Abstract
There is increasing evidence that the neutrophil chemoattractant proline-glycine-proline (PGP), derived from the breakdown of the extracellular matrix, plays an important role in neutrophil recruitment to the lung. PGP formation is a multistep process involving neutrophils, metalloproteinases (MMPs), and prolyl endopeptidase (PE). This cascade of events is now investigated in the development of lung emphysema. A/J mice were whole body exposed to cigarette smoke for 20 wk. After 20 wk or 8 wk after smoking cessation, animals were killed, and bronchoalveolar lavage fluid and lung tissue were collected to analyze the neutrophilic airway inflammation, the MMP-8 and MMP-9 levels, the PE activity, and the PGP levels. Lung tissue degradation was assessed by measuring the mean linear intercept. Additionally, we investigated the effect of the peptide l-arginine-threonine-arginine (RTR), which binds to PGP sequences, on the smoke-induced neutrophil influx in the lung after 5 days of smoke exposure. Neutrophilic airway inflammation was induced by cigarette smoke exposure. MMP-8 and MMP-9 levels, PE activity, and PGP levels were elevated in the lungs of cigarette smoke-exposed mice. PE was highly expressed in epithelial and inflammatory cells (macrophages and neutrophils) in lung tissue of cigarette smoke-exposed mice. After smoking cessation, the neutrophil influx, the MMP-8 and MMP-9 levels, the PE activity, and the PGP levels were decreased or reduced to normal levels. Moreover, RTR inhibited the smoke-induced neutrophil influx in the lung after 5 days' smoke exposure. In the present murine model of cigarette smoke-induced lung emphysema, it is demonstrated for the first time that all relevant components (neutrophils, MMP-8, MMP-9, PE) involved in PGP formation from collagen are upregulated in the airways. Together with MMPs, PE may play an important role in the formation of PGP and thus in the pathophysiology of lung emphysema.
Keywords: chronic obstructive pulmonary disease, metalloproteinases, proline-glycine-proline
chronic obstructive pulmonary disease (COPD) is a term referring to two separate chronic lung disorders: emphysema and chronic bronchitis (3). The pathogenesis of COPD is complex and multifactorial, where neutrophilic airway inflammation and protease-anti-protease imbalance play a pivotal role (1, 47). The inflammatory response of the lungs to noxious particles and gases, predominantly characterized by increased neutrophil numbers, contributes to the progressive airflow limitation (30). Besides neutrophils, macrophages and CD8+ T lymphocytes have also been implicated in the development and progression of COPD (17, 28, 38, 41, 42). Since tobacco smoking is the major risk factor in the development of COPD (25), the pathogenesis of COPD is strongly linked to the effects of cigarette smoke on the lungs. The general hypothesis on COPD states that cigarette smoke initiates an inflammatory immune response, characterized by a cascade of events that culminate in alveolar wall destruction, a characteristic of lung emphysema. First, cigarette smoke can act on airway epithelial cells and alveolar macrophages to release several inflammatory mediators and chemoattractants, such as tumor necrosis factor TNFα and IL-8, CXCL8 (12). Subsequently, the chemoattractants facilitate the migration of neutrophils and other cell types, like CD8+ T cells, to the site of inflammation (33). As a consequence, the activated neutrophils and macrophages release a variety of proteolytic enzymes, like matrix metalloproteinases (MMPs), finally resulting in degradation of the lung tissue (5, 16). It is proposed that the increased protease activity leads not only to the lung matrix breakdown, but also to the generation of the tripeptide proline-glycine-proline (PGP) from collagen (18, 19). PGP is chemotactic for neutrophils in vivo as well as in vitro (39, 53) and can also stimulate neutrophils to release CXCL8 (S. A. Overbeek, unpublished data). At this point, both CXCL8 and PGP might be involved in the continuous recruitment and activation of neutrophils in the airways, which will lead to an excessive release of proteases and an ongoing PGP formation. This process finally results in a chronic airway inflammation with tissue destruction and remodeling (19). Furthermore, it has been shown that airway exposure to PGP can induce lung emphysema in mice as indicated by alveolar enlargement and right ventricle heart hypertrophy (49, 53). Clinical data demonstrated that PGP was detected in the bronchoalveolar lavage fluid (BALF) and sputum of COPD patients, but not in asthmatics or controls (36, 53). PGP generation is a multistep process involving members of the MMP family, MMP-9 and/or MMP-8, and the serine protease family, prolyl endopeptidase (PE) (21). MMP-8 and MMP-9 proteolytically cleave collagen to smaller fragments and create an optimal substrate for PE. These collagen fragments are then further cleaved to PGP by PE. To our knowledge, PE is the only enzyme directly capable of cleaving PGP from shorter portions of collagen (4, 45).
The aims of this study were to investigate whether the different components involved in the proteolytic cascade generating the chemoattractant PGP were indeed present in the lungs of mice chronically exposed to cigarette smoke with emphasis on the PE and PGP levels in the lung, and we were interested in the effect of smoking cessation on the different components. First, we determined whether neutrophils were present in the airways of mice after cigarette smoke exposure. Second, MMP-8 and MMP-9 levels, necessary to cleave collagen into smaller fragments for PE, were measured in the lung. Then, PE activity in the airways was examined to confirm that the necessary enzyme for PGP generation was present. Finally, the presence of PGP was determined in the BALF of mice exposed to cigarette smoke.
MATERIALS AND METHODS
Animals.
Female A/J mice, 9–14 wk old, and male Balb/c mice, 5–6 wk old (Charles River Laboratories), were housed under controlled conditions in standard laboratory cages. They were provided free access to water and food. All in vivo experimental protocols were approved by the local Ethics Committee and were performed under strict governmental and international guidelines on animal experimentation.
Chronic cigarette smoke exposure.
Female A/J mice were divided into three groups. The first group was exposed to room air for 20 wk, the second group was exposed to cigarette smoke for 20 wk, and the third group was exposed to cigarette smoke for 20 wk followed by a period of 8 wk without cigarette smoke exposure. The mice were exposed in whole body chambers to air (sham) or to diluted mainstream cigarette smoke from the reference cigarettes 2R4F (Univ. of Kentucky, Lexington, KY) using a smoking apparatus (7). Exposures were conducted 4 h/day (with a 30/60-min fresh air break after each hour of exposure), 5 days/wk, for 20 wk to a target cigarette smoke concentration of 750 μg total particulate matter/l (TPM/l). This TPM concentration was reached after an adaptation period of 2 wk, starting with a TPM concentration of 125 μg TPM/l. The mass concentration of cigarette smoke TPM was determined by gravimetric analysis of Cambridge filter samples. The carbon monoxide (CO) was monitored continuously and was around 800 ppm. The nicotine concentration in the smoke was ∼40 μg/l. The sample sites were located in the middle of the exposure chamber at the breathing zone. The carboxyhemoglobin level measured via blood gas analysis was determined as 50% on average in the smoke-exposed mice. The mice were killed 16–24 h after the last air or smoke exposure, or after the smoke-free period of 8 wk. A/J mice were used in the present chronic COPD model, since this strain is characterized as moderately susceptible to the development of lung emphysema and to the lung inflammatory response after acute cigarette smoke exposure (23, 55).
1-Wk cigarette smoke exposure.
To confirm a smoke-related effect in another strain, Balb/c mice were exposed in whole body chambers to air (sham) or to diluted mainstream cigarette smoke from the reference cigarettes 2R4F using a peristaltic pump. Just before the experiments, filters were cut from the cigarettes. Each cigarette was smoked in 5 min at a rate of 5 l/h in a ratio with 60 l/h air. The mice were exposed to cigarette smoke using five cigarettes twice daily for five consecutive days, except for the first day when they were exposed to three cigarettes. The mice were killed 16 h after the last air or smoke exposure.
l-arginine-threonine-arginine administration.
The mice received vehicle (50 μl sterile PBS) or l-arginine-threonine-arginine (RTR) (50 μg RTR/50 μl sterile PBS) by oropharyngeal aspiration under light isoflurane anesthesia twice daily (15).
Bronchoalveolar lavage.
