Abstract
Background and Aims
Cross-pollination and satiation of seed predators are often invoked to explain synchronous mast reproduction in long-lived plants. However, explanations for the synchronous death of parent plants are elusive. The roles of synchronous seeding and post-reproductive mortality were investigated in a perennial monocarpic herb (Isoglossa woodii) in coastal dune forest in South Africa.
Methods
Pre-dispersal seed predation and seed production were assessed by measuring fruit and seed set of inflorescences sprayed with insecticide or water and with no spray treatments. Seed predation was measured at different densities of I. woodii plants by monitoring removal rates of seed from the forest floor. The influence of adult plants on establishment of I. woodii seedlings was assessed by monitoring growth and survivorship of seedlings in caged and uncaged 1 × 1 m plots in understorey gaps and thickets.
Key Results
Fruit and seed set were similar between spray treatments. An I. woodii stem produced 767·8 ± 160·8 seeds (mean ± s.e.) on dune crests and 1359·0 ± 234·4 seeds on the foredune. Seed rain was greater on the foredune than in other topographic locations. Seed predation rates were 32 and 54 % on dune crests and in dune slacks, respectively, and decreased with seed abundance, number of inflorescences per stem and plant height. Seedling recruitment was greater beneath synchronously dying adult plants than in natural understorey gaps (no I. woodii). However, seedling growth rate beneath I. woodii mid-way through its life-cycle was less than in gaps, although survivorship was similar.
Conclusions
The selective advantage of masting in I. woodii derives from satiation of both pre- and post-dispersal seed predators. In addition, post-seeding mortality of adult plants facilitates seedling establishment. Satiation of seed predators and the benefits of seedling establishment are strong drivers of the evolution of synchronous monocarpy in I. woodii.
Keywords: Synchronous flowering, understorey gaps, Isoglossa woodii, seed predation, seedling establishment, monocarpy
INTRODUCTION
The best known evolutionary advantages of synchronous reproduction or masting in plants are pollination efficiency for wind-pollinated species and satiation of seed predators (Silvertown, 1980; Ims, 1990; Smith et al., 1990; Kelly, 1994; Kelly and Sork, 2002; Schauber et al., 2002). In many perennial plants exhibiting synchronous reproduction, reproduction is supra-annual, with intervening periods ranging from 3 to >100 years (Janzen, 1976; Keeley and Bond, 1999; Sharma et al., 2008). Masting supra-annually gains an evolutionary advantage if longer reproductive cycles result in higher fitness compared with annually reproducing plants. In masting monocarpic species of Acanthaceae, satiation of seed predators and facilitation of seedling establishment by the synchronous death of parent plants have been proposed as the ultimate causes of synchronous reproduction (Janzen, 1976; Struhsaker, 1997). We examine these propositions by investigating the effect of the density and the synchronicity of mortality of adult plants on rates of seed predation and seedling establishment, respectively, of a dominant monocarpic herb (Isoglossa woodii) in sub-tropical forests of eastern South Africa.
Isoglossa woodii is a widespread herbaceous to semi-woody broad-leafed plant that dominates the understorey of sub-tropical coastal dune forests of eastern South Africa and south-eastern Mozambique (Griffiths et al., 2007; Tsvuura et al., 2007). Isolated patches of I. woodii can be found up to 10 km or more from the coast, varying in extent from a few stems covering a few square metres to thickets extending over several hundred hectares (Fig. 1) covering at least 65 % of the forest floor (Griffiths et al., 2007). The species exhibits synchronous reproduction and mortality events on a 4–7 year cycle (Griffiths et al., 2010). Following flowering and seed dispersal, I. woodii dies back and the population regenerates from seed. Such synchronous monocarpy is a feature of many species of the Acanthaceae (Tweedie, 1965, 1976; Janzen, 1976; Struhsaker, 1997; Sharma et al., 2008).
Fig. 1.
Isoglossa woodii growth form, habitat and cover. (A, B) Developing inflorescences of I. woodii; (C) example of experimental 1 × 1 m cage in a natural gap in I. woodii cover; (D) 4-year-old I. woodii on a foredune was typically tall (cf. with height of Megan Griffiths), while (E) 5-year-old stands on dune crests were short (cf. with height of Charmaine Meyer). (F) Isoglossa woodii die-back in April 2008 with regeneration beneath dead stems.
