Abstract
Reactive oxygen species (ROS) are generated as a result of normal cellular metabolism, mainly through the mitochondria and peroxisomes, but their release is enhanced by the activation of oxidant enzymes such as NADPH oxidases or downregulation of endogenous antioxidant enzymes such as manganese-superoxide dismutase (MnSOD) and catalase. Transforming growth factor-β (TGF-β), found to be overexpressed in airway smooth muscle (ASM) from asthmatic and chronic obstructive pulmonary disease patients, may be a pivotal regulator of abnormal ASM cell (ASMC) function in these diseases. An important effect of TGF-β on ASMC inflammatory responses is the induction of IL-6 release. TGF-β also triggers intracellular ROS release in ASMCs by upregulation of NADPH oxidase 4 (Nox4). However, the effect of TGF-β on the expression of key antioxidant enzymes and subsequently on oxidant/antioxidant balance is unknown. Moreover, the role of redox-dependent pathways in the mediation of the proinflammatory effects of TGF-β in ASMCs is unclear. In this study, we show that TGF-β induced the expression of Nox4 while at the same time inhibiting the expression of MnSOD and catalase. This change in oxidant/antioxidant enzymes was accompanied by elevated ROS levels and IL-6 release. Further studies revealed a role for Smad3 and phosphatidyl-inositol kinase-mediated pathways in the induction of oxidant/antioxidant imbalance and IL-6 release. The changes in oxidant/antioxidant enzymes and IL-6 release were reversed by the antioxidants N-acetyl-cysteine (NAC) and ebselen through inhibition of Smad3 phosphorylation, indicating redox-dependent activation of Smad3 by TGF-β. Moreover, these findings suggest a potential role for NAC in preventing TGF-β-mediated pro-oxidant and proinflammatory responses in ASMCs. Knockdown of Nox4 using small interfering RNA partially prevented the inhibition of MnSOD but had no effect on catalase and IL-6 expression. These findings provide novel insights into redox regulation of ASM function by TGF-β.
Keywords: Smad, phosphatidyl-inositol kinases, reactive oxygen species, N-acetyl cysteine, manganese-superoxide dismutase, transforming growth factor-β, NADPH oxidase 4 (Nox4)
reactive oxygen species (ROS) are highly reactive molecules, generated as a result of reduction of oxygen by electrons, which can act as signaling mediators of normal cellular function. They are primarily released by the mitochondrial electron transport chain, although other important cellular sources of ROS include the peroxisomes and NADPH oxidases (18). Cellular ROS are maintained at normal levels by enzymatic and nonenzymatic antioxidant mechanisms. Manganese superoxide dismutase (MnSOD) and catalase are key enzymatic antioxidants, protecting cells from mitochondrial and peroxisomal ROS, respectively (35). If ROS levels are high enough to overwhelm the antioxidant defenses or if the antioxidant protective mechanisms are compromised, then the resulting oxidative stress may lead to aberrant cell signaling through oxidative modification of redox-sensitive signaling proteins (18). Such proteins include proinflammatory transcription factors and kinases such as NF-κB, activating protein-1 (AP-1), and MAPKs (40). Oxidant/antioxidant imbalance is believed to be a key pathogenic factor in chronic inflammatory airway diseases such as asthma and chronic obstructive pulmonary disease (COPD) (31).
Elevated expression of the immunomodulatory and fibrogenic cytokine transforming growth factor-β (TGF-β) is evident in the airway smooth muscle (ASM) of asthmatic and COPD patients (50, 54). TGF-β is a key factor contributing to the abnormal ASM function in asthma and COPD by inducing ASM cell proliferation, hypertrophy, and release of angiogenic, fibrogenic, and inflammatory mediators (9, 20, 43, 54, 57). TGF-β transduces its effects through binding to specific serine/threonine kinase receptors. The signaling cascade is initiated by binding of TGF-β to a type II TGF-β receptor (TβRII), leading to formation of a complex with type I TβR (TβRI) and subsequently activation of TβRI serine/threonine kinase. Activated TβRI kinase phosphorylates Smad2 and -3, which then form a complex with Smad4 and translocate to the nucleus where they modulate gene transcription. Smad2 and -3 can both induce or inhibit gene expression depending on complexes they form with coactivator or corepressor proteins and transcription factors (14). The pathway is subject to negative-feedback regulation by the inhibitory Smad7, whose expression is induced by TGF-β through Smad3 activation (37). Interplay of Smad proteins with other signaling molecules such as the phosphatidylinositol 3-kinase (PI3Ks) and MAPKs increases the complexity of TGF-β-mediated responses (15).
An important inflammatory effect of TGF-β on ASMCs is the induction of IL-6 release (16). IL-6 acts on ASMCs to induce the release of eotaxin and VEGF and is also involved in ASMC-mediated activation of mast cells (1, 24). TGF-β induces many of its effects on different cell types by activation of redox-dependent signaling pathways (10, 23, 25). TGF-β induces intracellular ROS release in ASMCs through upregulation of the Nox catalytic subunit [NADPH oxidase 4 (Nox4)], leading to hyperplasia and hypertrophy (46), while it acts as an inhibitor of antioxidant and cytoprotective genes in liver and kidney cells (2, 28, 42). Therefore, we hypothesized that TGF-β disrupts oxidant/antioxidant balance and induces proinflammatory effects in ASMCs, by activating redox-dependent signaling pathways. To address this hypothesis we examined the effect of TGF-β on the expression of the pro-oxidant enzyme Nox4, the antioxidant enzymes MnSOD and catalase, as well as IL-6 release. We investigated the role of Nox4 and of the Smad and PI3K pathways in mediating these effects. We investigated the effect of antioxidant compounds on these effects, to determine whether they are mediated by redox-dependent pathways.
MATERIALS AND METHODS
Reagents and antibodies.
Recombinant human TGF-β1 was purchased from R&D Systems Europe (Abingdon, UK). N-acetyl-cysteine (NAC), ebselen, and methylthiazolyldiphenyl-tetrazolium bromide (MTT) were purchased from Sigma (Paisley, UK), and the PI3K inhibitor LY294002 was purchased from Merck (Beeston, UK). Antibodies against human Nox4, MnSOD, catalase, phospho-S423-S425-Smad3, total Smad3, and β-actin were purchased from AbCam (Cambridge, UK).
ASM cell isolation and culture.
ASM cells (ASMCs) were dissected from main or lobar brochus removed from healthy transplant donor lungs and were cultured in DMEM supplemented with 4 mM L-glutamine, 20 U/l penicillin, 20 μg/ml streptomycin, and 2.5 μg/ml amphotericin B and 10% FBS. Presence of ASMCs was confirmed by identifying the characteristic hill and valley morphology using light microscopy. Cell stocks were kept in 150 cm2 flasks at 37°C, 5% CO2, and humidified atmosphere. Cells between passages 3 and 6 were used for experiments. Tests to rule out the presence of mycoplasmal contamination were not performed. Before treatment, cells were incubated for 24 h in serum free medium containing phenol-free DMEM supplemented with 1 mM sodium pyruvate, 4 mM L-glutamine, 1:100 nonessential amino acids, 1% insulin-transferrin-selenium-X supplement, 0.1% BSA, and antibiotics as described above.
