Abstract
Background and Aims
Despite long-held interest, knowledge on why leaf size varies widely among species is still incomplete. This study was conducted to assess whether abiotic factors, phylogenetic histories and multi-trait interactions act together to shape leaf size.
Methods
Fifty-seven pairs of altitudinal vicariant species were selected in northern Spain, and leaf area and a number of functionally related leaf, shoot and whole plant traits were measured for each pair. Structural equation modelling helped unravel trait interactions affecting leaf size, and Mantel tests weighed the relative relevance of phylogeny, environment and trait interactions to explain leaf size reduction with altitude.
Key Results
Leaves of highland vicariants were generally smaller than those of lowlands. However, the extent of leaf size reduction with increasing altitude was widely variable among genera: from approx. 700 cm2 reduction (96 % in Polystichum) to approx. 30 cm2 increase (37 % in Sorbus). This was partially explained by shifts in leaf, shoot and whole plant traits (35–64 % of explained variance, depending on models), with size/number trade-offs more influential than shifts in leaf form and leaf economics. Shifts in traits were more important than phylogenetic distances or site-specific environmental variation in explaining the degree of leaf size reduction with altitude.
Conclusions
Ecological filters, constraints due to phylogenetic history (albeit modest in the study system), and phenotypic integration contribute jointly to shape single-trait evolution. Here, it was found that phenotypic change was far more important than shared ancestry to explaine leaf size differences of closely related species segregated along altitudes.
Keywords: Leaf size evolution, leaf economics, phylogeny, traits, altitude, indirect selection, morphological correlates, structural equation models
INTRODUCTION
Body and organ sizes of plants and animals are relevant attributes from ecological and physiological perspectives. The size of plant leaves, in particular, varies greatly among plant species and is subject to multiple sources of environmental and developmental control, which has promoted the development of a large body of empirical work and theoretical advancement on the topic (e.g. Givnish, 1979; Körner, 1999; Niklas et al., 2007; Price and Enquist, 2007). An important source of interspecies variation in leaf size is the segregation of taxa to habitats with contrasting thermal regimes. Species from colder sites display leaves that extend more slowly and end up with low cell numbers, which, linked to the fact that individual cells are of similar size to those of species from warmer habitats, generates a tendency for cold-adapted species to bear small leaves (Körner, 1999; Farrell et al., 2006). Although variation in leaf size is affected by a number of different environmental forces, there is a tendency for a reduction in leaf size with decreasing average temperatures, which is generally observed when examined across geographical gradients of altitude or latitude and across and within species (Körner et al., 1989; Joel et al., 1994; Sun et al., 2006).
Thus, an overall reduction of leaf size at colder habitats is a common pattern, but when examined in detail it shows ample variation among taxa and regions. Multimodal, variable, and non-linear geographic trends of leaf size variation with temperature or elevation have been identified (e.g. Dolph and Dilcher, 1980; Ehleringer, 1988). For example, in a previous report making use of some of the leaf size data used in this current paper, together with data compiled from literature for a large number of species, it was shown that different pairs of congeneric altitude vicariants showed a variable degree of leaf size reduction with increasing elevation (Milla, 2009). This indicates that, at least at the interspecies level, the adaptive pressure for leaf size reduction is not of similar strength for all taxa. What, then, drives variation in the ability of ancestors to generate vicariant species with contrasting leaf sizes at different thermal regimes? Here we suggest and test that phylogenetic factors and multi-trait interactions might generate the above variation.
Although there is some information on how divergences in leaf size evolved in co-ordination with other traits (Westoby and Wright, 2003; Sun et al., 2006; Yang et al., 2008), previous reports on whether leaf size shows phylogenetic inertia are surprisingly scarce (given long interest in the leaf size issue), and suggests that leaf size divergence may show little to moderate phylogenetic inertia and, instead, a certain tendency for convergent evolution (Ackerly and Reich, 1999; Whitman and Aarssen, 2010). This highlights the plastic evolutionary nature of this trait (Ackerly and Donohue, 1998). However, even if leaf size scores of species show little dependence on phylogenetic history, the diversity of leaf sizes within genera may be different among the different highest level taxa (i.e. families, orders or basal clades). This is a different level of phylogenetic analysis largely ignored in terms of its outcome for traits such as leaf size. Here, the focal trait will be the ability of different genera to diversify on species with contrasting leaf sizes when segregating across altitudes, and we will ask if genera belonging to different families, orders or basal clades do differ in that ability (i.e. whether there is phylogenetic signal in the genus-level variation in leaf size between altitudinal vicariants).
Multiple trait correlations may also account for discrepancies in the evolutionary divergence of a given trait within a given clade. First, and of particular relevance to the topic of this paper, leaf size is subject to allometric constraints with plant size, shoot size, leaf number, leaf morphology or with the size of reproductive organs (Cornelissen, 1999; Herrera, 2002; Westoby and Wright, 2003; Kleiman and Aarssen, 2007; Milla and Reich, 2007). Second, evolution selects on wholly integrated viable phenotypes, rather than on single, separate phenotypic traits (Lande and Arnold, 1983). Selection on a particular trait produces not only a direct effect on the frequency distribution of that character in subsequent generations, but also produces indirect effects on the distribution of correlated traits. Therefore, since multiple traits are interrelated to maintain phenotype viability, variation in a given trait can be buffered, or increased synergistically through selection on another trait to which it is functionally linked. Multiple examples exist in this regard (e.g. Herrera, 2002; Geber and Griffen, 2003).
In Milla (2009) an attempt was made to evaluate whether variation in leaf size could be partially accounted for by a morphological correlate [leafing intensity, i.e. number of leaves displayed per unit shoot size, sensu Kleiman and Aarssen (2007)], but that work did not investigate how multiple developmental correlates may have jointly facilitated/constrained the evolution of divergent leaf sizes in sister species. Additionally, although Milla (2009) examined how phylogenetic history influenced the inverse co-ordinated evolution of leafing intensity and leaf size, he did not examine the effect of common ancestry of genera on the extent of leaf size divergence with altitude per se. Here we make use of an expanded data set, including data on morphological correlates of leaf size from the leaf to the whole plant level, to investigate whether variation in leaf size reduction with altitude among an ample set of congeneric vicariants can be accounted for by phylogenetic and/or developmental correlates.
In particular, we sought to address the following specific questions. (a) Does the extent of leaf size reduction with altitude depend on co-variation in traits functionally related to leaf size? We anticipate that altitudinal adjustments in body size, shoot traits, leaf shape and functional leaf traits will influence the extent of leaf size reduction with elevation. A detailed account on the biological rationale of our multi-trait relationships hypothesis, which was explored using structural equation modelling, is provided in Materials and methods. (b) Is there a phylogenetic signal on the ability of genera to diversify on species with contrasting leaf sizes when segregating across altitudes? (c) What is the relative importance of phylogeny versus co-variation of traits to account for different degrees of leaf size reduction with altitude?
