Abstract
Background and Aims
Aluminium (Al) toxicity is one of the factors limiting crop production on acid soils. However, genotypic differences exist among plant species or cultivars in response to Al toxicity. This study aims to investigate genotypic differences among eight cultivars of tatary buckwheat (Fagopyrum tataricum) for Al resistance and explore the possible mechanisms of Al resistance.
Methods
Al resistance was evaluated based on relative root elongation (root elongation with Al/root elongation without Al). Root apex Al content, pectin content and exudation of root organic acids were determined and compared.
Key Results
Genotypic differences among the eight cultivars were correlated with exclusion of Al from the root apex. However, there was a lack of correlation between Al exclusion and Al-induced oxalate secretion. Interestingly, cell-wall pectin content of the root apex was generally lower in Al-resistant cultivars than in Al-sensitive cultivars. Although we were unable to establish a significant correlation between Al exclusion and pectin content among the eight cultivars, a strong correlation could be established among six cultivars, in which the pectin content in the most Al-resistant cultivar ‘Chuan’ was significantly lower than that in the most Al-sensitive cultivar ‘Liuku2’. Furthermore, root apex cell-wall pectin methylesterase activity (PME) was similar in ‘Chuan’ and ‘Liuku2’ in the absence of Al, but Al treatment resulted in increased PME activity in ‘Liuku2’ compared with ‘Chuan’. Immunolocalization of pectins also showed that the two cultivars had similar amounts of either low-methyl-ester pectins or high-methyl-ester pectins in the absence of Al, but Al treatment resulted in a more significant increase of low-methyl-ester pectins and decrease of high-methyl-ester pectins in ‘Liuku2’.
Conclusions
Cell-wall pectin content may contribute, at least in part, to differential Al resistance among tatary buckwheat cultivars.
Keywords: Aluminium resistance, cell wall, exclusion mechanism, Fagopyrum tataricum, pectin, pectin methylesterase, oxalate, toxicity
INTRODUCTION
Aluminium (Al) toxicity is a major factor limiting crop production on acid soils worldwide (von Uexküll and Mutert, 1995). Although liming is effective for the amelioration of Al toxicity, the cost of lime application precludes it as an economic strategy in areas where a large proportion of farmers are poor. Furthermore, it is also ineffective in correcting subsoil acidity. However, among plant species or cultivars within the same species there is genetic variation in response to Al toxicity, which provides an alternative solution to improve crop productivity on acid soils by selecting and breeding Al-resistant cultivars. Therefore, an understanding of the genetic and molecular mechanisms underlying Al resistance is essential to speed up the development of new Al-resistant cultivars. Identification of the major physiological mechanisms involved will also be valuable for the characterization of the major Al resistance genes. For example, only modest success has been made based on over-expression of genes associated with antioxidants (Ezaki et al., 2000), because oxidative stress is not a primary cause of Al-induced root growth inhibition in plants (Yamamoto et al., 2003).
Two strategies for the detoxification of Al by plants have been proposed (Taylor, 1991; Kochian, 1995): the exclusion of Al from the root apex (exclusion mechanism) and intracellular tolerance by sequestration of Al in the symplasm (internal tolerance mechanism). The latter is usually employed by Al-accumulating species such as Hydrangea macrophylla (Ma et al., 1997a) and buckwheat (Ma et al., 1997b). In most cases, exclusion of Al from the root apex via exudation of root organic acids is the most important mechanism of Al resistance (Kochian et al., 2004; Delhaize et al., 2007). However, Al resistance in some plant species cannot be explained solely by exudation of root organic acids. For example, in addition to Al-induced malate efflux from the root tip, constitutive phosphate exudation has also beenj related to Al resistance in the Al-resistant wheat cultivar ‘Atlas’ (Pellet et al., 1996). Also, efflux of organic acids is not responsible for Al resistance in signalgrass (Wenzl et al., 2001), maize (Piñeros et al., 2005) or buckwheat (Zheng et al., 2005). Therefore, it is likely that multiple mechanisms of resistance to Al are functioning in these plant species. Recently, a novel Al exclusion mechanism which relies on the exclusion of Al by root apex cell-wall pectin was proposed in rice, as rice does not show Al-induced secretion of organic acids (Yang et al., 2008). However, evidence supporting cell-wall pectin, in particular, as an important Al exclusion mechanism was weak as only two contrasting genotypes were compared in the above study.
Tatary buckwheat (Fagopyrum tataricum) is an important economic crop in Asia. It is a member of the Polygonaceae and is related to common buckwheat (F. esculentum Moench). Although genotypic differences in Al responses (Yang et al., 2005; Zheng et al., 2005) and Al resistance mechanisms have been recognized in common buckwheat (Klug and Horst, 2010), no information is available for tatary buckwheat. This study therefore focused on screening of genotypic differences among eight tatary buckwheat cultivars, and investigating whether and how the Al exclusion mechanism is responsible for their different Al resistance. We demonstrate that cell-wall pectin contributes to the differential Al resistance in tatary buckwheat.
MATERIALS AND METHODS
Plant materials and growth condition
Eight tatary buckwheat (Fagopyrum tataricum) cultivars collected from four provinces of southern China, where acid soils predominate, were used (for list of cultivar names see figures). Seeds were surface-sterilized for 20 min in a 1 % (v/v) sodium hypochlorite solution, washed three times with de-ionized water and soaked in de-ionized water overnight. They were then transferred to an incubator at 25 °C for germination. The germinated seeds were transferred to a net tray floated on a container filled with 5 L of 0·5 mm CaCl2 solution at pH 4·5. The solution was renewed daily. After 3 d of culture, the seedlings were placed in a compartmental hydroponic system for various treatments according to Yang et al. (2005). All the experiments were conducted in a growth chamber with a 14-h/26 °C day and a 10-h/23 °C night regime, a light intensity of 300 µmol m−2 s−1 and a relative humidity of 60 %.
Evaluation of Al resistance
Al resistance was examined by measuring root elongation of primary roots of 3-d-old seedlings grown in 0·5 mm CaCl2 solution, pH 4·5, containing 0 or 25 µm AlCl3. Root length was measured with a ruler before and after treatments (24 h). Relative root elongation was defined as the percentage root elongation of the Al treatment to that of the Al-free control. We used a simple salt solution (0·5 mm CaCl2) that has been widely used for screening of genotypic Al resistance in many species, such as rice (Ma et al., 2005), wheat (Yang et al., 2005) and buckwheat (Zheng et al., 1998). Boron (B) is essential for cell-wall development, but because only 3-day-old seedlings were tested in our experiments, B contained in the seeds was sufficient to sustain root growth, and addition of 5 µm B to 0·5 mk CaCl2 solution also had no effect on root growth of F. tataricum (data not shown).
