Abstract
Background and Aims
A detailed knowledge of cytotype distribution can provide important insights into the evolutionary history of polyploid systems. This study aims to explore the spatial distribution of different cytotypes in Pilosella echioides at various spatial scales (from the whole distributional range to the population level) and to outline possible evolutionary scenarios for the observed geographic pattern.
Methods
DNA-ploidy levels were estimated using DAPI flow cytometry in 4410 individuals of P. echioides from 46 populations spread over the entire distribution range in central Europe. Special attention was paid to the cytotype structure in the most ploidy-diverse population in south-west Moravia.
Key Results
Five different cytotypes (2x, 3x, 4x, 5x and 6x) were found, the last being recorded for the first time. Although ploidy-uniform (di- or tetraploid) sites clearly prevailed, nearly one-quarter of the populations investigated harboured more (up to all five) cytotypes. Whereas penta- and hexaploids constituted only a minority of the samples, a striking predominance of the triploid cytotype was observed in several populations.
Conclusions
The representative sampling confirmed previous data on cytotype distribution, i.e. the spatial aggregation of mixed-ploidy populations in south-west Moravia and Lower Austria and the predominance of ploidy-uniform populations in other parts of the area investigated. Recurrent origin of polyploids from diploid progenitors via unreduced gametes and their successful establishment are considered the key factors promoting intrapopulational ploidy mixture (‘primary hybrid zones’). As an alternative to the generally accepted theory of cytotype co-existence based on the development of different means of inter-ploidy reproductive isolation, it is suggested that a long-term ploidy mixture can also be maintained in free-mating populations provided that the polyploids originate with a sufficient frequency. In addition, the prevalence (or subdominance) of the triploid cytotype in several mixed-ploidy populations represents the first evidence of such a phenomenon in plant systems with exclusively sexual reproduction.
Keywords: Pilosella echioides, cytotype co-existence, cytotype distribution, flow cytometry, free mating, ploidy variation, triploid, sympatry
INTRODUCTION
A substantial fraction of angiosperm species (30–70 %) are at least ancient polyploids (Stebbins, 1950; Grant, 1981; Masterson, 1994; Bennett, 2004; Soltis, 2005), but contemporary studies point to the presence of a whole duplication genome event, i.e. polyploidization, at the base of almost all angiosperms (Buggs et al., 2009; Soltis et al., 2009). Principle questions in the research of polyploids relate to the mechanisms of their origin, establishment relative to putative diploid progenitors, and coexistence of the different cytotypes (Petit et al., 1999). There is increasing evidence that rates of polyploidization are often high and recurrent origins of both allo- and autopolyploids within diploid taxa are common (e.g. Brochmann et al., 1992; Soltis and Soltis, 1993, 1999, 2000; Wendel, 2000; Sharbel and Mitchell-Olds, 2001; Ramsey and Schemske, 2002; Guo et al., 2005; Wu et al., 2010). Special attention is also paid to the coexistence of cytotypes, namely within complexes of diploids and their autopolyploid derivatives, that is usually explained as either (a) a transitional stage in which one cytotype will outcompete the other, e.g. as a result of frequency dependent mating (cf. the minority cytotype exclusion principle; Levin, 1975; Fowler and Levin, 1984; Rodriguez, 1996; Petit et al., 1999; Husband, 2000; Kennedy et al., 2006), or (b) a more stable system with different cytotypes maintained by their different responses to spatial environmental variation (‘ecogeographic preferences’; Lewis, 1980). The latter can include ecological sorting, e.g. better adaptation of polyploids to harsh environment (cold, drought), competitive superiority of polyploids associated with higher growth rates (Lewis, 1980; Lumaret et al., 1987; Maceira et al., 1993; Felber-Girard et al., 1996; Husband and Schemske, 1998; Johnson et al., 2003), divergence in flowering time, divergence in behaviour and preferences of pollinators, mechanical isolation due to differences in floral morphology (van Dijk et al., 1992; Petit et al., 1997; Nuismer and Cunningham, 2005; Jersáková et al., 2010), and increased self-fertilization in polyploids (e.g. Husband and Schemske, 1997; Cook and Soltis, 2000; Barringer, 2007), all of which might lead to reproductive isolation and separation in space and time. Nonrandom insect attacks on genotypes with different ploidy levels can also affect cytotype frequencies, although it is strongly affected by the population size, site and herbivores (e.g. Münzbergová, 2006; Halverson et al., 2008a).
Distribution patterns of ploidy variation differ considerably among species. Some can consist of vicariant parapatric or allopatric populations of a single cytotype arranged along geographical or ecological gradients (e.g. Borgen and Hultgård, 2003; Mandáková and Münzbergová, 2006; Mráz et al., 2008; Trávníček et al., 2010). In other cases, mixed-ploidy populations occur with various frequencies across the geographic range of the species, often in variously wide contact zones between cytotypes (Borrill and Lindner, 1971; Husband and Schemske, 1998; Garnatje et al., 2001; Weiss et al., 2002; Keeler, 2004; Stuessy et al., 2004; Kao, 2008; Schönswetter et al., 2007; Suda et al., 2007b; Halverson et al., 2008b; Duchoslav et al., 2010). Hybrid zones arising in the area of inter-cytotype contact are traditionally divided into primary and secondary. A primary hybrid zone is formed as a direct consequence of the emergence of a neopolyploid within a diploid population, secondary hybrid zones arise following contact of cytotypes after phases of geographic separation (for review, see Petit et al., 1999).
Recent cytotype distributions can reflect the evolutionary past of particular species and various environmental or man-made factors that have co-determined it. Therefore knowledge of exact cytotype distribution patterns on geographical scales is helpful for understanding environmental conditions or evolutionary processes affecting such patterns (allopatric vs. sympatric occurrence; primary vs. secondary hybrid zone, etc.), coexistence of cytotypes within mixed populations (e.g. niche differentiation, assortative mating), and the frequency of inter-cytotype crossing (gene flow between cytotypes). The use of flow cytometry has allowed for cytotype evaluation of several-fold more individuals than conventional chromosome counting (Doležel, 1997) and is the most efficient tool for large screening studies of cytotype distribution (for review, see Kron et al., 2007).
Pilosella echioides is a perennial sexual allogamous and partly agamospermous species. Its geographic range stretches from south Russia westwards to central Europe (Bräutigam, 1992) where the occurrence is split into more or less isolated areas of various sizes. Distribution of P. echioides is closely associated with steppe grasslands that reached their widest distribution repeatedly in cold periods of Pleistocene glaciation, but it often occupies open sands, sandy grasslands, heathlands, rocks and light pine forests. Previous studies on P. echioides revealed the presence of four ploidy levels from diploids (2n = 18) to pentaploids (2n = 45) (Rotreklová et al., 2002, 2005) with diploid populations prevailing in Europe while mixed-ploidy populations with two to four cytotypes were found in south-west Moravia (Rotreklová et al., 2002, 2005), in the adjacent region of Lower Austria (Schuhwerk and Lippert, 1997) and in central and north-west Bohemia (Rotreklová et al., 2002). Diploids and triploids have also been shown to be sexual, tetraploids either sexual or (in some populations in north-west Hungary and south Slovakia) agamospermous, whereas the reproductive strategy of pentaploids is still poorly understood (Rotreklová et al., 2002, 2005; Peckert et al., 2005; Peckert and Chrtek, 2006; P. Trávníček et al., unpubl. res.). Both sexual and agamospermous populations (morphologically slightly different from each other) were reported from the Saratov region in Russia (Kashin and Cherishova, 1997).
The main goal of this study was to explore the spatial distribution of different cytotypes of P. echioides at various spatial scales (from the European part of the total geographic area to the population level) and to outline possible evolutionary and biogeographic scenarios for the observed geographic pattern. Special attention was paid to relatively large mixed-ploidy populations in south-west Moravia consisting exclusively of sexual plants (Rotreklová et al., 2002, 2005), which can serve as a model system for studies concerning mating interactions between diploids and polyploids, fitness differences among ploidy levels in various ontogenetic stages, and modelling dynamics of mixed-ploidy populations.
MATERIALS AND METHODS
Sampling of populations
A total of 46 Central European populations and 4410 individuals of Pilosella echioides (Lumn.) F.W. Schultz & Sch. Bip. (Hieracium echioides Lumn.) were surveyed for cytotype variation (Table 1). The scope of this study covered almost all contemporary known populations from Austria, Czech Republic, Germany, Hungary, Poland and Slovakia, comprising the geographic range 46°14′ to 53°54′N and 13°56′ to 21°48′E (Table 1). Plants from the rest of the distribution area (i.e. Asia and a part of the eastern Europe) were not included in this study due to their uncertain origin (hybridogenous origin cannot be ruled out) associated with taxonomical complexity (P. echioides s.l. is split into several minor species here). All samples included in this study could be straightforwardly indentified with taxonomic concept adopted for this species in central Europe. At all but one site, fresh leaves of 1–108 (45 in average) randomly selected plants were sampled, according to the abundance of plants at a particular locality and previously assessed cytotype composition (Table 1).
