Abstract
The diaphragm, the main muscle of inspiration, is constantly subjected to mechanical loading. One of the very few occasions during which diaphragm loading is arrested is during controlled mechanical ventilation in the intensive care unit. Recent animal studies indicate that the diaphragm is extremely sensitive to unloading, causing rapid muscle fiber atrophy: unloading-induced diaphragm atrophy and the concomitant diaphragm weakness has been suggested to contribute to the difficulties in weaning patients from ventilatory support. Little is known about the molecular triggers that initiate the rapid unloading atrophy of the diaphragm, although proteolytic pathways and oxidative signaling have been shown to be involved. Mechanical stress is known to play an important role in the maintenance of muscle mass. Within the muscle's sarcomere titin is considered to play an important role in the stress-response machinery. Titin is the largest protein known to date and acts as a mechanosensor that regulates muscle protein expression in a sarcomere strain-dependent fashion. Thus, titin is an attractive candidate for sensing the sudden mechanical arrest of the diaphragm when patients are mechanically ventilated, leading to changes in muscle protein expression. Here, we provide a novel perspective on how titin, and its biomechanical sensing and signaling, might be involved in the development of mechanical unloading-induced diaphragm weakness.
Keywords: respiratory muscle disuse, connectin, muscle wasting
the diaphragm is the main muscle of respiration. It contracts during each breathing cycle, thereby changing the anatomic configurations of the chest wall so that air flows into the lungs. Thus, the diaphragm is indispensable for life and is constantly subjected to mechanical loading.
Recent studies show that the diaphragm is remarkably sensitive to changes in mechanical loading. For instance, elevated diaphragm loading may cause profound diaphragm atrophy, injury, and weakness (25, 39, 40, 45, 52). Conversely, unloading of the diaphragm, as occurs in patients in the intensive care unit during controlled mechanical ventilation, also rapidly affects the inspiratory muscles. Levine et al. (31) revealed that brain-dead patients develop >50% atrophy of diaphragm fibers after only several days of mechanical ventilation, whereas non-respiratory muscles were unaffected. Mechanical ventilation-induced atrophy compromises the capacity of the diaphragm to generate force and contributes significantly to the difficulties in weaning these patients from ventilatory support (5). Weaning failure represents a large financial burden and is associated with several side effects including ventilator-associated pneumonia and thrombosis (23). Accordingly, clinicians aim to liberate patients from the ventilator as soon as possible.
Little is known about the molecular triggers that initiate the rapid disuse atrophy of the diaphragm. Although ventilation-induced systemic effects cannot be ruled out (26), the majority of existing data points toward local mediators within the diaphragm. For instance, mechanical ventilation induces oxidative stress in the diaphragm, which enhances proteolysis leading to atrophy and muscle weakness (3, 50). Here, we postulate that the reduction of mechanical stress per se during mechanical ventilation might be involved as well, with a mediating role for the giant sarcomeric protein titin.
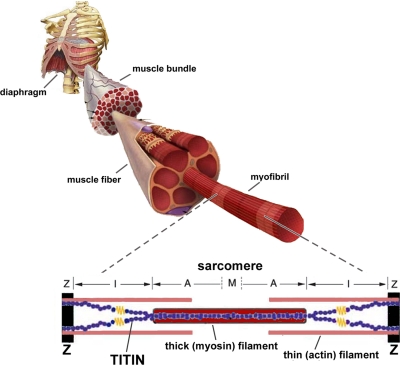
Titin is the largest protein known to date (3–4 MDa) (28) and spans the entire length of the sarcomere from Z-disk to M-band (Fig. 1). The central I-band region of titin is extensible and functions as a spring element that gives rise to the development of passive tension when a sarcomere is stretched. The COOH-terminal region of titin is located in the A-band, is inextensible, and contains near the M-band a kinase domain. As in the Z-disk, where titin filaments from opposite sarcomeres overlap, titin filaments from opposite half-sarcomeres overlap within the M-band, where they are interconnected by M-band proteins. Thus, titin filaments with opposite polarity overlap in both Z-disk and M-band, forming a contiguous filament along the myofibril. This layout of titin within the muscle's sarcomere makes it ideally suited to sense changes in mechanical loading. Indeed, various lines of research now suggest that titin plays an important role in the stress-response machinery. Below, we highlight these properties of titin, to build a framework for a novel, titin-based, perspective on ventilator-induced diaphragm weakness.
Fig. 1.
