Abstract
Background and Aims
Calotropis procera and Calotropis gigantea, originally from warm parts of Africa and Asia, are now pan-tropical and in ecological terms considered an indicator of overgrazed, disturbed lands; they grow successfully in dry areas. Variations in water relations, morphology and photosynthesis of the two species growing in the same habitat were studied to assess possible mechanisms of tolerance to drought and how these relate to their ecophysiological success. Also the hypothesis that their photosynthetic rate (A) under drought would be affected by stomatal and non-stomatal limitations was tested.
Methods
Water relations, gas exchange, water use efficiency (WUE), fluorescence parameters, pubescence and specific leaf area (SLA) of Calotropis procera and C. gigantea plants growing in the field were evaluated during the wet (WS) and dry (DS) seasons.
Results
The xylem water potential (ψ) was similar in both species during the WS and DS; drought caused a 28 % decrease of ψ. In C. procera, A, stomatal conductance (gs) and carboxylation efficiency (CE) were higher in the WS with half the values of those during the DS, this species being more affected by drought than C. gigantea. A high δ13C of C. gigantea (–26·2 ‰) in the WS indicated a higher integrated WUE, in agreement with its lower gs. Leaves of C. gigantea were more pubescent than C. procera. Relative stomatal and non-stomatal limitation of A increased with drought in both species; no changes in maximum quantum yield of photosystem II (PSII; Fv/Fm) were observed. The decrease in the relative quantum yield of PSII (φPSII) and in the photochemical quenching coefficient (qP) was more pronounced in C. procera than in C. gigantea.
Conclusions
The photosynthetic capacity of C. procera was higher than that of C. gigantea. During the DS, A was regulated by stomatal and non-stomatal factors in a coordinated manner and drought did not cause chronic photoinhibition. A higher density of trichomes and leaf angle in C. gigantea may contribute to the maintenance of A and confer more efficient protection of photochemical activity in the DS. Ecophysiological traits such as high photosynthetic rate throughout the year even during the DS, and high WUE, highly pubescent leaves and low SLA observed in both species contribute to the establishment and growth of Calotropis in dry conditions.
Keywords: Photosynthesis, Calotropis procera, Calotropis gigantea, fluorescence, non-stomatal limitation, stomatal limitation, drought
INTRODUCTION
Global climatic change, with increased ambient CO2 concentration and temperature of the planet, is predicted to alter precipitation patterns significantly, causing an increase in aridity in the semi-arid areas of the world (Lawlor, 2001; IPCC, 2007). High temperatures and dry conditions may be important factors in changing the success and regeneration of plant species (Wei et al., 2009). Understanding how plants respond to drought can play a major role in the protection of natural vegetation in these areas. In most terrestrial ecosystems, water availability is the main environmental factor limiting photosynthesis, growth and productivity (Schulze et al., 1987; Wullschleger et al., 2002) even in plants well adapted to arid conditions, and influences the distribution and the abundance of many species of plants (Schulze et al., 1987).
Seasonal loss of leaves, smaller photosynthetic leaf area and increased importance of photosynthetic stems, together with high trichome density, succulence, development of a deep root system, low osmotic potential and high water use efficiency (WUE), are, among others, the main adaptations of genera and of species within genera to dry zones (Solbrig and Orians, 1977). For example, within a genus, Eucalyptus cloeziana maintains its water status under drought due to its deeper root system and a greater capacity to extract water from soil than Eucalyptus argophloia (Ngugi et al., 2003). Also, photoprotective mechanisms involve physiological leaf adjustments in biochemistry, photochemistry, leaf morphology and anatomy (Havaux and Niyogi, 1999). Leaf pubescence has been reported to be an adaptation to arid environments by reducing the radiant energy absorbed by leaves, affecting their energy balance (Ehleringer, 1983), reducing transpiration whilst maintaining a favourable leaf temperature and so helping to keep a favourable water balance (Savé et al., 2000; Galmés et al., 2007). Variations between species within a genus have been shown in photosynthetic parameters of Mosla and Salix (Liu et al., 2003; Ge et al., 2004); transpiration rate (E) in shrubs of Caragana (Ma et al., 2004) and morphology and photosynthesis in two varieties of Digitalis minor growing in the Balearic Islands (Galmés et al., 2007).
To analyse differences between genera or species which may be responsible for their ecological behaviour in relation to drought it is necessary to consider a number of potential mechanisms. Drought causes reductions in stomatal conductance (gs) which may limit the photosynthetic rate (A) due to a direct effect on CO2 availability to chloroplasts by limiting diffusion through stomata (Cornic, 2000) and/or reduction of the mesophyll conductance, gm (Flexas et al., 2008; Hassiotou et al., 2009), or diminishing metabolic processes (Tezara et al., 1999, 2008; Lawlor, 2002; Nunes et al., 2008; Lawlor and Tezara, 2009) and/or photosystem II (PSII) activity and electron transport (Tezara et al., 2003). To identify differences between species, an increase in the relative stomatal limitation (Ls) may be calculated from the response curve of A to the intercellular CO2 concentration (Ci) to determine whether A is reduced only because of decreased gs or if there as an increase in non-stomatal limitation (Lm; Farquhar and Sharkey, 1982). Jacob and Lawlor (1991) defined Lm as the proportional reduction in Ci-saturated A (ACO2-sat) of plants subjected to stress relative to unstressed plants. Changes in Lm due to water deficit may reflect changes in photosynthetic processes such as Rubisco activity, ribulose bisphosphate (RuBP) production, ATP supply, electron transport rate (J) and light capture efficiency (Lawlor and Cornic, 2002; Tezara et al., 2005; Lawlor and Tezara, 2009). Therefore, Lm is also affected by parameters such as chlorophyll a fluorescence and carboxylation efficiency (CE), among others. Measurement of some of these plant characteristics using such techniques may be used to identify the physiological and other factors responsible for the ecological differences between species.
They have been applied to analyse the response of two species of Calotropis (Apocynaceae), known as milkweed, to seasonally dry conditions. Calotropis is a wasteland weed of world-wide distribution but most abundant in the sub-tropics and tropics, and rare in cold countries (Singh et al., 1996). Calotropis procera (Aiton) WT Aiton is native to Asia, Arabia and tropical Africa, and C. gigantea (L.) WT Aiton is native to southeast Asia (Malaysia, the Philippines, Sri Lanka, Thailand, India and China), and both were introduced into South America and the islands of the Caribbean (Lebrun, 1998). Calotropis procera grows up to an altitude of 1200 m (Sayed and Mohamed, 2000), while C. gigantea reaches 900 m (Usmani and Kushwaha, 2010). The genus Calotropis consists of common weeds which occur in arid ecosystems but have become naturalized in warm climates, where they grow commonly in disturbed areas. In Venezuela, C. procera shows a wider geographical distribution than C. gigantea. Both species are commonly found growing in hot and dry zones, generally next to the sea or in open and sunny places (Steyermark, 1994). Both are successful invaders, particularly in old fields. The two species of Calotropis studied here are perennial evergreen shrubs or small trees reaching 3–5 m tall, with large silver-green leaves, opposite, sub-sessile, clusters of waxy purple-tipped flowers, and inflated pale green seed pods (Kleinschmidt and Johnson, 1977; Nicholson, 1991). These species differ in characteristics, particularly in leaf pubescence which may be important, as mentioned above: C. procera is less pubescent (Colombo et al., 2007).
