Abstract
Doxorubicin, a common chemotherapeutic agent, causes respiratory muscle weakness in both patients and rodents. Tumor necrosis factor-α (TNF), a proinflammatory cytokine that depresses diaphragm force, is elevated following doxorubicin chemotherapy. TNF-induced diaphragm weakness is mediated through TNF type 1 receptor (TNFR1). These findings lead us to hypothesize that TNF/TNFR1 signaling mediates doxorubicin-induced diaphragm muscle weakness. We tested this hypothesis by treating C57BL/6 mice with a clinical dose of doxorubicin (20 mg/kg) via intravenous injection. Three days later, we measured contractile properties of muscle fiber bundles isolated from the diaphragm. We tested the involvement of TNF/TNFR1 signaling using pharmaceutical and genetic interventions. Etanercept, a soluble TNF receptor, and TNFR1 deficiency protected against the depression in diaphragm-specific force caused by doxorubicin. Doxorubicin stimulated an increase in TNFR1 mRNA and protein (P < 0.05) in the diaphragm, along with colocalization of TNFR1 to the plasma membrane. These results suggest that doxorubicin increases diaphragm sensitivity to TNF by upregulating TNFR1, thereby causing respiratory muscle weakness.
Keywords: chemotherapy, inflammation, skeletal muscle, cancer
symptoms of respiratory muscle insufficiency are evident in cancer patients undergoing chemotherapy. Patients experience decreased maximal inspiratory pressures, an indicator of respiratory muscle weakness (53), along with dyspnea (27) and exercise intolerance (16). Over half of patients undergoing doxorubicin chemotherapy report dyspnea, closely associated with impaired physical performance (38). Doxorubicin may contribute to these symptoms by depressing the function of respiratory muscles. Doxorubicin administered in vivo depresses specific force of murine diaphragm (20), the primary muscle of inspiration. This study addresses the cellular mechanism by which doxorubicin depresses diaphragm-specific force.
A candidate mechanism for doxorubicin-induced diaphragm weakness involves TNFα (TNF), a proinflammatory cytokine, and the TNF receptor subtype 1 (TNFR1). Doxorubicin stimulates TNF expression by immune cells (56) and cardiac muscle (46) and increases serum TNF levels in both humans and rodents (45, 52). Most cellular responses to TNF are the result of TNFR1 activation, localizing TNFR1 to the plasma membrane and amplifying downstream signaling (59). In diaphragm, TNF acts via TNFR1 to depress specific force (24).
Integrating these facts, we hypothesized that diaphragm weakness stimulated by doxorubicin is mediated via TNF/TNFR1 signaling. We tested this hypothesis using a murine model of doxorubicin chemotherapy (10, 20, 52). TNF/TNFR1 signaling was interrupted using the anti-TNF drug etanercept, a soluble TNF receptor, and genetically engineered mice deficient in TNFR1 (TNFR1−/−). Mice received a single intravenous injection of doxorubicin. After 72 h, diaphragm fiber bundles were excised and contractile function was measured ex vivo.
MATERIALS AND METHODS
Materials.
Doxorubicin was purchased from Bedford Laboratories (Bedford, OH). Etanercept was purchased from Immunex (Thousand Oaks, CA). (+)-Tubocurarine chloride hydrate (25 μM) was purchased from Sigma (St. Louis, MO). Mouse TNFα DuoSet ELISA kit was purchased from R&D Systems (Minneapolis, MN). The ELAST ELISA Amplification System was purchased from PerkinElmer LAS, (Waltham, MA). Primers for GAPDH (acc. no. NM_008084.2; forward and reverse 5′-CATGGCCTTCCGTGTTCCTA-3′, 5′-GCGGCACGTCAGATCCA-3′), TNF (acc. no. NM_013693.2; 5′-TCATGCACCACCATCAAGGA-3′, 5′-GACATTCGAGGCTCCAGTGAA-3′), and TNFR1 (acc. no. NM_011609.4; 5′-TCCGCTTGCAAATGTCACA-3′, 5′-GGCAACAGCACCGCAGTAC-3′) were purchased from Invitrogen (Carlsbad, CA). The TNF antibody was purchased from Chemicon (Millipore, Bedford, MA). The TNFR1 antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The annexin II antibody was purchased from ECM Biosciences (Versailles, KY). Fluorescence-conjugated secondary antibodies were purchased from Jackson ImmunoResearch Laboratories (West Grove, PA).
Animal care.
All experiments were approved by the Institutional Animal Care and Use Committee of the University of Kentucky. Studies were conducted at the University of Kentucky using 6- to 8-wk-old male C57BL/6 mice (Harlan, Indianapolis, IN) and TNFR1 receptor-deficient mice (TNFR1−/−; B6.129-Tnfrsf1atm1Mak; The Jackson Laboratory, Bar Harbor, ME) with background strain C57BL/6 as wild types. Animals were maintained in the Division of Laboratory Animal Resources facility on a 12:12-h dark:light cycle and provided food and water ad libitum.
Drug administration.
Mice were given an intravenous injection of doxorubicin (20 mg/kg). This dose is equivalent to 60 mg/m2 based on the conversion factor established by Freireich (17), which is derived from the relationship between body weight and surface area of the animal. This falls within the clinical dosing regimen for treatment of hematological malignancies (60–75 mg/m2) (22). Control animals received the same volume of vehicle (PBS). The diaphragm was excised for analysis at 24-h time points following a single injection (24, 48, and 72 h). For etanercept experiments, there were four experimental groups: vehicle, vehicle + etanercept, doxorubicin, and doxorubicin + etanercept. Mice were given two subcutaneous injections of etanercept (5 mg/kg): 24 h pre- and 36 h post-doxorubicin injection. For all endpoints, no statistical difference existed between the vehicle and vehicle + etanercept groups. Those groups were combined into one control group for further statistical analyses.
Contractile function.