Model of the sarcomere, the smallest contractile unit of striated muscle. The sarcomere comprises 3 major filaments: the thin (mostly actin) filaments, the thick (mostly myosin) filaments, and the giant filamentous molecule titin. The thin filaments are anchored in the Z-disk, where they are cross-linked by α-actinin. The thick filaments are centrally located in the sarcomere and constitute the sarcomeric A-band. The myosin heads, or cross-bridges, on the thick filament interact with actin during activation. Titin spans the half-sarcomeric distance from the Z-band to the M-band, thus forming a third sarcomeric filament. In the I-band region, titin is extensible and functions as a molecular spring that develops passive tension upon stretch. In the A-band, titin is inextensible due to its strong interaction with the thick filament.
Titin acts as a mechanosensor.
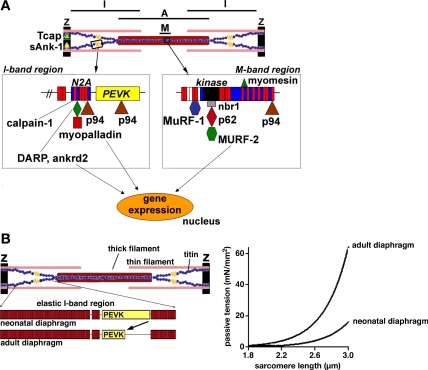
Recent studies provide evidence for the notion that titin acts as a mechanosensor that regulates protein expression in a sarcomere strain-dependent fashion (29, 47). This makes titin an attractive candidate for sensing the sudden mechanical arrest of the diaphragm during mechanical ventilation, leading to changes in muscle protein synthesis. Myosin synthesis is markedly reduced already 6 h after onset of controlled mechanical ventilation (15, 49). A central role in the regulation of protein synthesis has been ascribed to Akt, which can be activated through phosphorylation downstream of IGF1 to induce hypertrophy (48). Both IGF1 (17) and phosphorylated Akt (34) have been shown to be downregulated in the diaphragm of ventilated rats, suggesting a role for the Akt pathway in reducing protein synthesis in the diaphragm during mechanical ventilation. A downstream target of Akt is the serum response transcription factor (SRF) (57). SRF serves as a versatile protein that regulates gene expression and muscle hypertrophy (32). In addition to being a target of Akt signaling, SRF links titin-based signaling at the sarcomere's M-line to protein turnover in a muscle load-dependent fashion (29). At the periphery of the M-line, titin contains a serine/threonine kinase domain (TK) (16), which is ideally positioned to sense mechanical load. A signaling complex has been identified (see Fig. 2A) that interacts with the titin kinase, consisting of the zinc-finger protein nbr1, which interacts with the TK through a mechanically inducible conformation and that targets the ubiquitin-associated p62 to sarcomeres (29). p62 in turn interacts with MuRF2, a muscle-specific RING-B-box protein and ligand of the transactivation domain of the SRF. Importantly, nuclear translocation of MuRF2 was induced by mechanical inactivity and caused reduction of nuclear SRF and repression of transcription. Known SRF target genes include the sarcomeric proteins actin (6) and myosin heavy chain IIa (1), as well as IGF1 (8). Notably, a human mutation in the titin kinase has been shown to disrupt this titin kinase-signaling complex leading to muscle disease with early involvement of the diaphragm, causing respiratory failure as a frequent cause of death (29). Thus, an attractive hypothesis is that the abrupt arrest of diaphragm loading in mechanically ventilated patients impairs protein turnover due to effects on titin kinase signaling. Finally, it is worth highlighting that during controlled mechanical ventilation, the diaphragm is not only unloaded but also continuously passively shortened from its resting length and then restretched. In contrast to the effects of passive lengthening, so far the effects of passive shortening on titin kinase signaling have not been investigated.
Fig. 2.
A: a schematic representation of the 3 “hot spots” for titin-based sensing and signaling at the Z-disk, I-band, and M-line of the sarcomere (for a detailed discussion of these titin-binding proteins, see Ref. 20). Note that at the periphery of the M-line, titin contains a kinase domain which senses mechanical load. Moreover, a signaling complex has been identified that interacts with the titin kinase, consisting of nbr1, which interacts with the titin kinase and that targets the ubiquitin-associated p62 to the M-line. p62 in turn interacts with MuRF2, a muscle-specific RING-B-box protein and ligand of the transactivation domain of the serum response transcription factor. Mechanical inactivity of muscle induces nuclear translocation of MuRF2 to reduce nuclear serum response transcription factor and repress transcription. B: titin-based skeletal muscle stiffness can be altered via alternative splicing of the titin gene. For simplicity, only 2 titin molecules per half-sarcomere are depicted (the real number is likely to be 6). During skeletal muscle development, titin isoform size has been shown to decrease, with the magnitude of decrease depending on muscle type and ranging from ∼100 to ∼500 kDa (42). Titin microarray analysis revealed that the decrease in titin isoform size during development is largely caused by restructuring of titin's extensible I-band region, in particular the PEVK segment. Mechanical studies revealed that the loss of PEVK exons during development increases the passive tension generation upon fiber stretch (right) (44).
