Abstract
Background and Aims
To date, the structure of the nectary spur of Aeridinae has not been studied in detail, and data relating to the nectaries of ornithophilous orchids remain scarce. The present paper compares the structural organization of the floral nectary in a range of Aeridinae species, including both entomophilous and ornithophilous taxa.
Methods
Nectary spurs of Ascocentrum ampullaceum (Roxb.) Schltr. var. aurantiacum Pradhan, A. curvifolium (Lindl.) Schltr., A. garayi Christenson, Papilionanthe vandarum (Rchb.f.) Garay, Schoenorchis gemmata (Lindl.) J.J. Sm., Sedirea japonica (Rchb.f.) Garay & H.R. Sweet and Stereochilus dalatensis (Guillaumin) Garay were examined by means of light microscopy, scanning electron microscopy and transmission electron microscopy.
Key Results and Conclusions
The diverse anatomy of the nectary is described for a range of Aeridinae species. All species of Ascocentrum investigated displayed features characteristic of ornithophilous taxa. They have weakly zygomorphic, scentless, red or orange flowers, display diurnal anthesis, possess cryptic anther caps and produce nectar that is secluded in a relatively massive nectary spur. Unicellular, secretory hairs line the lumen at the middle part of the spur. Generally, however, with the exception of Papilionanthe vandarum, the nectary spurs of all entomophilous species studied here (Schoenorchis gemmata, Sedirea japonica, Stereochilus dalatensis) lack secretory trichomes. Moreover, collenchymatous secretory tissue, present only in the nectary spur of Asiatic Ascocentrum species, closely resembles that found in nectaries of certain Neotropical species that are hummingbird-pollinated and assigned to subtribes Maxillariinae Benth., Laeliinae Benth. and Oncidiinae Benth. This similarity in anatomical organization of the nectary, regardless of geographical distribution and phylogeny, indicates convergence.
Keywords: Aeridinae, collenchyma, entomophily, floral anatomy, micromorphology, nectar, nectary spur, Orchidaceae, ornithophily, trichomes
INTRODUCTION
Aeridinae is a large and diverse subtribe containing some 1350 species in 103 genera distributed throughout the warm-temperate and tropical regions of Asia, Australia and eastern Pacific Islands, with Acampe Lindl. and Taeniophyllum Blume extending as far west as tropical Africa. Mostly epiphytes, members of this subtribe are characterized by their monopodial growth and highly developed velamen radicum. Pollinaria comprise two or four hard pollinia with obvious stipe and viscidium, and many genera possess a column-foot and spurred labellum (Topik et al., 2005, 2006). Although circumscription of Aeridinae has now been clearly defined (e.g. Cameron et al., 1999; Chase et al., 2003), prior to the use of molecular techniques (Topik et al., 2005, and references therein; Kocyan et al., 2008) our understanding of relationships within this subtribe was frustrated by morphological diversification and parallelism (Garay, 1972; Dressler, 1993).
Molecular, phylogenetic analyses tentatively support the monophyly of Aeridinae. However, the position of Sedirea Garay & H.R. Sweet is unresolved and it is claimed that molecular analyses and pollinaria studies indicate that it is sister to the whole subtribe and to the Phalaenopsis alliance, respectively (Topik et al., 2005, 2006; Kocyan et al., 2008). On the basis of phylogenetic analyses, Ascocentrum Schltr. and Papilionanthe Schltr. are currently assigned to the Aerides alliance and Schoenorchis Reinw. ex Blume is placed in the Pelatantheria alliance (Topik et al., 2005). Stereochilus Lindl. is also closely related to Aerides Lour. and belongs to the Stereochilus–Smitinandia–Vandopsis–Pomatocalpa–Trichoglottis clade (Kocyan et al., 2008).
Despite the enormity of Aeridinae and the morphological diversity of its flowers, generally, anatomical investigation of the nectary spur, which is so characteristic of many of its members, has been neglected. A short spur (<3 cm) is thought to represent the apomorphic state for this particular subtribe and both loss and elongation (>3 cm) of the nectary spur are considered to have occurred several times in its evolution (Topik et al., 2005). Spur length, however, although reflecting the overall length of the flower, is often related to the length of pollinator mouthparts (T. Yukawa, Tsukuba Botanical Garden, National Science Museum, Tsukuba, Japan, unpubl. res. – cited in Topik et al., 2005). In Aerides, two different flower types occur; those with open apertures and those with hidden apertures to the nectary spur, and these appear to have evolved at least twice in geographically distinct regions. Even so, floral morphology of this genus is probably determined more by pollinator-driven selection than by phylogeny (Kocyan et al., 2008).
Reports of Aeridinae pollination are scarce. However, it appears that their pollinators are diverse and that these orchids employ a wide range of pollination strategies. Most species are entomophilous. For example, Phalaenopsis Ridl., Vanda Jones ex R. Br. and Aerides odorata Lour. are said to be pollinated by carpenter bees (Xylocopinae), and Papilionanthe teres (Roxb.) Schltr. by Xylocopa latipes (Carr, 1928; Dressler, 1990; van der Cingel, 2001; Kocyan et al., 2008). Sarcochilus R. Br. and Pomatocalpa Breda, Kuhl & Hasselt are pollinated by Carbonaria bees, whereas Amesiella Schltr. ex Garay and Neofinetia Hu are night-scented and possibly moth-pollinated (Ackerman, 1984; Dressler, 1990; van der Cingel, 2001; T. Yukawa, Tsukuba Botanical Garden, National Science Museum, Tsukuba, Japan, unpubl. res. – cited in Topik et al., 2005). Beetles pollinate short-spurred or spur-less species, as well as Peristeranthus T.E. Hunt and Trudelia cristata (Vanda cristata Wall. ex Lindl.) and, in the case of Cottonia Wight., this may involve pseudocopulation (van der Cingel, 2001). Other genera, such as Ascocentrum Schltr. and Renanthera Lour., are said to be ornithophilous (Dressler, 1990; van der Cingel, 2001), and Slade (1980) reported the pollination of the hybrid orchid Ascocentrum ‘Sagarik Gold’ [A. miniatum (Lindl.) Schltr. × A. curvifolium (Lindl.) Schltr.] by an unidentified honeyeater (Meliphagidae). Autogamy is apparently common and occurs in Schoenorchis paniculata Blume, Taeniophyllum hasseltii Rchb.f. and a species of Thrixspermum Lour., whereas in Cleisostoma parishii (Hook.f.) Garay and Papilionanthe longicornu [Aerides uniflora (Lindl.) Garay], it is wind-assisted (van der Cingel, 2001).
In the present paper, we compare the anatomical organization of the nectary spur of a range of Aeridinae taxa (Ascocentrum, Papilionanthe, Schoenorchis, Sedirea, Stereochilus) that differ in flower size, relative spur dimensions and pollinator, as a prelude to a wider investigation based on phylogeny. Furthermore, we compare the spur anatomy of ornithophilous Ascocentrum spp. with that of Neotropical, bird-pollinated Maxillariinae Benth., Laeliinae Benth. and Oncidiinae Benth. to determine whether these unrelated taxa exhibit pollinator-mediated convergence (Davies and Stpiczyńska, 2008, and references therein).
MATERIALS AND METHODS
Nectary spurs of seven species of Aeridinae were investigated: Ascocentrum ampullaceum (Roxb.) Schltr. var. aurantiacum Pradhan (accession no. KLD200701), Ascocentrum curvifolium (Lindl.) Schltr. (accession no. KLD200901), Ascocentrum garayi Christenson (accession no. KLD200702), Papilionanthe vandarum (Rchb.f.) Garay (accession no. S19990240), Schoenorchis gemmata (Lindl.) J.J. Sm. (accession no. KLD200703), Sedirea japonica (Rchb.f.) Garay & H.R. Sweet (accession no. S20070249) and Stereochilus dalatensis (Guillaumin) Garay (accession no. KLD200704). Those specimens whose accession numbers are prefixed ‘S’ were obtained from the Swansea Botanical Complex, UK, whereas the remainder came from the second author's collection. Abbreviations for authority names of plants follow Brummitt and Powell (1992). Nectar spurs were examined by light microscopy (LM), scanning electron microscopy (SEM) and transmission electron microscopy (TEM).
In each case, nectary tissue was fixed in 2·5 % glutaraldehyde/4 % formaldehyde in phosphate buffer (pH 7·4; 0·1 m) for 4 h at 4 °C and carefully washed three times in phosphate buffer. It was then post-fixed in 1 % osmium tetroxide solution at 0 °C for 1·5 h, washed in distilled water and dehydrated using a graded ethanol series. Finally, material was infiltrated and embedded in LR White resin. Following polymerization at 60 °C, sections were cut at 60 nm for TEM using a Reichert Ultracut-S ultramicrotome and a glass knife, stained with uranyl acetate and lead citrate (Reynolds, 1963), and examined using either an FEI Technai G2 Spirit Bio TWIN or Zeiss Leo 912AB transmission electron microscope, at an accelerating voltage of 120 kV.