Experiments were performed as described previously (19). In brief, mice were anesthetized with isoflurane and euthanized by cervical dislocation. The diaphragm was excised and placed in Krebs-Ringer solution (in mM: 137 NaCl, 5 KCl, 1 MgSO4, 1 NaH2PO4, 24 NaHCO3, 2 CaCl2) equilibrated with 95% O2-5% CO2 (pH ∼7.4). A fiber bundle with its associated rib and central tendon was isolated from the costal diaphragm. The muscle was attached to a force transducer (BG Series 100g; Kulite, Leonia, NJ) using 4–0 silk suture. The force transducer was mounted on a micrometer used to adjust muscle length. The muscle was placed in a temperature-controlled organ bath between platinum wire stimulating electrodes and stimulated to contract isometrically using electrical field stimulation (Grass S48; Quincy, MA). The output of the force transducer was recorded using an oscilloscope (546601B; Hewlett-Packard, Palo Alto, CA) and computer software (Axoscope 9.2; Molecular Devices, Sunnyvale, CA). In each experiment, the muscle was adjusted to the length where twitch force was maximal (optimal length, Lo) at room temperature, and Lo was measured using an electronic caliper (CD-6″ CS; Mitutoyo America, Aurora, IL). The bath temperature was then increased to 37°C, followed by an equilibration period of 30 min. For direct doxorubicin exposure, muscles were incubated in 2 μg/ml (2 μM) doxorubicin for 1 h. One minute before stimulation, 25 μM (+)-tubocurarine chloride hydrate was added to the organ bath. The force-frequency relationship was determined using contractions evoked at 2-min intervals using stimulus frequencies of 1 (twitch stimulus), 15, 30, 50, 80, 120, 150, 250, and 300 Hz. Pulse and train durations were 0.3 and 250 ms. Time-to-peak twitch force (TPT) and twitch half-relaxation time (1/2 RT) were also measured. Following each experiment, the muscle was removed, blotted dry, and weighed. Cross-sectional area was determined as described by Close (11). Specific forces were expressed as N/cm2.
ELISA.
TNF was measured in serum using a mouse-TNFα DuoSet ELISA kit according to the manufacturer's recommendations with the following modifications: 1) serum samples were incubated with the capture antibody at room temperature, 2) biotinylated detection antibody was incubated for 2 h at 37°C, and 3) streptavidin-horseradish peroxidase (HRP) was incubated for 20 min at 37°C. The HRP signal was amplified using the ELAST ELISA Amplification System according to the manufacturer's recommendations. A microplate reader (Spectramax M2, Molecular Devices) was used to detect optical density of the colorimetric signal. The concentration of TNF was calculated using a standard curve (recombinant mouse TNFα).
Relative quantification real-time PCR.
Reverse transcription was performed using Murine-Moloney Leukemia Virus Reverse Transcriptase and random hexamers (Promega, Madison, WI) plus 20 mg of total RNA isolated from diaphragm muscle homogenates with TRI Reagent (Molecular Research Center, Cincinnati, OH). Primer sequences were as described in Materials. PCR was performed using Applied Biosystems 7500 Real Time PCR system. Targets were amplified from 50 ng of cDNA using SYBR Green Master Mix reagent (stage 1, 1 cycle, 50°C, 2 min; stage 2, 1 cycle, 95°C, 10 min; stage 3, 40 cycles, 95°C, 15 s, 60°C, 1 min; Applied Biosystems). Reactions were performed in duplicate or triplicate for each cDNA sample. The abundance of target mRNA (TNF, TNFR1) relative to GAPDH mRNA was determined using the comparative cycle threshold method (21, 37).
Western blot analysis.
Diaphragm muscles were homogenized in 2× lysis buffer (20 mM Tris, pH 7.2, 2% SDS) and then diluted 1:1 in 2× sample loading buffer (120 mM Tris, pH 7.5, 200 mM DTT, 20% glycerol, 4% SDS, and 0.002% bromphenol blue). Proteins were fractionated on 15% SDS-polyacrylamide gels (Criterion precast gels; Bio-Rad, Hercules, CA) and transferred to reduced-fluorescence PVDF membrane (Immobilon-FL; Millipore, Bedford, MA). Membranes with transferred proteins were blocked for 1 h at room temperature in Odyssey blocking buffer (LI-COR, Lincoln, NE). Membranes were incubated with primary antibodies overnight at room temperature in Odyssey blocking buffer mixed 1:1 with PBS plus 0.2% Tween, followed by four 5-min washes. Membranes were incubated with fluorescence-conjugated secondary antibodies in Odyssey/PBS/0.2% Tween plus 0.01% SDS for 45 min. Fluorescence was imaged, and results were quantified using the Odyssey Infrared Imaging System (LI-COR). Results were normalized for total protein using MemCode Reversible Protein Stain (Pierce, Rockford, IL) and expressed as a percentage of the time-matched control.
Immunofluorescence microscopy.
Cross sections of diaphragm bundles were cut on a cryostat (6 μm) and stored at −80°C. Sections were fixed in 4% paraformaldehyde and permeabilized with Triton X-100, as described previously (43). Diaphragm sections were probed with antibodies to TNFR1 and annexin. Detection of antibody binding was performed using fluorescence-conjugated secondary antibodies. Sections were mounted with ProLong Gold antifade reagent with DAPI (Invitrogen) and examined by fluorescence microscopy (Zeiss, LSM 5 Live Microscope).
Statistical analyses.
Differences in body weight and force-frequency curves were analyzed using two-way repeated measures ANOVA with post hoc Bonferroni tests. Diaphragm muscle characteristics were analyzed using Student's t-tests. Data for mRNA and protein were expressed as % change relative to diaphragm of time-matched, vehicle-injected control animals and were analyzed using one-way ANOVA with post hoc Bonferroni tests. Statistical calculations were performed using commercial software (Prism 5.0a, Graphpad Software, La Jolla, CA; Microsoft Excel, Redmond, WA). Statistical significance was accepted at the P < 0.05 level. Results are reported as means ± SE.
RESULTS
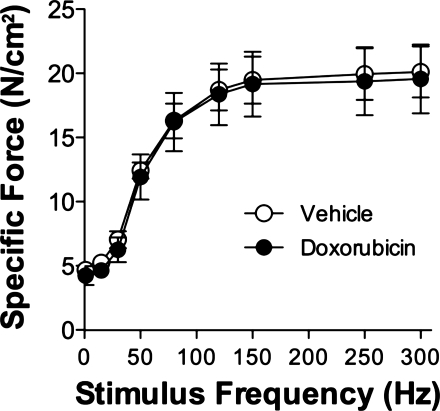
Diaphragm bundles were exposed for 1 h to 2 μg/ml of doxorubicin, a similar concentration found in the serum of patients undergoing doxorubicin chemotherapy (49). We saw no differences in specific force (Fig. 1) following direct doxorubicin exposure.