Al content in root apex
For determination of total Al of the root apices, the excised root apices (apical 10-mm root segments; ten apices per sample) were placed in a 1·5-mL Eppendorf tube containing 1 mL of 2 m HCl. The tubes were stood for at least 24 h with occasional shaking. Al concentrations in the extracts were determined by inductively coupled plasma atomic emission spectrometry (ICP-AES; Thermo Jarrel Ash, San Jose, CA, USA).
Al in roots and shoots
Al content in roots and shoots was determined by digesting the roots and shoots, which were harvested after treating the seedlings with 25 µm Al for 24 h and dried in an oven for 2 d at 70 °C, in an HNO3/HClO4 mixture (4 : 1, v/v). The Al concentration was determined by ICP-AES after appropriate dilution.
Collection of root exudes and analysis of organic acids
For analysis of root exudates, about 200 germinated seeds were transferred to the net tray as described above. After 3 d of culture, the net tray was transferred to a container filled with 5 L of 0·5 mm CaCl2 solution (pH 4·5) for 3 h, and the solution containing root exudates was collected. The net tray was then transferred to another container filled with 5 L of 0·5 mm CaCl2 solution (pH 4·5) containing 25 µm Al for another 3 h, and the solution containing root exudates was also collected and the number of seedlings was counted. As 3-d-old seedlings of tatary buckwheat have only one seminal root, the number of root apices is equal to the number of seedlings. The collected solution was set to pass first through a cation exchange column (16 mm × 14 cm) filled with 5 g Amerlite IR-120B resin (H+ form; Muromachi Chemical, Tokyo, Japan) and then through an anion exchange column filled with 1·5 g Dowex 1 × 8 resin (100–200 mesh, formate form). The organic acid anions retained in the anion exchange resin were eluted with 15 mL of 1 m HCl, and the eluent was concentrated to dryness using a rotary evaporator at 40 °C. The residue was re-dissolved in 1 mL of 30 mm NaOH and filtered (0·2 µm) before analysis. The organic acid anions were detected by ion chromatography (ICS 3000; Dionex, Beijing, China) equipped with an IonPac AS11 anion-exchange analytical column (4 × 250 mm) and a guard column (4 × 50 mm). The mobile phase was 30 mm NaOH at a flow rate of 0·6 mL min−1.
Cell-wall extraction and measurement of pectin content
Root apices (0–10 mm) were excised and collected in 96 % ethanol in Eppendorf reaction vials (four apices for each sample). Root samples were thoroughly homogenized in ethanol using a plastic grinder for 3 min. Homogenization was repeated twice. Cell-wall material was prepared as the alcohol-insoluble residue after repeated washing with ethanol. After every ethanol addition, the sample was centrifuged at 23 000g for 10 min and the supernatant was discarded. The remaining cell-wall material was dried at 60 °C, and hydrolysed according to Ahmed and Labavitch (1977) except that the incubation time was extended to 10 min in concentrated H2SO4 and 2 h after each water addition step. The uronic acid content was determined colorimetrically according to Blumenkrantz and Asboe-Hansen (1973). Galacturonic acid was used as a calibration standard, and thus the root pectin content was expressed as galacturonic acid equivalents (GaE).
PME activity assay
For extraction of pectin methylesterase (PME), cell-wall materials were suspended in an extraction buffer containing 10 mm Tris buffer (pH 7·7) and 1 m NaCl at 4 °C for 1 h with repeated vortexing (20 s for 20 min each). Extracts were centrifuged (14 000g, 10 min) and the supernatant was collected. The protein was desalted using Microcon YM-10 centrifugal filter units (Millipore, Billerica, MA, USA) into 10 mm Tris buffer (pH 7·7). PME activity was determined according to Anthon and Barrett (2004).
Immunofluorescence
Three-day-old seedlings were placed in a compartmental hydroponic system (Yang et al., 2005) containing 0·5 mm CaCl2 with or without 25 µm AlCl3. After 24 h of treatment, fresh roots were hand-sectioned from the root zone 1–3 mm behind the apex and localization of pectin via immunofluorescence was conducted as previously described (Yang et al., 2008).
Statistical analysis
Experiments were arranged in a randomized design and data were statistically evaluated by Duncan's multiple range test.
RESULTS
As relative root elongation (RRE) has been shown to be a suitable phenotypic criterion to assess Al resistance in a wide range of plant species, we used this parameter to screen tatary buckwheat cultivars for Al resistance (Fig. 1). As expected, there were genotypic differences in Al resistance among these cultivars. For instance, root elongation of ‘Chuan’ was inhibited by 36 % after 24 h exposure to 25 µm AlCl3, whereas the same treatment resulted in 57 % inhibition of root elongation in ‘Liuku2’ (Fig. 1). We thus termed ‘Chuan’ as an Al-resistant cultivar and ‘Liuku2’ as an Al-sensitive cultivar.
Fig. 1.
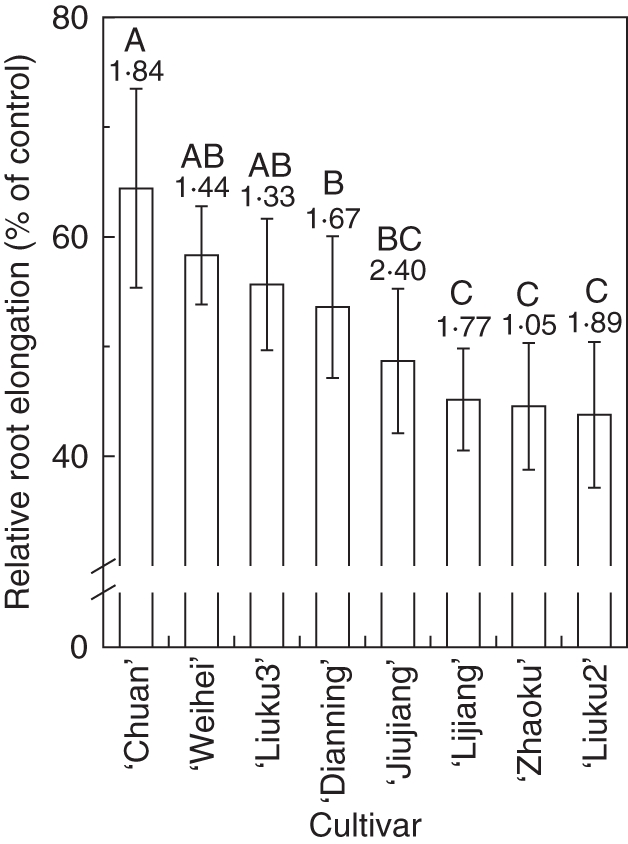
Screening of eight tatary buckwheat cultivars for Al resistance. Three-day-old seedlings were subjected to a 0·5 mm CaCl2 solution (pH 4·5) containing 0 or 25 µm AlCl3 for 24 h. Root growth was measured by a ruler before and after treatment. Relative root elongation was the percentage of root elongation in Al-free solution to that in Al-containing solution. Absolute control growth rates [cm (24 h)−1] are indicated above the columns. Error bars represent ± s.d. (n = 10). Means with different letters are statistically different at P < 0·05. Three replicate experiments were conducted and data from one set are presented.