Smad virus transduction.
Adenoviral Smad constructs carrying the vector expressing Flag-tagged Smad2, Smad3, Smad7, dominant-negative Smad3 (dn-Smad3), or β-galactosidase (null virus) were a kind gift from Dr. A. Moustakas, Ludwig Institute for Cancer Research (Uppsala, Sweden). Virus stocks were diluted in 10% FBS/DMEM to a multiplicity of infection (MOI) of 30 (30 virus particles/per cell) before infection of ASMCs. This dose of virus had no effect on cell viability, and ∼95% transduction efficiency in ASMCs was obtained as detected by green fluorescent protein expressing adenovirus (6). Cells were infected with the virus at MOI 30 for 24 h at 37°C, 5% CO2, and humidified atmosphere and then serum deprived for a further 24 h before stimulation with TGF-β for the indicated periods.
Small interfering RNA transfection.
ASMCs were transiently transfected with nontargeting small interfering RNA (siRNA) or Nox4 siRNA (Dharmacon, Lafayette, CO) for 18 h using Amaxa nucleofection (Lonza AG, Cologne, Germany). After the transfection, ASMCs were serum deprived for 6 h and incubated with TGF-β (1 ng/ml) for a further 48 h.
Intracellular ROS detection.
Intracellular ROS production was determined by 2′-7′- dichlorodihydrofluorescein diacetate (DCF-DA) staining. After stimulation, the medium was removed and the cells were washed with Kreb's-Ringer-HEPES (KRH) solution containing (in mM) 129 NaCl, 5 NaHCO3, 4.8 KCl, 1.2 KH2PO4, 1 CaCl2, 1.2 MgCl2, 2.8 glucose, 10 HEPES, 4 L-glutamine (pH 7.4) and incubated with 10 μM DCF-DA (Invitrogen) for 30 min in serum-free DMEM. At the end of the incubation period, the DCF-DA solution was removed and replaced with KRH solution. Fluorescence was measured using a microplate fluorescence reader at excitation wavelength of 485 nm and emission wavelength of 530 nm. To control for the mitogenic activity of TGF-β, relative fluorescence units were normalized to changes in cell number as determined by MTT assay.
cDNA preparation and real-time PCR.
Total RNA was isolated from ASMCs by using the RNeasy Mini Kit (Qiagen, West Sussex, UK) and reverse transcribed with random primers and AMV reverse transcriptase (Promega, Southampton, UK). mRNA expression was quantified by means of quantitative real-time PCR (Rotor Gene 3000; Corbett Research, Sydney, Australia) with SYBR Green PCR Master Mix Reagent (Qiagen, UK). Specific primers for Nox4, catalase, MnSOD, and 18S were designed according to their published sequences using the GenScript online primer design software and synthesized by Sigma-Genosys. The primer specificity was assessed by using the BLAST software. Melting curve analysis and agarose gel electrophoresis were carried out to ensure the presence of one specific PCR product. The sequences of the gene specific primer sets were: Nox4, 5′-TCTGGCTCT-CCATGAATGTC-3′ and 5′CTGCTTGGAACCTTCTGTGA-3′; MnSOD, 5′ACAGG CCTTATTCCACTGCT-3′ and 5′-CAGCATAACGATCGTGGTTT-3′; catalase, 5′-TAAGACTGACCAGGGCA-TC-3′ and 5′-CAAACCTTGGTGAGATCGAA-3′; 18SrRNA, 5′-CTTAGAGGGACAAGTGGCG-3′ and 5′-ACGCTGAGCCAGTCAGTGTA-3′. Data from the reaction were analyzed by using the computer software Rotor-Gene6 (Corbett Research) using a standard curve. Relative abundance of gene expression was normalized to 18S rRNA expression.
Western blotting.
Total cell protein was extracted with RIPA buffer containing (in %) 1 Igepal CA-630, 0.5 sodium deoxycholate, and 0.1 SDS in PBS (pH 7.4) containing 1 mM PMSF and protease inhibitor cocktail (Roche Applied Science). Protein extracts (20 μg/lane) were fractionated by SDS-PAGE on a 4–12% bis-tris precast gel (Invitrogen) and then transferred to a nitrocellulose membrane (Amerham Biosciences). The membrane was blocked with 5% non-fat dry milk in Tris-buffered saline containing 0.1% Tween (TBS-Tween) for 1 h at 4°C, followed by overnight incubation with the primary antibody in 5% milk at 4°C. The membranes were then washed with TBS-Tween and incubated with anti-rabbit- horseradish peroxidase antibody in 5% milk for 45 min at room temperature and visualized by incubating with ECL solution (Amersham Biosciences). Band densities were quantified by scanning densitometric analysis. Protein loading was controlled for by normalizing to β-actin levels.
Chemokine release.
Measurement of IL-6 concentrations in supernatants was performed using an ELISA (R&D Systems, Abingdon, UK), according to the manufacturer's instructions.
Statistical analysis.
Data are expressed as means ± SE. Results were analyzed using one-way ANOVA for repeated measures, followed by Dunnet post hoc test to determine differences between treatment groups.
RESULTS
Effect of TGF-β on Nox4, MnSOD and catalase expression, and IL-6 release.
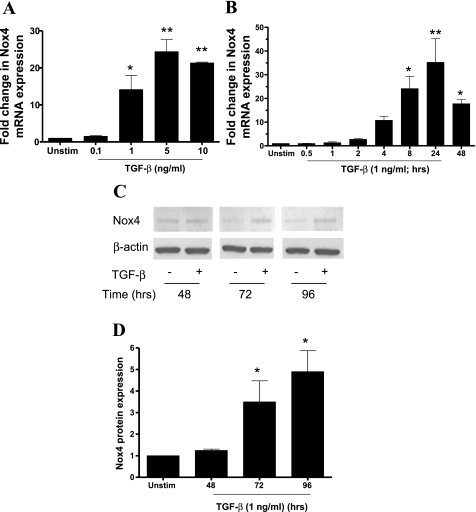
Stimulation with TGF-β for 24 h increased Nox4 mRNA expression in a concentration-dependent manner. Significant induction of Nox4 mRNA was observed at 1 ng/ml and reached maximal levels at 5 ng/ml (Fig. 1A). A fourfold increase in Nox4 mRNA was observed as early as 2 h after TGF-β (1 ng/ml) stimulation. The increase in Nox4 mRNA peaked after 24 h (∼35-fold) but remained elevated at 48 h (Fig. 1B). In agreement with the changes in mRNA, TGF-β led to an elevation in Nox4 protein levels between 48 and 96 h poststimulation (Fig. 1, C and D).
Fig. 1.