MATERIALS AND METHODS
Study species and sites
The study area is in northern Spain (Cantabrian mountain range). A sharp altitude gradient was located within this region, situated on the western massif of the Picos de Europa mountains. This gradient spans from 0 to 2640 m a.s.l. across only 40 km, from the Cantabrian sea shores to the highest summit of the Picos de Europa. The climate of the region is oceanic type, with mild winters and warm summers in the lowlands, turning colder, and only a bit more humid, as altitude increases.
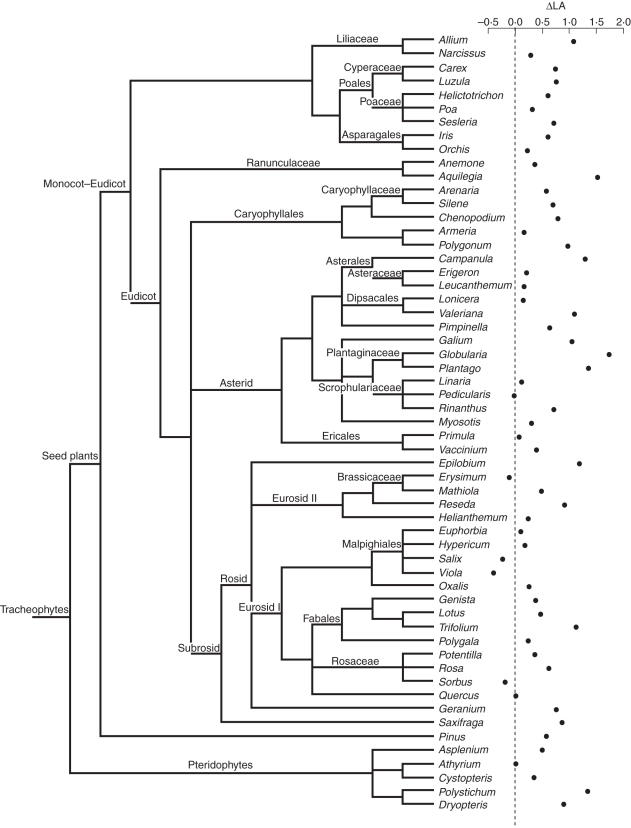
The study region was selected because of its high floristic richness, which allowed the selection of a large set of phylogenetically diverse altitude vicariants. To build this set, separate selection criteria were established for the group of genera as a whole, and for each single pair of vicariants. While selecting families and genera, the aim was to include most of the high-order taxonomic diversity of vascular plants of temperate regions. While selecting species within genera, care was taken to ensure that each pair of congeneric altitude vicariants shared growth form, leaf habit and microsite affinities (i.e. species and field sites were sampled in a stratified way so that species within genera differed mostly in altitude range). Following these procedures, we ended up with a set of 114 species, spanning 57 congeneric pairs. The species list is shown in Supplementary Data 1 (available online; note that all vicariants were congeneric except the two representatives of Orchidaceae, and that all pairs were composed of two taxonomically distinct species, except four sub-specific contrasts). This set includes 42 plant families, which take in most vascular plant lineages from temperate latitudes (Fig. 1).
Fig. 1.
Phylogenetic tree of the vascular plants, pruned for the 57 genera used in this study. The topology displayed was obtained from the maximally resolved seed plant tree available in Phylomatic (www.phylodiversity.net/phylomatic) and completed manually for fern species. For simplicity, branch lengths are not proportional to time since evolutionary divergence. The plot on the right side is the ΔLA score for each genus. The dotted line is the ΔLA = 0 reference line (i.e. the lowland and highland congeners have the same average leaf size).
Sampling and measurement of traits
For each vicariant, a site representative of its most typical habitat was located during 2005 and spring–summer 2006. Date of sampling varied depending on each specieś phenology, and occurred roughly from May to August 2006 in the lowlands and from July to August 2006 in the highlands.
The sampling strategy described below was intended to obtain species-specific average measures of the following traits: (a) leaf size, measured as one-sided projected surface area (LA, cm2); (b) leaf outline complexity [(leaf outline perimeter)2 × LA−1, dimensionless); (c) canopy maximum height (cm), measured as the shortest distance between the upper layer of leaves and the soil ground level, as a surrogate for plant size; (d) number of leaves per unit stem volume [leafing intensity per unit of stem volume sensu Kleiman and Aarssen (2007), n × mm−3]; (e) leaf nitrogen concentration (mg N × g−1 of leaf dry mass); and (f) leaf density (g leaf dry mass × cm−3 of leaf volume). Leaf nitrogen concentration and leaf density were chosen as appropriate representatives of axes of variation defined by leaf economics spectrum (sensu Wright et al., 2004). Leaf density, instead of the more common specific leaf area (SLA), was chosen because SLA is more correlated with leaf nitrogen than leaf density, and thus the selected traits encompass a wider portion of the variation in leaf traits related to leaf economics. Protocols follow Cornelissen et al. (2003) for leaf size, leaf density, leaf nitrogen concentration and canopy height measurements. Leafing intensity measurement followed Kleimman and Aarssen (2007), and calculation of leaf outline complexity followed Yonekawa et al. (1996).
Five plants per population, located at some distance of each other (variable depending on growth form and population size, but with a minimum of 3 m distance among individuals), were randomly selected, and their canopy maximum height measured to the nearest 0·5 cm. Shoot sampling was performed subsequently by randomly harvesting two fully developed and foliated shoots from each plant. Shoots were current-year, not-branching, shoot growth increments for woody perennials. For herbaceous species ‘shoots’ were upright and foliated not-branching shoots which might be the whole yearly growth increment in some species pairs (e.g. Rhinanthus), or a fraction of it in species with both basal rosettes and upright and branched shoots (e.g. Hypericum). Care was taken to sample exactly the same growth units in the two species composing each pair of vicariants. Individual and shoot selection in the field avoided very large and small plants and shoots, and standardized canopy exposure and type of shoot in case of shoot heteromorphism. An extra sample of leaves was harvested and amalgamated per population, oven-dried at 70 °C for 3 d and tested for total nitrogen concentration with an elemental autoanalyser (Elementar varioMAX N/CN, Hanau, Germany). Nitrogen concentrations were calculated on a mass basis (mg N g−1).