Al content in the root apex was measured to test whether the mechanism of Al exclusion accounted for the genotypic differences among these tatary buckwheat cultivars. There was a large difference in Al content (mean ± s.d.) in the root apices, ranging from 0·046 ± 0·002 µg per root apex in ‘Weihei’ to 0·133 ± 0·0098 µg per root apex in the most Al-sensitive ‘Liuku2’ after exposure to 25 µm Al for 24 h (Fig. 2A). Al content in the root apex of the most Al-resistant ‘Chuan’ was also lower (0·052 ± 0·0079 µg per root apex; Fig. 2A). The Al content of the root apex correlated negatively with RRE (Fig. 2B), suggesting an Al exclusion mechanism is contributing to the differential Al resistance in tatary buckwheat.
Fig. 2.
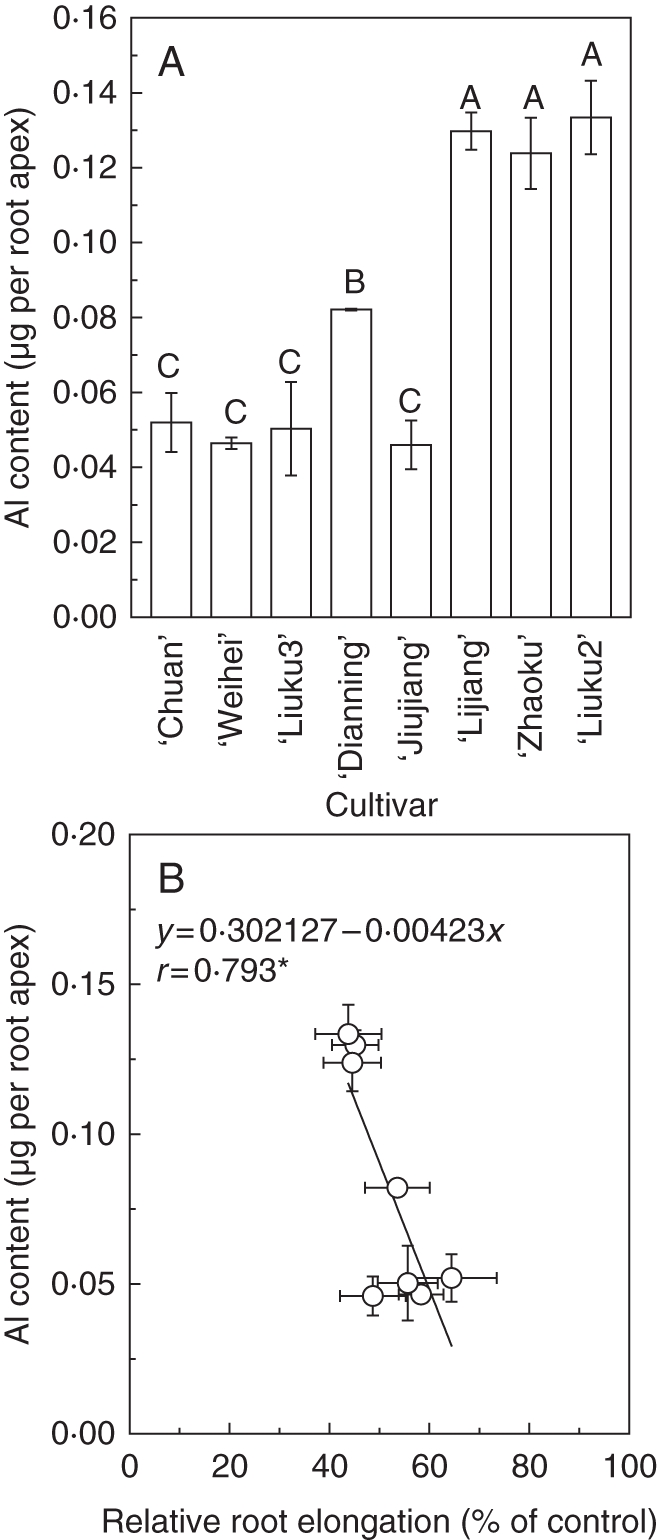
(A) Al content in root apex (B) and correlation between relative root elongation and root apex Al content among eight tatary buckwheat cultivars. *P < 0·05. Root apices (apical 10 mm) were cut with a razor blade after 24 h of treatment with 0·5 mm CaCl2 solution (pH 4·5) containing 25 µm AlCl3. Ten root apices were combined to one sample. Error bars for Al content represent s.d. of three replicates. Means with different letters are statistically different at P < 0·05.
To determine whether the differences in Al exclusion in the root apex are due to the Al-induced secretion of organic acids, we analysed organic acids in the root exudates. Without Al treatment, a trace amount (in some cases below the lowest detection limit) of malate and citrate was detected, and oxalate was the most abundant organic acid (data not shown). Moreover, oxalate was the only organic acid whose response was dependent on the presence of Al. Al-activated oxalate exudation rates ranged from 33·4 ± 14·6 ng per root apex h−1 in ‘Lijiang’ to 98·3 ± 1·5 ng per root apex h−1 in ‘Weihei’. The Al-activated oxalate exudation rates for ‘Chuan’ and ‘Liuku2’ were 56·1 ± 2·8 and 48·8 ± 7·1 ng per root apex h−1, respectively (Fig. 3). Overall, we could not establish a relationship between root Al-activated oxalate exudation and root apex Al content (Fig. 4).
Fig. 3.