Immediately after intraperitoneal injection with an overdose of pentobarbital, the lungs of separate groups of mice (n = 4–5) were lavaged four times through a tracheal cannula with 1 ml of saline (NaCl 0.9%), prewarmed at 37°C. After centrifuging the bronchoalveolar lavage fluid at 4°C (400 g, 5 min), the supernatant of the first milliliter was used for MMP-8, MMP-9 and PGP analysis, and the cell pellets of the four lavages were used for cell counts. The four cell pellets, kept on ice, were pooled per animal and resuspended in 150 μl of cold saline.
Neutrophil accumulation in BALF.
After staining with Türk solution, total cell counts per lung were made under light microscopy using a Burker-Turk chamber. Differential cell counts were performed on air-dried cytospin preparations stained by DiffQuick (Dade, Düdingen, Switzerland). Cells were identified as macrophages, neutrophils, and lymphocytes according to standard morphology. At least 200 cells were counted, and the absolute number of neutrophils was calculated.
Preparation of lung homogenates.
In brief, lung samples were homogenized in a potter glass tube with a Teflon pestle in 1 ml of ice-cold PBS. Homogenates were centrifuged at 14,000 g for 5 min, and supernatants were collected. The protein concentration of each sample was assayed using the Pierce BCA protein assay kit standardized to BSA according to the manufacturer's protocol (Thermo Fisher Scientific, Rockford, IL). The homogenates were diluted to a final concentration of 2 μg of protein/μl.
MPO activity in lung homogenates.
Lung homogenates were assessed biochemically for the neutrophil marker enzyme MPO according to a previously reported method (26). Fifty microliters of lung homogenate (2 μg protein/μl) was incubated with 50 μl of 50 mM KH2PO4/K2HPO4 buffer (pH 5.5) containing 0.5% hexadecyltrimethylammonium bromide (HTAB) for 30 min at room temperature. The enzymatic reaction was started by mixing the sample (100 μl) with 150 μl of 50 mM phosphate buffer (pH 5.5) containing 0.26 mg/ml o-dianisidine dihydrochloride and 0.52 mM 30% hydrogen peroxide (37°C). The enzyme activities were determined spectrophotometrically. The changes in absorbance were measured at 450 nm over 20 min with an iMark Microplate absorbance reader (Bio-Rad). The reaction was standardized by a series of pooled human neutrophils, and MPO activity was expressed in arbitrary units.
MMP-8 and MMP-9 ELISA.
Total MMP-9 (pro- and active MMP-9) and pro-MMP-9 levels were measured in BALF and lung homogenates by sandwich ELISA using the Quantikine mouse total and pro-MMP-9 ELISA kit (R&D Systems) according to the manufacturer's instructions. Active MMP-9 levels were calculated by subtracting the pro-MMP-9 levels from the total MMP-9 levels. Total MMP-8 levels were measured in BALF by ELISA using the mouse MMP-8 ELISA kit (Cusabio Biotech) according to the manufacturer's instructions.
Gelatin zymography.
Presence of active and latent forms of MMP-9 was analyzed by zymography on 11% polyacrylamide gels containing 1% gelatin (Sigma Aldrich) as previously described (51). Samples were diluted in nonreducing sample buffer (0.5 M Tris-HCl, pH 6.8, 8% sucrose, 20% SDS, and 0.05% bromophenol blue). Sample volumes were adjusted to obtain a uniform protein content of 15 μg/sample, and 10 μl of sample was added to each lane. Gels were electrophoresed at 20 mA at 4°C until the bromophenol blue-stained front reached the bottom of the gel. After running, the gels were washed twice in 2.5% Triton X-100 for 15 min at room temperature to remove the SDS, followed by two washes of 5 min in 50 mM Tris-HCl, pH 8.0, containing 5 mM CaCl2, 10 μM ZnCl2 and incubated overnight in the same buffer at 37°C. The gels were stained for 1 h with 0.5% Coomassie Brilliant Blue R-250 dissolved in 50% methanol and 5% acetic acid and subsequently destained for 2 h. Proteolytic activities were visualized by clear zones against a dark blue background indicating lysis of gelatin.
MMP-8 Western blot analysis.
Equal amounts (20 μl) of boiled BALF samples were subjected to 11% SDS-PAGE under reducing conditions and blotted to nitrocellulose membranes (Whatman, Dassel, Germany). Blots were blocked with PBS/0.1% Tween 20 (PBST) containing 5% milk proteins (Hercules, CA) for 1 h at room temperature and afterwards incubated overnight at 4°C with primary antibody (rabbit anti-MMP-8 polyclonal antibody, 1:500, Lifespan Biosciences) in PBST containing 2% milk proteins. After subsequent incubation with horseradish peroxidase (HRP)-conjugated secondary antibody (1:1,000; Dako, Glostrup, Denmark) for 1 h in PBST containing 2% milk proteins, the antibodies were visualized with commercial ECL reagents (Amersham Biosciences, Roosendaal, The Netherlands) and exposed to photographic film. Films were scanned on a GS710 Calibrated Imaging Densitometer (Bio-Rad Laboratories), and the optical density (OD) of the immunoreactive bands was quantified.
PE activity assay.
PE activity was measured in the lung homogenates using the fluorogenic substrate Z-Gly-Pro-7-amido-4-methylcoumarin (2-G-P-AMC) (Bachem) as previously described (22). Twenty microliters of the lung homogenate (1 μg/μl) was added to each well in a black 96-well flat-bottom plate, followed by addition of 80 μl of assay buffer (25 mM Tris, 0.25 M NaCl, pH 7.5, 2 mM DTT) containing 100 μM substrate Z-Gly-Pro-AMC. The fluorescence from liberated AMC was monitored every 1 min over 60 min at 37°C using a Fluostar reader at excitation wavelength of 355 nm and an emission wavelength of 460 nm. Fluorometric intensities observed were converted to pmol AMC released per minute using appropriate AMC standard curves. The enzyme activity is defined as pmol AMC released per minute at 37°C per 20 μg of protein.
Electrospray ionization liquid chromatography-MS/MS for PGP detection.
PGP and N-α-PGP were measured using a MDS Sciex (Applied Biosystems, Foster City, CA) API-4000 spectrometer equipped with a Shimadzu HPLC (Columbia, MD). HPLC was done using a 2.0 × 150-mm Jupiter 4u Proteo column (Phenomenex, Torrance, CA) with buffer A: 0.1% HCOOH and buffer B: MeCN + 0.1% HCOOH: 0 min-0.5 min 5% buffer B/95% buffer A, then increased over 0.5–2.5 min to 100% buffer B/0% buffer A. Background was removed by flushing with 100% isopropanol/0.1% formic acid. Positive electrospray mass transitions were at 270–70, 270–116, and 270–173 for PGP and 312–140 and 312–112 of AcPGP.
Histology and morphometric analysis.
Mice (n = 4–5), used for morphometric analysis, were killed by an intraperitoneal injection with an overdose of pentobarbital (Nembutal; Ceva Santé Animale, Naaldwijk, The Netherlands). The lungs were fixed with a 10% formalin infusion through a tracheal cannula at a constant pressure of 25 cmH2O (8). The left lung was immersed in fresh fixative for at least 24 h, after which it was embedded in paraffin. After paraffin embedding, 5-μm sections were cut and stained with hematoxylin/eosin (H&E) according to standard methods. Morphometric assessment of emphysema, including determination of the average interalveolar distance, was estimated by the mean linear intercept (Lm) analysis. The Lm was determined by light microscopy at a total magnification of ×100, whereby 24 random photomicroscopic images per left lung tissue section were evaluated by microscopic projection onto a reference grid. By dividing total grid length by the number of alveolar wall-grid line intersections, the Lm (in μm) was calculated (48).
Immunohistochemistry.
