Abstract
Ozone causes persistent airway hyperreactivity in humans and animals. One day after ozone exposure, airway hyperreactivity is mediated by release of eosinophil major basic protein that inhibits neuronal M2 muscarinic receptors, resulting in increased acetylcholine release and increased smooth muscle contraction in guinea pigs. Three days after ozone, IL-1β, not eosinophils, mediates ozone-induced airway hyperreactivity, but the mechanism at this time point is largely unknown. IL-1β increases NGF and the tachykinin substance P, both of which are involved in neural plasticity. These experiments were designed to test whether there is a role for NGF and tachykinins in sustained airway hyperreactivity following a single ozone exposure. Guinea pigs were exposed to filtered air or ozone (2 parts per million, 4 h). In anesthetized and vagotomized animals, ozone potentiated vagally mediated airway hyperreactivity 24 h later, an effect that was sustained over 3 days. Pretreatment with antibody to NGF completely prevented ozone-induced airway hyperreactivity 3 days, but not 1 day, after ozone and significantly reduced the number of substance P-positive airway nerve bundles. Three days after ozone, NK1 and NK2 receptor antagonists also blocked this sustained hyperreactivity. Although the effect of inhibiting NK2 receptors was independent of ozone, the NK1 receptor antagonist selectively blocked vagal hyperreactivity 3 days after ozone. These data confirm mechanisms of ozone-induced airway hyperreactivity change over time and demonstrate 3 days after ozone that there is an NGF-mediated role for substance P, or another NK1 receptor agonist, that enhances acetylcholine release and was not present 1 day after ozone.
Keywords: neural plasticity, pollution, tachykinin receptors
exposure to ozone induces airway hyperreactivity in humans (15, 44) and animals (50, 56) that occurs immediately after exposure and lasts several days. Acute, ozone-induced hyperreactivity is mediated by release of major basic protein from eosinophils resident in the lungs (65). Eosinophil major basic protein blocks inhibitory M2 muscarinic receptors that normally limit acetylcholine release from parasympathetic nerves, increasing acetylcholine release and causing airway hyperreactivity (14, 17). Airway smooth muscle is not immediately affected by ozone (65), thus acute ozone-induced hyperreactivity is solely a function of increased acetylcholine release from parasympathetic nerves. This explains why ozone-induced hyperreactivity in animals is prevented by anticholinergics (64), vagal sectioning (40), and depleting eosinophils and eosinophil major basic protein (65). Parasympathetic nerves are also involved in ozone-induced hyperreactivity in humans since airway hyperreactivity is prevented by atropine (3, 19).
There is also a secondary, systemic response to ozone. Environmental exposure to ozone is associated with increased ischemic stroke (37), ventricular arrhythmias (46), myocardial infarction (47), and increased cardiovascular mortality (32). However, these events occur largely several days after exposure to ozone (37, 47), suggesting a systemic inflammatory response that evolves over several days. Evidence for a systemic inflammatory response also comes from a study demonstrating that ozone significantly increases C-reactive protein, fibrinogen, and plasminogen activator fibrinogen inhibitor-1 in the blood 1–3 days after exposure (9). In the lungs, the mechanisms of hyperreactivity change over 3 days after ozone exposure, from eosinophil dependent 1 day postozone to eosinophil independent 3 days postozone (66), suggesting that different inflammatory responses also occur in the lungs over this lag period.
The mechanisms of hyperreactivity 3 days after ozone exposure are unknown, but in contrast to day 1, they involve increased smooth muscle contraction in addition to increased vagally mediated bronchoconstriction (49, 66). The systemic nature of this later response suggests a role for inflammatory mediators, including IL-1β, which is increased in the bone marrow 3 days after ozone (56). Blocking IL-1β inhibits hyperreactivity 3 days after ozone but has no effect on hyperreactivity 1 day after ozone. Since IL-1β stimulates expression of NGF (16) and substance P (16, 31, 63), both of which can mediate neural plasticity and hyperreactivity (26), these experiments were carried out to test whether NGF and substance P contribute to ozone-induced hyperreactivity 3 days after ozone exposure.
METHODS
Animals.
Pathogen-free guinea pigs were used (males and females, 300–450 g; from both Hilltop Lab Animals, Scottsdale, PA, and Elm Hill Breeding Labs, Chelmsford, MA). All guinea pigs were shipped in filtered crates, housed in high-efficiency particulate-filtered air, and fed a normal diet (Prolab; Agway, Syracuse, NY). Protocols were approved by Johns Hopkins University Bloomberg School of Public Health and Oregon Health & Science University Animal Care and Use Committees.
Ozone exposure.
Guinea pigs were placed in individual wire cages and exposed in a 700-l stainless steel exposure chamber with laminar airflow to either ozone (2.0 parts per million) or filtered air for 4 h as previously described (69, 70). Ozone was generated by an ultraviolet light generator (Orec, Glendale, CA) and was introduced into the chamber airflow at a rate of 2 l/min. Ozone concentrations within the chamber were monitored (model 1008 AH; Dasibi Environmental, Glendale, CA), calibrated, and recorded. The air supply within the chamber was replaced at a rate of 20 times/h. Control animals were exposed to filtered air under identical conditions. Following exposure, animals were maintained in particulate-filtered air until physiological measurements were made.
AbNGF or neurokinin receptor antagonist.
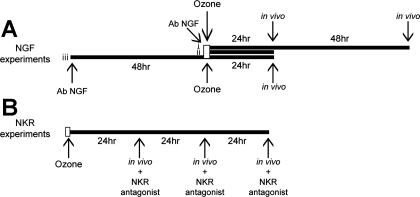
Some guinea pigs were treated with either antibody to NGF (AbNGF; 10 μg/kg ip) or goat IgG (10 μg/kg ip) 1 h before ozone exposure. Other guinea pigs were given AbNGF 2 days before ozone exposure (which is 3 days before physiological measurements). Other guinea pigs were treated after ozone exposure with either the neurokinin NK1 receptor antagonist CP-96,345 [3 mg/kg iv (28)] or the NK2 receptor antagonist SR48968 [0.1 mg/kg iv (18)] 30 min before physiological measurements (Fig. 1). Some animals were also treated with the NK1 receptor antagonist SR140333 [1 mg/kg iv (6)]. These results were not different from CP-96,345 and are not shown here.
Fig. 1.
The antibody to NGF (AbNGF) was administered 1 h before ozone exposure (A, i and ii) or 2 days before ozone exposure (A, iii). Physiological measurements were made either 1 or 3 days after ozone exposure. To test the role of neurokinin receptors in ozone-induced airway hyperreactivity, neurokinin receptor antagonists were administered during physiological measurements 1, 2, or 3 days after ozone exposure (B).
Anesthesia and measurement of pulmonary inflation pressure.