The outcrossing hypothesis (Janzen, 1976; Augspurger, 1980; Stephenson, 1982) suggests that a large visual display associated with synchronous flowering increases the chances of cross-pollination and benefits the parent plant by producing high quality seed. In self-compatible species, synchronous flowering may also lead to geitonogamous self-pollination, which provides reproductive assurance but conflicts with the benefits of outcrossing. As I. woodii is both self-compatible and animal pollinated (Griffiths et al., 2010), the out-crossing hypothesis is not a compelling explanation for synchronous monocarpy and is not examined here.
The predator satiation hypothesis (Janzen, 1976; Silvertown, 1980; Augspurger, 1981) proposes that synchronous reproduction in long-lived species produces more seed than can be consumed by seed predators in mast years, and starves the seed predators in non-seeding years. Where predation pressure is high, a fitness advantage is predicted for individual plants that flower synchronously with the rest of the population. If predation rates are high then asynchronous lineages will eventually be eliminated from the population. By removing asynchronously flowering individuals from the tails of the time-of-seeding distribution, predation pressure, through stabilizing selection, effectively selects for synchronous reproduction.
A third hypothesis is that interspecific competition, particularly regarding the timing of establishment of seedlings, may have driven the evolution of reproductive synchrony in monocarpic species (Struhsaker, 1997). The dominance of monocarpic species in the community is maintained by synchronized mass reproduction and seedling establishment, which excludes seedlings of other species beneath the parent plants. As the parent plants die, their seedlings are released from competition. Individuals that release their seedlings from competition in this way gain a fitness advantage leading to synchronized seedling establishment and ultimately synchronized reproduction, which in turn outcompetes other species, thereby maintaining herb dominance. The evolution of facilitation of seedling establishment through post-reproductive mortality of adult plants requires that seeds, and eventually seedlings, of an individual plant fall directly below the parent (Janzen, 1976), which is feasible and potentially adaptive in species that passively disperse their seed (Foster, 1977; Gadgil and Prasad, 1984). In I. woodii, fruits dehisce explosively on drying so that seeds are actively dispersed, but for relatively short distances in comparison with other vectors.
In this study two hypotheses of the evolution of synchronous flowering in I. woodii were experimentally examined, and mechanisms that determine its ubiquitous cover in sub-tropical coastal forest were explored. To explore the efficacy of the predator satiation hypothesis, we determined whether insect consumers limit seed production by feeding on flowers and developing seed and fruit. Seed production was measured and the predator satiation hypothesis was tested at a local scale by monitoring removal of I. woodii seed from seed stations located in areas of different I. woodii densities. For seed predation to be a driver of synchronous monocarpy we predicted that seed predation would be greater at sites where the number of seed in the soil seed bank was lower. We also tested the hypothesis that the mortality of parent plants facilitates seedling establishment (‘seedling facilitation hypothesis’, sensu Foster, 1977; Struhsaker, 1997) using a seedling transplant experiment situated at two locations where the I. woodii population was in vegetative or post-reproductive phases. We predicted that seedling establishment post-reproduction would be greater in areas with I. woodii cover than in areas with no I. woodii cover.
MATERIALS AND METHODS
Study species populations
Several populations of Isoglossa woodii (C.B.Clarke, Acanthaceae) occur in the iSimangaliso Wetland Park in KwaZulu-Natal Province, South Africa. Two of these, at Cape Vidal (28°16′S, 32°29′E) and Mapelane Nature Reserve (28°24′32″S, 32°25′17″E), cover hundreds of hectares of the understorey in the coastal dune forest. The Cape Vidal population flowered from March to October 2007, and seedling establishment after flowering was measured at this site. The Mapelane population last had a mass flowering event in 2003, was in mid-cycle at the time of this study and in this population seedling establishment was measured under established I. woodii stands.
Seed set, pre-dispersal flower and seed predation
Pre-dispersal flower and seed predation were measured using a modification of the design of Louda and Potvin (1995). Thirty I. woodii plants at least 5 m apart were tagged on the foredune, dune crest, backdune and in dune slacks. Density, height and basal diameters of the plants were measured, and on six inflorescences on each plant three treatments were applied randomly: insecticide spray [Chlorpirifos, Agro-Serve (Pty) Ltd, Silverton, South Africa]; water spray (as a control for the physical effects of spraying); and no spray. Treatments were initiated when inflorescences were still developing before any flower buds were evident. Spraying was carried out once per fortnight, during which the number of insect-damaged flowers and fruits was also recorded. After the main flowering event and before the dispersal of seed, all inflorescences were harvested and each was kept separately in paper envelopes. In the laboratory, the numbers of flowers and fruits developed on each inflorescence was counted. Eight fruits were dissected from each inflorescence and the number of seeds in each was counted. Fruit set (number of fruits/number of flowers) was calculated for each inflorescence as well as seed set (number of seeds/number of ovules) for each fruit.
