Abstract
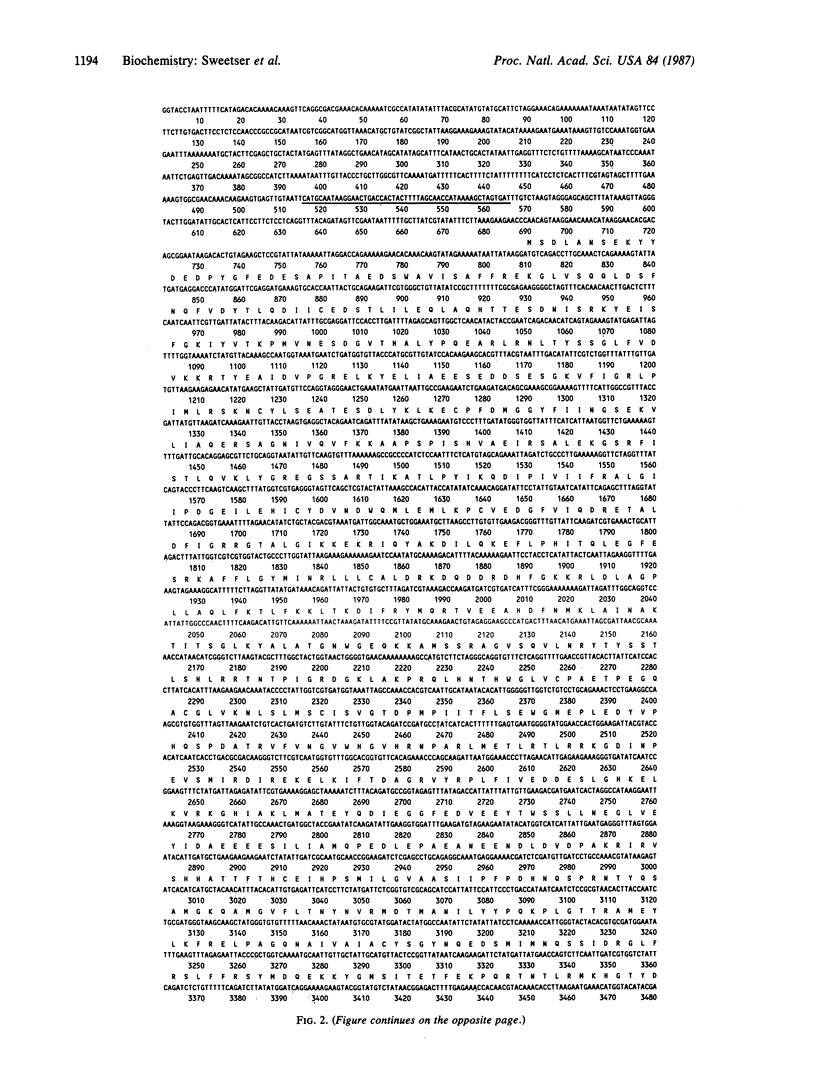
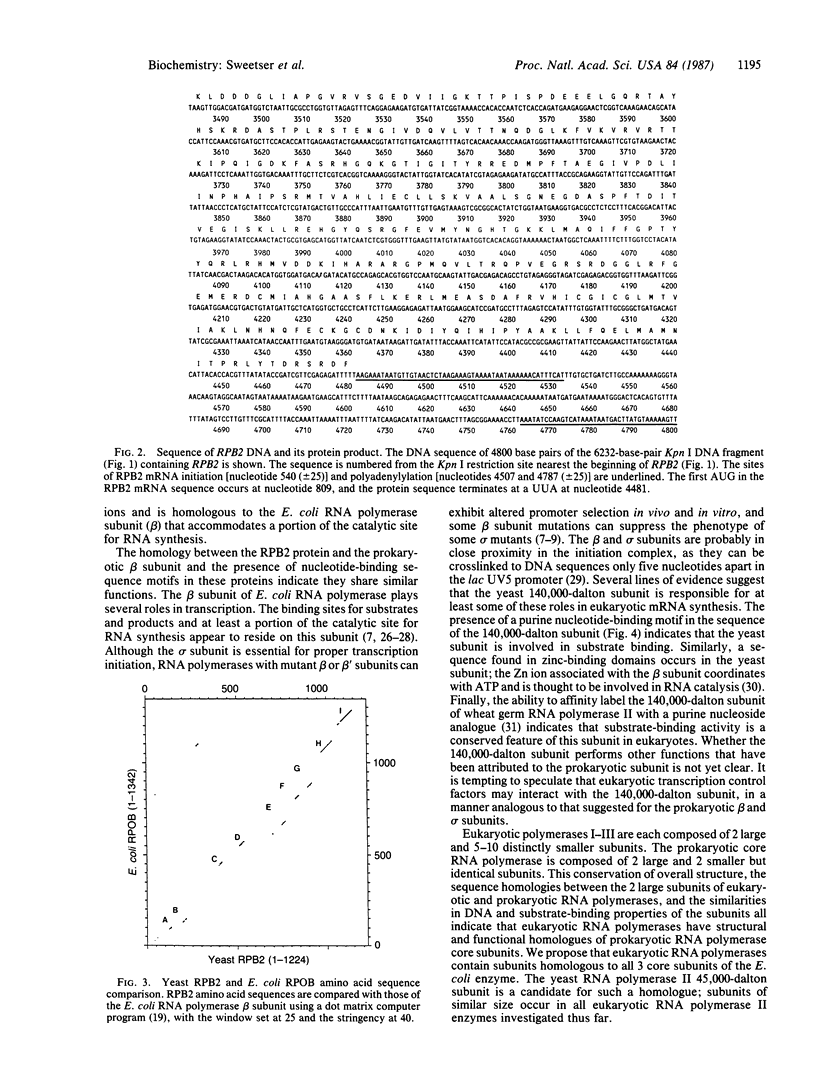
Eukaryotic RNA polymerases are complex aggregates whose component subunits are functionally ill-defined. The gene that encodes the 140,000-dalton subunit of Saccharomyces cerevisiae RNA polymerase II was isolated and studied in detail to obtain clues to the protein's function. This gene, RPB2, exists in a single copy in the haploid genome. Disruption of the gene is lethal to the yeast cell. RPB2 encodes a protein of 138,750 daltons, which contains sequences implicated in binding purine nucleotides and zinc ions and exhibits striking sequence homology with the beta subunit of Escherichia coli RNA polymerase. These observations suggest that the yeast and the E. coli subunit have similar roles in RNA synthesis, as the beta subunit contains binding sites for nucleotide substrates and a portion of the catalytic site for RNA synthesis. The subunit homologies reported here, and those observed previously with the largest RNA polymerase subunit, indicate that components of the prokaryotic RNA polymerase "core" enzyme have counterparts in eukaryotic RNA polymerases.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison L. A., Moyle M., Shales M., Ingles C. J. Extensive homology among the largest subunits of eukaryotic and prokaryotic RNA polymerases. Cell. 1985 Sep;42(2):599–610. doi: 10.1016/0092-8674(85)90117-5. [DOI] [PubMed] [Google Scholar]
- Berg J. M. Potential metal-binding domains in nucleic acid binding proteins. Science. 1986 Apr 25;232(4749):485–487. doi: 10.1126/science.2421409. [DOI] [PubMed] [Google Scholar]
- Biggs J., Searles L. L., Greenleaf A. L. Structure of the eukaryotic transcription apparatus: features of the gene for the largest subunit of Drosophila RNA polymerase II. Cell. 1985 Sep;42(2):611–621. doi: 10.1016/0092-8674(85)90118-7. [DOI] [PubMed] [Google Scholar]