Titin-based signaling and proteolysis.
Titin-based signaling plays a role in protein degradation. Gene expression profiling in different models of muscle atrophy lead to the identification of a subset of genes that are commonly upregulated in atrophying muscle (4). These so-called atrogenes include the muscle-specific ubiquitin ligases atrogin-1/MAFbx and MuRF-1. MAFbx and MuRF-1 conjugate ubiquitin molecules to proteins to mark these for degradation by the proteasome (36), and recent studies have identified myosin heavy chains as targets of MuRF-1-mediated ubiquitination and degradation (9, 12). MuRF-1 and MAFbx have been shown to be consistently upregulated in animal models of mechanical ventilation (34, 53), as well as in the diaphragm of mechanically ventilated brain-dead patients (31). However, the upregulation of MuRF-1 in unloaded diaphragm is not only more pronounced than in other conditions associated with muscle atrophy, but also more pronounced than the upregulation of MAFbx (11, 30, 31, 34, 53), whereas in conditions associated with elevated diaphragm loading, such as COPD, MuRF-1 expression has been found to be unaltered, but MAFbx significantly upregulated (43, 52). Perhaps this exaggerated response of MuRF-1 to diaphragm unloading is related to its titin-binding properties. MuRF-1 is a well-established titin-binding protein (7), and titin and titin-based protein complexes are recently recognized as integral parts of the mechanosensitive protein network and as critical components in loading-induced muscle signaling (see also the previous paragraph). Titin's binding sites for signaling proteins are not randomly distributed along the titin filament but seem restricted to “hot spots”: one in and near the Z-disk, another in the central I-band, and a third in the M-band region (for a schematic overview, see Fig. 2A). The M-line region of titin (for a more detailed discussion of Z-disk and I-band signaling, see Ref. 20) contains a number of sites that may be involved in sensing and signaling. These include a binding site for MuRF-1 (7, 37). Although MuRF-1 binds titin, ubiquitination of titin itself appears to be regulated by MuRF-1-independent pathways (58). Considering that MuRF-1 binds in the vicinity of the titin kinase, it has been proposed that MuRF-1 exerts its influence in a mechanical strain-dependent fashion (19). Thus, in loaded muscle, part of the MuRF-1 pool might be sequestered by titin, which is released upon muscle unloading to induce proteolysis.
A role for titin in mechanical ventilation-induced proteolysis might involve effects on calpain activity as well. After 18 h of mechanical ventilation of rats (50), calpain-like activity was >2-fold upregulated, and inhibition of calpain activity attenuated protein degradation and diaphragm atrophy. The calpain family is a group of intracellular cysteine proteases requiring calcium for activity; calpain-1 and -2 are expressed ubiquitously, and calpain-3, also known as p94, is striated muscle specific. Evidence indicates that activation of calpains is an initial step in myofilament proteolysis by cleavage of myosin and actin (38). In this way, calpains yield fragments that are degradable by the ubiquitin-proteasome pathway. p94 is very sensitive to autolysis, and its stability and activity is thought to be regulated by binding to titin. p94 has been shown to bind to the PEVK region, the M-line region, and the N2A element of titin (22, 27, 51) (see Fig. 2A). In vitro studies revealed that the p94-N2A interaction suppresses p94 autolysis and protects titin from proteolysis (22). These observations suggest that p94 and titin function as a complex in skeletal muscle sarcomeres. Calpain-1 (or u-calpain) has been found to be upregulated in rat diaphragm during mechanical ventilation (35). Recent work indicated that ∼40% of total calpain-1 is tightly bound to the extensible I-band region of titin (10) rendering it inactive. Importantly, calcium binding to titin increased the amount of bound calpain-1, and it was suggested that the bound calpain-1 constitutes a reservoir for this protease. Although the mechanisms underlying calpain-1 release from titin are unknown, it seems plausible that when the diaphragm muscle is inactive and cytosolic calcium levels are low, calpain-1 is released from titin to become active in cleaving cytoskeletal proteins. Whether the affinity of calpain-1 for titin is stretch dependent has not been investigated, but certainly warrants investigation.
Titin isoform size and proteolysis.