Table 1.
List of studied Pilosella echioides populations complemented by brief locality descriptions, geographical coordinates, altitude, DNA-ploidy levels, number of plants and the relative frequencies for particular cytotypes.
| Locality code | Locality details | Geographic coordinates (WGS 84) | Altitude (m a.s.l.) | DNA ploidy level | No. of plants | Relative frequency | Cited ploidy levels |
|---|---|---|---|---|---|---|---|
| D1 | Germany, Brandenburg, distr. Barnim: village of Niederfinow, waste sandy places and heathland along the road near the Schiffhebewerk | 52°50′50″N 13°56′50″E | 30–80 | 2x | 70 | 1·00 | 2x (Rotreklová et al., 2002) |
| D2 | Germany, Brandenburg, distr. Barnim: Falkenberg Hills, pastures above the road from village of Amalienhof to village of Struwenberg ca 0·4 km NW of Amalienhof | 52°49′30″N 13°56′10″E | 40 | 2x | 50 | 1·00 | 2x (Rotreklová et al., 2002) |
| PL1 | Poland, Wyżyna Małopolska upland, distr. Sandomierz, Góry Pieprzowe nature reserve near Sandomierz, loess slopes above the Wisła River, 4·8 km E of the city center | 50°41′03″N 21°48′15″E | 200 | 2x | 51 | 1·00 | 2x (Rotreklová et al., 2005) |
| PL2 | Poland, Wyżyna Małopolska upland, distr. Busko-Zdrój, Skorocice village, Skorocice nature reserve, grassland on gypseous rocks | 50°25′40″N 20°40′06″E | 210 | 2x | 51 | 1·00 | 2x (Rotreklová et al., 2005) |
| PL3 | Poland, Lower Odra valley, distr. Szczecin, Cedynia village, sandy soils near the road between villages of Bielinek and Dolny Lubiechów ca. 0·8 km of Bielinek | 52°55′40″N 14°09′30″E | 50 | 2x | 40 | 1·00 | 2x (Rotreklová et al., 2002) |
| PL4 | Poland, Lower Wisła valley, distr. Chełmno, Góra św. Wawrzyńca 1·3 km WNW of the village Kałdusy, sandy grassland | 50°19′38″N 18°22′55″E | 80 | 2x | 15 | 1·00 | |
| PL5 | Poland, Lower Wisła valley, distr. Sztum, Biała Góra nature reserve, ca. 0·8 km SE of the village Biała Góra, sandy slopes, very rare (1 plant) | 53°54′43″N 18°54′03″E | 32 | 2x | 1 | 1·00 | |
| PL6 | Poland, Wyżyna Małopolska upland, distr. Radom, Iłża, 0·2 km E of the castle ruines, mesophilous grassland (pasture) on loess | 51°09′47″N 21°14′31″E | 210 | 2x | 15 | 1·00 | |
| PL7 | Poland, Wyżyna Małopolska upland, distr. Ostrowiec Świętokrzyski, Ćmielów, 0·5 km S of the village, loess slopes on the road to Krzczonowice | 50°53′06″N 21°30′27″E | 185 | 2x | 15 | 1·00 | |
| PL8 | Poland, Wyżyna Małopolska upland, distr. Ostrowiec Świętokrzyski, Goździelin, 0·6 km S of the village, loess slopes on the road to Moczydło | 50°53′50″N 21°21′31″E | 190 | 2x | 15 | 1·00 | |
| SK1 | Slovakia, distr. Trebišov: Streda nad Bodrogom village, Tarbucka hill (277 m), sands on the NW slope (‘Veterné piesky’) | 48°22′41″N 21°46′59″E | 150 | 2x | 52 | 1·00 | 2x (Rotreklová et al., 2005) |
| SK2 | Slovakia, distr. Malacky: Borský Mikuláš village, a pinewood along the road from Borský Mikuláš village to Šaštín village | 48°37′45″N 17°11′17″E | 200 | 2x | 49 | 0·98 | 2x (Rotreklová et al., 2005) |
| 3x | 1 | 0·02 | |||||
| SK3 | Slovakia, Nitriansky kraj, distr. Nové Zámky: Čenkov, grassland on sand (nature reserve), 0·8 km NW of the village | 47°46′07″N 18°31′11″E | 116 | 4x | 4 | 1·00 | 4x (Peckert et al., 2005) |
| SK4 | Slovakia, Nitriansky kraj, distr. Nové Zámky: Jurský Chlm, near the road 0·5 km S of the village, grassland | 47°47′59″N 18°32′08″E | 150 | 4x | 2 | 1·00 | 4x (Peckert et al., 2005) |
| A1 | Austria, Burgenland, Leithagebirge: Winden am See, Mt. Zeilerberg (302 m) ca 2 km N of the village of Winden am See, grassland | 47°58′18″N 16°45′25″E | 260 | 2x | 32 | 1·00 | 2x (Rotreklová et al., 2002) |
| A2 | Austria, Niederösterreich: Retz, Windmühlenberg ca 0·7 km NW of the town Retz, dry grassland | 48°45′42″N 15°56′27″E | 317 | 2x | 11 | 0·17 | 2x, 3x, 4x (Schuhwerk and Lippert, 1997) |
| 3x | 27 | 0·43 | |||||
| 4x | 25 | 0·40 | |||||
| A3 | Austria, Niederösterreich, distr. Gänserndorf: slopes above the road near the railway station Helmahof | 48°18′37″N 16°35′47″E | 160 | 2x | 50 | 1·00 | |
| A4 | Austria, Niederösterreich, distr. Gänserndorf: open sandy sites ca. 1 km N of the village of Markhof | 48°16′43″N 16°50′19″E | 150 | 2x | 100 | 0·93 | 2x, 4x (Rotreklová et al., 2002) |
| 3x | 1 | 0·01 | |||||
| 4x | 7 | 0·06 | |||||
| A5 | Austria, Niederösterreich: Retz, hill Gollitsch (325 m) ca 0·5 km SW of the town of Retz, dry grassland | 48°45′15″N 15°56′37″E | 320 | 2x | 81 | 0·79 | |
| 3x | 17 | 0·16 | |||||
| 4x | 4 | 0·04 | |||||
| 5x | 1 | 0·01 | |||||
| A6 | Austria, Niederösterreich: Retz, slopes of Talberg hill (302 m) ca 1·0 km W of the village of Obernalb, dry grassland | 48°44′51″N 15°55′17″E | 390 | 4x | 50 | 1·00 | |
| H1 | Hungary, Komárom-Esztergom megye: Tokod-altáró, N margin of a sand-pit 1 km S of the village (Oldal-földek), sandy grassland | 47°43′23″N 18°41′42″E | 200 | 4x | 52 | 1·00 | |
| H2 | Hungary, Komárom-Esztergom megye: Dorog, Arany-hegy ca 2 km SE of the railway station, dry grassland | 47°42′20″N 18°44′22″E | 170 | 4x | 50 | 1·00 | |
| H3 | Hungary, Komárom-Esztergom megye: Dorog, Sátor-kő hill, 1 km SE of the railway station in the town | 47°43′13″N 18°45′05″E | 165 | 4x | 50 | 1·00 | |
| H4 | Hungary, Komárom-Esztergom megye: Leányvár, Kalap-hegyi-dűlő NW of the village, near Vaskapupuszta (Tapétagyár) | 47°41′40″N 18°45′35″E | 170 | 4x | 50 | 1·00 | |
| H5 | Hungary, Somogy megye: Nagybajom, Homokpuszta (near the road to Böhönye), 3·5 km NW of the village, sandy grassland | 46°24′16″N 17°28′34″E | 150 | 2x | 50 | 1·00 | |
| H6 | Hungary, Baranya megye: Nagyharsány, Villányi hegység, Mt. Szársomlyó, Szoborpark, upper margin of a quarry, along a path | 45°51′25″N 18°25′45″E | 150 | 4x | 20 | 1·00 | |
| H7 | Hungary, Veszprém megye: Lesenceistvánd, near a main road to Sümeg, near a sand-pit, ca 1·5 km NE of the village, sandy grassland | 46°52′58″N 17°21′35″E | 140 | 2x | 30 | 1·00 | |
| H8 | Hungary, Bakony Mts.: Fenyőfő village, the sands on the southwest border of the village | 47°17′N 17°45′E | 320 | 2x | 65 | 1·00 | 2x (Rotreklová et al., 2005) |