Paraffin sections were deparaffinized, and endogenous peroxidase activity was blocked with 0.3% H2O2 (Merck, Darmstadt, Germany) in methanol for 30 min at room temperature and rehydrated in a graded ethanol series to PBS. For antigen retrieval, the slides were boiled in 10 mM citrate buffer (pH 6.0) for 10 min in a microwave. The slides were cooled down to room temperature, rinsed with PBS (3×), and blocked with 5% goat serum (Dakocytomation, Glostrup, Denmark) in 1% bovine serum albumin in PBS for 30 min at room temperature. Sections were incubated with the primary antibody (rabbit anti-PE, 6 μg/ml, PREP antibody, ProteinTech Group) in 1% bovine serum albumin/PBS overnight at 4°C. The slides were rinsed with PBS (3×) and incubated with the biotinylated secondary antibody (goat anti-rabbit, 1:200, Dakocytomation) in 1% bovine serum albumin/PBS for 45 min at room temperature. The slides were rinsed with PBS (3×), and the biotinylated proteins were visualized by incubation with streptavidin-biotin complex/horseradish peroxidase (Vectastain Elite ABC, Vector Laboratories) for 45 min at room temperature, followed by 0.015% H2O2/0.05% diaminobenzidene (Sigma, Schneldorf, Germany)/0.05 M Tris-HCl (pH 7.6) for 10 min at room temperature. Sections were counterstained with Mayer hematoxylin (Merck), dehydrated, and mounted in Permount (Fisher Scientific). Negative controls without the primary antibody and normal rabbit IgG (AB-105-C, R&D Systems) were included as controls. Photomicrographs were taken with an Olympus BX50 microscope equipped with a Leica DFC 320 digital camera.
Statistical analysis.
Experimental results are expressed as means ± SE. Differences between groups were statistically determined by an unpaired two-tailed Student's t-test using GraphPad Prism (version 4.0). Pearson's correlation was used to compare the relationship between the different parameters. Calculations were made using SPSS version 16. Results were considered statistically significant when P < 0.05.
RESULTS
Cigarette smoke exposure induces neutrophilic airway inflammation.
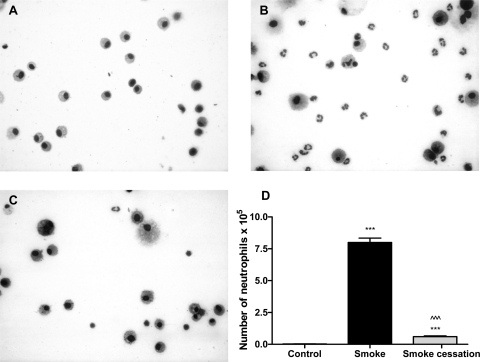
To investigate whether chronic smoke exposure induces neutrophilic airway inflammation, the alveolar lumen (by BALF) and the lung tissue of air- and smoke-exposed mice was analyzed. In cytospins, neutrophils were absent in the BALF of air-exposed mice (Fig. 1A), whereas the smoke-exposed mice developed a BALF neutrophilia (Fig. 1B). After the smoking cessation period of 8 wk, almost no neutrophils in the BALF were detected (Fig. 1C). Quantification of neutrophil cell counts on these cytospin preparations confirmed the observed increase of BALF neutrophils after smoke exposure and a decrease in neutrophil numbers after smoking cessation (Fig. 1D).
Fig. 1.
Neutrophil influx in the bronchoalveolar lavage (BALF) is related to smoke exposure. Representative photomicrographs of DiffQuick-stained cytospins on glass slides of BALF from air-exposed mice (A), smoke-exposed mice (B), and smoke-exposed mice 8 wk after smoke cessation (C). Magnification, ×1000. D: absolute neutrophil numbers in the BALF of mice exposed to air, mice exposed to cigarette smoke for 20 wk (black bar), and mice exposed to cigarette smoke for 20 wk + a smoking cessation period of 8 wk (gray bar). N = 4–5 animals/group. Values are expressed as means ± SE. ***P ≤ 0.001; significantly different from the control group. ^^^P ≤ 0.001; significantly different from the smoke group.
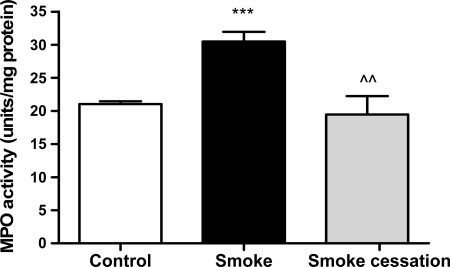
Neutrophil accumulation in lung tissue was quantitated by measuring MPO activity in lung homogenates. The MPO activity in the lung homogenates of mice exposed to cigarette smoke was significantly higher compared with control animals (Fig. 2). After a smoking cessation period of 8 wk, the smoke-induced increase in MPO activity was restored towards normal levels.
Fig. 2.
Elevated MPO activity in lung homogenates after chronic smoke exposure. MPO activity in lung homogenates of mice exposed to air (white bar), mice exposed to cigarette smoke for 20 wk (black bar), and mice exposed to cigarette smoke for 20 wk + a smoking cessation period of 8 wk (gray bar). N = 4–6 animals/group. Values are expressed as means ± SE. ***P ≤ 0.001; significantly different from the control group. ^^P ≤ 0.01; significantly different from the smoke group.
Cigarette smoke-induced increase in MMP-8 and MMP-9 levels in BALF and lung homogenates.
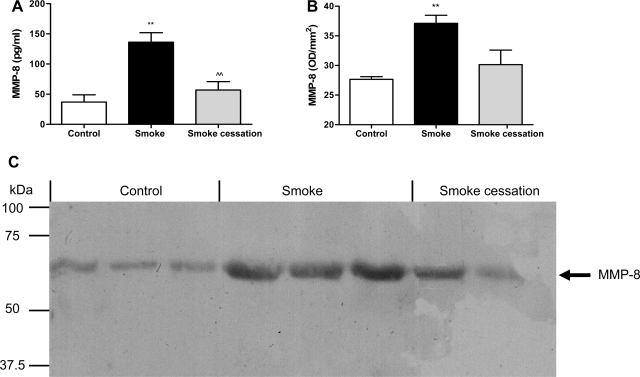
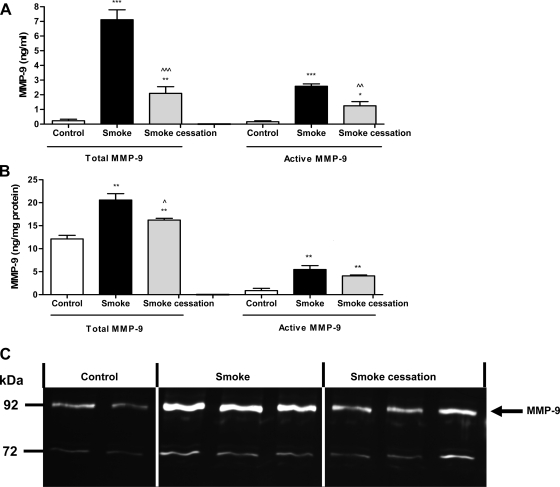
MMP-8 and MMP-9 are normally present in the granules of neutrophils and can be released following stimulation (6, 10, 11). Since an increased neutrophil cell count and MPO activity was observed in the mice exposed to cigarette smoke, the next step was measuring the MMP-8 and MMP-9 levels in the BALF and lung tissue homogenates of air- and smoke-exposed mice. An ELISA was performed to detect the total MMP-8 and total and pro-MMP-9 levels. The mice exposed to cigarette smoke for 20 wk showed significantly increased total MMP-8 levels in the BALF measured via ELISA (Fig. 3A). After smoking cessation, the concentrations of MMP-8 in the BALF were significantly decreased compared with smoke-exposed mice (Fig. 3A). To further support these data, BALF samples were also analyzed for MMP-8 levels via Western blotting. Via Western blot analysis, elevated MMP-8 levels were observed after a 20-wk smoke exposure, and, after a smoking cessation period of 8 wk, the MMP-8 levels were reduced (Fig. 3C). The molecular weight bands at ∼65 kDa likely represent active MMP-8. The optical density of the molecular weight bands was measured and depicted in Fig. 3B. Unfortunately, no clear differences in MMP-8 levels were observed in the lung homogenates. The mice exposed to cigarette smoke for 20 wk also showed increased total MMP-9 levels in the BALF (Fig. 4A) as well as in the lung homogenates (Fig. 4B). The active form of MMP-9 was also significantly elevated after smoke exposure (Fig. 4, A and B). After smoke cessation, the BALF and lung homogenate concentrations of both total and active MMP-9 were decreased compared with the smoke-exposed mice (Fig. 4, A and B). To further support these data, lung homogenates were analyzed for the presence and activity of MMP-9 by gelatin zymography. In lung homogenates of cigarette smoke-exposed mice, the MMP-9 gelatinase activity was higher than in lung homogenates of the control mice. After a smoking cessation period of 8 wk, reduced MMP-9 activity in gelatin zymography was observed compared with the smokers (Fig. 4C). Since the BALF (and lung homogenates) showed elevated activity of MMP-8 and MMP-9, the first necessary proteases to generate PGP from collagen are present during chronic smoke exposure.