Fig. 1.
Doxorubicin exposure in vitro does not alter diaphragm force. Specific force 1 h following doxorubicin (2 μg/ml) exposure. Data are means ± SE; n = 3/group.
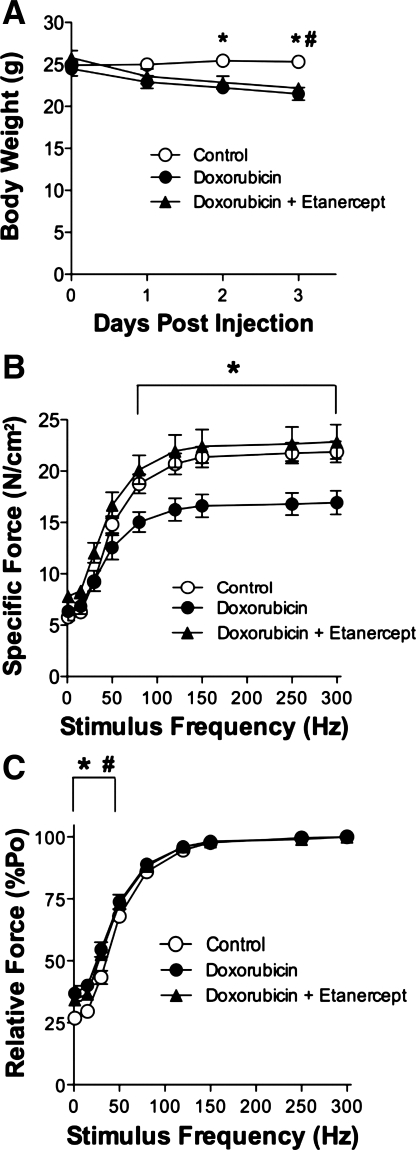
Doxorubicin depresses diaphragm force (Fig. 2), confirming our previous results (20). To test TNF as a mediator of doxorubicin action, mice were injected with etanercept in combination with doxorubicin. Doxorubicin causes a loss in body weight (20), which is not protected with etanercept treatment (Fig. 2A). This loss in weight did not affect the physical dimensions of diaphragm fiber bundles. We saw no differences between groups in fiber bundle weight (doxorubicin 2.5 ± 0.2 mg, doxorubicin + etanercept 2.7 ± 0.1 mg, control 2.9 ± 0.2 mg, P > 0.3) or cross-sectional area (doxorubicin 0.26 ± 0.04 mm2, doxorubicin + etanercept 0.29 ± 0.01 mm2, control 0.31 ± 0.02 mm2, P > 0.2). Lo was not different between groups (P > 0.3). The depression in diaphragm-specific force caused by doxorubicin was abolished by etanercept treatment (Fig. 2B). The relative force-frequency curve was shifted leftward (Fig. 2C). In both doxorubicin-treated groups the twitch:tetanus ratio was significantly increased (doxorubicin 0.37 ± 0.03, doxorubicin + etanercept 0.34 ± 0.01, control 0.27 ± 0.01, P < 0.01). TPT was not altered (doxorubicin 18 ± 1 ms, doxorubicin + etanercept 19 ± 1 ms, control 19 ± 1 ms, P > 0.5) nor was ½ RT changed (doxorubicin 18 ± 1 ms, doxorubicin + etanercept 16 ± 2 ms, control 16 ± 1 ms, P > 0.7).
Fig. 2.
Etanercept, a soluble TNF receptor, abolishes doxorubicin-induced diaphragm dysfunction 72 h following injection. A: body weight over 3 days following doxorubicin administration. Diaphragm-specific force (B) and relative force (C) 72 h following injection. Control refers to vehicle for doxorubicin group and vehicle + etanercept group. Data are means ± SE; n = 9 (control), 4 (doxorubicin), 5 (doxorubicin + etanercept). For all panels, P < 0.01 for overall difference by repeated-measures ANOVA; *P < 0.01 (control vs. doxorubicin) or #P < 0.05 (control vs. doxorubicin + etanercept) by Bonferroni test.
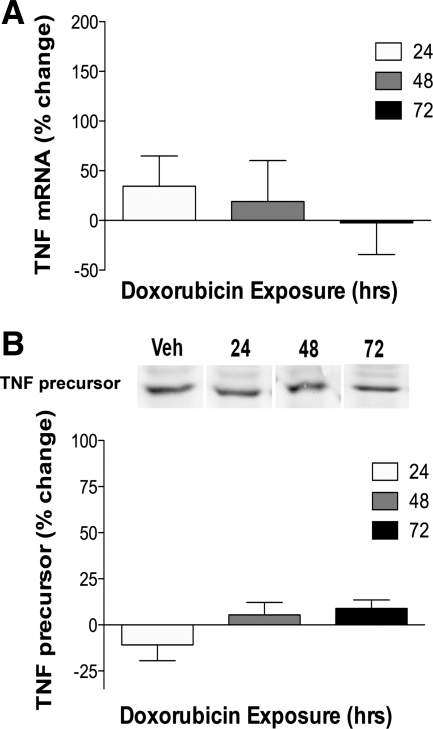
The protective effect of etanercept, a soluble TNF receptor, suggested circulating TNF might mediate the doxorubicin-induced dysfunction. Circulating TNF was measured using a standard ELISA kit, with a tyramide amplification system that enabled detection of TNF at levels <15 pg/ml. The linear regression slope of the standard curve was greater with amplification, suggesting greater sensitivity (amplified 5.2 × 10−3, unamplified 2.0 × 10−3). Despite amplification, serum TNF levels fell below the detection limit of our assay in both groups. Nor did doxorubicin alter TNF mRNA or protein levels in the diaphragm (Fig. 3).
Fig. 3.
Doxorubicin does not alter diaphragm TNF mRNA or protein. A: TNF mRNA in the diaphragm following doxorubicin administration (24 h: vehicle n = 5, treated n = 6; 48 h: vehicle n = 5, treated n = 5; 72 h: vehicle n = 6, treated n = 6). B: TNF protein with representative lanes (24 h: vehicle n = 3, treated n = 3; 48 h: vehicle n = 3, treated n = 3; 72 h: vehicle n = 3, treated n = 3). Data are expressed as % change from time-matched control values.