Most research regarding Calotropis has been concentrated on its medicinal properties (Lewis and Elvin-Lewis, 1977; Oudhia and Dixit, 1994; Oudhia, 1999a, b) as it is an important source of pharmaceutical compounds (Longanga et al., 2000; Ahmed et al., 2005; Usmani and Kushwaha, 2010). Also, Calotropis yields a durable fibre useful for ropes, carpets, fishing nets and sewing thread (Pérez-Arbeláez, 1978; Tuntawiroon and Samootsakorn, 1984). The effect of water deficit on the growth of C. procera in greenhouse-grown and cultivated plants has been evaluated (Boutraa, 2010), but there are few ecophysiological data on natural populations of species of this genus (Colombo et al., 2007; Boutraa, 2010). Both species show high A throughout the year even during the dry season (DS), suggesting the occurrence of particular strategies of drought resistance (Khan and Beena, 2002; Colombo et al., 2007; Khan et al., 2007; Boutraa, 2010).
In order to gain insight into the possible mechanisms of tolerance to drought and assess differences in physiological responses to environmental variables of these two species of Calotropis, the seasonal changes in gas exchange, water relations and chlorophyll a fluorescence parameters of plants growing in their natural habitat were measured. The climate in the study area is typical of a tropical semi-arid ecosystem, characterized by high radiation, high temperatures, annual rainfall of ≤800 mm, with evaporation exceeding precipitation and so limiting water availability for vegetation growth during seasonal drought (Wilson, 1989). The following four hypotheses were tested: (1) that differences in ecophysiological traits related to maintenance of water balance and photosynthetic capacity contribute to the greater success of C. procera than C. gigantea in seasonally dry areas; (2) that the higher density of trichomes in C. gigantea provides a photoprotective mechanism that improves its photosynthetic performance compared with C. procera; (3) that C. procera is more widely adapted to short-term changes in temperature than C. gigantea; and (4) that limitation of photosynthesis to drought in xerophytic, successfully adapted species is caused by stomatal closure and not by metabolic limitation.
MATERIALS AND METHODS
Plant material and field site climate
The study was carried out in a thorn scrub on the coast of Vargas State in Venezuela at 10°34′ N, 67°09′ W and 38 m. The area has been defined as extreme, semi-desert, dry coastal xerophytic scrub vegetation (Huber and Alarcon, 1988). The species studied, Calotropis procera and C. gigantea, are important evergreen components of these ecosystem, although C. procera is more abundant than C. gigantea (a proportion of approx. 3:1 C. procera:C. gigantea plants in the 1000 m2 study area). Ten plants of each of the two Calotropis species were sampled from May 2004 to April 2005, three times during the DS and three during the wet season (WS). The years of the study were characterized by a precipitation pattern with a DS from December to April with 70 mm rainfall and a WS (May–November) with 617 mm. The mean monthly air temperatures (Ta) ranged from 26 to 34 °C (Colombo et al., 2007).
Microclimatic parameters
The photosynthetic photon flux (PPF) was measured with a quantum sensor and meter (LI-185; LI-COR Inc., Lincoln, NE, USA). Air (Ta) and leaf temperature (TL) were measured with YSI 405 and 409 B thermistors, respectively, connected to a telethermometer (Yellow Springs, OH, USA), and relative humidity was measured with a hair strand hygrometer (Abbeon model AB167B, Santa Barbara, CA, USA).
Physiological measurements
All physiological measurements described below were carried out on the fourth fully expanded intact leaf.
Water relations
Xylem water potential (ψ) was measured on leaf discs taken at 0630 h (n = 4) with a Wescor 5000 osmometer (Wescor, Inc., Logan, UT, USA). Leaf water content (LWC) was determined in leaf discs from ten leaves taken at 0700 h as LWC = [fresh weight (f. wt) – leaf dry weight (d. wt)]/leaf d. wt.
Soil samples (n = 4) were collected near the plants studied at a depth of 30 cm. The samples were placed in metal containers, weighed, dried at 100 °C for 72 h, re-weighed and the soil water content (SWC) calculated as: (soil f. wt – soil d. wt)/soil d. wt. Also, soil texture was determined by Bouyoucos analysis as described by Anderson and Ingram (1993).
Specific leaf area and pubescence
Specific leaf area (SLA = disc area/disc mass) was measured on discs (n = 10) from the same leaves used for the gas exchange determinations, dried to constant weight at 70 °C.
Leaf trichomes of each species were carefully removed with a brush, weighed and the pubescence was expressed as a proportion of the total leaf weight (n = 15). The PPF absorbed by the leaf was measured in leaves (n = 20) of each species with the 1800-12 integrating sphere (LI-COR). Also, leaf angle relative to the horizontal was determined using a protractor.
Leaf gas exchange
Gas exchange in attached leaves was measured with a portable infrared gas analyser [IRGA; CIRAS 2 with a PLC (B) assimilation chamber; PP Systems plc, Hitchin, UK]. Measurements were done at a CO2 concentration (Ca) of 370 µmol mol−1, unless otherwise stated. Instantaneous A was measured at 1000 h when the daily maximum A occurred, at a PPF = 1200 µmol m−2 s−1 and a TL of 32 ± 0·5 °C. The integrated daily net photosynthetic rate (AD) was calculated as the integration of the area below the curves of six daily courses of A. The A/Ci curves were generated during the WS (n = 4) and DS (n = 6) by reducing Ci as calculated by the program in the IRGA after Farquhar et al. (1980) from approx. 220 µmol mol−1, when A at Ca = 370 µmol mol−1 was initially measured, to zero and then progressively increasing Ci to 1400 µmol mol−1. CO2 was provided by a cylinder filled with pure gas inserted into the IRGA. The A/Ci curves were fitted to the empirical equation A = b + deKCi, where b = ACO2-sat and (b + d) = y-intercept (Tezara et al., 1998). The CE was calculated from the initial slope of the curve. The Ls was calculated as Ls = 100 × (Ao – A)/Ao, where Ao is A at Ci = Ca (Farquhar and Sharkey, 1982). The Lm was calculated as Lm = 100 × (AC – AD)/AC, where AC is the A of WS leaves at Ci = 700 µmol mol−1 and AD is the rate of droughted leaves (DS) at the same Ci (Jacob and Lawlor, 1991). Response curves of A vs. PPF and A vs. TL were generated between 0900 and 1100 h at ambient Ca, using a leaf microclimate control system connected to an LCA-4 IRGA (Analytical Development Co., Hoddesdon, UK). The PPF was varied using neutral filters (Balzers, Handelsbank, Zürich, Switzerland); the light source was either the sun or a 50 W dichroic lamp (Phillips, Caracas, Venezuela). No gas exchange measurements were done on leaves without trichomes due to a strong wilting (loss of turgor) and stomatal closure half an hour after pubescence was removed.
