Abstract
Background and Aims
The effects of cotyledon damage on seedling growth and survival are relatively well established, but little is known about the effects on aspects of plant fitness such as seed number and size. Here the direct and indirect mechanisms linking cotyledon damage and plant fitness in the annual species Medicago lupulina are examined.
Methods
Growth and reproductive traits, including mature plant size, time to first flowering, flower number, seed number and individual seed mass were monitored in M. lupulina plants when zero, one or two cotyledons were removed at 7 d old. Structural equation modelling (SEM) was used to examine the mechanisms linking cotyledon damage to seed number and seed mass.
Key Results
Cotyledon damage reduced seed number but not individual seed mass. The primary mechanism was a reduction in plant biomass with cotyledon damage that in turn reduced seed number primarily through a reduction in flower numbers. Although cotyledon damage delayed flower initiation, it had little effect on seed number. Individual seed mass was not affected by cotyledon removal, but there was a trade-off between seed number and seed mass.
Conclusions
It is shown how a network of indirect mechanisms link damage to cotyledons and fitness in M. lupulina. Cotyledon damage had strong direct effects on both plant size and flowering phenology, but an analysis of the causal relationships among plant traits and fitness components showed that a reduction in plant size associated with cotyledon damage was an important mechanism influencing fitness.
Keywords: Cotyledon damage, herbivory, fitness, Medicago lupulina L., alpine grassland, structural equation modelling (SEM), plant growth, flowering phenology
INTRODUCTION
The seedling stage is the most vulnerable phase in a plant's life history. Competition, pathogens and resource limitation are common challenges facing seedlings (Maron, 1997; Stinchcombe, 2002; Bell et al., 2006; Hanley et al., 2007), but chief amongst the factors influencing seedling recruitment is herbivory (Moles and Westoby, 2004). Moreover, selective seedling herbivory can significantly alter community composition in established vegetation (Hanley et al., 1995; Howe et al., 2002).
Numerous studies have examined the impacts of herbivore attack on seedling survival, growth potential, competitive ability and fitness (Mabry and Wayne, 1997; Husheer et al., 2006; Hanley and Sykes, 2009), but, despite these studies, the ecological consequences of cotyledon herbivory are not well understood. The impacts of cotyledon herbivory may differ from those of leaf herbivory due to the many functional differences between cotyledons and leaves (Kitajima, 2003). There is strong evidence that cotyledon damage can reduce the survival and the growth potential of plants (Bonfil, 1998; Kitajima, 2003; Bisognin et al., 2005; Boege and Marquis, 2005), with the impact of such damage most severe in newly emerged seedlings (Hanley et al., 2004; Hanley and Fegan, 2007). Reduced size probably influences interactions between both seedlings and surrounding established vegetation (Frost and Rydin, 1997; del-Val and Crawley, 2005) and reproductive success (Stinchcombe, 2002; Hanley and May, 2006; Hanley and Fegan, 2007).
The mechanisms driving the fitness consequences of cotyledon damage remain poorly understood. Stinchcombe (2002), for example, showed that cotyledon herbivory indirectly affects fitness through plant size but did not investigate the mechanisms linking plant size with final fitness. Leaf herbivory can negatively affect fitness through decreasing floral size (Mothershead and Marquis, 2000), flower number (Mauricio et al., 1993), flowering period (Poveda et al., 2003), seed production (Vallius and Salonen, 2006) and delays in flower initiation (Kettenring et al., 2009). Whether cotyledon damage exerts similar effects remains unknown. Two recent studies have shown that cotyledon herbivory can reduce both growth and flowering potential (Hanley and May, 2006; Hanley and Fegan, 2007) but did not consider any subsequent effects on seed production. To our knowledge, no study has explicitly explored how cotyledon herbivory directly and indirectly influences plant fitness through traits including mature plant size, flowering phenology and flower number.
In this study, the mechanisms driving the impacts of cotyledon damage on fitness in the annual species Medicago lupulina L are examined. The effects of cotyledon damage on mature plant size, flower traits and final fitness components are quantified and the networks of interaction among these variables are examined using structural equation modelling (SEM).
MATERIALS AND METHODS
Study species
Medicago lupulina is a widely distributed annual species native to Eurasia. It is common in abandoned fields and open habitats in the alpine meadows of the Tibetan Plateau (2900–3000 m a.s.l.). Stems are thin and procumbent with 10–15 small bright yellow flowers borne in densely packed racemes. Blossoming in alpine habitats occurs from late July to mid-August, with seed maturity generally in late-August to mid-September. The cotyledons are frequently subjected to damage by a ground-dwelling beetle (Tenebrionidae).
