Abstract
We have previously described the physiological and morphological properties of the cough receptors and their sites of termination in the airways and centrally in the nucleus tractus solitarius (nTS). In the present study, we have addressed the hypothesis that the primary central synapses of the cough receptors subserve an essential role in the encoding of cough. We found that cough requires sustained, high-frequency (≥8-Hz) afferent nerve activation. We also found evidence for processes that both facilitate (summation, sensitization) and inhibit the initiation of cough. Sensitization of cough occurs with repetitive subthreshold activation of the cough receptors or by coincident activation of C-fibers and/or nTS neurokinin receptor activation. Desensitization of cough evoked by repetitive and/or continuous afferent nerve activation has a rapid onset (<60 s) and does not differentiate between tussive stimuli, suggesting a central nervous system-dependent process. The cough reflex can also be actively inhibited upon activation of other airway afferent nerve subtypes, including slowly adapting receptors and pulmonary C-fibers. The sensitization and desensitization of cough are likely attributable to the prominent, primary, and unique role of N-methyl-d-aspartate receptor-dependent signaling at the central synapses of the cough receptors. These attributes may have direct relevance to the presentation of cough in disease and for the effectiveness of antitussive therapies.
Keywords: solitary tract, capsaicin, N-methyl-d-aspartate, windup
the vagal afferent nerves regulating cough in anesthetized guinea pigs are insensitive to capsaicin, airway smooth muscle contraction, or changes in airway luminal pressure. Terminating primarily in the larynx, trachea, and main stem bronchi, the cough receptors are exquisitely sensitive to punctate mechanical stimuli and acid (6, 35). When these stimuli are delivered to the large extrapulmonary airways and main stem bronchi, coughing results, typically one or two powerful expiratory events each preceded by an enhanced inspiratory phase (5). The cough receptors have their cell bodies in the nodose ganglia, terminate rostral and lateral to obex in nucleus tractus solitarius (nTS), and utilize glutamate as their primary transmitter (6, 7, 33).
Relative to other visceral reflex effects, such as changes in heart rate, blood pressure, respiratory rate, and airway smooth muscle tone, the cough reflex is subject to more complex regulatory mechanisms that include conscious (cortical) control and cough threshold regulation that is manifest in both conscious and anesthetized preparations (4). Threshold, even suprathreshold, tussive stimuli will initiate unpredictable cough repetitions and forcefulness depending upon the stimulus intensity, perceptions of the urge to cough, and conscious modulation that can either enhance or completely prevent coughing depending on the social situation and perceived need to cough (11). Regulation of cough occurs within multiple brain structures, including, among others, nTS, ventral respiratory group, thalamus, and raphe (3, 22, 49, 50).
Our previous studies have defined the mechanisms by which cough is initiated in anesthetized guinea pigs and the likely areas of central and peripheral termination of the cough receptors (5–7, 35). In the present study, we addressed the hypothesis that a major site for cough pattern regulation is at the primary central synapses of the cough receptors in nTS. The results reveal complex actions of and interactions between afferent nerve subpopulations, with evidence for summation, sensitization, desensitization, and both facilitation and inhibition initiated by activating vagal afferent nerve subtypes innervating the intrapulmonary airways and even afferent nerve subtypes innervating other visceral organs such as the esophagus. We also describe a prominent, primary, and unique role of N-methyl-d-aspartate (NMDA) receptor-dependent signaling at the central synapses of the cough receptors.
METHODS
Experiments were carried out using anesthetized (1.5 g/kg urethane, ip) male Hartley guinea pigs (200–350 g, pathogen-free; Harlan) and were approved by the Johns Hopkins Animal Care and Use Committee. When the experiments were completed, animals were killed by asphyxiation in a vessel filled with carbon dioxide followed by exsanguination.
Once adequate anesthesia was induced, guinea pigs were secured supine on a warming pad. The trachea was cannulated at its caudal-most end with a leur stub adaptor that was subsequently attached to a length of tubing that terminated inside a chamber that warmed and humidified inspired room air (5, 6). A pressure transducer attached to a side port in the tracheal cannula monitored respiratory efforts, which were recorded digitally (Biopac data acquisition system, Santa Barbara, CA). The extrathoracic trachea was then opened lengthwise along its ventral-most aspect: retracted bilaterally and perfused with warmed, oxygenated Krebs buffer [composition (in mM) 118 NaCl, 5.4 KCl, 1 NaHPO4, 1.2 MgSO4, 1.9 CaCl2, 25 NaHCO3, and 11.1 dextrose, pH = 7.4, 3 ml/min]; introduced into the tracheal lumen at the caudal-most exposed segment of the trachea, and removed at the rostral end of the trachea by attaching PE tubing threaded through the upper airways to a gentle suction source. The buffer contained 3 μM indomethacin to block formation of neuromodulatory prostanoids.
Coughing was evoked by applying citric acid (0.001–2 M) topically to the tracheal mucosa, delivered in 100-μl aliquots at 1-min intervals. Concentration-response curves were constructed in an ascending fashion. Cough was defined based on visual confirmation of a cough-like respiratory effort, with associated signature pressures, including a ≥500% increase in peak expiratory pressure preceded by an enhanced inspiratory effort, all occurring in <1 s. Expiration reflexes, which resembled coughs but without the preceding enhanced inspiratory effort and a blunted expiratory effort, were rarely seen in response to tracheal stimulation (<10% of evoked responses) and were not counted as coughs if they occurred independent from coughs in the preceding respiratory cycle. Cough and other respiratory reflexes were also evoked by electrically stimulating (5–64 Hz, 1-ms pulse duration, 10-s train, 8 V) the tracheal mucosa using a custom-made platinum electrode or by adding the voltage-gated potassium channel blocker 4-aminopyridine (4-AP) to the tracheal perfusate. In some animals, coughing was evoked after unilateral cuts of either a recurrent laryngeal nerve (RLN) or a vagus nerve. The RLN were cut at their caudal-most location along the trachea. Regardless of the tussive stimulus used, the coughing evoked in this model has been shown to be largely C-fiber-independent, occurring secondary to the activation of the acid-sensitive, capsaicin-insensitive mechanoreceptors innervating the larynx, trachea, and main stem bronchi and arising bilaterally from the nodose ganglia (5, 6, 9, 34, 35). Diagrams of our preparation and the innervation of the guinea pig airways can be found elsewhere (6, 9, 32, 35).
We attempted to modulate citric acid-induced coughing and the other respiratory reflex effects by microinjection of glutamate NMDA [amino-5-phosphovaleric acid (AP-5) and SDZ-220581] or non-NMDA (CNQX and NBQX) receptor antagonists. These drugs were selected for study based on their 15- to 1,000-fold selectivity for their target receptors (7, 39, 40). We also studied the effects of microinjection of substance P, γ-aminobutyric acid (GABA), the GABAB receptor agonist baclofen, or the GABAA receptor antagonist bicucculline. After preparing guinea pigs surgically for cough, animals were then placed in a Kopf stereotaxic frame. The skull was exposed, and lambda was located and used as a stereotaxic reference point. Locations within nTS received microinjections as described elsewhere (7), delivered in 100-nl volumes through borosilicate micropipettes pulled sharp using a Flaming Brown micropipette puller (P-87; Sutter Instruments). Microinjection sites were evaluated at autopsy by visualizing the fluorescent dye RH421, which is included in our microinjectate as described elsewhere (7). Once the microinjections were completed (the whole procedure with bilateral injection takes <10 min), animals were quickly repositioned on the heating pad and prepared for cough studies as described above.
