Abstract
HER-2/neu is a valuable therapeutic and prognostic marker in primary breast carcinomas. The aim of this study was to evaluate the association between mammographic calcifications and HER-2/neu overexpression in primary breast carcinomas and assess its clinical perspective. A retrospective study of 152 preoperative mammograms in patients with breast carcinoma was performed. Expression of HER-2/neu was determined by immunohistochemical staining on 152 tissues that comprised specimens of 11 ductal carcinoma in situ (DCIS) and 141 primary invasive carcinomas. Mammographic calcifications were evaluated according to the Breast Imaging Reporting and Data System (BI-RADS), fourth edition. Calcifications were found in 73 (48.0%) out of 152 patients by mammography finding. Calcifications were more common in carcinomas with HER-2/neu overexpression (45:73, 61.6%) than in those without HER-2/neu overexpression (28:79, 35.4%; P = 0.001). Of the 73 carcinomas with calcifications on mammography, mass with spiculated margin as an associated finding of calcifications was more significantly frequent in carcinomas with HER-2/neu overexpression (15 of 45, 33.3%) than in those without HER-2/neu overexpression (2 of 28, 7.1%; P = 0.036). Fine linear morphology was more common in carcinomas with HER-2/neu overexpression (15:45, 33.3%) when compared with those without HER-2/neu overexpression (2:28, 7.1%; P = 0.036). Clustered distribution of calcifications was more common in carcinomas with HER-2/neu overexpression (26:45, 57.8%) compared with carcinomas without HER-2/neu overexpression (6:28, 21.4%; P = 0.048). Mammographic calcifications are correlated with HER-2/neu overexpression in primary breast carcinomas. Calcifications detected during screening mammography are not only of diagnostic value but of use in determining therapeutic options and prognosis.
Key words: Breast neoplasms, mammography, calcification, HER-2/neu
Introduction
Mammography contributes to the improvement of breast carcinoma survival through early detection and treatment of breast lesions. Calcifications represent one of the most important mammographic findings of breast carcinomas, playing an important role in the diagnosis of breast diseases. Therefore, identifying biological features associated with progression and tumor aggressiveness in conjunction with breast radiological features such as calcifications would help to make treatment decisions. Previous studies have attempted to evaluate and interpret the clinical perspective of the relationship between mammographic calcifications and the expression of selected biological markers, such as ER, PR, Bax, Fas, Bcl-2, and DNA fragmentation factor in breast carcinomas.1,2
Amplification of HER-2/neu is considered as an independent poor prognostic factor in invasive breast carcinomas and has been associated with altered clinical responsiveness to systemic breast cancer treatment such as chemotherapy and antiestrogens.3 The reported range of patients found to be overexpressed for HER-2/neu by immunohistochemistry is 20–68%.2,4,5 Some investigators have attempted to evaluate the association between mammographic calcifications and HER-2/neu overexpression. Two prior reports have shown a correlation between malignant-appearing microcalcifications and HER-2/neu overexpression in ductal carcinoma in situ (DCIS) and nonpalpable breast carcinomas. Malignant-appearing microcalcifications was the major mammographic finding in nonpalpable breast carcinomas, and statistically significant associations were found between malignant-appearing microcalcifications and HER-2/neu overexpression in the study by Karamauzis et al.2. Evans et al.5 reported that DCIS with HER-2/neu overexpression had higher frequency of calcifications than those without HER-2/neu overexpression, and HER-2/neu positive DCIS showed the following features more commonly than HER-2/neu negative disease: calcification, ductal distribution of calcification, rod-shaped calcification, and granular calcification.
As HER-2/neu overexpression has been shown to correlate with aggressive histological features and worse prognosis, the association of mammographic calcifications with HER-2/neu overexpression may have practical value. However, these clinical data focused on DCIS cases. Therefore, we investigated the correlation of HER-2/neu status with mammographic calcifications in consecutive primary breast carcinomas. The purpose of our study was to evaluate the association between mammographic calcifications and HER-2/neu overexpression in patients with primary breast carcinomas.