Shoots were taken to the laboratory, and the following measurements were performed. First, a representative leaf lamina was cut from each shoot, avoiding cataphylls, hypsophylls and particularly small nomophylls from the distal and proximal, short-internode, ends of the stem axis. To reach full hydration, leaves were placed overnight in a Petri dish containing water at 4 °C. Then, the leaves were scanned at 300 d.p.i. and lamina thickness was measured at full turgidity to the nearest 0·01 mm using a dial thickness gauge (Mitutoyo Co., Aurora, IL, USA). The leaves were subsequently oven-dried at 70 °C and weighed to the nearest 0·001 mg using a microbalance (MT XP6, Mettler-Toledo Inc., Westerville, OH, USA). Scanned leaves were processed with ImageJ software (http://rsb.info.nih.gov/ij) to obtain leaf area and leaf outline perimeter. Then, the ratio (leaf outline perimeter)2/leaf area was used as a proxy of leaf outline complexity (Yonekawa et al., 1996). Leaf density (Ld, g m−3) was further approximated as
Lamina volume at full turgidity was assumed to approximate accurately to the expression leaf area × leaf thickness for all genera, expect in Pinus. For Pinus, we assumed a half-circle shape of the cross-section of the needle, and corrected the denominator of the above equation accordingly to better approximate needle volume.
All mature-sized nomophylles in the shoot were counted (leaf number) and detached. Then the basal diameter of the stem segment was measured to the nearest 0·01 mm. using a dial thickness gauge (Mitutoyo Co.), and the length of that stem was measured to the nearest 0·1 mm with a digital calliper. Leaf number per shoot was standardized as a volume-based ‘leafing intensity’ index (number of leaves per unit of shoot volume), following Kleiman and Aarssen (2007). This provides a metric comparable among species, representing a measure of relative investment in leaf number. Stem volume was assumed to approximate to a cylinder shape, with basal stem diameter as the cylinder width, and stem length as cylinder height. Total leaf number in a shoot was divided by total volume of its sustaining stem (volume-based leafing intensity, n × mm−3; LIV hereafter).
Additionally, three 10-cm-deep soil core replicates were collected at each study site, in bare soil spots within the selected population. The three replicates were amalgamated into a single sample, air-dried, and tested for pH and percentage organic matter contents. Site-specific climate data were obtained from a regional climate model based on both topographical and long-term climatic records (S. Vicente and D. Nogués, Pyrenean Institute of Ecology, Zaragoza, Spain, unpubl. res.). We extracted long-term mean annual temperature, annual rainfall, and thermal amplitude for each site from this regional model at a precision level of 200 × 200 m.
Data analysis
The target metrics for all analyses described below are ΔTrait, which is the genus-level difference between the (log)score of the trait of the highland and that of the lowland vicariant. For example, ΔLA was
ΔTrait was not weighted per the specific difference in altitude within each pair of congeners. This is because differences among genera on environmental distances associated with altitude between highland and lowland congeners did not influence ΔTrait (see results of Mantel partial tests in Fig. 3). Thus, the above expression is kept for simplicity. Note that this does not mean that differences in altitude within each pair of vicariants were not important as to ΔTrait (see Supplementary Data 4, available online). Our questions focused on genus-specific metrics, and thus species-level results are not included in the main body of the paper but are provided as Supplementary Data (1, 4 and 5).
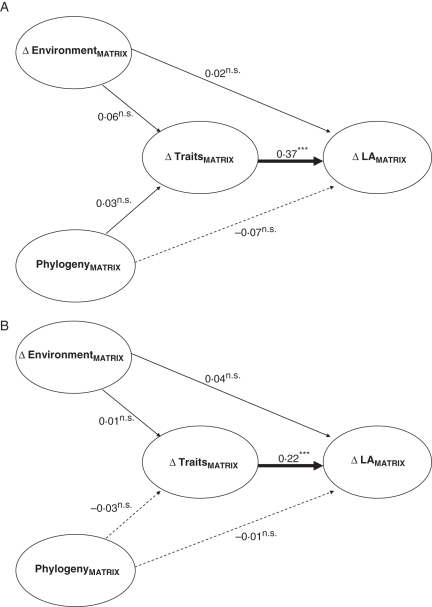
Fig. 3.
Path diagram showing partial regression coefficients obtained from partial Mantel tests among four distance matrices, using that of our focal trait, Δ LAMATRIX, as the response matrix. All matrices are genus-based. Δ EnvironmentMATRIX is a distance matrix made out of the differences in elevation between the lowland and highland vicariant of each genus. PhylogenyMATRIX is a phylogenetic distance matrix among all genera. Δ TraitsMATRIX is a distance matrix summarizing variation in the reduction of all other traits with altitude (i.e. leaf nitrogen, leaf density, leafing intensity, plant height and leaf outline complexity). Δ LAMATRIX is a distance matrix capturing the differences in leaf size-reduction-with-increasing-elevation among genera. (A) and (B) as in Fig. 2.
(a) Phylogenetic signal of leaf size reduction with altitude
Phylogenetic signals in the evolution of genus-level ΔLA scores were investigated by using the analysis of traits (AOT) module of the Phylocom package (Webb et al., 2006; for details, see Supplementary Data 2). In short, AOT produces estimations of significance of tree-wise and node-wise phylogenetic signals of the target trait, based on permutation tests of trait scores at the tips of the tree. A full account of the construction of the phylogenetic tree and the phylogenetic methods employed are provided in Supplementary Data 2.
(b) Structural equation models
Structural equation modelling (SEM) was used to investigate interactions between multiple traits and LA based on previous knowledge (Shipley, 2000). In this section, the datasets used to build the several SEMs and the rationale for model construction are described. In Supplementary Data 3, statistical details are provided on the estimation of goodness-of-fit, and on the statistical significance of path coefficients and semi-partial correlation coefficients. Also, in Supplementary Data 5, results are provided of additional SEM analyses of highland and lowlands species separately, and of multi-group analyses among altitudes.
SEMs were implemented for two separate data sets. First, a set of 30 genera was used (stem-bearing species dataset, hereon), after discarding those genera where at least one of the two species in the congeneric pair did not possess sufficiently internoded shoots, and instead displayed leaves mostly in a rossette-like structure (see Supplementary Data 1). This precluded the collection of LIV data for those species, and reduced to 30 genera the extent of the dataset for which complete data for all variables were available. Second, the complete set of 57 genera was used (all-species dataset, here on) for SEM models not including LIV as predictor variable.
First an overall causal structure relating the groups of variables was designed in a model. This structure included the following specific expectations.
Plant body size tends to decrease pronouncedly with increasing altitude, similarly to leaf size (Körner, 1999). However, regardless of whether body and leaf sizes are generally related allometrically (Niklas, 1994; Cornelissen, 1999), sharp vertical gradients in temperature in alpine ecosystems complicate the relationship of plant size to leaf size (and how it changes with elevation), because even small increases in plant height in the alpine imply sharp decreases in the atmospheric temperature experienced by plant organs (Körner, 1999). Therefore, we hypothesize that congeners that reduce plant height the least with increasing altitude will need to reduce leaf size the most to endure exposure to a harsher aerial microenvironment.
Likewise, shoot traits also vary as a function of altitude (Sun et al., 2006), and are related to leaf size variation (Westoby and Wright, 2003). Particularly, there is a strong trade-off between number and size of individual leaves, for which some studies have reported variation in intercept and displacement along common slopes for different altitudes (Kleiman and Aarssen, 2007; Milla, 2009). Given this tight trade-off, we expect that genera showing the highest increases in leaf number with altitude will also show sharper reductions in leaf size.