Statistical analyses.
A nonpaired experimental design had to be employed for most of the experiments described below. Results are presented as means ± SE of n experiments where n is a single animal. Differences among group means were assessed by one-way ANOVA and Sheffé's f-test for unplanned comparisons. P values of <0.05 were considered statistically significant. Rarely (<10% animals), guinea pigs did not cough during surgery or had basal respiratory rates of ≤45 breaths/min that were not attributable to some experimental intervention. These animals were excluded from subsequent analyses. Also, in the microinjection studies, if the targeted brain stem locations were missed with microinjection, these animals were excluded from further analysis.
Reagents.
Substance P (0.1–1 mM), SDZ-220581 (1 mM), NBQX (1 mM), indomethacin (30 mM), GABA (10 mM), CNQX (10 mM), bicuculline (0.2 mM), baclofen (0.25 mM), AP-5 (0.1–1 mM) and 4-AP (1 M) were purchased from either Sigma (St. Louis, MO) or Tocris (Ellisville, MO). All drugs were prepared as stock solutions in saline and diluted further on the day of experimentation in saline, except indomethacin, which was dissolved in ethanol before dilution in Krebs buffer or saline.
RESULTS
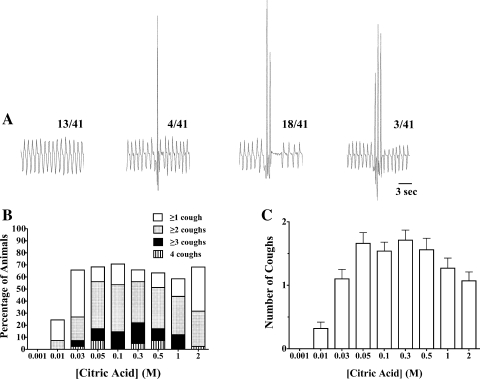
Citric acid applied topically in ascending concentrations (0.001–2 M) to the tracheal mucosa evoked 10 ± 1 coughs cumulatively in control animals (n = 41; Fig. 1). The first two doses of citric acid challenge studied (0.001 and 0.01 M) were mostly subthreshold for initiating cough (0 and 25% of animals coughed in response to 0.001 and 0.01 M citric acid). All higher doses of citric acid studied evoked coughing in ∼70% of animals. The number of coughs evoked by each suprathreshold dose of citric acid was largely concentration-independent, with the number of coughs evoked at each dose between 0.03 and 2 M ranging between zero and four coughs, with an average of about 1.5 coughs/dose. If coughing occurred (as mentioned above, ∼70% of challenges with 0.03–2 M citric acid resulted in coughing), the majority of animals coughed two or more times at each dose, but only rarely four or more coughs occurred.
Fig. 1.
Citric acid-evoked coughing in anesthetized guinea pigs. Citric acid was applied topically to the tracheal mucosa in 100-μl aliquots in concentrations of 0.001–2 M. The lowest doses of citric acid studied (0.001 and 0.01 M) were typically subthreshold for initiating cough. At higher threshold concentrations (≥0.03 M), citric acid evoked 0–4 coughs in a largely concentration-independent manner. A: representative traces of coughing evoked by a single concentration (0.05 M) of citric acid in 4 different animals. Cough is defined visually and by the characteristic forceful expiratory (upward deflection) pressure increase, preceded by an enhanced inspiration, with the entire cycle occurring in <1 s. The no. of all animals studied coughing 0, 1, 2, or 3 times in response to 0.05 M citric acid is indicated in the fractional ratio presented over each representative trace [3 of the 41 control animals studied coughed 4 times to 0.05 M citric acid (data not shown)]. B: distribution of cough patterns at each concentration of citric acid studied. Only 5 of 41 animals coughed 4 times to any dose of citric acid, and of the 10 bouts of 4 coughs observed, 1 animal accounted for 5 of these events. C: mean ± SE no. of coughs evoked at each dose of citric acid studied [Citric acid], citric acid concentration.
The observations that a cough threshold exists and that at suprathreshold concentrations cough frequency becomes largely stimulus intensity (concentration) independent implies several competing and parallel regulatory processes that encode cough frequencies. In subsequent studies, we used both electrical stimulation and citric acid challenges to elucidate the various mechanisms regulating cough thresholds and cough frequency.
Summation of and sensitization to cough receptor signaling.
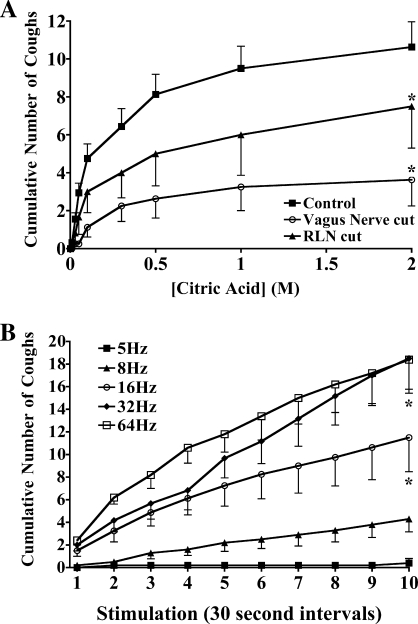
Summation of afferent input resulting in differential reflex effects can be assessed by altering the number of active units (with constant stimulus intensity) or by increasing the activity in each stimulated unit. The roughly symmetric innervation of the trachea by afferent nerves projecting to the airways through the right and left vagus nerves offers a simple approach to evaluating summation. Cutting a vagus nerve slightly reduced basal respiratory rate while unilateral RLN transection did not affect respiration. However, a unilateral cut of an RLN at the caudal-most end of the extrathoracic trachea or a unilateral vagotomy reduced by approximately one-half the number of coughs evoked cumulatively by tracheal citric acid challenge (Fig. 2A). The number of citric acid doses evoking cough was more affected than the number of coughs per dose.
Fig. 2.
Summation of afferent input regulates coughing in anesthetized guinea pigs. A: coughing was evoked by citric acid applied topically to the tracheal mucosa in intact animals and in animals undergoing a unilateral right (n = 4) or left (n = 4) vagus nerve or recurrent laryngeal nerve (RLN; n = 6) transection. Unilateral vagotomy modestly reduced respiratory rate relative to intact controls, which averaged 75 ± 5 and 55 ± 3 breaths/min in control (n = 16) and unilaterally vagotomized (n = 8) animals, respectively (P < 0.05). RLN transection was without effect on respiratory rate [76 ± 4 breaths/min in animals undergoing right (n = 3) or left (n = 3) RLN transection]. The nerve cuts had a statistically insignificant effect on the no. coughs evoked when coughing occurred (1.7 ± 0.2, 1.3 ± 0.2, and 1.8 ± 0.3 coughs/dose in control animals and in animals undergoing unilateral vagus nerve or RLN transection, respectively; n = 6–16; P > 0.1). These nerve cuts did, however, significantly reduce the no. of citric acid challenge doses that evoked coughing (6.2 ± 0.5, 2.6 ± 0.7, and 4.3 ± 1.4 doses evoking cough in control animals and in animals undergoing unilateral vagus or RLN transection, respectively; P < 0.05). B: coughing requires high-frequency afferent nerve stimulation. The tracheal mucosa was stimulated electrically in 10-s trains every 30 s at various frequencies, and the cumulative no. of coughs is presented. Stimulation of the tracheal mucosa at 5–8 Hz was typically subthreshold for evoking cough (>70% failure rate). Stimulation frequencies >10 Hz were required to reliably evoke coughing. The results are presented as means ± SE of 5–10 experiments.