Semi-thin sections (0·9–1·0 µm thick) were prepared for LM and stained with 0·25 % toluidine blue O in 0·25 % (w/v) aqueous sodium tetraborate solution (TBO). Hand-cut sections of fresh material were tested for the presence of starch and lignin with IKI solution and acidified phloroglucinol, respectively. Ruthenium red was used to test for the presence of acidic polysaccharides and mucilage, whereas alcoholic Sudan III was used to test for lipids (Jensen, 1962). Auramine O was also used to test for the presence of lipids, and the staining reaction was examined by means of a Nikon Eclipse 90i microscope equipped with fluorescein isothiocyanate filter. Autofluorescence of plastid chlorophyll in P. vandarum was investigated using a Nikon Optiphot II fluorescence microscope with UV-2B filter. In each case, control sections were used. The thickness of the spur wall was measured at its proximal end and micrometry and photomicrography were undertaken using a Nikon Eclipse 600 microscope with Screen Measurement version 4·21 software.
Fixed spurs were also cut longitudinally to examine the epidermis lining the lumen. They were subsequently dehydrated in acetone, subjected to critical-point drying using liquid CO2, sputter-coated with gold and examined by means of a TESCAN/VEGA LMU scanning electron microscope, at an accelerating voltage of 30 kV.
RESULTS
Those members of Aeridinae investigated differed in both their floral morphology (including that of the spur) and their spur anatomy. Spurs varied in the width of the lumen, and the thickness of their walls and that of the outer tangential wall of the secretory epidermis. Moreover, whereas in some species the epidermis lining the lumen was glabrous, in others it was papillose, pubescent or hirsute, and there were also obvious differences in the structure of the epidermal cuticle and spur vasculature. The distribution of these characters and quantitative data are presented in Table 1.
Table 1.
Comparison of micromorphological characters (mean with range in parentheses) of the spur in selected Aeridinae
| Character studied | Ascocentrum ampullaceum var. aurantiacum | Ascocentrum curvifolium | Ascocentrum garayi | Papilionanthe vandarum | Schoenorchis gemmata | Sedirea japonica | Stereochilus dalatensis |
|---|---|---|---|---|---|---|---|
| Spur length (mm) | 6·00 (5·69–6·30) | 6·00 (5·73–6·27) | 5·90 (5·66–6·20) | 16·00 (15·94–16·12) | 1·43 (1·30–1·58) | 12·50 (8·00–14·00) | 2·83 (2·76–2·92) |
| Thickness of spur wall (μm) | 250·92 (223·51–330·51) | 266·30 (249·70–281·60) | 354·80 (245·80–457·50) | 368·13 (285·05–454·45) | 97·3 (68·00–167·80) | 448·85 (379·30–590·00) | 227·19 (140·20–285·11) |
| Number of vascular bundles | 6–10 | 10 | 10 | 22 | 2 | 6–10 | 6 |
| Dimensions of secretory epidermal cells (μm) | 36·71 × 31·52 | 17·98 × 16·40 | 19·50 × 23·70 | 30·95 × 14·83 | 10·68 × 14·37 | 24·30 × 31·0 | 15·75 × 20·54 |
| Thickness of outer cell wall of secretory epidermis (μm) | 3·61 (2·17–6·36) | 7·40 (6·10–8·90) | 1·90 (0·80–2·10) | 1·05 (0·81–1·37) | 0·91 (0·40–1·29) | 3·39 (2·90–4·13) | 1·85 (1·04–2·33) |
| Presence of subepidermal collenchyma | + | + | + | – | – | – | – |
| Length of secretory hairs (μm) | 37·42 (16·30–56·80) | 46·22 (16·40–66·50) | 28·12 (12·50–34·70) | 260·55 (71·27–397·09) | – | – | – |
Ascocentrum
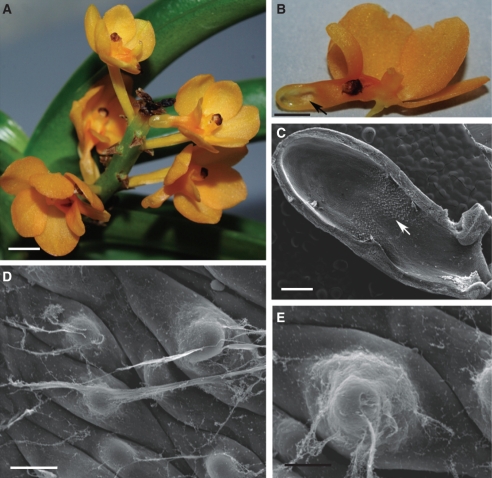
Ascocentrum spp. have features characteristic of ornithophilous orchids in that their flowers are weakly zygomorphic, diurnal, lack fragrance and nectar guides, and are pink, orange or red with cryptic anther caps (Figs 1A, B, 3A, B and 5A, B).
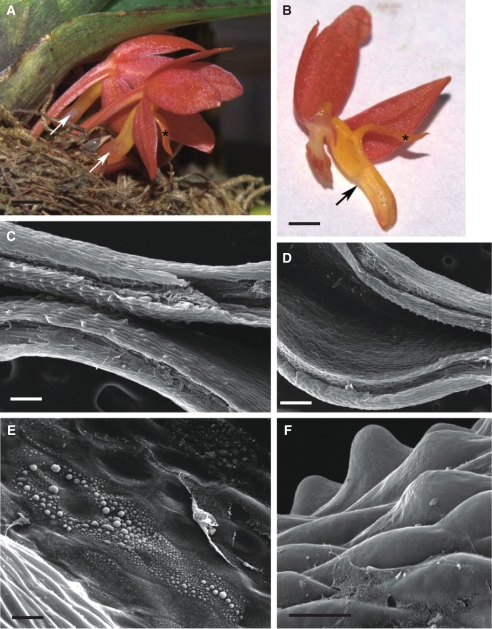
Fig. 1.
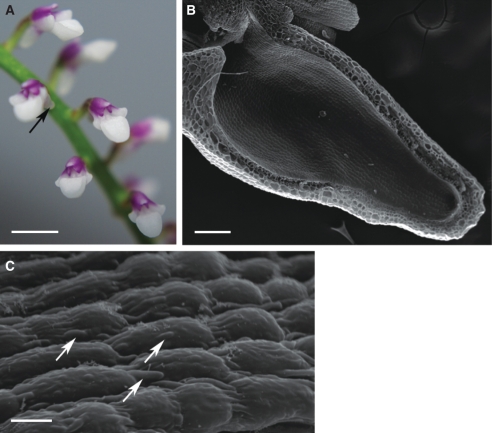
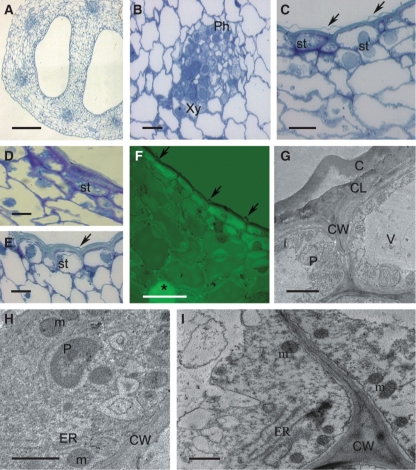
Habit of the flower and micromorphology of the spur of Ascocentrum ampullaceum var. aurantiacum; (C–F) scanning electron microgrpahs. (A) Red–orange flower showing saccate nectary spur (arrows). Labellum marked with an asterisk. Scale bar = 2·5 mm. (B) Bisected flower showing relative positions of column, labellum (asterisk) and nectary spur (arrow). Scale bar = 2 mm. (C) Middle region of spur with secretory hairs. Scale bar =150 µm. (D) Distal part of spur with glabrous epidermis. Scale bar = 300 µm. (E, F) Nectar residue on surface of glabrous and hirsute part of spur. Scale bars = 30 and 25 µm, respectively. Key to abbreviations used in all figures: C, cuticle; CL, cuticular layer; CW, cell wall; ER, endoplasmic reticulum; D, dictyosome; m, mitochondrion; N, nucleus; n, nectar residue; P, plastid; Ph, phloem; st, starch; sv, secretory vesicle; V, vacuole; Vb, vascular bundle; Xy, xylem.
Ascocentrum ampullaceum var. aurantiacum
This species has weakly zygomorphic, scentless, red–orange flowers with purple iridescence and orange–yellow column and spur. The labellum is simple and ligulate and uniformly orange–yellow (Fig. 1A, B). The entrance to the saccate spur is scarlet and laterally compressed (Fig. 1B, C). The anther cap is orange with a scarlet central region.