- Chambon P. Eukaryotic nuclear RNA polymerases. Annu Rev Biochem. 1975;44:613–638. doi: 10.1146/annurev.bi.44.070175.003145. [DOI] [PubMed] [Google Scholar]
- Chatterji D., Wu C. W., Wu F. Y. Nuclear magnetic resonance studies on the role of intrinsic metals in Escherichia coli RNA polymerase. Effect of DNA template on the nucleotide-enzyme interaction. J Biol Chem. 1984 Jan 10;259(1):284–289. [PubMed] [Google Scholar]
- Cho J. M., Kimball A. P. Probes of eukaryotic DNA-dependent RNA polymerase II-I. Binding of 9-beta-D-arabinofuranosyl-6-mercaptopurine to the elongation subsite. Biochem Pharmacol. 1982 Aug 15;31(16):2575–2581. doi: 10.1016/0006-2952(82)90703-1. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass R. E., Jones S. T., Nene V., Nomura T., Fujita N., Ishihama A. Genetic studies on the beta subunit of Escherichia coli RNA polymerase. VIII. Localisation of a region involved in promoter selectivity. Mol Gen Genet. 1986 Jun;203(3):487–491. doi: 10.1007/BF00422074. [DOI] [PubMed] [Google Scholar]
- Hanna M. M., Meares C. F. Topography of transcription: path of the leading end of nascent RNA through the Escherichia coli transcription complex. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4238–4242. doi: 10.1073/pnas.80.14.4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura T., Ishihama A., Kajitani M., Takahashi T., Nakada N., Yoshinaga K. Promoter selectivity of Escherichia coli RNA polymerase. II: Altered promoter selection by mutant holoenzymes. Mol Gen Genet. 1984;193(1):8–16. doi: 10.1007/BF00327407. [DOI] [PubMed] [Google Scholar]