Summation but not necessarily frequency-response relationships could also be demonstrated using an electrical stimulus to evoke cough (Fig. 2B). At a voltage (8 volts), pulse duration (1 ms), and train duration (10 s) found to be optimal in previous experiments, the ability of stimulation of the tracheal mucosa at frequencies between 5 and 64 Hz to evoke cough was assessed. Stimuli were delivered repetitively at 30-s intervals for 5 min, and the cumulative number of coughs evoked was determined. Stimulation at 5 Hz consistently failed to evoke coughing while 8 Hz was also largely ineffective, evoking cough in less than one-half of all trials over the 10-min period of stimulation. Consistent with studies performed in cats (2), stimulation frequencies of 10–64 Hz were nearly always effective, evoking one to two coughs on average at every minute interval over the entire 10-min period of stimulation.
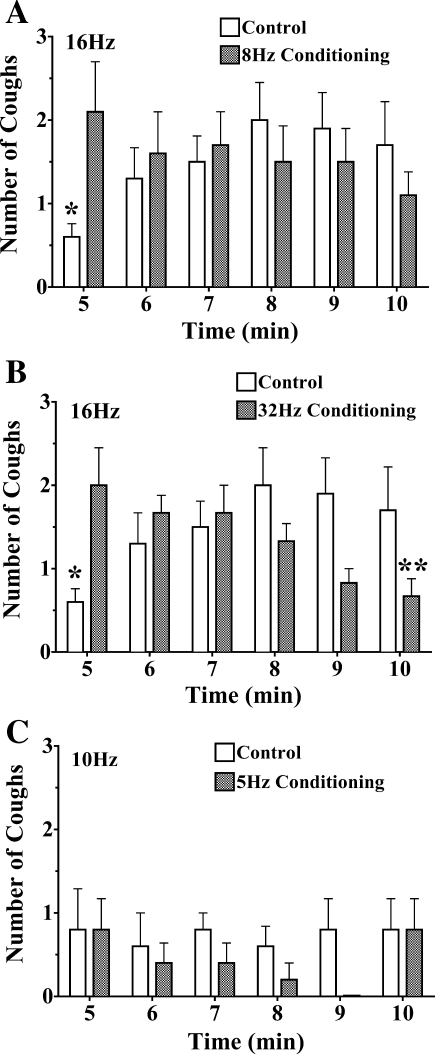
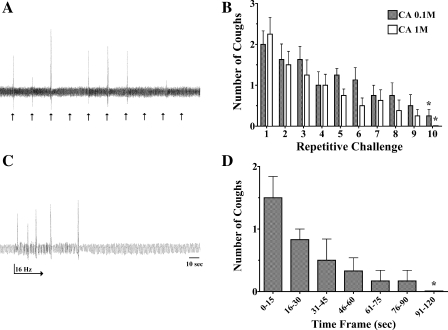
Sensitization of the cough reflex was evaluated in animals provoked electrically as described above (Fig. 3). Coughing induced by 16 Hz stimulation was assessed in animals that had first been repeatedly challenged with electrical stimuli at a sub- or near-threshold stimulation frequency of 8 Hz, or at a suprathreshold stimulation frequency of 32 Hz, both at 30-s intervals, or had been unchallenged for an equivalent duration before 16 Hz stimulation. In either preconditioning experimental design, the coughing evoked by 16 Hz stimulation was enhanced by nearly threefold relative to the number of coughs evoked without any conditioning stimuli. This conditioning effect of repetitive electrical stimulation was also apparent after several stimulations at 16 Hz, since the number of coughs evoked nearly tripled from the first to fourth stimulus. However, similar conditioning effects were not apparent when the conditioning and test stimuli were limited to sub- or near-threshold stimulation frequencies (5 and 10 Hz, respectively).
Fig. 3.
Sensitization/conditioning of the cough reflex by near- or suprathreshold stimulation of the afferent nerves regulating cough. The tracheal mucosa was stimulated electrically (16 Hz) in 10-s trains at 1-min intervals following a 5-min prestimulation period when the trachea was either unstimulated (control) or stimulated at near (8 Hz; n = 10 each; A)- or supra (32 Hz; n = 6; B)-threshold stimulation frequencies repetitively at 30-s intervals. Repetitive stimulation of the tracheal mucosa at near- or suprathreshold frequencies markedly increased the no. of coughs evoked by 16 Hz stimulation. Also note the progressive increase in cough no. produced in the control experiments by repetitive stimulation at 16 Hz. C: in contrast to the effects of near- and suprathreshold stimulation, changing stimulation frequencies from sub (5 Hz)- to near (10 Hz)-threshold frequencies did not sensitize coughing (n = 5/treatment group). All of the results described are presented as means ± SE of 5–10 experiments. *Initial evoked response in control preparations was smaller than the time-matched response evoked in conditioned preparations (P < 0.05). The response at 10 min in the 32 Hz conditioning preparations was significantly smaller (**P < 0.05) than the response evoked initially in this group.
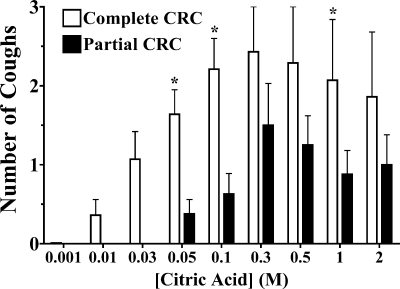
Conditioning/sensitization of the cough reflex could also be demonstrated with citric acid challenges (Fig. 4). The number of coughs evoked by each citric acid dose studied over a complete concentration-response curve (0.001–2 M) was compared with that when the initial sub- or near-threshold doses were omitted (0.05–2 M). The first three doses of the complete concentration-response curve evoked very few coughs (1.4 ± 0.2 coughs cumulatively) in animals receiving all challenge doses, but the next six subsequent doses studied evoked more coughing than in animals challenged with only the six suprathreshold concentrations of citric acid.
Fig. 4.
Citric acid evoked coughing using complete (n = 8) and truncated (n = 8) concentration-response curves (CRC). The mean ± SE no. of coughs evoked by each dose of citric acid is presented. The first 3 doses of the complete concentration-response curve evoked few coughs cumulatively in these experiments (1.4 ± 0.2 coughs). Subsequent doses of citric acid evoked significantly (*) more coughs than when the initial sub- or near-threshold challenges were not included.
Desensitization of the cough reflex.