The effect of transforming growth factor (TGF)-β on NADPH oxidase 4 (Nox4) expression. A and B: abnormal airway smooth muscle cells (ASMCs) were stimulated with TGF-β (A; 0.1–10 ng/ml) for 24 h or with TGF-β (B; 1 ng/ml) for different times over a 48-h period. Data are expressed as fold change in Nox4 mRNA normalized to 18S rRNA with respect to unstimulated cells (Unstim). C and D: ASMCs were incubated with TGF-β (1 ng/ml) for different times over a 96-h period. Nox4 protein expression was determined in whole cell lysates by Western blotting (C), and band densities were measured by densitometric analysis (D). For each time point data are expressed as fold change in Nox4 protein expression normalized to β-actin expression, with respect to unstimulated control. In C the samples were loaded on the gel in a different order. For simplicity, the picture of the blot has been cut and rearranged so that the treatment times are in ascending order. Bars represent means ± SE of 3 ASMC donors (A), 8 ASMC donors (B), and 3 ASMC donors (C). *P < 0.05; **P < 0.01 compared with unstimulated control.
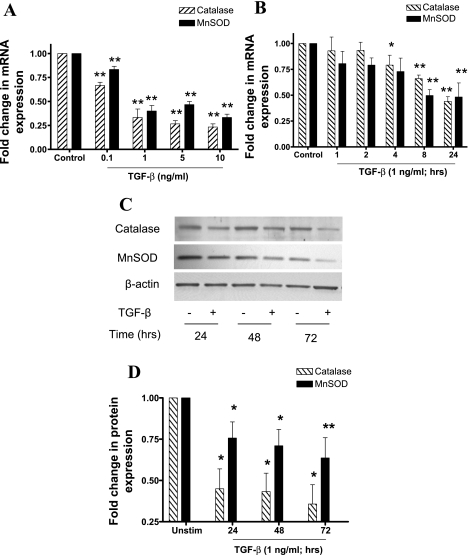
In contrast with its effect on Nox4 expression, TGF-β strongly inhibited MnSOD and catalase mRNA in a concentration and time-dependent manner. For both enzymes, significant inhibition was observed at 0.1 ng/ml, whereas maximal inhibition was observed at 10 ng/ml (Fig. 2A). Maximal inhibition of catalase mRNA occurred 24 h poststimulation, whereas maximal inhibition of MnSOD occurred after 8 h. The mRNA expression of both enzymes was reduced by ∼50% after 24 h stimulation (Fig. 2B). Consistent with the observed reduction in mRNA expression, TGF-β (1 ng/ml) inhibited catalase and MnSOD protein expression in a time-dependent manner, with maximum inhibition occurring after 72 h stimulation. Maximum inhibition was ∼70% for catalase and 40% for MnSOD (Fig. 2, C and D).
Fig. 2.
The effect of TGF-β on catalase and manganese-superoxide dismutase (MnSOD) expression. A and B: ASMCs were stimulated with TGF-β (A; 0.1–10 ng/ml) for 24 h or TGF-β (B; 1 ng/ml) for different times over a 24-h period. Data are expressed as fold change in mRNA expression normalized to 18S rRNA with respect to unstimulated cells. C and D: ASMCs were incubated with TGF-β (1 ng/ml) for different times over a 72-h period. MnSOD and catalase protein expression was determined in whole cell lysates by Western blotting (C), and band densities were measured by densitometric analysis (D). For each time point, data are expressed as fold change in protein expression normalized to β-actin expression, with respect to unstimulated control. Bars represent means ± SE of 3 ASMC (A), 5 ASMC donors (B), and 6 ASMC donors (C). *P < 0.05 and **P < 0.01 compared with unstimulated control.
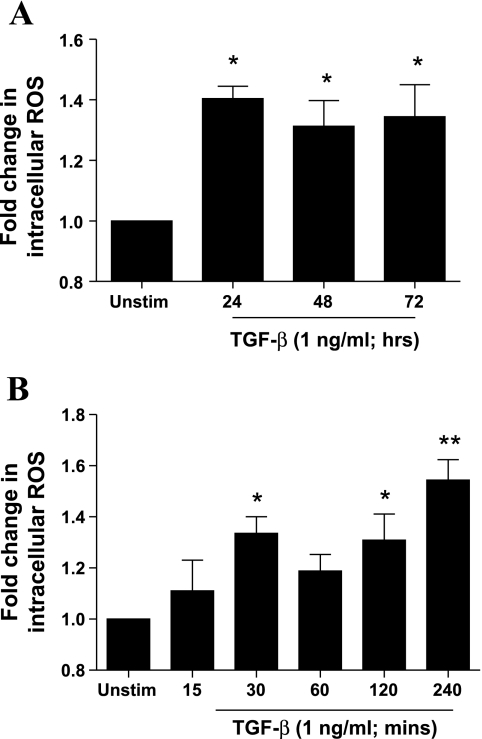
In agreement with the observed changes in oxidant/antioxidant enzyme expression, stimulation with TGF-β (1 ng/ml) for 24–72 h led to an increase in intracellular ROS levels as determined by DCF-DA staining (Fig. 3A), demonstrating pro-oxidant consequences of TGF-β in ASMCs. Moreover, stimulation with TGF-β (1 ng/ml) for 15–240 min led to increased intracellular ROS levels, peaking at 30 min and 240 min poststimulation, indicating that TGF-β induces both early and late ROS release (Fig. 3B). Changes in the oxidant/antioxidant balance and increased intracellular ROS release were accompanied by an increase in IL-6 production 24–72 h after TGF-β stimulation (data not shown). Therefore, TGF-β drives ASMCs toward a pro-oxidant and proinflammatory phenotype.
Fig. 3.
Effect of TGF-β on intracellular reactive oxygen species (ROS) levels and IL-6 release. ASMCs were incubated with TGF-β (1 ng/ml) for different times over a 72-h period (A) or 240-min period (B). At the end of the stimulation period cells were incubated with 2′-7′-dichlorodihydrofluorescein diacetate (10 μM) and fluorescence was measured at 485/530 nm and normalized to changes in cell number as determined by methylthiazolyldiphenyl-tetrazolium bromide assay. Data shown represent relative fluorescence units normalized for cell number and are expressed as fold change with respect to unstimulated control. Bars represent means ± SE of 3 ASMC donors (A) and 5 ASMC donors (B). *P < 0.05 and **P < 0.01 compared with unstimulated control.
Role of Smad-dependent pathways in the modulation of Nox4, catalase and MnSOD expression, and IL-6 release.
