Abstract
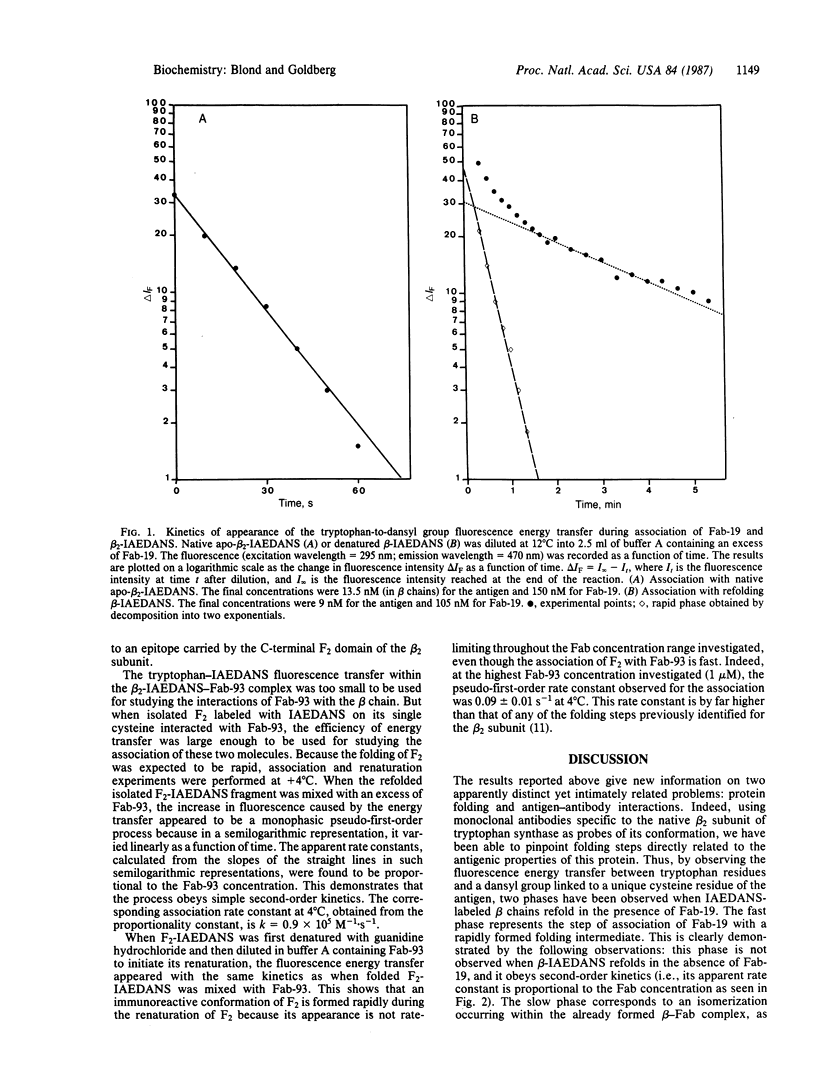
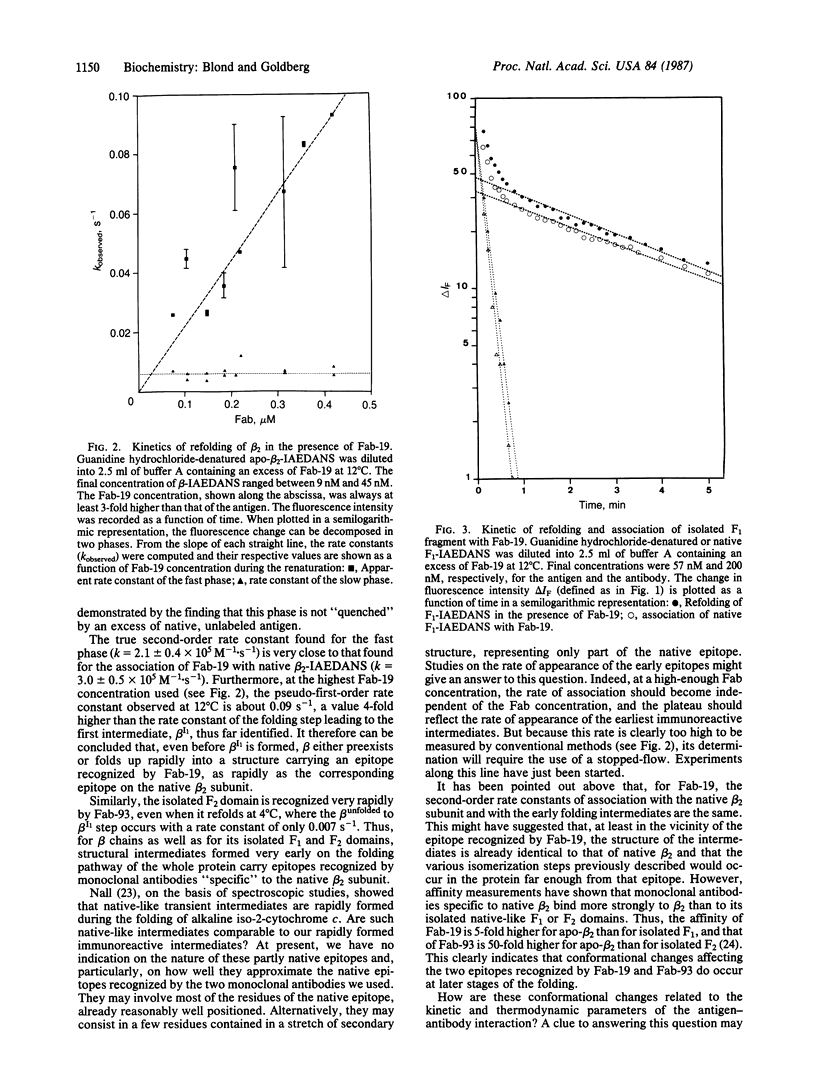
Two monoclonal antibodies directed against the native beta 2 subunit of Escherichia coli tryptophan synthase [L-serine hydro-lyase (adding indoleglycerol-phosphate), EC 4.2.1.20], one recognizing the C-terminal F1 domain and the other the N-terminal F2 domain, were used to detect immunoreactive intermediates in the folding of the protein. For that purpose, the association of the monoclonal antibodies with either the beta 2 subunit or its isolated domains was studied by using fluorescence energy transfer between tryptophan residues of the antibodies and a dansyl group covalently linked to the antigen. It is shown that the association of both monoclonal antibodies with the antigen occurs within a few seconds after initiation of the renaturation, whereas complete refolding of the beta 2 subunit requires several minutes under the same experimental conditions. Thus, immunoreactive intermediates appear to be formed at an early stage of the folding process. While the isolated F1 domain alone is able to rapidly refold into a conformational intermediate already well recognized by the anti-native-beta 2 antibody, it cannot, in the absence of the F2 domain, reach its native conformation. However, its association with the anti-native-beta 2 antibody induces a structural change of F1 that brings it closer to the conformation it normally adopts when interacting with F2 inside the native beta 2 subunit.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amit A. G., Mariuzza R. A., Phillips S. E., Poljak R. J. Three-dimensional structure of an antigen-antibody complex at 6 A resolution. Nature. 1985 Jan 10;313(5998):156–158. doi: 10.1038/313156a0. [DOI] [PubMed] [Google Scholar]
- Blond S., Goldberg M. E. Kinetics and importance of the dimerization step in the folding pathway of the beta 2 subunit of Escherichia coli tryptophan synthase. J Mol Biol. 1985 Apr 20;182(4):597–606. doi: 10.1016/0022-2836(85)90245-1. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chaffotte A. F., Goldberg M. E. Immunochemical evidence for conformational flexibility and its modulation by specific ligands in the beta 2 subunit of Escherichia coli tryptophan synthase. Biochemistry. 1983 May 24;22(11):2708–2714. doi: 10.1021/bi00280a019. [DOI] [PubMed] [Google Scholar]
- Djavadi-Ohaniance L., Friguet B., Goldberg M. E. Structural and functional influence of enzyme-antibody interactions: effects of eight different monoclonal antibodies on the enzymatic activity of Escherichia coli tryptophan synthase. Biochemistry. 1984 Jan 3;23(1):97–104. doi: 10.1021/bi00296a016. [DOI] [PubMed] [Google Scholar]