Seed production
Seed production was measured before dispersal when fruits were maturing. Thirty I. woodii plants of different sizes were randomly selected for measurement, ten each from the foredune, dune crest and dune slack. For each plant, its size (number of stems, stem diameter and height) was measured and the number of inflorescences was counted. Ten inflorescences representing the range of sizes (i.e. number of flowers per inflorescence) present on the plant were selectively harvested from each plant. The number of flowers and fruits developed on each inflorescence was counted. For five inflorescences from each plant five fruits were dissected and the number of seeds in each was counted. The mean fruit and seed set for each plant was calculated. The number of seeds produced by each plant was estimated as the product of the number of inflorescences and the mean fruit set and mean seed set per inflorescence.
Seed predation
Post-dispersal seed predation was investigated using the modified method of Wurm (1998) of placing seeds in Petri dishes at sites and monitoring their removal rate. Seeds were collected during the I. woodii flowering event and the experiment was conducted towards the end of I. woodii seed fall in July–August 2007. Seed removal rates were compared in areas with different stem and inflorescence densities, including I. woodii gaps. Each sampling unit consisted of a 1 m2 quadrat where stems were counted, their heights and numbers of inflorescences were measured and the level of browsing damage was estimated. Then seed densities were measured in the top 1 cm of soil by sieving four Petri dish (circle with diameter = 9 cm) samples covering 0·25 m2. In the centre of the quadrat two Petri dishes were placed 20 cm apart, filled with seed-free sand. One of the two Petri dishes was randomly selected for placing seed for measurement of removal rates. The other dish was retained as a control with no seed to allow estimation of seed fall into the treatment dish. Eight seeds were placed at known positions on the soil surface of the dish. Seed stations were marked, and the dishes were checked for removal or addition of seed three times at 10–13 d intervals between July and August 2007. Removed seeds were replaced while surplus seeds were removed.
There were 28–46 seed stations in use at each sampling interval. Stations were spaced at least 10 m apart at two sites on the foredunes, two on dune crests and two in dune slacks. Each site had 4–21 stations. The site with four stations was established in the second and third sampling intervals to increase sample size and to increase area coverage of the experiment.
Establishment of I. woodii seedlings in gaps during die-back
At Cape Vidal, the understorey consists of large areas of I. woodii cover and some patches that are naturally free of such cover (Griffiths et al., 2007). To investigate the factors limiting I. woodii recruitment at favourable locations, four 1 × 1 m plots of I. woodii seedlings were planted in each of 15 sites located around natural gaps between 9 September and 29 November 2007. Two plots were in the gap, at least 5 m from the edge, and two were in the nearby I. woodii thicket and 10 m from the edge of the gap. Each plot contained 16 seedlings in a grid layout. One plot in the gap and one in the thicket was covered by a cage to prevent large mammalian herbivores from disturbing the seedlings (Fig. 1). Seedlings that died within 10 d after transplanting were assumed to have died of transplant shock and were replaced. Seedling survival was recorded at 3 month intervals and evidence of browsing (apex of shoot removed) and severe herbivory (≥50 % of leaf eaten) was also recorded. Final measurements were carried out in early November 2008.
Establishment of I. woodii seedlings beneath adult conspecific plants
To test the hypothesis that synchronous reproduction and the associated synchronous mortality of adult plants facilitates recruitment, we examined whether I. woodii seedlings were capable of establishing beneath mature plants. In late March 2008 I. woodii seedlings from Cape Vidal were transplanted to ten sites 40 km south at Mapelane Nature Reserve. Each site consisted of three 1 × 1 m plots whose centres formed the corners of a 5 m-sided equilateral triangle. One sub-plot was always located in a natural gap in the I. woodii thicket, another in the centre of an artificial 2 × 2 m gap created by removing the I. woodii to ground level and a third located under stands of mature I. woodii. The number of stems removed in the artificial gap and the size of the natural gap were recorded. In each plot, 16 I. woodii seedlings from Cape Vidal, with heights of 80–120 mm (but choosing similarly sized seedlings in any one sub-plot), were planted in a grid layout. Seedlings that died of transplant shock were replaced after 10 d. Isoglossa woodii stems re-growing into artificial gaps were removed regularly. Eight months after transplanting, survivorship and height growth of seedlings were measured.
Data analysis
Flower, fruit set and seed set data from pre-dispersal flower and seed predation were analysed using a general linear model with the spray treatment as a fixed factor, topographic location a random factor and I. woodii stem density, height and basal diameter as covariates. For seed production data, Pearson correlation coefficients were calculated to explore the nature of relationships between plant size (stem diameter and height) and each of seed set, fruit set and number of inflorescences per stem.