One, two, or three days after a single ozone exposure, guinea pigs were anesthetized with 2.0 g/kg ip urethane (21). Both jugular veins were cannulated for administration of drugs. A carotid artery was cannulated and connected to a transducer (DTX; Spectramed, Oxnard, CA) to measure blood pressure, and heart rate was derived electronically from the blood pressure signal. Guinea pigs were tracheostomized and ventilated with a positive-pressure, constant-volume rodent ventilator (Harvard Apparatus, South Natick, MA) at a tidal volume of 1.0 ml/100 g body wt and 100 breaths/min. Animals were paralyzed with succinylcholine (10 μg·kg−1·min−1 iv). Pulmonary inflation pressure was measured at the trachea by using a pressure transducer (DTX; Spectramed). A positive pressure of 100–200 mmH2O was needed to adequately ventilate animals. Signals were recorded on a polygraph (Grass Instrument, Quincy, MA).
Bronchoconstriction was measured as an increase in pulmonary inflation pressure over baseline inflation pressure produced by the ventilator (36). The sensitivity of measurement was increased by recording baseline inflation pressure on one channel and increases/changes in inflation pressure over baseline on a separate channel at a higher sensitivity. Increases in inflation pressure as small as 2–3 mmH2O above the baseline can be accurately measured with this method. All animals were treated with guanethidine (5 mg/kg iv) to deplete norepinephrine at least 20 min before the start of each experiment.
Measurement of vagally mediated bronchoconstriction.
Both vagus nerves were cut, and distal ends were placed on platinum electrodes and covered with mineral oil. Electrical stimulation (1–25 Hz, 0.2 ms, 5-s train stimulus, 10 V at 1- to 2-min intervals) produced frequency-dependent bronchoconstriction (measured as an increase in inflation pressure) and bradycardia. These responses were blocked by atropine (1 mg/kg iv), indicating that they were mediated by acetylcholine release onto muscarinic receptors.
Measurement of M3 muscarinic receptor function on airway smooth muscle.
The function of M3 muscarinic receptors on airway smooth muscle was tested in vagotomized animals by measuring bronchoconstriction induced by acetylcholine (1–10 μg/kg iv) or methacholine (1–10 μg/kg iv). Both acetylcholine and methacholine were used since acetylcholine is the endogenous neurotransmitter and is metabolized by acetylcholinesterases. To control for decreases in acetylcholinesterase activity as has been shown after ozone inhalation (20), methacholine responsiveness was also measured because it is less susceptible to acetylcholinesterases. Animals were vagotomized to eliminate the reflex component of intravenous muscarinic agonists (5, 29, 57).
Bronchoalveolar lavage.
Following physiological measurements, the lungs were lavaged via the tracheal cannula with 5 aliquots of 10-ml warm PBS. The recovered cells were centrifuged, resuspended in PBS, and counted using a hemocytometer. Differential cell counts were obtained from aliquots of the bronchoalveolar lavage (BAL) suspension, which were spun onto glass slides and stained (Diff-Quik, Scientific Products, McGraw Park, IL, or Hemacolor, EMD Chemicals, Gibbstown, NJ).
Immunofluorescent staining for substance P in airway nerves.
Tissue was fixed with 4% formaldehyde, cryoprotected in 18% sucrose in PBS, then 9% sucrose in 50% optimal cutting temperature (OCT; Sakura Finetek, Torrance, CA), and finally covered with OCT and frozen. Sections were cut 12 μm thick, collected on slides, and air-dried. Slides were rinsed in PBS and incubated in antigen unmasking solution (Vector Laboratories, Burlingame, CA). Slides were blocked with 3% bovine serum albumin, 15% normal goat serum, and 0.1% Tween 20 in PBS for 1 h at room temperature and then incubated overnight at 4°C with a rabbit polyclonal antibody to substance P (diluted to 1:500; AB1566; Chemicon, Temecula, CA) and with mouse monoclonal antiserum to the neuronal marker protein gene product 9.5 (diluted 1:50; PGP9.5; AbD Serotec, Oxford, United Kingdom). Control slides were incubated without primary antibody. All slides were incubated with secondary antibodies conjugated to Alexa fluorophores (goat anti-mouse Alexa 488 or goat anti-rabbit Alexa 555; Molecular Probes) for 2 h at room temperature.
The slides were photographed with an epifluorescence microscope equipped with appropriate filters to visualize fluorescein or rhodamine. Exposure times were fixed to allow comparison between treatment groups. Exposure limits were chosen to be short enough to not collect any background fluorescence in sections not stained with primary antibodies and were then kept constant throughout the collection of data. Every nerve bundle adjacent to airway smooth muscle was photographed for each cartilaginous airway. Separately, nerve fibers within the airway smooth muscle were also photographed, but the number of fibers that were positive for substance P were so few that quantitative analysis proved difficult. Therefore, to quantify substance P expression in nerve bundles, bundles were identified by PGP9.5 staining, photographed under the fluorescein filter (to visualize PGP9.5-stained bundles), and then photographed again under the rhodamine filter (to visualize substance P). Pictures were overlaid, and nerve bundles were scored by three independent reviewers for presence or absence of substance P. Both photographs and subsequent analysis were done blindly.
Drugs.
Guanethidine, methacholine, acetylcholine, succinylcholine, atropine, goat IgG, and urethane were purchased from Sigma (St. Louis, MO). CP-96,345 was purchased from Pfizer (Groton, CT), and SR140333 and SR48968 were generous gifts from Dr. X. Emonds-Alt (Sanofi Recherche, Montpellier, France). AbNGF was purchased from R&D Systems (Minneapolis, MN). All drugs were dissolved in 0.9% NaCl.
Statistics.
All data are expressed as means ± SE. Frequency, acetylcholine, and methacholine responses were analyzed using two-way ANOVA for repeated measures (GraphPad Prism 5.0a; GraphPad Software, La Jolla, CA). Baseline heart rates, blood pressures, baseline inflation pressure, and BAL were analyzed by ANOVA (StatView 4.5; Abacus Concepts, Berkeley, CA). Substance P-positive nerve bundles were analyzed by two-way ANOVA (GraphPad Prism 5.0a). A P value of ≤0.05 was considered significant.