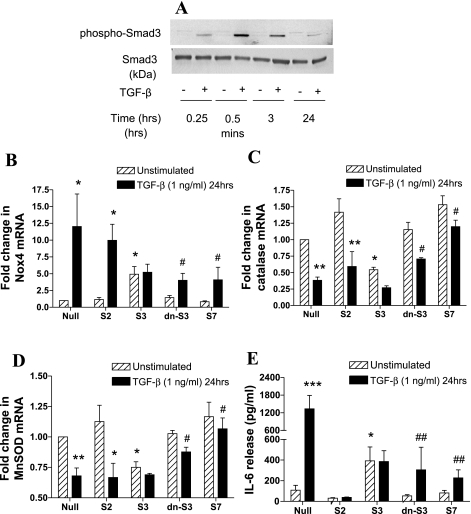
To determine whether the changes in oxidant/antioxidant enzymes and IL-6 release induced by TGF-β are mediated by common signaling mechanisms, the role of Smad proteins in these effects was investigated. We confirmed the activation of Smad-dependent signaling, by determining the effect of TGF-β on Smad3 phosphorylation in whole cell lysates. TGF-β (1 ng/ml) strongly increased Smad3 phosphorylation in a time-dependent manner, peaking after 30 min, but remaining elevated after 24 h stimulation (Fig. 4A).
Fig. 4.
Role of Smad-dependent signaling in the regulation of Nox4, MnSOD, catalase expression, and IL-6 release by TGF-β. A: effect of TGF-β on Smad3 phosphorylation over time. ASMCs were treated with TGF-β (1 ng/ml) for different times over a 24-h period. Phosphorylated and total Smad3 protein expression was determined in whole cell lysates by Western blotting. The blot is representative of experiments on 4 ASMC donors. B–D: ASMCs were infected with null virus (Null), wt-Smad2 (S2), wt-Smad3 (S3), wt-Smad7 (S7), or dn-Smad3 (dn-S3) adenoviral constructs for 72 h and stimulated with TGF-β (1 ng/ml) for the last 24 h of the infection period. Data are expressed as fold change in Nox4 (B), catalase (C), MnSOD (D) mRNA expression, and IL-6 release (E) with respect to null virus control. Bars represent means ± SE of 3 ASMC donors. *P < 0.05, **P < 0.01, ***P < 0.001 compared with null; #P < 0.05 and ##P < 0.01 compared with null, TGF-β-stimulated cells.
We studied the role of Smad signaling in the regulation of Nox4, catalase, MnSOD gene expression, and IL-6 release using adenoviral constructs expressing wild-type Smad2, -3, -7, and the mutant dn-Smad3 genes. Overexpression of Smad3 in unstimulated cells led to a fivefold increase in Nox4 mRNA levels, whereas overexpression of Smad2, -7, or dn-Smad3 had no effect. Consistent with this finding, overexpression of dn-Smad3 or Smad7 inhibited TGF-β (1 ng/ml)-induced upregulation of Nox4 mRNA (Fig. 4B). In contrast, overexpression of Smad3 in unstimulated cells led to an ∼50% reduction in catalase mRNA, whereas dn-Smad3 or Smad7 partially reversed TGF-β-mediated inhibition of catalase mRNA (Fig. 4C). Similarly, overexpression of Smad3 induced a ∼25% decrease in MnSOD mRNA in unstimulated cells, whereas TGF-β (1 ng/ml)-mediated inhibition of MnSOD was partly reversed by dn-Smad3 and completely reversed by Smad7 (Fig. 4D). Furthermore, overexpression of Smad3 in unstimulated cells induced IL-6 release, whereas overexpression of dn-Smad3 or Smad7 inhibited TGF-β-induced IL-6 release (Fig. 4E). Interestingly, the effect of TGF-β on Nox4, catalase and MnSOD mRNA, and IL-6 release in cells overexpressing wt-Smad3 was not augmented possibly because of maximal activation of the pathway. Our data show that activation of Smad3 downstream of TGF-β leads to the associated changes in oxidant/antioxidant enzyme expression and IL-6 release.
Role of PI3K-dependent pathways in the regulation of Nox4, catalase and MnSOD expression, and IL-6 release.
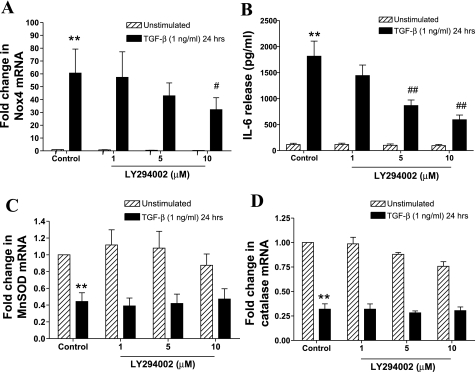
PI3Ks are another important downstream target of TβR1 and mediate several effects of TGF-β on ASMCs. The pan-PI3K inhibitor LY294002 significantly inhibited TGF-β-induced Nox4 mRNA expression (Fig. 5A) and IL-6 release (Fig. 5B) but did not reverse TGF-β-mediated inhibition of catalase and MnSOD mRNA expression (Fig. 5, C and D). Thus PI3K is required for augmentation of pro-oxidant enzyme expression and cytokine release, but not the suppression of antioxidant enzyme expression, indicating that these pathways are not necessarily coregulated.
Fig. 5.
Role of phosphatidylinositol 3-kinase (PI3K)-dependent signaling in the regulation of Nox4, MnSOD and catalase mRNA expression, and IL-6 release by TGF-β. ASMCs were pretreated with LY294002 (1–10 μM) for 1 h and then incubated with TGF-β (1 ng/ml) for 24 h. Nox4 (A), MnSOD (C), and catalase (D) mRNA expression was determined by real-time PCR and expressed as fold change with respect to unstimulated control. IL-6 release (B) was determined by ELISA. Bars represent means ± SE of 4 ASMC donors **P < 0.01 compared with unstimulated control; #P < 0.05 and ##P < 0.01 compared with TGF-β-stimulated cells.
Role of ROS in TGF-β-induced regulation of oxidant/antioxidant enzyme expression and IL-6 release.
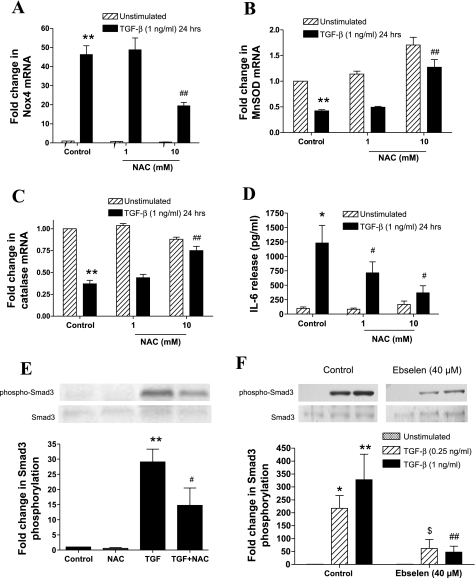
The role of redox-dependent mechanisms in the modulation of Nox4 and MnSOD and catalase expression, as well as in the release of IL-6 in response to TGF-β, was investigated using the antioxidant NAC. NAC (10 mM) inhibited TGF-β-induced Nox4 mRNA expression (Fig. 6A) and reversed the inhibitory effects of TGF-β on MnSOD (Fig. 6B) and catalase mRNA expression (Fig. 6C). Finally, NAC (1–10 mM) strongly inhibited TGF-β (1 ng/ml)-induced IL-6 release (Fig. 6D). To determine at which point in the TGF-β-mediated pathways this inhibitory effect occurs, the effect of NAC (10 mM) on TGF-β-induced Smad3 phosphorylation was determined. NAC inhibited TGF-β (1 ng/ml)-induced Smad3 phosphorylation by ∼50% (Fig. 6E). To confirm that the effects of NAC are due to its ROS-scavenging capacity, we determined the effect of another antioxidant compound ebselen (40 μM) on TGF-β (0.25–1 ng/ml)-mediated Smad3 phosphorylation. Ebselen led to an approximate fivefold inhibition of Smad3 phosphorylation (Fig. 6F), suggesting that a redox-dependent activation of Smad3 is involved in the disruption in oxidant/antioxidant enzyme expression and induction of IL-6 release by TGF-β.