Fig. 3.
Smoke-related MMP-8 increase in BALF. The total MMP-8 levels were determined in the BALF (A) after 20-wk air exposure (white bar), after 20-wk smoke exposure (black bar), and after 20-wk smoke exposure + a smoking cessation period of 8 wk (gray bar) via ELISA. N = 4–5 animals/group. Values are expressed as means ± SE. **P ≤ 0.01; significantly different from the control group. ^^P ≤ 0.01; significantly different from the smoke group. BALF samples from 3 control mice, 3 smoke-exposed mice, and 2 smoke-exposed mice after a smoking cessation period were randomly chosen and analyzed via Western blotting (C). The molecular weight bands at ∼65 kDa likely represent active MMP-8, and the optical density of these molecular weight bands was measured (B). The position of the bands for MMP-8 is indicated by an arrow.
Fig. 4.
Smoke-related MMP-9 increase in BALF and lung homogenates. The total and active MMP-9 levels were determined in the BALF (A) and lung homogenates (B) after 20-wk air exposure (white bars), after 20-wk smoke exposure (black bars), and after 20-wk smoke exposure + a smoking cessation period of 8 wk (gray bars). N = 4–5 animals/group. Values are expressed as means ± SE. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001; significantly different from the control group. ^P ≤ 0.05, ^^P ≤ 0.01, ^^^P ≤ 0.001; significantly different from the smoke group. Lung homogenates from 2 control mice, 3 smoke-exposed mice, and 3 smoke-exposed mice after a smoking cessation period were randomly chosen and analyzed by gelatin zymography (C). Gelatinolytic activity of MMP-9 was increased in the lung homogenates after 20 wk of smoke exposure compared with the air-exposed mice, whereas after smoking cessation, the activity of MMP-9 was decreased. The position of the bands for MMP-9 is indicated by an arrow.
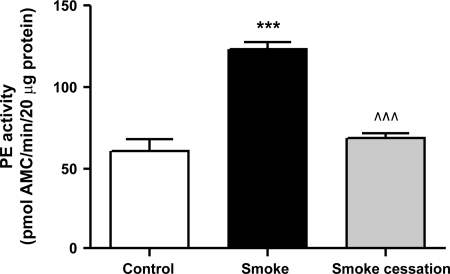
PE activity is elevated in lung homogenates after chronic smoke exposure.
The smaller collagen fragments generated by proteases, such as MMP-9, are hypothesized to be further cleaved by PE to the formation of PGP (4, 45). To determine whether PE may play a role in PGP generation related to lung emphysema, lung homogenates of the mice were examined for PE activity using a colorimetric assay. We found a twofold increase in PE activity in lung homogenates of smoke-exposed mice compared with air-exposed mice (Fig. 5). This increase returned to normal levels after a smoking cessation period of 8 wk. It can be concluded that PE activity is strongly related to cigarette smoke exposure.
Fig. 5.
Prolyl endopeptidase (PE) activity was elevated in lung homogenates after chronic smoke exposure. PE activity in lung homogenates of mice exposed to air (white bar), mice exposed to cigarette smoke for 20 wk (black bar), and mice exposed to cigarette smoke for 20 wk + a smoking cessation period of 8 wk (gray bar). N = 4–5 animals/group. Values are expressed as means ± SE. ***P ≤ 0.001; significantly different from the control group. ^^^P ≤ 0.001; significantly different from the smoke group.
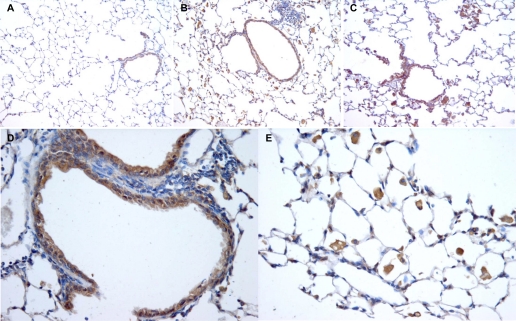
Various cell types in the lung express PE after chronic cigarette smoke exposure.
Immunohistochemical analysis was performed to identify which cell types in the lung tissue express PE and to compare the PE expression in the lung tissue of PBS-exposed mice, cigarette smoke-exposed mice, and cigarette smoke-exposed mice after a smoking cessation period of 8 wk. In the lung tissue of the air-exposed mice, the epithelial cells express PE (Fig. 6A), whereas in the lung tissue of the smoke-exposed mice, the epithelial cells as well as the inflammatory cells (macrophages and neutrophils) highly express PE (Fig. 6B). The inflammatory cells were still present in the lung tissue of the smoke-exposed mice after a smoking cessation period of 8 wk, and PE was expressed in the epithelial cells and in the inflammatory cells in the lung tissue of these animals (Fig. 6C). In Fig. 6D it is clearly demonstrated that murine lung epithelial cells contain PE, and Fig. 6E shows a profound staining of PE in inflammatory cells in the lung tissue.
Fig. 6.
Localization of PE in the lung. Representative photomicrographs of an immunohistological staining for PE (brown color, DAB staining) in lung tissue of air-exposed mice (A), smoke-exposed mice (B), and smoke-exposed mice after a smoking cessation period of 8 wk (C). Representative photomicrographs of lung tissue of a smoke-exposed mouse with a pronounced expression of PE in the epithelial cells (D) and the inflammatory cells (E). Magnification, ×200 (A–C), ×400 (D and E). N = 3 animals/group.
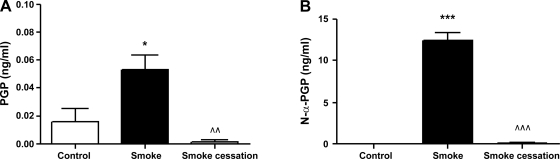
PGP and N-α-PGP levels are increased in BALF after chronic smoke exposure.
The components necessary for the PGP formation, namely MMP-8, MMP-9 and PE, were present in the airways of the mice after chronic smoke exposure. At this point, the MS technique of ESI-LC/MS/MS was used for examining PGP and N-α-PGP levels in BALF from mice before and after smoking cessation and healthy controls. PGP levels were significantly increased in the BALF of mice exposed to cigarette smoke compared with the control animals. After smoking cessation, the PGP levels were returned to basal levels (Fig. 7A). Figure 7B shows that the BALF of all cigarette smoke-exposed mice were positive for N-α-PGP above our detection limit of 10 pg/ml, but all control BALF samples and the samples of the mice after smoking cessation were negative. These data pointed out that the PGP generation is related to chronic cigarette smoke exposure.
Fig. 7.
Proline-glycine-proline (PGP) and N-α-PGP were detectable in BALF after chronic smoke exposure. The PGP (A) and N-α-PGP (B) levels were determined in the BALF after 20-wk air exposure (white bars), after 20-wk smoke exposure (black bars), and after 20-wk smoke exposure + a smoking cessation period of 8 wk (gray bars). N = 4–5 animals/group. Values are expressed as means ± SE. *P ≤ 0.05, ***P ≤ 0.001; significantly different from the control group. ^^P ≤ 0.01, ^^^P ≤ 0.001; significantly different from the smoke group.
Chronic cigarette smoke exposure induces irreversible alveolar enlargement.