We had reported previously that, when citric acid concentration-response curves were repeated 10 min after an initial concentration-response curve, few coughs were evoked, implying desensitization of the cough reflex, either through effects of acid at the peripheral nerve terminals or by a central process (5). We have studied this phenomenon further in the present study using three separate approaches. In initial experiments, we evaluated the ability of citric acid to evoke cough when delivered at a single concentration repetitively at 1-min intervals over 10 min (Fig. 5, A and B). At concentrations of 0.1 and 1 M, citric acid reliably evoked coughing when administered consecutively over the first few minutes of the experiments. However, with each consecutive challenge, the number of coughs evoked by these concentrations of citric acid progressively declined, and, by 10 min, only 2/16 animals coughed to either citric acid concentration. The rate of decline in cough number was essentially identical for the two concentrations of citric acid studied. In a second series of experiments, we evoked cough chemically with the voltage-gated potassium channel blocker 4-AP, which is known to induce a sustained (>10 min) high-frequency activation of the cough receptors in vitro and which we had shown previously evokes coughing when applied topically to the tracheal mucosa (5, 36). Once coughing started during the 1 mM 4-AP challenge, 9.6 ± 3.4 coughs were observed over the ensuing 5 min, but only 2.8 ± 1.5 coughs were recorded over the subsequent 5 min and 0.4 ± 0.4 coughs over the next 5-min interval despite continuous perfusion of the tracheal lumen with the potassium channel opener (n = 5). Finally, we evoked cough by continuously stimulating the tracheal mucosa with electrical impulses shown in prior studies to be of optimal intensity (8 volts, 1-ms pulse duration) and frequency (16 Hz). As with 4-AP challenge, coughing was evoked repetitively over the first 30 s of stimulation, but, by 90 s, coughing ceased and never reappeared despite continuous electrical stimulation for 5 min (Fig. 5, C and D).
Fig. 5.
The cough reflex desensitizes with repetitive tussive challenge. Coughing was evoked repetitively in 1-min intervals by citric acid challenges (0.1 or 1 M in 100-μl aliquots) or by 5 min continuous electrical stimulation (16 Hz, 1-ms pulse duration) of the tracheal mucosa. A: representative trace showing coughs evoked by 0.1 M citric acid challenges delivered repetitively at 1-min intervals. By the 8th consecutive challenge, few if any coughs were observed. B: mean ± SE coughs evoked by repetitive citric acid challenges (n = 8/treatment group). C: representative trace showing the repetitive coughing evoked initially by electrical stimulation of the tracheal mucosa but the rapid onset of desensitization and cessation of coughing despite continued stimulation. D: mean ± SE coughs evoked in 15- to 30-s epochs during the continuous electrical stimulation of the tracheal mucosa (n = 6). *Significant reduction in response relative to that evoked at the initial time points studied (P < 0.05).
Pharmacology of cough receptor signaling and interactions with pulmonary stretch receptors.
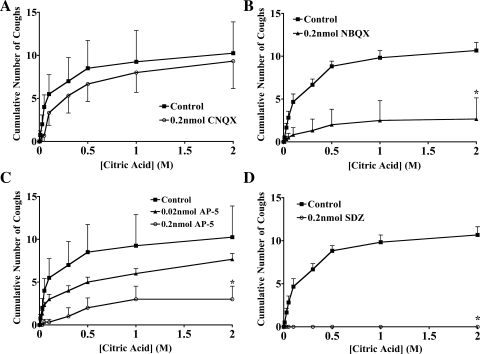
Contrary to most if not all previously published studies of airway afferent nerve signaling in nTS, our results suggest a predominant role for NMDA receptor activation during cough and a modulatory role for non-NMDA receptors. Thus CNQX microinjection at the likely sites of cough receptor termination did not inhibit citric acid-evoked coughing while NBQX inhibited but did not abolish the cough responses. By contrast, AP-5 and SDZ-220581 inhibited and even abolished citric acid-evoked coughing while having no effect on basal respiratory rate (Fig. 6). When both NMDA and non-NMDA receptor antagonists were administered, a synergistic inhibition of evoked coughing was observed. Thus, whereas neither 0.02 nmol AP-5 nor 0.02 nmol CNQX (0.01 nmol bilaterally) reduced the number of coughs evoked cumulatively by citric acid (0.001–2 M citric acid; Fig. 6), when administered simultaneously, they essentially abolished citric acid-evoked coughing [11 ± 1 vs. 1 ± 1 coughs following microinjection of vehicle (n = 10) or AP-5 and CNQX (n = 3), respectively; P < 0.05].
Fig. 6.
Inhibition of citric acid evoked coughing by microinjection of glutamate N-methyl-d-aspartate (NMDA) and non-NMDA receptor antagonists. Citric acid was applied topically to the tracheal mucosa in ascending concentrations (0.001–2 M) in animals first microinjected with vehicle (n = 4–8) or CNQX (n = 3; A), NBQX (n = 6; B), amino-5-phosphovaleric acid (AP-5, n = 3–4; C), or SDZ-220581 (n = 3; D). Microinjections were delivered bilaterally in nucleus tractus solitarius (nTS) locations identified as primary sites of cough receptor termination (7). All of the results described are presented as means ± SE of 3–8 experiments. *Significant reduction in cumulative coughs evoked relative to control experiments (P < 0.05).
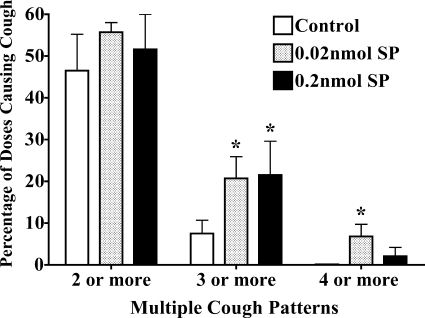
We had reported previously that jugular C-fiber activation from the airways sensitizes the cough reflex such that significantly lower stimulation voltages were required to evoke cough from the tracheal mucosa (34). This effect of airway jugular C-fiber activation was prevented by neurokinin receptor antagonists and mimicked by substance P microinjection in the commissural subnucleus of nTS. We speculated that this sensitization may be due to the convergence of jugular C-fibers and the cough receptors on a single population of nTS relay neurones. At the time of those previous experiments, we had little specific information about the likely sites of termination of the cough receptors. Guided by our previous study in which tracheal cough receptor termination was found to be in nTS nuclei rostral and lateral to the commissural subnucleus (7), we repeated our previous studies with substance P microinjection (Fig. 7). We used citric acid to evoke coughing. As with our previous studies, we found that coughing could be enhanced by substance P microinjection. The enhancement of coughing produced by substance P microinjection was apparent by analysis of the number of cough challenges that resulted in multiple coughs. Substance P microinjection increased by approximately threefold the number of citric acid challenges that evoked three or more coughs and resulted in several instances of four coughs in response to a single citric acid challenge. Vehicle injections in this set of experiments were associated with no single citric acid challenge causing four or more coughs. The threshold concentration evoking cough and the number of citric acid doses evoking cough were not statistically significantly altered by substance P microinjection. At the location targeted, substance P microinjection was also without effect on respiratory rate.
Fig. 7.