Titin isoform size has been suggested to depend on mechanical stress. Multiple splice pathways in the I-band encoding region of the titin gene give rise to isoforms with distinct spring compositions (13). In humans, the titin gene contains 363 exons coding for a protein that is maximally ∼4.2 MDa in size (2): alternative splicing of the titin gene results in isoforms that range from 3 to 4 MDa. Recent work from our lab revealed that neonatal diaphragm expresses a single large titin isoform that as a function of age gradually becomes smaller (unpublished observations). Although similar results were found in skeletal muscles from the leg (42), the isoform half-transition time was shortest in diaphragm muscle with significant changes in isoform size detected within 48 h. Interestingly, titin isoform size in embryonic tissue was comparable to neonatal tissue immediately after birth, suggesting that titin isoform switching might be triggered by the increased muscle loading after birth. As diaphragm loading undergoes a profound increase after birth, this can potentially explain the rapid isoform transition in the diaphragm compared with leg muscles. The decrease in titin isoform size during development is largely caused by restructuring of titin's extensible I-band region, in particular the PEVK segment, leading to increased passive stiffness of muscle fibers (for a schematic, see Fig. 2B). Changes in the diaphragm are apparent already after 6–12 h of controlled mechanical ventilation of rodents (49). Although detailed studies that address whether titin isoform switching occurs on a time scale this fast have not been conducted, we believe that it is more likely that initially titin exerts an effect on diaphragm function through changes in titin-based signaling pathways (as discussed above), whereas titin isoform switching contributes at a later stage. Future studies should address this issue and study titin isoform size and exon composition in the diaphragm of mechanically ventilated patients at various time points following unloading. In addition to modulating titin isoform size, mechanical unloading is known to induce titin degradation. For instance, recent studies revealed preferential loss of titin during soleus unloading, which was associated with reduced passive tension of muscle fibers (54, 55). Both an increase of titin isoform size and preferential loss of titin with mechanical unloading could have significant consequences for the diaphragm of mechanically ventilated patients. For instance, more compliant titin isoforms could 1) reduce the sarcomere's ability to maintain the thick filament centrally located (24), 2) reduce calcium sensitivity of force generation by increasing the distance between the thick and thin filaments (14), and 3) reduce muscle protein synthesis by reducing the strain on the titin kinase domain (29).
Summary and future directions.
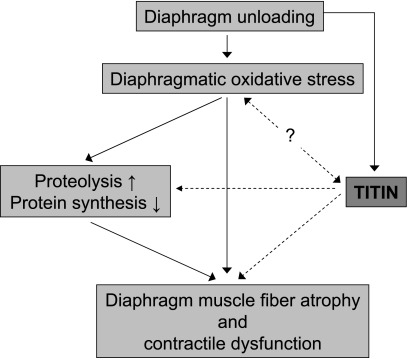
Whereas titin's functional roles were once thought to be restricted to providing myofibrillar elasticity, recent studies suggest that titin may function as a mechanical load sensor. Titin together with its ligands in the Z-disk, I-band, and M-line region of the sarcomere could act as a “tensiometer” that when it senses that stress levels rise or fall beyond physiological limits, triggers downstream signaling events leading to changes in muscle gene expression and proteolysis, altering the trophic status of muscle. We propose that titin and its signalosomes are intimately involved in ventilator-induced structural and functional changes in the diaphragm muscle (for a schematic, see Fig. 3). These ideas could be tested by studying the effects of mechanical ventilation on diaphragm function and structure in mice, which harbor genetically engineered changes in titin's properties [e.g., absence of the titin kinase domain (18, 41) or shorter spring elements rendering stiffer titin molecules (21)]. Such studies will elucidate whether titin plays a primary role in diaphragm remodeling during mechanical ventilation.
Fig. 3.
Schematic illustrating the potential role of titin in the development of diaphragm dysfunction during controlled mechanical ventilation. Prolonged mechanical ventilation and the concomitant diaphragm unloading results in diaphragm muscle fiber atrophy and contractile dysfunction. The current dogma regarding the pathogenesis of the diaphragmatic changes upon unloading includes key roles for diaphragmatic oxidative stress and increased proteolysis (through activation of the ubiquitin-proteasome pathway, calpains, and capases) and decreased protein synthesis (for a review see Ref. 46). Recent studies suggest that titin's properties respond to changes in muscle loading and that it acts as a mechanosensor that regulates the muscle's trophic status by regulation of protein expression and degradation. Furthermore, although little information is available regarding the effect of titin signaling on oxidative stress and vice versa, previous work suggests that titin's stiffness can be modulated by oxidative modification (33). We propose that titin, through its mechanosensing and signaling, contributes to the development of diaphragm contractile dysfunction and atrophy during mechanical ventilation-induced diaphragm unloading. Solid lines, established pathways involved in the pathogenesis of ventilator-induced diaphragm weakness; dashed lines, the novel titin-based pathways proposed in this Perspective.