- Panka D., Dennis D. RNA polymerase. Direct evidence for two active sites involved in transcription. J Biol Chem. 1985 Feb 10;260(3):1427–1431. [PubMed] [Google Scholar]
- Riva M., Memet S., Micouin J. Y., Huet J., Treich I., Dassa J., Young R., Buhler J. M., Sentenac A., Fromageot P. Isolation of structural genes for yeast RNA polymerases by immunological screening. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1554–1558. doi: 10.1073/pnas.83.6.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shortle D., Haber J. E., Botstein D. Lethal disruption of the yeast actin gene by integrative DNA transformation. Science. 1982 Jul 23;217(4557):371–373. doi: 10.1126/science.7046050. [DOI] [PubMed] [Google Scholar]
- Simpson R. B. The molecular topography of RNA polymerase-promoter interaction. Cell. 1979 Oct;18(2):277–285. doi: 10.1016/0092-8674(79)90047-3. [DOI] [PubMed] [Google Scholar]
- Young R. A., Bloom B. R., Grosskinsky C. M., Ivanyi J., Thomas D., Davis R. W. Dissection of Mycobacterium tuberculosis antigens using recombinant DNA. Proc Natl Acad Sci U S A. 1985 May;82(9):2583–2587. doi: 10.1073/pnas.82.9.2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Yeast RNA polymerase II genes: isolation with antibody probes. Science. 1983 Nov 18;222(4625):778–782. doi: 10.1126/science.6356359. [DOI] [PubMed] [Google Scholar]
- Yura T., Ishihama A. Genetics of bacterial RNA polymerases. Annu Rev Genet. 1979;13:59–97. doi: 10.1146/annurev.ge.13.120179.000423. [DOI] [PubMed] [Google Scholar]
- la Cour T. F., Nyborg J., Thirup S., Clark B. F. Structural details of the binding of guanosine diphosphate to elongation factor Tu from E. coli as studied by X-ray crystallography. EMBO J. 1985 Sep;4(9):2385–2388. doi: 10.1002/j.1460-2075.1985.tb03943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]