- Dolgikh D. A., Kolomiets A. P., Bolotina I. A., Ptitsyn O. B. 'Molten-globule' state accumulates in carbonic anhydrase folding. FEBS Lett. 1984 Jan 2;165(1):88–92. doi: 10.1016/0014-5793(84)80020-4. [DOI] [PubMed] [Google Scholar]
- Dyson H. J., Cross K. J., Houghten R. A., Wilson I. A., Wright P. E., Lerner R. A. The immunodominant site of a synthetic immunogen has a conformational preference in water for a type-II reverse turn. Nature. 1985 Dec 5;318(6045):480–483. doi: 10.1038/318480a0. [DOI] [PubMed] [Google Scholar]
- Friguet B., Chaffotte A. F., Djavadi-Ohaniance L., Goldberg M. E. Measurements of the true affinity constant in solution of antigen-antibody complexes by enzyme-linked immunosorbent assay. J Immunol Methods. 1985 Mar 18;77(2):305–319. doi: 10.1016/0022-1759(85)90044-4. [DOI] [PubMed] [Google Scholar]
- Friguet B., Djavadi-Ohaniance L., Goldberg M. E. Conformational changes induced by domain assembly within the beta 2 subunit of Escherichia coli tryptophan synthase analysed with monoclonal antibodies. Eur J Biochem. 1986 Nov 3;160(3):593–597. doi: 10.1111/j.1432-1033.1986.tb10079.x. [DOI] [PubMed] [Google Scholar]
- Friguet B., Djavadi-Ohaniance L., Goldberg M. E. Some monoclonal antibodies raised with a native protein bind preferentially to the denatured antigen. Mol Immunol. 1984 Jul;21(7):673–677. doi: 10.1016/0161-5890(84)90053-1. [DOI] [PubMed] [Google Scholar]
- Friguet B., Djavadi-Ohaniance L., Pages J., Bussard A., Goldberg M. A convenient enzyme-linked immunosorbent assay for testing whether monoclonal antibodies recognize the same antigenic site. Application to hybridomas specific for the beta 2-subunit of Escherichia coli tryptophan synthase. J Immunol Methods. 1983 Jun 10;60(3):351–358. doi: 10.1016/0022-1759(83)90292-2. [DOI] [PubMed] [Google Scholar]
- Holmes N. J., Parham P. Enhancement of monoclonal antibodies against HLA-A2 is due to antibody bivalency. J Biol Chem. 1983 Feb 10;258(3):1580–1586. [PubMed] [Google Scholar]
- Hudson E. N., Weber G. Synthesis and characterization of two fluorescent sulfhydryl reagents. Biochemistry. 1973 Oct 9;12(21):4154–4161. doi: 10.1021/bi00745a019. [DOI] [PubMed] [Google Scholar]
- Högberg-Raibaud A., Goldberg M. E. Isolation and characterization of independently folding regions of the beta chain of Escherichia coli tryptophan synthetase. Biochemistry. 1977 Sep 6;16(18):4014–4020. doi: 10.1021/bi00637a012. [DOI] [PubMed] [Google Scholar]
- Kim P. S., Baldwin R. L. Specific intermediates in the folding reactions of small proteins and the mechanism of protein folding. Annu Rev Biochem. 1982;51:459–489. doi: 10.1146/annurev.bi.51.070182.002331. [DOI] [PubMed] [Google Scholar]