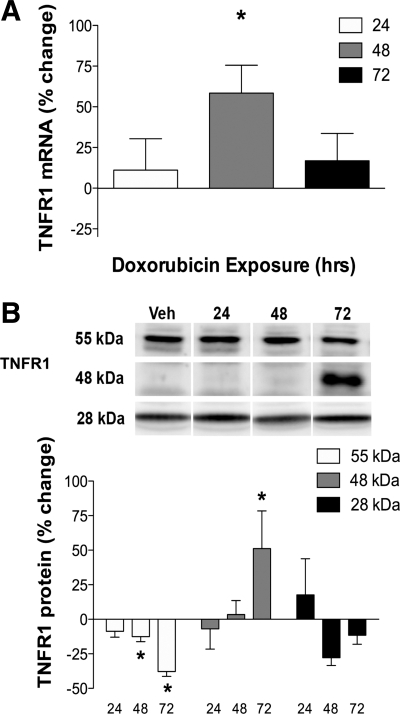
TNFR1 signaling mediates TNF-induced skeletal muscle weakness (24). Diaphragm TNFR1 mRNA levels were 50% greater than control 48 h after doxorubicin exposure (Fig. 4A). TNFR1 protein exists in three different isoforms (25), all of which were detected in the diaphragm. The full-length 55-kDa TNFR1 was decreased 48–72 h following doxorubicin administration, with no change in the soluble 28-kDa isoform (Fig. 4B). The 48-kDa TNFR1 isoform was increased at 72 h following doxorubicin (Fig. 4B).
Fig. 4.
TNFR1 is elevated in the diaphragm following doxorubicin administration. A: TNFR1 mRNA (24 h: vehicle n = 6, treated n = 6; 48 h: vehicle n = 4, treated n = 8; 72 h: vehicle n = 7, treated n = 8). B: TNFR1 protein (24 h: vehicle n = 6, treated n = 6; 48 h: vehicle n = 5, treated n = 5; 72 h: vehicle n = 6, treated n = 6) in the diaphragm following doxorubicin administration. Data are % change of time-matched vehicles represented as means ± SE. For all panels, P < 0.05 for overall difference by repeated-measures ANOVA. A: *P < 0.05 (48 h); B: *P < 0.05 (55 kDa, 48 kDa) by Bonferroni test.
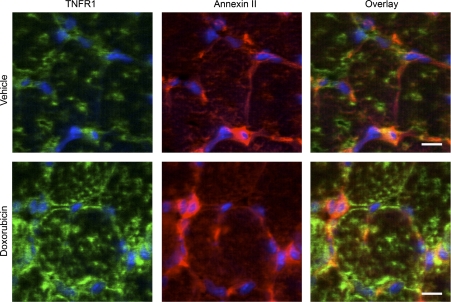
The majority of TNFR1 resides in the golgi apparatus and is translocated to the plasma membrane upon stimulation by TNF (8, 33). We observed TNFR1 staining within diaphragm fibers and in close approximation to annexin II, a plasma membrane marker (Fig. 5). Figure 5 is a representative image of our observations in three animals, four diaphragm sections per animal. TNFR1 staining is less prominent in vehicle-treated animals.
Fig. 5.
TNFR1 localization after doxorubicin exposure. Panels show representative confocal images of transverse sections from diaphragms of mice treated with vehicle (top) or doxorubicin (bottom); muscle was collected and processed 72 h postinjection. Sections were stained for anti-TNFR1 (green) and counterstained with anti-annexin II (red) and DAPI (blue), a nuclear stain. Scale bar = 10 μm.
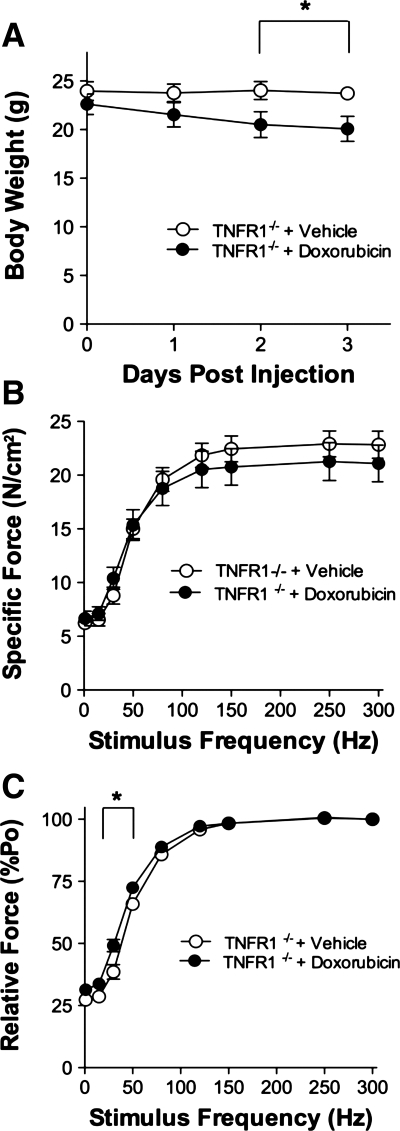
TNFR1 deficiency did not protect against doxorubicin-induced loss of body weight (Fig. 6A). As in the etanercept experiments (above), doxorubicin did not affect the size of diaphragm fiber bundles from TNFR1−/− mice. Reductions in fiber bundle weight (doxorubicin 2.7 ± 0.4 mg vs. vehicle 3.4 ± 0.3, P > 0.2) and cross-sectional area (doxorubicin 0.28 ± 0.03 mm2 vs. vehicle 0.35 ± 0.03, P > 0.1) were not significant. Nor was Lo different (P > 0.9).
Fig. 6.
TNFR1 deficiency protects against doxorubicin-induced diaphragm dysfunction. A: body weight over 3 days following doxorubicin administration. Specific force (B) and relative force (C) measured 72 h following injection. Data are means ± SE; n = 8 (vehicle) or 7 (doxorubicin); for A and C, P < 0.01 for overall difference by repeated-measures ANOVA; *P < 0.01 by Bonferroni test.