Interspecies variation in leaf form, particularly leaf outline complexity, is known to change as climatic regimes do (Wing et al., 2005). Lobed leaves are more effective convection-heat dissipaters than straight-edged leaves, and thus tend to be more abundant at warmer sites (Vogel, 1970). This relationship has long been used for paleoclimatic inference (Royer et al., 2005). Moreover, leaf size and shape both influence heat dissipation properties of leaf blades (Givnish, 1979). Accommodation of heat dissipation needs through modification of leaf form at high elevations may compensate for modest leaf size reduction with altitude. In fact, leaf form adjustments have already been investigated as putative alternatives to leaf size reduction across other environmental gradients (McDonald et al., 2003). Therefore, we also expect a negative relationship between the response of leaf outline complexity and that of leaf size to increasing elevation.
Finally, traits related to leaf economics (sensu Wright et al., 2004) will react in a predictable manner to the altitude gradient, with high altitude congeners bearing leaves tougher but with higher mass-based nutrient concentration (Körner, 1999), which we view as one of the deviations with altitude from the multiple leaf trait co-ordination reported in Wright et al. (2004). Also, it has been reported that, for some species, leaf size and leaf economic traits change co-ordinately in response to varying thermal regimes (Royer et al., 2005; Milla et al., 2008). For example, leaf size reduction with decreasing temperature was generally accompanied by an increase in specific leaf area in Kudo et al. (1996). Therefore, we hypothesized that variations in leaf economic traits will influence the degree of leaf size reduction with altitude in our set of congeneric vicariants.
All the above traits will not only influence leaf size, but will also mutually constrain and interrelate with each other. For example, it is a reasonable expectation that plant size and leaf number per stem volume are inversely correlated, provided that stems that expand in short internodes should grow into shorter canopies, and into compacter and thus smaller plant bodies.
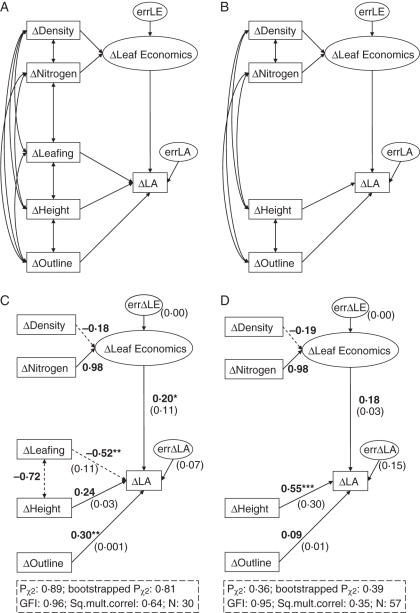
The model finally selected by goodness-of-fit estimates should (a) address the extent to which variation in the four separate types of traits influence ΔLA as explained above; (b) account for likely among-trait relationships that occur during plant development (e.g. compromises between leaf size and number strongly affect leaf display strategy, which is likely to influence plant stature). Considering the above a priori constraints, several tentative specific models (not shown) were generated, and the models which received the highest statistical support are shown in Fig. 2. Models in Fig. 2A and B are nested, with LIV being excluded in the latter. Model 2A was fitted to the set of 30 genera, and model 2B to the complete 57-genus dataset. This structure hypothesized that leaf nitrogen concentration and leaf density will influence leaf size jointly, through the unobserved latent variable ‘leaf economics’. Latent variables causing effects over their indicators are the most common type of construct or unobserved variable in SEM. However, when measured variables might be determinants of the construct rather than effects, and the construct is allowed to have residual variance due to putative unmeasured sources of variation, this type of construct is recommended, rather than classical latents or composites (Grace and Bollen, 2008). All other predictors were assumed to affect leaf size independently and directly through their corresponding observed variables. Correlations among external variables were only included in the model if significant.
Fig. 2.
Structural equation model of the causal relationships among ΔTraits and ΔLA that received the highest statistical support for (A) the subset of vicariants including only those genera for which data on all metrics were available (n = 30 genera) and (B) the whole set of vicariants, including those genera for which data on leafing intensity were not available (n = 57 genera). (C, D) Fit of model A and B, respectively, to the data. Δ is the genus-level variation between lowland and highland congeners. LA is one-sided projected surface area (cm2). Outline is leaf outline complexity [(leaf outline perimeter)2 × LA−1, dimensionless]. Height is canopy maximum height (cm). Leafing is number of leaves per unit stem volume (n × mm−3); Density is leaf density (g leaf dry mass × cm−3 of leaf volume). Nitrogen is leaf nitrogen concentration (mg N × g−1 of leaf dry mass). Solid and dashed arrows indicate positive and negative effects, respectively. Standardized path coefficients are written in bold type letter, and squared semi-partial correlation coefficients are given in parenthesis. Unexplained variance (U) is also shown in parenthesis beside the errLA box. Statistically significant paths are denoted as follows: ***, P < 0·001; **, P < 0·05; or *, P < 0·1. Fit statistics of the model (Pχ2 as calculated with MCX2, bootstrapped Pχ2 and GFI, see Supplementary Data 3) and an estimate of total explained variance (squared multiple correlations) are given in the insets at the bottom. Pearson correlation coefficients matrix for the six variables used to build the structural equation models are given in Supplementary Data 6 (available online).
(c) Relative importance of environment, phylogeny and multi-trait interactions
To evaluate explicitly the relative relevance of phylogeny, of differences in elevation within pairs of vicariants, and of co-variation in plant traits, over the degree of leaf size reduction with altitude, we used a procedure that allowed us to (a) evaluate all drivers of ΔLA jointly, in a single analysis; and (b) summarize the influence of all developmental traits in a single distance matrix, directly comparable to phylogeny, and to differences in altitude. First, four Euclidean distance matrices were built. Δ EnviromentMATRIX was made out of the differences in pH, percentage organic matter, mean annual temperature, annual rainfall, and thermal amplitude between the site of the lowland and that of the highland vicariant of each genus. PhylogenyMATRIX was the phylogenetic distance matrix among all genera. Δ TraitsMATRIX summarized the variation of all other traits with altitude (i.e. leaf nitrogen, leaf density, leafing intensity, plant height and leaf outline complexity). Δ LAMATRIX captured the variation in leaf size-reduction-with-increasing-elevation among genera. Variables were z-standardized prior to the building of the distance matrices to ensure that each trait contributes similarly to the Δ TraitsMATRIX. Then a path diagram was drawn relating all three possible drivers (Δ EnvironmentMATRIX, PhylogenyMATRIX, and Δ TraitsMATRIX) directly to Δ LAMATRIX, taking into consideration the indirect effects that Δ EnvironmentMATRIX and PhylogenyMATRIX may exert on Δ LAMATRIX through their influence over Δ TraitsMATRIX (see Fig. 3). The relevance effect size of each predictor matrix, controlled for the effect of the other matrices connected with the response, was assessed through Mantel partial tests, using the partial regression coefficients as path coefficients (Smouse et al., 1986). These coefficients were our measure of relative relevance of ΔEnvironment, Phylogeny and ΔTraits over ΔLA. The PhylogenyMATRIX was obtained using the PHYDIST function of the Phylocom package (Webb et al., 2006). The calculation of all other distance matrices, and the execution of partial Mantel tests, was done using the ECODIST package (Goslee and Urban, 2007) implemented in R 2·6·2 (R-Project; www.r-project.org).