Materials and Methods
Patients
The institutional review board at the University of Shandong approved this study. One hundred fifty-two patients with preoperative mammograms underwent mastectomy or radical mastectomy with lymph node dissection at Ji’nan Central Hospital of Shandong University (Ji’nan, China) from May 2003 to August 2006. Breast carcinoma is routinely divided into invasive carcinoma and DCIS. The tumor subtypes of invasive carcinoma were classified as ductal carcinoma and others, which included medullary, mucinous, or lobular carcinomas. The tumor grade of invasive carcinomas was classified according to the Scarff–Bloom–Richardson system.6 Based on the frequency of cell mitosis, tubule formation, and nuclear pleomorphism, invasive carcinoma was graded as grade 1 (low), grade 2 (moderate), or grade 3 (high grade). The presence of lymph node metastases was reviewed for each patient. The tumor stage was determined according to the American Joint Committee on Cancer (AJCC) Cancer Staging Manual, sixth edition.7 The median age of the patients was 51 years (range, 27–82 years).
Mammography
The mammograms were obtained with a digital mammography system (Gold Standard, Pan-pacific Enterprises Inc., USA). Standard craniocaudal and lateral views were carried out in all patients. Cone-down magnification views were obtained when necessary. The mammograms were reviewed by an experienced breast radiologist (M.H.), who was not aware of any of the clinical data or HER-2/neu status. All of the mammograms were then appraised by another radiologist (A.O.). Mammograms were reviewed according to the analytic criteria of Breast Imaging Reporting and (BI-RADS), 4th edition.8 Mammographic findings were categorized as mass, calcifications, architectural distortions, and asymmetric densities. According to the associated findings with calcification, mammographic calcifications were categorized as associated with mass, architectural distortions, and asymmetric densities. Furthermore, we took the “associated mass with spiculated margin” variable into account. Calcifications were classified into four groups according to their morphologies: fine linear, punctate, coarse, and fine pleomorphicsi. The four analytic criteria chosen for calcification distribution were clustered, segmental, regional, linear, and diffuse.
Immunohistochemistry Analysis
HER-2/neu status was determined by immunohistochemical staining of tissue sections with monoclonal antibody (Dako, no. K5204). Detection was carried through with strepavidin–peroxidase complex. Histopathological assessment was performed on paraffin sections of excision specimens cut at 4–6 μm. Immunohistochemistry was performed on sections incubated in 3% H2O2 in methanol for 10 min, followed by washing in 0.01 mmol/l phosphate-buffered saline and treatment with 1% normal goat serum for 10 min at room temperature. The slides were then incubated with the primary antibody (HER-2/neu, 1:100) for 2 h at 4°C. Sites of primary antibody binding were identified by incubating blots with horse radish peroxidase-conjugated anti-rabbit IgG followed by reaction with substrate, 3,3′-diaminobenzidine tetrahydrochloride (DAB). As a negative control, preimmune goat serum was used in place of the primary antibody.
The immunohistochemistry test examines the protein on the cell surface according to HercepTest (DakoCytomation, Carpinteria, CA, USA). Two pathologists without access to the patient’s clinical information judged the degree of color change in the cell against a nonstandardized chart and graded it as 0, 1+, 2+, or 3+. Carcinomas showing 0 or 1+ staining were defined as “negative” for overexpression. Carcinomas showing 2+ or 3+ staining were defined as “positive” for overexpression.
Statistical Analysis
χ2 test was used to evaluate the association between mammographic findings and HER-2/neu overexpression in breast carcinomas. All statistical tests were two-sided, while the statistical significance of observed difference was set at P < 0.05, and all data was analyzed using Statistical Package for the Social Sciences statistical software (version 10.0, SPSS Inc., Chicago, IL, USA).