TNFR1 deficiency abolished the depression in specific force caused by doxorubicin (Fig. 6B) but the leftward shift in the relative force-frequency curve persisted (Fig. 6C). An increase in the twitch:tetanus ratio approached statistical significance (doxorubicin 0.32 ± 0.02 vs. vehicle 0.27 ± 0.02, P > 0.06), but we observed no change in TPT (doxorubicin 19 ± 1 ms vs. vehicle 20 ± 2, P > 0.6) or ½ RT (doxorubicin 15 ± 1 ms vs. vehicle 17 ± 1, P > 0.3).
DISCUSSION
These studies demonstrate that TNF/TNFR1 signaling mediates diaphragm weakness induced by doxorubicin. Etanercept, a soluble TNF receptor, prevented the depression in force caused by doxorubicin. We detected no changes in circulating or muscle-derived TNF. Rather, doxorubicin appears to stimulate expression and sarcolemmal localization of TNFR1. Genetic TNFR1 deficiency protected the diaphragm against doxorubicin-induced weakness, confirming an essential role for TNF/TNFR1 signaling.
Direct effects of doxorubicin.
Circulating levels of doxorubicin are ∼1.25 μg/ml following an intravenous doxorubicin injection (12 mg/kg) (32). Extrapolating from our 20 mg/kg dose, circulating levels of doxorubicin in our model are expected to be 2 μg/ml, approximately two times the concentration of circulating doxorubicin in patients following chemotherapy (∼1 μg/ml) (14, 49). We show that direct, short-term exposure to 2 μg/ml of doxorubicin in vitro does not alter the force of diaphragm fiber bundles. It is possible that with long-term exposure, doxorubicin itself could alter muscle function directly. However, an indirect mechanism appears more likely. Other studies (36, 46) and our current results suggest that doxorubicin causes muscle dysfunction through secondary mediators.
Roles of TNF and TNFR1.
One potential mediator is TNF, which is elevated in cancer patients undergoing doxorubicin chemotherapy (45) and in healthy rodents that receive doxorubicin injections (19, 52). TNF is implicated in numerous inflammatory diseases and closely associated with the loss of muscle function (12, 42, 60). Exposure to TNF in vivo depresses skeletal muscle-specific force (1, 24), linking the inflammatory cytokine with skeletal muscle dysfunction.
In our study we found no changes in circulating or muscle-derived TNF following doxorubicin administration. This difference from other studies of healthy rodents is likely due to the different method of doxorubicin administration, intravenous vs. intraperitoneal injection. We have shown that intraperitoneal injection of doxorubicin causes an exacerbated inflammatory response, most likely drug-induced peritonitis. This localized inflammatory response is absent after intravenous injection of doxorubicin, although diaphragm weakness still persists (20).
The data that implicate TNF are from our studies of etanercept, a soluble TNF receptor that blocks the functional activity of circulating TNF (23). Rheumatoid arthritis patients are prescribed etanercept to block chronic inflammation (2). Etanercept administration also attenuates the inflammatory response in other conditions, diminishing TNF in the circulation and mimicking TNFR1 deficiency (31, 40, 54). While serum TNF levels fell below the limits of our assay, diaphragm protection by etanercept suggests TNF is essential for doxorubicin-induced dysfunction.
TNFR1 mediates TNF signaling in a vast majority of cells (59). For example, selective deletion of this receptor subtype protects against carrageenan-induced inflammation of the lungs (40), proinflammatory signaling in macrophages (13), and TNF-induced muscle weakness (24). Our current data show that TNFR1 deficiency also protects diaphragm against doxorubicin-induced weakness.
Cellular mechanism.
Doxorubicin may weaken the diaphragm by altering TNFR1 expression. We found that doxorubicin increases TNFR1 mRNA, suggesting alterations of transcriptional regulation in response to doxorubicin. For example, members of the CCAAT/enhancer binding protein (C/EBP) family regulate the TNFR1 promoter (9) suggesting C/EBP proteins as potential gene regulators by which doxorubicin might increase TNFR1 expression. Alternatively, doxorubicin might increase TNFR1 mRNA by promoting mRNA stabilization.
Doxorubicin selectively altered tissue levels of all three TNFR1 isoforms. Doxorubicin increased the 48-kDa isoform, an exosome-associated TNFR1 receptor that promotes TNFR1 signaling among cells, thereby altering function (61). For example, circulating exosomes from patients in septic shock are presumed to contain the 48-kDa TNFR1 isoform and have been shown to depress contractile function of healthy cardiac muscle (5). Similarly, by stimulating the exosome-associated isoform, doxorubicin could promote TNFR1 signaling among diaphragm muscle fibers and thereby induce contractile dysfunction. The 55-kDa full-length receptor is found on the plasma membrane and undergoes proteolytic cleavage to form the 28-kDa soluble TNFR1 (25, 47). Doxorubicin stimulated loss of the full-length receptor from the diaphragm over time, suggesting cleavage and release of the soluble 28-kDa isoform. By binding to free TNF, soluble TNFR1 may neutralize the cytokine or may function as a carrier protein, increasing TNF half-life and biological activity (29, 40).
A second mechanism of doxorubicin action appears to be TNFR1 translocation. Under basal conditions, TNFR1 is housed in the golgi and trafficked to the plasma membrane in response to TNF stimulation (8, 25, 33). Similarly, doxorubicin treatment increased TNFR1 expression in the diaphragm and stimulated translocation of TNFR1. Greater TNFR1 availability at the cell surface favors ligand binding, increasing diaphragm sensitivity to TNF, and promoting respiratory muscle weakness.
The intracellular mechanism by which TNFR1 activation impairs contractile function appears to involve redox signaling. TNF stimulates skeletal muscle to produce oxidants via a TNFR1-dependent mechanism (24). This increase in oxidants can cause contractile dysfunction by affecting myofibrillar proteins (3, 35) or calcium homeostasis (55, 58). Permeabilized muscle fibers from TNF-treated animals show a depression in calcium-activated force with no alterations in calcium sensitivity or cross-bridge cycling rate (24). Myofilament proteins are susceptible to oxidation, which can alter structure and impair contractile function (7, 26). These findings suggest oxidants downstream of TNFR1 signaling could alter the myofibrillar lattice, leading to contractile dysfunction.
Translational relevance.