- Mariuzza R. A., Boulot G., Guillon V., Poljak R. J., Berek C., Jarvis J. M., Milstein C. Preliminary crystallographic study of the Fab fragments of two monoclonal anti-2-phenyloxazolone antibodies. J Biol Chem. 1985 Aug 25;260(18):10268–10270. [PubMed] [Google Scholar]
- Nall B. T. Native or nativelike species are transient intermediates in folding of alkaline iso-2 cytochrome c. Biochemistry. 1986 May 20;25(10):2974–2978. doi: 10.1021/bi00358a036. [DOI] [PubMed] [Google Scholar]
- Ohgushi M., Wada A. 'Molten-globule state': a compact form of globular proteins with mobile side-chains. FEBS Lett. 1983 Nov 28;164(1):21–24. doi: 10.1016/0014-5793(83)80010-6. [DOI] [PubMed] [Google Scholar]
- Onoue K., Yagi Y., Grossberg A. L., Pressman D. Number of binding sites of rabbit macroglobulin antibody and its subunits. Immunochemistry. 1965 Dec;2(4):401–415. doi: 10.1016/0019-2791(65)90039-x. [DOI] [PubMed] [Google Scholar]
- Richardson J. S. The anatomy and taxonomy of protein structure. Adv Protein Chem. 1981;34:167–339. doi: 10.1016/s0065-3233(08)60520-3. [DOI] [PubMed] [Google Scholar]
- Sachs D. H., Schechter A. N., Eastlake A., Anfinsen C. B. An immunologic approach to the conformational equilibria of polypeptides. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3790–3794. doi: 10.1073/pnas.69.12.3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westhof E., Altschuh D., Moras D., Bloomer A. C., Mondragon A., Klug A., Van Regenmortel M. H. Correlation between segmental mobility and the location of antigenic determinants in proteins. Nature. 1984 Sep 13;311(5982):123–126. doi: 10.1038/311123a0. [DOI] [PubMed] [Google Scholar]
- Wetlaufer D. B. Folding of protein fragments. Adv Protein Chem. 1981;34:61–92. doi: 10.1016/s0065-3233(08)60518-5. [DOI] [PubMed] [Google Scholar]
- Wilson D. A., Crawford I. P. Purification and properties of the B component of Escherichia coli tryptophan synthetase. J Biol Chem. 1965 Dec;240(12):4801–4808. [PubMed] [Google Scholar]
- Wilson I. A., Niman H. L., Houghten R. A., Cherenson A. R., Connolly M. L., Lerner R. A. The structure of an antigenic determinant in a protein. Cell. 1984 Jul;37(3):767–778. doi: 10.1016/0092-8674(84)90412-4. [DOI] [PubMed] [Google Scholar]
- Zetina C. R., Goldberg M. E. Kinetics of renaturation and self-assembly of intermediates on the pathway of folding of the beta 2-subunit of Escherichia coli tryptophan-synthetase. J Mol Biol. 1982 May 5;157(1):133–148. doi: 10.1016/0022-2836(82)90516-2. [DOI] [PubMed] [Google Scholar]