PGP and N-α-PGP levels were observed in the BALF of smoke-exposed mice, which indicates that collagen breakdown occurs in these airways. To ensure that tissue degradation was present after chronic smoke exposure, the mean linear intercept, a quantification method for alveolar size, was used to quantify the presence and severity of emphysema. Significant air space enlargement was observed in the mice after 20 wk of cigarette smoke exposure (Lm of the air-exposed mice: 42.5 ± 0.8 μm vs. the Lm of the smoke-exposed mice: 52.6 ± 1.7 μm, P < 0.01). Furthermore, the air space enlargement induced by cigarette smoke exposure was not reversible, since the increased Lm was not reduced after a period of 8 wk without cigarette smoke exposure (Lm after smoking cessation: 49.6 ± 1.4 μm).
Correlations between different parameters.
The air-exposed mice and the smoke-exposed mice were grouped together, and the relationship between the various parameters was calculated. The most relevant correlations are described in Table 1. First, when we correlated the amount of neutrophils in the BALF and the MMP8 and MMP-9 levels in the BALF, the correlation coefficient (R2) was high (Table 1). The MPO activity and the MMP-9 levels in the lung homogenates were also strongly correlated. A highly significant correlation was found between the neutrophils in the BALF and the PE activity. Furthermore, a tight correlation between the neutrophil influx in the BALF and the N-α-PGP levels in the BALF was observed (Table 1). The neutrophil influx was also significantly correlated with the PGP levels in the BALF. Moreover, the MMP-8 and MMP-9 levels as well as the PE activity revealed strong correlation with the N-α-PGP levels in the BALF, which indicates that both proteases are related to N-α-PGP formation. The MMP-9 levels, the PE activity, and the N-α-PGP levels showed a significant association with the lung destruction measured by the mean linear intercept (Table 1), indicating that MMP-9, PE, and N-α-PGP are related to the development of lung emphysema.
Table 1.
Correlations between the different parameters measured in the airways of air- and smoke-exposed mice
| Parameter | Versus | R2 |
|---|---|---|
| Neutrophils | MMP-8 in BALF | 0.863*** |
| Neutrophils | MMP-9 in BALF | 0.977*** |
| Neutrophils | PE activity | 0.957*** |
| Neutrophils | N-α-PGP | 0.987*** |
| MMP-8 in BALF | N-α-PGP | 0.823*** |
| MMP-9 in BALF | N-α-PGP | 0.961*** |
| PE activity | N-α-PGP | 0.925*** |
| MMP-9 in BALF | Lm | 0.890** |
| PE activity | Lm | 0.821** |
| N-α-PGP | Lm | 0.916** |
Lm, mean linear intercept.
P < 0.01;
P < 0.001.
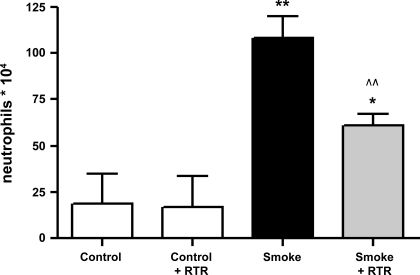
Cigarette smoke-induced neutrophil influx in the BALF is significantly decreased after RTR administration.
To investigate whether inhibition of PGP in the airways can inhibit or prevent the neutrophil migration to the lung, the complementary peptide l-arginine-threonine-arginine (RTR), which can bind to PGP sequences (49), was tested in a murine smoking model of 5 days' cigarette smoke exposure. We observed a significant increase of BALF neutrophils after 5 days' smoke exposure compared with the air-exposed mice. The cigarette smoke-induced neutrophil influx in the BALF was significantly decreased after RTR administration (Fig. 8).
Fig. 8.
Cigarette smoke-induced neutrophil influx in the BALF was significantly decreased after l-arginine-threonine-arginine (RTR) administration. Absolute neutrophil numbers in the BALF of mice exposed to air, RTR-treated mice exposed to air, mice exposed to cigarette smoke, and RTR-treated mice exposed to cigarette smoke for 5 days. The mice received vehicle (PBS) or RTR by oropharyngeal aspiration (50 μg/50 μl PBS) twice daily. N = 4–6 animals/group. Values are expressed as means ± SE. *P ≤ 0.05, **P ≤ 0.01; significantly different from the control group. ^^P ≤ 0.01; significantly different from the smoke group.
DISCUSSION
In this report, we describe the involvement of PE in the development of lung emphysema. Since lung emphysema is characterized by the destruction of alveolar walls, the role of proteases is extensively studied in this disease (13). Neutrophil elastase and MMPs have attracted the most attention, whereas little is known about PE in inflammation biology or lung pathology. PE activity has been detected in a variety of tissues and body fluids, and various physiological roles are suggested (31), such as neuropeptide processing and secretion related to psychiatric diseases (29, 43) and affecting the renin-angiotensin system (54). Gaggar et al. (21) were the first who demonstrated that PE could be involved in airway inflammation, showing an elevated PE activity in sputum from cystic fibrosis patients. They identified a novel pathway for generating the tripeptide PGP from collagen, in which PE is involved. This pathway is based on in vitro experiments, where sputum from cystic fibrosis and COPD patients has the ability to generate PGP de novo from collagen, and they demonstrated a role for MMP-1, MMP-8, MMP-9, and PE in this process. These data were further expanded with in vivo murine data, where PGP production in the BALF was examined after intratracheal administration of MMPs or human neutrophil elastase (HNE) with or without PE (36). At first, this pathway for generating chemotactic PGP from collagen can be activated via an inflammatory stimulus, such as CXCL8, which will lead to the recruitment and activation of neutrophils. In lung emphysema, this cascade will start with cigarette smoke exposure to the lungs, which was mimicked in the present murine lung emphysema model. After chronic cigarette smoke exposure, an excessive neutrophil influx in the BALF and an increased MPO activity in the lung tissue was observed, which is in agreement with other in vivo lung emphysema studies (14, 20, 32). Second, when neutrophils are activated, they will release different proteases, like MMP-8 and MMP-9, since neutrophils are a rich source of these MMPs (11, 35). There is an abundance of literature regarding MMP-9 related to lung pathology, reviewed in Atkinson and Senior (2). In our study, the total and active MMP-8 and MMP-9 levels were increased in the BALF of the smoke-exposed mice compared with the control animals. Other in vivo studies also demonstrated increased MMP-9 levels in the BALF of mice after cigarette smoke exposure (44, 52). Additionally, elevated MMP-8 and MMP-9 levels were observed in the airways of COPD patients compared with healthy controls (6, 9, 51). In this study, the MMP-8 and MMP-9 levels in the airways are most probably related to the amount of neutrophils as reflected in the high correlation coefficient. Besides neutrophils, alveolar macrophages and lymphocytes can also synthesize and release MMP-9 (2), which could explain the difference between the normalized MPO activity in the lung tissue after 8 wk of smoking cessation and the MMP-9 levels in the lung homogenates, which did not return completely to basal level. MMP-8 and MMP-9 have been proposed to induce airway remodeling, because of their capacity to cleave structural proteins, such as collagens and elastin (34). At this point, collagen in the lung can be cleaved in smaller fragments caused by the release of MMP-8 and MMP-9 in the airways after smoke exposure. MMPs alone are not sufficient to generate PGP from collagen. Therefore, the next step was measuring PE activity in the lung homogenates. To our knowledge, PE is the only enzyme directly capable of cleaving PGP from shorter portions of collagen, like peptides and oligopeptides, generated by the prior digestion of MMPs (4, 45). In this lung emphysema model, a significant increase in PE activity was observed in the lung homogenates of smoke-exposed mice compared with air-exposed mice. Furthermore, we can emphasize that both MMP-8/MMP-9 and PE are important for the formation of N-α-PGP from collagen, since the MMP-8/MMP-9 levels and the PE activity were strongly correlated to the N-α-PGP levels in the BALF. From our immunohistological staining for PE, it can be concluded that the PE expression is increased in lung tissue of cigarette smoke-exposed mice compared with air-exposed mice. This increase in PE expression is caused by the inflammatory cell influx in the lungs of the smoke-exposed mice, since the inflammatory cells (macrophages and neutrophils) highly express PE. The epithelial cells in the lung tissue of both the air-exposed mice and the cigarette smoke-exposed mice contain PE, concluding that epithelial cells are an important source for PE in the lung. Exposure of human bronchial epithelial cells to cigarette smoke extract for 16 h in vitro leads to an elevated PE activity in the supernatant (E. Mortaz, unpublished data), suggesting that cigarette smoke exposure might lead to a release of PE. However, PE could also be released as a result of airway epithelial necrosis or necrosis of other inflammatory cell types (macrophages and neutrophils) after cigarette smoke exposure. After smoking cessation, the inflammatory cells were still present in the lung tissue and express PE, but the PE activity in the lung homogenates returned to normal levels. One explanation could be that the activity of PE is decreased in the inflammatory cells despite that they contain PE. However, Polgar (40) described that PE does not have a zymogen (or proenzyme) form and is synthesized as an active peptidase. Second, the PE activity could also be related to the amount of neutrophils in the lung tissue, which was also reduced after smoking cessation, since the MPO activity in these lung homogenates was returned to normal levels. Recently, O'Reilly et al. (37) described that neutrophils contain PE and are capable themselves to generate PGP from collagen after LPS exposure. In our study, we found a strong correlation between the neutrophils in the BALF and the PE activity, indicating that the PE activity is related to the neutrophil influx. As with MMPs, PE can be released by neutrophils through degranulation in response to proinflammatory stimuli and could both play a role in a wide variety of chronic inflammatory diseases. Whether cigarette smoke exposure is able to activate this pathway for generating PGP from collagen is the subject of ongoing studies. Furthermore, it has also been demonstrated that PE is present in both macrophages and lymphocytes (27, 50), while its presence in other cell types is as yet unknown.