Results
Clinicopathological Features of Breast Carcinoma According to Mammographic Calcifications
There were a total of 152 breast carcinomas in our study, of which 121 were invasive ductal subtype, 20 were other invasive subtype (among which nine were mucinous, six lobular, and five medullary carcinomas), and 11 were DCIS. Tumor grading result was grade 1 in 33, grade 2 in 88, and grade 3 in 20 of 141 invasive breast tumors. Clinical staging result was stage I, 41 patients (29.1%); stage II, 63 patients (44.7%); and stage III, 37 patients (26.2%). Ninety-four out of 141 breast carcinomas had axillary lymph node metastasis.
There was a significant difference in invasive tumor subtypes between carcinomas with mammographic calcifications and those without mammographic calcifications (χ2 = 6.389, P = 0.011). Ductal subtype was more frequent in carcinomas with calcifications, and other invasive subtypes (lobular, mucinous, and medullary) were more frequent in carcinomas without calcifications. Tumor–node–metastases (TNM) III stage and axillary lymph node metastasis were more common in carcinomas with mammographic calcifications than in those without calcifications (χ2 = 11.60, P = 0.003; χ2 = 9.647, P = 0.002, respectively). There was no significant difference in tumor types and grades between carcinomas with calcifications and those without calcifications (P > 0.05; Table 1).
Table 1.
Clinical–Pathological Features of Primary Breast Carcinoma According to Mammographic Calcifications
| Clinical-Pathological Features | With Calcifications (%) | Without Calcifications (%) | P value |
|---|---|---|---|
| Tumor type (n = 152) | |||
| Invasive carcinoma | 65 (89.0) | 76 (96.2) | |
| DCIS | 8 (11.0) | 3 (3.8) | 0.089 |
| Total | 73 (100) | 79 (100) | |
| Tumor subtypes in invasive carcinoma (n = 141) | |||
| Ductal | 61 (93.8) | 60 (78.9) | |
| Others | 4 (6.2) | 16 (21.1) | 0.011 |
| Total | 65 (100) | 76 (100) | |
| TNM stage in invasive carcinomas (n = 141) | |||
| I | 12 (18.5) | 29 (39.7) | |
| II | 28 (43.0) | 35 (47.9) | |
| III | 25 (38.5) | 12 (16.4) | 0.003 |
| Total | 65 (100) | 76 (100) | |
| Axillary lymph node metastasis (n = 141) | |||
| Yes | 52 (80) | 42 (55.2) | |
| No | 13 (20) | 34 (44.8) | 0.002 |
| Total | 65 (100) | 76 (100) | |
| Tumor grade in invasive carcinomas (n = 141) | |||
| G1 | 14 (21.5) | 19 (25.0) | |
| G2 | 42 (64.6) | 46 (60.5) | |
| G3 | 9 (13.9) | 11 (14.5) | 0.868 |
| Total | 65 (100) | 76 (100) | |
DCIS ductal carcinoma in situ, G1 grade 1, G2 grade 2, G3 grade 3
Correlation Between Mammographic Features and HER-2/neu Overexpression
Seventy-three out of 152 patients with breast carcinoma displayed HER-2/neu overexpression. Of the 152 breast carcinomas, 60 presented as mass, 73 as calcifications, 20 as architectural distortion, and eight as asymmetric density. Calcifications (arrows) were more significantly frequent in carcinomas with HER-2/neu overexpression (45 of 73, 61.6%) than in those without HER-2/neu overexpression (28 of 79, 38.4%; P = 0.001; Figs. 1, 2, 3, and 4). There was no significant difference in other mammographic features such as mass, architectural distortion, and asymmetric density between the carcinomas with or without HER-2/neu overexpression (P > 0.05; Table 2).