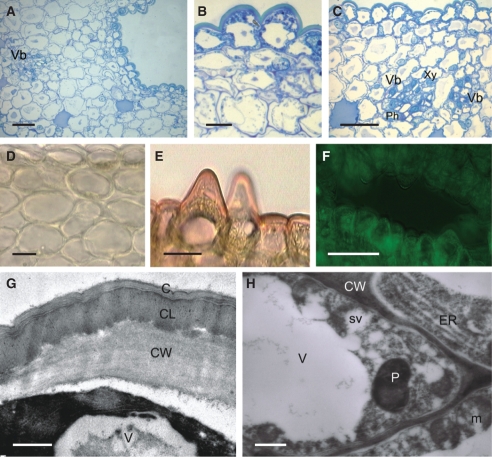
The wall of the spur is composed of several layers of cells. Short, unicellular hairs (mean length = 37·42 µm) occur predominantly in the middle part of the spur, whereas distally, the epidermis lining the lumen is glabrous and composed of flattened cells covered with non-striated cuticle (Fig. 1C–F). Nectar residues were observed on the surface of epidermal cells, especially those at the distended, distal part of the spur (Fig. 1E, F). The secretory hairs are densely distributed (Fig. 2A). Epidermal cells (both flattened cells and unicellular hairs) have thick cellulosic walls, and the outer, tangential walls are particularly pronounced (Fig. 2B–G). The cuticle stains with Sudan III, TBO and auramine O (Fig. 2C–G), but with auramine O, fluorescence of the cuticle is much greater for the flat, epidermal cells than for the hairs (Fig. 2D). The nucleus is located in the basal part of the hair, and the parietal cytoplasm contains leucoplasts (Fig. 2B, G). Epidermal cells located at the bottom of the spur contain numerous vacuoles that stain purple with TBO, indicating the presence of phenolic compounds. Similar compounds are also present in the small, thick-walled collenchyma cells that compose the one- to two-layered, subepidermal region (Fig. 2E, F). Primary pit-fields, containing numerous plasmodesmata, connect epidermal and subepidermal collenchymatous cells (Fig. 2H). Deep-seated parenchyma contains elongated idioblasts, whose vacuolar contents stain intensely with TBO. The spur is supplied with several collateral vascular bundles comprising xylem and phloem in equal proportions (Fig. 2E).
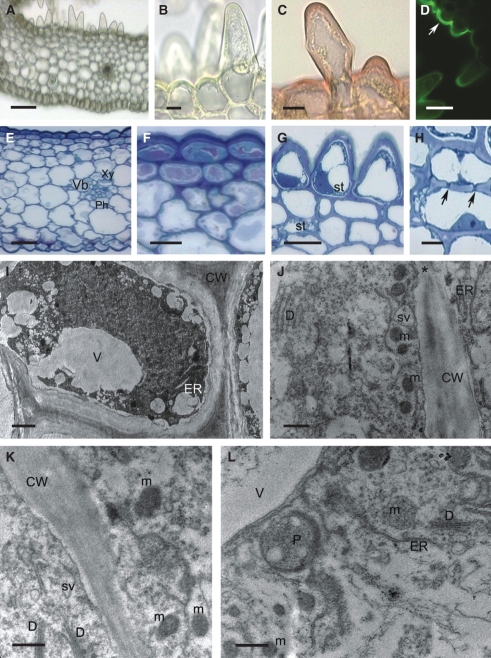
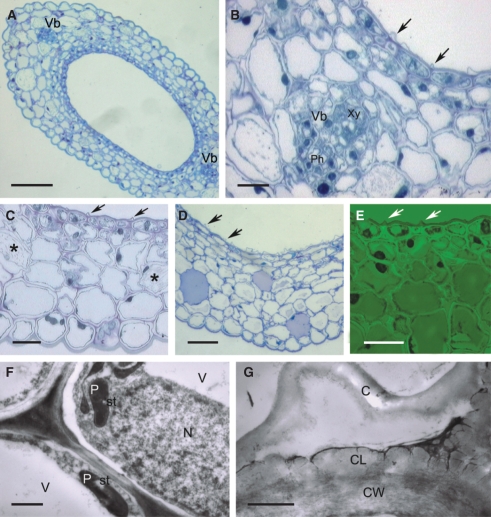
Fig. 2.
Histology and ultrastructure of the spur of A. ampullaceum var. aurantiacum: (A–H) light micrographs; (I–L) transmission electron micrographs. (A) Hand-cut section of spur wall showing vascular bundle and secretory hairs. Scale bar = 50 µm. (B) Detail of thick-walled epidermis and secretory hair with granular cytoplasm and basally positioned nucleus. Scale bar = 10 µm. (C) Cuticle covering cell walls of secretory epidermis stained with Sudan III. Scale bar = 10 µm. (D) Cuticle covering flat epidermal cells stained intensely with auramine O (arrow), whereas secretory hairs stained only slightly. Scale bar = 25 µm. (E) Nectary spur with glabrous epidermis and vascular bundle containing an equal proportion of phloem and xylem. Scale bar = 20 µm. (F) Detail of thick-walled epidermis with thick cuticle and subepidermal cells. Vacuoles are stained violet. Scale bar = 25 µm. (G) Short secretory hairs with parietal cytoplasm, leucoplasts containing starch and thick walls with cuticle that stains blue–green with TBO. Scale bar = 20 µm. (H) Subepidermal cells with pitted, thick walls (arrows). Scale bar =10 µm. (I) Granular cytoplasm of secretory epidermis with vacuole, ER, mitochondria and irregular plastid profiles. The thick cell wall has a reticulate cuticle. Scale bar = 1 µm. (J) Detail of cytoplasm with dictyosomes, ER, secretory vesicles near plasmalemma and primary pit-field in cell wall (asterisk). (K) Parietal cytoplasm with mitochondria and dictyosomes. (L). Subepidermal cell containing plastid with small starch grains, dictyosomes, ER and mitochondria. (J–L) Scale bars = 0·5 µm. Abbreviations: see Fig. 1.
The secretory epidermis contains granular cytoplasm with numerous mitochondria, rough endoplasmic reticulum (RER) profiles, dictyosomes and abundant secretory vesicles (Fig. 2I–K), as well as leucoplasts of irregular shape that contain small starch grains. The latter also occur in subepidermal cells (Fig. 2L).
Ascocentrum curvifolium
Ascocentrum curvifolium also has weakly zygomorphic flowers (Fig. 3A) lacking fragrance. All tepals are orange–red with purple iridescence (Fig. 3A, B). The throat of the flower, as well as the lateral lobes of the labellum, is yellow. The latter are inflated, permitting only a narrow channel into the spur. The mid-lobe of the labellum is immovable and arranged at 90° to the floral tube. Proximally, the nectary spur is yellow, but orange with purple iridescence distally (Fig. 3B).
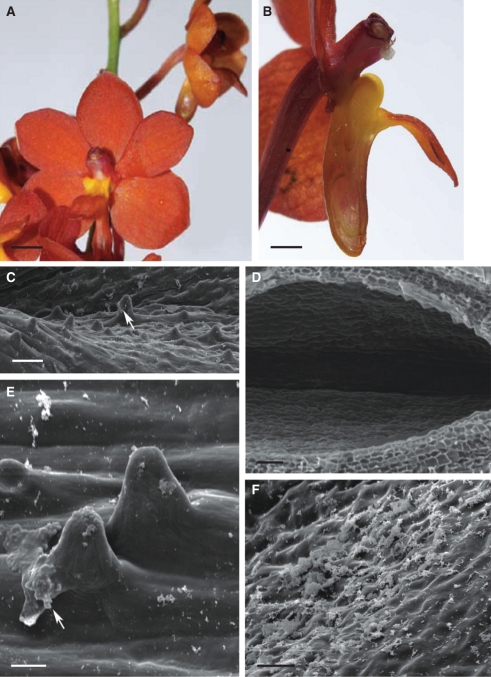
Fig. 3.
Habit of flower and micromorphology of the spur of Ascocentrum curvifolium; (C–F) scanning electron micrographs. (A) Inflorescence bearing weakly zygomorphic, orange–red flowers with purple iridescence. Scale bar = 4 mm. (B) Bisected flower showing relative positions of column, labellum and nectary spur. Scale bar =1·5 mm. (C) Epidermis lining nectary spur with hairs and nectar residues (arrow). Scale bar = 50 µm. (D) Glabrous epidermis lining distal part of spur. Scale bar = 50 µm. (E) Secretory hairs with smooth cuticle and nectar residues (arrow). Scale bar = 20 µm. (F) Smooth cuticle covering surface of secretory epidermis with nectar residues. Scale bar = 25 µm. Abbreviations: see Fig. 1.
As in A. ampullaceum var. aurantiacum, hairs up to 66·5 µm in length are found only at the middle part of the spur, the rest of the lumen being glabrous and lined by flattened epidermal cells (Fig. 3C–F). Nectar residues are present on the surface of the hairs and at the bottom of the spur (Fig. 3C, E, F).