All the components (neutrophils, MMPs, and PE) necessary for the generation of PGP from lung collagen were present in the airways of the mice after chronic smoke exposure. Consequently, PGP and N-α-PGP levels were detected in the BALF of cigarette smoke-exposed mice. Low levels of PGP were observed in the BALF of air-exposed mice, which could be a result of normal collagen turnover. Unlike PGP, no N-α-PGP was detected in the BALF of air-exposed mice, suggesting that the acetylation of PGP might be an important step in the pathogenesis of lung emphysema. In vitro, it was shown that N-α-PGP is four to seven times more potent as a neutrophil chemoattractant than PGP (24). Moreover, in sputum of COPD patients, the PGP levels were also significantly increased compared with healthy controls (58 ± 12 ng/ml vs. 22 ± 12 ng/ml, P < 0.05) (36). The N-α-PGP levels were much higher in the BALF of smoke-exposed mice compared with the levels in sputum of COPD patients (36). A possible explanation for this could be that the subjects were stable outpatients with advanced COPD, and most PGP generation might occur early when matrix destruction is most active. Snelgrove et al. (46) observed that acetylation of PGP occurred when PGP was incubated with different concentrations cigarette smoke condensate. The extent of smoking will be related to the amount of N-α-PGP, which could be another possible explanation for the differences between the human and mice observations. However, it is still very difficult to compare the results from sputum of COPD patients with the results of the BALF of the smoke-exposed mice.
The correlation between N-α-PGP/PGP and neutrophils in the BALF could imply that N-α-PGP and PGP are neutrophil chemoattractants or are both generated due to neutrophilic airway inflammation. It is difficult to conclude that the observed increase in PGP levels is only neutrophil related; however, different results pointed out that the neutrophils play an important role in this process. First, the amount of neutrophils is extremely increased after cigarette smoke exposure. Moreover, the PE staining pointed out that neutrophils are an important source of PE. Furthermore, we found strong correlations between the neutrophil influx, the PE activity, and the N-α-PGP levels. It would be very interesting for future research to repeat this study in neutrophil-depleted animals to investigate whether this cascade of events leading to PGP formation is really neutrophil dependent.
The importance of PGP in chronic inflammatory diseases, characterized by neutrophils as potential target cells, is reinforced by several other studies. First, in COPD patients, PGP was observed in the BALF, but also in sputum and serum (36, 53), suggesting a local as well as a systemic effect for PGP in COPD. In vivo research pointed out that PGP generation correlates well with a neutrophil influx in the BALF (21). Furthermore, neutrophil infiltration and lung emphysema were observed in mice after chronic airway exposure to PGP (49, 53). PGP was also measured in the BALF of mice after exposure to aerosolized LPS (53). Finally, PGP derived from collagen was identified as being chemotactic for neutrophils in vitro as well as in vivo (39, 53).
The importance of PGP in neutrophil migration to the lung in a cigarette smoke exposure model is now enforced, since the cigarette smoke-induced neutrophil influx was significantly decreased in the BALF after RTR administration. In addition, van Houwelingen et al. (49) found that RTR reduces the emphysema-like changes in the airways after LPS and PGP exposure. We propose that after PGP formation from the extracellular matrix, the neutrophils in the airways of the smoke-exposed mice are attracted and activated by both PGP and the mouse equivalents of CXCL8. Subsequently, the neutrophils will release more proteases, like MMP-8, MMP-9 and PE, ultimately leading to lung tissue degradation and ongoing PGP formation. This vicious circle (Fig. 9) of events initiated by cigarette smoking is a continuous process and may potentially end in the development of lung emphysema, which was also observed in the present murine model via Lm analysis. All the different components (MMP-8, MMP-9, PE, and N-α-PGP) were significantly correlated to the alveolar enlargement, suggesting a role for these components in the pathophysiology of lung emphysema.
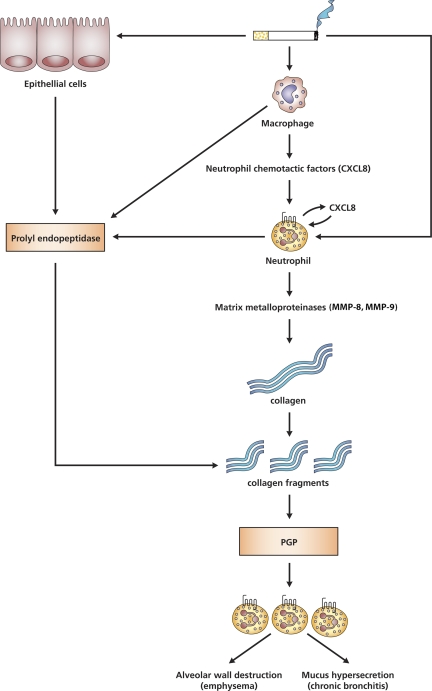
Fig. 9.
PGP generation is a multistep process. In lung emphysema, the cascade of events leading to PGP formation will start with cigarette smoke exposure. Cigarette smoke can stimulate alveolar macrophages to release several chemoattractants, such as CXCL8. Subsequently, CXCL8 facilitates the migration of neutrophils to the site of inflammation. The activated neutrophils are also capable themselves to produce CXCL8, and the cigarette smoke exposure will also affect these neutrophils. PGP formation is a multistep process initially involving release of proteases from the MMP family, like MMP-8 and MMP-9. MMP-8 and MMP-9 are released by activated neutrophils and can proteolytically cleave collagen to smaller fragments resulting in an optimal substrate for PE activity. These collagen fragments are then further cleaved to PGP by PE, a member of the serine protease family. Various cell types, like neutrophils, macrophages, and epithelial cells, express PE. The generated PGP is chemotactic for neutrophils and results in an environment of chronic inflammation with proteolytic damage and PGP formation. Finally, this will lead to alveolar wall destruction (emphysema) and mucus hypersecretion (chronic bronchitis).
After a smoking cessation period of 8 wk, the neutrophilic airway inflammation, the MMP-8 and MMP-9 levels, the PE activity, and the PGP formation in the lungs are all decreased or reduced to normal levels. Other studies also pointed out that after smoking cessation, the neutrophil levels and MMP-9 levels in the BALF were diminished (32, 44). Unlike all other parameters in our study, lung emphysema was still present after smoking cessation, suggesting that the alveolar wall destruction is not reversible. Consequently, smoking cessation can interrupt the vicious circle of an ongoing neutrophil influx into the lung. This will result in a decrease in neutrophils and proteases, which will stop the lung matrix breakdown, and thus the PGP formation, while the lung emphysema stays present. This suggests that PE activity and PGP formation are associated with cigarette smoke exposure and not directly with emphysema. Additionally, after 1 wk of smoke exposure, the PE activity and PGP levels were also increased in the lung (data not shown), but not as obviously as after 20 wk of smoke exposure. This indicates that PE and N-α-PGP could be biomarkers for the state of inflammation during chronic inflammatory diseases.