| H9 | Hungary, distr. Heves, Bükk Mts: stony slopes 0·8 km NW of the village of Szarvaskö (railway station) | 47°59′35″N 20°19′48″E | 340 | 2x | 53 | 1·00 | 2x (Rotreklová et al., 2002) |
| H10 | Hungary. Borsod-Abaúj-Zemplén megye: Tállya, Patócs hill, grassland on the top, 2·7 km NW of the village | 48°14′56″N 21°12′05″E | 200 | 4x | 43 | 0·90 | |
| 5x | 2 | 0·04 | |||||
| 6x | 3 | 0·06 | |||||
| H11 | Hungary, Pest megye: Újhartyán, disturbed grassland at the highway exit (Exit 44) NE of the village | 47°13′29″N 19°24′27″E | 118 | 4x | 50 | 1·00 | |
| H12 | Hungary, Székesfehérvár: Balinka, bottom of a deserted limestone quarry near the road from Balinka to Bodajk | 47°19′00″N 18°12′24″E | 172 | 4x | 41 | 1·00 | 4x (Peckert et al., 2005) |
| H13 | Hungary, Komárom-Esztergom megye: Szomód, Les-hegy hill, around the top, 2·2 km NW of the village | 47°41′56″N 18°19′24″E | 230 | 4x | 49 | 0·98 | |
| 6x | 1 | 0·02 | |||||
| H14 | Hungary, Bács-Kiskun megye: Kelebia, pine forest margin on sand 5 km NW of the village | 46°14′09″N 19°38′21″E |
130 | 4x | 25 | 1·00 | |
| H15 | Hungary, Pest megye, Szentendrei sziget: Tótfalu, dry grassland on sand dunes 4·3 km NNW of the village center | 47°47′30″N 19°04′42″E |
103 | 4x | 26 | 1·00 | |
| CZ1 | Czech Republic, distr. Mělník: village of Tišice, the sands along the railway Tišice – Neratovice, ca 100 m from railway station Tišice | 50°15′57″N 14°33′07″E | 160 | 2x | 62 | 1·00 | 2x (Rotreklová et al., 2005) |
| CZ2 | Czech Republic, České středohoří Mts., distr. Litoměřice: Kalvárie (‘Tříkřížová hora’) hill, rocks above the Labe river, 1·5 km NW of the village of Velké Žernoseky | 50°32′50″N 14°03′01″E | 214 | 3x | 18 | 0·19 | 3x, 4x (Rotreklová et al., 2002) |
| 4x | 35 | 0·38 | |||||
| 5x | 40 | 0·43 | |||||
| CZ3 | Czech Republic, distr. Beroun: ‘Trubínský vrch’ hill at the W margin of the village of Trubín | 49°56′37″N 13°59′47″E | 325 | 2x | 27 | 1·00 | 2x (Rotreklová et al., 2002) |
| CZ4 | Czech Republic, distr. Třebíč: nature reserve ‘Mohelenská hadcová step’, serpentine rocks near the village of Mohelno | 49°06′30″N 16°11′10″E | 350 | 2x | 19 | 1·00 | 2x (Rotreklová et al., 2002) |
| CZ5 | Czech Republic, distr. Znojmo: hill called ‘U Michálka’ at the S margin of the village of Bohutice, dry grassland | 48°59′03″N 16°21′31″E | 270 | 4x | 15 | 1·00 | 4x (Rotreklová et al., 2002) |
| CZ6 | Czech Republic, distr. Znojmo: hill at the NE margin of the village of Hoštěradice (place called ‘U kapličky’), dry grassland | 48°57′12″N 16°15′46″E | 240 | 4x | 46 | 0·94 | 2x, 3x (Rotreklová et al., 2002) |
| 6x | 3 | 0·06 | |||||
| CZ7 | Czech Republic, distr. Znojmo: slopes in the valley of the Dyje river SE of the village of Dyje | 48°50′26″N 16°07′27″E | 232 | 2x | 37 | 1·00 | 2x (Rotreklová et al., 2002) |
| CZ8 | Czech Republic, distr. Znojmo: heathland and waste places S of the village of Konice (nature reserve ‘Popické kopečky’) | 48°49′44″N 16°01′11″E | 300 | 3x | 4 | 0·27 | 3x, 4x (Rotreklová et al., 2002) |
| 4x | 10 | 0·67 | |||||
| 5x | 1 | 0·06 | |||||
| CZ9 | Czech Republic, distr. Znojmo: Skalky hill 0·6 km S of the church in the village of Havraníky | 48°48′18″N 16°00′31″E | 304 | 4x | 100 | 1·00 | 4x (Rotreklová et al., 2002) |
| CZ10 | Czech Republic, distr. Znojmo: Hnanice, S slopes of the ‘Staré vinice’ hill (339 m) ca 0·8 km N of the village of Hnanice | 48°48′23″N 15°59′28″E | 318 | 3x | 65 | 0·60 | |
| 4x | 30 | 0·28 | |||||
| 5x | 13 | 0·12 | |||||
| CZ11 | Czech Republic, distr. Znojmo: Havranické vřesoviště heathland 1·0 km W to NW of the village of Havraníky For more details and data on subpopulation level see Fig. 3 and Table 2. |
48°49′10″N 16°00′10″E | 325 | 2x | 134 | 0·06 | 2x, 3x, 4x (Rotreklová et al., 2002) |
| 3x | 1745 | 0·73 | |||||
| 4x | 483 | 0·20 | |||||
| 5x | 25 | 0·01 | |||||
| 6x | 1 | 0·00 |
The large population at the Havranické vřesoviště heathland (a former pasture) is currently fragmented into more or less isolated subpopulations (at least in terms of pollinator movements). The fragmentation is due to terrain ruggedness and the invasion of Arrhenatherum elatius (L.) J. Presl & C. Presl or trees and shrubs. Altogether 19 subpopulations and 2388 individuals (54·1 % of total amount) were selected here and screened for small-scale ploidy level variation (Table 2). Depending on the abundance of plants, approx. 29–179 individuals per subpopulation were sampled to assess cytotype composition. The only exception was subpopulation Ha13, where a fine-scale distribution analysis was performed and altogether 979 plants were screened for ploidy level with their position recorded on an orthogonal grid (Table 2 and Fig. 4A).
Table 2.
List of Pilosella echioides subpopulations from the Havranické vřesoviště heathland (population CZ11), their cytotype composition, number of plants and relative frequencies of particular cytotypes
| Subpopulation code | DNA ploidy level | No. of plants | Relative frequency |
|---|---|---|---|
| Ha1 | 2x | 18 | 0·13 |
| 3x | 108 | 0·80 | |
| 4x | 8 | 0·06 | |
| 5x | 1 | 0·01 | |
| Ha2 | 3x | 36 | 0·72 |
| 4x | 14 | 0·28 | |
| Ha3 | 2x | 9 | 0·05 |
| 3x | 145 | 0·81 | |
| 4x | 20 | 0·11 | |
| 5x | 5 | 0·03 | |
| Ha4 | 3x | 19 | 0·38 |
| 4x | 31 | 0·62 | |
| Ha5 | 2x | 6 | 0·09 |
| 3x | 57 | 0·85 | |
| 4x | 4 | 0·06 | |
| Ha6 | 2x | 3 | 0·05 |
| 3x | 61 | 0·94 | |
| 4x | 1 | 0·02 | |
| Ha7 | 3x | 4 | 0·08 |
| 4x | 46 | 0·92 | |
| Ha8 | 2x | 2 | 0·03 |
| 3x | 44 | 0·67 | |
| 4x | 20 | 0·30 | |
| Ha9 | 3x | 71 | 1·00 |
| Ha10 | 2x | 10 | 0·17 |
| 3x | 24 | 0·41 | |
| 4x | 25 | 0·42 | |
| Ha11 | 2x | 47 | 0·96 |
| 3x | 2 | 0·04 | |
| Ha12 | 2x | 20 | 0·69 |
| 3x | 9 | 0·31 | |
| Ha13 | 2x | 8 | 0·01 |
| 3x | 874 | 0·89 | |
| 4x | 97 | 0·10 | |
| Ha14 | 2x | 1 | 0·01 |
| 3x | 93 | 0·85 | |
| 4x | 15 | 0·14 | |
| 5x | 1 | 0·01 | |
| Ha15 | 3x | 13 | 0·35 |
| 4x | 15 | 0·41 | |
| 5x | 9 | 0·24 | |
| Ha16 | 3x | 55 | 0·80 |
| 4x | 13 | 0·19 | |
| 5x | 1 | 0·01 | |
| Ha17 | 2x | 10 | 0·08 |
| 3x | 57 | 0·46 | |
| 4x | 57 | 0·46 | |
| 6x | 1 | 0·01 | |
| Ha18 | 4x | 49 | 1·00 |
| Ha19 | 3x | 73 | 0·49 |
| 4x | 68 | 0·46 | |
| 5x | 8 | 0·05 |
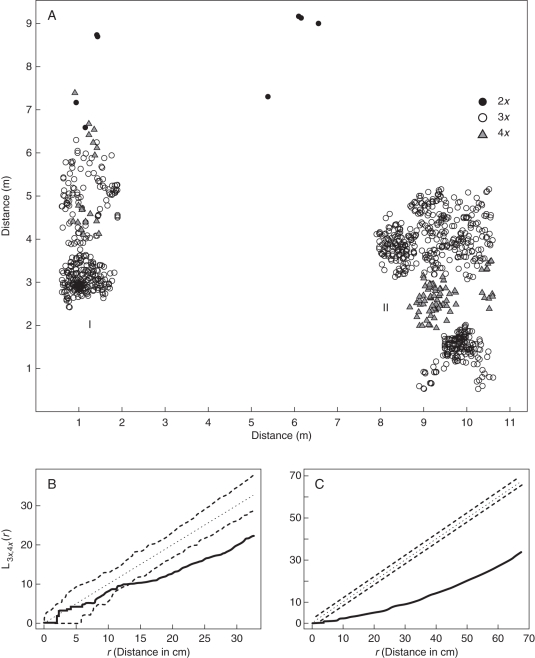
Fig. 4.