Carbon isotope ratio (δ13C)
Leaf samples were ground and then analysed for δ13C (n = 4) with an isotope ratio mass spectrometer at the Ecology and Evolution Department of Biological Sciences (University of Illinois at Chicago).
Chlorophyll a fluorescence
Chlorophyll fluorescence was measured on attached dark-adapted leaves (n = 6) with a Mini PAM fluorometer (Walz, Effeltrich, Germany) using the protocol described by Genty et al. (1989). The Fv/Fm was measured in situ at the minimum dawn PPF. The ϕPSII at steady state A was calculated as ϕPSII = (F'm – Fs)/F'm, where Fs and F'm are fluorescence at steady-state A and maximum fluorescence in the light, respectively. The photochemical quenching coefficient qP and non-photochemical quenching (NPQ) were calculated from measurements of fluorescence. The electron transport rate of PSII was estimated by the method of Krall and Edwards (1992) as J = ϕPSII × PPF × a × 0·5, where a is the fraction of incident PPF absorbed by the leaf.
Statistical analysis
Results are presented as means ± s.e. Significance at P < 0·05 was assessed by one- and two-way analysis of variance (ANOVA) using the statistical package Statistica. Curves were fitted using the Sigmaplot package.
RESULTS
Microclimatic conditions
No differences were observed in microclimatic parameters between seasons. The PPF between 0700 and 1600 h ranged from 100 to 1500 µmol m−2 s−1, Ta from 25 ± 0·5 to 32·0 ± 0·2 °C and relative humidity from 85 ± 2 to 65 ± 1 %. The daily course of TL was the same for both species and did not change between seasons; from 1000 to 1500 h TL was maintained at 31 ± 0·5 °C; leaf–air water vapour deficit pressure (Δw) ranged between 0·5 and 1·6 kPa for both species.
Water status and gas exchange
The soil at the site was sandy, consisting of 71 % sand, 14 % clay and 16 % lime. Table 1 shows the seasonal changes in SWC and several leaf variables. In the DS, SWC decreased 83 % where C. procera grows, in contrast to a 69 % reduction where C. gigantea grows. Both species showed a similar LWC in the two seasons and, after several months of drought (from August 2004 to April 2005), LWC was significantly reduced by 14 and 9 % in C. procera and C. gigantea, respectively. The SLA was significantly lower in C. procera than in C. gigantea, decreasing 69 % with drought in C. gigantea and remaining unaffected in C. procera. Pubescence was unaffected by season in both species; in C. gigantea, trichome mass was approx. 2·5 times higher than in C. procera. The fraction of PPF absorbed by leaves of C. procera was significantly higher than by those of C. gigantea, while the leaf angle was smaller in the former.
Table 1.
Seasonal changes in soil water content (SWC; n = 24), leaf water content (LWC; n = 10), specific leaf area (SLA; n = 10), pubescence (weight of trichomes; n = 15), fraction of incident PPF absorbed by the leaf (a; n = 20) and leaf angle (n = 50) of plants of Calotropis procera and C. gigantea growing in the field
|
C. procera |
C. gigantea |
|||
|---|---|---|---|---|
| Parameter | WS | DS | WS | DS |
| SWC (% dry matter) | 8·3 (0·4)c | 1·4 (0·2)a | 4·3 (0·1)b | 1·3 (0·2)a |
| LWC (% dry matter) | 86 (4)b | 74 (3)a | 86 (3)b | 78 (3)a |
| SLA (cm2 g−1) | 127 (5)a | 126 (6)a | 203 (9)c | 167 (2)b |
| mg trichomes g−1 | 1·3 (0·1)a | 1·8 (0·4)a | 3·3 (0·2)b | 4·4 (0·5)b |
| a | 0·82 (0·01)b | 0·79 (0·01)b | 0·75 (0·00)a | 0·74 (0·00)a |
| Leaf angle (°) | 20 (1)a | 23 (0·7)a | 48 (1)b | 51 (1·1)b |
Results are presented as means (s.e.). Different letters indicate significant differences between seasons and species for each parameter at P < 0·05.
The δ13C was higher in C. gigantea (–26·2 ± 0·4 ‰) than in C. procera (–27·4 ± 0·06 ‰) during the WS.
No seasonal differences in ψ between the species were observed; a decrease of –0·4 MPa on average occurred with drought (Fig. 1). Differences in gas exchange were observed between species and seasons (Fig. 1). Leaves of C. procera showed values of A and gs approx. 28 and 66 % higher than C. gigantea during the WS, this difference disappearing during the DS. During the DS, A and gs decreased 48 and 49 %, respectively in C. procera, while in C. gigantea A was reduced by 34 % and gs was unchanged. In C. gigantea, E was significantly lower than in C. procera and during the DS a reduction of 48 % was observed in both species (Fig. 1). The WUE was higher in C. gigantea and remained unaffected in both species during the DS.
Fig. 1.
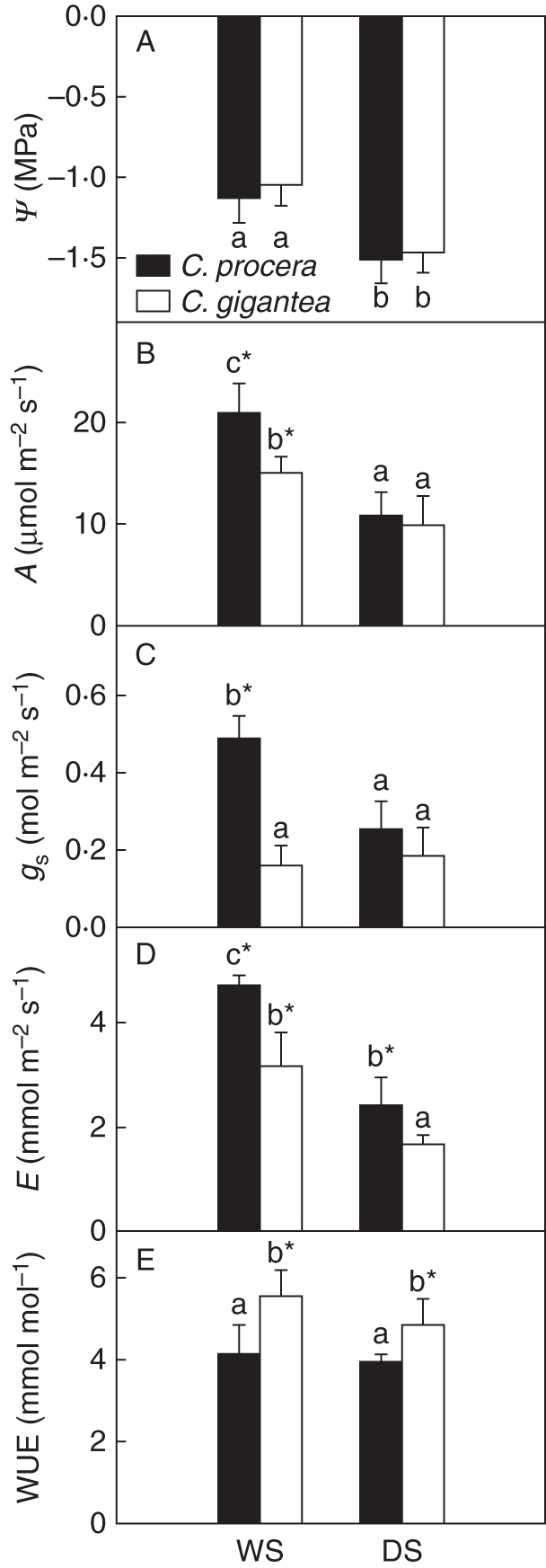
Seasonal changes in (A) xylem water potential, (B) photosynthetic rate, (C) stomatal conductance, (D) transpiration rate and (E) water use efficiency of leaves of Calotropis procera and Calotropis gigantea, as indicated, during the wet (WS) and dry seasons (DS). Values are means ± s.e. (n = 36) of measurements made three times during the DS and three times during the WS. Gas exchange was measured between 1000 and 1100 h under a PPF of 1200 ±70 µmol m−2 s−1, 32 ± 0·5 °C leaf temperature, and ambient CO2 concentration of 370 µmol mol−1. Different letters indicate significant differences between seasons, and asterisks indicate significant differences between species for each parameter at P < 0·05.