Clinicians are presently unsure whether the diaphragm of patients with acute respiratory failure should be completely unloaded by the ventilator or whether some level of activation must be imposed (56). If, indeed, disuse of the diaphragm does induce rapid structural modification of titin, it could be argued that at least some level of diaphragm activation should be maintained at any time during mechanical ventilation either by using a supportive ventilatory mode, instead of controlled mechanical ventilation, or by diaphragm muscle pacing. Clearly, more work, including a better understanding of the role of titin, is needed in this fascinating and clinically important area of research.
GRANTS
This work was financially supported by a VENI grant (016.096.043) from the Dutch Organization for Scientific Research (C. A. C. Ottenheijm) and National Heart, Lung, and Blood Institute Grant HL-061497 (H. Granzier).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Allen DL, Weber JN, Sycuro LK, Leinwand LA. Myocyte enhancer factor-2 and serum response factor binding elements regulate fast myosin heavy chain transcription in vivo. J Biol Chem 280: 17126–17134, 2005 [DOI] [PubMed] [Google Scholar]
- 2. Bang ML, Centner T, Fornoff F, Geach AJ, Gotthardt M, McNabb M, Witt CC, Labeit D, Gregorio CC, Granzier H, Labeit S. The complete gene sequence of titin, expression of an unusual approximately 700-kDa titin isoform, and its interaction with obscurin identify a novel Z-line to I-band linking system. Circ Res 89: 1065–1072, 2001 [DOI] [PubMed] [Google Scholar]
- 3. Betters JL, Criswell DS, Shanely RA, Van GD, Falk D, Deruisseau KC, Deering M, Yimlamai T, Powers SK. Trolox attenuates mechanical ventilation-induced diaphragmatic dysfunction and proteolysis. Am J Respir Crit Care Med 170: 1179–1184, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Bodine SC, Latres E, Baumhueter S, Lai VK, Nunez L, Clarke BA, Poueymirou WT, Panaro FJ, Na E, Dharmarajan K, Pan ZQ, Valenzuela DM, DeChiara TM, Stitt TN, Yancopoulos GD, Glass DJ. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 294: 1704–1708, 2001 [DOI] [PubMed] [Google Scholar]
- 5. Boles JM, Bion J, Connors A, Herridge M, Marsh B, Melot C, Pearl R, Silverman H, Stanchina M, Vieillard-Baron A, Welte T. Weaning from mechanical ventilation. Eur Respir J 29: 1033–1056, 2007 [DOI] [PubMed] [Google Scholar]
- 6. Carson JA, Schwartz RJ, Booth FW. SRF and TEF-1 control of chicken skeletal alpha-actin gene during slow-muscle hypertrophy. Am J Physiol Cell Physiol 270: C1624–C1633, 1996 [DOI] [PubMed] [Google Scholar]
- 7. Centner T, Yano J, Kimura E, McElhinny AS, Pelin K, Witt CC, Bang ML, Trombitas K, Granzier H, Gregorio CC, Sorimachi H, Labeit S. Identification of muscle specific ring finger proteins as potential regulators of the titin kinase domain. J Mol Biol 306: 717–726, 2001 [DOI] [PubMed] [Google Scholar]
- 8. Charvet C, Houbron C, Parlakian A, Giordani J, Lahoute C, Bertrand A, Sotiropoulos A, Renou L, Schmitt A, Melki J, Li Z, Daegelen D, Tuil D. New role for serum response factor in postnatal skeletal muscle growth and regeneration via the interleukin 4 and insulin-like growth factor 1 pathways. Mol Cell Biol 26: 6664–6674, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Clarke BA, Drujan D, Willis MS, Murphy LO, Corpina RA, Burova E, Rakhilin SV, Stitt TN, Patterson C, Latres E, Glass DJ. The E3 ligase MuRF1 degrades myosin heavy chain protein in dexamethasone-treated skeletal muscle. Cell Metab 6: 376–385, 2007 [DOI] [PubMed] [Google Scholar]
- 10. Coulis G, Becila S, Herrera-Mendez CH, Sentandreu MA, Raynaud F, Richard I, Benyamin Y, Ouali A. Calpain 1 binding capacities of the N1-line region of titin are significantly enhanced by physiological concentrations of calcium. Biochemistry 47: 9174–9183, 2008 [DOI] [PubMed] [Google Scholar]