Fine-scale distribution of Pilosella echioides cytotypes in subpopulation Ha13 from the Havranické vřesoviště heathland (population CZ11). (A) Spatial arrangement of Pilosella plants – note distinct intra-cytotype clustering and structuring of the subpopulation into separate areas I and II. (B, C) Pairwise inter-cytotype association between triploid and tetraploid plants in both areas (I in B and II in C). Values of L3x,4x(r)-function are shown by a thick continuous line and dashed lines delimit a 95 % confidence interval. The negative association of cytotypes is indicated by smaller values of L(r)-function than is the lower threshold of the confidence interval which is obvious in both subplots. It means that both cytotypes are strongly clumped together and spatially separated from each other. Diploids were excluded from pairwise comparisons due to their low abundance.
Voucher specimens of selected plants from each population are deposited in herbarium PRA.
Flow cytometry
All 4410 collected plants were subjected to DNA-ploidy level estimation via flow cytometry (Suda et al., 2006). Young, intact leaf tissue of the analysed plant(s) and an appropriate amount of leaf tissue of the internal reference standard [Bellis perennis L., 2C-value set to 3·96 pg following Leong-Škorničková et al. (2007)] were co-chopped using a sharp razor blade in a plastic Petri-dish containing 0·5 mL of ice-cold Otto I buffer (0·1 m citric acid, 0·5 % Tween 20) (Otto, 1990; Doležel et al., 2007). The crude suspension was filtered through a 0·42-μm nylon mesh to remove tissue debris and then incubated for at least 10 min at room temperature. Isolated nuclei were stained with 1 mL of Otto II buffer (0·4 m Na2HPO4·12H2O) supplemented with the AT-selective fluorochrome 4′,6-diamidino-2-phenylindole (DAPI) and β-mercaptoethanol at final concentrations of 4 µg mL−1 and 2 µL mL−1, respectively. Immediately after staining, the relative fluorescence intensity of at least 3000 particles was recorded with a PA-II flow cytometer (Partec GmbH, Münster, Germany) equipped with a mercury lamp for UV excitation. Resulting histograms were evaluated with FloMax software (Partec GmbH) and DNA ploidy levels were determined on the basis of the sample/standard ratio. The Pilosella tissue was processed within 7 d of collection. Usually, bulk samples from three (but for ploidy-uniform populations up to ten) Pilosella plants (0·5 cm2 of leaf from each plant) were measured. Previous trial analyses confirmed the reliability of such a protocol (i.e. the lack of endopolyploidy and the low mitotic activity in the Pilosella tissues selected for flow analyses). In addition, good congruency between the number of nuclei in particular peaks and the number of analysed individuals with different ploidy levels allowed the proportions of the cytotypes in mixed samples to be estimated with high accuracy. Only histograms with coefficients of variation of G0/G1 peaks of both the bulked sample and the standard below 3·5 % were considered. If the quality of analyses did not meet this criterion, all plants from the bulked sample were re-analysed separately (to detect potential between-plant differences in fluorescence intensity).
Chromosome counts are available from all ploidy (but hexaploid) levels and a representative subset of populations included in the present study (Table 1). A standard procedure to correct evaluation of ploidy level via flow cytometry in concordance with chromosome counts has been already set (Rotreklová et al., 2002, 2005). Genome size of di-, tri-, tetra- and pentaploids was published by Suda et al. (2007a).
Spatial analyses
For fine-scale spatial analysis of cytotype distribution, the position of all individuals in the mapped subpopulation (Ha13; population CZ11) was analysed using the K-function (Ripley, 1977) in the R-package ‘spatstat’ (Baddeley and Turner, 2005). Both, the intensity and the type (random, clumped or regular) of clustering of an individual cytotype distribution are detected with the K-function and the computation is based on comparison of the number of neighbours within a radius r of each individual from the plot with the expected unit density calculated from the number of all individuals and study plot area. For inter-cytotype comparison, the bivariate K12(r)-function (Cressie, 1993) was calculated to assess positive, neutral or negative associations based on counting all neighbours of the other cytotype within r. Transformation of K-functions to L-functions (Doležal et al., 2006) is used to calculate the spatial patterns at various scales, i.e. for increasing radius r. Graphic visualization of L12(r) vs. r was determined by Monte Carlo permutations with 1000 replications to assess the type of individual association by comparing the behaviour of the L-function in relation to the 95 % confidence interval. Values of L12(r)-function above, within and below the limits of confidence interval point out positive, neutral and negative association of compared cytotypes, respectively.
RESULTS
Cytotype distribution on a large scale
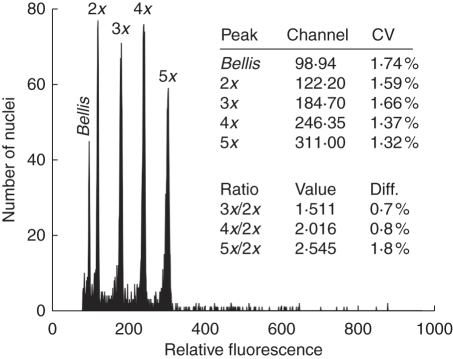
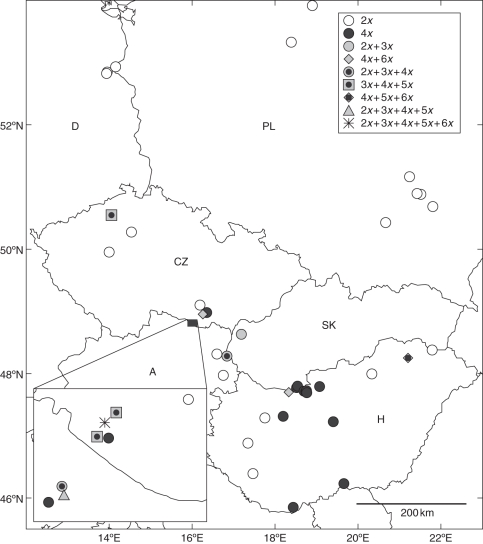
Five DNA-ploidy levels were discovered among the 4410 plants within 46 populations. A representative histogram bearing the four most common ploidy levels is provided in Fig. 1. While diploids, triploids, tetraploids and pentaploids were known from previous research (Table 1), hexaploids are reported here for the first time. Altogether 11 populations (23·9 %) were assessed as mixed and 35 (76·1 %) as cytotypically uniform, possessing either diploids (21 populations) or tetraploids (14). Besides the two types of uniform populations (diploid or tetraploid), seven different population types (with respect to cytotype composition) were also found. They consisted of two (three populations), three (six), four (one) and five (one) cytotypes (for details, see Table 1). Whereas populations SK2, H13 and CZ6 had two intermingled cytotypes, where one was dominant and the second one in frequencies below 0·10, complex patterns and more or less balanced compositions of cytotypes were usually observed in populations with sympatric growth of three and more cytotypes (except for populations A4 and H10; Table 1). Overall the distribution patterns (Fig. 2) point to the accumulation of mixed-ploidy populations in south-west Moravia and adjacent regions of Lower Austria, several of which were rare in other parts of the area investigated (i.e. populations CZ2, H10 and H13). While cytotypically uniform populations prevail in the study area, diploid populations were assessed as ubiquitous and only tetraploid ones were shown to have some spatial ties to Hungary (Table 1).
Fig. 1.
Flow cytometric histogram of four most common cytotypes (2x, 3x, 4x and 5x) of Pilosella echioides from the Havranické vřesoviště heathland (populations CZ11) and the internal reference standard (Bellis perennis). Nuclei from all plants were isolated, stained with DAPI and analysed simultaneously. Note low coefficients of variance of resulting peaks and virtually identical size of the monoploid genome of all Pilosella cytotypes.
Fig. 2.
Distribution of Pilosella echioides cytotypes in the area investigated, based on flow-cytometric analysis of 4410 individuals from 46 populations. The upper-right inset shows symbols used for populations with different cytotype composition. The lower-left inset shows the spatial distribution of populations in south-west Moravia (Czech Republic) and Lower Austria. Country abbreviations: A, Austria; CZ, Czech Republic; D, Germany; H, Hungary; PL, Poland; SK, Slovakia. Population CZ11, which is often mentioned in the text, is marked by an asterisk.