RESULTS
Here, leaf area was used as a metric for leaf size, but all results reported in this section were highly congruent if leaf mass, instead of area, was the target metric (results not shown). Species-specific average scores for each trait studied here, together with altitude of study populations and family and growth-form affiliations of single species, are shown in Supplementary Data 1. Species average leaf area (LA, cm2) spanned five orders of magnitude across the 114 species sampled, from 0·03 cm2 of the smallest-leaved species, Galium pyrenaicum, to 1298·80 cm2 of the largest-leaved species, Athyrium filix-femina (Supplementary Data 1). This includes most of the range of variation reported world-wide for this trait (Niklas et al., 2007), particularly for the small-leaved side of the spectrum. We lack super-large leaves of approx. 2250 cm2 reported in Niklas et al. (2007).
Leaves of the highland vicariants were generally smaller. There was a consistent trend for the highland vicariant of each pair (e.g. Aguilegia pyrenaica) to have smaller leaves than its lowland congener (e.g. Aquilegia vulgaris) (P < 0·01; Fig. 1). However, there was considerable scatter as to the magnitude of this tendency. The range spans from genera that modestly enlarge leaf size with increasing altitude (e.g. Salix) to those that diminish leaf size several orders of magnitude (e.g. Allium, Polystichum) (Fig. 1 and Supplementary Data 4). Regarding other traits, overall, highland congeners tended to be smaller plants, to bear more densely leaved shoots, to have simpler leaf outline shapes, and to contain more nitrogen in their leaves per unit dry mass, but to have leaves as dense as those of their lowland relatives (Supplementary Data 4).
Phylogeny of leaf size variation among altitudinal vicariants
In Fig. 1 the phylogenetic tree of vascular plants, pruned for the genera used in this study, is displayed. Figure 1 also shows the ΔLA typical of each genus. No major phylogenetic trend is readily observable from this pattern. Only several node divergences showed significant (P < 0·05) node-wise estimates of phylogenetic signal in this regard (e.g. seedplants, subrosids2, Cyperaceae and Asteraceae). Also, only a few nodes showed significant (P < 0·05) divergence in ΔLA among children nodes associated to that radiation event (e.g. Ranunculaceae). When evaluated tree-wise, only a modest trend of tree-wide phylogenetic inertia estimate arose (P = 0·09, obtained through Phylocom procedures).
Relationships among traits
Figure 2 depicts the SEM models for inter-trait relationships that were selected by goodness-of-fit measures and their adjustment to data. Bivariate correlations among variables are accesible in Supplementary Data 5. Both models in Fig. 2A and B received high statistical support, as indicated by the several goodness-of-fit metrics, including those specifically robust to low sample sizes.
Size number-related variables (i.e. ΔLIV and ΔCanopy height) were the most relevant predictors of ΔLA in these models. Explained variance was higher in the model that used the smaller 30-genus dataset and thus included ΔLIV as a predictor variable. Also, added variance explained by each predictor uniquely (scores of squared semi-partial correlation coefficients) was always much lower than total explained variance, which indicates that explanatory power shared by multiple predictors was large.
When all genera were entered in the model (model 2D) it was found that the extent of canopy height reduction from the lowland to the highland vicariant was accompanied by reductions in leaf size to a similar degree. Variations in leaf economics and in leaf outline complexity were of little importance as predictors of leaf size variation in this model. However, when only the stem-bearing species were considered (model 2C), and ΔLIV was thus entered in the model, a different picture arose. ΔLIV was, in this case, the predictor more tightly related to ΔLA. Genera showing the most pronounced increment in LIV with altitude tended to be those showing the smallest reduction in leaf size (negative path coefficient). The relevance of ΔCanopy height dropped in this model to a non-significant score, and ΔLeaf economics and ΔLeaf outline complexity became significant and related to ΔLA directly (i.e. the more the reduction in leaf density, nitrogen concentration or leaf outline complexity with altitude, the more the reduction in leaf size). ΔLIV also overrides the relevance of ΔCanopy height when it is included in models built with only the smaller stem-bearing species data set (not shown). This indicates that peculiarities of the 27 non-stemmed genera are not the origin of the discrepancy among models.
Relative relevance of phylogeny and multi-trait interactions to explain leaf size variation with altitude
The results of partial Mantel tests indicate that the adjustment of correlated traits with increasing elevation was much more relevant than shared ancestry on influencing the degree of leaf size reduction with altitude (Fig. 3). In neither of the two models did phylogenetic distances account for a significant portion of variation in either leaf size or other traits (n.s. path coefficients in Fig. 3). In contrast, the partial Mantel coefficients between ΔTraitsMATRIX and ΔLAMATRIX were highly significant in both the 30- and 57-genus models (0·37 and 0·22, respectively; see Fig. 3). Also, the differences between genera in the environmental distance among the highland and lowland vicariant (i.e. ΔEnvironment) explained almost nothing about inter-genera differences in leaf size reduction with elevation.
DISCUSSION
Leaf size is a function of developmental, environmental and, to a lesser extent, phylogenetic drivers, acting jointly
The initial motivation for this project was to ascertain whether pronounced differences among genera as to the extent of leaf size reduction with increasing elevation could be attributed to co-variation in developmental traits and/or to phylogenetic affinities. It was found that 64 % (Fig. 2C) to 35 % (Fig. 2D) of the between-genera variation in leaf size reduction with altitude could be accounted for by parallel or inverse variation with other leaf, shoot or whole plant traits. In addition, a very modest tendency was detected for genera sharing ancestry to produce species with similar degree of divergence in leaf size.