Fig. 6.
Effect of the antioxidant N-acetyl-cysteine (NAC) on the regulation of Nox4, MnSOD and catalase mRNA expression, and IL-6 release by TGF-β. A–D: AMSCs were pretreated with NAC (1–10 mM) for 1 h and then incubated with TGF-β (1 ng/ml) for 24 h. Nox4 (A), MnSOD (B) and catalase (C) mRNA expression, and IL-6 release (D) were determined. E and F: ASMCs were pretreated with NAC (10 mM; E) or ebselen (40 μM; F) for 1 h and then stimulated with TGF-β (0.25–1 ng/ml) for 15 min. Phosphorylated and total Smad3 protein expression was determined in whole cell lysates by Western blotting. Data are expressed as fold change in phosphorylated-Smad3 protein expression normalized to total Smad3 expression, with respect to unstimulated control. In the experiments using ebselen, the effect of different concentrations (10, 20, and 40 μM) of the antioxidant on Smad3 phosphorylation was determined. Due to their large number the samples were loaded on 2 different gels. For simplicity only the sections of the blots showing the effect of control and 40 μM of ebselen are depicted in F. Bars represent means ± SE of 3 ASMC donors. *P < 0.05 and **P < 0.01 compared with unstimulated control; #P < 0.05 and ##P < 0.01 compared with TGF-β (1 ng/ml)-stimulated cells; $P < 0.01 compared with TGF-β (0.25 ng/ml)-stimulated cells.
Role of Nox4 in TGF-β-induced regulation of antioxidant enzyme expression and IL-6 release.
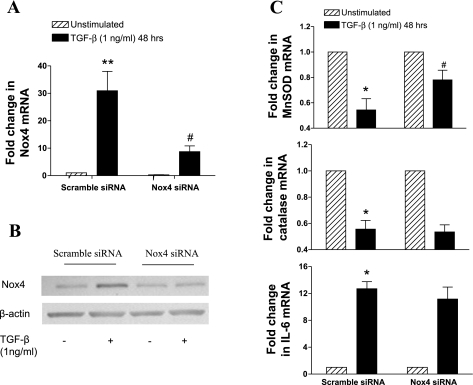
The role of Nox4 in the inhibition of MnSOD and catalase expression, as well as in the induction of IL-6, was investigated by using Nox4 siRNA. Nox4 siRNA (250 nM) strongly inhibited TGF-β (1 ng/ml)-induced Nox4 mRNA and protein (Fig. 7, A and B). The inhibition of Nox4 partially prevented the inhibition of MnSOD by TGF-β although it did not have any significant effect on the modulation of catalase and IL-6 expression (Fig. 7C). Thus the induction of Nox4 amplifies the inhibitory effect of TGF-β on MnSOD expression.
Fig. 7.
Effect of Nox4 small-interfering RNA (siRNA) on the regulation of MnSOD, catalase, and IL-6 expression by TGF-β. AMSCs were transfected with Nox4 siRNA (250 nM) for 18 h, serum deprived for 6 h, and then incubated with TGF-β (1 ng/ml) for 48 h. Nox4 mRNA was determined by real-time PCR (A), and protein was determined in whole cell extracts by Western blotting (B). MnSOD, catalase, and IL-6 (C) mRNA were determined by real-time PCR. mRNA data were expressed as fold change with respect to unstimulated control. Bars represent means ± SE of 4 ASMC donors. *P < 0.05 and **P < 0.01 compared with unstimulated control; #P < 0.05 compared with TGF-β (1 ng/ml)-stimulated cells.
DISCUSSION
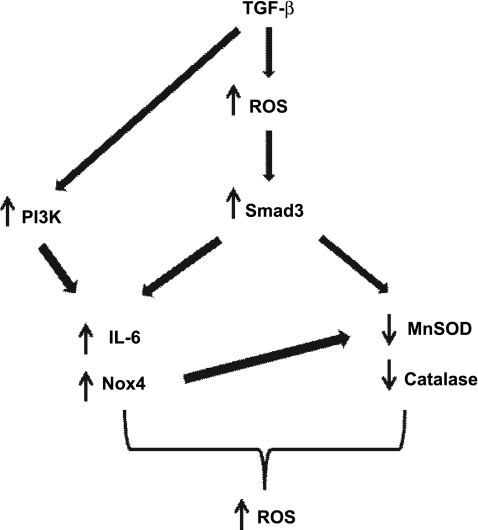
We have shown that TGF-β increases the expression of the Nox catalytic subunit Nox4 while at the same time inhibits the expression of the antioxidants MnSOD and catalase in human ASMCs. Deregulation of the endogenous oxidant/antioxidant balance by TGF-β leads to elevated intracellular levels of ROS and is accompanied by increased IL-6 release. Activation of Smad3 is involved in driving the pro-oxidant and proinflammatory effects of TGF-β in ASMCs. On the other hand, activation of the PI3K pathway is involved in the induction of Nox4 expression and IL-6 release but does not appear to be involved in the inhibition of antioxidant enzymes. Moreover, the antioxidant NAC prevents the disruption in oxidant/antioxidant enzyme balance and attenuates IL-6 release in response to TGF-β through inhibition of Smad3 activation. Our findings, summarized in Fig. 8, demonstrate an important role for TGF-β/Smad/PI3K signaling in the induction of oxidative stress and inflammatory mediator release in human ASMCs. Moreover, they suggest a potential role of NAC in preventing these effects by specifically targeting the TGF/Smad pathway.
Fig. 8.
Schematic summary of the proposed mechanism for TGF-β-mediated regulation of oxidant/antioxidant balance and IL-6 release in ASMCs. Early release of ROS in response to TGF-β activates the Smad pathway leading to upregulation of Nox4 and IL-6 expression and downregulation of catalase and MnSOD expression. Activation of PI3K also contributes to the upregulation of Nox4 and IL-6. The upregulation of Nox4 may also contribute to the regulation of MnSOD expression but not of catalase or of IL-6. The resulting oxidant/antioxidant imbalance leads to increased levels of intracellular ROS.