Cytotype distribution on a small- and fine-scale
Detailed investigation of the Havranické vřesoviště heathland (south-west Moravia, population CZ11) revealed a high degree of differentiation in cytotype composition and their relative frequencies at the subpopulation level (Fig. 3 and Table 2). Generally, out of 19 subpopulations examined only two were assessed as cytotype-uniform (i.e. subpopulations 9 and 18 hosting triploids and tetraploids, respectively). The majority of populations were of mixed ploidy levels with two (five subpopulations), three (eight) and four (four) cytotypes co-occurring. Altogether eight different patterns of cytotype composition were identified with the prevailing type of 2x, 3x and 4x individuals growing in sympatry (five subpopulations, 26·3 %). Such extensive insight into this particular population enables reliable interpretations of cytotype composition at the individual level (across all subpopulations) and points to a huge overall predominance of triploids (73·1 % out of almost 2400 plants analysed). In contrast, only 5·6 %, 20·2 % and 1·1 % representation was ascertained for diploids, tetraploids and pentaploids, respectively. In addition just a single plant was estimated as hexaploid.
Fig. 3.
Distribution of Pilosella echioides cytotypes in spatially isolated subpopulations in the Havranické vřesoviště heathland (population CZ11, south-west Moravia, Czech Republic). The lower-right inset shows symbols used for populations with different cytotype composition. Numbers refer to detailed information on subpopulations in Table 2. (Orthophoto used with the permission of the Podyjí National Park Administration, ©GEODIS Brno 2008.)
Although all subpopulations exhibit specific combinations and frequencies of particular cytotypes (Table 2), some tendencies should be highlighted. Unlike other parts of the area investigated, diploid and tetraploid cytotypes were predominant in only four subpopulations (two per ploidy level). In contrast, triploids, which were usually very rare, were detected as the dominant cytotype in ten subpopulations (52·6 %), and in another five subpopulations they comprise the main admixture. In agreement with the generally observed pattern, pentaploids were a minority component (except for subpopulation Ha15, where there was almost equal representation of triploids, tetraploids and pentaploids; Table 2).
The fine-scale cytotype distribution was studied in one randomly chosen subpopulation (Ha13, one of the 12 subpopulations harbouring at least three different cytotypes) to reveal spatial patterns among ploidy levels at the individual level (Fig. 4A). Remarkably plants of the same cytotype were clumped together and thus a high degree of negative intercytotype association (even at distance of <5 cm) was documented (Fig. 4B and C).
DISCUSSION
Large-scale cytotype distribution
The diploid cytotype of Pilosella echioides prevails in central Europe and is the only one discovered so far in regions north of the Sudeten Mts and the Carpathians (north Germany, Poland). In contrast, both diploid and tetraploid populations with more or less pronounced differences in ecological preferences (sandy places and rocks vs. dry grasslands, respectively) and geographical distribution were found in the Pannonian Basin (samples from Slovakia, Austria and Hungary; Fig. 2 and Table 1). However, such spatial separation could be at least partially caused by the presence of exclusively agamospermous tetraploid populations in this area (Peckert et al., 2005). In agreement with previously published papers (Schuhwerk and Lippert, 1997; Rotreklová et al., 2002, 2005), considerable cytotype variation and the common occurrence of mixed-ploidy populations were found in south-west Moravia and in the adjacent region of Lower Austria (Fig. 2 and Table 1). On the other hand, the presence of hexaploids (populations H10 and H13) and pentaploids (population H10) in otherwise tetraploid Hungarian populations was hitherto unknown. The overall distribution pattern showed only poor spatial structuring of cytotypes in contrast to previous studies from the same area examining polyploid complexes of Vicia cracca (Trávníček et al., 2010), Pilosella officinarum (Mráz et al., 2008), Knautia arvensis (Kolář et al., 2009) and Allium oleraceum (Duchoslav et al., 2010). Nevertheless, a similar pattern, showing the non-structured distribution of ploidy-uniform and the scattered presence of mixed-ploidy populations, has previously been reported in Gymnadenia conopsea (Trávníček et al., 2011). Moreover, Pilosella echioides with up to five ploidy levels within a single population joins Gymnadenia conopsea and Senecio carniolicus (Schönswetter et al., 2007; Suda et al., 2007b; Hülber et al., 2009) in being one of the most diverse polyploid complexes ever detected.
Small-scale cytotype distribution and sympatric occurrence
The present detailed research of the most diverse mixed-ploidy population (Havranické vřesoviště heathland, CZ11) revealed stochastic spatial arrangement of cytotypes at the entire population level and a pronounced cytotype coexisting ability in various types of composition and frequencies (Fig. 3 and Table 2). This challenges theoretical predictions suggesting that the co-occurrence of ploidy types should be unstable (minority cytotype extinction) except for the evolution of reproductive barriers (e.g. Rieseberg and Willis, 2007) both pre-zygotic (e.g. differences in pollinator's assemblages and/or selective pollinator's foraging behaviour; Thompson et al., 2004; Nuismer and Cunningham, 2005; Thompson and Merg, 2008; Husband and Sabara, 2004; Kennedy et al., 2006) and/or post-zygotic (e.g. triploid block; Baack, 2005a; Köhler at al., 2010). However, reproductive barriers and assortative mating have not evolved in Pilosella echioides since all cytotypes can hybridize and produce viable offspring without dramatically reduced fitness or pollen preferences (Peckert and Chrtek, 2006; P. Trávníček et al., unpubl. res.). A high proportion (even dominance) of triploids indicates the frequent occurrence of inter-cytotype hybridization and the lack of triploid block. Moreover, the cytotypes do not differ from each other in their morphological characters, flower display and flowering time. Thus assortative mating caused by pollinator movements within rather than between cytotypes very likely should be ruled out. A similar system lacking pre-mating barriers, despite morphological differences between cytotypes, was found in Gymnadenia conopsea s.l. (Jersáková et al., 2010), but post-zygotic mating barriers acting in those populations restricted the possibility of inter-cytotype hybrids (Trávníček et al., 2011).
The fine-scale spatial distribution of cytotypes showed outstanding intra-cytotype aggregation and inter-cytotype separation (Fig. 4) that cannot be easily explained by one simple hypothesis. Vegetative spread by daughter rosettes could easily explain the intra-cytotype aggregation but it is rather rare (P. Trávníček et al., unpubl. res.). The short-distance achene dispersal can be suggested as another possible explanation as it could contribute to cytotype coexistence (Baack, 2005b) and to accumulation of particular cytotypes (e.g. seeds from 2x × 4x crosses are almost exclusively triploid; Peckert and Chrtek, 2006). On the other hand, the achenes are adapted to long-distance dispersal due to the presence of a pappus. An alternative hypothesis is spatial separation (pre-mating barrier), i.e. microhabitat differentiation of cytotypes (Felber-Girard et al., 1996; Šafářová and Duchoslav, 2010) which can significantly increase the probability of successful intra-cytotype mating and, thus, decrease the minority cytotype disadvantage (Levin, 1975; Felber, 1991). However, selection pressures leading to spatial separation are lacking due to the aforementioned free mating, producing viable and usually fertile inter-cytotype hybrids. In addition, aggregation of triploids is contrariwise due to the absence of recruitment ability by reciprocal hybridization (Peckert and Chrtek, 2006). Furthermore, separation on such a fine-scale is not likely to prevent pollen movement between cytotypes (the pollinators have longer cruising ranges). Last but not least, a preliminary study (P. Trávníček et al., unpubl. res.) does not show correlations between cytotype occurrence and vegetation features (density, structure, species composition), although other factor(s) can drive the fine-scale distribution of cytotypes. Clearly the available data on cytotype coexistence in Pilosella echioides are insufficient to completely address such curious phenomena and future in-depth studies are needed.
Origin of populations with ploidal heterogeneity
Although the present study corroborates the occurrence of mixed-ploidy populations of Pilosella echioides in south-west Moravia and Lower Austria, their origin is still puzzling. Nevertheless, the hypothesis that the polyploids arose within diploid populations, i.e. establishment of a so-called ‘primary hybrid zone’ (e.g. Felber, 1991; Ramsey and Schemske, 1998; Petit et al., 1999) appears to be likely. It is possible that the scarce presence of triploids and/or tetraploids in otherwise pure diploid populations outside this area (e.g. populations SK2 and A4) could point to the first stages of polyploid establishment (Husband, 2004; Yamauchi et al., 2004; Baack, 2005a), analogous to past processes in populations in south-west Moravia. However, an alternative explanation, namely long-distance seed dispersal (promoted by achenes with pappus) from polyploid populations, can be suggested. A detailed study of Pilosella aurantiaca, a species with seeds nearly identical to P. echioides, revealed that up to 95 % achenes were dispersed within 6 m distance from the mother plant (Stergios, 1976). Similar dispersal distances were observed in another Asteraceae species, e.g. in Senecio inaequidens where 99·8 % of achenes were dispersed within 100 m with a maximum deposition rate of 5·2 m from the maternal plant (Monty et al., 2008). Although short-distance achene dispersal seems to prevail within close relatives, the long-distance achene dispersal is inherently difficult to quantify and cannot be simply ruled out as a contributory element to gene flow in Pilosella echioides. It can also be argued that polyploid achenes (embryos) were observed in neither hand-pollinated diploids (Peckert and Chrtek, 2006) nor natural diploid populations (P. Trávníček et al., unpubl. res.) therefore long-distance seed dispersal is a plausible explanation.