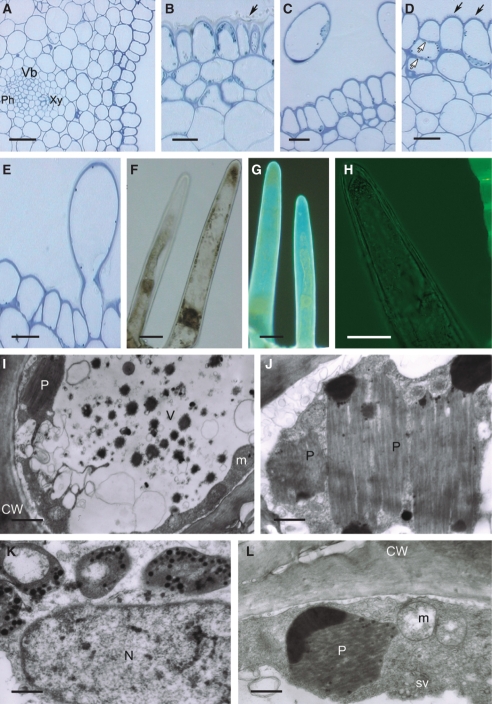
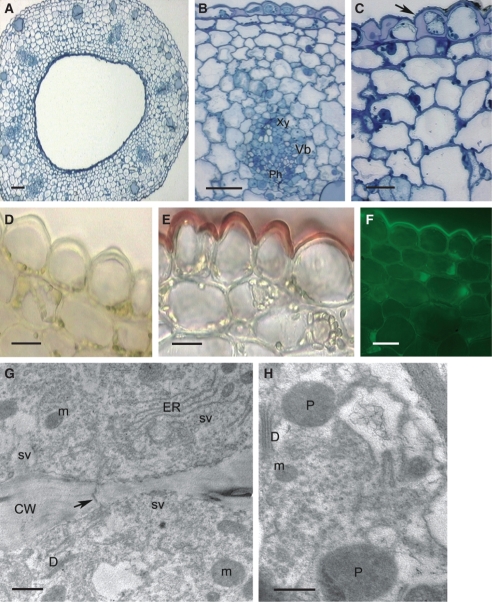
In transverse section, the wall of the spur is fleshy and multilayered (Fig. 4A; Table 1). Small epidermal cells and hairs lining the lumen of the spur display extraordinarily thick, cellulosic walls and a thick cuticle (Fig. 4A–F). The cuticle is smooth, without visible pores and cracks (Fig. 3E). It stains intensely with TBO, Sudan III and auramine O (Fig. 4C–F) and, as in the previous species, it is the cuticle overlying flat, epidermal cells that fluoresces most (Fig. 4E, F). Cells comprising the 1–2 layers directly beneath the epidermis are collenchymatous, with thick walls (Fig. 4A–F). These walls are not lignified and stain intensely with ruthenium red. Epidermal and subepidermal cells have dense cytoplasm with relatively large nuclei (Fig. 4C). The granular, vesiculate cytoplasm contains numerous mitochondria and RER profiles (Fig. 4G, H), whereas leucoplasts sometimes contain starch (Fig. 4H).
Fig. 4.
Histology and ultrastructure of spur of A. curvifolium: (A–F) light micrographs; (G,H) transmission electron micrographs. (A) Hand-cut section of spur wall showing epidermis with secretory hairs, underlying tissues and vascular bundle. Scale bar =50 µm. (B) Hand-cut section of spur treated with IKI and showing glabrous, thick-walled epidermal cells from proximal part of spur and thick-walled subepidermal cells. Scale bar = 20 µm. (C) Detail of thick-walled epidermis with secretory hairs and thick cuticle, together with subepidermal cells. Note that walls are pitted (arrows). Scale bar = 25 µm. (D) Cuticle covering glabrous epidermal cells stains intensely with Sudan III, but that covering the secretory hairs stains only slightly. Scale bar =10 µm. (E) Thick-walled epidermal cells from distal part of spur treated with auramine O and showing thick, strongly fluorescent cuticle (arrow). (F) Secretory hair with weakly fluorescent cuticle following staining with auramine O. Note that elsewhere, the cuticle fluoresces strongly (arrow). (E,F) Scale bars = 25 µm. (G) Detail of cytoplasm with dictyosomes, ER and irregular plastid profiles. (H) Secretory cell showing mitochondria and plastid with starch grains. (G,H) Scale bars = 1 µm. Abbreviations: see Fig. 1.
Numerous small, collateral, vascular bundles, comprising equal proportions of xylem and phloem, occur in deeply seated parenchyma, whereas idioblasts containing phenolic compounds are found close to the outer epidermis.
Ascocentrum garayi
Flowers of A. garayi are yellow–orange with dark brown anther caps (Fig. 5A, B). They lack fragrance and nectar guides and the entrance to the spur, which contains copious nectar, is narrow (Fig. 5B, C). Nectar is secreted by trichomes arising from the epidermis lining the lumen of the saccate spur. They are present only in the middle part of the spur, where the latter becomes constricted (Fig. 5C–E). The rest of the lumen is lined with glabrous epidermal cells. Trichomes are conical, unicellular and very short (mean = 28·12 µm). SEM reveals that, distally, trichomes bear nectar residues (Fig. 5D, E). The secretory epidermis is supported by 1–2 layers of underlying, small parenchyma cells with dense cytoplasm and relatively large nuclei (Fig. 6A–C). The subepidermal cells are thin-walled in the hirsute region of the spur, whereas proximally, they are collenchymatous (Fig. 6D). Cell walls of the secretory epidermis are relatively thick (mean = 1·9 µm), composed of cellulose and have a thick cuticular layer (Fig. 6B–E, G). The cuticle overlying both epidermal cells and trichomes is smooth but lamellate (Fig. 6G), without visible striations, and lacks pores and cracks. Although it stained with Sudan III, it did not fluoresce following treatment with auramine O (Fig. 6E, F).
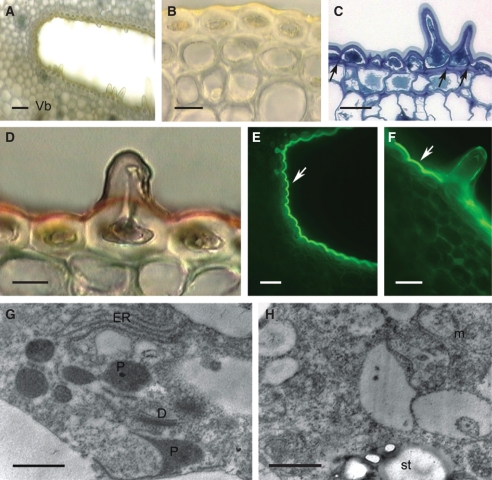
Fig. 5.
Habit of flower and spur of Ascocentrum garayi; (C–E) scanning electron micrographs. (A) Inflorescence bearing weakly zygomorphic, orange flowers. Scale bar = 3 mm. (B) Bisected flower showing relative positions of column, labellum, nectary spur and displaced, darkly coloured, cryptic, anther cap. Arrow indicates position of secretory hairs. Scale bar = 2 mm. (C) Spur cut longitudinally showing hairs (arrow) concentrated towards its centre, and coinciding with a constriction in its wall. Scale bar = 1 mm. (D) Short conical hairs with nectar residues at their tips. Scale bar = 30 µm. (E) Smooth cuticle of secretory hairs, with nectar residues. Scale bar = 20 µm. Abbreviations: see Fig. 1.
Fig. 6.
Sections through nectary spur of A. garayi; (A–F) light micrographs; (G, H) transmission electron micrographs. (A) Lumen and secretory epidermis, as well as subsecretory parenchyma containing vascular strand and idioblasts. Scale bar = 40 µm. (B) Detail of nucleated, secretory, epidermal papillae with thick, outer tangential walls. Scale bar = 10 µm. (C) Section showing idioblasts (with selectively stained vacuoles) and detail of vascular strands. Scale bar =50 µm. (D) Section through proximal part of spur showing thick-walled epidermis and subepidermal cells. Scale bar = 20 µm. (E) Slightly stained epidermal cuticle following treatment with Sudan III. Scale bar = 20 µm. (F). Secretory epidermis following treatment with auramine O, but lacking fluorescence. Scale bar = 25 µm. (G) Detail of outer tangential wall with lamellate cuticle and an inner, homogeneous cuticular layer. Scale bar = 0·5 µm. (H) Secretory cells showing ER and plastid with darkly stained stroma but few lamellae. Scale bar = 1 µm. Abbreviations: see Fig. 1.
Both epidermal cells and trichomes have dense, parietal cytoplasm and relatively large nuclei (Fig. 6A, B–D). The plastids do not accumulate starch (Fig. 6H). The nectary is supplied with collateral vascular bundles (Fig. 6A, C), but here, unlike the other Ascocentrum spp. investigated, phloem predominates. The ground parenchyma contains idioblasts whose vacuoles stain strongly with TBO (Fig. 6A, C) and sometimes these contain raphides.