Modulation of citric acid-evoked coughing by substance P (SP) microinjection. Citric acid was applied topically to the tracheal mucosa in ascending concentrations (0.001–2 M) in animals first microinjected with vehicle or substance P. Microinjections were delivered bilaterally in nTS locations identified as primary sites of cough receptor termination (7). The no. of doses of citric acid evoking cough was unaffected by substance P microinjection [citric acid (9 doses from 0.001 to 2 M) evoked coughing in 5.6 ± 0.5, 6.1 ± 0.4, and 5.3 ± 0.6 out of 9 challenge doses in preparations microinjected with vehicle (n = 13), 0.02 nmol substance P (n = 10), and 0.2 nmol substance P (n = 8), respectively; P > 0.1]. By contrast, when coughing occurred, the no. of coughs/dose of citric acid was increased significantly. Substance P microinjection was without effect on respiratory rate in these studies (just before citric acid challenge, respiratory rate averaged 67 ± 4, 63 ± 4, and 59 ± 3 breaths/min in preparations microinjected with vehicle, 0.02 nmol substance P, and 0.2 nmol substance P, respectively). Data are presented as means ± SE of 8–13 experiments. *Statistically significant enhancement in response relative to control (P < 0.05).
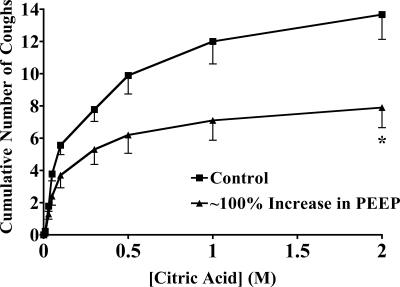
Not all afferent nerve interactions with cough receptor signaling promote or enhance coughing. In a separate study, we have found that nodose C-fiber activation profoundly inhibits citric acid-evoked coughing in anesthetized guinea pigs (Y. L. Chou and B. J. Canning, unpublished observations). Similar inhibitory interactions have been reported between rapidly and slowly adapting receptor (SAR) relay neurones (15, 16). We studied the effects of stretch receptor activation on citric acid-evoked coughing in anesthetized guinea pigs (Fig. 8). Increasing end-expiratory pressures by ∼100% reduced respiratory rate and significantly reduced citric acid-evoked coughing. A further increase in end-expiratory pressure to ∼200% of baseline produced no further decrease in respiratory rate or reduction in citric acid-evoked coughing.
Fig. 8.
Citric acid evoked coughing in the absence and presence of positive end-expiratory pressure (PEEP) created by attaching an air pump to a side port of the artificial nose of the preparation. Citric acid was applied topically to the tracheal mucosa in ascending concentrations (0.001–2 M) in animals breathing with minimal (1–2 cmH2O) PEEP or breathing with an elevated PEEP (122 ± 14% of peak expiratory pressure at baseline). Respiratory rate decreased from 73 ± 4 to 62 ± 3 breaths/min with the application of PEEP. *Statistically significant decrease in cumulative coughs. All of the results described are presented as means ± SE of 10–13 experiments.
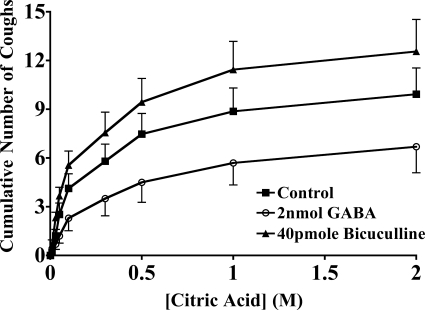
The inhibitory effects of SAR relay neurones on rapidly adapting receptor (RAR) relay neurone excitability have been attributed to the actions of GABAergic pump cells (15). We attempted to evaluate the role of GABA in cough (Fig. 9). Bilateral bicuculline microinjection had modest but variable effects on citric acid-induced coughing. The GABAA antagonist did significantly decrease the threshold for citric acid-evoked coughing but failed to modify any other aspect of the cough response. The effects of GABA microinjection on citric acid-evoked coughing were also highly variable and ultimately failed to produce any statistically significant effect on any measure of citric acid-evoked coughing. However, the GABAB receptor selective agonist baclofen (50 pmol/injection) markedly inhibited coughing when microinjected bilaterally in the nTS of anesthetized guinea pigs (10 ± 2 vs. 2 ± 1 coughs in control and baclofen-treated animals, respectively; n = 5–6 each; P < 0.05).
Fig. 9.
The role of γ-aminobutyric acid (GABA) in modulating citric acid-evoked coughing. Citric acid was applied topically to the tracheal mucosa in ascending concentrations (0.001–2 M) in animals first microinjected with vehicle, bicuculline, or GABA. Microinjections were delivered bilaterally in nTS locations identified as primary sites of cough receptor termination (7). Bicuculline produced a statistically significant increase in the no. of coughs evoked by threshold concentrations of citric acid. Thus the first 3 doses of citric acid evoked 0.9 ± 0.3 and 2.3 ± 0.3 coughs following microinjection of vehicle (n = 8) or bicuculline (n = 9), respectively (P < 0.05). Indeed, 4 of 9 and 9 of 9 (P < 0.05) animals microinjected with bicuculline coughed in response to 0.01 and 0.03 citric acid, respectively, whereas only 0 of 8 and just 4 of 8 animals microinjected with vehicle coughed in response to 0.01 and 0.03 M citric acid, respectively. Overall, however, bicuculline was without effect on the cumulative no. of coughs evoked by citric acid. The effects of GABA were highly variable and failed to reach statistical significance. By contrast, microinjection of the GABAB agonist baclofen nearly abolished citric acid-evoked coughing (10 ± 2 vs. 2 ± 1 coughs in control and baclofen-treated animals, respectively; n = 5–6 each; P < 0.05). None of these interventions had an effect on respiratory rate (data not shown). The results are presented as means ± SE of 9–15 experiments.
DISCUSSION
Coughing is unique among bronchopulmonary reflexes in that it is highly dependent on threshold regulation and requires an epileptiform-like change in respiratory motor neuron activity to produce the characteristic enhanced inspiratory and expiratory efforts, separated by glottis closing during the compressive phase of the reflex (3, 4). All of these phases occur at an accelerated rate relative to those of a tidal breath, and the entire motor pattern can be differentiated from that of a sigh, gasp, or sneeze. Coughing is also heavily influenced by consciousness. Humans can create a cough motor pattern independent of any tussive stimulus or can willfully and entirely prevent coughing in response to suprathreshold stimulation (13, 52). These characteristics imply a complex regulation involving reconfiguration of inspiratory and expiratory motorneurone firing patterns, integration of peripheral sensory input and cortical inputs related to perception and emotion, gating mechanisms that regulate the timing, forcefulness, repetitions, and termination of cough efforts, and peripheral regulation of afferent nerve action potential patterning driving cough.
Studying the reconfiguration processes required to change a respiratory motor pattern to a cough motor pattern is not amenable to reduced methods of unitary electrophysiological analysis. The all-important timing of activation and inactivation among the various regulatory elements is lost with unitary analysis and can only be discerned with multichannel recordings and modeling (3, 49, 50). However, understanding the integration of synaptic input within each regulatory element may help develop more complex and realistic models of cough motor pattern generation (47). Insights into how coughing changes in disease and with therapy may also be easier to evaluate in the more reduced settings of in vitro experimentation or with in vivo recordings from single units within the various components of the cough reflex circuitry. We set out to establish some characteristic features of synaptic regulation at the central terminals of the cough receptors.
We have identified the likely central and peripheral termination sites of tracheal and laryngeal cough receptors in nTS (7, 35). We have also described various attributes of the encoding of cough that may be regulated in this population of primary vagal afferent synapses (5, 6, 34). Knowing in advance these attributes will help subsequent, more mechanistic studies of the transduction and encoding of cough. Although the validity of our conclusions about the pharmacology and physiology of synaptic transmission at the primary central synapse of the cough receptors awaits a more direct analysis, we speculate that the results presented are an accurate depiction of the synaptic process at the central terminals of the cough receptors.