The Ci/Ca ratio decreased little in C. procera, while no change was observed in C. gigantea (Table 2). The integrated daily net photosynthetic rate (AD) followed the same trend observed in A, i.e. higher in C. procera during the WS than in C. gigantea; AD decreased by 39 and 31 % in C. procera and C. gigantea, respectively, during the DS (Table 2).
Table 2.
Seasonal changes in intercellular CO2/ambient CO2 concentration ratio (Ci/Ca ratio; n = 36), integrated daily net photosynthetic rate (AD; n =18). CO2-saturated photosynthetic rate (ACO2-sat), carboxylation efficiency (CE), CO2 compensation concentration (Γ), relative stomatal and mesophyll limitations (Ls and Lm; n = 4 for the WS and n = 6 for the DS for A/Ci parameters), in leaves of Calotropis procera and C. gigantea growing in the field
|
C. procera |
C. gigantea |
|||
|---|---|---|---|---|
| Parameter | WS | DS | WS | DS |
| Ci/Ca ratio | 0·63 (0·03)a | 0·51 (0·02)b | 0·61 (0·003)a | 0·58 (0·02)a |
| AD (mmol m−2 d−1) | 271c | 167a | 234b | 162a |
| ACO2-sat (μmol m−2 s−1) | 38·9 (1·9)b | 26·7 (3·9)a | 53·7 (5·6)c | 24 (5·7)a |
| CE (mol m−2 s−1) | 0·29 (0·05)b | 0·16 (0·02)a | 0·18 (0·01)a | 0·16 (0·02)a |
| Γ (μmol mol−1) | 62·7 (12)a | 117·6 (7)b | 51·3 (8)a | 125·3 (7)b |
| Ls (%) | 31·6 (5)a | 42·1 (3)bc | 35·1 (1)ab | 46·7 (2)c |
| Lm (%) | 0 | 45 (8)a | 0 | 58 (9)a |
Results are presented as means (s.e.). Different letters indicate significant differences between seasons and species for each parameter at P < 0·05.
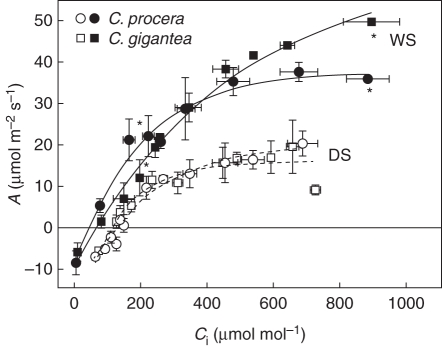
Through the analysis of A/Ci curves it was found that both ACO2-sat and CE declined with drought in C. procera, whereas CE was unaffected in C. gigantea (Fig. 2; Table 2). During drought, the CO2 compensation point (Γ) increased approx. 2 and 2·4 times in C. procera and C. gigantea, respectively. The Ls increased 33 % in both species, whereas Lm increased with drought by 45 and 58 %, in C. procera and C. gigantea, respectively.
Fig. 2.
Response of photosynthetic rate to intercellular CO2 concentration in leaves of Calotropis procera and Calotropis gigantea, as indicated, growing in the field during the wet (WS, filled symbols, n = 4) and dry season (DS, open symbols, n = 6). Values are means ± s.e.; standard errors are shown when larger than the symbols. Asterisks indicate significant differences at P < 0·05 of ACO2-sat and carboxylation efficiency between species in the WS.
During the WS, A (APPF-sat) was saturated at a PPF of 1500 µmol m−2 s−1 in both species and was higher in C. procera (Table 3). Significant differences were observed between the species in ϕCO2 and the light compensation point (LCP), while no differences were observed in the dark respiration rate (RD).
Table 3.
Photosynthetic photon flux-saturated photosynthetic rate (APPF-sat), apparent quantum yield of CO2 fixation (ϕCO2), light compensation point (LCP) and dark respiration (RD) in leaves of plants of Calotropis procera and C. gigantea growing in the field during the WS
| Parameter | C. procera | C. gigantea |
|---|---|---|
| APPF-sat (μmol m−2 s−1) | 18·4 (2)b | 10·8 (0·5)a |
| ϕCO2 (μmol μmol photon−1) | 0·017 (0·000)b | 0·014 (0·002)a |
| LCP (μmol m−2 s−1) | 140 (28)a | 216 (20)b |
| RD (μmol m−2 s−1) | 2·2 (0·3)a | 2·6 (0·3)a |
Results are presented as means (s.e.) (n = 4). Different letters represent significant differences between species for each parameters at P < 0·05.
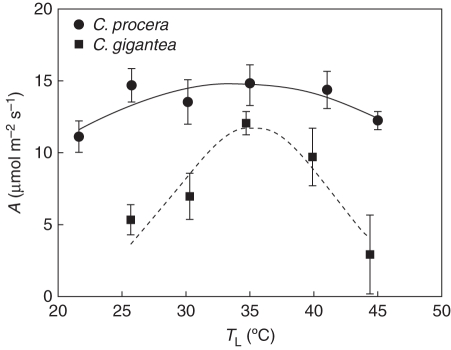
The optimum temperature for A, when 90 % of maximum A occurs, showed a wider range (25–40 °C) in C. procera than in C. gigantea (33–38 °C). A higher value of A, measured at 35 °C TL, was observed in C. procera than in C. gigantea (Fig. 3).
Fig. 3.
Response of photosynthetic rate to leaf temperature of Calotropis procera and Calotropis gigantea, as indicated, growing in the field during the wet season, WS. Values are means ± s.e. (n = 4); standard errors are shown when larger than the symbols. Measurements were done under a PPF of 1200 ± 70 µmol m−2 s−1 and ambient CO2 concentration of 370 µmol mol−1.