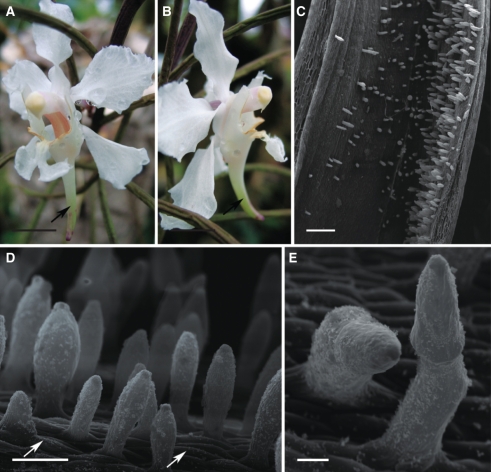
Papilionanthe vandarum
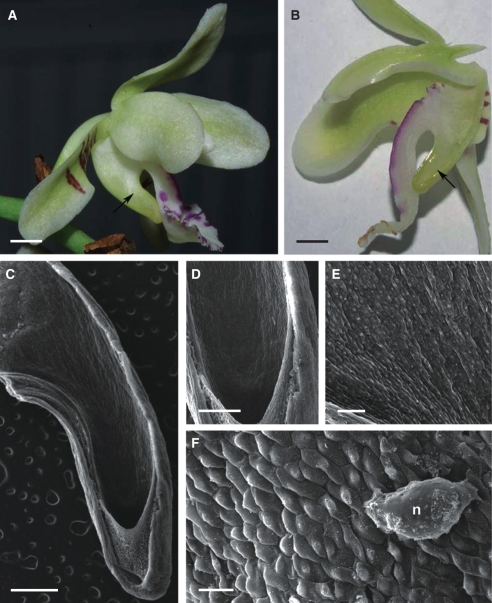
The predominantly white flowers of P. vandarum are fragrant and are thought to be pollinated by moths. The labellum is three-lobed and the spur is long, straight or curved, and slender (Fig. 7A, B). Long, unicellular, clavate hairs (up to 397·09 µm in length) are present throughout the spur. However, they are particularly long and more densely arranged along the inner, adaxial surface of the spur (Fig. 7C, D). Between these are occasional, bicellular hairs (Fig. 7E). Epidermal cells lining the spur lumen are small and covered by cuticle with evident striations. Striations are also present at the base of the hairs, whereas the cuticle covering the rest of the hair is almost smooth and lacks pores and distensions. Nectar residues occur on the surface of both hairs and epidermal cells (Fig. 7D, E).
Fig. 7.
Habit of flower and spur of Papilionanthe vandarum; (C–E) scanning electron micrographs. (A, B) Zygomorphic, white flower with cream anther cap and curved nectary spur (arrow). Scale bars = 5 mm. (C) Spur lumen showing relative position of secretory hairs. Scale bar = 0·5 mm. (D) Secretory hairs with nectar residues on their surface and epidermal cells with striated cuticle (arrows). Scale bar = 80 µm. (E) Bicellular, secretory hairs with nectar residues. Scale bar = 25 µm. Abbreviations: see Fig. 1.
In transverse section, the wall of the nectary spur is thick and multilayered (Table 1). Epidermal cells, and hair cells lining the lumen, contain parietal cytoplasm and numerous, but starchless, leucoplasts (Fig. 8A–G). The cuticle covering the secretory hairs and flat epidermal cells does not fluoresce with auramine O (Fig. 8H). Below the secretory epidermis are 2–3 layers of small, parenchymatous cells. Cellulosic cell walls are relatively thin (Fig. 8A–D) and contain numerous primary pit-fields with plasmodesmata that connect the epidermis and subepidermal parenchyma. The ground parenchyma contains numerous, large vascular bundles with prominent phloem (Fig. 8A).
Fig. 8.
Histology and ultrastructure of spur of Papilionanthe vandarum: (A–H) light micrographs; (I–L) transmission electron micrographs. (A) Section of spur wall showing epidermis, underlying tissues and vascular bundle. Scale bar = 50 µm. (B) Glabrous epidermis cells with nectar residues upon its surface (arrow). (C) Transverse section through epidermis and secretory hairs showing parietal cytoplasm with plastids. (D) Epidermal cells with striated cuticle (solid arrows). Pit-fields are present in epidermal and subepidermal cells (open arrows). (E) Epidermis with secretory, unicellular hair cut longitudinally and showing smooth cuticle. (B–E) Scale bars = 20 µm. (F) Unicellular, secretory hairs with large nuclei and numerous plastids in parietal cytoplasm. (G) Hairs showing autofluorescence and that plastids lack chlorophyll. (F, G) Scale bars = 25 µm. (H) Secretory hair following treatment with auramine O, yet showing no fluorescence. Scale bar = 25 µm. (I) Transverse section of hair showing cytoplasm with mitochondria and plastids. Vacuole contains vesicles and dark precipitates. Scale bar = 4 µm. (J) Plastid with numerous, dense lamellae and osmiophilic regions. Scale bar =1·5 µm. (K) Detail of cytoplasm with nucleus and chromoplasts. Scale bar = 1 µm. (L) Parietal cytoplasm with plastid, mitochondria and secretory vesicles. Scale bar = 1·5 µm. Abbreviations: see Fig. 1.
Epidermal cells and hairs contain dense cytoplasm with abundant mitochondria and vesicles (Fig. 8I–L). Vesicles are also present in the periplasmic space (Fig. 8J). As well as typical chromoplasts, large leucoplasts with very densely and regularly packed membranes and numerous plastoglobuli, or peripherally situated osmiophilic regions, may be present (Fig. 8I–L). Vacuoles may also contain small, dark precipitates and vesicles (Fig. 8I).
Schoenorchis gemmata
The white mid-lobe of the labellum predominates in this very small, fragrant, rose–purple flower. Sepals are white or purple with white apices, and the spur and lateral lobes of the labellum are white, marked with purple. This species is entomophilous, the labellum forming a platform for alighting pollinators. The nectary spur, although short, is proportionally large and has a narrow entrance (Fig. 9A, B). It is straight, but expanded proximally. The column is very short and fleshy.
Fig. 9.
Inflorescence and spur of Schoenorchis gemmata; (B, C) scanning electron micrographs. (A) Zygomorphic, white and purple flower with broad labellum and spur (arrow). Scale bar = 1·5 mm. (B) Spur showing lumen with glabrous epidermis. Scale bar = 200 µm. (C) Epidermal cells showing distension of cuticle (arrows). Scale bar = 10 µm.
The spur wall is significantly thinner than that of other species investigated (Table 1). The epidermis lining the lumen is glabrous, and the cuticle is distended (Figs 9B, C and 10A–E). These distensions occur on the cell surface, but are particularly abundant at cell junctions (Figs 9C and 10B–E). Epidermal cells, like the underlying parenchyma, are small with dense cytoplasm and large nuclei, leucoplasts and several small vacuoles (Fig. 10A–C, F). The spur is supplied by only two vascular bundles, each containing more phloem than xylem (Fig. 10A, B). Ground parenchyma contains numerous idioblasts with phenolic contents and/or prominent raphides (Fig. 10C–E). TEM shows that lecuoplasts have an electron-dense stroma, with small starch grains (Fig. 10F). The thin, cellulosic wall has a reticulate cuticular layer, as well as a layer that becomes detached (Fig. 10G). The cuticle does not fluorescence on treatment with auramine O (Fig. 10E).
Fig. 10.
Histology and ultrastructure of spur of Schoenorchis gemmata; (A–E) light micrographs; (F, G) transmission electron micrographs. (A) Section through nectary spur of S. gemmata showing lumen lined with glabrous epidermis and two vascular strands embedded in the ground parenchyma. Scale bar = 100 µm. (B) Detail of thin-walled, secretory epidermal cells showing distended cuticle (arrows), subepidermal layers and vascular bundle with prominent phloem. Scale bar = 20 µm. (C) Section showing epidermal cells with distended cuticle (arrows) and idioblasts containing raphides (asterisks). Scale bar = 20 µm. (D) Section showing idioblasts with selectively stained vacuoles and epidermis with distended cuticle (arrows). Scale bar = 50 µm. (E) Distended cuticle (arrows) that does not fluoresce following treatment with auramine O. Scale bar = 25 µm. (F) Detail of nucleated, thin-walled secretory, epidermal cell with plastids containing small starch grains and plastoglobuli. Scale bar = 1 µm. (G) Detail of outer tangential wall of secretory epidermal cell with thick, reticulate, cuticular layer and distended cuticle. Scale bar = 2 µm. Abbreviations: see Fig. 1.
Sedirea japonica
Like all the other entomophilous species investigated, flowers of Sedirea japonica are more strongly zygomorphic than those of Ascocentrum spp. They have pale, greenish white tepals and column. However, whereas the dorsal sepal and petals are entirely greenish white, bases of lateral sepals are barred purple–brown. The white labellum is marked at the margin with rose-coloured blotches that perhaps function as nectar guides, and is developed to a greater extent than the labella of Ascocentrum spp., the mid-lobe being expanded distally (Fig. 11A, B). The greenish white spur is approximately twice as long as it is wide at its widest point, and forwardly curving. The anther cap is cream-coloured. Flowers are very fragrant by day.
Fig. 11.
Habit of flower and spur of Sedirea japonica; (C–F) scanning electron micrographs. (A) Greenish white, zygomorphic flower, marked purple–brown and rose, with broad labellum and curved spur (arrow). Scale bar = 4 mm. (B) Bisected flower showing relative positions of column, labellum and nectary spur (arrow). Scale bar = 3 mm. (C, D) Spur cut longitudinally, showing glabrous, inner epidermis. Scale bars = 1 and 0·5 mm, respectively. (E) Lining of spur showing minutely papillose secretory cells. Scale bar = 200 µm. (F) Smooth cuticle of secretory cells bearing nectar residues. Scale bar = 50 µm. Abbreviations: see Fig. 1.