Summation and sensitization of cough receptor input.
We found evidence for both summation and sensitization of cough receptor actions. Summation was apparent in frequency-response analysis and by quantifying the effects of unilateral vagus or RLN transection on cough. These interventions accomplish similar effects but by different means, with altered electrical stimulation frequencies targeting the activity/unit with a constant number of units activated while unilateral nerve cuts preserve a constant activity/unit level but reduce by roughly 50% the number of active units. Summation is not a particularly novel attribute of the cough reflex, but, because it can alter stimulus thresholds for evoking cough, it illustrates potential modes of altering the cough reflex in disease and with therapeutic intervention. Given the profound differences in coughing evoked by electrical stimulation at 8 and 16 Hz, we speculate that antitussive therapy need not completely anesthetize airway afferent nerves or block entirely synaptic transmission at nTS to have profound effects on coughing frequency and intensity, or perceived urge to cough.
Sensitization of the cough reflex has been observed following allergen challenge, cigarette smoke exposure, and challenge with autacoids such as bradykinin, thromboxane, histamine, and ATP (14, 17, 18, 23–25, 30, 31, 54). These effects have been attributed at least in part to an effect on afferent nerve excitability. We have also shown sensitization of cough evoked in anesthetized guinea pigs following activation of capsaicin-sensitive C-fibers innervating the airways and lungs (34). This effect of C-fiber activation could be mimicked by microinjection of either capsaicin or substance P in the commissural subnucleus of nTS and could be reversed by centrally administered neurokinin receptor antagonists. Similar results have been reported elsewhere (23, 40). We repeated the experiments with substance P microinjection in the present study, targeting the primary central termination sites of the cough receptors. As in our previous study, substance P again enhanced cough reflex sensitivity. These results are consistent with the notion that cough receptors and bronchopulmonary C-fibers may converge centrally in nTS and act synergistically to initiate coughing.
We observed another form of sensitization. Subthreshold cough receptor activation by electrical stimulation (8 Hz) or with citric acid (≤0.03 M) was generally ineffective at evoking cough but enhanced by three- to fourfold the number of coughs evoked when a suprathreshold stimulus was applied. A similar potentiation was apparent with repetitive suprathreshold electrical stimulation. This process is different from wind up in that it may not be C-fiber dependent and requires higher frequencies than that used to produce wind up in the spinal cord (53). However, the effects are quite pronounced and may, along with the convergent interactions described above, have relevance to the encoding of cough. The prominent role of NMDA receptor signaling in the encoding of cough may contribute to the appearance of this stimulus-evoked potentiation.
NMDA receptor regulation of the cough reflex: potential role in desensitization.
With each breath in animals as different as sheep, dogs, guinea pigs, pigeons, and lizards, lung stretch receptors regulate respiratory rate by signaling the brain stem information about lung volume and stretch (1, 9, 26, 48). Cutting the vagi in most animals results in an immediate and precipitous fall in respiratory rate coupled with a marked increase in tidal volume. RARs, especially those in smaller animals with higher basal respiratory rates, also signal information about lung volume, inflation rates, and stretch and tonically influence both respiratory rate and autonomic outflow to the airways (12, 19, 27). Even bronchopulmonary C-fibers, aortic baroreceptors, and peripheral chemoreceptors tonically transmit information to the brain, regulating cardiopulmonary autonomic tone as well as respiration (10, 29, 41, 45, 46). Signaling at the central synapses of these afferent nerves is known to be primarily non-NMDA receptor-dependent and must be largely protected from desensitization. It is thus a striking observation from the present study that signaling triggered by cough receptor activation very rapidly desensitizes and likely does so through effects in the central nervous system (CNS).
We speculate that the profound desensitization associated with repetitive tussive challenge may be related to the prominent role of NMDA receptors in the encoding of cough in the nTS (present study and Refs. 39 and 40). Unlike non-NMDA receptor signaling, NMDA receptor function is characterized by regulatory mechanisms, including Mg2+ block, Ca2+-dependent sensitization/unblocking, and nitric oxide-mediated desensitization processes that determine the variable role of this receptor subtype in regulating synaptic transmission in the CNS (8, 37, 38). A nitric oxide-dependent desensitization would explain the rapid loss of cough responsiveness to a variety of stimuli (acid, 4-AP, electrical stimulation) that have been shown in previous studies to produce little if any afferent nerve desensitization (28, 36). Regardless of the mechanism, the process of desensitization may be every bit as relevant to changes in cough presentation in disease as would be sensitization, either peripherally or centrally mediated.
Afferent nerve interactions and the encoding of cough.
We have shown previously that C-fiber activation acts synergistically with other airway afferent nerve subpopulations to initiate coughing and reflex bronchospasm (32, 34). In these studies, the data suggested that C-fibers were not able to initiate these reflex effects independently but produced changes in cough and airway autonomic outflow by amplifying the actions of other airway afferent nerves. A similar permissive role for slowly adapting stretch receptors in cough has been proposed based on the results of studies carried out in rabbits (20). In these studies, the authors took advantage of the peculiar sensitivity of rabbit SARs to an acute, toxic effect of sulfur dioxide. When SAR activity was diminished and/or eliminated, subsequent attempts at evoking cough were less effective. From these results, the authors came to the reasonable conclusion that basal SAR activity is permissive to subsequently evoked coughing.
We have found no relationship between respiratory rate, which is highly correlated with SAR activity, and cough responsiveness (5). We also report here that increasing end-expiratory pressure reduces coughing evoked subsequently by citric acid challenge. The increase in end-expiratory pressure was accompanied by a decrease in respiratory rate, consistent with an enhanced activation of pulmonary stretch receptors. At a minimum, then, we find no evidence for a permissive effect of SARs in cough. Other studies have also struggled to find evidence supportive of the notion that SARs are permissive in cough (42, 43, 51). If indeed SAR activation can inhibit coughing, it would not be an unprecedented interaction between airway afferent nerve subtypes. SARs have been shown to tonically regulate RAR relay neurone excitability (16). This inhibitory effect of SAR activation is dependent on a descending projection of GABAergic pump cells to RAR relay neurones in the commissural subnucleus of nTS (15). We found that bicuculline microinjection modestly enhanced coughing at threshold acid concentrations while the GABAB-selective agonist baclofen dose-dependently inhibits citric acid-evoked coughing upon microinjection. GABA microinjection had inconsistent effects on cough, which could reflect a combination of inhibitory actions on relay neurones but perhaps counteracted by excitatory effects on the cough receptor terminals (5).
Implications for cough in disease and for therapeutic interventions.