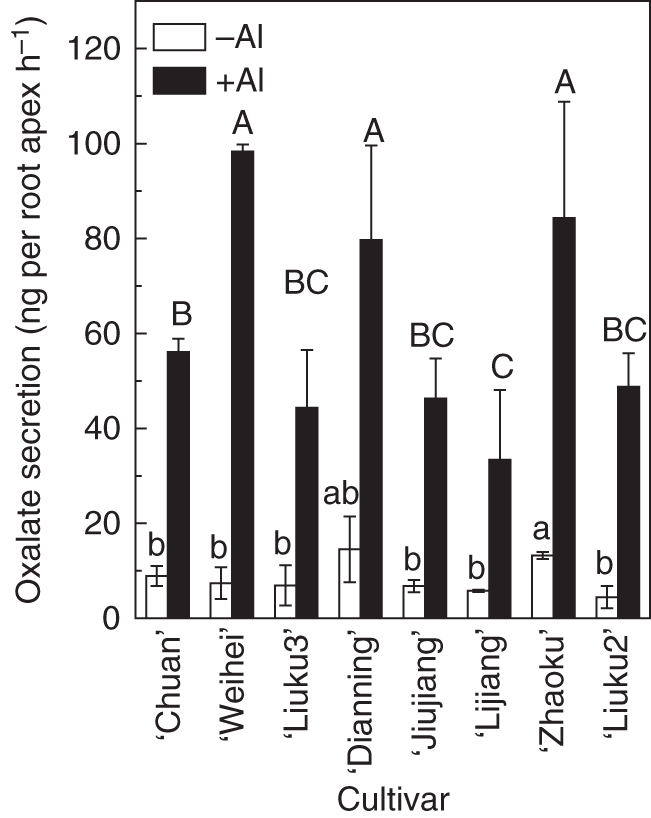
Al-induced secretion of oxalate in eight tatary buckwheat cultivars. Three-day-old seedlings were exposed to 0·5 mm CaCl2 solution (pH 4·5) for 3 h, and root exudates were collected. Seedlings were then exposed to 0·5 mm CaCl2 solution (pH 4·5) containing 25 µm Al for 3 h, and root exudates were also collected. Oxalate was analysed by ion chromatography. Error bars represent ± s.d. (n = 3). Means with different letters are statistically different among cultivars at P < 0·05.
Fig. 4.
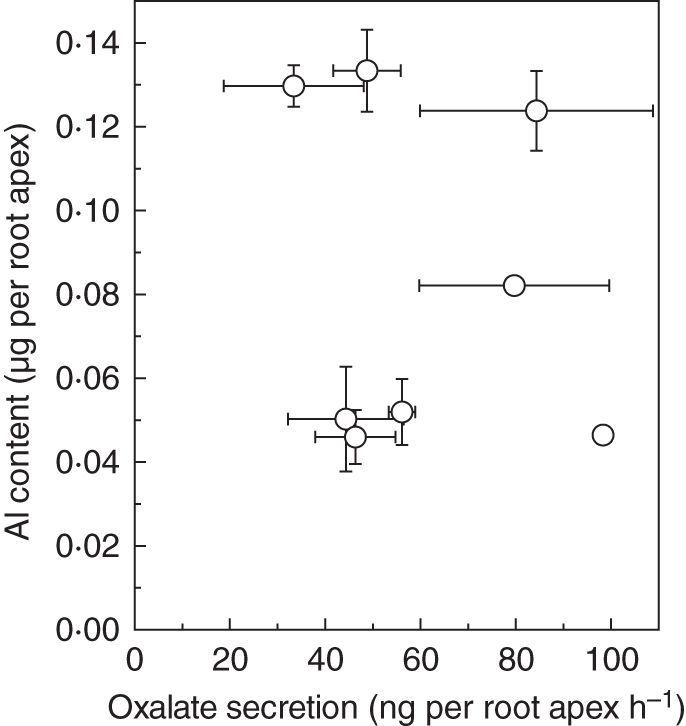
Correlation between Al content and oxalate secretion rate of eight tatary buckwheat cultivars. Data for Al content (n = 3) and oxalate secretion rate (n = 3) were taken from Figs 2 and 3, respectively.
The lack of correlation between Al-activated oxalate exudation and Al exclusion (Al content) implies that other exclusion mechanisms might be operating. In rice, exudation of organic acids does not respond to Al stress and is not responsible for Al exclusion (Yang et al., 2008; Famoso et al., 2010). Interestingly, cell-wall polysaccharides play an important role in exclusion of Al from the root apex of rice (Yang et al., 2008). Thus, whether the cell-wall polysaccharides also have a similar function in plants that show Al-induced secretion of organic acids will be of interest. As shown in Fig. 5A, the root apex pectin content ranged from 1·80 ± 0·39 to 3·38 ± 0·41 µg per root apex in the eight tatary buckwheat cultivars. In general, pectin content in Al-resistant cultivars was lower than that in Al-sensitive cultivars. However, a moderately Al-resistant cultivar, ‘Dianning’, had the lowest pectin content. Therefore, we could not establish a correlation between pectin content and Al content among the eight cultivars (dashed regression line in Fig. 5B), but a significant positive correlation could be established if ‘Dianning’ and ‘Lijiang’ were excluded (solid regression line in Fig. 5B). Among the cultivars, the pectin content of the most Al-sensitive ‘Liuku2’ (3·38 ± 0·41 µg per root apex) was significantly higher than that of the most Al-resistant ‘Chuan’ (2·38 ± 0·52 µg per root apex; Fig. 5A), indicating that pectin content could partly explain the differential ability of Al exclusion among tatary buckwheat cultivars.
Fig. 5.
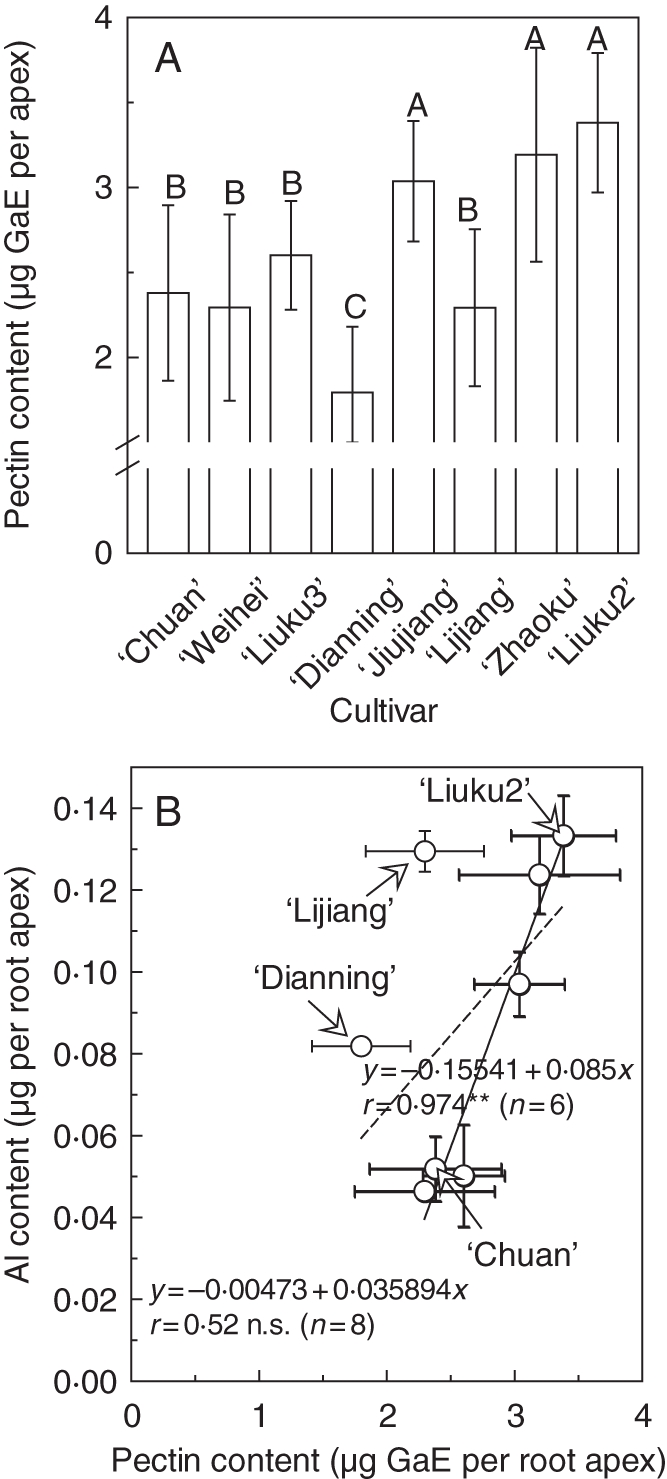
Pectin content in root apex of eight tatary buckwheat cultivars (A) and correlation between root apical Al contents and pectin contents (B). For pectin content determination, 3-d-old seedlings were exposed to 0·5 mM CaCl2 solution (pH 4·5) for 24 h, and root apices were excised. Data are means ± s.d. (n = 3). Means with different letters are statistically different at P < 0·05. In (B), data for Al content (n = 3) were the same as shown in Fig. 2. Solid and dashed regression lines represent correlation between Al content and pectin content among six (excluding cultivars ‘Dianning’ and ‘Lijiang’) and all eight cultivars respectively. **P < 0·01; n.s., not significant. GaE, galacturonic acid equivalents.