RESULTS
Ozone significantly increased baseline pulmonary inflation pressure 1 and 3 days after exposure compared with air-exposed controls (Table 1). Neither treatment with AbNGF (2 days or 1 h before ozone) prevented the ozone-induced increase in pulmonary inflation pressure 1 day after ozone. However, AbNGF, but not control IgG, significantly attenuated the baseline rise in pulmonary inflation pressure 3 days after ozone. Treatment with the NK1 and NK2 receptor antagonists also did not prevent ozone-induced increase in pulmonary inflation pressure at day 3. Resting heart rate in four different groups of controls (controls for ozone, both protocols for AbNGF, and neurokinin antagonist-treated) ranged from 260 ± 6 to 328 ± 10 beats/min. As previously reported (66), ozone did not cause any consistent effect on resting heart rate. Similarly, resting blood pressure in all control groups ranged from 46 ± 2 mmHg systolic/22 ± 2 mmHg diastolic to 51 ± 2 mmHg systolic/26 ± 3 mmHg diastolic, and exposure to ozone did not affect resting blood pressure. None of the treatments (AbNGF, IgG, and tachykinin antagonists) altered resting heart rate or blood pressure in ozone or control animals.
Table 1.
Baseline pulmonary parameters
| Treatment | Group | n | Pulmonary Inflation Pressure, mmH2O |
|---|---|---|---|
| 1 Day | Air | 5 | 106 ± 2 |
| Ozone | 5 | 242 ± 17* | |
| Ozone + AbNGF 1 h before O3 | 5 | 208 ± 8* | |
| 1 Day | Air | 5 | 132 ± 13 |
| Ozone | 5 | 250 ± 26* | |
| Ozone + AbNGF 2 days before O3 | 5 | 210 ± 10* | |
| 3 Days | Air | 5 | 92 ± 6 |
| Ozone | 5 | 155 ± 14* | |
| Ozone + AbNGF | 6 | 118 ± 19 | |
| Ozone + IgG | 5 | 160 ± 12* | |
| 3 Days | Air | 12 | 114 ± 9 |
| Ozone | 5 | 156 ± 11* | |
| Ozone + NK1 antagonist | 6 | 174 ± 9* | |
| Ozone + NK2 antagonist | 5 | 172 ± 9* |
Values are means ± SE. Baseline pulmonary inflation pressure increased 1 and 3 days postozone. The antibody to NGF (AbNGF) prevented the ozone-induced increase in baseline pulmonary inflation pressure 3 days after exposure.
Significantly different from air-exposed controls.
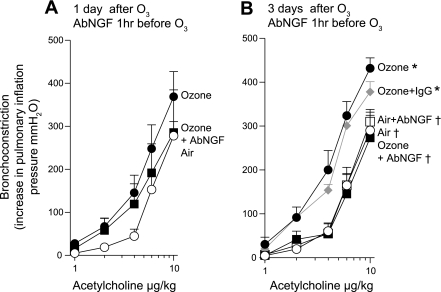
Stimulation of both vagus nerves (1–25 Hz) caused frequency-dependent bronchoconstriction in all animals that was significantly potentiated 1 and 3 days after a single exposure to ozone (Fig. 2) and was completely blocked by 1 mg/kg atropine (data not shown). Pretreatment with AbNGF 1 h before ozone exposure did not significantly inhibit ozone-induced airway hyperreactivity 1 day later (Fig. 2A), but it did completely prevent ozone-induced airway hyperreactivity 3 days after exposure (Fig. 2B). Pretreatment with AbNGF had no effect on vagally mediated bronchoconstriction in air-exposed controls (Fig. 2B), nor did control IgG inhibit vagally induced bronchoconstriction in ozone-exposed animals (Fig. 2B). To test whether AbNGF only worked 3 days after ozone because it required time to have an effect, AbNGF was administered 2 days before ozone exposure (3 days before physiological measurements; Fig. 1A). In these experiments, AbNGF also did not inhibit ozone-induced hyperreactivity (Fig. 2C).
Fig. 2.
NGF mediates ozone-induced airway hyperreactivity 3 days after exposure but does not contribute to airway hyperreactivity 1 day after ozone. Electrical stimulation of both vagus nerves in anesthetized guinea pigs causes frequency-dependent bronchoconstriction, measured as an increase in pulmonary inflation pressure, in air-exposed controls (A–C, ○) that is significantly potentiated both 1 and 3 days after ozone exposure (A–C, ●). Pretreatment with AbNGF 1 h before ozone exposure did not prevent airway hyperreactivity 1 day later (A, ■; these data are not significantly different from ozone alone) but was completely protective 3 days after ozone (B, ■). Blocking NGF had no effect on vagally mediated bronchoconstriction in air-exposed controls (B, □). An isotype control (IgG) had no effect in ozone-exposed animals (B, gray diamonds). Blocking NGF 3 days before physiological testing [2 days before exposure to ozone (2d before O3)] did not prevent ozone-induced airway hyperreactivity (C, ▾). Thus the protective effect of AbNGF depends on the days postozone, not on the days postantibody administration. *Significantly different from air-exposed guinea pigs. †Significantly different from ozone-exposed guinea pigs. Data are expressed as means ± SE, n = 5. Note, there are differences among A, B, and C controls and ozone hyperreactivity due to variability between batches of guinea pigs. As such, each set of data has its own controls, and data were compared statistically only within each experiment (not across experiments).
Intravenous acetylcholine in vagotomized animals bypasses the nervous system and directly induces bronchoconstriction via a direct effect at M3 muscarinic receptors on airway smooth muscle. Acetylcholine-induced bronchoconstriction was not changed 1 day after ozone but was slightly although significantly potentiated (by 33%) 3 days after ozone compared with air-exposed controls (Fig. 3). This potentiation at 3 days was also inhibited by AbNGF (Fig. 3B). The control IgG had no effect on acetylcholine-induced bronchoconstriction in ozone-exposed animals (Fig. 3B).
Fig. 3.
Three days after ozone, airway smooth muscle is hyperreactive to acetylcholine, and this effect is blocked with AbNGF. In vagotomized guinea pigs, acetylcholine causes dose-related bronchoconstriction in air-exposed animals (A and B, ○). Acetylcholine-induced bronchoconstriction was not altered 1 day after ozone but was significantly potentiated 3 days after ozone compared with air-exposed controls (A and B, ●). AbNGF did not affect acetylcholine-induced bronchoconstriction 1 day after ozone (A, ■) but did inhibit acetylcholine-induced bronchoconstriction 3 days after ozone (B, ■). Neither IgG (B, gray diamonds) in ozone-exposed animals nor AbNGF (B, □) in air-exposed animals had any effect on acetylcholine-induced bronchoconstriction. *Significantly different from air-exposed guinea pigs. †Significantly different from ozone-exposed guinea pigs. Data are expressed as means ± SE, n = 4–6.
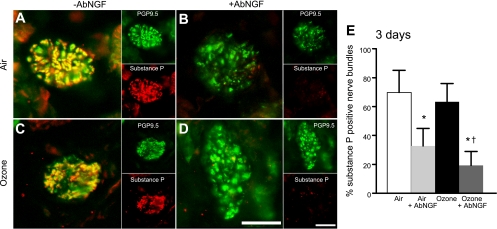
There was no difference in substance P-positive nerve bundles between lungs of ozone- and air-exposed controls 3 days after exposure (Fig. 4, A, C, and E). However, AbNGF significantly reduced the number of substance P-positive nerve bundles 3 days later, regardless of whether they were air- or ozone-exposed (Fig. 4, B, D, and E).