Experimental design
Seeds were collected in September 2008. In late May 2009, the seeds were germinated on moistened filter paper and seedlings were transferred to cylindrical pots (350 mm diameter by 500 mm deep) containing field soil. Twenty-four pots with ten seedlings each were planted and placed outside at the Alpine Meadow Ecosystem Scientific Research Station in the north-east of the Tibetan Plateau (E102°53′, N34°55′). The site has an average annual temperature of 1·2 °C with a minimum monthly mean of –10·7°C in January and a maximum monthly mean of 11·7°C in July. The site has 270 frost-free days and a mean annual precipitation of 562 mm. Seven days after germination, the pots were randomly divided into three groups of eight pots. The pots were placed in three rows with two pots per group arranged randomly in each row. There was a 1 m distance between neighbouring pots. To simulate insect damage, plants in one group had both cotyledons removed at the nodes using scissors, while plants in a second group had one cotyledon removed. The remaining plants were left with their cotyledons intact as a control. Simulated herbivory can be an imperfect proxy for actual herbivory, however simulated herbivory permits much closer control over experimental conditions (Tiffin and Inouye, 2000; Lehtilä, 2003). The beetles in this case are chewing herbivores that produce patterns of damage to the cotyledons of M. lupulina that are visually very similar to those produced by scissors. While this approach cannot account for effects such as a plant response to insect saliva, we believe that the losses of nutrient reserves caused by our method closely mimic the effects of the beetles on plant nutrient reserves. To ensure that natural herbivory did not also occur in this experiment any ground-dwelling beetles present were removed from the soil when pots were filled, and insecticide was used to prevent beetles from moving into the pots.
After cotyledon removal, the seedlings were grown for 35 d, by which time any undamaged cotyledons were lost or had withered. At this stage, thinning was carried out to avoid competition, leaving 96 individual plants (32 individuals per treatment and four individuals per pot). Plants were harvested in mid-September (60 d after thinning). Two plants had died in each of the two cotyledon damage treatments, leaving a total sample size of 92. A range of plant reproductive traits were measured including the number of days to the first flower opening, the total number of inflorescences, total number of viable seeds and total seed mass for each plant. Plant shoot biomass (oven-dried at 80 °C for 24 h) was measured at harvest.
Statistical analysis
Structural equation modelling was used to examine the direct and indirect effects of cotyledon damage on plant performance at maturity (Grace, 2006). Structural equation modelling is a powerful tool for examining relationships among causally linked intercorrelated variables. Each single-headed arrow in an SEM represents a causal relationship where the variable at the tail of the arrow is a direct cause of the variable at the head. A double-headed arrow indicates an unresolved correlation between two variables. An initial SEM is specified based on prior theoretical knowledge, and a χ2 test is used to determine whether the covariance structures implied by the model adequately fit the actual covariance structures of the data. A non-significant χ2 test (P > 0·05) indicates adequate model fit. If the initial model does not adequately fit then model modification indices provide a strong tool for data exploration and hypothesis generation.
An initial path model based on exploratory analyses was developed to examine the direct and indirect effects of cotyledon removal on components of plant fitness. The experimental cotyledon treatments were represented by the degree of cotyledon damage (0 = control, 0·5 = one cotyledon removed and 1 = two cotyledons removed). Direct paths from the cotyledon treatment to mature biomass and time to flower initiation represented the direct effects of cotyledon damage on plant size and flowering phenology. Direct paths from biomass to the timing of flowering and flower number were included to account for the effect of plant size on both the timing and ability to produce flowers. Seed number and individual seed mass received arrows from all other variables in the model. Together these paths represented all potential direct and indirect mechanisms through which cotyledon damage could impact fecundity and seed quality. Finally, a double-headed arrow between seed number and seed mass was used to represent the trade-off between total seed number and individual seed mass (Smith and Fretwell, 1974; Venable, 1992). Pot was treated as a random factor in the analysis to account for the clustering of four plants per pot. All variables except the proportion of cotyledons removed were log-transformed to ensure linear relationships and normal distributions. Correlations between all transformed variables used in this study are presented in Table 1.
Table 1.