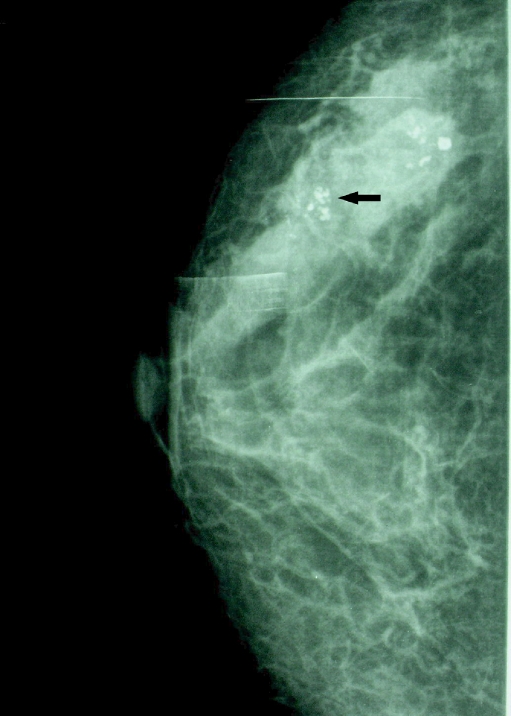
Fig 1.
Mammogram (CC) showing pleomorphic calcifications associated asymmetric density in a ductal distribution because of HER-2/neu-positive invasive ductal carcinoma.
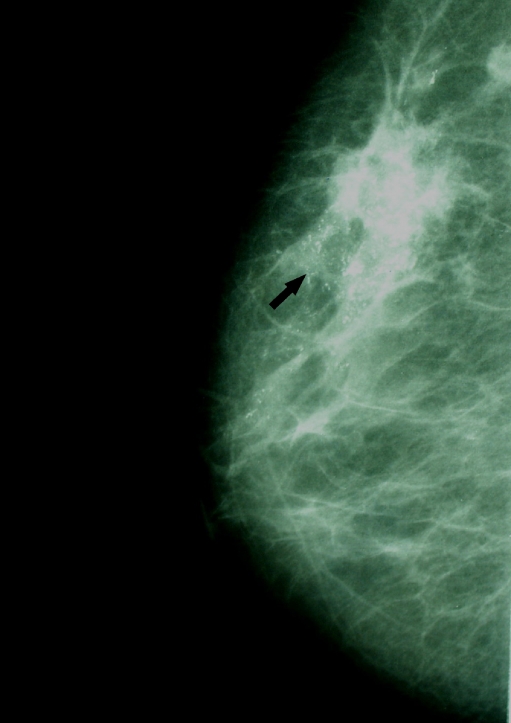
Fig 2.
Mammogram (MLO) showing punctate and fine linear calcifications because of HER-2/neu-positive invasive ductal carcinoma.
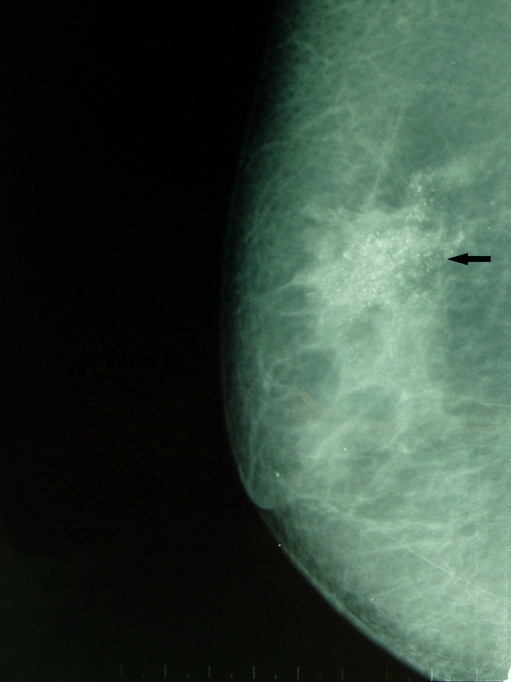
Fig 3.