Fig. 4.
Blocking NGF significantly decreased the percentage of substance P-positive nerve bundles in guinea pig airways. Representative images of airway nerve bundles in guinea pig lung stained with antibodies to pan-neuronal marker PGP9.5 (green) and substance P (red) are shown with their merged overlay (A–D). Quantification of substance P-positive bundles is shown in E. Three days after ozone (C and E, black bar), there is no change in the percentage of substance P-positive nerve bundles compared with air-exposed controls (A and E, white bar). Pretreatment with AbNGF significantly reduced the number of substance P-positive nerve bundles in both air (B and E, light gray bar)- and ozone (D and E, dark gray bar)-exposed animals. Scale bar is 50 μm. *Significantly different from air-exposed controls. †Significantly different from ozone-exposed animals. Data are expressed as means ± SE, n = 3–5.
Two and three days after ozone, guinea pigs were hyperreactive to vagal nerve stimulation compared with air-exposed controls (Figs. 5 and 6A). An NK1 receptor antagonist completely prevented vagally mediated airway hyperreactivity 2 and 3 days after ozone (Figs. 5 and 6A) while having no effect in air-exposed controls (Fig. 6A). Whereas both intravenous acetylcholine and methacholine-induced bronchoconstriction were significantly potentiated 3 days after ozone, treatment with the NK1 receptor antagonist did not prevent hyperreactivity to intravenous acetylcholine (Fig. 6, B and C). In contrast, the NK2 receptor antagonist significantly inhibited vagally induced bronchoconstriction in both ozone- and air-exposed animals (Fig. 7A). However, the NK2 receptor antagonist only decreased bronchoconstriction to acetylcholine and methacholine in ozone-exposed animals (Fig. 7, B and C).
Fig. 5.
Vagally induced hyperreactivity 2 days after a single exposure to ozone is prevented by the neuronal NK1 receptor antagonist (NK1R Ant.), CP-96,345. Electrical stimulation of the vagus nerves produced frequency-dependent bronchoconstriction in air-exposed controls (○) that was significantly potentiated 2 days after a single exposure to ozone (●). The NK1 receptor antagonist prevented ozone-induced hyperreactivity (▴). *Significantly different from air-exposed controls. †Significantly different from ozone. Data are expressed as means ± SE, n = 3–5.
Fig. 6.
Three days after ozone, vagally mediated hyperreactivity is mediated by NK1 receptors. Electrical stimulation of both vagus nerves produced frequency-dependent bronchoconstriction (A, ○) that was significantly potentiated 3 days after a single exposure to ozone (A, ●). The NK1 receptor antagonist CP-96,345 (3 mg/kg iv) prevented ozone-induced hyperreactivity (A, ▴). Bronchoconstriction induced by both acetylcholine (B) and methacholine (C) was potentiated by ozone (●) but was not inhibited by the NK1 receptor antagonist (▴). Treatment of air-exposed controls with the NK1 receptor antagonist had no effect on vagally, acetylcholine-, or methacholine-induced bronchoconstriction (A–C, ▵). *Significantly different from air controls. †Significantly different from ozone. Data are expressed as means ± SE, n = 4–12.
Fig. 7.
The NK2 receptor antagonist SR48968 (0.1 mg/kg iv) prevented vagally induced bronchoconstriction (A) regardless of whether the animals were exposed to ozone (3 days after ozone, ● vs. ▾) or air (○ vs. ▿). Bronchoconstriction induced by both acetylcholine (B) and methacholine (C) was also potentiated 3 days after a single exposure to ozone (●) and was inhibited by the NK2 receptor antagonist (B and C, ▾). However, the NK2 receptor antagonist did not inhibit bronchoconstriction induced by acetylcholine and methacholine in air controls (▿). Air- and ozone-exposed animals are the same as in Fig. 5. *Significantly different from air controls. †Significantly different from ozone. Data are expressed as means ± SE, n = 4–12.
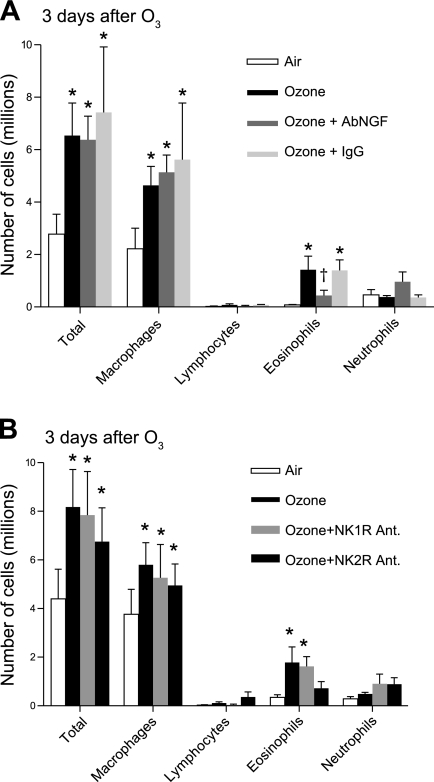
One day after ozone, the only cells significantly increased in BAL were neutrophils, and AbNGF had no effect on this increase (data not shown). Three days after ozone, macrophages and eosinophils were significantly increased in the BAL (Fig. 8). The increase in macrophages was not blocked by AbNGF, NK1, or NK2 receptor antagonists. The increase in eosinophils was significantly inhibited by the AbNGF 3 days after ozone (Fig. 8A). Ozone still caused significant eosinophilia 3 days later in the presence of the NK1 receptor antagonist but not in the presence of the NK2 receptor antagonist (Fig. 8B).
Fig. 8.
Eosinophils, macrophages, and total cells in the bronchoalveolar lavage are significantly increased 3 days after ozone (black bars) compared with air controls (white bars). AbNGF reduced eosinophils in ozone-exposed animals (A, dark gray bars). Treatment with the NK1 receptor antagonist (B, medium gray bars) did not affect the number of inflammatory cells in bronchoalveolar lavage. Treatment with the NK2 receptor antagonist (B, dark gray bars) decreased eosinophils 3 days after ozone. *Significantly different from air controls. †Significantly different from ozone animals. Data are expressed as means ± SE, n = 5.
DISCUSSION
One day after ozone exposure, airway hyperreactivity is mediated by release of eosinophil major basic protein that blocks neuronal M2 muscarinic receptors, resulting in increased acetylcholine release from parasympathetic nerves (66). Three days after ozone exposure, hyperreactivity is no longer mediated by eosinophils (66) but is blocked by an antagonist to IL-1β receptors (56, 66). Here, we show that the sustained hyperreactivity 3 days after a single exposure to ozone is also inhibited by pretreatment with AbNGF, which had no effect on hyperreactivity 1 day after ozone (Fig. 2).