- 11. Deruisseau KC, Kavazis AN, Deering MA, Falk DJ, Van Gammeren D, Yimlamai T, Ordway GA, Powers SK. Mechanical ventilation induces alterations of the ubiquitin-proteasome pathway in the diaphragm. J Appl Physiol 98: 1314–1321, 2005 [DOI] [PubMed] [Google Scholar]
- 12. Fielitz J, Kim MS, Shelton JM, Latif S, Spencer JA, Glass DJ, Richardson JA, Bassel-Duby R, Olson EN. Myosin accumulation and striated muscle myopathy result from the loss of muscle RING finger 1 and 3. J Clin Invest 117: 2486–2495, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Freiburg A, Trombitas K, Hell W, Cazorla O, Fougerousse F, Centner T, Kolmerer B, Witt C, Beckmann JS, Gregorio CC, Granzier H, Labeit S. Series of exon-skipping events in the elastic spring region of titin as the structural basis for myofibrillar elastic diversity. Circ Res 86: 1114–1121, 2000 [DOI] [PubMed] [Google Scholar]
- 14. Fukuda N, Wu Y, Farman G, Irving TC, Granzier H. Titin-based modulation of active tension and interfilament lattice spacing in skinned rat cardiac muscle. Pflügers Arch 449: 449–457, 2005 [DOI] [PubMed] [Google Scholar]
- 15. Futier E, Constantin JM, Combaret L, Mosoni L, Roszyk L, Sapin V, Attaix D, Jung B, Jaber S, Bazin JE. Pressure support ventilation attenuates ventilator-induced protein modifications in the diaphragm. Crit Care 12: R116, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gautel M, Leonard K, Labeit S. Phosphorylation of KSP motifs in the C-terminal region of titin in differentiating myoblasts. EMBO J 12: 3827–3834, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gayan-Ramirez G, de Paepe K, Cadot P, Decramer M. Detrimental effects of short-term mechanical ventilation on diaphragm function and IGF-I mRNA in rats. Intensive Care Med 29: 825–833, 2003 [DOI] [PubMed] [Google Scholar]
- 18. Gotthardt M, Hammer RE, Hubner N, Monti J, Witt CC, McNabb M, Richardson JA, Granzier H, Labeit S, Herz J. Conditional expression of mutant M-line titins results in cardiomyopathy with altered sarcomere structure. J Biol Chem 278: 6059–6065, 2003 [DOI] [PubMed] [Google Scholar]
- 19. Granzier HL, Labeit S. The giant protein titin: a major player in myocardial mechanics, signaling, and disease. Circ Res 94: 284–295, 2004 [DOI] [PubMed] [Google Scholar]
- 20. Granzier HL, Labeit S. The giant protein titin: a major player in myocardial mechanics, signaling, and disease. Circ Res 94: 284–295, 2004 [DOI] [PubMed] [Google Scholar]
- 21. Granzier HL, Radke MH, Peng J, Westermann D, Nelson OL, Rost K, King NM, Yu Q, Tschope C, McNabb M, Larson DF, Labeit S, Gotthardt M. Truncation of titin's elastic PEVK region leads to cardiomyopathy with diastolic dysfunction. Circ Res 105: 557–564, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hayashi C, Ono Y, Doi N, Kitamura F, Tagami M, Mineki R, Arai T, Taguchi H, Yanagida M, Hirner S, Labeit D, Labeit S, Sorimachi H. Multiple molecular interactions implicate the connectin/titin N2A region as a modulating scaffold for p94/calpain 3 activity in skeletal muscle. J Biol Chem 283: 14801–14814, 2008 [DOI] [PubMed] [Google Scholar]
- 23. Herridge MS, Cheung AM, Tansey CM, Matte-Martyn A, Diaz-Granados N, Al Saidi F, Cooper AB, Guest CB, Mazer CD, Mehta S, Stewart TE, Barr A, Cook D, Slutsky AS. One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med 348: 683–693, 2003 [DOI] [PubMed] [Google Scholar]
- 24. Horowits R, Podolsky RJ. The positional stability of thick filaments in activated skeletal muscle depends on sarcomere length: evidence for the role of titin filaments. J Cell Biol 105: 2217–2223, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jiang TX, Reid WD, Road JD. Free radical scavengers and diaphragm injury following inspiratory resistive loading. Am J Respir Crit Care Med 164: 1288–1294, 2001 [DOI] [PubMed] [Google Scholar]
- 26. Jubran A. Critical illness and mechanical ventilation: effects on the diaphragm. Respir Care 51: 1054–1061, 2006 [PubMed] [Google Scholar]
- 27. Kinbara K, Sorimachi H, Ishiura S, Suzuki K. Skeletal muscle-specific calpain, p49: structure and physiological function. Biochem Pharmacol 56: 415–420, 1998 [DOI] [PubMed] [Google Scholar]