Mammogram MLO showing punctate calcifications because of HER-2/neu-positive invasive mucinous carcinoma.
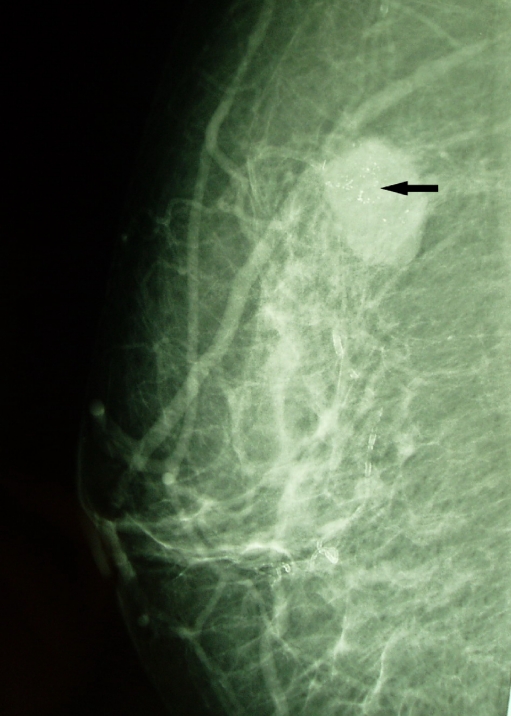
Fig 4.
Mammogram (MLO) showing a mass associated with fine linear calcifications because of HER-2/neu-negative invasive lobular carcinoma.
Table 2.
Association Between Mammographic Features and HER-2/neu Overexpression
| Mammographic Features | n | HER-2/neu Overexpression (%) | P value | |
|---|---|---|---|---|
| Yes (n = 73) | No (n = 79) | |||
| Mass | ||||
| + | 60 | 33 (45.2) | 27 (34.2) | |
| − | 92 | 40 (54.8) | 52 (65.8) | 0.165 |
| Calcification | ||||
| + | 73 | 45 (61.6) | 28 (35.4) | |
| − | 79 | 28 (38.4) | 51 (64.6) | 0.001 |
| Architectural distortion | ||||
| + | 20 | 11 (15.1) | 9 (11.4) | |
| − | 132 | 62 (84.9) | 70 (88.6) | 0.804 |
| Asymmetric density | ||||
| + | 8 | 3 (4.1) | 5 (6.3) | |
| − | 144 | 70 (95.9) | 74 (93.7) | 0.503 |
Positive sign presence of each mammographic feature, negative sign absence of each mammographic feature
Correlation Between Findings Associated with Calcifications and HER-2/neu Overexpression
In the 73 carcinomas with calcifications on mammography, calcifications were associated with a mass in 30 (17 with spiculated margin), architectural distortion in six and asymmetric density in three. There was no significant difference in calcifications associated with masses between carcinomas with HER-2/neu overexpression (18 of 45, 40.0%) and those without HER-2/neu overexpression(17 of 28, 60.7%; P = 0.314). However, mass with spiculated margin as an associated finding of calcifications was more frequent in carcinomas with HER-2/neu overexpression (15 of 45, 33.3%) than in those without HER-2/neu overexpression (2 of 28, 7.1%; P = 0.036). There was no significant difference in either architectural distortion or asymmetric density as an associated finding of the calcification between the carcinomas with or without HER-2/neu overexpression (P > 0.05; Table 3).
Table 3.