Since sustained ozone-induced hyperreactivity was blocked by AbNGF, we tested whether it was mediated via substance P because NGF increases substance P in rabbit lung (34) and mouse lung (30) and increases both neurokinin A and substance P expression in dorsal root ganglia (55). In vivo, NGF causes hyperreactivity to electrical stimulation of airway nerves that is blocked by a NK1 receptor antagonist (60). Collectively, these data suggest that NGF mediates airway hyperreactivity by increasing substance P or other tachykinin receptor agonists.
Acute airway hyperreactivity to ozone is mediated by eosinophils and not by substance P. Any treatment that interferes with eosinophils, eosinophil major basic protein, or acetylcholine release is protective in these animals (66). Although substance P can activate eosinophils (13), this is unlikely a mechanism of acute ozone hyperreactivity since ozone does not activate capsaicin-sensitive transient receptor potential vanilloid 1 (TRPV1) channels (51). Furthermore, acute, ozone-induced hyperreactivity is not inhibited by capsaicin ablation of sensory nerves (33).
However, substance P is well-known to increase under inflammatory conditions. It is increased in lungs with viral infection (8) and antigen challenge (10, 53). Ozone increases substance P in BAL of humans (27) and increases substance P-positive nerve fibers in ferret tracheas (61, 62). Although ozone did not increase the number of substance P-positive nerves in guinea pig lungs exposed to ozone, AbNGF significantly decreased the number of substance P-positive airway nerves, independently of ozone exposure (Fig. 4), and in AbNGF-treated guinea pigs, ozone failed to cause sustained airway hyperreactivity (Fig. 2B). Conversely, guinea pigs were hyperreactive to vagal nerve stimulation 1 day after ozone exposure regardless of whether guinea pigs were treated with AbNGF immediately before ozone exposure (Fig. 2A) or treated 2 days before ozone exposure (Fig. 2C). These data show that the protective effect of the AbNGF depends on the days postozone, not on the days postantibody administration. Thus the mechanism of ozone-induced hyperreactivity is changed over time. Here, we show that 2 and 3 days after ozone airway hyperreactivity is mediated by an NK1 receptor agonist, likely substance P since it has the highest affinity for NK1 receptors of the tachykinin family (43).
One, two, and three days after ozone, vagally induced bronchoconstriction is abolished by the muscarinic receptor antagonist, atropine; thus acetylcholine, not substance P, is directly contracting airway smooth muscle. Although NK1 receptors are present on airway parasympathetic nerves (39), pharmacological data show they do not increase acetylcholine release under normal conditions (7, 22, 25, 52). However, enhanced neurotransmission by substance P has been demonstrated (7, 25), and we and others have shown that substance P is present in nerve bundles supplying the large airways of nonozone-exposed guinea pigs and ferrets (Fig. 4) (42, 62). We have shown that substance P increases acetylcholine release indirectly by activating resident eosinophils and releasing the M2 receptor antagonist, major basic protein (13). However, ozone increases substance P in airways (27, 48, 61, 62) including in nerve fibers and in nerve cell bodies, that are probably parasympathetic nerve cells, in ferret airways (61). Three days after ozone, when eosinophils are no longer involved in airway hyperreactivity (66), the effectiveness of NK1 receptor antagonists (Fig. 6) suggests substance P or another tachykinin receptor agonist is directly increasing acetylcholine release from parasympathetic nerves.
An alternative possibility is NK1 receptors were unmasked in the 3 days following ozone. Receptor unmasking has been described for NK2 receptors on sensory nerves in antigen-challenged guinea pigs where receptor activation occurs within minutes of antigen challenge (58). The mechanisms for receptor unmasking are unclear but may occur through posttranslational modification or trafficking of receptors to the cell membrane rather than new receptor production. Thus enhanced substance P or enhanced NK1 receptor activation on parasympathetic nerves could increase acetylcholine release, resulting in ozone-induced airway hyperreactivity.
In contrast to the NK1 receptor antagonist, the NK2 receptor antagonist inhibited vagally induced bronchoconstriction both in air- and ozone-exposed guinea pigs (Fig. 7). NK2 receptors are present on nerves and on airway smooth muscle (1, 24, 38, 42). However, given that there is no effect of NK2 receptor agonists on sensory nerves in control guinea pigs (41), the NK2 receptors are probably on parasympathetic nerves and function independently of ozone to enhance vagally mediated bronchoconstriction.
Another important difference between hyperreactivity 1 and 3 days after ozone is that airway smooth muscle is slightly but significantly hypercontractile 3 days after ozone (49, 66). This increased contractility is not mediated by a reflex triggered by acetylcholine since the vagus nerves were cut (5, 57). Although acetylcholine-induced bronchoconstriction is mediated through M3 muscarinic receptors and is blocked by atropine, blocking NK2 receptors prevented the increased bronchoconstriction to acetylcholine, without affecting the baseline constriction (Fig. 7, B and C), suggesting a positive interaction between NK2 and M3 receptors on airway smooth muscle 3 days after ozone. The presence of functional NK2 receptors on airway smooth muscle has been demonstrated (67), and others have also suggested a synergistic interaction between M3 and NK2 receptors (45). Increased substance P would also explain the ozone-induced smooth muscle responsiveness since release of substance P alone can potentiate airway contractility to muscarinic agonists (54).
In conclusion, the mechanisms of ozone-induced airway hyperreactivity change between 1 and 3 days. The initial response to ozone is mediated by degranulation of eosinophils, release of major basic protein, blockade of inhibitory M2 muscarinic receptors on parasympathetic nerves, increased acetylcholine release, and increased vagally mediated bronchoconstriction (65). Three days later, there has been a phenotypic change in the mechanisms of hyperreactivity so that eosinophils are no longer the cause of ozone-induced hyperreactivity. Three days postozone, hyperreactivity is mediated by IL-1 (56), NGF, and substance P. These are related because blocking IL-1β decreases ozone-induced NGF (2), whereas blocking NGF prevents IL-1β-induced airway hyperreactivity to substance P (16), suggesting that NGF is an intermediary between IL-1β and substance P. Our data suggest this pathway mediates airway hyperreactivity 3 days after ozone in guinea pigs (Fig. 9). The end result is that sustained airway hyperreactivity after ozone is mediated by tachykinins, probably substance P. The dominant effect of substance P is at NK1 receptors on parasympathetic nerves to enhance acetylcholine release, but substance P also enhances smooth muscle contraction to acetylcholine via NK2 receptors on airway smooth muscle.
Fig. 9.