The results of this and our previous studies highlight key elements of experimental design when evaluating interventions that are expected to modulate coughing. Anesthesia is often necessary when studying the cough reflex, but anesthesia modifies coughing, especially C-fiber-dependent cough (5, 6, 9). Anesthesia prevents C-fiber-dependent cough. Also, once threshold levels of stimulation are attained with acid challenge, the coughing evoked in anesthetized animals becomes stimulus intensity-independent. This may be peripherally regulated, perhaps because of the involvement of rapidly inactivating acid-sensing ion channels in the response to acid (5, 28). Central mechanisms also likely play a role, as shown in the present study. Interventions that are expected to enhance coughing are thus competing against regulatory processes that actively inhibit repetitive coughing events. Analysis might then focus on changes in threshold sensitivity or on the number of doses evoking repetitive coughing events, as opposed to the cumulative number of coughs over a complete concentration-response curve. A similar approach has been employed in studying cough evoked by mechanical stimulation of the airway mucosa (21). With interventions expected to inhibit coughing, changes in peak afferent nerve frequency seem to be more important than the total number of action potentials evoked. Similarly, interventions acting centrally to inhibit cough may have profound effects on coughing and yet only subtle effects on nTS relay neurone excitation. This may serve as a cautionary note for drug development, to avoid dismissing the utility of a therapeutic entity based on its modest effects on peak action potential frequencies and/or modest effects on synaptic transmission.
That coughing is preceded by an urge to cough could have been predicted from studies in which summation of afferent inputs define sub- and suprathreshold stimulus intensities for the initiation of cough. These observations predict mechanisms of enhanced or depressed coughing in disease and a rationale for therapeutic intervention. Even a subtle increase in afferent drive or responsiveness to afferent drive can create a greatly enhanced cough response. It follows logically that therapeutics or diseases that decrease, even modestly, afferent drive or responsiveness to afferent drive could render a chronic, problematic cough with frequent bouts of coughing more tolerable and potentially absent entirely. This also predicts utility of therapeutics that target the especially high stimulation frequencies required for cough initiation, perhaps by reducing peak activation frequency of the cough receptors, or use dependent blocking of NMDA receptor signaling, which differentially inhibits high-frequency activation (8, 44).
DISCLOSURES
No conflicts of interest are declared by the authors.
ACKNOWLEDGMENTS
The research described in this manuscript was funded by National Heart, Lung, and Blood Institute Grant HL-083192.
REFERENCES
- 1. Al-Ghamdi MS, Jones JF, Taylor EW. Evidence of a functional role in lung inflation for the buccal pump in the agamid lizard, Uromastyx aegyptius microlepis. J Exp Biol 204: 521–531, 2001 [DOI] [PubMed] [Google Scholar]
- 2. Bolser DC. Fictive cough in the cat. J Appl Physiol 71: 2325–2331, 1991 [DOI] [PubMed] [Google Scholar]
- 3. Bolser DC, Poliacek I, Jakus J, Fuller DD, Davenport PW. Neurogenesis of cough, other airway defensive behaviors and breathing: A holarchical system? Respir Physiol Neurobiol 152: 255–265, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Canning BJ. Encoding of the cough reflex. Pulm Pharmacol Ther 20: 396–401, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Canning BJ, Farmer DG, Mori N. Mechanistic studies of acid-evoked coughing in anesthetized guinea pigs. Am J Physiol Regul Integr Comp Physiol 291: R454–R463, 2006 [DOI] [PubMed] [Google Scholar]
- 6. Canning BJ, Mazzone SB, Meeker SN, Mori N, Reynolds SM, Undem BJ. Identification of the tracheal and laryngeal afferent neurones mediating cough in anaesthetized guinea-pigs. J Physiol 557: 543–558, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Canning BJ, Mori N. Central terminations of the afferent nerves regulating cough in guinea pigs. FASEB J 24: 3916–3926, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen HS, Lipton SA. The chemical biology of clinically tolerated NMDA receptor antagonists. J Neurochem 97: 1611–1626, 2006 [DOI] [PubMed] [Google Scholar]
- 9. Chou YL, Scarupa MD, Mori N, Canning BJ. Differential effects of airway afferent nerve subtypes on cough and respiration in anesthetized guinea pigs. Am J Physiol Regul Integr Comp Physiol 295: R1572–R1584, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Coleridge HM, Coleridge JC, Schultz HD. Afferent pathways involved in reflex regulation of airway smooth muscle. Pharmacol Ther 42: 1–63, 1989 [DOI] [PubMed] [Google Scholar]
- 11. Davenport PW. Clinical cough I: the urge-to-cough: a respiratory sensation. Handb Exp Pharmacol 187: 263–276, 2009 [DOI] [PubMed] [Google Scholar]
- 12. Davies A, Sant'Ambrogio F, Sant'Ambrogio G. Onset of inspiration in rabbit during artificial ventilation. J Physiol 318: 17–23, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Eccles R. The powerful placebo in cough studies? Pulm Pharmacol Ther 15: 303–308, 2002 [DOI] [PubMed] [Google Scholar]
- 14. El-Hashim AZ, Amine SA. The role of substance P and bradykinin in the cough reflex and bronchoconstriction in guinea-pigs. Eur J Pharmacol 513: 125–133, 2005 [DOI] [PubMed] [Google Scholar]
- 15. Ezure K, Tanaka I. GABA, in some cases together with glycine, is used as the inhibitory transmitter by pump cells in the Hering-Breuer reflex pathway of the rat. Neuroscience 127: 409–417, 2004 [DOI] [PubMed] [Google Scholar]
- 16. Ezure K, Tanaka I. Lung inflation inhibits rapidly adapting receptor relay neurons in the rat. Neuroreport 11: 1709–1712, 2000 [DOI] [PubMed] [Google Scholar]
- 17. Fox AJ, Lalloo UG, Belvisi MG, Bernareggi M, Chung KF, Barnes PJ. Bradykinin-evoked sensitization of airway sensory nerves: a mechanism for ACE-inhibitor cough. Nat Med 2: 814–817, 1996 [DOI] [PubMed] [Google Scholar]
- 18. Gatti R, Andre E, Amadesi S, Dinh TQ, Fischer A, Bunnett NW, Harrison S, Geppetti P, Trevisani M. Protease-activated receptor-2 activation exaggerates TRPV1-mediated cough in guinea pigs. J Appl Physiol 101: 506–511, 2006 [DOI] [PubMed] [Google Scholar]
- 19. Green JF, Kaufman MP. Pulmonary afferent control of breathing as end-expiratory lung volume decreases. J Appl Physiol 68: 2186–2194, 1990 [DOI] [PubMed] [Google Scholar]
- 20. Hanacek J, Davies A, Widdicombe JG. Influence of lung stretch receptors on the cough reflex in rabbits. Respiration 45: 161–168, 1984 [DOI] [PubMed] [Google Scholar]
- 21. House A, Celly C, Skeans S, Lamca J, Egan RW, Hey JA, Chapman RW. Cough reflex in allergic dogs. Eur J Pharmacol 492: 251–258, 2004 [DOI] [PubMed] [Google Scholar]