Association Between Associated Findings of Calcifications and HER-2/neu Overexpression
| Associated Findings | n | HER-2/neu Overexpression (%) | P value | |
|---|---|---|---|---|
| Yes (n = 45) | No (n = 28) | |||
| Mass | 35 | 18 (40.0) | 17(60.7) | 0.314 |
| Mass with spiculated margin | 17 | 15 (33.3) | 2 (7.1) | 0.036 |
| Architectural distortion | 8 | 5 (13.3) | 2 (7.1) | 0.721 |
| Asymmetric density | 3 | 2 (4.4) | 1 (3.6) | 0.347 |
Association Between Mammographic Calcification Features and HER-2/neu Overexpression
Of the 73 carcinomas with calcifications on mammography, clustered distribution of calcifications was more common in carcinomas with HER-2/neu overexpression (26 of 45, 57.8%) compared with carcinomas without HER-2/neu overexpression (6 of 28, 21.4%; P = 0.048). Fine linear morphology was significantly more frequent in carcinomas with HER-2/neu overexpression (15 of 45, 33.3%) compared to those without HER-2/neu overexpression (2 of 28, 7.1%; P = 0.036; Table 4).
Table 4.
Association Between Morphology and Distribution of Calcifications and HER-2/neu Overexpression
| Features | n | HER-2/neu Overexpression (%) | P value | |
|---|---|---|---|---|
| Yes (n = 45) | No (n = 28) | |||
| Distribution | ||||
| Clustered | 32 | 26 (57.8) | 6 (21.4) | 0.048 |
| Segmental | 12 | 7 (15.6) | 5 (17.9) | 0.872 |
| Regional | 6 | 2 (4.4) | 4 (14.3) | 0.355 |
| Linear | 10 | 5 (11.1) | 5 (17.9) | 0.718 |
| Diffuse | 13 | 5 (11.1) | 8 (28.6) | 0.188 |
| Morphologic aspects | ||||
| Fine linear | 17 | 15 (33.3) | 2 (7.1) | 0.036 |
| Punctate | 20 | 9 (20.0) | 11 (39.3) | 0.181 |
| Fine pleomorphic | 32 | 18 (40.0) | 14 (50.0) | 0.604 |
| Coarse | 4 | 3 (6.7) | 1 (3.6) | 0.591 |
Discussion
Until now, mammography is the mainstay of image diagnosis of breast disease. Calcifications represent one of the most important features of breast carcinoma. It is well documented that calcifications represent one of the earliest mammographically detectable changes associated with in situ and invasive breast carcinomas in asymptomatic women.2,9 Fondrinier and co-workers10 reported a 47% incidence of malignancy in 204 women with mammographic files showing clustered calcifications.
Two prospective randomized trials that compared breast-conserving therapy (BCT) with modified radical mastectomy (RM) for patients with early-stage carcinoma of the breast revealed no significant difference in overall survival (OS) or disease-free survival (DFS).11,12 However, local recurrence is also seen after RM, the risk being equal to or only slightly smaller than after conservative surgery and radiotherapy.13 Thus, there is a considerable need to identify tumor markers associated with tumor aggressiveness and progression in conjunction with mammographic features such as calcifications, providing more clues that would help breast specialists make treatment decisions.
Overexpression of HER-2/neu is the greatest abnormality in breast cancer and has been thoroughly analyzed as prognostic factor for breast cancer.14,15 Recently, Seo et al.16 reported that HER-2/neu overexpression in primary breast carcinoma was correlated with patients’ age and calcifications at mammography. However, Likaki-Karatza et al.1 found that HER-2/neu positivity was statistically correlated to asymmetric density with poorly defined margins without calcifications in high-grade breast carcinomas. In the current study, calcifications were more frequent in carcinomas with HER-2/neu overexpression (61.6%) than in those without HER-2/neu overexpression (45.4%). Therefore, mammographic calcifications are related to HER-2/neu overexpression.