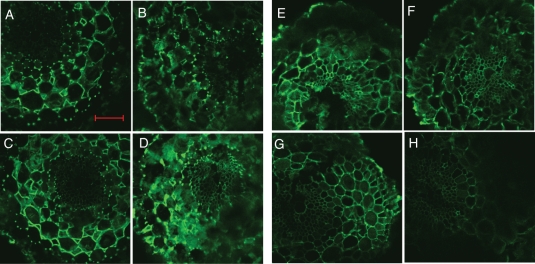
Several lines of evidence indicate that not only does pectin content contribute to Al accumulation in plants but so too does its degree of methylation (Eticha et al., 2005; Yang et al., 2008). Therefore, we determined and compared PME activity between ‘Chuan’ and ‘Liuku2’, because these two cultivars exhibited the greatest genotypic difference and pectin content difference. First, we analysed PME activity using a sensitive colorimetric assay method based on determination of the amount of methanol released from pectin by PME extracts. As shown in Fig. 6, PME activity was not significantly different between ‘Chuan’ and ‘Liuku2’ in the absence of Al, but Al treatment resulted in a significant increase of PME activity especially in ‘Liuku2’. Second, monoclonal antibodies (JIM5 and JIM7), which are specific for cell-wall pectin differing in the degree of methylation, were used for immunofluorescence localization of different cell-wall pectins. JIM5 stains low-methyl-ester pectins, and the intensity of JIM5 fluorescence did not differ between ‘Chuan’ and ‘Liuku2’ in the absence of Al (Fig. 7A, C). Fluorescence intensity was enhanced by Al treatment in the two cultivars, but was more evident in ‘Liuku2’ (Fig. 7B, D). JIM7 stains high-methyl-ester pectins, and the intensity of JIM7 fluorescence was not different in the two cultivars in the absence of Al (Fig. 7E, G). However, Al treatment resulted in a decrease of fluorescence in the two cultivars but was more evident in the Al-sensitive ‘Liuku2’ (Fig. 7F, H).
Fig. 6.
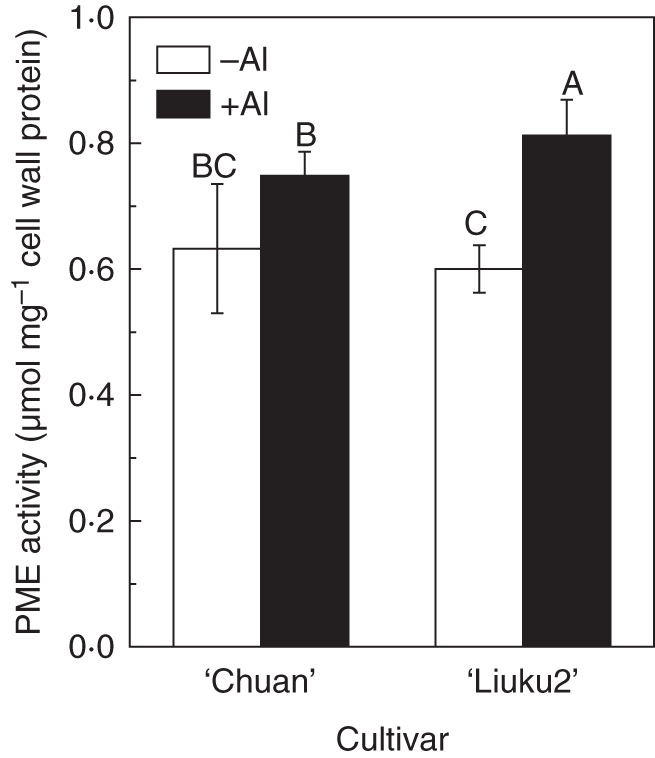
Pectin methylesterase (PME) activity in the root apex of two tatary buckwheat cultivars. Three-day-old seedlings were exposed to 0·5 mm CaCl2 solution (pH 4·5) containing 0 or 25 µm Al for 24 h. Root apices were excised for cell wall and PME extraction. PME activity was determined colorimetrically. Data are means ± s.d. (n = 3). Means with different letters are statistically different at P < 0·05.
Fig. 7.
Immunolocalization of low-methyl-ester pectin (JIM5 epitope; A–D) and high-methyl-ester pectin (JIM7 epitope; E–H) in root cross-sections of two tatary buckwheat cultivars. Three-day-old seedlings of Al-resistant ‘Chuan’ (A, B, E, F) or Al-sensitive ‘Liuku2’ (C, D, G, H) were subjected to 0·5 mm CaCl2 solution with (B, D, F, H) or without (A, C, E, G) 25 µm Al for 24 h. Root sections were taken from 1 to 3 mm behind the apex. Scale bar = 50 µm, for all images.