Chlorophyll a fluorescence
Seasonal changes in fluorescence parameters were observed (Fig. 4). The Fv/Fm for both species was 0·84 and was not affected by drought. During the DS, decreases in φPSII of 42 and 32 % at high PPF were observed in C. procera and C. gigantea, respectively; consequently, J was reduced by 32 and 28 % in C. procera and C. gigantea, respectively. The qP followed the same trend as ϕPSII, decreasing 30 % with drought, while NPQ increased 60 % in C. procera and approx. 100 % in C. gigantea in the DS, although NPQ was lower in C. gigantea. The J was higher in C. procera due to higher ϕPSII and qP.
Fig. 4.
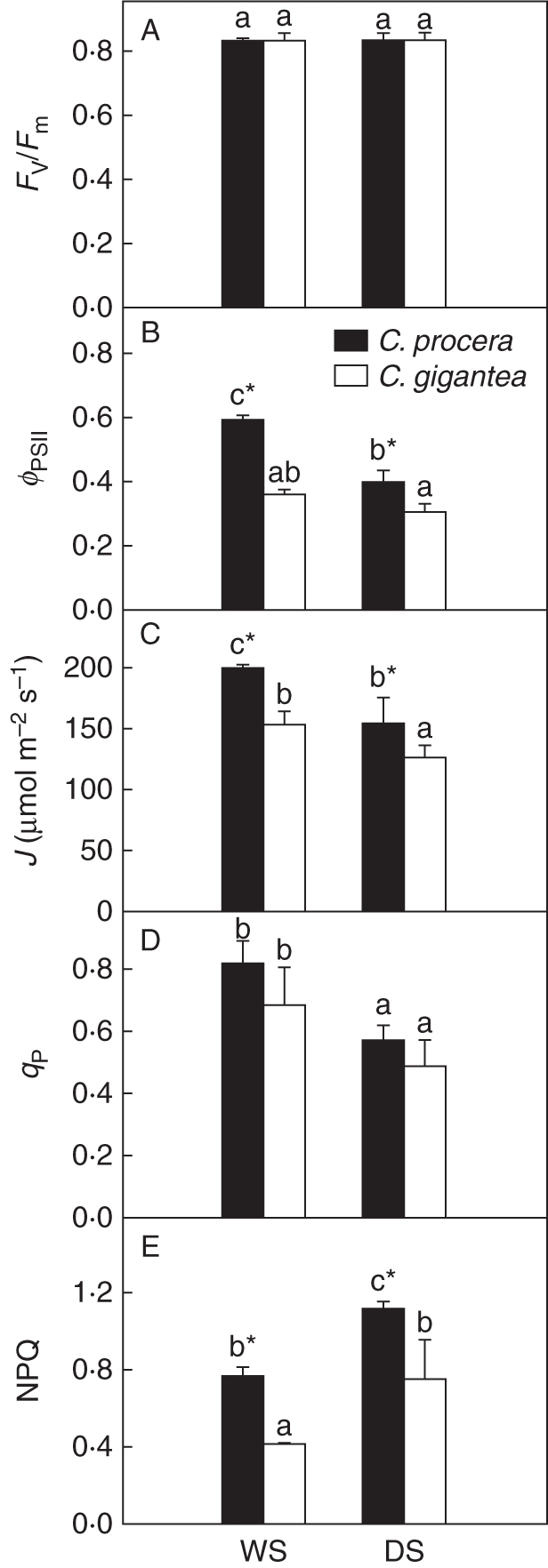
Seasonal changes in (A) maximum quantum yield, (B) relative quantum yield of PSII, (C) total electron transport rate, (D) coefficient of photochemical quenching and (E) coefficient of non-photochemical quenching of fluorescence in plants of Calotropis procera and Calotropis gigantea, as indicated. Values are means ± s.e. (n = 36). Fluorescence parameters were measured three times during the dry season (DS) and three times during the wet season (WS) at 1000 h under a PPF of 1170 ± 40 µmol m−2 s−1. Asterisks indicate significant differences at P < 0·05 of Amax and carboxylation efficiency between species in the WS.
DISCUSSION
Calotropis species are drought (Colombo et al., 2007) and salt tolerant (Khan et al., 2007); however, the mechanism behind their successful distribution across arid regions is not well understood. Analysis of the responses of two closely related species, C. procera and C. gigantea, which have invaded seasonally dry desert areas of Venezuela, in a continent from which they did not originate, was aimed at increasing understanding of their mechanisms of adaptation to dry conditions and how these, and differences between the two species, may affect their ecology. Most studies on Calotropis focus on the pharmacological and medical aspects, and studies on the ecophysiology of Calotropis are needed to improve the basis for management of this plant species. In addition, analysis of the changes to leaf physiology caused by water deficit and effects of temperature enhances understanding of the way in which drought under natural conditions affects plants of semi-desert areas. Basic physiological responses of desert plants are not well understood, and such information may be compared with responses of crop plants which have been somewhat better studied (Boutraa, 2010). Also the potential pharmacological value of Calotropis species, and the need for management to decrease their impact on invaded ecosystems, warrant these and further studies.
Responses of C. procera and C. gigantea during the wet season
Values of ψ of C. procera and C. gigantea during the WS were the same, lower than in other xerophytic species (Tezara et al., 2005) but similar to those reported for some evergreen trees and drought-avoiding shrubs (Tezara et al., 1998). In contrast, very low values of ψ (−5 MPa) have been reported for shrubs from a dry-deciduous ecosystem (Smith and Nobel, 1986) and herbs in a tropical thorn scrub during the DS (Tezara et al., 1998). There were differences between the photosynthetic performances of the two species: A, gs and AD were greater (larger photosynthetic capacity) in C. procera than in C. gigantea, but these interspecific photosynthetic differences disappeared in the DS. Also, in C. procera, both ϕCO2 and the PPF-saturated photosynthetic rate (APPF-sat) were higher than in C. gigantea, which suggests that the former has either a larger light-harvesting capacity or a greater electron transport rate. Also, the CE was larger in C. procera than in C. gigantea, indicating that it has a higher activity and/or amount of Rubisco. Differences in light absorption (a) due to the variation in leaf pubescence may explain photochemical differences between the two species: leaves of C. gigantea with a greater trichome density and a higher angle are less prone to overheating and have a smaller NPQ. Changes in NPQ associated with differences in pubescence have been reported in species of Caragana (Ma et al., 2004), Encelia (Ehleringer and Björkman, 1978) and varieties of D. minor (Galmés et al., 2007).
The larger δ13C of C. gigantea than C. procera in the WS indicated a better WUE (long term) in this species, related to its lower gs. The δ13C for both species in this study agree with data of C. procera (–27·9 ‰) in southern Sinai, supporting the conclusion that the photosynthetic metabolism is C3 (Sayed and Mohamed, 2000).
In the WS, C. procera showed a broad temperature optimum (Topt; short-term responses) for A and higher values compared with C. gigantea, allowing a greater carbon gain over a wider range of environmental temperatures. The leaf temperature for both species was around 31 ± 0·5 °C from 1000 to 1500 h, where A was 98 % of maximum for C. procera; in contrast C. gigantea showed 75 % of maximum A during the same time interval, contributing to the lower AD of C. gigantea (14 % lower than C. procera). Differences between species within a genus with respect to the Topt for A have been reported for the genus Caragana (Ma et al., 2004). In contrast, species from the same genus (Encelia farinosa and E. californica) showed a similar Topt (Ehleringer and Björkman, 1978).