The ‘primary hybrid zone’ hypothesis can be supported by the overall cytotype distribution pattern, i.e. by the lack of a transitional zone between the two main ploidy levels (di- and tetraploids), either in the entire area investigated or in the vicinity of populations showing an increased incidence of mixed-ploidy (Fig. 2). Therefore, the hypothesis of enhanced occurrence of populations with cytotype variability in such zones (e.g. Husband and Schemske, 1998; Petit et al., 1999, Trávníček et al., 2010) seems to be unlikely. Nevertheless, different past cytotype distribution patterns could boost the establishment of a contact zone between cytotypes and contribute to the present state.
Population history per se can play a crucial role in the formation of cytotype co-occurrence. Several populations (including the cytotypically diverse CZ2, CZ8, CZ10 and especially CZ11) inhabit relict areas, such as rocks in canyons of big rivers (Labe, Dyje), where a permanent forest-free area has been hypothesized (Chytrý and Vicherek, 1995, 2003; Ložek, 2007). The long-term occurrence of different cytotypes in such habitats, leading to the evolution of mechanisms allowing sympatric growth is one possible explanation. This agrees with the unusual occurrence of sexual hexaploids in closely related Pilosella officinarum in the same habitats (Mráz et al., 2008). Alternatively, relict habitats may have facilitated the long-term occurrence of diploids and tetraploids in close vicinity and their equal adaptation to local conditions. More recently, as new secondary habitats are created by man-made changes (including deforestation and maintenance of suitable forest-free areas by pasture) both cytotypes may have been able to spread out widely and form mixed-ploidy populations. If so, then the relatively short-term coexistence of cytotypes may explain the absence of any kind of pre-zygotic isolation. Clearly, all outlined scenarios need further in-depth study and no definitive conclusions can be drawn at present.
Besides the mixed-ploidy populations comprising diploids and tetraploids, it is noted that diploids are absent in several populations (e.g. populations CZ2, CZ8 and CZ10), and here only polyploids (tetraploids and higher) coexist (populations H10, H13 and CZ6). That such populations were founded by tetraploids is very likely and their further diversification may have been triggered by the fusion of reduced and unreduced gametes and subsequent backcrossing. Nevertheless, an allopolyploid origin of higher ploidy levels via introgression from coexisting Pilosella species cannot be ruled out completely despite the morphological identity of cytopes and the fact that Pilosella species differ distinctly in their genome size (and thus interspecific hybridization is detectable by flow cytometry; Suda et al., 2007a).
The role of triploids in mixed-ploidy populations
One of the most challenging findings of the present study is the prevalence of fertile (though with lower fecundity in comparison with di- and tetraploids; Peckert and Chrtek, 2006) triploids in several cytotypically diverse populations. The role of triploids has hitherto been understood to be crucial in promoting autotetraploid establishment (Husband, 2004), despite the observation of lower fitness and fecundity of triploids (Felber, 1991; Burton and Husband, 2000; Baack, 2005a). This has resulted in the evolution of various pre-zygotic barriers between newly arisen tetraploids and their diploid progenitors constraining triploid genesis based on the principle of minority cytotype disadvantage (Levin, 1975; Husband, 2000; Burton and Husband, 2001; Baack, 2005a). Contrary to this broadly accepted model, the present research indicates that triploids are essentially equivalent to diploids and tetraploids, if free inter-cytotype mating and sufficient triploid fitness are ensured. Under these conditions triploids even produced the highest cytotype seed variation and contributed significantly to the maintenance of mixed-ploidy populations (Peckert and Chrtek, 2006).
Conclusions and future prospects
Pilosella echioides, with up to five different ploidy levels growing in sympatry, represents a species with one of the most complex patterns of cytotype coexistence so far detected. The uniqueness of this plant system also lies in (a) a presumable lack of inter-cytotype breeding barriers and (b) a high frequency of triploid individuals. These findings stand in stark contrast to generally accepted theories of cytotype coexistence and call for further research aimed at understanding the evolutionary forces governing formation, establishment and further fate of different cytotypes. The possibility of a long-term ploidy co-existence due to free inter-cytotype mating interactions reshapes our views on (a) the evolution of pre- and/or post-zygotic breeding barriers among cytotypes, (b) the patterns and dynamics of inter-cytotype interactions and (c) the processes maintaining mixed-ploidy populations. In addition, the abundance of triploid plants coupled with their fertility provides qualitatively new information on the evolutionary potential of this odd-ploidy level and its role in maintaining ploidy mixtures. Collectively, Pilosella echioides represents a unique system for detailed investigation of evolutionary dynamics of populations with ploidy heterogeneity.
ACKNOWLEDGEMENTS
This work was supported by the Academy of Sciences of the Czech Republic (grant numbers KJB601110813 and AV0Z60050516) and the Charles University in Prague (grant number 1207/2007). Further support was provided by the Ministry of Education, Youth and Sports of the Czech Republic (grant numbers MSM 0021620828 and MSM 6007665806), Ministry of Culture of the Czech Republic (grant no. MK00002327201) and Czech Science Foundation (grant number 206/08/H049). We would like to thank Hana Chudáčková (Prague), Jana Rauchová (Průhonice), Pavla Růžičková (Prague), Tomáš Urfus (Prague), Petr Vít (Prague) and Jaroslav Zahradníček (Prague) for their asistance in the field and laboratory, Filip Kolář (Prague) for his assistance with fine-scale spatial analysis, Zoltán Barina (Budapest) for his help during the sampling in Hungary, Tomáš Peckert (Přimda) for his valuable comments at the beginning of the project and Jan Suda (Prague) for his help with finalizing the manuscript. Our special thanks go to Podyjí National Park Administration for their every support and permission needed for part of the project.
LITERATURE CITED
- Baack EJ. Ecological factors influencing tetraploid establishment in snow buttercups (Ranunculus adoneus): minority cytotype exclusion and barriers to triploid formation. American Journal of Botany. 2005a;92:1827–1835. doi: 10.3732/ajb.92.11.1827. [DOI] [PubMed] [Google Scholar]
- Baack EJ. To succeed globally, disperse locally: effect of local pollen and seed dispersal on tetraploid establishment. Heredity. 2005b;94:538–546. doi: 10.1038/sj.hdy.6800656. [DOI] [PubMed] [Google Scholar]
- Baddeley A, Turner R. Spatstat: an R package for analyzing spatial point patterns. Journal of Statistical Software. 2005;12:1–42. [Google Scholar]
- Barringer BC. Polyploidy and self-fertilization in flowering plants. American Journal of Botany. 2007;94:1527–1533. doi: 10.3732/ajb.94.9.1527. [DOI] [PubMed] [Google Scholar]
- Bennett MD. Perspectives on polyploidy in plants – ancient and neo. Biological Journal of Linnean Society. 2004;82:411–423. [Google Scholar]
- Borgen L, Hultgård U-M. Parnassia palustris: a genetically diverse species in Scandinavia. Botanical Journal of the Linnean Society. 2003;142:347–372. [Google Scholar]
- Borrill M, Lindner R. Diploid–tetraploid sympatry in Dactylis (Gramineae) New Phytologist. 1971;70:1111–1124. [Google Scholar]
- Bräutigam S. In: Vergleichende Chorologie der zentraleuropäischen Flora 3. Meusel H, Jäger EJ, editors. Jena: Gustav Fischer, 325–333; 1992. pp. 550–560. Hieracium L. [Google Scholar]
- Brochmann C, Soltis PS, Soltis DE. Recurrent formation and polyphyly of Nordic polyploids in Draba (Brassicaceae) American Journal of Botany. 1992;79:673–688. [Google Scholar]
- Buggs RJA, Soltis PS, Soltis DE. Does hybridization between divergent progenitors drive whole-genome duplication? Molecular Ecology. 2009;18:3334–3339. doi: 10.1111/j.1365-294X.2009.04285.x. [DOI] [PubMed] [Google Scholar]