Given the relative importance of an internal tolerance mechanism in common buckwheat, a species closely related to tatary buckwheat, we measured Al accumulation in roots and shoots of ‘Chuan’ and ‘Liuku2’. Table 1 shows the results for roots and shoots when grown in 25 µm Al for 24 h. The Al content in the roots of the two cultivars was quite similar, although the difference was significant at P < 0·05. The Al content in the shoots of ‘Chuan’ exceeded 100 µg g−1 d. wt. This cultivar was thus also ranked as an Al-accumulator.
Table 1.
Al content in roots and shoots of tatary buckwheat cultivars
| Al content (μg Al g−1 d. wt) |
||
|---|---|---|
| Cultivar | Root | Shoot |
| ‘Chuan’ | 3303·2 ± 1·2b | 117·6 ± 6·2a |
| ‘Liuku2’ | 3473·7 ± 153·4a | 63·6 ± 1·5b |
Three-day-old seedlings were grown in 0·5 mm CaCl2 solution containing 25 µm Al3+ for 24 h. Data are means ± s.d. (n = 3). Values followed by different letters within the same column are statistically different at P < 0·05.
DISCUSSION
Genotypic differences in tatary buckwheat are associated with Al exclusion from root apex
One of the initial effects and visible symptoms of Al toxicity in plants is the rapid inhibition of root elongation (Kochian et al., 2004; Zheng and Yang, 2005). The extent of root growth inhibition has been used extensively as an criterion for Al toxicity (Foy, 1988) and thus RRE was applied in screening genotypic Al differences among plant species or cultivars within the same species (Yang et al., 2005). In the present study, we screened genotypic differences in Al resistance in terms of RRE among eight tatary buckwheat cultivars collected from southern China where acid soils are dominant. The RRE results clearly showed that there were significant genotypic differences in response to Al stress among the eight cultivars (Fig. 1). For instance, ‘Chuan’ was the most resistant cultivar, with RRE as high as 64 %, while ‘Liuku2’ the most sensitive cultivar, with an RRE of 43 %.
The genotypic differences in response to Al stress among cultivars could be attributed to two mechanisms (Taylor, 1991; Kochian, 1995). One is based on exclusion of Al from the root apex, the major site of Al toxicity (Ryan et al., 1993), whereas the other relies on the ability to tolerate symplastic Al. In studies of common buckwheat, it is clear that both exclusion of Al from the root apex and internal detoxification by forming an Al–oxalate complex in a molar ratio of 1 : 3 are operating to produce Al resistant (Ma et al., 1997b; Zheng et al., 1998, 2005). In the present study, less Al was generally found in the apices of Al-resistant cultivars than in Al-sensitive cultivars (Fig. 2A). And the degree of Al resistance for all eight cultivars was negatively correlated with the root tip Al content (Fig. 2B), indicating that an Al-exclusion mechanism most likely underlies Al resistance in tatary buckwheat too.
Oxalate secretion is not responsible for genotypic Al differences
It is widely accepted that Al resistance relies on the secretion of organic acids from the root apex in a diverse range of plant species (Kochian et al., 2004). For example, a strong correlation was found between the degree of Al resistance and the magnitude of Al-activated root malate secretion in a large number of wheat genotypes (Ryan et al., 1995). However, Al-induced secretion of root organic acids may be inadequate to explain genotypic Al differences in certain plant species. For example, Piñeros et al. (2005) demonstrated that the magnitude of Al-induced secretion of root organic acids cannot explain the degree of Al resistance among six maize genotypes differing significantly in their genetic background. A recent study of common buckwheat, a closely related species of tatary buckwheat, has suggested that Al-induced root oxalate secretion could not fully explain the genotypic differences in Al resistance because the Al-sensitive buckwheat cultivar investigated also secreted oxalate in a similar pattern and comparable amount (Zheng et al., 2005). In the present study, we found that tatary buckwheat secreted oxalate from the roots in response to Al stress (Fig. 3). However, there was no correlation between Al-induced oxalate secretion and root apex Al accumulation (Fig. 4). For example, the most Al-resistant cultivar ‘Chuan’ had a similar oxalate secretion rate to the most Al-sensitive cultivar ‘Liuku2’. Another Al-resistance cultivar ‘Weihei’ had a lower oxalate secretion rate than the Al-sensitive cultivar ‘Zhaoku’. Therefore, the present results clearly suggest that secretion of root organic acids is not responsible for the genotypic differences in Al resistance among tatary buckwheat cultivars, and novel, as yet unknown, Al resistance mechanisms must be operating in certain plant species.
The role of cell-wall pectin in Al resistance
Al easily binds to the cell wall of roots and the majority of Al resides in the cell wall (Horst, 1995; Horst et al., 2010). Therefore, it is logical to speculate that the cell wall plays a critical role in Al resistance. Recent molecular evidence also supports the view of cell-wall properties affecting Al resistance (Horst et al., 2010). For example, a map-based clone technique has led Huang et al. (2009) to isolate two genes, STAR1 (sensitive to Al rhizotoxicity 1) and STAR2, which encode two interacting proteins that form an ABC transporter complex. The STAR1–STAR2 complex had efflux transport activity for UDP-glucose into the apoplast, which led the authors to speculate that cell-wall modification may play a role in rice Al resistance (Huang et al., 2009). Al forms electrostatic bonds preferentially with oxygen donor ligands such as carboxylate and Pi (Macdonald and Martin, 1988). As the primary cell-wall component, pectin with its carboxylate groups is considered as a major binding site of Al (Chang et al., 1999; Taylor et al., 2000), and thus pectin content may be related to accumulation of Al. We analysed pectin content in eight tatary buckwheat cultivars, and found that the pectin content of the root apex differ significantly among these cultivars. In general, the Al-resistant cultivars had lower pectin content than the Al-sensitive cultivars. However, two cultivars with moderate Al sensitivity had the lowest pectin content (Fig. 5A). Nonetheless, a strong positive correlation could be established between the pectin content and Al content of tatary buckwheat cultivars except ‘Dianning’ and ‘Lijiang’ (Fig. 5B), suggesting that the pectin had a significant effect on root Al accumulation in tatary buckwheat. Schmohl and Horst (2000) also reported that salt-adapted maize suspension cells contained more pectin and were more Al-sensitive than normal cells, while pectolyase-treated cells were more tolerant.