Three main messages were extract from these general patterns. (1) The evidence shown here demonstrates that different taxa confronted with a similar ecological problem can arrive at a plethora of contrasting evolutionarily viable solutions for adjusting a given single trait (i.e. a wide range of leaf size reduction in this case). (2) The above plethora exists, to a large extent, because other traits are modulated co-ordinately with leaf size to result in viable complete phenotypes (Coleman et al., 1994). This is illustrated by the contrasting phenotypes of Pedicularis and Campanula. The species of Campanula living in the highlands has leaves remarkably smaller than those of its lowland vicariant, and lower leaf outline complexity and canopy height, though higher leafing intensity and leaf nitrogen concentration. In contrast, the highland vicariant of Pedicularis has leaves even slightly larger than those of its lowland congener, but with more complex outlines, and somewhat taller canopy height, though lower leafing intensity and nitrogen concentration. Both species conform to the general pattern of trait co-variation outlined by the SEM model in Fig. 2A, but lie at opposite ends of the spectrum of variation. (3) This evolutionary potential is, to a very modest degree in our study cases, limited by the phylogenetic history of each genus. Child taxa of each clade share a common ancestral phenotypic design, and are more prone to evolve into similarly shaped new phenotypes (Blomberg and Garland, 2002). However, here only a slight trend was found for leaf size divergence to show phylogenetic signal, which means that several genera such as Geranium, Lonicera or Sorbus are less prone to evolve species with small Linaria-like leaves, even if in very high and cold environments, and vice versa. This signal, nonetheless, exerted a very modest effect when directly compared with the effect of morphological correlates (see subheading below).
The present results are in line with recent work that highlights the importance of morphological correlates to interpret leaf size variation in a quantitative way (e.g. Sun et al., 2006; Kleiman and Aarssen, 2007; Poorter and Rozendaal, 2008; Olson et al., 2009). However, here we show and suggest that explanatory factors need to be hierarchically ordered to arrive at a synthetic understanding. First, however important developmental traits might be, environmental constraints pose a remarkable initial ecological filter on leaf size per se; there is a clear-cut and statistically significant trend here for small leaves to be favoured by selection at high altitudes, irrespective of developmental correlates. This is in accordance with earlier theory on leaf size variation and its adaptive value (Givnish, 1979; etc.). This is downplayed in recent literature, where the direct adaptive role of modulating leaf size is frequently downgraded to the level of a mere correlate (see, for example, Kleiman and Aarssen, 2007). Then, phylogenetic constraints or modulations in the complete phenotype that facilitate the adjustment of the plant body to the highland environment may enter into play.
Relative relevance of phylogeny and trait interactions, and of the several morphological correlates
The transformation of raw data to distance matrices permitted the direct comparison in a path model context of the relative strength of phylogeny and multi-trait interactions as drivers of leaf size reduction with altitude. Most previous efforts in this field have focused on the debate on whether to consider the effects of phylogenetic signal in trait-to-trait interactions (i.e. PIC approach) or stick to raw cross-species comparison (see synthesis in Carvalho et al., 2006). Some authors have claimed that explicit consideration of the comparative method might be avoided, since the inclusion of nearly negligible effects of phylogeny do not compensate for the associated complications of phylogenetic methods (e.g. Ricklefs and Starck, 1996). Here, it was found that co-variation with other plant traits was much more influential than phylogenetic distances in accounting for leaf size reduction differences among pairs of vicariants. This result supports phenotypic integration as a more powerful driver of variation in single traits than phylogenetic effects, at least to account for the macroevolution of leaf size. However, even if phylogenetic signal (which did in fact exist in our leaf size data to a moderate extent) exerted a negligible effect in comparison with phenotypic integration, we think that much more evidence in this sense should be accumulated before ruling out general effects of shared ancestry. Following Ackerly (1999), we advocate the routine use of the comparative method when analysing cross species data sets that include traits with phylogenetic signal.
It is also of interest to comment here on the approach used to partition phylogenetic, environmental and developmental effects. It is a common challenge for research programmes to be able to weigh the relative importance of historical and developmental effects over the evolution of single plant traits. One available procedure is to include phylogenetic signal vectors as additional variables in models (Diniz-Filho et al., 1998, 2007). These synthetic vectors only inform on the degree of general isolation or clustering of a terminal taxon in the context of the whole phylogenetic tree, thus compressing and reducing most information available in a phylogenetic distance matrix. This approach was not suitable for our purposes, because it does not account for genus-to-genus phylogenetic distances, which is a more appropriate metric to be explicitly compared with genus-to-genus differences in traits or environment. To our knowledge, the usage of partial Mantel tests embedded into a path model as a way of dissecting the direct and indirect effects of phylogenetic and morphological drivers is novel in this context but see Leduc et al. (1992), Zmyslony and Gagnon (2000) or Castillo-Monroy et al. (2010) for similar procedures in spatial and ecosystems ecology.
Size/number trade-offs are more relevant than leaf form and leaf economics to explain leaf size variation with altitude
Regarding the dissection of the specific developmental correlates that helped to account for variation in the degree of divergence in leaf size with altitude, size/number related variables were the most relevant. LIV, for stem-bearing species, especially for those in the lowlands, was a significant predictor of leaf size. A tight trade-off between leaf size and LIV has been reported consistently in several geographic regions and for widely different growth forms (Kleiman and Aarssen, 2007; Yang et al., 2008, Whitman and Aarssen, 2010), and even a theoretical proposal of leaf size evolution has been coined out of this remarkably pervasive trade-off (Kleiman and Aarssen, 2007; but see Milla, 2009). Here, it was found that the commonly observed pattern of reduced leaf size with increasing altitude was accompanied by a less-known pattern of increase in leafing intensity. Kleiman and Aarssen (2007) suggested several hypothetical functional implications of high leafing intensity, like increased phenotypic plasticity, improved ability to recover after herbivore damage, or higher opportunity for fecundity allocation. All these have not been suggested as traits typical of high mountain plants (Körner, 1999). However, in the light of the results found here (LIV much higher at highlands, see Supplementary Data 4), the inclusion of those traits as part of the high altitude adaptive syndrome should be explored. Shifts in canopy height also accounted for a significant portion of leaf size variation, especially when species without measurable stems were considered and LIV was excluded from the models. The genera that reduced plant height the most at the highlands tended to be those reducing leaf size the most as well. This opposes our initial expectation that because leaves displayed further away from the soil surface will experience a harsher microclimate in the alpine environment (sensu Körner, 1999), they will display smaller leaf areas to endure wider thermal amplitudes (Givnish, 1979) (explained in full in Materials and methods). However, from a biomechanical viewpoint, small body sizes cannot support axes thick enough to bear large leaves that conform to Corner's rules (Corner, 1949). This suggests that developmental rules relating the size of whole bodies to that of their individual appendages constrained the extent of leaf size reduction in the harsh microenvironment experienced by plant organs displayed far from the soil surface in the highlands. Interspecific variation in plant height also affects leaf traits other than leaf size, like leaf economic traits (Reich, 2000), discussed below.