- 28. Labeit S, Kolmerer B, Linke WA. The giant protein titin. Emerging roles in physiology and pathophysiology. Circ Res 80: 290–294, 1997 [DOI] [PubMed] [Google Scholar]
- 29. Lange S, Xiang F, Yakovenko A, Vihola A, Hackman P, Rostkova E, Kristensen J, Brandmeier B, Franzen G, Hedberg B, Gunnarsson LG, Hughes SM, Marchand S, Sejersen T, Richard I, Edstrom L, Ehler E, Udd B, Gautel M. The kinase domain of titin controls muscle gene expression and protein turnover. Science 308: 1599–1603, 2005. [DOI] [PubMed] [Google Scholar]
- 30. Levine S, Biswas C, Dierov J, Barsotti R, Shrager JB, Nguyen T, Sonnad S, Kucharchzuk JC, Kaiser LR, Singhal S, Budak MT. Increased proteolysis, myosin depletion and atrophic AKT-FOXO signaling in human diaphragm disuse. Am J Respir Crit Care Med. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Levine S, Nguyen T, Taylor N, Friscia ME, Budak MT, Rothenberg P, Zhu J, Sachdeva R, Sonnad S, Kaiser LR, Rubinstein NA, Powers SK, Shrager JB. Rapid disuse atrophy of diaphragm fibers in mechanically ventilated humans. N Engl J Med 358: 1327–1335, 2008 [DOI] [PubMed] [Google Scholar]
- 32. Li S, Czubryt MP, McAnally J, Bassel-Duby R, Richardson JA, Wiebel FF, Nordheim A, Olson EN. Requirement for serum response factor for skeletal muscle growth and maturation revealed by tissue-specific gene deletion in mice. Proc Natl Acad Sci USA 102: 1082–1087, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mayans O, Wuerges J, Canela S, Gautel M, Wilmanns M. Structural evidence for a possible role of reversible disulphide bridge formation in the elasticity of the muscle protein titin. Structure 9: 331–340, 2001 [DOI] [PubMed] [Google Scholar]
- 34. McClung JM, Kavazis AN, Whidden MA, Deruisseau KC, Falk DJ, Criswell DS, Powers SK. Antioxidant administration attenuates mechanical ventilation-induced rat diaphragm muscle atrophy independent of protein kinase B (PKB Akt) signalling. J Physiol 585: 203–215, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. McClung JM, Van Gammeren D, Whidden MA, Falk DJ, Kavazis AN, Hudson MB, Gayan-Ramirez G, Decramer M, Deruisseau KC, Powers SK. Apocynin attenuates diaphragm oxidative stress and protease activation during prolonged mechanical ventilation. Crit Care Med 37: 1373–1379, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mitch WE, Goldberg AL. Mechanisms of muscle wasting. The role of the ubiquitin-proteasome pathway. N Engl J Med 335: 1897–1905, 1996 [DOI] [PubMed] [Google Scholar]
- 37. Mrosek M, Labeit D, Witt S, Heerklotz H, von Castelmur E, Labeit S, Mayans O. Molecular determinants for the recruitment of the ubiquitin-ligase MuRF-1 onto M-line titin. FASEB J 21: 1383–1392, 2007 [DOI] [PubMed] [Google Scholar]
- 38. Neti G, Novak SM, Thompson VF, Goll DE. Properties of easily releasable myofilaments: are they the first step in myofibrillar protein turnover? Am J Physiol Cell Physiol 296: C1383–C1390, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Orozco-Levi M, Lloreta J, Minguella J, Serrano S, Broquetas JM, Gea J. Injury of the human diaphragm associated with exertion and chronic obstructive pulmonary disease. Am J Respir Crit Care Med 164: 1734–1739, 2001 [DOI] [PubMed] [Google Scholar]
- 40. Ottenheijm CA, Heunks LM, Dekhuijzen PN. Diaphragm muscle fiber dysfunction in chronic obstructive pulmonary disease: toward a pathophysiological concept. Am J Respir Crit Care Med 175: 1233–1240, 2007 [DOI] [PubMed] [Google Scholar]