However, the quantity of pectin is not the only factor determining its Al binding ability as the degree of methylesterification affects the number of free carboxyl groups in pectin. For example, Schmohl et al. (2000) reported that the Al sensitivity of Zea mays cell suspension cultures was negatively related to the degree of pectin methylesterification and they concluded that the degree of pectin methylesterification and the activity of PME are important in the expression of Al toxicity and resistance. Using immunofluorescence localization of pectins, Eticha et al. (2005) also found that an Al-sensitive maize cultivar had a higher proportion of low-methylated pectin than an Al-resistant cultivar. Yang et al. (2008) found that pectins in sensitive rice cultivar root tips had a higher degree of de-methylesterification that might result in a greater ability to bind Al. Here, we found that PME activities were not significantly different between the ‘Chuan’ ‘Liuku2’cultivars in the absence of Al (Fig. 6), although Al treatment resulted in an increase of PME activity in both (more evident in ‘Liuku2’). Using immunofluorescence localization of pectin with two kinds of antibodies to detect both low- and high-methyl-ester pectins, we found that the Al-resistant cultivar ‘Chuan’ had similar amounts of both low- and of high-methyl-ester pectins to those of the Al-sensitive cultivar ‘Liuku2’ in the absence of Al (Fig. 7). These results suggest that it is pectin content rather than the degree of methylation that contributes to the initial Al accumulation in the root apex of tatary buckwheat cultivars. With greater exposure time, the Al-sensitive cultivar accumulated increasing Al, and consequently more pectin, setting up a vicious circle. The greater sensitivity of PME activity to Al stress in Al-sensitive ‘Liuku2’ could be the result of more Al binding to the cell wall and thereby more severe Al toxicity.
Multiple Al resistance mechanisms have been proposed in several plant species (Kochian et al., 2004). Apart from the well-known Al-activated exudation of root organic acids, there is little information on the nature of other Al resistance mechanisms. Recently, accumulating evidence supports the view that root apoplast, especially cell-wall pectin, plays an important role in Al resistance or toxicity in plants (Horst et al., 2010). For example, an interesting correlation between root apex pectin content or the degree of pectin methylation and root apex Al content has been observed in maize and rice (Eticha et al., 2005; Yang et al., 2008). Furthermore, Li et al. (2009) reported that the disorganized distribution of pectin epitopes was related to Al-induced root growth inhibition in maize. These results imply that cell-wall pectin could play a major role in determining not only the extent of Al binding but also root growth inhibition at least in monocotyledonous plants. In this study, we provided evidence that cell-wall pectin could play an important role in Al exclusion in tatary buckwheat, a dicotyledonous plant species. This reinforces the view that cell-wall properties could be considered as a novel Al resistance mechanism at least at a given growth stage (Yang et al., 2008), even though Al-induced organic acid secretion coexists in tatary buckwheat.
It is interesting to note that tatary buckwheat was able to accumulate a substantial amount (about 100 µg Al g−1 d. wt) of Al in shoots (Table 1) after 24 h of exposure to 25 µm. The ability of tatary buckwheat to accumulate Al was similar with that of common buckwheat, a known Al accumulator (data not shown). Why therefore is Al exclusion important for Al-accumulating plants? Al strongly interacts with the negative binding sites of the apoplast of either Al excluder or Al accumulator species, and binding of Al to cell walls will decrease the extensibility of the cell wall (Tabuchi and Matsumoto, 2001; Ma et al., 2004). A recent review by Horst et al. (2010) stated that even in an Al accumulator, detoxification of Al apoplastically is still important for protecting roots from Al injury. The most representative example is common buckwheat, an Al accumulator, in which both exclusion and internal tolerance mechanisms cooperate to detoxify Al (Ma et al., 1997b).
In summary, eight cultivars of tatary buckwheat showed genotypic Al differences and an Al exclusion mechanism was shown to be involved in Al resistance. Although Al-induced exudation of root organic acids is well established as an Al resistance mechanism in a diverse range of plant species, this could not explain the different Al resistance in tatary buckwheat. Instead, root apex cell-wall pectin was, in general, associated with accumulation of root apical Al.
ACKNOWLEDGEMENTS
We are grateful to Dr J. Paul Knox (Centre for Plant Science, University of Leeds, UK) for kindly donating the monoclonal antibodies specific for pectins (JIM5 and JIM7). This work was financially supported by the Natural Science Foundation of China (Grant nos. 30830076 and J20090207), Changjiang Scholarship and China Postdoctoral Science Special Foundation (Grant no. 20081474).
LITERATURE CITED
- Ahmed AER, Labavitch JM. A simplified method for accurate determination of cell wall uronide content. Journal of Food Biochemistry. 1977;1:361–365. [Google Scholar]
- Anthon GE, Barrett DM. Comparison of three colorimetric reagents in the determination of methanol with alcohol oxidase. Application to the assay of pectin methylesterase. Journal of Agricultural and Food Chemistry. 2004;52:3749–3753. doi: 10.1021/jf035284w. [DOI] [PubMed] [Google Scholar]
- Blumenkrantz N, Asboe-Hansen G. New method for quantitative determination of uronic acids. Analytical Biochemistry. 1973;54:484–489. doi: 10.1016/0003-2697(73)90377-1. [DOI] [PubMed] [Google Scholar]
- Chang YC, Yamamoto Y, Mastsumoto H. Accumulation of aluminum in the cell wall pectin in cultured tobacco (Nicotiana tabacum L.) cells treated with a combination of aluminum and ion. Plant Cell and Environment. 1999;22:1009–1017. [Google Scholar]
- Delhaize E, Gruber BD, Ryan PR. The roles of organic acid permeases in aluminium resistance and mineral nutrition. FEBS Letters. 2007;581:2255–2262. doi: 10.1016/j.febslet.2007.03.057. [DOI] [PubMed] [Google Scholar]
- Eticha D, Stass A, Horst WJ. Cell-wall pectin and its degree of methylation in the maize root-apex: significance for genotypic differences in aluminium resistance. Plant Cell and Environment. 2005;28:1410–1420. [Google Scholar]
- Ezaki B, Gardner RC, Ezaki Y, Matsumoto H. Expression of aluminum-induced genes in transgenic Arabidopsis plants can ameliorate aluminum stress and/or oxidative stress. Plant Physiology. 2000;122:657–665. doi: 10.1104/pp.122.3.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Famoso AN, Clark RT, Shaff JE, Craft E, McCouch SR, Kochian LV. Development of a novel aluminum tolerance phenotyping platform used for comparisons of cereal aluminum tolerance and investigations into rice aluminum tolerance mechanisms. Plant Physiology. 2010;153:1678–1691. doi: 10.1104/pp.110.156794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foy CD. Plant adaptation to acid, aluminum-toxic soils. Communications in Soil Science and Plant Analysis. 1988;19:959–987. [Google Scholar]