Leaf economics was of lesser importance than size/number traits in influencing leaf size variation with altitude. Neither leaf density, nor leaf nitrogen concentration, separately, correlated significantly with leaf size (P > 0·1, not shown). However, one of the SEM models yielded a significant path coefficient of leaf economics on leaf size. Some recent work points out that increasing leaf size incurs diminishing returns of investments in leaf tissue, as indicated by allometric relationships between leaf size and several leaf economic traits (Niklas et al., 2007; Milla and Reich, 2007), and by metabolic models indicating that mass-based physiological rates of leaves decrease with increasing leaf size (Price and Enquist, 2007). Here, it was found that, irrespective of the fact that diminishing returns pose a limit to the evolution of extra-large leaves, when put into the context of a multivariate model, its relevance compared with that of size/number variables is small, at least in our study system. This view is in accord with results in tropical tree species (Poorter and Rozendaal, 2008). Regarding changes in leaf outline complexity, we did not find evidence in support of it acting as a compensatory mechanism for low reductions in leaf size with altitude, in contrast with initial expectations. McDonald et al. (2003) examined whether changes in leaf lobing worked as an alternative to low leaf size reduction at the arid and low fertility ends of rainfall and soil quality gradients. Similarly to the pattern encountered here for an altitude gradient, McDonald et al. (2003) did not find an interaction between the response of leaf size and that of leaf outline complexity. Together, both studies suggest that the fact that both leaf size and shape control heat-dissipation needs of leaf blades (Givnish, 1979), which surely vary across gradients of rainfall or temperature, does not result in mutual developmental compensation between both traits.
In summarizing the overall results of the present study, it was found that a fair amount of the variability in the response of leaf size-reduction-with-altitude can be accounted for by co-variation with size/number developmental correlates, such as LIV, canopy height and, to a lesser extent, by leaf level correlates like leaf outline complexity, nitrogen concentration or tissue density. Phylogenetic constraints had a very minor role in limiting the amplitude of evolutionary responses of ancestors to the ecological pressure of reducing leaf size with altitude. This study provides some guidance as to the quantitative exploration of which factors contribute to explain leaf size variation among species. Expansion to other geographic areas, other environmental contrasts and exploration of alternative predictors of leaf size is clearly needed if we are to understand holistically why plant species display units of foliage in such diverse ways.
SUPPLEMENTARY DATA
ACKNOWLEDGEMENTS
We thank Mar Triguero and Luis Giménez-Benavides for help during field work, Oscar Godoy, Charles Price, David Ackerly, Lonnie Aarssen, James Grace and anonymous referees for their useful comments on early stages of this manuscript, and Matthew Bowker for statistical advice on the usage of path analysis of Mantel tests. We also thank Herminio Nava, Sara Robinson and Amparo Mora, who helped to locate study populations and also to select species, the Departamento de Biologia de Organismos y Sistemas (Universidad de Oviedo, Spain) for letting us use their facilities to process field samples, Melchor Maestro (IPE-CSIC, Zaragoza, Spain) for kindly analysing leaf nitrogen, and the officials of the ‘Parque Nacional Picos de Europa’ for granting permission to carry out the study. RM was supported by the Minisiterio de Educación y Ciencia (Spain).
LITERATURE CITED
- Ackerly DD. Comparative plant ecology and the role of phylogenetic information. In: Press MC, Scholes JD, Barker MG, editors. Physiological plant ecology. Oxford: Blackwell Science; 1999. pp. 391–413. [Google Scholar]
- Ackerly DD, Donoghue MJ. Leaf size, sapling allometry, and Corner's rules: phylogeny and correlated evolution in maples (Acer) American Naturalist. 1998;152:767–791. doi: 10.1086/286208. [DOI] [PubMed] [Google Scholar]
- Ackerly DD, Reich PB. Convergence and correlations among leaf size and function in seed plants: a comparative test using independent contrasts. American Journal of Botany. 1999;86:1272–1281. [PubMed] [Google Scholar]
- Blomberg SP, Garland T. Tempo and mode in evolution: phylogenetic inertia, adaptation and comparative methods. Journal of Evolutionary Biology. 2002;15:899–910. [Google Scholar]
- Carvalho P, Diniz-Filho JAF, Bini LM. Factors influencing changes in trait correlations across species after using phylogenetic independent contrasts. Evolutionary Ecology. 2006;20:591–602. [Google Scholar]
- Castillo-Monroy AP, Bowker MA, Maestre FT, et al. Relationships between biological soil crust, bacterial diversity and abundance and ecosystem functioning: Insights from a semi-arid Mediterranean environment. Journal of Vegetation Science. 2010 in press. [Google Scholar]
- Coleman JS, McConnaughay KDM, Ackerly DD. Interpreting phenotypic variation in plants. Trends in Ecology and Evolution. 1994;9:187–191. doi: 10.1016/0169-5347(94)90087-6. [DOI] [PubMed] [Google Scholar]
- Cornelissen JHC. A triangular relationship between leaf size and seed size among woody species: allometry, ontogeny, ecology and taxonomy. Oecologia. 1999;118:248–255. doi: 10.1007/s004420050725. [DOI] [PubMed] [Google Scholar]
- Cornelissen JHC, Lavorel S, Garnier E, et al. Handbook of protocols for standardised and easy measurements of plant functional traits worldwide. Australian Journal of Botany. 2003;51:335–380. [Google Scholar]
- Corner EJH. The durian theory, or the origin of the modern tree. Annals of Botany. 1949;13:368–414. [Google Scholar]
- Diniz-Filho JAF, Santana CER, Gini LM. An eigenvector method for estimating phylogenetic inertia. Evolution. 1998;52:1247–1262. doi: 10.1111/j.1558-5646.1998.tb02006.x. [DOI] [PubMed] [Google Scholar]
- Diniz-Filho JAF, Santana CER, Gini LM. Seeing the forest for the trees: partitioning ecological and phylogenetic components of Bergmann's rule in European carnivora. Ecography. 2007;30:598–608. [Google Scholar]
- Dolph GE, Dilcher DL. Variation in leaf size with respect to climate in Costa Rica. Biotropica. 1980;12:91–99. [Google Scholar]
- Ehleringer JR. Changes in leaf characteristics of species along elevational gradients in the Wasatch Front Utah USA. American Journal of Botany. 1988;75:680–689. doi: 10.1002/j.1537-2197.1988.tb13490.x. [DOI] [PubMed] [Google Scholar]
- Farrell AD, Ougham HJ, Tomos AD. The effect of gibberellic acid on the response of leaf extension to low temperature. Plant, Cell & Environment. 2006;29:1329–1337. doi: 10.1111/j.1365-3040.2006.01513.x. [DOI] [PubMed] [Google Scholar]