The epidermis lining the spur is glabrous to minutely papillose (Figs 11C–F and 12A–F). These epidermal cells are elongate, and the centrally placed, conical papillae (Fig. 11E, F) stain with TBO (Fig. 12A–C). The cuticle is finely striated (Fig. 12C), but lacks pores (Fig. 12D–F). It stains intensely with Sudan III, but only slightly with auramine O (Fig. 12E, F). Cell walls, in particular radial walls and outer tangential walls adjacent to the lumen, are thick (Fig. 12B, C). The cytoplasm of epidermal cells and underlying parenchyma contains many large chloroplasts (Fig. 12C–E). Large idioblasts, close to the outer epidermis, stain strongly with TBO and often contain raphides (Fig. 12A). The spur is supplied with several large, vascular bundles and phloem predominates. Generally, xylem parenchyma occurs in each vascular bundle, but only 4–5 small, xylem vessels are present (Fig. 12B).
Fig. 12.
Histology and ultrastructure of spur of Sedirea japonica; (A–F) light micrographs; (G, H) transmission electron micrographs. (A) Section through nectary spur showing lumen lined with glabrous epidermis and vascular strands embedded in parenchyma. Scale bar = 100 µm. (B) Detail of spur wall with secretory epidermis and vascular bundle. Scale bar = 50 µm. (C) Epidermal cells with thick radial walls and fine cuticular striations (arrow). (D) Hand-cut section treated with IKI, showing epidermal and subepidermal cells containing chloroplasts. (E) A similar section stained with Sudan III and showing epidermis with thick cuticle. (C–E) Scale bars =20 µm. (F) Weakly fluorescent cuticle covering secretory epidermis. Scale bar = 25 µm. (G) Parietal cytoplasm of secretory epidermis and subepidermal cell containing ER profiles, mitochondria, dictyosomes, secretory vesicles and plasmodesmata in cell wall (arrow). Scale bar = 1 µm. (H) Detail of parietal cytoplasm of secretory epidermal cell containing plastids, dictyosomes and mitochondria. Scale bar = 0·5 µm. Abbreviations: see Fig. 1.
TEM reveals that the cytoplasm is granular, with abundant mitochondria, RER profiles, dictyosomes and vesicles that accumulate in the outermost cytoplasm (Fig. 12G, H). Leucoplasts have few internal membranes, and osmiophilic precipitates can be found in vacuoles. Primary pit-fields with plasmodesmata are frequent in the walls of the epidermis and adjoining parenchyma (Fig. 12G).
Stereochilus dalatensis
This entomophilous species produces simple racemes of small, fragrant, pale pink flowers with rose-coloured labella. The tepals are strongly reflexed and the column is vertical, so that the angle between it and the more or less horizontal, fleshy labellum approaches 90°. The anther cap is rose-coloured, whereas the nectary spur is white, flushed pink, relatively wide, saccate, fleshy and conical (Fig. 13A).
Fig. 13.
Inflorescence and spur of Stereochilus dalatensis; (B–E) scanning electron micrographs. (A) Zygomorphic, pink and rose-coloured flowers with saccate spurs (arrows). Scale bar = 5 mm. (B) Bisected spur showing two loculi (lumina) separated by a median, longitudinal septum with proximal protuberance (arrow). Scale bar = 0·5 mm. (C) Detail of the two spur lumina lined with glabrous epidermis. Scale bar = 200 µm. (D) Epidermal cells showing distension of cuticle at cell junctions (arrows). Scale bar = 50 µm. (E) Secretory epidermis showing distension of cuticle (arrows) and nectar residues. Scale bar = 10 µm. Abbreviations: see Fig. 1.
In S. dalatensis, a median septum divides the nectary spur longitudinally into two loculi or lumina (Figs 13B, C and 14A). A small, proximal protuberance is present in the loculus (Fig. 13B). The epidermis lining the lumen is glabrous and covered with a smooth cuticle. Where rows of adjacent epidermal cells run parallel to each other, the cuticle often becomes distended, and this coincides with the position of longitudinal walls, thus emphasizing cell shape (Fig. 13D, E). Furthermore, nectar residues may be present on the surface of epidermal cells (Fig. 13E).
The wall of the spur is multilayered and relatively thick (Fig. 14A; Table 1). The spur is supplied by six, collateral vascular bundles, with equal development of xylem and phloem (Fig. 14B). Epidermal cells have a thick outer wall that is heavily cuticularized (Fig. 14C–G), but the cuticle does not fluoresce with auramine O (Fig. 14F). These cells contain parietal cytoplasm with mitochondria, vesicles, free ribosomes and ER profiles, but dictyosomes are seldom encountered. Large nuclei and amyloplasts are frequently present (Fig. 14C–E), and chromoplasts occur in the underlying parenchyma. TEM observations confirmed the presence of cutinized, reticulate layers in the outer, tangential cell wall of epidermal cells, and that the distended cuticle lacks pores and cracks (Fig. 14G).
Fig. 14.
Histology and ultrastructure of spur of Stereochilus dalatensis; (A–F) light micrographs; (G–I) transmission electron micrographs. (A) Section through bilocular nectary spur showing lumina lined with glabrous epidermis and vascular bundles embedded in parenchyma. Scale bar = 300 µm. (B) Detail of vascular bundle with phloem and xylem in equal proportions. Scale bar = 30 µm. (C–E) Epidermal cells with thick, outer walls and distended cuticle (arrows), large nuclei and plastids containing starch grains. Subepidermal cells are thin-walled. Scale bars = 20 µm. (F) Cuticle covering secretory epidermis following treatment with auramine O, but lacking fluorescence. Idioblast with raphides marked with asterisk. Scale bar = 25 µm. (G). Parietal cytoplasm of secretory epidermal cell, showing outer cell wall with distended cuticle. Scale bar = 2 µm. (H) Granular cytoplasm of secretory epidermal cell containing mitochondria, ER and plastids. Scale bar = 1 µm. (I) Detail of cytoplasm with ER and mitochondria. Scale bar = 1 µm. Abbreviations: see Fig. 1.
A ‘Saftdecke’ or nectar cover to the spur appears to be present in Ascocentrum garayi, Schoenorchis gemmata and Stereochilus dalatensis (Figs 5C, 9B and 13B).
DISCUSSION
Nectary spurs of the species investigated differ greatly in shape and length, from very short, saccate spurs in Stereochilus and Schoenorchis, moderately long spurs in Ascocentrum and Sedirea, to long spurs in Papilionanthe. Furthermore, spur morphology is considered taxonomically significant (Kocyan et al., 2008), with spur length being correlated with that of the mouth-parts of the pollinator. Remarkably, the presence of a spur, even in deceptive species lacking a reward, can be sufficient to attract pollinators (Bell et al., 2009).
Species of Ascocentrum possess weakly zygomorphic, red or orange flowers with cryptic anther caps. Their flowers lack nectar guides and fragrance. These features are characteristic of ornithophilous species (van der Pijl and Dodson, 1969; Proctor and Yeo, 1973; Proctor et al., 1996; van der Cingel, 2001; Ortega-Olivencia et al., 2005; Cronk and Ojeda, 2008; Davies and Stpiczyńska, 2008, and references therein; Stpiczyńska et al., 2009). Cryptic anther caps may also facilitate pollination. The presence of anther caps and pollinaria on beaks of birds usually evokes a bill-cleaning response and thus pollinia are often lost or destroyed. However, many ornithophilous species (some 50 % of hummingbird-pollinated taxa) have blue, grey, brown, cream or greyish white, cryptic anther caps, and these are thought to illicit a lesser response than their more conspicuous, yellow counterparts (Dressler, 1971; Topik et al., 2006). As a result, transfer of pollinaria to the stigma is more likely. Moreover, the nectary of all Ascocentrum species studied has a collenchymatous subepidermis, similar to that found in other ornithophilous species (Stpiczyńska and Davies, 2006; Stpiczyńska et al., 2004, 2005, 2009). Thus, morphologically, anatomically and in terms of colour [which in A. ampullaceum var. aurantiacum and A. curvifolium resembles the floral colour combinations of presumed hummingbird-pollinated Ornithidium coccineum (Jacq.) Salisb. ex R. Br., O. sophronitis Rchb.f. and Hexisea imbricata (Lindl.) Rchb.f. (Stpiczyńska et al., 2004, 2005, 2009)], all the Ascocentrum species studied are thought to be ornithophilous. Unfortunately, direct observations of bird pollination are rare. Nevertheless, Slade (1980) recorded an unidentified honeyeater pollinating flowers of the hybrid Ascocentrum ‘Sagarik Gold’ growing in his garden in Vanuatu (New Hebrides), and Whitten et al. (2007) assert that certain brightly coloured species of Ornithidium (Salisb.) ex R. Br. are indeed bird-pollinated.