Dyspnea, or shortness of breath, is a sign of respiratory muscle insufficiency in humans (6, 48, 57) and a common symptom found in cancer patients undergoing chemotherapy (15, 53). Multiple factors, both drug and disease related, may contribute to dyspnea and related side effects resulting in serious clinical consequences. Patients may become too ill to receive chemotherapy, delaying clinic visits, decreasing treatment efficacy, and lessening the probability of a successful outcome (18, 30, 39, 50). These side effects also cause discomfort and distress that significantly degrade the quality of life (4, 44). This can affect cancer progression where decrements in the quality of life are correlated with increased morbidity and mortality (34, 50, 51). Moreover, these side effects are persistent. They can continue for months or years after a course of chemotherapy is completed (28, 41). Clearly, interventions to lessen dyspnea and related side effects would benefit patients and facilitate treatment.
Our study can help guide the development of an intervention that preserves respiratory muscle function. Our current findings help focus this effort. We have identified a specific ligand (TNF) and cell surface receptor (TNFR1) that appear to be essential for doxorubicin-induced weakness. By inference, this identifies a cascade of postreceptor signaling events that mediate the cellular response to TNFR1 activation. The TNF/TNFR1 pathway now becomes a target for new therapies to preserve diaphragm function and lessen the most debilitating side effects of cancer chemotherapy.
GRANTS
This study was supported by a predoctoral fellowship from the American Heart Association (to L. A. A. Gilliam), National Institutes of Health Training Grant T32-HL-086341 (to M. B. Reid and to L. A. A. Gilliam, predoctoral scholar), and a postdoctoral fellowship from the American Heart Association (to L. F. Ferreira).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Drs. Carole Moncman and Francisco Andrade for assistance with the immunofluorescence experiments.
REFERENCES
- 1. Adams V, Mangner N, Gasch A, Krohne C, Gielen S, Hirner S, Thierse HJ, Witt CC, Linke A, Schuler G, Labeit S. Induction of MuRF1 is essential for TNF-alpha-induced loss of muscle function in mice. J Mol Biol 384: 48–59, 2008 [DOI] [PubMed] [Google Scholar]
- 2. Alonso-Ruiz A, Pijoan JI, Ansuategui E, Urkaregi A, Calabozo M, Quintana A. Tumor necrosis factor alpha drugs in rheumatoid arthritis: systematic review and metaanalysis of efficacy and safety. BMC Musculoskelet Disord 9: 52, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Andrade FH, Reid MB, Allen DG, Westerblad H. Effect of hydrogen peroxide and dithiothreitol on contractile function of single skeletal muscle fibres from the mouse. J Physiol 509: 565–575, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Arndt V, Stegmaier C, Ziegler H, Brenner H. A population-based study of the impact of specific symptoms on quality of life in women with breast cancer 1 year after diagnosis. Cancer 107: 2496–2503, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Azevedo LC, Janiszewski M, Pontieri V, Pedro Mde A, Bassi E, Tucci PJ, Laurindo FR. Platelet-derived exosomes from septic shock patients induce myocardial dysfunction. Crit Care 11: R120, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Basso SM, Caratozzolo E, Massani M, Antoniutti M, di Pinto FC, Callegari P, Bonariol L, Bassi N. Nocturnal dyspnea in a young adult male patient: a typical case of an unrecognized traumatic rupture of the diaphragm. J Trauma 56: 720, 2004 [DOI] [PubMed] [Google Scholar]
- 7. Beckman JS. Oxidative damage and tyrosine nitration from peroxynitrite. Chem Res Toxicol 9: 836–844, 1996 [DOI] [PubMed] [Google Scholar]
- 8. Bradley JR, Thiru S, Pober JS. Disparate localization of 55-kd and 75-kd tumor necrosis factor receptors in human endothelial cells. Am J Pathol 146: 27–32, 1995 [PMC free article] [PubMed] [Google Scholar]
- 9. Bristol JA, Morrison TE, Kenney SC. CCAAT/enhancer binding proteins alpha and beta regulate the tumor necrosis factor receptor 1 gene promoter. Mol Immunol 46: 2706–2713, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chabner BA, Ryan DP, Paz-Ares L, Garcia-Carbonero R, Calabresi P, Hardman JG, Limbird LE, Gilman AG. Goodman & Gilman's The pharmacologic Basis of Therapeutics. New York: McGraw-Hill, Medical Publishing Division, 2001 [Google Scholar]
- 11. Close RI. Dynamic properties of mammalian skeletal muscles. Physiol Rev 52: 129–197, 1972 [DOI] [PubMed] [Google Scholar]
- 12. Conraads V. Pro-inflammatory cytokines and their receptors in chronic heart failure: do they really matter? Acta Cardiol 61: 161–168, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Crisafulli C, Galuppo M, Cuzzocrea S. Effects of genetic and pharmacological inhibition of TNF-alpha in the regulation of inflammation in macrophages. Pharmacol Res 60: 332–340, 2009 [DOI] [PubMed] [Google Scholar]
- 14. Delgado G, Potkul RK, Treat JA, Lewandowski GS, Barter JF, Forst D, Rahman A. A phase I/II study of intraperitoneally administered doxorubicin entrapped in cardiolipin liposomes in patients with ovarian cancer. Am J Obstet Gynecol 160: 812–817; discussion 817–819, 1989 [DOI] [PubMed] [Google Scholar]
- 15. Dy SM, Lorenz KA, Naeim A, Sanati H, Walling A, Asch SM. Evidence-based recommendations for cancer fatigue, anorexia, depression, and dyspnea. J Clin Oncol 26: 3886–3895, 2008 [DOI] [PubMed] [Google Scholar]