- 22. Jakus J, Poliacek I, Halasova E, Murin P, Knocikova J, Tomori Z, Bolser DC. Brainstem circuitry of tracheal-bronchial cough: c-fos study in anesthetized cats. Respir Physiol Neurobiol 160: 289–300, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Joad JP, Munch PA, Bric JM, Evans SJ, Pinkerton KE, Chen CY, Bonham AC. Passive smoke effects on cough and airways in young guinea pigs: role of brainstem substance P. Am J Respir Crit Care Med 169: 499–504, 2004 [DOI] [PubMed] [Google Scholar]
- 24. Kamei J, Takahashi Y. Involvement of ionotropic purinergic receptors in the histamine-induced enhancement of the cough reflex sensitivity in guinea pigs. Eur J Pharmacol 547: 160–164, 2006 [DOI] [PubMed] [Google Scholar]
- 25. Karlsson JA, Zackrisson C, Lundberg JM. Hyperresponsiveness to tussive stimuli in cigarette smoke-exposed guinea-pigs: a role for capsaicin-sensitive, calcitonin gene-related peptide-containing nerves. Acta Physiol Scand 141: 445–454, 1991 [DOI] [PubMed] [Google Scholar]
- 26. Kashani M, Haigh AL. The effects of vagotomy on ventilation and blood gas composition in dog, sheep and rabbit. Q J Exp Physiol Cogn Med Sci 60: 285–298, 1975 [DOI] [PubMed] [Google Scholar]
- 27. Kesler BS, Canning BJ. Regulation of baseline cholinergic tone in guinea-pig airway smooth muscle. J Physiol 518: 843–855, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kollarik M, Undem BJ. Mechanisms of acid-induced activation of airway afferent nerve fibres in guinea-pig. J Physiol 543: 591–600, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kumar P, Prabhakar N. Sensing hypoxia: carotid body mechanisms and reflexes in health and disease. Respir Physiol Neurobiol 157: 1–3, 2007 [DOI] [PubMed] [Google Scholar]
- 30. Lewis CA, Ambrose C, Banner K, Battram C, Butler K, Giddings J, Mok J, Nasra J, Winny C, Poll C. Animal models of cough: literature review and presentation of a novel cigarette smoke-enhanced cough model in the guinea-pig. Pulm Pharmacol Ther 20: 325–333, 2007 [DOI] [PubMed] [Google Scholar]
- 31. Liu Q, Fujimura M, Tachibana H, Myou S, Kasahara K, Yasui M. Characterization of increased cough sensitivity after antigen challenge in guinea pigs. Clin Exp Allergy 31: 474–484, 2001 [DOI] [PubMed] [Google Scholar]
- 32. Mazzone SB, Canning BJ. Synergistic interactions between airway afferent nerve subtypes mediating reflex bronchospasm in guinea pigs. Am J Physiol Regul Integr Comp Physiol 283: R86–R98, 2002 [DOI] [PubMed] [Google Scholar]
- 33. Mazzone SB, McGovern AE. Immunohistochemical characterization of nodose cough receptor neurons projecting to the trachea of guinea pigs (Abstract). Cough 4: 9, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mazzone SB, Mori N, Canning BJ. Synergistic interactions between airway afferent nerve subtypes regulating the cough reflex in guinea-pigs. J Physiol 569: 559–573, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mazzone SB, Reynolds SM, Mori N, Kollarik M, Farmer DG, Myers AC, Canning BJ. Selective expression of a sodium pump isozyme by cough receptors and evidence for its essential role in regulating cough. J Neurosci 29: 13662–13671, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. McAlexander MA, Undem BJ. Potassium channel blockade induces action potential generation in guinea-pig airway vagal afferent neurones. J Auton Nerv Syst 78: 158–164, 2000 [DOI] [PubMed] [Google Scholar]
- 37. McBain CJ, Traynelis SF. Malevolent lurkers no more: NMDA receptors come of age. J Physiol 575: 317–318, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mustafa AK, Kumar M, Selvakumar B, Ho GP, Ehmsen JT, Barrow RK, Amzel LM, Snyder SH. Nitric oxide S-nitrosylates serine racemase, mediating feedback inhibition of D-serine formation. Proc Natl Acad Sci USA 104: 2950–2955, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mutolo D, Bongianni F, Cinelli E, Fontana GA, Pantaleo T. Modulation of the cough reflex by antitussive agents within the caudal aspect of the nucleus tractus solitarii in the rabbit. Am J Physiol Regul Integr Comp Physiol 295: R243–R251, 2008 [DOI] [PubMed] [Google Scholar]
- 40. Mutolo D, Bongianni F, Fontana GA, Pantaleo T. The role of excitatory amino acids and substance P in the mediation of the cough reflex within the nucleus tractus solitarii of the rabbit. Brain Res Bull 74: 284–293, 2007 [DOI] [PubMed] [Google Scholar]
- 41. Nadel JA, Widdicombe JG. Effect of changes in blood gas tensions and carotid sinus pressure on tracheal volume and total lung resistance to airflow. J Physiol 163: 13–33, 1962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nishino T, Hasegawa R, Ide T, Isono S. Hypercapnia enhances the development of coughing during continuous infusion of water into the pharynx. Am J Respir Crit Care Med 157: 815–821, 1998 [DOI] [PubMed] [Google Scholar]
- 43. Nishino T, Sugimori K, Hiraga K, Hond Y. Influence of CPAP on reflex responses to tracheal irritation in anesthetized humans. J Appl Physiol 67: 954–958, 1989 [DOI] [PubMed] [Google Scholar]
- 44. Paoletti P, Neyton J. NMDA receptor subunits: function and pharmacology. Curr Opin Pharmacol 7: 39–47, 2007 [DOI] [PubMed] [Google Scholar]
- 45. Pilowsky PM, Goodchild AK. Baroreceptor reflex pathways and neurotransmitters: 10 years on. J Hypertens 20: 1675–1688, 2002 [DOI] [PubMed] [Google Scholar]
- 46. Pisarri TE, Yu J, Coleridge HM, Coleridge JC. Background activity in pulmonary vagal C-fibers and its effects on breathing. Respir Physiol 64: 29–43, 1986 [DOI] [PubMed] [Google Scholar]
- 47. Poliacek I, Corrie LW, Wang C, Rose MJ, Bolser DC. Microinjection of DLH into the region of the caudal ventral respiratory column in the cat: evidence for an endogenous cough-suppressant mechanism. J Appl Physiol 102: 1014–1021, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Richards SA. Vagal control of thermal panting in mammals and birds. J Physiol 199: 89–101, 1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Shannon R, Baekey DM, Morris KF, Li Z, Lindsey BG. Functional connectivity among ventrolateral medullary respiratory neurones and responses during fictive cough in the cat. J Physiol 525: 207–224, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Shannon R, Baekey DM, Morris KF, Lindsey BG. Ventrolateral medullary respiratory network and a model of cough motor pattern generation. J Appl Physiol 84: 2020–2035, 1998 [DOI] [PubMed] [Google Scholar]
- 51. Suzuki H, Kondo T, Yamabayashi H, Kobayashi I, Ohta Y. Influence of central respiratory activity on the cough response in anesthetized dogs. Jpn J Physiol 41: 879–891, 1991 [DOI] [PubMed] [Google Scholar]
- 52. Widdicombe J, Eccles R, Fontana G. Supramedullary influences on cough. Respir Physiol Neurobiol 152: 320–328, 2006 [DOI] [PubMed] [Google Scholar]
- 53. Woolf CJ. Windup and central sensitization are not equivalent. Pain 66: 105–108, 1996 [PubMed] [Google Scholar]
- 54. Xiang A, Uchida Y, Nomura A, Iijima H, Sakamoto T, Ishii Y, Morishima Y, Masuyama K, Zhang M, Hirano K, Sekizawa K. Involvement of thromboxane A(2) in airway mucous cells in asthma-related cough. J Appl Physiol 92: 763–770, 2002 [DOI] [PubMed] [Google Scholar]