Bivariate correlations between variables included in the structural equation model linking the proportion of cotyledons removed (CT) in Medicago lupulina to plant mature biomass (PMB), days to first flowering (TFF), flower number (FN) and the fitness components of seed number (SN) and individual seed mass (ISM)
| CT | PMB | TFF | ISM | SN | FN | |
|---|---|---|---|---|---|---|
| CT | 1 | |||||
| PMB | –0·44239*** | 1 | ||||
| TFF | 0·42047*** | –0·17027 | 1 | |||
| ISM | 0·04987 | –0·10817 | 0·06182 | 1 | ||
| SN | –0·39235*** | 0·92123*** | –0·11053 | –0·25731* | 1 | |
| FN | –0·32229** | 0·90776*** | –0·07018 | –0·14968 | 0·95543*** | 1 |
All variables except the cotyledon treatment were log-transformed.
Asterisks indicate the significance of the correlation coefficients (*P < 0·05; **P < 0·01; ***P < 0·001).
The SEM models were fit using M-Plus 6 (Muthén and Muthén, 2010). Each path coefficient was divided by its standard error to assess significance. Coefficients with P <0·05 were considered significant, and non-significant paths were retained in the final model. Model fitting was by maximum likelihood with standard errors and a χ2 test that are robust to non-normality and non-independence of samples (MLR). The type = ‘Complex’ option in the model statement with clustering by pots was used to account for the grouping of four plants per pot. This approach is analogous to the use of a random factor in a mixed model.
RESULTS
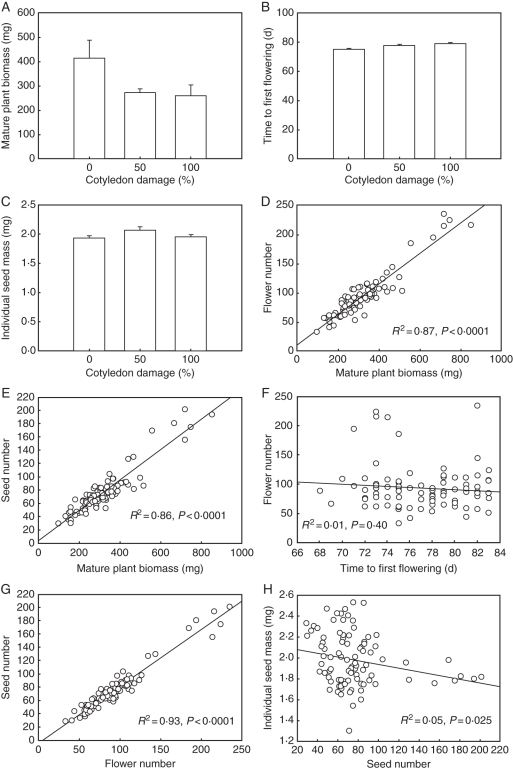
The initial SEM adequately fit our data (χ21 = 1·37, P = 0·24; Fig. 1, Table 2). Cotyledon damage led to reduced mature plant size and a delay in the initiation of flowering (Fig. 2). Cotyledon damage reduced seed number indirectly through plant size and the number of flowers. Surprisingly, delayed flower initiation did not affect seed number directly but did have a weak positive effect on flower number. The negative correlation between seed number and seed mass suggests a physiological or genetic trade-off between seed number and seed mass. The lack of significant paths from either cotyledon damage or plant biomass and timing of flowering to individual seed size indicates that the mechanisms controlling individual seed size and the seed size–number trade-off were not represented by variables included in the model.
Fig. 1.
The final structural equation model linking cotyledon damage (number of cotyledons removed) in Medicago lupulina to plant biomass, days to flowering, flower number and the fitness components of seed number and seed mass. Each arrow represents a causal relationship such that a change in the variable at the tail of the arrow is a direct cause of a change in the variable at the head. Non-significant paths are indicated by dotted arrows. The thickness of the solid arrows reflects the magnitude of the standardized SEM coefficients. Larger standardized coefficients (listed beside each significant path) indicate that the variable at the tail has a stronger effect on the variable at the head. The variance explained (R2) for each endogenous variable is listed beside that variable.
Table 2.