- Burton TL, Husband BC. Fitness differences among diploids and tetraploids and their triploid progeny in Chamerion angustifolium (Onagraceae): mechanisms of inviability and implications for polyploid evolution. Evolution. 2000;54:1182–1191. doi: 10.1111/j.0014-3820.2000.tb00553.x. [DOI] [PubMed] [Google Scholar]
- Burton TL, Husband BC. Fecundity and offspring ploidy in matings among diplod, triploid and tetraploid Chamerion angustifolium (Onagraceae): consequences for tetraploid establishment. Heredity. 2001;87:573–582. doi: 10.1046/j.1365-2540.2001.00955.x. [DOI] [PubMed] [Google Scholar]
- Chytrý M, Vicherek J. Lesní vegetace Národního parku Podyjí. Die Waldvegetation des Nationalparks Thayatal. Prague: Academia; 1995. [Google Scholar]
- Chytrý M, Vicherek J. Travinná, keříčková a křovinná vegetace Národního parku Podyjí [Grassland, heathland, and scrub vegetation of the Podyjí/Thayatal National Park] Thayensia. 2003;5:11–84. [Google Scholar]
- Cook LM, Soltis PS. Mating systems of diploid and allotetraploid populations of Tragopogon (Asteraceae). II. Artificial populations. Heredity. 2000;84:410–415. doi: 10.1046/j.1365-2540.2000.00654.x. [DOI] [PubMed] [Google Scholar]
- Cressie NAC. Statistics for spatial data. New York, NY: J. Wiley and Sons; 1993. [Google Scholar]
- van Dijk P, Hartog M, van Delden W. Single cytotype areas in autopolyploid Plantago media L. Biological Journal of the Linnean Society. 1992;46:315–331. [Google Scholar]
- Doležel J. Application of flow cytometry for the study of plant genomes. Journal of Applied Genetics. 1997;38:285–302. [Google Scholar]
- Doležal J, Šrůtek M, Hara T, Sumida A, Penttilä T. Neighborhood interactions influencing tree population dynamics in nonpyrogenous boreal forest in northern Finland. Plant Ecology. 2006;185:135–150. [Google Scholar]
- Doležel J, Greilhuber J, Suda J. Estimation of nuclear DNA content in plants using flow cytometry. Nature Protocols. 2007;2:2233–2244. doi: 10.1038/nprot.2007.310. [DOI] [PubMed] [Google Scholar]
- Duchoslav M, Šafářová L, Krahulec F. Complex distribution patterns, ecology and coexistence of ploidy levels of Allium oleraceum (Alliaceae) in the Czech Republic. Annals of Botany. 2010;105:719–735. doi: 10.1093/aob/mcq035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felber F. Establishment of a tetraploid cytotype in a diploid population: effect of relative fitness of the cytotypes. Journal of Evolutionary Biology. 1991;4:195–207. [Google Scholar]
- Felber-Girard M, Felber F, Buttler A. Habitat differentiation in a narrow hybrid zone between diploid and tetraploid Anthoxanthum alpinum. New Phytologist. 1996;133:531–540. [Google Scholar]
- Fowler NL, Levin DA. Ecological constraints on the establishment of a novel polyploid in competition with its diploid progenitor. American Naturalist. 1984;124:703–711. [Google Scholar]
- Garnatje T, Garcia-Jacas N, Vilatersana R. Natural triploidy in Centaurea and Cheirolophus (Asteraceae) Botanica Helvetica. 2001;111:25–29. [Google Scholar]
- Grant V. Plant speciation. New York, NY: Columbia University Press; 1981. [Google Scholar]
- Guo YP, Saukel J, Mettermayr R, Ehrendorfer F. AFLP analyses demonstrate genetic divergence, hybridisation, and multiple polyploidisation in the evolution of Achillea (Asteraceae-Anthemideae) New Phytologist. 2005;166:273–289. doi: 10.1111/j.1469-8137.2005.01315.x. [DOI] [PubMed] [Google Scholar]
- Halverson K, Heard SB, Nason JD, Stireman JO. Differential attack on diploid, tetraploid, and hexaploid Solidago altissima L. by five insect gallmakers. Oecologia. 2008a;154:755–761. doi: 10.1007/s00442-007-0863-3. [DOI] [PubMed] [Google Scholar]
- Halverson K, Heard SB, Nason JD, Stireman JO. Origins, distribution, and local co-occurrence of polyploid cytotypes in Solidago altissima (Asteraceae) American Journal of Botany. 2008b;95:50–58. doi: 10.3732/ajb.95.1.50. [DOI] [PubMed] [Google Scholar]
- Hülber K, Sonnleitner M, Flatscher R, et al. Ecological segregation drives fine-scale cytotype distribution of Senecio carniolicus in the Eastern Alps. Preslia. 2009;81:309–319. [PMC free article] [PubMed] [Google Scholar]
- Husband BC. Constraints on polyploid evolution: a test of the minority cytotype exclusion principle. Proceedings of the Royal Society of London, Series B – Biological Sciences. 2000;267:217–223. doi: 10.1098/rspb.2000.0990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husband BC. The role of triploid hybrids in the evolutionary dynamics of mixed-ploidy populations. Biological Journal of the Linnean Society. 2004;82:537–546. [Google Scholar]
- Husband BC, Sabara HA. Reproductive isolation between autotetraploids and their diploid progenitors in fireweed, Chamerion angustifolium. New Phytologist. 2004;161:703–713. doi: 10.1046/j.1469-8137.2004.00998.x. [DOI] [PubMed] [Google Scholar]
- Husband BC, Schemske DW. The effect of inbreeding in diploid and tetraploid populations of Epibolium angustifolium (Onagraceae): implications for the genetic basis of inbreeding depression. Evolution. 1997;51:737–746. doi: 10.1111/j.1558-5646.1997.tb03657.x. [DOI] [PubMed] [Google Scholar]
- Husband BC, Schemske DW. Cytotype distribution at a diploid-tetraploid contact zone in Chamerion (Epilobium) angustifolium (Onagraceae) American Journal of Botany. 1998;85:1688–1694. [PubMed] [Google Scholar]
- Jersáková J, Castro S, Sonk N, et al. Absence of pollinator-mediated premating barriers in mixed-ploidy populations of Gymnadenia conopsea s.l. (Orchidaceae) Evolutionary Ecology. 2010;24:1199–1218. [Google Scholar]
- Johnson MTJ, Husband BC, Burton TL. Habitat differentiation between diploid and tetraploid Galax urceolata (Diapensiaceae) International Journal of Plant Sciences. 2003;164:703–710. [Google Scholar]
- Kao RH. Origins and widespread distribution of co-existing polyploids in Arnica cordifolia (Asteraceae) Annals of Botany. 2008;101:145–152. doi: 10.1093/aob/mcm271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashin AS, Chernishova MP. Chastota apomiksisa v populjatsiyach nekotorykh vidov Taraxacum i Hieracium (Asteraceae) Botanicheskii Zhurnal. 1997;82:14–24. [Google Scholar]
- Keeler KH. Impact of intraspecific polyploidy in Andropogon gerardii (Poaceae) populations. American Midland Naturalist. 2004;152:63–74. [Google Scholar]
- Kennedy BF, Sabara HA, Haydon D, Husband BC. Pollinator-mediated assortative mating in mixed ploidy populations of Chamerion angustifolium (Onagraceae) Oecologia. 2006;150:398–408. doi: 10.1007/s00442-006-0536-7. [DOI] [PubMed] [Google Scholar]
- Köhler C, Scheid OM, Erilova A. The impact of triploid block on the origin and evolution of polyploid plants. Trends in Genetics. 2010;26:142–148. doi: 10.1016/j.tig.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Kolář F, Štech M, Trávníček P, et al. Towards resolving the Knautia arvensis agg. (Dipsacaceae) puzzle: primary and secondary contact zones and ploidy segregation at landscape and microgeographic scales. Annals of Botany. 2009;103:963–974. doi: 10.1093/aob/mcp016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kron P, Suda J, Husband BC. Applications of flow cytometry to evolutionary and population biology. Annual Review of Ecology, Evolution and Systematics. 2007;38:847–876. [Google Scholar]
- Leong-Škorničková J, Šída O, Jarolímová V, et al. Chromosome numbers and genome size variation in Indian species of Curcuma (Zingiberaceae) Annals of Botany. 2007;100:505–526. doi: 10.1093/aob/mcm144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin DA. Minority cytotype exclusion in local plant populations. Taxon. 1975;24:35–43. [Google Scholar]
- Lewis WH. Polyploidy in species population. In: Lewis WH, editor. Polyploidy, biological relevance. New York, NY: Plenum Press; 1980. pp. 104–143. [Google Scholar]
- Ložek V. Zrcadlo minulosti. Česká a slovenská krajina v kvartéru. Prague: Dokořán; 2007. [Google Scholar]
- Lumaret R, Guillerm JL, Delay J, Loutfi A, Izco J, Jay M. Polyploidy and habitat differentiation in Dactylis glomerata L. from Galicia (Spain) Oecologia. 1987;73:436–446. doi: 10.1007/BF00385262. [DOI] [PubMed] [Google Scholar]
- Maceira NO, Jacquard P, Lumaret R. Competition between diploid and derivative autotetraploid Dactylis glomerata L. from Galicia: implications for the establishment of novel polyploid populations. New Phytologist. 1993;124:321–328. doi: 10.1111/j.1469-8137.1993.tb03822.x. [DOI] [PubMed] [Google Scholar]