Our data confirm previous findings in ASMCs, vascular smooth muscle cells (VSMCs), and cardiac fibroblasts, showing specific induction of Nox4 expression in response to TGF-β (10, 45, 46). The differences in the magnitude of induction of Nox4 mRNA observed between experiments are likely to be the result of the variability between the different subjects and not due to experimental conditions. We extend the above studies to show that the increase in Nox4 expression is accompanied by a decrease in the expression of the antioxidant enzymes catalase and MnSOD. These findings are in agreement with previous reports in fibroblasts and hepatocytes showing inhibition of antioxidant enzyme activity and expression (30, 34). Taking these findings together, we can deduce that TGF-β leads to an increase in Nox4-dependent ROS release while at the same time compromises the protection against mitochondrial and peroxisomal derived ROS by inhibiting the expression of MnSOD and catalase, respectively. This is supported by our findings showing increased intracellular ROS in response to TGF-β in the same time frame as the observed changes in pro- and antioxidant enzyme expression. Considering that DCF-DA can be oxidized by a number of oxidative/nitrosative species, these findings show an increase in the general oxidant status of ASMCs in response to TGF-β (22). Moreover, there is evidence in VSMCs demonstrating an inhibitory effect of catalase and MnSOD on cell proliferation, suggesting that their downregulation by TGF-β may amplify the proliferative effect Nox4 on ASMCs (32, 33). Our results demonstrate for the first time the ability of TGF-β to induce the release of ROS while at the same time inhibiting the expression of antioxidant enzymes in ASMCs. This observation provides an important new insight into the cellular mechanisms leading to the pro-oxidant effects of TGF-β in ASMCs.
Activation of Smads and particularly Smad3 has been implicated in the induction of oxidative stress in different cell types (2, 4, 46). We showed that overexpression of wt-Smad3 in unstimulated cells induces Nox4 expression and IL-6 release, whereas it inhibits MnSOD and catalase expression, thus mimicking the effect of TGF-β. Moreover, expression of the inactive dn-Smad3 prevented the effects of TGF-β on oxidant/antioxidant gene expression and IL-6 release, directly implicating Smad3 in the mediation of these effects. Overexpression of Smad7 reversed the effects of TGF-β on Nox4, catalase and MnSOD expression, and IL-6 release, consistent with its role as an endogenous inhibitor of Smad signaling (37). Since TGF-β induces Smad7 expression, this may provide a mechanism to prevent excessive accumulation of ROS and release of IL-6 in ASMCs through negative-feedback regulation. Unexpectedly, the effect of TGF-β on the modulation of oxidant/antioxidant enzymes and the induction of IL-6 release was not augmented in cells overexpressing Smad3. On the contrary, the effect of TGF-β on Nox4 mRNA and IL-6 release was attenuated. This unexpected result is possibly due to induction of the inhibitory Smad7. Smad7 gene is induced by TGF-β in a Smad2/3-dependent manner (37). Therefore, overactivation of Smad3 through overexpression and TGF-β stimulation may lead to strong upregulation of Smad7, which overwhelms the effect of TGF-β. Our data show for the first time a pivotal dual role of the TGF-β-Smad pathway in both inducing oxidative stress and inflammatory cytokine release while compromising antioxidant protection. Therefore, TGF-Smad-mediated pathways play a pivotal role in driving ASMCs toward a pro-oxidant and proinflammatory phenotype.
PI3Ks have been shown to be activated by TGF-β, leading to hyperplasia, hypertrophy, and VEGF release in ASMCs (20, 43, 54). Using the pharmacological inhibitor LY294002, we have demonstrated a role for PI3K activation in the induction of Nox4 expression in agreement with findings by Sturrock et al. (46). Moreover, we showed that LY294002 also prevents the release of IL-6 in response to TGF-β, demonstrating for the first time the involvement of PI3Ks in TGF-β-induced IL-6 release. LY294002 was effective at 5 and 10 μM, concentrations shown to potently inhibit PI3K activity in human ASMCs (17). It is possible that Smad3 and PI3K may act synergistically to induce Nox4 expression and IL-6 release. PI3K-Smad3 synergism in response to TGF-β has been reported in mesangial cells (41). The same concentrations of LY294002 failed to reverse the inhibition of catalase and MnSOD expression. These findings are in contrast with findings by Kato et al. (28), in mouse mesangial cells where a role for PI3K in TGF-β-induced inhibition of MnSOD has been demonstrated. These discrepancies may be due to cell-specific differences or due to the fact that in that study LY294002 was used at a concentration of 25 μM, at which it may have off-target effects.
The thiol-containing antioxidant NAC is known to reduce oxidative biomarkers in the airways of COPD patients (13), while in vitro studies show that it prevents oxidant-induced ASM contraction (7) and ASMC proliferation and inflammatory mediator release (5, 51, 53). NAC acts as an antioxidant by directly removing cellular ROS through its thiol group but also by acting as a precursor for glutathione biosynthesis (8). Our data suggest that NAC prevents the activation of Smad3 by TGF-β, thus attenuating the resulting imbalance in oxidant/antioxidant enzyme expression and elevated IL-6 release. The activation of Smad3 was also attenuated by ebselen, an antioxidant compound that is structurally distinct from NAC, indicating the involvement of ROS in the mediation of these effects by TGF-β. Nox4-dependent ROS have been implicated in the prolonged activation of Smad3 in cardiac fibroblasts (10). Because we have shown that TGF-β also induces increased intracellular ROS levels 15–240 min poststimulation, our data show that the initial activation of Smad3 may occur in response to a very early induction of ROS release by TGF-β. This is also supported by findings in human lung fibroblasts showing induction of ROS release occurring only minutes after TGF-β stimulation (25). Nonetheless, based on these data we cannot exclude the possibility that the inhibitory effect of NAC on TGF-β-induced IL-6 release may also be due to its effect on other redox-dependent transcription factors such as MAPKs, NF-κB, and/or AP-1 (25, 51). Furthermore, ROS produced in response to the changes in oxidant/antioxidant enzymes triggered by TGF-β may be involved in Smad3 activation in a positive-feedback manner, thus leading to amplification of oxidative stress. The inhibitory effect of NAC on Smad3 activation may also reflect a role of the glutathione redox status in the phosphorylation of Smad3. This is consistent with recent reports in human lung fibroblasts and bronchial smooth muscle cells, demonstrating inhibition of TGF-β-mediated fibrogenic effects by glutathione, through inhibition of Smad3 activation (36). Our findings suggest that antioxidant compounds such as NAC and ebselen can specifically prevent TGF-β from driving ASMCs toward a pro-oxidant and proinflammatory phenotype, thus indicating a potential role of these compounds in preventing aberrant ASM function in chronic inflammatory airway disease.