Unstandardized and standardized path coefficients, and the standard error of the unstandardized coefficients from the final structural equation model linking cotyledon damage (number of cotyledons removed) in Medicago lupulina to plant biomass, days to flowering, flower number and the fitness components of seed number and seed mass
| Unstandardized coefficients | Standard error | P-value | Standardized coefficients | |
|---|---|---|---|---|
| Seed number | ||||
| Cotyledon treatment | –0·020 | 0·012 | 0·085 | –0·052 |
| Mature plant biomass | 0·255 | 0·048 | <0·001 | 0·265 |
| Flower number | 0·744 | 0·053 | <0·001 | 0·697 |
| Time to first flowering | 0·042 | 0·210 | 0·842 | 0·006 |
| Individual seed mass | ||||
| Cotyledon treatment | 0·001 | 0·014 | 0·968 | 0·004 |
| Mature plant biomass | 0·067 | 0·098 | 0·493 | 0·204 |
| Flower number | –0·120 | 0·089 | 0·179 | –0·329 |
| Time to first flowering | 0·181 | 0·328 | 0·553 | 0·072 |
| Flower number | ||||
| Mature plant biomass | 0·832 | 0·052 | <0·001 | 0·932 |
| Time to first flowering | 0·603 | 0·275 | 0·028 | 0·087 |
| Time to first flowering | ||||
| Cotyledon treatment | 0·023 | 0·004 | <0·001 | 0·429 |
| Mature plant biomass | 0·003 | 0·009 | 0·784 | 0·020 |
| Mature plant biomass | ||||
| Cotyledon treatment | –0·180 | 0·024 | <0·001 | –0·442 |
| Seed number – individual seed mass covariance | –0·001 | 0·000 | <0·001 | –0·473 |
The P-values are from a one-sample t-test of whether the unstandardized path coefficient differed significantly from zero. The paths described in each section of the table were from the indented variables to the variable above.
Fig. 2.
Bar charts and bivariate scatterplots for all relationships identified as significant in the final structural equation model linking cotyledon damage (number of cotyledons removed) in Medicago lupulina to plant biomass, days to flowering, flower number and the fitness components of seed number and seed mass. (A) Cotyledon damage (%) vs. mature plant biomass (mg). (B) Cotyledon damage (%) vs. time to first flowering (d). (C) Cotyledon damage (%) vs. individual seed mass (mg). (D) Mature plant biomass (mg) vs. flower number. (E) Mature plant biomass (mg) vs. seed number. (F) Time to first flowering (d) vs. flower number. (G) Flower number vs. seed number. (H) Individual seed mass (mg) vs. seed number. In the barplots, error bars are + 1 s.e. and n = 30 for 50 and 100 % cotyledon damage and n = 32 in the 0 % treatment. In the scatterplots, n = 92. Trend lines were plotted using univariate linear regressions. Note that the R2 and P-values are from the linear regressions and a non-significant bivariate relationship can be significant when placed in a multivariate SEM.
DISCUSSION
Consistent with other studies (Stinchcombe, 2002), damage to cotyledons in M. lupulina had indirect effects on fitness through plant biomass. Our results provide some of the first insights into the mechanisms driving the relationship between cotyledon damage, biomass and fitness. The primary mechanism was a strong effect of plant size on the ability to produce flowers. The relationship between plant size and flower number is well established (Schmitt, 1983; Vallius and Salonen, 2006; Gómez et al., 2009). There was, however, also a weaker direct effect of biomass on seed number independent of flower number. A potential explanation for this direct effect is an increased rate of flower abortion in smaller plants, as reduced resources can induce abortions at the flower, seed and fruit stages (Marcelis et al., 2004). In alpine grasslands with a short growing season, smaller plants may need to abort some flowers or seeds to ensure that at least some seeds reach maturity.
Cotyledons are critical sources of energy and nutrients, and thus cotyledon damage or loss can have detrimental effects on plant growth both at the seedling stage and carrying on to maturity (Lamont and Groom, 2002; Kitajima, 2003; Hanley et al., 2004; Hanley and May, 2006; Hanley and Fegan, 2007). Ipomoea hederacea, for example, can completely compensate for damage to mature leaves but not cotyledons (Stinchcombe, 2002). Similarly, Boege and Marquis (2005) concluded that plants should generally invest relatively more resources in mechanisms to tolerate or deter damage at the cotyledon stage. Our results showed that plants with one cotyledon removed produced 32 % fewer seeds and those with both cotyledons removed produced 40 % less than intact plants.