Proposed mechanism for how ozone causes airway hyperreactivity 1 and 3 days after exposure. In air animals, NK2 receptors appear to enhance release of acetylcholine from parasympathetic nerves. One day after ozone exposure, we have previously shown [Sar9,Met(O2)11]-substance P (SM Substance P) stimulates release of eosinophil major basic protein (MBP) that inhibits neuronal M2 muscarinic receptors and enhances acetylcholine release from parasympathetic nerves leading to airway hyperreactivity. Three days after ozone, eosinophils no longer mediated airway hyperreactivity. NK1 receptors on parasympathetic nerves now appear to mediate enhanced release of acetylcholine, and a role for NK2 receptors on airway smooth muscle has been unmasked that enhances acetylcholine-mediated smooth muscle contraction. NKA, neurokinin A.
Multiple, often unrelated mechanisms for airway hyperreactivity have been identified for specific insults to the lung. These include changes in cytokines, inflammatory cells (14, 35, 65), neural plasticity (11, 62), and smooth muscle hyperresponsiveness (49, 59, 66). Here, we show that mechanisms of airway hyperreactivity change over 3 days following a specific insult, in this case ozone. New or increased expression of substance P or neurokinin receptors in nerves supplying the lungs that are mediated by IL-1β and NGF and that lag 3 days behind exposure to ozone may account for the delayed decline in lung function and increased morbidity and mortality in humans seen days after environmental exposure to ozone (4, 12, 23). Thus data presented here have implications in identifying treatments for airway hyperreactivity since target receptors may be quite different immediately and in the days following exposure to ozone or other environmental pollutants.
GRANTS
This work was supported by the American Heart Association Grant 0810148Z (K. C. Verhein) and the National Institutes of Health Grants HL-55543 (A. D. Fryer), ES-014601 (A. D. Fryer), ES-017592 (A. D. Fryer), HL-61013 (D. B. Jacoby), HL-071795 (D. B. Jacoby), AI-075064 (D. B. Jacoby), and RR-023424 (D. B. Jacoby).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
Present address of K. C. Verhein: National Institute of Environmental Health Sciences, Research Triangle Park, NC 27709.
Present address of M. S. Hazari: Environmental Protection Agency, Research Triangle Park, NC 27711.
REFERENCES
- 1. Baluk P, Nigel W, McDonald B, McDonald DM. Localization of tachykinin NK1, NK2, and NK3 receptors in airways by immunohistochemistry (Abstract). Am J Respir Crit Care Med 153: A161, 1996 [Google Scholar]
- 2. Barker J, Wu Z, Dey R. Interleukin (IL)-1 regulates ozone-induced nerve growth factor (NGF) release in bronchoalveolar lavage fluid (BALF) in mice (Abstract). FASEB J 23: 1009.6, 2009 [Google Scholar]
- 3. Beckett WS, McDonnell WF, Horstman DH, House DE. Role of the parasympathetic nervous system in acute lung response to ozone. J Appl Physiol 59: 1879–1885, 1985 [DOI] [PubMed] [Google Scholar]
- 4. Bell ML, McDermott A, Zeger SL, Samet JM, Dominici F. Ozone and short-term mortality in 95 US urban communities, 1987–2000. JAMA 292: 2372–2378, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Belmonte KE, Fryer AD, Costello RW. Role of insulin in antigen-induced airway eosinophilia and neuronal M2 muscarinic receptor dysfunction. J Appl Physiol 85: 1708–1718, 1998 [DOI] [PubMed] [Google Scholar]
- 6. Biyah K, Molimard M, Emonds-Alt X, Advenier C. SR 140333 prevents potentiation by citric acid of plasma exudation induced by histamine in airways. Eur J Pharmacol 308: 325–328, 1996 [DOI] [PubMed] [Google Scholar]
- 7. Canning BJ, Reynolds SM, Anukwu LU, Kajekar R, Myers AC. Endogenous neurokinins facilitate synaptic transmission in guinea pig airway parasympathetic ganglia. Am J Physiol Regul Integr Comp Physiol 283: R320–R330, 2002 [DOI] [PubMed] [Google Scholar]
- 8. Carr MJ, Hunter DD, Jacoby DB, Undem BJ. Expression of tachykinins in nonnociceptive vagal afferent neurons during respiratory viral infection in guinea pigs. Am J Respir Crit Care Med 165: 1071–1075, 2002 [DOI] [PubMed] [Google Scholar]
- 9. Chuang KJ, Chan CC, Su TC, Lee CT, Tang CS. The effect of urban air pollution on inflammation, oxidative stress, coagulation, and autonomic dysfunction in young adults. Am J Respir Crit Care Med 176: 370–376, 2007 [DOI] [PubMed] [Google Scholar]
- 10. Chuaychoo B, Hunter DD, Myers AC, Kollarik M, Undem BJ. Allergen-induced substance P synthesis in large-diameter sensory neurons innervating the lungs. J Allergy Clin Immunol 116: 325–331, 2005 [DOI] [PubMed] [Google Scholar]
- 11. Coleridge JC, Coleridge HM, Schelegle ES, Green JF. Acute inhalation of ozone stimulates bronchial C-fibers and rapidly adapting receptors in dogs. J Appl Physiol 74: 2345–2352, 1993 [DOI] [PubMed] [Google Scholar]
- 12. Desqueyroux H, Pujet JC, Prosper M, Le Moullec Y, Momas I. Effects of air pollution on adults with chronic obstructive pulmonary disease. Arch Environ Health 57: 554–560, 2002 [DOI] [PubMed] [Google Scholar]
- 13. Evans CM, Belmonte KE, Costello RW, Jacoby DB, Gleich GJ, Fryer AD. Substance P-induced airway hyperreactivity is mediated by neuronal M2 receptor dysfunction. Am J Physiol Lung Cell Mol Physiol 279: L477–L486, 2000 [DOI] [PubMed] [Google Scholar]
- 14. Evans CM, Fryer AD, Jacoby DB, Gleich GJ, Costello RW. Pretreatment with antibody to eosinophil major basic protein prevents hyperresponsiveness by protecting neuronal M2 muscarinic receptors in antigen-challenged guinea pigs. J Clin Invest 100: 2254–2262, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Foster WM, Brown RH, Macri K, Mitchell CS. Bronchial reactivity of healthy subjects: 18–20 h postexposure to ozone. J Appl Physiol 89: 1804–1810, 2000 [DOI] [PubMed] [Google Scholar]