The involvement of Nox4 in the inhibition of MnSOD and catalase expression, and the induction of IL-6 by TGF-β, was investigated using siRNA approach. Reduction of TGF-β-mediated Nox4 expression by transfection with Nox4 siRNA was accompanied by an attenuation of TGF-β-mediated inhibition of MnSOD expression. On the other hand, the inhibition of catalase and induction of IL-6 release was not affected. This finding indicates that upregulation of Nox4 by TGF-β is directly involved in the concomitant downregulation of MnSOD. Because Nox4 protein expression is increased 48 h after stimulation, whereas MnSOD is already inhibited after 24 h, it is possible that Nox4 activity sustains rather than stimulates the inhibition of MnSOD. Moreover, our data indicate that although TGF-β-mediated regulation of catalase and IL-6 is redox-dependent these effects may depend solely on the early release of ROS leading to Smad3 activation and not on the induction of Nox4, which occurs later. Therefore, apart from its pro-oxidant effect, Nox4 also contributes to the reduction in antioxidant enzyme expression by TGF-β, thus enhancing intracellular oxidative stress.
In conclusion, our data demonstrate that TGF-β disrupts oxidant/antioxidant balance and increases IL-6 release in ASMCs, through Smad and PI3K-dependent pathways. These changes are reversed by the antioxidant NAC through inhibition of Smad3 phosphorylation. Smad3 phosphorylation is also reduced by the antioxidant ebselen, suggesting the involvement of redox-dependent mechanisms in the activation of Smad3 and consequently the mediation of oxidant/antioxidant imbalance and IL-6 release. Moreover, the induction of Nox4 was found to be functionally linked with the inhibition of MnSOD expression. These findings serve to further our understanding of the mechanisms underlying abnormal ASM function in chronic inflammatory airway disease.
GRANTS
This work was funded by Wellcome Trust Grant 085935 and was supported by the National Institute for Health Research Respiratory Disease Biomedical Research Unit at the Royal Brompton and Harefield National Health Service Foundation Trust and Imperial College London.
DISCLOSURES
No conflicts of interest, financial or otherwise are declared by the author(s).
REFERENCES
- 1. Ammit AJ, Moir LM, Oliver BG, Hughes JM, Alkhouri H, Ge Q, Burgess JK, Black JL, Roth M. Effect of IL-6 trans-signaling on the pro-remodeling phenotype of airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 292: L199–L206, 2007 [DOI] [PubMed] [Google Scholar]
- 2. Bakin AV, Stourman NV, Sekhar KR, Rinehart C, Yan X, Meredith MJ, Arteaga CL, Freeman ML. Smad3-ATF3 signaling mediates TGF-beta suppression of genes encoding Phase II detoxifying proteins. Free Radic Biol Med 38: 375–387, 2005. [DOI] [PubMed] [Google Scholar]
- 4. Black D, Lyman S, Qian T, Lemasters JJ, Rippe RA, Nitta T, Kim JS, Behrns KE. Transforming growth factor beta mediates hepatocyte apoptosis through Smad3 generation of reactive oxygen species. Biochimie 89: 1464–1473, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brar SS, Kennedy TP, Whorton AR, Murphy TM, Chitano P, Hoidal JR. Requirement for reactive oxygen species in serum-induced and platelet-derived growth factor-induced growth of airway smooth muscle. J Biol Chem 274: 20017–20026, 1999 [DOI] [PubMed] [Google Scholar]
- 6. Catley MC, Sukkar MB, Chung KF, Jaffee B, Liao SM, Coyle AJ, Haddad el-B, Barnes PJ, Newton R. Validation of the anti-inflammatory properties of small-molecule IkappaB Kinase (IKK)-2 inhibitors by comparison with adenoviral-mediated delivery of dominant-negative IKK1 and IKK2 in human airways smooth muscle. Mol Pharmacol 70: 697–705, 2006 [DOI] [PubMed] [Google Scholar]
- 7. Cortijo J, Marti-Cabrera M, de la Asuncion JG, Pallardo FV, Esteras A, Bruseghini L, Vina J, Morcillo EJ. Contraction of human airways by oxidative stress protection by N-acetylcysteine. Free Radic Biol Med 27: 392–400, 1999 [DOI] [PubMed] [Google Scholar]
- 8. Cotgreave IA. N-acetylcysteine: pharmacological considerations and experimental and clinical applications. Adv Pharmacol 38: 205–227, 1997 [PubMed] [Google Scholar]
- 9. Coutts A, Chen G, Stephens N, Hirst S, Douglas D, Eichholtz T, Khalil N. Release of biologically active TGF-β from airway smooth muscle cells induces autocrine synthesis of collagen. Am J Physiol Lung Cell Mol Physiol 280: L999–L1008, 2001 [DOI] [PubMed] [Google Scholar]
- 10. Cucoranu I, Clempus R, Dikalova A, Phelan PJ, Ariyan S, Dikalov S, Sorescu D. NAD(P)H oxidase 4 mediates transforming growth factor-beta1-induced differentiation of cardiac fibroblasts into myofibroblasts. Circ Res 97: 900–907, 2005 [DOI] [PubMed] [Google Scholar]
- 13. De BF, Aceto A, Dragani B, Spacone A, Formisano S, Pela R, Donner CF, Sanguinetti CM. Long-term oral n-acetylcysteine reduces exhaled hydrogen peroxide in stable COPD. Pulm Pharmacol Ther 18: 41–47, 2005 [DOI] [PubMed] [Google Scholar]
- 14. Derynck R, Zhang Y, Feng XH. Smads: transcriptional activators of TGF-beta responses. Cell 95: 737–740, 1998 [DOI] [PubMed] [Google Scholar]
- 15. Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature 425: 577–584, 2003 [DOI] [PubMed] [Google Scholar]
- 16. Elias JA, Wu Y, Zheng T, Panettieri R. Cytokine- and virus-stimulated airway smooth muscle cells produce IL-11 and other IL-6-type cytokines. Am J Physiol 273: L648–L655, 1997 [DOI] [PubMed] [Google Scholar]
- 17. Farghaly HS, Blagbrough IS, Medina-Tato DA, Watson ML. Interleukin 13 increases contractility of murine tracheal smooth muscle by a phosphoinositide 3-kinase p110delta-dependent mechanism. Mol Pharmacol 73: 1530–1537, 2008 [DOI] [PubMed] [Google Scholar]
- 18. Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature 408: 239–247, 2000 [DOI] [PubMed] [Google Scholar]
- 20. Goldsmith AM, Bentley JK, Zhou L, Jia Y, Bitar KN, Fingar DC, Hershenson MB. Transforming growth factor-beta induces airway smooth muscle hypertrophy. Am J Respir Cell Mol Biol 34: 247–254, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Halliwell B, Whiteman M. Measuring reactive species and oxidative damage in vivo and in cell culture: how should you do it and what do the results mean? Br J Pharmacol 142: 231–255, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Herrera B, Murillo MM, varez-Barrientos A, Beltran J, Fernandez M, Fabregat I. Source of early reactive oxygen species in the apoptosis induced by transforming growth factor-beta in fetal rat hepatocytes. Free Radic Biol Med 36: 16–26, 2004 [DOI] [PubMed] [Google Scholar]