Recently, a retrospective study showed that calcification descriptors and the categorization of calcification descriptors in the BI-RADS fourth edition help stratify the risk of malignancy.17 The calcification descriptors and categories in BI-RADS fourth edition help predict the risk of malignancy for suspicious calcifications. In the current study, we evaluated mammographic calcifications according to the BI-RADS fourth edition and determined whether there was any difference in distribution and morphology of mammographic calcifications between the two groups of breast carcinomas with or without HER-2/neu overexpression. We did not assess the density of calcifications. Concerning the morphologic category of mammographic calcifications, our study showed that fine linear calcifications were more common in carcinomas with HER-2/neu overexpression than in those without HER-2/neu overexpression. In addition, we found a significant association between clustered distribution of calcifications and HER-2/neu overexpression, and our finding was in contrast to another report.16 One potential weakness of the current study is the potential for underestimation given the stratification of the data and subsequent low numbers in some of the categories. Another potential weakness of the study is that we have had only one breast radiologist review the mammograms and score them according to BI-RADS. Further study with a larger group of patients is recommended to confirm these results and could offer more clues about their prognostic value in primary breast carcinoma.
It was reported that mammographic calcifications as an associated finding of mass were more frequent in carcinomas with HER-2/neu overexpression than in those without HER-2/neu overexpression16. In our study, there was no significant difference in these two kind carcinomas. It is worth noting, though, that mass with speculated margin as an associated finding of calcifications was more common in carcinomas with HER-2/neu overexpression compared to those without HER-2/neu overexpression. However, Evans et al.18 reported that mammographic spiculation (the presence of either a spiculated mass or distortion) was associated with more prolonged breast-cancer-specific survival. To our knowledge, the relationship between mammographic-calcification-associated spiculated mass and HER-2/neu status has not been previously reported. Thus, further study is needed to determine the relationship between the associated findings of calcifications including spiculated mass and HER-2/neu overexpression.
In conclusion, mammographic calcifications are correlated with HER-2/neu overexpression. Morphologic features, distribution, and associated findings of mammographic calcifications may be related to HER-2/neu overexpression. Calcifications detected during screening mammography are not only of diagnostic value but also of use in determining therapeutic options and prognosis.
Reference
- 1.Likaki-Karatza E, Ravazoula P, Androutsopoulos G, Michail G, Batistatou A, Tzorakoleftherakis E, Kalofonos H, Kourounis G. Correlation of mammographic appearance and molecular prognostic factors in high-grade breast carcinomas. Eur J Gynaecol Oncol. 2006;27:39–41. [PubMed] [Google Scholar]
- 2.Karamouzis MV, Likaki-Karatza E, Ravazoula P, Badra FA, Koukouras D, Tzorakoleftherakis E, Papavassiliou AG, Kalofonos HP. Non-palpable breast carcinomas: correlation of mammographically detected malignant-appearing microcalcifications and molecular prognostic factors. Int J Cancer. 2002;102:86–90. doi: 10.1002/ijc.10654. [DOI] [PubMed] [Google Scholar]
- 3.Menard S, Pupa SM, Campiglio M, Tagliabue E. Biologic and therapeutic role of HER2 in cancer. Oncogene. 2003;22:6570–6578. doi: 10.1038/sj.onc.1206779. [DOI] [PubMed] [Google Scholar]
- 4.Walker RA, Dearing SJ, Brown LA. Comparison of pathological and biological features of symptomatic and mammographically detected ductal carcinoma in situ of the breast. Hum Pathol. 1999;30:943–948. doi: 10.1016/S0046-8177(99)90248-4. [DOI] [PubMed] [Google Scholar]