- Geber MA, Griffen LR. Inheritance and natural selection on functional traits. International Journal of Plant Sciences. 2003;164:S21–S42. [Google Scholar]
- Givnish TJ. On the adaptive significance of leaf form. In: Solbrig OT, Jain S, Johnson GB, Raven PH, editors. Topics in plant population biology. New York, NY: Columbia University Press; 1979. pp. 375–407. [Google Scholar]
- Goslee SC, Urban DL. The ecodist package for dissimilarity-based analysis of ecological data. Journal of Statistical Software. 2007;22:1–19. [Google Scholar]
- Grace JB, Bollen KA. Representing general theoretical concepts in structural equation models: the role of composite variables. Environmental and Ecological Statistics. 2008;15:191–213. [Google Scholar]
- Herrera CM. Correlated evolution of fruit and leaf size in bird-dispersed plants: species-level variance in fruit traits explained a bit further? Oikos. 2002;97:426–432. [Google Scholar]
- Joel G, Aplet G, Vitousek PM. Leaf morphology along environmental gradients in Hawaiian Metrosideros polymorpha. Biotropica. 1994;26:17–22. [Google Scholar]
- Kleiman D, Aarssen LW. The leaf size/number trade-off in trees. Journal of Ecology. 2007;95:376–382. [Google Scholar]
- Körner C. Alpine plant life, functional plant ecology of high mountain ecosystems. Berlin: Springer–Verlag; 1999. [Google Scholar]
- Körner C, Neumayer M, Menendez-Riedl SP, Smeets-Scheel A. Functional morphology of mountain plants. Flora. 1989;182:353–383. [Google Scholar]
- Kudo G, Nordenhall U, Molau U. Effects of snowmelt timing on leaf traits, leaf production, and shoot growth of alpine plants: comparisons along a snowmelt gradient in northern Sweden. Ecoscience. 1996;6:439–450. [Google Scholar]
- Lande R, Arnold SJ. The measurement of selection on correlated characters. Evolution. 1983;37:1210–1226. doi: 10.1111/j.1558-5646.1983.tb00236.x. [DOI] [PubMed] [Google Scholar]
- Leduc A, Drapeau P, Bergeron Y, Legendre P. Study of spatial components of forest cover using partial Mantel tests and path analysis. Journal of Vegetation Science. 1992;3:69–78. [Google Scholar]
- McDonald PG, Fonseca CR, Overton JM, Westoby M. Leaf size divergence along rainfall and soil–nutrient gradients: is the method of size reduction common among clades? Functional Ecology. 2003;17:50–57. [Google Scholar]
- Milla R. The leafing intensity premium hypothesis tested across clades, growth forms and altitudes. Journal of Ecology. 2009;97:972–983. [Google Scholar]
- Milla R, Reich PB. The scaling of leaf area and mass: the cost of light interception increases with leaf size. Proceedings of the Royal Society Series B. 2007;274:2109–2114. doi: 10.1098/rspb.2007.0417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milla R, Reich PB, Niinemets U, Castro-Díez P. Environmental and developmental controls on specific leaf area are little modified by leaf allometry. Functional Ecology. 2008;22:565–576. [Google Scholar]
- Niklas KJ. Plant allometry, the scaling of form and process. Chicago, IL: University of Chicago Press; 1994. [Google Scholar]
- Niklas KJ, Cobb ED, Niinemets Ü, et al. ‘Diminishing returns’ in the scaling of functional leaf traits across and within species groups. Proceedings of the National Academy of Sciences of the USA. 2007;104:8891–8896. doi: 10.1073/pnas.0701135104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson M, Aguirre-Hernández R, Rosell JA. Universal foliage–stem scaling across environments and species in dicot trees: plasticity, biomechanics and Corner's rules. Ecology Letters. 2009;12:210–219. doi: 10.1111/j.1461-0248.2008.01275.x. [DOI] [PubMed] [Google Scholar]
- Poorter L, Rozendaal DMA. Leaf size and leaf display of 38 tropical tree species. Oecologia. 2008;158:35–46. doi: 10.1007/s00442-008-1131-x. [DOI] [PubMed] [Google Scholar]
- Price CA, Enquist BJ. Scaling mass and morphology in leaves: an extension of the WBE model. Ecology. 2007;88:1132–1141. doi: 10.1890/06-1158. [DOI] [PubMed] [Google Scholar]
- Reich PB. Do tall trees scale physiological heights? Trends in Ecology and Evolution. 2000;15:41–42. doi: 10.1016/s0169-5347(99)01734-6. [DOI] [PubMed] [Google Scholar]
- Ricklefs RE, Starck JM. Applications of phylogenetically independent contrasts: a mixed progress report. Oikos. 1996;77:167–172. [Google Scholar]
- Royer DL, Wilf P, Janesko DA, Kowalski EA, Dilcher DL. Correlations of climate and plant ecology to leaf size and shape: potential proxies for the fossil record. American Journal of Botany. 2005;92:1141–1151. doi: 10.3732/ajb.92.7.1141. [DOI] [PubMed] [Google Scholar]
- Shipley B. Cause and correlation in biology: a user's guide to path analysis, structural equations and causal inference. Cambridge: Cambridge University Press; 2000. [Google Scholar]
- Smouse PE, Long JC, Sokal RR. Multiple regression and correlation extensions of the Mantel Test of matrix correspondence. Systematic Zoology. 1986;35:627–632. [Google Scholar]
- Sun SC, Jin DM, Shi PL. The leaf size–twig size spectrum of temperate woody species along an altitudinal gradient: an invariant allometric scaling relationship. Annals of Botany. 2006;97:97–107. doi: 10.1093/aob/mcj004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel S. Convective cooling at low airspeeds and the shapes of broad leaves. Journal of Experimental Botany. 1970;21:91–101. [Google Scholar]
- Webb CO, Ackerly DD, Kembel SW. Phylocom: software for the analysis of community phylogenetic structure and trait evolution. Version 4·0. 2006 doi: 10.1093/bioinformatics/btn358. http://www.phylodiversity.net/phylocom/ . Last successful access: 2 September 2010. [DOI] [PubMed] [Google Scholar]
- Westoby M, Wright IJ. The leaf size–twig size spectrum and its relationship to other important spectra of variation among species. Oecologia. 2003;135:621–628. doi: 10.1007/s00442-003-1231-6. [DOI] [PubMed] [Google Scholar]
- Wing SL, Harrington GJ, Smith FA, Block JI, Boyer DM, Freeman KH. Transient floral change and rapid global warming at the Paleocene–Eocene boundary. Science. 2005;310:993–996. doi: 10.1126/science.1116913. [DOI] [PubMed] [Google Scholar]
- Whitman T, Aarssen LW. The leaf size/number trade-off in herbaceous angiosperms. Journal of Plant Ecology. 2010;3:49–58. [Google Scholar]
- Wright IJ, Reich PB, Westoby M, et al. The worldwide leaf economics spectrum. Nature. 2004;428:821–827. doi: 10.1038/nature02403. [DOI] [PubMed] [Google Scholar]
- Yang D, Li G, Sun S. The generality of leaf size versus number trade-off in temperate woody species. Annals of Botany. 2008;102:623–629. doi: 10.1093/aob/mcn135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonekawa S, Sakai N, Kitani O. Identification of idealized leaf types using simple dimensionless shape factors by image analysis. Transactions of the American Society of Agricultural Engineers. 1996;39:1525–1533. [Google Scholar]
- Zmyslony J, Gagnon D. Path analysis of spatial predictors of front-yard landscape in an anthropogenic environment. Landscape Ecology. 2000;15:357–371. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.