Consistent with other studies, cotyledon damage delayed the initiation of flowering (Stinchcombe, 2002; Hanley and May, 2006; Hanley and Fegan, 2007). The delay in flowering had a slight positive effect on flower number, suggesting that the plants were attempting to compensate for the negative effects of reduced biomass on flower number by achieving a larger size before flowering. Later-flowering individuals can achieve a larger size before flowering and thus may have more resources available for seed production (Winn and Gross, 1993). This positive effect was small relative to the effects of biomass on flower number, suggesting that time to complete seed set before the first frosts is a major limiting factor. Pollinator availability may be a second mechanism limiting the delay in flowering, as consistent flowering phenology is critical to ensure sufficient pollinator visitation in many species (Cariveau et al., 2004; Weis and Kossler, 2004). The importance of this mechanism is unclear as annual M. lupulina is dominantly self-fertilizing, with outcrossing generally by bee pollination (Yan et al., 2009). Even in a largely self-fertilized species an absence of pollinators could have longer term fitness effects by reducing the number of outbreeding events, as increased inbreeding can reduce seed and seedling fitness (Ferrer et al., 2009; Hirao, 2010).
In contrast to the strong effects of cotyledon damage on seed number, we found few impacts of cotyledon damage on individual seed mass. The timing and type of defoliation can affect the impacts on the components of plant fitness. For example, early defoliation can alter flower number and seed number while late defoliation can affect seed mass (García and Ehrlén, 2002; Marshall et al., 2005). In the case of M. lupulina there was a trade-off between seed number and seed size that may explain the lack of direct effects on seed size. The seed size–number trade-off is well known both among and within plant species (Smith and Fretwell, 1974; Venable, 1992; Paul-Victor and Turnbull, 2009). The negative relationship between seed number and mass in M. lupulina suggests that when cotyledon herbivory reduces overall plant vigour, this species allocates resources to fewer seeds to maintain seed quality. Large seed size is frequently linked to enhanced seedling survival and regeneration success, particularly in highly nutrient-deficient habitats (Moles and Westoby, 2004; Hanley et al., 2007). The alpine grasslands of the Tibetan Plateau where M. lupulina is found are very infertile, suggesting that the maintenance of consistent seed size is important for this species even when cotyledon damage reduces the resources available to a plant. The constraints on seed size in this species are probably not linked to dispersal, however, since the seeds are generally dispersed within their fruits by adhesion or ingestion of the rough-surfaced indehiscent pods of M. lupulina by birds and mammals.
In conclusion, our analysis of the network of causal relationships among plant traits and fitness components in M. lupulina shows that a reduction in plant size is associated with cotyledon damage and is an important mechanism influencing reproductive fitness. Cotyledon damage had strong direct effects on both plant size and flowering phenology, but affected reproductive fitness largely through changes in seed number rather than individual seed mass. Declines in seed number in damaged plants were driven by flower number and rate of seed set through reduced plant biomass. Delays in flower initiation had a slight positive effect on flower number, but were not sufficient to compensate for the negative effects of reduced biomass on flower number and fitness.
ACKNOWLEDGEMENTS
We thank the editor and three anonymous referees for helpful comments on the manuscript. This work was supported by the National Basic Research Program of China (973 2007CB108904 to S.Z.) and the Canadian Foundation for Innovation (24642 to E.G.L.).
LITERATURE CITED
- Bell T, Freckleton RP, Lewis OT. Plant pathogens drive density-dependent seedling mortality in a tropical tree. Ecology Letters. 2006;9:569–574. doi: 10.1111/j.1461-0248.2006.00905.x. [DOI] [PubMed] [Google Scholar]
- Bisognin DA, Velasquez L, Widders I. Cucumber seedling dependence on cotyledonary leaves for early growth. Pesquisa Agropecuária Brasileira. 2005;40:531–539. [Google Scholar]