- 5.Evans AJ, Pinder SE, Ellis IO, Sibbering DM, Elston CW, Poller DN, Wilson AR. Correlations between the mammographic features of ductal carcinoma in situ (DCIS) and C-erbB-2 oncogene expression. Clin Radiol. 1994;49:559–562. doi: 10.1016/S0009-9260(05)82937-X. [DOI] [PubMed] [Google Scholar]
- 6.Doussal V, Tubiana-Hulin M, Friedman S, Hacene K, Spyratos F, Brunet M. Prognostic value of histologic grade nuclear components of Scarff–Bloom–Richardson (SBR): an improved score modification based on a multivariate analysis of 1262 invasive ductal breast carcinomas. Cancer. 1989;64:1914–1921. doi: 10.1002/1097-0142(19891101)64:9<1914::AID-CNCR2820640926>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 7.Greene FI, Page DI, Fleming ID. AJCC Cancer Staging Manual. 6. New York: Springer; 2002. [Google Scholar]
- 8.Breast Imaging Reporting and Data System (BI-RADS)–Mammography. 4. Reston: Am College Radiol; 2003. [Google Scholar]
- 9.Evans AJ, Kutt E, Record C, Waller M, Moss S. Radiological findings of screen-detected cancers in a multi-centre randomized, controlled trial of mammographic screening in women from age 40 to 48 years. Clin Radiol. 2006;61:784–788. doi: 10.1016/j.crad.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 10.Fondrinier E, Lorimier G, Guerin-Boblet V, Bertrand AF, Mayras C, Dauver N. Breast microcalcifications. Multivariate analysis of radiologic and clinical factors for carcinoma. World J Surg. 2002;26:290–296. doi: 10.1007/s00268-001-0220-3. [DOI] [PubMed] [Google Scholar]
- 11.Straus K, Lichter A, Lippman M, Danforth D, Swain S, Cowan K, deMoss E, MacDonald H, Steinberg S, d’Angelo T. Research of the National Cancer Institute early breast cancer trial. Monogr Natl Cancer Inst. 1992;11:27–32. [PubMed] [Google Scholar]
- 12.Early Breast Cancer Trialists, Collaborative Group Effects of radiotherapy and surgery in early breast cancer: an overview of randomized trials. N Engl J Med. 1995;33:1444–1445. doi: 10.1056/NEJM199511303332202. [DOI] [PubMed] [Google Scholar]
- 13.Voogd AC, Nielsen M, Peterse JL, Blichert-Toft M, Bartelink H, Overgaard M, Tienhoven G, Andersen KW, Sylvester RJ, Dongen JA. Difference in risk factors for local and distant recurrence after breast-conserving therapy or mastectomy for stage I and II breast cancer: pooled results of two large European randomized trials. J Clin Oncol. 2001;19:1688–1697. doi: 10.1200/JCO.2001.19.6.1688. [DOI] [PubMed] [Google Scholar]
- 14.Ariga R, Zarif A, Korasick J, Reddy V, Siziopikou K, Gattuso P. Correlation of her-2/neu gene amplification with other prognostic and predictive factors in female breast carcinoma. Breast J. 2005;11:278–280. doi: 10.1111/j.1075-122x.2005.21463.x. [DOI] [PubMed] [Google Scholar]
- 15.Rodrigues NA, Dillon D, Carter D, Parisot N, Haffty BG. Differences in the pathologic and molecular features of intraductal breast carcinoma between younger and older women. Cancer. 2003;97:1393–1403. doi: 10.1002/cncr.11204. [DOI] [PubMed] [Google Scholar]
- 16.Seo BK, Pisano ED, Kuzimak CM, Koomen M, Pavic D, Lee Y, Cole EB, Lee J. Correlation of HER-2/neu overexpression with mammography and age distribution in primary breast carcinomas. Acad Radiol. 2006;13:1211–1218. doi: 10.1016/j.acra.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 17.Burnside ES, Ochsner JE, Fowler KJ, Fine JP, Salkowski LR, Rubin DL, Sisney GA. Use of microcalcification descriptors in BI-RADS 4th edition to stratify risk of malignancy. Radiology. 2007;242:388–395. doi: 10.1148/radiol.2422052130. [DOI] [PubMed] [Google Scholar]
- 18.Evans AJ, Pinder SE, James JJ, Ellis IO, Cornford E. Is mammographic spiculation an independent, good prognostic factor in screening-detected invasive breast cancer? AJR Am J Roentgenol. 2006;187:1377–1380. doi: 10.2214/AJR.05.0725. [DOI] [PubMed] [Google Scholar]