- Mandáková T, Münzbergová Z. Distribution and ecology of cytotypes of the Aster amellus aggregates in the Czech Republic. Annals of Botany. 2006;98:845–856. doi: 10.1093/aob/mcl165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masterson J. Stomatal size in fossil plants – evidence for polyploidy in majority of angiosperms. Science. 1994;264:421–424. doi: 10.1126/science.264.5157.421. [DOI] [PubMed] [Google Scholar]
- Monty A, Stainier C, Lebeau F, Pieret N, Mahy G. Seed rain pattern of the invasive weed Senecio inaequidens (Asteraceae) Belgian Journal of Botany. 2008;141:51–63. [Google Scholar]
- Mráz P, Šingliarová B, Urfus T, Krahulec F. Cytogeography of Pilosella officinarum (Compositae): altitudinal and longitudinal differences in ploidy level distribution in the Czech Republic and Slovakia and the general pattern in Europe. Annals of Botany. 2008;101:59–71. doi: 10.1093/aob/mcm282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Münzbergová Z. Ploidy level interacts with population size and habitat conditions to determine degree of herbivory damage in plant populations. Oikos. 2006;115:443–452. [Google Scholar]
- Nuismer SL, Cunningham BM. Selection for phenotypic divergence between diploid and autotetraploid Heuchera grossulariifolia. Evolution. 2005;59:1928–1935. [PubMed] [Google Scholar]
- Otto F. DAPI staining of fixed cells for high-resolution flow cytometry of nuclear DNA. In: Crissman HA, Darzynkiewicz Z, editors. Methods in cell biology: flow cytometry. San Diego, CA: Academic Press; 1990. pp. 105–110. [DOI] [PubMed] [Google Scholar]
- Peckert T, Chrtek J. Mating interactions among coexisting diploid, triploid and tetraploid cytotypes of Hieracium echioides (Asteraceae) Folia Geobotanica. 2006;41:323–334. [Google Scholar]
- Peckert T, Chrtek J, Plačková I. Genetic variation in agamospermous populations of Hieracium echioides in southern Slovakia and northern Hungary (Danube Basin) Preslia. 2005;77:307–315. [Google Scholar]
- Petit C, Lesbros P, Ge X, Thompson JD. Variation in flowering phenology and selfing rate across a contact zone between diploid and tetraploid Arrhenatherum elatius (Poaceae) Heredity. 1997;79:31–40. [Google Scholar]
- Petit C, Bretagnolle F, Felber F. Evolutionary consequences of diploid-polyploid hybrid zones in wild species. Trends in Ecology and Evolution. 1999;14:306–311. doi: 10.1016/s0169-5347(99)01608-0. [DOI] [PubMed] [Google Scholar]
- Ramsey J, Schemske DW. Pathways, mechanisms, and rates of polyploid formation in flowering plants. Annual Review of Ecology and Systematics. 1998;29:467–501. [Google Scholar]
- Ramsey J, Schemske DW. Neopolyploidy in flowering plants. Annual Review of Ecology and Systematics. 2002;33:589–639. [Google Scholar]
- Rieseberg LH, Willis JH. Plant speciation. Science. 2007;317:910–914. doi: 10.1126/science.1137729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripley BD. Modelling spatial patterns. Journal of the Royal Statistical Society, Series B. 1977;39:172–212. [Google Scholar]
- Rodriguez DJ. A model for the establishment of polyploidy in plants. American Naturalist. 1996;147:33–46. [Google Scholar]
- Rotreklová O, Krahulcová A, Vaňková D, Peckert T, Mráz P. Chromosome numbers and breeding systems in some species of Hieracium subgen. Pilosella from Central Europe. Preslia. 2002;74:27–44. [Google Scholar]
- Rotreklová O, Krahulcová A, Mráz P, et al. Chromosome numbers and breeding systems in some species of Hieracium subgen. Pilosella from Europe. Preslia. 2005;77:177–195. [Google Scholar]
- Šafářová L, Duchoslav M. Cytotype distribution in mixed populations of polyploid Allium oleraceum measured at a microgeographic scale. Preslia. 2010;82:107–126. [Google Scholar]
- Schönswetter P, Lachmayer M, Lettner C, et al. Sympatric diploid and hexaploid cytotypes of Senecio carniolicus (Asteraceae) in the Eastern Alps are separated along an altitudinal gradient. Journal of Plant Research. 2007;120:721–725. doi: 10.1007/s10265-007-0108-x. [DOI] [PubMed] [Google Scholar]
- Schuhwerk F, Lippert W. Chromozomenzahlen von Hieracium L. (Compositae, Lactuceae) Teil 1. Sendtnera. 1997;4:181–206. [Google Scholar]
- Sharbel TF, Mitchell-Olds T. Recurrent polyploid origins and chloroplast phylogeography in the Arabis holboellii complex (Brassicaceae) Heredity. 2001;87:59–68. doi: 10.1046/j.1365-2540.2001.00908.x. [DOI] [PubMed] [Google Scholar]
- Soltis DE, Soltis PS. Molecular data and the dynamic nature of polyploidy. Critical Reviews in Plant Sciences. 1993;12:243–273. [Google Scholar]
- Soltis DE, Soltis PS. Polyploidy: recurrent formation and genome evolution. Trends in Ecology & Evolution. 1999;14:348–352. doi: 10.1016/s0169-5347(99)01638-9. [DOI] [PubMed] [Google Scholar]
- Soltis DE, Albert VA, Leebens-Mack J, et al. Polyploidy and angiosperm diversification. American Journal of Botany. 2009;96:336–348. doi: 10.3732/ajb.0800079. [DOI] [PubMed] [Google Scholar]
- Soltis PS. Ancient and recent polyploidy in angiosperms. New Phytologist. 2005;166:5–8. doi: 10.1111/j.1469-8137.2005.01379.x. [DOI] [PubMed] [Google Scholar]
- Soltis PS, Soltis DE. The role of genetic and genomic attributes in the success of polyploids. Proceedings of the National Academy of Sciences of the USA. 2000;97:7051–7057. doi: 10.1073/pnas.97.13.7051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stebbins GL. Variation and evolution in plants. New York, NY: Columbia University Press; 1950. [Google Scholar]
- Stergios BG. Achene production, dispersal, seed germination, and seedling establishment of Hieracium aurantiacum in an abandoned field community. Canadian Journal of Botany. 1976;54:1189–1197. [Google Scholar]
- Stuessy TF, Weiss-Schneeweiss H, Keil DJ. Diploid and polyploid cytotype distribution in Melampodium cinereum and M. leucanthum (Asteraceae, Heliantheae) American Journal of Botany. 2004;91:889–898. doi: 10.3732/ajb.91.6.889. [DOI] [PubMed] [Google Scholar]
- Suda J, Krahulcová A, Trávníček P, Krahulec F. Ploidy level versus DNA ploidy level: an appeal for consistent terminology. Taxon. 2006;55:447–450. [Google Scholar]
- Suda J, Krahulcová A, Trávníček P, Rosenbaumová R, Peckert T, Krahulec F. Genome size variation and species relationships in Hieracium subgen. Pilosella (Asteraceae) as inferred by flow cytometry. Annals of Botany. 2007a;100:1323–1335. doi: 10.1093/aob/mcm218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suda J, Weiss-Schneeweiss H, Tribsch A, Schneeweiss GM, Trávníček P, Schönswetter P. Complex distribution patterns of di-, tetra-, and hexaploid cytotypes in the European high mountain plant Senecio carniolicus (Asteraceae) American Journal of Botany. 2007b;94:1391–1401. doi: 10.3732/ajb.94.8.1391. [DOI] [PubMed] [Google Scholar]
- Thompson JN, Merg KF. Evolution of polyploidy and the diversification of plant–pollinator interactions. Ecology. 2008;89:2197–2206. doi: 10.1890/07-1432.1. [DOI] [PubMed] [Google Scholar]
- Thompson JN, Nuismer SL, Merg K. Plant polyploidy and the evolutionary ecology of plant/animal interactions. Biological Journal of the Linnean Society. 2004;82:511–519. [Google Scholar]
- Trávníček P, Eliášová A, Suda J. The distribution of cytotypes of Vicia cracca in Central Europe: the changes that have occurred over the last four decades. Preslia. 2010;82:149–163. [Google Scholar]
- Trávníček P, Kubátová B, Čurn V, et al. Remarkable coexistence of multiple cytotypes of the fragrant orchid (Gymnadenia conopsea agg.): evidence from flow cytometry. Annals of Botany. 2011;107:77–87. doi: 10.1093/aob/mcq217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss H, Dobeš C, Schneeweiss GM, Greimler J. Occurrence of tetraploid and hexaploid cytotypes between and within populations in Dianthus sect. Plumaria (Caryophyllaceae) New Phytologist. 2002;156:85–94. [Google Scholar]
- Wendel JF. Genome evolution in polyploids. Plant Molecular Biology. 2000;42:225–249. [PubMed] [Google Scholar]
- Wu LL, Cui XK, Milne RI, Sun YS, Liu JQ. Multiple autopolyploidization and range expansion of Allium przewalskianum Regel (Alliaceae) in the Qinghai-Tibetan Plateau. Molecular Ecology. 2010;19:1691–1704. doi: 10.1111/j.1365-294X.2010.04613.x. [DOI] [PubMed] [Google Scholar]
- Yamauchi A, Hosokawa A, Nagata H, Shimoda M. Triploid bridge and role of parthenogenesis in the evolution of autopolyploidy. American Naturalist. 2004;164:101–112. doi: 10.1086/421356. [DOI] [PubMed] [Google Scholar]