Responses of C. procera and C. gigantea during the dry season
Plants of C. procera as well as C. gigantea were able to withstand a severe reduction in SWC without a marked decrease in water status, as denoted by maintenance of rather high values of ψ compared with the wet, but drought did cause a slight reduction in ψ and LWC in both species. Maintenance of the water status in both species is probably due to their deep root systems, enabling water absorption from deep soil. Sharma (1968) showed that C. procera has a very deep, stout taproot reaching depths of 1·7–3·0 m with few or no near-surface lateral roots in Indian sandy desert soils. We measured smaller SLA in C. procera than C. gigantea, but this seems to have little correlation with the responses, despite SLA being used as an index of scleromorphism; low values have been related to a high capacity to resist low water availability (Ogaya and Peñuelas, 2006).
Despite the xeromorphic characteristics and the relatively small decrease in water potential and LWC, water deficit did inhibit gas exchange of both species, but more so in C. procera than in C. gigantea. The photosynthetic capacity [instantaneous photosynthetic rate, APPF-sat, A(at 35 °C), CE, AD and ϕCO2] of C. procera was higher than that of C. gigantea. The reduction in both ACO2-sat and AD may be attributed to stomatal closure as well as a reduction in mesophyll capacity, with similar responses from both species. Some of the ecophysiological traits observed in the two species of Calotropis (e.g. a positive and high photosynthesis rate throughout the year in the field and a high instantaneous WUE even during the DS, very pubescent leaves and a low SLA) may contribute to their successful growth in areas with lack of water. Similar results have been reported for C. procera growing in the Karachi desert (Khan and Beena, 2002). Values of AD throughout the year were greater than observed in other xerophytic C3 species (Tezara et al., 1998) and less affected by drought, suggesting why this plant is successful in dry conditions and thus invasive. The positive effects of pubescence on decreasing the radiation load on the photosynthetic apparatus may contribute to photoprotection and so stress did not cause chronic photoinhibition. Also, it clearly reduces water loss by transpiration so can also help both species to avoid substantial loss of water and photosynthetic activity.
In both species of Calotropis photosynthesis is limited by both stomatal and non-stomatal factors. In this respect, the response is similar to that of crop plants where a relatively rapid decrease in water supply leads to dehydration of leaves with lower RWC and ψ and loss of turgor, which can result in stomatal closure (Lawlor, 2001; Lawlor and Tezara, 2009). Such mechanisms may not apply to plants of dry regions such as C. procera and C. gigantea, adapted over a long period to the DS. However, drought led to partial stomatal closure (gs reduced by 58 %) in C. procera but not in C. gigantea, probably due to its greater pubescence and leaf angles, similar to the response of other plants of such habitats, e.g. E. farinosa (Ehleringer and Björkman, 1978) which decrease water loss by transpiration.
Water deficit markedly reduced ACO2-sat, and CE in C. procera, while CE was unaffected in C. gigantea. The lower values of CE and ACO2-sat suggest loss of Rubisco amount and/or activity with decreasing ψ. The amount and activity of Rubisco and the availability of RuBP affect CE, and thus A (Lawlor and Tezara, 2009). Similar results were found for Medicago truncatula (Nunes et al., 2008), Ipomoea carnea and Jatropha gossypifolia (Tezara et al., 2005), and Lycium nodosum (Tezara et al., 2003). In contrast, CE was unaffected by drought in E. californica and E. farinosa (Ehleringer and Björkman, 1978). The changes in ACO2-sat support the earlier conclusion that factors associated with decreased ψ which progressively reduced A increase non-stomatal limitations of A (Lawlor, 2002; Lawlor and Cornic, 2002; Lawlor and Tezara, 2009).
The increase in Ls and Lm with declining ψ shows that stomatal (Farquhar and Sharkey, 1982) and mesophyll (Lawlor, 2002; Nunes et al., 2008; Lawlor and Tezara, 2009) factors limit A under drought conditions. Also, the nearly constant value of Ci with an average Ci/Ca = 0·58 in both species and seasons suggests that there is a balance between the stomata limitation and the mespohyll activities even under the longer term drought (Schulze and Hall, 1982; Lawlor, 2002). Many studies have reported that both stomatal and metabolic components are responsible for decreased A, not only in mesophytic crop plants under relatively rapid drought [e.g. Helianthus annuus (Tezara et al., 1999; Lawlor, 2002)] but also of Amaranthus palmeri (Ehleringer, 1983), E. farinosa (Ehleringer and Cook, 1984), and L. nodosum, I. carnea and J. gossypifolia (Tezara et al., 2003, 2005).
An increase in Lm probably reflects reductions in photochemical activity related to ATP supply which determines RuBP regeneration, thereby decreasing CE. It has also been suggested that increased Lm under stress is caused by decreased activity of some Calvin cycle enzymes, for example Rubisco, which would be seen as a decrease in CE, but evidence for this is weak (Lawlor and Tezara, 2009). Another factor that may contribute to increased Lm is shrinkage of tissue with decreasing RWC (Hassiotou et al., 2009). This could be the case in C. gigantea, in which SLA was reduced by 20 %. A more compact mesophyll would decrease gaseous diffusion pathways, thus preventing ready access of CO2 to the mesophyll cells. However, in mesophytes, increasing the ambient CO2 does not overcome the limitation (which may be partly caused by shrinkage), although the relative magnitude of the mesophyll limitation might be smaller because of shrinkage. Maintenance of relatively constant Ci/Ca could also reflect long-term adaptation to conditions in metabolism and with the stomata.
Water deficit often does not affect Fv/Fm (Lawlor and Cornic, 2002; Tezara et al., 2003; Nunes et al., 2008) and did not do so in this study, so photoinhibition was not observed during the DS, suggesting that PSII activity is very drought resistant in C. procera and C. gigantea. However, Fv/Fm was decreased and photoinhibition occurred in the shrubs I. carnea and J. gossypifolia under natural conditions (Tezara et al., 2005) and in the herb D. minor (glabrous variety) (Galmés et al., 2007). The high leaf trichome density in Calotropis species could be an efficient mechanism of photochemical photoprotection.
The lower ϕPSII of droughted plants of both species of Calotropis was accompanied by a lower qP and J, i.e. with a higher reduction state of the QA pool, compared with plants in the WS: this is related to the increase in Lm. This is similar to the decreased ϕPSII, indicating that J was affected by drought, in other xerophytic species (Tezara et al., 2003, 2005). Drought increased NPQ in both species of Calotropis, suggesting that a greater proportion of the energy was thermally dissipated, thus accounting for the apparent downregulation of PSII and supporting the photoprotective role of NPQ.