- 16. Frossard N, Naline E, Olgart Hoglund C, Georges O, Advenier C. Nerve growth factor is released by IL-1beta and induces hyperresponsiveness of the human isolated bronchus. Eur Respir J 26: 15–20, 2005 [DOI] [PubMed] [Google Scholar]
- 17. Fryer AD, Jacoby DB. Function of pulmonary M2 muscarinic receptors in antigen-challenged guinea pigs is restored by heparin and poly-l-glutamate. J Clin Invest 90: 2292–2298, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Girard V, Yavo JC, Emonds-Alt X, Advenier C. The tachykinin NK2 receptor antagonist SR 48968 inhibits citric acid-induced airway hyperresponsiveness in guinea pigs. Am J Respir Crit Care Med 153: 1496–1502, 1996 [DOI] [PubMed] [Google Scholar]
- 19. Golden JA, Nadel JA, Boushey HA. Bronchial hyperirritability in healthy subjects after exposure to ozone. Am Rev Respir Dis 118: 287–294, 1978 [DOI] [PubMed] [Google Scholar]
- 20. Gordon T, Taylor BF, Amdur MO. Ozone inhibition of tissue cholinesterase in guinea pigs. Arch Environ Health 36: 284–288, 1981 [DOI] [PubMed] [Google Scholar]
- 21. Green C. Animal Anesthesia. London: Laboratory Animals, 1982 [Google Scholar]
- 22. Grumann-Junior A, Dias MA, Alves RV, Boteon JE, Calixto JB. Mechanisms mediating substance P-induced contraction in the rat iris in vitro. Invest Ophthalmol Vis Sci 41: 1861–1870, 2000 [PubMed] [Google Scholar]
- 23. Gryparis A, Forsberg B, Katsouyanni K, Analitis A, Touloumi G, Schwartz J, Samoli E, Medina S, Anderson HR, Niciu EM, Wichmann HE, Kriz B, Kosnik M, Skorkovsky J, Vonk JM, Dortbudak Z. Acute effects of ozone on mortality from the “air pollution and health: a European approach” project. Am J Respir Crit Care Med 170: 1080–1087, 2004 [DOI] [PubMed] [Google Scholar]
- 24. Guard S, Watson SP. Tachykinin receptor types: classification and membrane signalling mechanisms. Neurochem Int 18: 149–165, 1991 [DOI] [PubMed] [Google Scholar]
- 25. Hall AK, Barnes PJ, Meldrum LA, Maclagan J. Facilitation by tachykinins of neurotransmission in guinea-pig pulmonary parasympathetic nerves. Br J Pharmacol 97: 274–280, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hazari MS, Pan JH, Myers AC. Nerve growth factor acutely potentiates synaptic transmission in vitro and induces dendritic growth in vivo on adult neurons in airway parasympathetic ganglia. Am J Physiol Lung Cell Mol Physiol 292: L992–L1001, 2007 [DOI] [PubMed] [Google Scholar]
- 27. Hazbun ME, Hamilton R, Holian A, Eschenbacher WL. Ozone-induced increases in substance P and 8-epi-prostaglandin F2 alpha in the airways of human subjects. Am J Respir Cell Mol Biol 9: 568–572, 1993 [DOI] [PubMed] [Google Scholar]
- 28. Hey JA, Danko G, del Prado M, Chapman RW. Augmentation of neurally evoked cholinergic bronchoconstrictor responses by prejunctional NK2 receptors in the guinea-pig. J Auton Pharmacol 16: 41–48, 1996 [DOI] [PubMed] [Google Scholar]
- 29. Holtzman MJ, McNamara MP, Sheppard D, Fabbri LM, Hahn HL, Graf PD, Nadel JA. Intravenous versus inhaled atropine for inhibiting bronchoconstrictor responses in dogs. J Appl Physiol 54: 134–139, 1983 [DOI] [PubMed] [Google Scholar]
- 30. Hoyle GW, Graham RM, Finkelstein JB, Nguyen KP, Gozal D, Friedman M. Hyperinnervation of the airways in transgenic mice overexpressing nerve growth factor. Am J Respir Cell Mol Biol 18: 149–157, 1998 [DOI] [PubMed] [Google Scholar]
- 31. Hurst SM, Stanisz AM, Sharkey KA, Collins SM. Interleukin 1 beta-induced increase in substance P in rat myenteric plexus. Gastroenterology 105: 1754–1760, 1993 [DOI] [PubMed] [Google Scholar]
- 32. Jerrett M, Burnett RT, Pope CA, 3rd, Ito K, Thurston G, Krewski D, Shi Y, Calle E, Thun M. Long-term ozone exposure and mortality. N Engl J Med 360: 1085–1095, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jimba M, Skornik WA, Killingsworth CR, Long NC, Brain JD, Shore SA. Role of C fibers in physiological responses to ozone in rats. J Appl Physiol 78: 1757–1763, 1995 [DOI] [PubMed] [Google Scholar]
- 34. Larsen GL, Loader J, Nguyen DD, Fratelli C, Dakhama A, Colasurdo GN. Mechanisms determining cholinergic neural responses in airways of young and mature rabbits. Pediatr Pulmonol 38: 97–106, 2004 [DOI] [PubMed] [Google Scholar]
- 35. Lee AM, Fryer AD, van Rooijen N, Jacoby DB. Role of macrophages in virus-induced airway hyperresponsiveness and neuronal M2 muscarinic receptor dysfunction. Am J Physiol Lung Cell Mol Physiol 286: L1255–L1259, 2004 [DOI] [PubMed] [Google Scholar]
- 36. Lee LY, Bleecker ER, Nadel JA. Effect of ozone on bronchomotor response to inhaled histamine aerosol in dogs. J Appl Physiol 43: 626–631, 1977 [DOI] [PubMed] [Google Scholar]
- 37. Lisabeth LD, Escobar JD, Dvonch JT, Sanchez BN, Majersik JJ, Brown DL, Smith MA, Morgenstern LB. Ambient air pollution and risk for ischemic stroke and transient ischemic attack. Ann Neurol 64: 53–59, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Maggi CA. Tachykinin receptors and airway pathophysiology. Eur Respir J 6: 735–742, 1993 [PubMed] [Google Scholar]
- 39. Mapp CE, Miotto D, Braccioni F, Saetta M, Turato G, Maestrelli P, Krause JE, Karpitskiy V, Boyd N, Geppetti P, Fabbri LM. The distribution of neurokinin-1 and neurokinin-2 receptors in human central airways. Am J Respir Crit Care Med 161: 207–215, 2000 [DOI] [PubMed] [Google Scholar]
- 40. Mitchell HW, Adcock J. Vagal mechanisms and the effect of indomethacin on bronchoconstrictor stimuli in the guinea-pig. Br J Pharmacol 94: 522–527, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Moore KA, Undem BJ, Weinreich D. Antigen inhalation unmasks NK-2 tachykinin receptor-mediated responses in vagal afferents. Am J Respir Crit Care Med 161: 232–236, 2000 [DOI] [PubMed] [Google Scholar]
