google Scholar]
- 29. Tateishi Y, Oda S, Nakamura M, Watanabe K, Kuwaki T, Moriguchi T, Hirasawa H. Depressed heart rate variability is associated with high IL-6 blood level and decline in the blood pressure in septic patients. Shock 28: 549–553, 2007 [DOI] [PubMed] [Google Scholar]
- 30. Tracey KJ. Physiology and immunology of the cholinergic antiinflammatory pathway. J Clin Invest 117: 289–296, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, Li JH, Wang H, Yang H, Ulloa L, Al-Abed Y, Czura CJ, Tracey KJ. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature 421: 384–388, 2003 [DOI] [PubMed] [Google Scholar]