- Boege K, Marquis RJ. Facing herbivory as you grow up: the ontogeny of resistance in plants. Trends in Ecology and Evolution. 2005;20:441–448. doi: 10.1016/j.tree.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Bonfil C. The effects of seed size, cotyledon reserves, and herbivory on seedling survival and growth in Quercus rugosa and Q. laurina (Fagaceae) American Journal of Botany. 1998;85:79–87. [PubMed] [Google Scholar]
- Cariveau D, Irwin RE, Brody AK, Garcia-Mayeya LS, Von der Ohe A. Direct and indirect effects of pollinators and seed predators to selection on plant and floral traits. Oikos. 2004;104:15–26. [Google Scholar]
- del-Val E, Crawley MJ. Are grazing increaser species better tolerators than decreasers? An experimental assessment of defoliation tolerance in eight British grassland species. Journal of Ecology. 2005;93:1005–1016. [Google Scholar]
- Ferrer MM, Good-Avila SV, Montaña C, Domínguez CA, Eguiarte LE. Effect of variation in self-incompatibility on pollen limitation and inbreeding depression in Flourensia cernua (Asteraceae) scrubs of contrasting density. Annals of Botany. 2009;103:1077–1089. doi: 10.1093/aob/mcp033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost I, Rydin H. Effects of competition, grazing and cotyledon nutrient supply on growth of Quercus robur seedlings. Oikos. 1997;79:53–58. [Google Scholar]
- García MB, Ehrlén J. Reproductive effort and herbivory timing in a perennial herb: fitness components at the individual and population levels. American Journal of Botany. 2002;89:1295–1302. doi: 10.3732/ajb.89.8.1295. [DOI] [PubMed] [Google Scholar]
- Gómez JM, Abdelaziz M, Muñoz-Pajares J, Perfectti F. Heritability and genetic correlation of corolla shape and size in Erysimum mediohispanicum. Evolution. 2009;63:1820–1831. doi: 10.1111/j.1558-5646.2009.00667.x. [DOI] [PubMed] [Google Scholar]
- Grace JB. Structural equation modeling and natural systems. Cambridge: Cambridge University Press; 2006. [Google Scholar]
- Hanley ME, Fegan EL. Timing of cotyledon damage affects growth and flowering in mature plants. Plant, Cell and Environment. 2007;30:812–819. doi: 10.1111/j.1365-3040.2007.01671.x. [DOI] [PubMed] [Google Scholar]
- Hanley ME, May OC. Cotyledon damage at the seedling stage affects growth and flowering potential in mature plants. New Phytologist. 2006;169:243–250. doi: 10.1111/j.1469-8137.2005.01578.x. [DOI] [PubMed] [Google Scholar]
- Hanley ME, Sykes RJ. Impacts of seedling herbivory on plant competition and implications for species coexistence. Annals of Botany. 2009;103:1347–1353. doi: 10.1093/aob/mcp081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanley ME, Fenner M, Edwards PJ. An experimental field study of the effects of mollusc grazing on seedling recruitment and survival in grassland. Journal of Ecology. 1995;83:621–627. [Google Scholar]
- Hanley ME, Fenner M, Whibley H, Darvill B. Early plant growth: identifying the end point of the seedling phase. New Phytologist. 2004;163:61–66. doi: 10.1111/j.1469-8137.2004.01094.x. [DOI] [PubMed] [Google Scholar]
- Hanley ME, Cordier PK, May OC, Kelly CK. Seed size and seedling growth: differential response of Australian and British Fabaceae to nutrient limitation. New Phytologist. 2007;174:381–388. doi: 10.1111/j.1469-8137.2007.02003.x. [DOI] [PubMed] [Google Scholar]
- Hirao AS. Kinship between parents reduces offspring fitness in a natural population of Rhododendron brachycarpum. Annals of Botany. 2010;105:637–646. doi: 10.1093/aob/mcq018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe HF, Brown JS, Zorn-Arnold B. A rodent plague on prairie diversity. Ecology Letters. 2002;5:30–36. [Google Scholar]
- Husheer SW, Robertson AW, Coomes DA, Frampton CM. Herbivory and plant competition reduce mountain beech seedling growth and establishment in New Zealand. Plant Ecology. 2006;183:245–256. [Google Scholar]
- Kettenring KM, Weekley CW, Menges ES. Herbivory delays flowering and reduces fecundity of Liatris ohlingerae (Asteraceae), an endangered, endemic plant of the Florida scrub. Journal of the Torrey Botanical Society. 2009;136:350–362. [Google Scholar]
- Kitajima K. Impact of cotyledon and leaf removal on seedling survival in three tree species with contrasting cotyledon functions. Biotropica. 2003;35:429–434. [Google Scholar]