- 41. Ottenheijm CA, Hidalgo C, Rost K, Gotthardt M, Granzier H. Altered contractility of skeletal muscle in mice deficient in titin's M-band region. J Mol Biol 393: 10–26, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ottenheijm CA, Knottnerus AM, Buck D, Luo X, Greer K, Hoying A, Labeit S, Granzier H. Tuning passive mechanics through differential splicing of titin during skeletal muscle development. Biophys J 97: 2277–2286, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ottenheijm CAC, Heunks LMA, Li YP, Jin B, Minnaard R, van Hees HWH, Dekhuijzen PNR. Activation of ubiquitin-proteasome pathway in the diaphragm in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 174: 997–1002, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ottenheijm CAC, Heunks LMA, Hafmans T, van der Ven PF, Benoist C, Zhou H, Labeit S, Granzier HL, Dekhuijzen PNR. Titin and diaphragm dysfunction in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 173: 527–534, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ottenheijm CAC, Heunks LMA, Sieck GC, Zhan WZ, Jansen SM, Degens H, de Boo T, Dekhuijzen PNR. Diaphragm dysfunction in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 172: 200–205, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Powers SK, Kavazis AN, Levine S. Prolonged mechanical ventilation alters diaphragmatic structure and function. Crit Care Med 37: S347–S353, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Puchner EM, Alexandrovich A, Kho AL, Hensen U, Schafer LV, Brandmeier B, Grater F, Grubmuller H, Gaub HE, Gautel M. Mechanoenzymatics of titin kinase. Proc Natl Acad Sci USA 105: 13385–13390, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sandri M. Signaling in muscle atrophy and hypertrophy. Physiology 23: 160–170, 2008 [DOI] [PubMed] [Google Scholar]
- 49. Shanely RA, Van Gammeren D, Deruisseau KC, Zergeroglu AM, McKenzie MJ, Yarasheski KE, Powers SK. Mechanical ventilation depresses protein synthesis in the rat diaphragm. Am J Respir Crit Care Med 170: 994–999, 2004 [DOI] [PubMed] [Google Scholar]
- 50. Shanely RA, Zergeroglu MA, Lennon SL, Sugiura T, Yimlamai T, Enns D, Belcastro A, Powers SK. Mechanical ventilation-induced diaphragmatic atrophy is associated with oxidative injury and increased proteolytic activity. Am J Respir Crit Care Med 166: 1369–1374, 2002 [DOI] [PubMed] [Google Scholar]
- 51. Sorimachi H, Kinbara K, Kimura S, Takahashi M, Ishiura S, Sasagawa N, Sorimachi N, Shimada H, Tagawa K, Maruyama K. Muscle-specific calpain, p94, responsible for limb girdle muscular dystrophy type 2A, associates with connectin through IS2, a p94-specific sequence. J Biol Chem 270: 31158–31162, 1995 [DOI] [PubMed] [Google Scholar]
- 52. Testelmans D, Crul T, Maes K, Agten A, Crombach M, Decramer M, Gayan-Ramirez G. Atrophy and hypertrophy signalling in the diaphragm of patients with COPD. Eur Respir J 35: 549–556, 2010 [DOI] [PubMed] [Google Scholar]
- 53. Testelmans D, Maes K, Wouters P, Gosselin N, Deruisseau K, Powers S, Sciot R, Decramer M, Gayan-Ramirez G. Rocuronium exacerbates mechanical ventilation-induced diaphragm dysfunction in rats. Crit Care Med 34: 3018–3023, 2006 [DOI] [PubMed] [Google Scholar]
- 54. Toursel T, Stevens L, Granzier H, Mounier Y. Passive tension of rat skeletal soleus muscle fibers: effects of unloading conditions. J Appl Physiol 92: 1465–1472, 2002 [DOI] [PubMed] [Google Scholar]
- 55. Udaka J, Ohmori S, Terui T, Ohtsuki I, Ishiwata S, Kurihara S, Fukuda N. Disuse-induced preferential loss of the giant protein titin depresses muscle performance via abnormal sarcomeric organization. J Gen Physiol 131: 33–41, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Vassilakopoulos T, Zakynthinos S, Roussos C. Bench-to-bedside review: weaning failure–should we rest the respiratory muscles with controlled mechanical ventilation? Crit Care 10: 204, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wang Y, Falasca M, Schlessinger J, Malstrom S, Tsichlis P, Settleman J, Hu W, Lim B, Prywes R. Activation of the c-fos serum response element by phosphatidyl inositol 3-kinase and rho pathways in HeLa cells. Cell Growth Differ 9: 513–522, 1998 [PubMed] [Google Scholar]
- 58. Witt SH, Granzier H, Witt CC, Labeit S. MURF-1 and MURF-2 target a specific subset of myofibrillar proteins redundantly: towards understanding MURF-dependent muscle ubiquitination. J Mol Biol 350: 713–722, 2005 [DOI] [PubMed] [Google Scholar]