In conclusion, in the present murine model of cigarette smoke-induced lung emphysema, it is demonstrated for the first time that all relevant components (neutrophils, MMP-8/MMP-9, and PE), which may contribute to the neutrophilic airway inflammation by generating PGP from lung collagen, were upregulated in the airways and were related to alveolar destruction.
GRANTS
This work was performed within the framework of the Dutch Top Institute Pharma Project T1-103. This project was supported in part by National Heart, Lung, and Blood Institute (NHLBI) Grants HL-07783, HL-090999, and HL-087824 (to J. E. Blalock).
DISCLOSURES
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NHLBI or National Institutes of Health. No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Kim Verheijden for excellent technical assistance.
REFERENCES
- 1. Abboud RT, Vimalanathan S. Pathogenesis of COPD. Part I. The role of protease-antiprotease imbalance in emphysema. Int J Tuberc Lung Dis 12: 361–367, 2008 [PubMed] [Google Scholar]
- 2. Atkinson JJ, Senior RM. Matrix metalloproteinase-9 in lung remodeling. Am J Respir Cell Mol Biol 28: 12–24, 2003 [DOI] [PubMed] [Google Scholar]
- 3. Barnes PJ. Chronic obstructive pulmonary disease. N Engl J Med 343: 269–280, 2000 [DOI] [PubMed] [Google Scholar]
- 4. Barrett AJ, Rawlings ND. Oligopeptidases, and the emergence of the prolyl oligopeptidase family. Biol Chem Hoppe Seyler 373: 353–360, 1992 [DOI] [PubMed] [Google Scholar]
- 5. Belvisi MG, Bottomley KM. The role of matrix metalloproteinases (MMPs) in the pathophysiology of chronic obstructive pulmonary disease (COPD): a therapeutic role for inhibitors of MMPs? Inflamm Res 52: 95–100, 2003 [DOI] [PubMed] [Google Scholar]
- 6. Betsuyaku T, Nishimura M, Takeyabu K, Tanino M, Venge P, Xu S, Kawakami Y. Neutrophil granule proteins in bronchoalveolar lavage fluid from subjects with subclinical emphysema. Am J Respir Crit Care Med 159: 1985–1991, 1999 [DOI] [PubMed] [Google Scholar]
- 7. Braber S, Henricks PA, Nijkamp FP, Kraneveld AD, Folkerts G. Inflammatory changes in the airways of mice caused by cigarette smoke exposure are only partially reversed after smoking cessation. Respir Res 11: 99, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Braber S, Verheijden KA, Henricks PA, Kraneveld AD, Folkerts G. A comparison of fixation methods on lung morphology in a murine model of emphysema. Am J Physiol Lung Cell Mol Physiol 299: L843–L851, 2010 [DOI] [PubMed] [Google Scholar]
- 9. Cataldo D, Munaut C, Noel A, Frankenne F, Bartsch P, Foidart JM, Louis R. MMP-2- and MMP-9-linked gelatinolytic activity in the sputum from patients with asthma and chronic obstructive pulmonary disease. Int Arch Allergy Immunol 123: 259–267, 2000 [DOI] [PubMed] [Google Scholar]
- 10. Chakrabarti S, Patel KD. Regulation of matrix metalloproteinase-9 release from IL-8-stimulated human neutrophils. J Leukoc Biol 78: 279–288, 2005 [DOI] [PubMed] [Google Scholar]
- 11. Chakrabarti S, Zee JM, Patel KD. Regulation of matrix metalloproteinase-9 (MMP-9) in TNF-stimulated neutrophils: novel pathways for tertiary granule release. J Leukoc Biol 79: 214–222, 2006 [DOI] [PubMed] [Google Scholar]
- 12. Chung KF. Cytokines in chronic obstructive pulmonary disease. Eur Respir J Suppl 34: 50s–59s, 2001 [PubMed] [Google Scholar]
- 13. Churg A, Wang RD, Tai H, Wang X, Xie C, Wright JL. Tumor necrosis factor-alpha drives 70% of cigarette smoke-induced emphysema in the mouse. Am J Respir Crit Care Med 170: 492–498, 2004 [DOI] [PubMed] [Google Scholar]
- 14. D'Hulst AI, Vermaelen KY, Brusselle GG, Joos GF, Pauwels RA. Time course of cigarette smoke-induced pulmonary inflammation in mice. Eur Respir J 26: 204–213, 2005 [DOI] [PubMed] [Google Scholar]
- 15. De Vooght V, Vanoirbeek JA, Haenen S, Verbeken E, Nemery B, Hoet PH. Oropharyngeal aspiration: an alternative route for challenging in a mouse model of chemical-induced asthma. Toxicology 259: 84–89, 2009 [DOI] [PubMed] [Google Scholar]
- 16. Demedts IK, Brusselle GG, Bracke KR, Vermaelen KY, Pauwels RA. Matrix metalloproteinases in asthma and COPD. Curr Opin Pharmacol 5: 257–263, 2005 [DOI] [PubMed] [Google Scholar]
- 17. Di Stefano A, Capelli A, Lusuardi M, Balbo P, Vecchio C, Maestrelli P, Mapp CE, Fabbri LM, Donner CF, Saetta M. Severity of airflow limitation is associated with severity of airway inflammation in smokers. Am J Respir Crit Care Med 158: 1277–1285, 1998 [DOI] [PubMed] [Google Scholar]
- 18. Djekic UV, Gaggar A, Weathington NM. Attacking the multi-tiered proteolytic pathology of COPD: new insights from basic and translational studies. Pharmacol Ther 121: 132–146, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Folkerts G, Kraneveld AD, Nijkamp FP. New endogenous CXC chemokine ligands as potential targets in lung emphysema. Trends Pharmacol Sci 29: 181–185, 2008 [DOI] [PubMed] [Google Scholar]
- 20. Foronjy RF, Mirochnitchenko O, Propokenko O, Lemaitre V, Jia Y, Inouye M, Okada Y, D'Armiento JM. Superoxide dismutase expression attenuates cigarette smoke- or elastase-generated emphysema in mice. Am J Respir Crit Care Med 173: 623–631, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gaggar A, Jackson PL, Noerager BD, O'Reilly PJ, McQuaid DB, Rowe SM, Clancy JP, Blalock JE. A novel proteolytic cascade generates an extracellular matrix-derived chemoattractant in chronic neutrophilic inflammation. J Immunol 180: 5662–5669, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Goossens F, De Meester I, Van hoof G, Scharpe S. A sensitive method for the assay of serum prolyl endopeptidase. Eur J Clin Chem Clin Biochem 30: 235–238, 1992 [PubMed] [Google Scholar]
- 23. Guerassimov A, Hoshino Y, Takubo Y, Turcotte A, Yamamoto M, Ghezzo H, Triantafillopoulos A, Whittaker K, Hoidal JR, Cosio MG. The development of emphysema in cigarette smoke-exposed mice is strain dependent. Am J Respir Crit Care Med 170: 974–980, 2004 [DOI] [PubMed] [Google Scholar]
- 24. Haddox JL, Pfister RR, Muccio DD, Villain M, Sommers CI, Chaddha M, Anantharamaiah GM, Brouillette WJ, DeLucas LJ. Bioactivity of peptide analogs of the neutrophil chemoattractant, N-acetyl-proline-glycine-proline. Invest Ophthalmol Vis Sci 40: 2427–2429, 1999 [PubMed] [Google Scholar]
- 25. Hogg JC. Pathophysiology of airflow limitation in chronic obstructive pulmonary disease. Lancet 364: 709–721, 2004 [DOI] [PubMed] [Google Scholar]
- 26. Kruidenier L, Kuiper I, Van Duijn W, Mieremet-Ooms MA, van Hogezand RA, Lamers CB, Verspaget HW. Imbalanced secondary mucosal antioxidant response in inflammatory bowel disease. J Pathol 201: 17–27, 2003 [DOI] [PubMed] [Google Scholar]