Conversely, zygomorphy was more pronounced in the flowers of all other species investigated. They were all fragrant, with a proportionally more expanded mid-lobe to the labellum than has Ascocentrum. They lacked red pigmentation, cryptic anther caps and subepidermal collenchyma, and this is consistent with characteristics of entomophilous species (van der Pijl and Dodson, 1969; van der Cingel, 2001; Davies and Stpiczyńska, 2008). Their nectary spurs showed considerable variation in length. Topik et al. (2005) reported that short-spurred species of Aeridinae are mainly pollinated by bees and beetles, whereas those species having long spurs are generally pollinated by moths. Petaloid spurs are also present in other plant groups such as Aquilegia L. and Impatiens L., and it has been shown that intra- and inter-specific variation in the spur length of Aquilegia is an adaptation for pollination by a variety of different pollinators (Hodges, 1997; Kramer and Hodges, 2010).
Consequently, it is speculated that the species of Stereochilus, Schoenorchis and Sedirea investigated here, all of which are brightly coloured in shades of purple, pink and white, and all of which produce nectar and fragrance by day, are mainly pollinated by hymenoptera. Flowers of Papilionanthe vandarum, however, are mainly white, with relatively long spurs, indicating that this species is probably pollinated by moths (van der Pijl and Dodson, 1969; van der Cingel, 2001).
A ‘Saftdecke’ or nectar cover, often formed by the lateral compression of the spur (Sprengel, 1793 – cited in Kocyan et al., 2008), appears to be present in certain species, including both ornithophilous (e.g. Ascocentrum garayi) and entomophilous (e.g. Schoenorchis gemmata and Stereochilus dalatensis) taxa. Once thought by Sprengel to protect against raindrops, these nectar covers have since been interpreted by others as protection against nectar thieves or evaporation.
Of the species investigated, only Stereochilus dalatensis has a bilocular nectary spur, the loculi or lumina being separated by a median, longitudinal septum whose function remains unknown. In all other cases, the spur was unilocular. The inner surface of the nectary spurs of Schoenorchis gemmata, Stereochilus dalatensis and Sedirea japonica is glabrous to minutely papillose. Spurs possessing a glabrous inner epidermis have also been recorded for other bee-pollinated species, such as Aerides crassifolia C.S.P. Parish ex Burb. (Kocyan et al., 2008). Moreover, S. dalatensis has a protuberance within the lumen, similar to that described for certain species of Aerides (Chen and Wood, 2009). By contrast, the internal surface of the nectary spur of Ascocentrum species and Papilionanthe vandarum is pubescent to hirsute. These hairs are thought to increase the total surface area for nectar secretion and re-absorption (Davies and Stpiczyńska, 2008, and references therein). That trichomes can occur within the spurs of certain rewardless species, such as Dactylorhiza fuchsii (Druce) Soó and Barlia robertiana (Loisel.) Greuter (Matthews et al., 2009; M. L. Matthews, Institute of Systematic Botany, University of Zürich, Switzerland, pers. comm., 2010), is thus of great interest. According to Bell et al. (2009), in deceptive species, such trichomes or papillae can provide tactile cues for pollinators. As in many orchids (Stpiczyńska, 1997, 2003; Stpiczyńska and Matusiewicz, 2001; Davies and Stpiczyńska, 2008; Bell et al., 2009; Matthews et al., 2009), the nectary trichomes of Ascocentrum species are unicellular and conical, whereas those of Papilionanthe are clavate and occasionally bicellular. Bicellular or multicellular trichomes also occur in the spurs of several species of Orchidinae (Matthews et al., 2009; M. L. Matthews, Institute of Systematic Botany, University of Zürich, Switzerland, pers. comm., 2010).
In Ascocentrum species, hairs are distributed as a wide band around the central region of the nectary spur. It is possible that their position deep within the spur prevents them from being destroyed during nectar probing. The arrangement of secretory hairs also appears to be correlated with that of the vascular bundles, the longer hairs usually occurring closer to the latter. The spurs of nearly all Aeridinae species studied here are supplied by two large and several smaller vascular strands. As a result, the distribution pattern, and thus density, of the hairs is relatively uniform. The only exception was Papilionanthe vandarum, where hairs occur mainly as a longitudinal band along the main vascular bundle that runs the length of the adaxial wall of the spur. This is similar to the arrangement found in the moth-pollinated Angraecum germinyanum Hook.f. (Davies and Stpiczyńska, 2008).
Generally, nectar sugars are transported to the nectary, as pre-nectar, via the phloem. From here, they pass to the secretory cells, along either the symplast or the apoplast. In those members of the Aeridinae investigated, both forms of transport can coexist, as the cells are interconnected by numerous plasmodesmata, and the relatively thick cellulosic cell walls, when tested histochemically, showed no evidence of barriers to apoplastic transport. Such an arrangement has been recorded for a number of other taxa (Fahn, 2000; Stpiczyńska et al., 2004; Nepi, 2007).
Anatomically, nectaries (nectary spurs) of Asiatic Ascocentrum species closely resemble the morphologically dissimilar nectaries of Neotropical species of Maxillariinae (Ornithidium coccineum, O. sophronitis), Laeliinae (Hexisea imbricata) and Oncidiinae [Symphyglossum sanguineum (Rchb.f.) Schltr.], especially in their possession of collenchyma (Stpiczyńska et al., 2004, 2005, 2009; Stpiczyńska and Davies, 2006). This tissue may not only function in protecting the delicate nectary tissue from the beaks of pollinating birds, but probably, simultaneously, provides an apoplastic route for nectar movement within the nectary.
Amyloplasts were absent from the nectary cells of many species, such as A. garayi. This is in contrast to the results obtained for most nectariferous species studied to date, where plastids differentiate to form amyloplasts and become implicated in nectar production. Typically, starch grains are abundant in amyloplasts at the presecretory stage, but as nectary secretory activity progresses, they disappear and plastids develop irregular profiles (Nepi, 2007). Starchless nectary plastids have also been observed in Gymnadenia conopsea (L.) R. Br. (Stpiczyńska and Matusiewicz, 2001), O. coccineum and O. sophronitis (Stpiczyńska et al., 2004, 2009) and here sugars present in the nectar are probably delivered in the phloem. The phloem component of vascular bundles supplying the nectary spur was particularly well developed for those species investigated here. This agrees with a number of previous studies which showed that a relationship exists between phloem supply and nectar carbohydrate production (Nepi, 2007, and references therein). Although plastids within the secretory epidermal cells of those Aeridinae species studied here rarely contain starch, they frequently contain osmophilic material. This was particularly evident in P. vandarum, where such plastids might be involved in the synthesis of secondary metabolites. The nectary cells of all species investigated, regardless of pollinator, have numerous mitochondria, ER profiles, dictyosomes and small vesicles: cellular characters concomitant with granulocrine secretion (Nepi, 2007, and references therein).
The relatively thick cuticle overlying the secretory layer of all Aeridinae species studied lacks pores and cracks, and is probably permeable to nectar. In all species, it stained with Sudan III, indicating the presence of lipids. However, the staining reaction with auramine O was more variable. For example, in hairless species, such as Schoenorchis gemmata and Stereochilus dalatensis, the cuticle did not stain at all with auramine O, whereas that of Sedirea japonica only stained slightly when compared with the staining reaction of Sudan III. The cuticle covering the secretory hairs also stained only slightly or, as in P. vandarum, did not stain at all with auramine O, whereas that overlying flat epidermal cells showed a greater uptake of stain. These results probably indicate that variation in the chemical composition of the cuticle results in localized differences in permeability to nectar, as has been recorded for Platanthera bifolia (L.) Rich. and P. chlorantha Custer ex Rchb. (Stpiczyńska, 1997, 2003). In Schoenorchis gemmata and Stereochilus dalatensis, however, nectar secretion is accompanied by distension of the cuticle. Although cuticular distension was not observed for other taxa, it is known to occur in certain ornithophilous Maxillariinae and Laeliinae (Davies and Stpiczyńska, 2008, and references therein; Stpiczyńska et al., 2009). On the basis of the results presented here, it would appear that distension of the cuticle is not confined to either ornithophilous or entomophilous species. Differences in the structure and thickness of the nectary cuticle were also observed for Aeridinae. For example, whereas the epidermal cuticle of Ascocentrum garayi is lamellate, that of Schoenorchis gemmata and Stereochilus dalatensis has a delicate, reticulate layer. Furthermore, fine cuticular striations, although present in nectariferous Sedirea japonica and Papilionanthe vandarum, are absent from Ascocentrum garayi, A. ampullaceum var. aurantiacum, A. curvifolium, Schoenorchis gemmata and Stereochilus dalatensis. Bell et al. (2009), however, observed cuticular striations on epidermal cells lining the spurs of nectarless Orchidinae. Therefore, whether a given species is nectariferous or rewardless is not necessarily related to the presence, or otherwise, of cuticular striations.
Despite the small number of Aeridinae species investigated here, it would appear that the nectary spur of this subtribe varies considerably in its structure. Some show modifications characteristic of ornithophilous species, whereas others display characteristics of insect-pollinated taxa. The occurrence of identical nectary trichomes and similar spur vasculature in both ornithophilous and entomophilous species of Aeridinae indicates that these structures evolved independently of pollination syndrome. Comparison of the data presented here with previous results (Stpiczyńska and Davies, 2006; Davies and Stpiczyńska, 2008; Stpiczyńska et al., 2004, 2005, 2009) shows that certain, ornithophilous, Asiatic Aeridinae have a number of features in common with Neotropical, hummingbird-pollinated species assigned to subtribes Maxillariinae, Laeliinae and Oncidiinae, most notably the presence of a protective, subepidermal collenchyma. The occurrence of similar anatomical organization in orchid taxa found on other continents and assigned to other subtribes is indicative of convergence and thus appears to be related more to pollinator-driven selection than to phylogeny.