- 42. Myers AC, Undem BJ. Electrophysiological effects of tachykinins and capsaicin on guinea-pig bronchial parasympathetic ganglion neurones. J Physiol 470: 665–679, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nakanishi S. Mammalian tachykinin receptors. Annu Rev Neurosci 14: 123–136, 1991 [DOI] [PubMed] [Google Scholar]
- 44. Peden DB. Pollutants and asthma: role of air toxics. Environ Health Perspect 110, Suppl 4: 565–568, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rice AJ, Reynolds PN, Reynolds AM, Holmes MD, Scicchitano R. Tachykinin-induced bronchoconstriction in sheep is NK-1 receptor mediated and exhibits tachyphylaxis. Respirology 6: 113–123, 2001 [DOI] [PubMed] [Google Scholar]
- 46. Rich DQ, Schwartz J, Mittleman MA, Link M, Luttmann-Gibson H, Catalano PJ, Speizer FE, Dockery DW. Association of short-term ambient air pollution concentrations and ventricular arrhythmias. Am J Epidemiol 161: 1123–1132, 2005 [DOI] [PubMed] [Google Scholar]
- 47. Ruidavets JB, Cournot M, Cassadou S, Giroux M, Meybeck M, Ferrieres J. Ozone air pollution is associated with acute myocardial infarction. Circulation 111: 563–569, 2005 [DOI] [PubMed] [Google Scholar]
- 48. Schierhorn K, Hanf G, Fischer A, Umland B, Olze H, Kunkel G. Ozone-induced release of neuropeptides from human nasal mucosa cells. Int Arch Allergy Immunol 129: 145–151, 2002 [DOI] [PubMed] [Google Scholar]
- 49. Schultheis AH, Bassett DJ, Fryer AD. Ozone-induced airway hyperresponsiveness and loss of neuronal M2 muscarinic receptor function. J Appl Physiol 76: 1088–1097, 1994 [DOI] [PubMed] [Google Scholar]
- 50. Shore SA, Schwartzman IN, Le Blanc B, Murthy GG, Doerschuk CM. Tumor necrosis factor receptor 2 contributes to ozone-induced airway hyperresponsiveness in mice. Am J Respir Crit Care Med 164: 602–607, 2001 [DOI] [PubMed] [Google Scholar]
- 51. Taylor-Clark TE, , Undem BJ. Ozone activates airway nerves via the selective stimulation of TRPA1 ion channels. J Physiol 588: 423–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Tramontana M, Patacchini R, Giuliani S, Lippi A, Lecci A, Santicioli P, Criscuoli M, Maggi CA. Characterization of the antibronchoconstrictor activity of MEN 11420, a tachykinin NK2 receptor antagonist, in guinea-pigs. Eur J Pharmacol 352: 279–288, 1998 [DOI] [PubMed] [Google Scholar]
- 53. Undem BJ, Hunter DD, Liu M, Haak-Frendscho M, Oakragly A, Fischer A. Allergen-induced sensory neuroplasticity in airways. Int Arch Allergy Immunol 118: 150–153, 1999 [DOI] [PubMed] [Google Scholar]
- 54. van Hoof HJ, den Hartog GJ, Voss HP, van Bree L, Bast A. Capsaicin treatment induces muscarinic hyperreactivity in guinea pig trachea: a warning. Eur J Pharmacol 347: 261–264, 1998 [DOI] [PubMed] [Google Scholar]
- 55. Vedder H, Affolter HU, Otten U. Nerve growth factor (NGF) regulates tachykinin gene expression and biosynthesis in rat sensory neurons during early postnatal development. Neuropeptides 24: 351–357, 1993 [DOI] [PubMed] [Google Scholar]
- 56. Verhein KC, Jacoby DB, Fryer AD. IL-1 receptors mediate persistent, but not acute, airway hyperreactivity to ozone in guinea pigs. Am J Respir Cell Mol Biol 39: 730–738, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wagner EM, Jacoby DB. Methacholine causes reflex bronchoconstriction. J Appl Physiol 86: 294–297, 1999 [DOI] [PubMed] [Google Scholar]
- 58. Weinreich D, Moore KA, Taylor GE. Allergic inflammation in isolated vagal sensory ganglia unmasks silent NK-2 tachykinin receptors. J Neurosci 17: 7683–7693, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. White SR, Ohno S, Munoz NM, Gleich GJ, Abrahams C, Solway J, Leff AR. Epithelium-dependent contraction of airway smooth muscle caused by eosinophil MBP. Am J Physiol Lung Cell Mol Physiol 259: L294–L303, 1990 [DOI] [PubMed] [Google Scholar]
- 60. Wu ZX, Dey RD. Nerve growth factor-enhanced airway responsiveness involves substance P in ferret intrinsic airway neurons. Am J Physiol Lung Cell Mol Physiol 291: L111–L118, 2006 [DOI] [PubMed] [Google Scholar]
- 61. Wu ZX, Maize DF, Jr, Satterfield BE, Frazer DG, Fedan JS, Dey RD. Role of intrinsic airway neurons in ozone-induced airway hyperresponsiveness in ferret trachea. J Appl Physiol 91: 371–378, 2001 [DOI] [PubMed] [Google Scholar]
- 62. Wu ZX, Satterfield BE, Dey RD. Substance P released from intrinsic airway neurons contributes to ozone-enhanced airway hyperresponsiveness in ferret trachea. J Appl Physiol 95: 742–750, 2003 [DOI] [PubMed] [Google Scholar]
- 63. Wu ZX, Satterfield BE, Fedan JS, Dey RD. Interleukin-1β-induced airway hyperresponsiveness enhances substance P in intrinsic neurons of ferret airway. Am J Physiol Lung Cell Mol Physiol 283: L909–L917, 2002 [DOI] [PubMed] [Google Scholar]
- 64. Yeadon M, Wilkinson D, Darley-Usmar V, O'Leary VJ, Payne AN. Mechanisms contributing to ozone-induced bronchial hyperreactivity in guinea-pigs. Pulm Pharmacol 5: 39–50, 1992 [DOI] [PubMed] [Google Scholar]
- 65. Yost BL, Gleich GJ, Fryer AD. Ozone-induced hyperresponsiveness and blockade of M2 muscarinic receptors by eosinophil major basic protein. J Appl Physiol 87: 1272–1278, 1999 [DOI] [PubMed] [Google Scholar]
- 66. Yost BL, Gleich GJ, Jacoby DB, Fryer AD. The changing role of eosinophils in long-term hyperreactivity following a single ozone exposure. Am J Physiol Lung Cell Mol Physiol 289: L627–L635, 2005 [DOI] [PubMed] [Google Scholar]
- 67. Yuan L, Burcher E, Nail B. Tachykinin receptors and non-cholinergic bronchoconstriction in the anaesthetized guinea-pig. Clin Exp Pharmacol Physiol 23: 119–124, 1996 [DOI] [PubMed] [Google Scholar]