- 27. Lesser M, Chang JC, Orlowski J, Kilburn KH, Orlowski M. Cathepsin B and prolyl endopeptidase activity in rat peritoneal and alveolar macrophages. Stimulation of peritoneal macrophages by saline lavage. J Lab Clin Med 101: 327–334, 1983 [PubMed] [Google Scholar]
- 28. Maeno T, Houghton AM, Quintero PA, Grumelli S, Owen CA, Shapiro SD. CD8+ T cells are required for inflammation and destruction in cigarette smoke-induced emphysema in mice. J Immunol 178: 8090–8096, 2007 [DOI] [PubMed] [Google Scholar]
- 29. Maes M, Goossens F, Scharpe S, Calabrese J, Desnyder R, Meltzer HY. Alterations in plasma prolyl endopeptidase activity in depression, mania, and schizophrenia: effects of antidepressants, mood stabilizers, and antipsychotic drugs. Psychiatry Res 58: 217–225, 1995 [DOI] [PubMed] [Google Scholar]
- 30. Mannino DM. Chronic obstructive pulmonary disease: definition and epidemiology. Respir Care 48: 1185–1191; discussion 1191–1193, 2003 [PubMed] [Google Scholar]
- 31. Mannisto PT, Venalainen J, Jalkanen A, Garcia-Horsman JA. Prolyl oligopeptidase: a potential target for the treatment of cognitive disorders. Drug News Perspect 20: 293–305, 2007 [DOI] [PubMed] [Google Scholar]
- 32. March TH, Wilder JA, Esparza DC, Cossey PY, Blair LF, Herrera LK, McDonald JD, Campen MJ, Mauderly JL, Seagrave J. Modulators of cigarette smoke-induced pulmonary emphysema in A/J mice. Toxicol Sci 92: 545–559, 2006 [DOI] [PubMed] [Google Scholar]
- 33. Mukaida N. Pathophysiological roles of interleukin-8/CXCL8 in pulmonary diseases. Am J Physiol Lung Cell Mol Physiol 284: L566–L577, 2003 [DOI] [PubMed] [Google Scholar]
- 34. Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res 69: 562–573, 2006 [DOI] [PubMed] [Google Scholar]
- 35. O'Connor CM, FitzGerald MX. Matrix metalloproteases and lung disease. Thorax 49: 602–609, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. O'Reilly P, Jackson PL, Noerager B, Parker S, Dransfield M, Gaggar A, Blalock JE. N-alpha-PGP and PGP, potential biomarkers and therapeutic targets for COPD. Respir Res 10: 38, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. O'Reilly PJ, Hardison MT, Jackson PL, Xu X, Snelgrove RJ, Gaggar A, Galin FS, Blalock JE. Neutrophils contain prolyl endopeptidase and generate the chemotactic peptide, PGP, from collagen. J Neuroimmunol 217: 51–54, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pesci A, Balbi B, Majori M, Cacciani G, Bertacco S, Alciato P, Donner CF. Inflammatory cells and mediators in bronchial lavage of patients with chronic obstructive pulmonary disease. Eur Respir J 12: 380–386, 1998 [DOI] [PubMed] [Google Scholar]
- 39. Pfister RR, Haddox JL, Sommers CI. Injection of chemoattractants into normal cornea: a model of inflammation after alkali injury. Invest Ophthalmol Vis Sci 39: 1744–1750, 1998 [PubMed] [Google Scholar]
- 40. Polgar L. The prolyl oligopeptidase family. Cell Mol Life Sci 59: 349–362, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Retamales I, Elliott WM, Meshi B, Coxson HO, Pare PD, Sciurba FC, Rogers RM, Hayashi S, Hogg JC. Amplification of inflammation in emphysema and its association with latent adenoviral infection. Am J Respir Crit Care Med 164: 469–473, 2001 [DOI] [PubMed] [Google Scholar]
- 42. Saetta M, Di Stefano A, Turato G, Facchini FM, Corbino L, Mapp CE, Maestrelli P, Ciaccia A, Fabbri LM. CD8+ T-lymphocytes in peripheral airways of smokers with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 157: 822–826, 1998 [DOI] [PubMed] [Google Scholar]
- 43. Schulz I, Zeitschel U, Rudolph T, Ruiz-Carrillo D, Rahfeld JU, Gerhartz B, Bigl V, Demuth HU, Rossner S. Subcellular localization suggests novel functions for prolyl endopeptidase in protein secretion. J Neurochem 94: 970–979, 2005 [DOI] [PubMed] [Google Scholar]
- 44. Seagrave J, Barr EB, March TH, Nikula KJ. Effects of cigarette smoke exposure and cessation on inflammatory cells and matrix metalloproteinase activity in mice. Exp Lung Res 30: 1–15, 2004 [DOI] [PubMed] [Google Scholar]
- 45. Shan L, Mathews II, Khosla C. Structural and mechanistic analysis of two prolyl endopeptidases: role of interdomain dynamics in catalysis and specificity. Proc Natl Acad Sci USA 102: 3599–3604, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Snelgrove RJ, Jackson PL, Hardison MT, Noerager BD, Kinloch A, Gaggar A, Shastry S, Rowe SM, Shim YM, Hussell T, Blalock JE. A critical role for LTA4H in limiting chronic pulmonary neutrophilic inflammation. Science 330: 90–94, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Stockley RA. Neutrophils and the pathogenesis of COPD. Chest 121: 151S–155S, 2002 [DOI] [PubMed] [Google Scholar]
- 48. Thurlbeck WM. Measurement of pulmonary emphysema. Am Rev Respir Dis 95: 752–764, 1967 [DOI] [PubMed] [Google Scholar]
- 49. van Houwelingen AH, Weathington NM, Verweij V, Blalock JE, Nijkamp FP, Folkerts G. Induction of lung emphysema is prevented by l-arginine-threonine-arginine. FASEB J 22: 3403–3408, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Van hoof G, Goossens F, Hendriks L, De Meester I, Hendriks D, Vriend G, Van Broeckhoven C, Scharpe S. Cloning and sequence analysis of the gene encoding human lymphocyte prolyl endopeptidase. Gene 149: 363–366, 1994 [DOI] [PubMed] [Google Scholar]
- 51. Vernooy JH, Lindeman JH, Jacobs JA, Hanemaaijer R, Wouters EF. Increased activity of matrix metalloproteinase-8 and matrix metalloproteinase-9 in induced sputum from patients with COPD. Chest 126: 1802–1810, 2004 [DOI] [PubMed] [Google Scholar]
- 52. Vlahos R, Bozinovski S, Jones JE, Powell J, Gras J, Lilja A, Hansen MJ, Gualano RC, Irving L, Anderson GP. Differential protease, innate immunity, and NF-κB induction profiles during lung inflammation induced by subchronic cigarette smoke exposure in mice. Am J Physiol Lung Cell Mol Physiol 290: L931–L945, 2006 [DOI] [PubMed] [Google Scholar]
- 53. Weathington NM, van Houwelingen AH, Noerager BD, Jackson PL, Kraneveld AD, Galin FS, Folkerts G, Nijkamp FP, Blalock JE. A novel peptide CXCR ligand derived from extracellular matrix degradation during airway inflammation. Nat Med 12: 317–323, 2006 [DOI] [PubMed] [Google Scholar]
- 54. Welches WR, Brosnihan KB, Ferrario CM. A comparison of the properties and enzymatic activities of three angiotensin processing enzymes: angiotensin converting enzyme, prolyl endopeptidase and neutral endopeptidase 24.11. Life Sci 52: 1461–1480, 1993 [DOI] [PubMed] [Google Scholar]
- 55. Yao H, Edirisinghe I, Rajendrasozhan S, Yang SR, Caito S, Adenuga D, Rahman I. Cigarette smoke-mediated inflammatory and oxidative responses are strain-dependent in mice. Am J Physiol Lung Cell Mol Physiol 294: L1174–L1186, 2008 [DOI] [PubMed] [Google Scholar]