Given the enormity of Aeridinae and the relatively few species presented here, it is important that ultrastructural work now be extended to include other taxa selected according to their phylogenetic position, so as to improve upon our current knowledge and understanding of nectary diversity in this subtribe.
ACKNOWLEDGEMENTS
We are grateful to Alan Gregg (Swansea Botanical Complex, Swansea, UK) for supplying some of the flowers that form the subject of this paper and for helping to prepare the manuscript, as well as to Dr Michał Rudaś, inż. Marek Wróbel (CLA University of Life Sciences in Lublin, Poland) and Mgr Julita Nowakowska (Laboratory of Electron Microscopy, University of Warsaw, Poland) for use of TEM and SEM facilities.
LITERATURE CITED
- Ackerman JD. Pollination of tropical and temperate orchids. In: Tan KW, editor. Proceedings of the Eleventh World Orchid Conference. Miami, FL: American Orchid Society; 1984. pp. 98–101. [Google Scholar]
- Bell AK, Roberts DL, Hawkins JA, Rudall PJ, Box MS, Bateman R. Comparative micromorphology of nectariferous and nectarless labellar spurs in selected clades of subtribe Orchidinae (Orchidaceae) Botanical Journal of the Linnean Society. 2009;160:369–387. [Google Scholar]
- Brummitt RK, Powell CE. Authors of plant names. Kew: Royal Botanical Gardens; 1992. [Google Scholar]
- Cameron KM, Chase MW, Whitten WM, et al. A phylogenetic analysis of Orchidaceae: evidence from RBCL nucleotide sequences. American Journal of Botany. 1999;86:208–224. [PubMed] [Google Scholar]
- Carr CE. Orchid pollination notes. Journal of the Malayan Branch of the Royal Asiatic Society. 1928;6:49–72. [Google Scholar]
- Chase MW, Barrett RL, Cameron KM, Freudenstein JV. DNA data and Orchidaceae systematics: a new phylogenetic classification. In: Dixon KM, Kell SP, Barrett RL, Cribb PJ, editors. Orchid conservation. Sabah, Malaysia: Natural History Publications; 2003. pp. 69–89. Kota Kinabalu. [Google Scholar]
- Chen X, Wood J. Stereochilus Lindley. In: Wu ZY, Raven PH, Hong DY, editors. Flora of China; Orchidaceae. Vol. 25. Beijing: Science Press/ St. Louis: Missouri Botanical Garden Press; 2009. pp. 463–464. [Google Scholar]
- Cronk Q, Ojeda I. Bird-pollinated flowers in a evolutionary and molecular context. Journal of Experimental Botany (Flowering Newsletter) 2008;59:715–727. doi: 10.1093/jxb/ern009. [DOI] [PubMed] [Google Scholar]
- Davies KL, Stpiczyńska M. The anatomical basis of floral, food-reward production in Orchidaceae. In: Texeira da Silva JA, editor. Floriculture, ornamental and plant biotechnology. London: Global Science Books; 2008. pp. 392–407. [Google Scholar]
- Dressler RL. Dark pollinaria in hummingbird-pollinated orchids or do hummingbirds suffer from strabismus? American Naturalist. 1971;105:80–83. [Google Scholar]
- Dressler RL. The orchids – natural history and classification. London: Harvard University Press; 1990. [Google Scholar]
- Dressler RL. Phylogeny and classification of the orchid family. Cambridge: Cambridge University Press; 1993. [Google Scholar]
- Fahn A. Structure and function of secretory cells. Advances in Botanical Research. 2000;31:37–75. [Google Scholar]
- Garay LA. On the systematics of the monopodial orchids. I. Botanical Museum Leaflets Harvard University. 1972;23:149–212. [Google Scholar]
- Hodges SA. Floral nectar spurs and diversification. International Journal of Plant Species. 1997;158:81–88. (Suppl. 6) [Google Scholar]
- Jensen WA. Botanical histochemistry: principle and practice. San Francisco: W.H. Freeman; 1962. [Google Scholar]
- Kocyan A, de Vogel EF, Conti E, Gravendeel B. Molecular phylogeny of Aerides (Orchidaceae) based on one nuclear and two plastid markers: a step forward in understanding the evolution of the Aeridinae. Molecular Phylogenetics and Evolution. 2008;48:422–443. doi: 10.1016/j.ympev.2008.02.017. [DOI] [PubMed] [Google Scholar]
- Kramer EM, Hodges SA. Aquilegia as a model system for the evolution and ecology of petals. Philosophical Transactions of the Royal Society B. 2010;365:477–490. doi: 10.1098/rstb.2009.0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews ML, Cozzolino S, Schiestl F. Nectary and spur structure and evolution in rewarding and deceit-pollinated Orchidinae (Orchidaceae) Conference Proceedings Charles Darwin's 200th Birthday 150 years “Origin of species”. 2009 University of Bern and Natural History Museum, 12–13 February 2009. Poster no. 45. http://www.botanica-helvetica.ch/d/Aktivitaeten_Agenda/biology08/documents/biology_09_posters.pdf. [Google Scholar]
- Nepi M. Nectary structure and ultrastructure. In: Nicolson SW, Nepi M, Pacini E, editors. Nectaries and nectar. Dordrecht: Springer; 2007. pp. 129–166. [Google Scholar]
- Ortega-Olivencia A, Rodriguez-Riano T, Valtuena FJ, Lopez J, Devesa JA. First confirmation of a native bird-pollinated plant in Europe. Oikos. 2005;110:578–590. [Google Scholar]
- Proctor M, Yeo P. The pollination of flowers. London: Collins; 1973. [Google Scholar]
- Proctor M, Yeo P, Lack A. The natural history of pollination. London: Harper Collins; 1996. [Google Scholar]
- Reynolds ES. The use of lead citrate at high pH as an electron-opaque stain for electron microscopy. Journal of Cell Biology. 1963;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slade GH. Dendrobium mohlianum – a case of pollination by birds. American Orchid Society Bulletin. 1980;49:870–871. [Google Scholar]
- Stpiczyńska M. The structure of the nectary of Platanthera bifolia L. (Orchidaceae) Acta Societatis Botanicorum Poloniae. 1997;66:5–11. [Google Scholar]
- Stpiczyńska M. Nectar resorption in the spur of Platanthera chlorantha (Custer) Rchb. – structural and microautoradiographical studies. Plant Systematics and Evolution. 2003;238:119–126. [Google Scholar]
- Stpiczyńska M, Davies KL. Nectary structure in Symphyglossum sanguineum (Rchb.f.) Schltr. (Orchidaceae) Acta Agrobotanica. 2006;59:7–16. [Google Scholar]
- Stpiczyńska M, Matusiewicz J. Anatomy and ultrastructure of the spur nectary of Gymnadenia conopsea L. (Orchidaceae) Acta Societatis Botanicorum Poloniae. 2001;70:267–272. [Google Scholar]
- Stpiczyńska M, Davies KL, Gregg A. Nectary structure and nectar secretion in Maxillaria coccinea (Jacq.) L.O. Williams ex Hodge (Orchidaceae) Annals of Botany. 2004;93:87–95. doi: 10.1093/aob/mch008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stpiczyńska M, Davies KL, Gregg A. Comparative account of nectary structure in Hexisea imbricata (Lindl.) Rchb. f. (Orchidaceae) Annals of Botany. 2005;95:749–756. doi: 10.1093/aob/mci081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stpiczyńska M, Davies KL, Gregg A. Nectary structure of Ornithidium sophronitis Rchb. f. (Orchidaceae: Maxillariinae) Acta Agrobotanica. 2009;62:3–12. [Google Scholar]
- Topik H, Yukawa T, Ito M. Molecular phylogenetics of subtribe Aeridinae (Orchidaceae): insights from plastid matK and nuclear ribosomal ITS sequences. Journal of Plant Research. 2005;118:271–284. doi: 10.1007/s10265-005-0217-3. [DOI] [PubMed] [Google Scholar]
- Topik H, Yukawa T, Ito M. Evolutionary analysis of pollinaria morphology of subtribe Aeridinae (Orchidaceae) Reinwardtia. 2006;12:223–235. [Google Scholar]
- van der Cingel NA. An atlas of orchid pollination – America, Africa, Asia and Australia. 2001 Rotterdam: A.A. Balkema. [Google Scholar]
- van der Pijl L, Dodson CH. Orchid flowers: their pollination and evolution. Coral Gables, FL: University of Miami Press; 1969. [Google Scholar]
- Whitten WM, Blanco MA, Williams NH, et al. Molecular phylogenetics of Maxillaria and related genera (Orchidaceae: Cymbidieae) based on combined molecular data sets. American Journal of Botany. 2007;94:1860–1889. doi: 10.3732/ajb.94.11.1860. [DOI] [PubMed] [Google Scholar]