- Horst WJ. The role of the apoplast in aluminium toxicity and resistance of higher plants: a review. Zeitschrift für Pflanzenernährung und Bodenkunde. 1995;158:419–428. [Google Scholar]
- Horst WJ, Wang Y, Eticha D. The role of the root apoplast in aluminium-induced inhibition of root elongation and in aluminium resistance of plants: a review. Annals of Botany. 2010;106:185–197. doi: 10.1093/aob/mcq053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CF, Yamaji N, Mitani N, Yano M, Nagamura Y, Ma JF. A bacterial-type ABC transporter is involved in aluminum tolerance in rice. The Plant Cell. 2009;21:655–667. doi: 10.1105/tpc.108.064543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klug B, Horst WJ. Oxalate exudation into the root-tip water free space confers protection from aluminum toxicity and allows aluminum accumulation in the symplast in buckwheat (Fagopyrum esculentum) New Phytologist. 2010;187:380–391. doi: 10.1111/j.1469-8137.2010.03288.x. [DOI] [PubMed] [Google Scholar]
- Kochian LV. Cellular mechanisms of aluminum toxicity and resistance in plants. Annual Review of Plant Physiology and Plant Molecular Biology. 1995;46:237–260. [Google Scholar]
- Kochian LV, Hoekenga AO, Piñeros MA. How do crop plants tolerate acid soils? Mechanisms of aluminum tolerance and phosphorous efficiency. Annual Review of Plant Biology. 2004;55:459–493. doi: 10.1146/annurev.arplant.55.031903.141655. [DOI] [PubMed] [Google Scholar]
- Li YY, Yang JL, Zhang YJ, Zheng SJ. Disorganized distribution of homogalacturonan epitopes in cell walls as one possible mechanism for aluminium-induced root growth inhibition in maize. Annals of Botany. 2009;104:235–241. doi: 10.1093/aob/mcp123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma JF, Hiradate S, Nomoto K, Iwashita T, Matsumoto H. Internal detoxification mechanism of Al in Hydrangea. Plant Physiology. 1997a;113:1033–1039. doi: 10.1104/pp.113.4.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma JF, Zheng SJ, Matsumoto H, Hiradate S. Detoxifying aluminium with buckwheat. Nature. 1997b;390:569–570. [Google Scholar]
- Ma JF, Shen R, Nagao S, Tanimoto E. Aluminum targets elongating cells by reducing cell wall extensibility in wheat roots. Plant and Cell Physiology. 2004;45:583–589. doi: 10.1093/pcp/pch060. [DOI] [PubMed] [Google Scholar]
- Ma JF, Nagao S, Huang CF, Nishimura M. Isolation and characterization of a rice mutant hypersensitive to Al. Plant and Cell Physiology. 2005;46:1054–1061. doi: 10.1093/pcp/pci116. [DOI] [PubMed] [Google Scholar]
- Macdonald TL, Martin RB. Aluminum ion in biological systems. Trends in Biochemistry Science. 1988;13:15–19. doi: 10.1016/0968-0004(88)90012-6. [DOI] [PubMed] [Google Scholar]
- Pellet DM, Papernik L, Kochian LV. Multiple aluminum-resistance mechanism in wheat. Roles of root apical phosphate and malate exudation. Plant Physiology. 1996;112:591–597. doi: 10.1104/pp.112.2.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piñeros MA, Shaff JE, Manslank HS, Carvalho Alves VM, Kochian LV. Aluminum resistance in maize cannot be solely explained by root organic acid exudation. A comparative physiological study. Plant Physiology. 2005;137:231–241. doi: 10.1104/pp.104.047357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan PR, DiTomaso JM, Kochian LV. Aluminium toxicity in roots: an investigation of spatial sensitivity and the role of the root cap. Journal of Experimental Botany. 1993;44:437–446. [Google Scholar]
- Ryan PR, Delhaize E, Randall PJ. Malate efflux from root apices and tolerance to aluminium are highly correlated in wheat. Australian Journal of Plant Physiology. 1995;22:531–536. [Google Scholar]
- Schmohl N, Horst WJ. Cell wall pectin content modulates aluminium sensitivity of Zea mays (L.) cells grown in suspension culture. Plant Cell and Environment. 2000;23:735–742. [Google Scholar]
- Schmohl N, Ppilling J, Fisahn J, Horst WJ. Pectin methylesterase modulates aluminium sensitivity in Zea mays and Solanum tuberosum. Physiologia Plantarum. 2000;109:419–427. [Google Scholar]
- Tabuchi A, Matsumoto H. Changes in cell-wall properties of wheat (Triticum aestivum) roots during aluminium-induced growth inhibition. Physiologia Plantarum. 2001;112:353–358. doi: 10.1034/j.1399-3054.2001.1120308.x. [DOI] [PubMed] [Google Scholar]
- Taylor GJ. Current views of the aluminum stress response: the physiological basis of tolerance. In: Randall DD, Blevins DG, Miles CD, editors. Current topics in plant biochemistry and physiology. Columbia, MO: University of Missouri; 1991. pp. 57–93. [Google Scholar]
- Taylor GJ, McDonald-Stephens JL, Hunter DB, et al. Direct measurement of aluminum uptake and distribution in single cells of Chara coralline. Plant Physiology. 2000;123:987–996. doi: 10.1104/pp.123.3.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Uexküll HR, Mutert E. Global extent, development and economic impact of acid soils. In: Date RA, Grundon NJ, Raymet GE, Probert ME, editors. Plant–soil interactions at low pH: principles and management. Dordrecht: Kluwer Academic Publishers; 1995. pp. 5–19. [Google Scholar]
- Wenzl P, Patiño GR, Chaves AL, Mayer JE, Rao IM. The high levels of aluminum resistance in signalgrass is not associated with known mechanisms of external aluminum detoxification in root apices. Plant Physiology. 2001;122:1473–1484. doi: 10.1104/pp.125.3.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y, Kobayashi Y, Devi SR, Rikiishi S, Matsumoto H. Oxidative stress triggered by aluminum in plant roots. Plant and Soil. 2003;255:239–243. [Google Scholar]
- Yang JL, Zheng SJ, He YF, Tang C, Zhou GD. Genotypic differences among plant species in response to aluminum stress. Journal of Plant Nutrition. 2005;28:949–961. [Google Scholar]
- Yang JL, Li YY, Zhang YJ, et al. Cell wall polysaccharides are specifically involved in the exclusion of aluminum from the rice root apex. Plant Physiology. 2008;146:602–611. doi: 10.1104/pp.107.111989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng SJ, Yang JL. Target sites of Al phytotoxicity. Biologia Plantarum. 2005;49:321–331. [Google Scholar]
- Zheng SJ, Ma JF, Matsumoto H. High aluminum resistance in buckwheat. I. Al-induced specific secretion of oxalic acid from root tips. Plant Physiology. 1998;117:745–751. doi: 10.1104/pp.117.3.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng SJ, Yang JL, He YF, et al. Immobilization of aluminum with phosphorous in roots is associated with high aluminum resistance in buckwheat. Plant Physiology. 2005;138:297–303. doi: 10.1104/pp.105.059667. [DOI] [PMC free article] [PubMed] [Google Scholar]