- 16. Elbl L, Vasova I, Tomaskova I, Jedlicka F, Kral Z, Navratil M, Smardova L, Wagnerova B, Vorlicek J. Cardiopulmonary exercise testing in the evaluation of functional capacity after treatment of lymphomas in adults. Leuk Lymphoma 47: 843–851, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Freireich EJ, Gehan EA, Rall DP, Schmidt LH, Skipper HE. Quantitative comparison of toxicity of anticancer agents in mouse, rat, hamster, dog, monkey, and man. Cancer Chemother Rep 50: 219–244, 1966 [PubMed] [Google Scholar]
- 18. Ganz PA, Bower JE. Cancer related fatigue: a focus on breast cancer and Hodgkin's disease survivors. Acta Oncol 46: 474–479, 2007 [DOI] [PubMed] [Google Scholar]
- 19. Gilliam LA, Ferreira LF, Bruton JD, Moylan JS, Westerblad H, St Clair DK, Reid MB. Doxorubicin acts through tumor necrosis factor receptor subtype 1 to cause dysfunction of murine skeletal muscle. J Appl Physiol 107: 1935–1942, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gilliam LA, Moylan JS, Callahan LA, Sumandea MP, Reid MB. Doxorubicin causes diaphragm weakness in murine models of cancer chemotherapy. Muscle Nerve. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Giulietti A, Overbergh L, Valckx D, Decallonne B, Bouillon R, Mathieu C. An overview of real-time quantitative PCR: applications to quantify cytokine gene expression. Methods 25: 386–401, 2001 [DOI] [PubMed] [Google Scholar]
- 22. Glass B, Kloess M, Bentz M, Schlimok G, Berdel WE, Feller A, Trumper L, Loeffler M, Pfreundschuh M, Schmitz N. Dose-escalated CHOP plus etoposide (MegaCHOEP) followed by repeated stem cell transplantation for primary treatment of aggressive high-risk non-Hodgkin lymphoma. Blood 107: 3058–3064, 2006 [DOI] [PubMed] [Google Scholar]
- 23. Grounds MD, Davies M, Torrisi J, Shavlakadze T, White J, Hodgetts S. Silencing TNFalpha activity by using Remicade or Enbrel blocks inflammation in whole muscle grafts: an in vivo bioassay to assess the efficacy of anti-cytokine drugs in mice. Cell Tissue Res 320: 509–515, 2005 [DOI] [PubMed] [Google Scholar]
- 24. Hardin BJ, Campbell KS, Smith JD, Arbogast S, Smith J, Moylan JS, Reid MB. TNF-alpha acts via TNFR1 and muscle-derived oxidants to depress myofibrillar force in murine skeletal muscle. J Appl Physiol 104: 694–699, 2008 [DOI] [PubMed] [Google Scholar]
- 25. Hawari FI, Rouhani FN, Cui X, Yu ZX, Buckley C, Kaler M, Levine SJ. Release of full-length 55-kDa TNF receptor 1 in exosome-like vesicles: a mechanism for generation of soluble cytokine receptors. Proc Natl Acad Sci USA 101: 1297–1302, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hernandez OM, Jones M, Guzman G, Szczesna-Cordary D. Myosin essential light chain in health and disease. Am J Physiol Heart Circ Physiol 292: H1643–H1654, 2007 [DOI] [PubMed] [Google Scholar]
- 27. Hirsch A, Vander Els N, Straus DJ, Gomez EG, Leung D, Portlock CS, Yahalom J. Effect of ABVD chemotherapy with and without mantle or mediastinal irradiation on pulmonary function and symptoms in early-stage Hodgkin's disease. J Clin Oncol 14: 1297–1305, 1996 [DOI] [PubMed] [Google Scholar]
- 28. Hjermstad MJ, Oldervoll L, Fossa SD, Holte H, Jacobsen AB, Loge JH. Quality of life in long-term Hodgkin's disease survivors with chronic fatigue. Eur J Cancer 42: 327–333, 2006 [DOI] [PubMed] [Google Scholar]
- 29. Idriss HT, Naismith JH. TNF alpha and the TNF receptor superfamily: structure-function relationship(s). Microsc Res Tech 50: 184–195, 2000 [DOI] [PubMed] [Google Scholar]
- 30. Jacobsen PB, Hann DM, Azzarello LM, Horton J, Balducci L, Lyman GH. Fatigue in women receiving adjuvant chemotherapy for breast cancer: characteristics, course, and correlates. J Pain Symptom Manage 18: 233–242, 1999 [DOI] [PubMed] [Google Scholar]
- 31. Jiang Y, Deacon R, Anthony DC, Campbell SJ. Inhibition of peripheral TNF can block the malaise associated with CNS inflammatory diseases. Neurobiol Dis 32: 125–132, 2008 [DOI] [PubMed] [Google Scholar]
- 32. Johansen PB. Doxorubicin pharmacokinetics after intravenous and intraperitoneal administration in the nude mouse. Cancer Chemother Pharmacol 5: 267–270, 1981 [DOI] [PubMed] [Google Scholar]
- 33. Jones SJ, Ledgerwood EC, Prins JB, Galbraith J, Johnson DR, Pober JS, Bradley JR. TNF recruits TRADD to the plasma membrane but not the trans-Golgi network, the principal subcellular location of TNF-R1. J Immunol 162: 1042–1048, 1999 [PubMed] [Google Scholar]
- 34. Kramer JA, Curran D, Piccart M, de Haes JC, Bruning P, Klijn J, Van Hoorebeeck I, Paridaens R. Identification and interpretation of clinical and quality of life prognostic factors for survival and response to treatment in first-line chemotherapy in advanced breast cancer. Eur J Cancer 36: 1498–1506, 2000 [DOI] [PubMed] [Google Scholar]
- 35. Lamb GD, Posterino GS. Effects of oxidation and reduction on contractile function in skeletal muscle fibres of the rat. J Physiol 546: 149–163, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lien YC, Noel T, Liu H, Stromberg AJ, Chen KC, St Clair DK. Phospholipase C-delta1 is a critical target for tumor necrosis factor receptor-mediated protection against adriamycin-induced cardiac injury. Cancer Res 66: 4329–4338, 2006 [DOI] [PubMed] [Google Scholar]
- 37. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2[-Delta Delta C(T)] Method. Methods 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- 38. Lund MB, Kongerud J, Boe J, Nome O, Abrahamsen AF, Ihlen H, Forfang K. Cardiopulmonary sequelae after treatment for Hodgkin's disease: increased risk in females? Ann Oncol 7: 257–264, 1996 [DOI] [PubMed] [Google Scholar]