- Lamont BB, Groom PK. Green cotyledons of two Hakea species control seedling mass and morphology by supplying mineral nutrients rather than organic compounds. New Phytologist. 2002;153:101–110. [Google Scholar]
- Lehtilä K. Precision of herbivore tolerance experiments with imposed and natural damage. Evolution. 2003;57:677–680. doi: 10.1111/j.0014-3820.2003.tb01559.x. [DOI] [PubMed] [Google Scholar]
- Mabry CM, Wayne PW. Defoliation of the annual herb Abutilon theophrasti: mechanisms underlying reproductive compensation. Oecologia. 1997;111:225–232. doi: 10.1007/s004420050229. [DOI] [PubMed] [Google Scholar]
- Marcelis LFM, Heuvelink E, Baan Hofman-Eijer LR, Den Bakker J, Xue LB. Flower and fruit abortion in sweet pepper in relation to source and sink strength. Journal of Experimental Botany. 2004;55:2261–2268. doi: 10.1093/jxb/erh245. [DOI] [PubMed] [Google Scholar]
- Maron JL. Interspecific competition and insect herbivory reduce bush lupine (Lupinus arboreus) seedling survival. Oecologia. 1997;110:284–290. doi: 10.1007/s004420050161. [DOI] [PubMed] [Google Scholar]
- Marshall DL, Abrahamson NJ, Avritt JJ, et al. Differences in plastic responses to defoliation due to variation in the timing of treatments for two species of Sesbania (Fabaceae) Annals of Botany. 2005;95:1049–1058. doi: 10.1093/aob/mci116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauricio R, Bowers MD, Bazzaz FA. Pattern of leaf damage affects fitness of the annual plant Raphanus sativus (Brassicaceae) Ecology. 1993;74:2066–2071. [Google Scholar]
- Moles AT, Westoby M. What do seedlings die from and what are the implications for evolution of seed size? Oikos. 2004;106:193–199. [Google Scholar]
- Mothershead K, Marquis RJ. Fitness impacts of herbivory through indirect effects on plant–pollinator interactions in Oenothera macrocarpa. Ecology. 2000;81:30–40. [Google Scholar]
- Muthén LK, Muthén BO. Mplus users guide. 6th edn. Los Angeles: Muthén and Muthén; 2010. [Google Scholar]
- Paul-Victor C, Turnbull LA. The effect of growth conditions on the seed size/number trade-off. PLoS ONE. 2009;4:e6917. doi: 10.1371/journal.pone.0006917. doi:10.1371/journal.pone.0006917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poveda K, Steffan-Dewenter I, Scheu S, Tscharntke T. Effects of below- and above-ground herbivores on plant growth, flower visitation and seed set. Oecologia. 2003;135:601–605. doi: 10.1007/s00442-003-1228-1. [DOI] [PubMed] [Google Scholar]
- Schmitt L. Individual flowering phenology, plant size, and reproductive success in Linanthus androsaceus, a California annual. Oecologia. 1983;59:135–140. doi: 10.1007/BF00388084. [DOI] [PubMed] [Google Scholar]
- Smith CC, Fretwell SD. The optimal balance between size and number of offspring. American Naturalist. 1974;108:499–506. [Google Scholar]
- Stinchcombe JR. Fitness consequences of cotyledon and mature-leaf damage in the ivyleaf morning glory. Oecologia. 2002;131:220–226. doi: 10.1007/s00442-002-0871-2. [DOI] [PubMed] [Google Scholar]
- Tiffin P, Inouye BD. Measuring tolerance to herbivory: accuracy and precision of estimates made using natural versus imposed damage. Evolution. 2000;54:1024–1029. doi: 10.1111/j.0014-3820.2000.tb00101.x. [DOI] [PubMed] [Google Scholar]
- Vallius E, Salonen V. Allocation to reproduction following experimental defoliation in Platanthera bifolia (Orchidaceae) Plant Ecology. 2006;183:291–304. [Google Scholar]
- Venable DL. Size–number trade-offs and the variation of seed size with plant resource status. American Naturalist. 1992;140:287–304. [Google Scholar]
- Weis AE, Kossler TM. Genetic variation in flowering time induces phenological assortative mating: quantitative genetic methods applied to Brassica rapa. American Journal of Botany. 2004;91:825–836. doi: 10.3732/ajb.91.6.825. [DOI] [PubMed] [Google Scholar]
- Winn AA, Gross KL. Latitudinal variation in seed weight and flower number in Prunella vulgaris. Oecologia. 1993;93:55–62. doi: 10.1007/BF00321191. [DOI] [PubMed] [Google Scholar]
- Yan J, Chu HJ, Wang HC, Li JQ, Tao S. Population genetic structure of two Medicago species shaped by distinct life form, mating system and seed dispersal. Annals of Botany. 2009;103:825–834. doi: 10.1093/aob/mcp006. [DOI] [PMC free article] [PubMed] [Google Scholar]