- 24. Hollins F, Kaur D, Yang W, Cruse G, Saunders R, Sutcliffe A, Berger P, Ito A, Brightling CE, Bradding P. Human airway smooth muscle promotes human lung mast cell survival, proliferation, and constitutive activation: cooperative roles for CADM1, stem cell factor, and IL-6. J Immunol 181: 2772–2780, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Junn E, Lee KN, Ju HR, Han SH, Im JY, Kang HS, Lee TH, Bae YS, Ha KS, Lee ZW, Rhee SG, Choi I. Requirement of hydrogen peroxide generation in TGF-beta 1 signal transduction in human lung fibroblast cells: involvement of hydrogen peroxide and Ca2+ in TGF-beta 1-induced IL-6 expression. J Immunol 165: 2190–2197, 2000 [DOI] [PubMed] [Google Scholar]
- 28. Kato M, Yuan H, Xu ZG, Lanting L, Li SL, Wang M, Hu MC, Reddy MA, Natarajan R. Role of the Akt/FoxO3a pathway in TGF-beta1-mediated mesangial cell dysfunction: a novel mechanism related to diabetic kidney disease. J Am Soc Nephrol 17: 3325–3335, 2006 [DOI] [PubMed] [Google Scholar]
- 30. Kayanoki Y, Fujii J, Suzuki K, Kawata S, Matsuzawa Y, Taniguchi N. Suppression of antioxidative enzyme expression by transforming growth factor-beta 1 in rat hepatocytes. J Biol Chem 269: 15488–15492, 1994 [PubMed] [Google Scholar]
- 31. Kirkham P, Rahman I. Oxidative stress in asthma and COPD: antioxidants as a therapeutic strategy. Pharmacol Ther 111: 476–494, 2006 [DOI] [PubMed] [Google Scholar]
- 32. Lin SJ, Shyue SK, Shih MC, Chu TH, Chen YH, Ku HH, Chen JW, Tam KB, Chen YL. Superoxide dismutase and catalase inhibit oxidized low-density lipoprotein-induced human aortic smooth muscle cell proliferation: role of cell-cycle regulation, mitogen-activated protein kinases, and transcription factors. Atherosclerosis 190: 124–134, 2007 [DOI] [PubMed] [Google Scholar]
- 33. Madamanchi NR, Moon SK, Hakim ZS, Clark S, Mehrizi A, Patterson C, Runge MS. Differential activation of mitogenic signaling pathways in aortic smooth muscle cells deficient in superoxide dismutase isoforms. Arterioscler Thromb Vasc Biol 25: 950–956, 2005 [DOI] [PubMed] [Google Scholar]
- 34. Marklund SL. Regulation by cytokines of extracellular superoxide dismutase and other superoxide dismutase isoenzymes in fibroblasts. J Biol Chem 267: 6696–6701, 1992 [PubMed] [Google Scholar]
- 35. Moldovan L, Moldovan NI. Oxygen free radicals and redox biology of organelles. Histochem Cell Biol 122: 395–412, 2004 [DOI] [PubMed] [Google Scholar]
- 36. Ono A, Utsugi M, Masubuchi K, Ishizuka T, Kawata T, Shimizu Y, Hisada T, Hamuro J, Mori M, Dobashi K. Glutathione redox regulates TGF-beta-induced fibrogenic effects through Smad3 activation. FEBS Lett 583: 357–362, 2009 [DOI] [PubMed] [Google Scholar]
- 37. Park SH. Fine tuning and cross-talking of TGF-beta signal by inhibitory Smads. J Biochem Mol Biol 38: 9–16, 2005 [DOI] [PubMed] [Google Scholar]
- 40. Rahman I, Adcock IM. Oxidative stress and redox regulation of lung inflammation in COPD. Eur Respir J 28: 219–242, 2006 [DOI] [PubMed] [Google Scholar]
- 41. Runyan CE, Schnaper HW, Poncelet AC. The phosphatidylinositol 3-kinase/Akt pathway enhances Smad3-stimulated mesangial cell collagen I expression in response to transforming growth factor-beta1. J Biol Chem 279: 2632–2639, 2004 [DOI] [PubMed] [Google Scholar]
- 42. Sancho P, Bertran E, Caja L, Carmona-Cuenca I, Murillo MM, Fabregat I. The inhibition of the epidermal growth factor (EGF) pathway enhances TGF-beta-induced apoptosis in rat hepatoma cells through inducing oxidative stress coincident with a change in the expression pattern of the NADPH oxidases (NOX) isoforms. Biochim Biophys Acta 1793: 253–263, 2009 [DOI] [PubMed] [Google Scholar]
- 43. Shin JH, Shim JW, Kim DS, Shim JY. TGF-beta effects on airway smooth muscle cell proliferation, VEGF release and signal transduction pathways. Respirology 14: 347–353, 2009 [DOI] [PubMed] [Google Scholar]
- 45. Sturrock A, Cahill B, Norman K, Huecksteadt TP, Hill K, Sanders K, Karwande SV, Stringham JC, Bull DA, Gleich M, Kennedy TP, Hoidal JR. Transforming growth factor-β1 induces Nox4 NAD(P)H oxidase and reactive oxygen species-dependent proliferation in human pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 290: L661–L673, 2006 [DOI] [PubMed] [Google Scholar]
- 46. Sturrock A, Huecksteadt TP, Norman K, Sanders K, Murphy TM, Chitano P, Wilson K, Hoidal JR, Kennedy TP. Nox4 mediates TGF-β1-induced retinoblastoma protein phosphorylation, proliferation, and hypertrophy in human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 292: L1543–L1555, 2007 [DOI] [PubMed] [Google Scholar]
- 50. Vignola AM, Chanez P, Chiappara G, Merendino A, Pace E, Rizzo A, la Rocca AM, Bellia V, Bonsignore G, Bousquet J. Transforming growth factor-beta expression in mucosal biopsies in asthma and chronic bronchitis. Am J Respir Crit Care Med 156: 591–599, 1997 [DOI] [PubMed] [Google Scholar]
- 51. Wuyts WA, Vanaudenaerde BM, Dupont LJ, Demedts MG, Verleden GM. N-acetylcysteine reduces chemokine release via inhibition of p38 MAPK in human airway smooth muscle cells. Eur Respir J 22: 43–49, 2003 [DOI] [PubMed] [Google Scholar]
- 53. Wuyts WA, Vanaudenaerde BM, Dupont LJ, Van Raemdonck DE, Demedts MG, Verleden GM. N-acetylcysteine inhibits interleukin-17-induced interleukin-8 production from human airway smooth muscle cells: a possible role for anti-oxidative treatment in chronic lung rejection? J Heart Lung Transplant 23: 122–127, 2004 [DOI] [PubMed] [Google Scholar]
- 54. Xie S, Sukkar MB, Issa R, Khorasani NM, Chung KF. Mechanisms of induction of airway smooth muscle hyperplasia by transforming growth factor-β. Am J Physiol Lung Cell Mol Physiol 293: L245–L253, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Xie S, Sukkar MB, Issa R, Oltmanns U, Nicholson AG, Chung KF. Regulation of TGF-β1-induced connective tissue growth factor expression in airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 288: L68–L76, 2005 [DOI] [PubMed] [Google Scholar]