We conclude that hypothesis 1 is substantiated, but the responses are complex. During the WS both species of Calotropis had a similar water status, but A, AD and gs were higher in C. procera than in C. gigantea, showing a higher photosynthetic capacity, possibly related to its greater leaf thickness. Also C. procera had a greater and broader short-term temperature optimum (substantiating hypothesis 3). The larger photosynthetic capacity of C. procera shown by these short-term measurements is an explanation of its wider and more frequent distribution than C. gigantea. Both species showed high (instantaneous) WUE compared with other xerophytic species in the north of Venezuela, indicating that they are well adapted to seasonally dry habitats. The presence of highly pubescent leaves together with a deep root system probably helps these two species to tolerate drought, particularly C. gigantea during drought when photosynthesis was less inhibited that that of C. procera (supporting hypothesis 2). In the DS, water potential and RWC decreased in both species, but not as severely as in other xerophytes due, probably, to the deep root system attested to by the literature, hairs (which favour C. gigantea, reducing the effect on stomata, etc.) and the large instantaneous WUE, so water deficit did not cause chronic photoinhibition and some photosynthesis was maintained during the DS. Photosynthesis was co-limited by stomatal and non-stomatal factors (not supporting hypothesis 4). Co-limitation would operate as a mechanism that optimizes water use and resource allocation when carbon acquisition is impaired. Tolerance to high irradiance allows the plants to maintain a positive carbon balance and growth in semi-arid coastal habitats characterized by large temporal and spatial variations in rainfall.
ACKNOWLEDGEMENTS
This work was supported by CDCH grants 03-33-5383·2004 and 03-33-5383·2006 (Universidad Central de Venezuela). We wish to thank P. Taylor, R. Urich and A. Herrera for critically reading the manuscript, and E. Rengifo for her help in the measurements of the A/Ci response curve during the DS.
LITERATURE CITED
- Ahmed KK, Rana AC, Dixit VK. Calotropis species (Ascelpediaceae), a comprehensive review. Pharmacognosy Magazine. 2005;1:48–52. [Google Scholar]
- Anderson JM, Ingram JS. Tropical soil biology and fertility. A handbook of methods. Wallingford, UK: CAB International; 1993. [Google Scholar]
- Boutraa T. Growth performance and biomass partitioning of the desert shrub Calotropis procera under water stress conditions. Research Journal of Agriculture and Biological Sciences. 2010;6:20–26. [Google Scholar]
- Colombo R, Marín O, Irazábal S, Tezara W. Relaciones hídricas, fotosíntesis y anatomía foliar de dos especies del género Calotropis. Interciencia. 2007;32:791–796. [Google Scholar]
- Cornic G. Drought stress inhibits photosynthesis by decreasing stomatal aperture – not by affecting ATP synthesis. Trends in Plant Science. 2000;5:187–188. [Google Scholar]
- Ehleringer J. Ecophysiology of Amaranthus palmeri, a sonoran desert summer annual. Oecologia. 1983;57:107–112. doi: 10.1007/BF00379568. [DOI] [PubMed] [Google Scholar]
- Ehleringer J, Björkman O. A comparison of photosynthetic characteristics of Encelia species possessing glabrous and pubescent leaves. Plant Physiology. 1978;62:185–190. doi: 10.1104/pp.62.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehleringer J, Cook C. Photosynthesis in Encelia farinosa Gray in response to decreasing leaf water potential. Plant Physiology. 1984;75:688–693. doi: 10.1104/pp.75.3.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquhar GD, Sharkey TD. Stomatal conductance and photosynthesis. Annual Review of Plant Physiology. 1982;33:317–345. [Google Scholar]
- Farquhar GD, von Caemmerer S, Berry JA. A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta. 1980;149:78–90. doi: 10.1007/BF00386231. [DOI] [PubMed] [Google Scholar]
- Flexas J, Ribas-Carbó M, Díaz-Espejo A, Galmés J, Medrano H. Mesophyll conductance to CO2: current knowledge and future prospects. Plant, Cell and Environment. 2008;31:602–621. doi: 10.1111/j.1365-3040.2007.01757.x. [DOI] [PubMed] [Google Scholar]
- Galmés J, Medrano H, Flexas J. Photosynthesis and photoinhibition in response to drought in a pubescent (var. minor) and a glabrous (var. palaui) variety of Digitalis minor. Environmental and Experimental Botany. 2007;60:105–111. [Google Scholar]
- Ge Y, Lu J, Liao J, Guan B, Chang J. Photosynthetic parameters of Mosla hangchowensis and M. dianthera as affected by soil moisture. Photosynthetica. 2004;42:387–391. [Google Scholar]
- Genty B, Briantais JM, Baker NR. The relationships between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochimica et Biophysica Acta. 1989;990:87–92. [Google Scholar]
- Hassiotou F, Ludwig M, Renton M, Veneklaas EJ, Evans JR. Influence of leaf dry mass per area, CO2, and irradiance on mesophyll conductance in sclerophylls. Journal of Experimental Botany. 2009;60:2303–2314. doi: 10.1093/jxb/erp021. [DOI] [PubMed] [Google Scholar]
- Havaux M, Niyogi KK. The violaxanthin cycle protects plants from photooxidative damage by more than one mechanism. Proceedings of the National Academic of Sciences, USA. 1999;96:8762–8767. doi: 10.1073/pnas.96.15.8762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber O, Alarcón C. Mapa de Vegetación de Venezuela. Caracas: Oscar Todtmann Editores; 1988. Escala 1:2·000·000. Ministerio del Ambiente y de los Recursos Naturales Renovables y The Nature Conservancy. [Google Scholar]
- IPCC. Fourth assessment report of Working Group I. Climate change 2007: the physical science basis, summary for policymakers. 2007 Intergovernmental Panel on Climate Change. [Google Scholar]
- Jacob J, Lawlor DW. Stomatal and mesophyll limitations of photosynthesis in phosphate deficient sunflower, maize and wheat plants. Journal of Experimental of Botany. 1991;42:1003–1011. [Google Scholar]
- Khan A, Beena N. Seasonal variation in water relations of desert shrubs from Karachi, Pakistan. Pakistan Journal of Botany. 2002;34:329–340. [Google Scholar]
- Khan R, Shahzad S, Choudhary MI, Khan SA, Ahmad A. Biodiversity of the endophytic fungi isolated from Calotropis procera (Ait.) R. Br. Pakistan Journal of Botany. 2007;39:2233–2239. [Google Scholar]
- Kleinschmidt HE, Johnson RW. Weeds of Queensland. SR Hampton: Government Printer; 1977. [Google Scholar]
- Krall JP, Edwards GE. Relationship between photosystem II activity and CO2 fixation in leaves. Physiologia Plantarum. 1992;86:180–187. [Google Scholar]
- Lawlor DW. Photosynthesis. Oxford: BIOS Scientific Publishers; 2001. [Google Scholar]