- 39. Marks LB, Halperin EC, Prosnitz LR, Ross M, Vredenburgh JJ, Rosner GL, Peters W. Post-mastectomy radiotherapy following adjuvant chemotherapy and autologous bone marrow transplantation for breast cancer patients with greater than or equal to 10 positive axillary lymph nodes. Cancer and Leukemia Group B. Int J Radiat Oncol Biol Phys 23: 1021–1026, 1992 [DOI] [PubMed] [Google Scholar]
- 40. Mazzon E, Esposito E, Di Paola R, Muia C, Crisafulli C, Genovese T, Caminiti R, Meli R, Bramanti P, Cuzzocrea S. Effect of tumour necrosis factor-alpha receptor 1 genetic deletion on carrageenan-induced acute inflammation: a comparison with etanercept. Clin Exp Immunol 153: 136–149, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Meeske KA, Siegel SE, Globe DR, Mack WJ, Bernstein L. Prevalence and correlates of fatigue in long-term survivors of childhood leukemia. J Clin Oncol 23: 5501–5510, 2005 [DOI] [PubMed] [Google Scholar]
- 42. Meldrum DR. Tumor necrosis factor in the heart. Am J Physiol Regul Integr Comp Physiol 274: R577–R595, 1998 [DOI] [PubMed] [Google Scholar]
- 43. Moncman CL, Wang K. Nebulette: a 107 kD nebulin-like protein in cardiac muscle. Cell Motil Cytoskeleton 32: 205–225, 1995 [DOI] [PubMed] [Google Scholar]
- 44. Moore AJ, Moxham J, Polkey MI. Diaphragm weakness as a cause of breathlessness after anatomically distant surgery. Thorax 60: 786–787, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Morsi MI, Hussein AE, Mostafa M, El-Abd E, El-Moneim NA. Evaluation of tumour necrosis factor-alpha, soluble P-selectin, gamma-glutamyl transferase, glutathione S-transferase-pi and alpha-fetoprotein in patients with hepatocellular carcinoma before and during chemotherapy. Br J Biomed Sci 63: 74–78, 2006 [DOI] [PubMed] [Google Scholar]
- 46. Mukherjee S, Banerjee SK, Maulik M, Dinda AK, Talwar KK, Maulik SK. Protection against acute adriamycin-induced cardiotoxicity by garlic: role of endogenous antioxidants and inhibition of TNF-alpha expression. BMC Pharmacol 3: 16, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Newton RC, Solomon KA, Covington MB, Decicco CP, Haley PJ, Friedman SM, Vaddi K. Biology of TACE inhibition. Ann Rheum Dis 60, Suppl 3: iii25–iii32, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Paulin E, Yamaguti WP, Chammas MC, Shibao S, Stelmach R, Cukier A, Carvalho CR. Influence of diaphragmatic mobility on exercise tolerance and dyspnea in patients with COPD. Respir Med 101: 2113–2118, 2007 [DOI] [PubMed] [Google Scholar]
- 49. Piscitelli SC, Rodvold KA, Rushing DA, Tewksbury DA. Pharmacokinetics and pharmacodynamics of doxorubicin in patients with small cell lung cancer. Clin Pharmacol Ther 53: 555–561, 1993 [DOI] [PubMed] [Google Scholar]
- 50. Ripamonti C. Management of dyspnea in advanced cancer patients. Support Care Cancer 7: 233–243, 1999 [DOI] [PubMed] [Google Scholar]
- 51. Roychowdhury DF, Hayden A, Liepa AM. Health-related quality-of-life parameters as independent prognostic factors in advanced or metastatic bladder cancer. J Clin Oncol 21: 673–678, 2003 [DOI] [PubMed] [Google Scholar]
- 52. Tangpong J, Cole MP, Sultana R, Joshi G, Estus S, Vore M, St CW, Ratanachaiyavong S, St Clair DK, Butterfield DA. Adriamycin-induced, TNF-alpha-mediated central nervous system toxicity. Neurobiol Dis 23: 127–139, 2006 [DOI] [PubMed] [Google Scholar]
- 53. Travers J, Dudgeon DJ, Amjadi K, McBride I, Dillon K, Laveneziana P, Ofir D, Webb KA, O'Donnell DE. Mechanisms of exertional dyspnea in patients with cancer. J Appl Physiol 104: 57–66, 2008 [DOI] [PubMed] [Google Scholar]
- 54. Tukov FF, Luyendyk JP, Ganey PE, Roth RA. The role of tumor necrosis factor alpha in lipopolysaccharide/ranitidine-induced inflammatory liver injury. Toxicol Sci 100: 267–280, 2007 [DOI] [PubMed] [Google Scholar]
- 55. Tupling AR, Vigna C, Ford RJ, Tsuchiya SC, Graham DA, Denniss SG, Rush JW. Effects of buthionine sulfoximine treatment on diaphragm contractility and SR Ca2+ pump function in rats. J Appl Physiol 103: 1921–1928, 2007 [DOI] [PubMed] [Google Scholar]
- 56. Ujhazy P, Zaleskis G, Mihich E, Ehrke MJ, Berleth ES. Doxorubicin induces specific immune functions and cytokine expression in peritoneal cells. Cancer Immunol Immunother 52: 463–472, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Versteegh MI, Braun J, Voigt PG, Bosman DB, Stolk J, Rabe KF, Dion RA. Diaphragm plication in adult patients with diaphragm paralysis leads to long-term improvement of pulmonary function and level of dyspnea. Eur J Cardiothorac Surg 32: 449–456, 2007 [DOI] [PubMed] [Google Scholar]
- 58. Viner RI, Williams TD, Schoneich C. Peroxynitrite modification of protein thiols: oxidation, nitrosylation, and S-glutathiolation of functionally important cysteine residue(s) in the sarcoplasmic reticulum Ca-ATPase. Biochemistry 38: 12408–12415, 1999 [DOI] [PubMed] [Google Scholar]
- 59. Wajant H, Pfizenmaier K, Scheurich P. Tumor necrosis factor signaling. Cell Death Differ 10: 45–65, 2003 [DOI] [PubMed] [Google Scholar]
- 60. Yende S, Waterer GW, Tolley EA, Newman AB, Bauer DC, Taaffe DR, Jensen R, Crapo R, Rubin S, Nevitt M, Simonsick EM, Satterfield S, Harris T, Kritchevsky SB. Inflammatory markers are associated with ventilatory limitation and muscle dysfunction in obstructive lung disease in well functioning elderly subjects. Thorax 61: 10–16, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zhang J, Hawari FI, Shamburek RD, Adamik B, Kaler M, Islam A, Liao DW, Rouhani FN, Ingham M, Levine SJ. Circulating TNFR1 exosome-like vesicles partition with the LDL fraction of human plasma. Biochem Biophys Res Commun 366: 579–584, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]