- Lawlor DW. Limitation to photosynthesis in water stressed leaves: stomata versus metabolism and the role of ATP. Annals of Botany. 2002;89:871–885. doi: 10.1093/aob/mcf110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawlor DW, Cornic G. Photosynthetic carbon assimilation and associated metabolism in relation to water deficits in higher plants. Plant, Cell and Environment. 2002;25:275–294. doi: 10.1046/j.0016-8025.2001.00814.x. [DOI] [PubMed] [Google Scholar]
- Lawlor DW, Tezara W. Causes of decreased photosynthetic rate and metabolic capacity in water-deficient leaf cells: a critical evaluation of mechanisms and integration of processes. Annals of Botany. 2009;103:561–579. doi: 10.1093/aob/mcn244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebrun J-P. Catalogue des plantes vasculaires de la Mauritanie et du Sahara occidental. Vol. 55. Genève: Boissiera; 1998. [Google Scholar]
- Lewis W, Elvin-Lewis M. Medical botany: plants affecting man's health. New York: John Wiley & Sons Publications; 1977. [Google Scholar]
- Liu M, Jiang G, Li Y, et al. Gas exchange, photochemical efficiency, and leaf water potential in three Salix species. Photosynthetica. 2003;41:393–398. [Google Scholar]
- Longanga OA, Vercruysse A, Foriers A. Contribution to the ethnobotanical, phytochemical and pharmacological studies of traditionally used medicinal plant in the treatment of dysentery and diarrhoea in Lomela area, Democratic Republic of Congo (DRC) Journal of Ethnopharmacology. 2000;71:411–423. doi: 10.1016/s0378-8741(00)00167-7. [DOI] [PubMed] [Google Scholar]
- Ma C, Gao Y, Guo H, Wang J. Photosynthesis, transpiration, and water use efficiency of Caragana microphylla, C. intermedia, and C. korshinskii. Photosynthetica. 2004;42:65–70. [Google Scholar]
- Ngugi M, Doley D, Hunt A, Dart P, Ryan P. Leaf water relations of Eucalyptus cloeziana and Eucalyptus argophloia in response to water deficit. Tree Physiology. 2003;23:335–343. doi: 10.1093/treephys/23.5.335. [DOI] [PubMed] [Google Scholar]
- Nicholson DH. Flora of Dominica, part 2: Dicotyledoneae. Smithsonian contributions to botany. Smithsonian Institution Scholarly Press; 1991. [Google Scholar]
- Nunes C, de Sousa SA, da Silva JM, Salema FMP, Bernardes da Silva A. Physiological responses of the legume model Medicago truncatula cv. Jemalong to water deficit. Environmental and Experimental Botany. 2008;63:289–296. [Google Scholar]
- Ogaya R, Peñuelas J. Contrasting foliar responses to drought in Quercus ilex and Pillyrea latifolia. Biología Plantarum. 2006;50:373–382. [Google Scholar]
- Oudhia P, Dixit A. Weeds in Ambikapur region (Madhya Pradesh) and their traditional use. Weeds News. 1994;1(2):19–21. [Google Scholar]
- Oudhia P. Studies on allelopahty and medicinal weeds in chickpea fields. International Chickpea and Pigeonpea Newsletter. 1999a;6:29–33. [Google Scholar]
- Oudhia P. Medicinal weeds in groundnut fields of Chhattisgarh (India) International Arachis Newsletter. 1999b;19:62–64. [Google Scholar]
- Pérez-Arbeláez E. Plantas útiles de Colombia. 4th edn. Bogotá: Litografía-Arco; 1978. [Google Scholar]
- Savé R, Biel C, de Herralde F. Leaf pubescence, water relations and chlorophyll fluorescence in two subspecies of Lotus creticus L. Biologia Plantarum. 2000;43:239–244. [Google Scholar]
- Sayed O, Mohamed M. Altitudinal changes in photosynthetic pathways of floristic elements in southern Sinai, Egypt. Photosynthetica. 2000;38:367–372. [Google Scholar]
- Schulze E, Robichaux R, Grace J, Rundel P, Ehleringer J. Plant water balance. BioScience. 1987;37:32–36. [Google Scholar]
- Schulze E-D, Hall AE. Stomatal responses, water loss and CO2 assimilation rates of plants in contrasting environments. In: Lange OL, Nobel PS, Osmond CB, Ziegler H, editors. Physiological plant ecology II. Water relations and carbon assimilation. Berlin: Springer-Verlag; 1982. pp. 181–230. [Google Scholar]
- Sharma BM. Root systems of some desert plants in Churu, Rajasthan. Indian Forester. 1968;94:240–246. [Google Scholar]
- Singh U, Wadhwani AM, Johri BM. Dictionary of economic plants of India. New Delhi: Indian Council of Agricultural Research; 1996. pp. 38–39. [Google Scholar]
- Smith S, Nobel P. Deserts. In: Baker N, Long S, editors. Photosynthesis in contrasting environments. Topics in photosynthesis. Vol. 7. Amsterdam: Elsevier; 1986. pp. 13–62. [Google Scholar]
- Solbrig OT, Orians GH. The adaptative characteristics of desert plants. American Scientist. 1977;65:412–421. [Google Scholar]
- Steyermark J. Flora del parque nacional Morrocoy. Caracas: Agencia Española de Cooperación Internacional (AECI) y Fundación Instituto Botánico de Venezuela; 1994. [Google Scholar]
- Tezara W, Fernández MD, Donoso C, Herrera A. Seasonal changes in photosynthesis and stomatal conductance of five plant species from a semiarid ecosystem. Photosynthetica. 1998;35:399–410. [Google Scholar]
- Tezara W, Mitchell VJ, Driscoll SD, Lawlor DW. Water stress inhibits plant photosynthesis by decreasing coupling factor and ATP. Nature. 1999;401:914–917. [Google Scholar]
- Tezara W, Martínez D, Rengifo E, Herrera A. Photosynthetic responses of the tropical spiny shrub Lycium nodosum (Solanaceae) to drought, soil salinity and saline spray. Annals of Botany. 2003;92:757–765. doi: 10.1093/aob/mcg199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tezara W, Marín O, Rengifo E, Martínez D, Herrera A. Photosynthesis and photoinhibition in two xerophytic shrubs during drought. Photosynthetica. 2005;43:37–45. [Google Scholar]
- Tezara W, Driscoll S, Lawlor DW. Partitioning of photosynthetic electron flow between CO2 assimilation and O2 reduction in sunflower plants under water deficit. Photosynthetica. 2008;46:127–134. [Google Scholar]
- Tuntawiroon N, Samootsakorn P, Theeraraj G. The environmental implications of the use of Calotropis gigantea as a textile fabric. Agriculture Ecosystems Environment. 1984;11:203–212. [Google Scholar]
- Usmani S, Kushwaha P. A study on hepatoprotective activity of Calotropis gigantea leaves extract. International Journal of Pharmacy and Pharmaceutical Sciences. 2010;2:101–103. [Google Scholar]
- Wei Y, Bai Y, Henderson DC. Critical conditions for successful regeneration of an endangered annual plant, Cryptantha minima: a modeling approach. Journal of Arid Environments. 2009;73:872–875. [Google Scholar]
- Wilson M. The Caribbean environment, geography for the CXC. Oxford: Oxford University Press; 1989. [Google Scholar]
- Wullschleger S, Tschaplinski T, Norby R. Plant water relations at elevated CO2 – implications for water-limited environments. Plant, Cell and Environment. 2002;25:319–331. doi: 10.1046/j.1365-3040.2002.00796.x. [DOI] [PubMed] [Google Scholar]