The proportion of seed lost to predation was arcsine square-root transformed to fit a normal distribution of residuals before analysis (Kéry and Hatfield, 2003). The data were analysed using a general linear model with site as a random factor nested in topographic location. Isoglossa woodii stem density and height, number of inflorescences per I. woodii stem, soil seed bank density and mean date of sampling were additional predictor variables (covariates). Because there was collinearity among the density predictor variables, a separate analysis was run for each. In case density effects on predation occur at coarser scales, neighbourhood-scale densities were also calculated by averaging the size of the soil seed bank, stem density and number of inflorescences for a station and its two nearest stations, and these variables were tried as alternatives to the fine-scale density variables.
In both transplant experiments, seedling growth was calculated as (final height – initial height)/initial height, while survivorship was the proportion of the 16 seedlings that survived in each sub-plot. Growth data were square-root transformed while survivorship data were arcsine square-root transformed to obtain normality of residuals.
At Cape Vidal, seedling growth and survivorship were analysed among the 15 sites using a split-plot analysis, with sites as blocks each containing two plots: gap and thicket. Each of these was split into a caged and uncaged plot. At Mapelane, growth and survivorship of seedlings beneath adult conspecific plants were compared among three I. woodii habitats: thickets, artificial clearings and natural gaps, using analysis of covariance with gap size, stem density and stem height of cleared I. woodii plants as covariates. All analyses were carried out in SPSS v. 15 (SPSS, 2007).
RESULTS
Seed set, pre-dispersal flower and seed predation
Flower production per inflorescence was similar between the natural condition (no spray) and the insecticide and water spray treatments (F2,153 = 1·45, P = 0·24). On both sprayed and non-sprayed inflorescences, only occasional (<3 %) flowers or fruits were insect damaged (petals or corolla tube browsed; petals, corolla tube or fruit with insect holes) so that fruit set was similarly high among treatments (F2,153 = 0·32, P = 0·73, Table 1) but varied among topographic locations (F3,153 = 3·09, P = 0·03). Fruit set was greater on the foredune and backdune than in the dune slack, while the density, height and basal diameter of I. woodii stems did not influence fruit set (data not shown). Seed set was also similar between the spray treatments (F2,163 = 0·53, P = 0·59, Table 1) and none of the covariates was significant (P > 0·05 in all cases). Of the 1247 fruits examined, 74·3 % contained only one seed, 4·5 % had two seeds and the rest were selectively aborted, which was also the case with the remaining two ovules that did not set seed.
Table 1.
Mean (± s.e., %) fruit and seed set of inflorescences of I. woodii treated with insecticide spray, water spray and no spray
| Treatment | Fruit set | n | Seed set |
|---|---|---|---|
| Insecticide | 61·13 ± 2·44 | 434 | 39·24 ± 1·60 |
| Water spray | 61·37 ± 2·34 | 427 | 42·33±1·57 |
| No spray | 61·14 ± 3·11 | 386 | 46·68 ± 1·37 |
Seed production
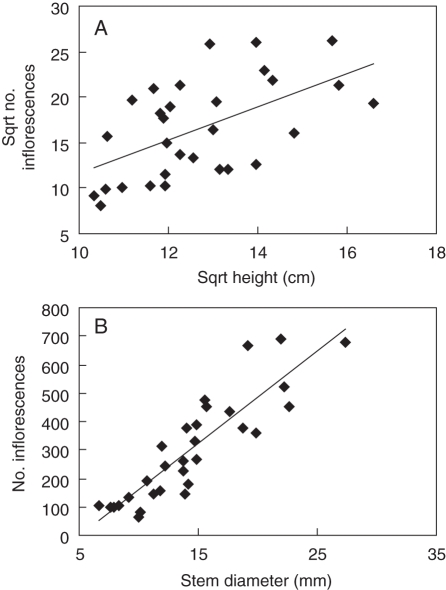
Both fruit set and seed set were not correlated with plant height and stem diameter (–0·035 < r < 0·235, P > 0·05, n = 30 in all cases). However, the number of inflorescences on an individual plant was positively correlated with both plant height and stem diameter (Fig. 2).
Fig. 2.
Number of inflorescences on various sizes of I. woodii plants. In (A) the relationship is y = 1·84x – 6·87 (F1,28 = 14·72, P = 0·001, r2 = 0·32), and in (B) y = 32·49x – 167·9 (F1,28 = 99·18, P < 0·0005, r2 = 0·78).
Individual I. woodii stems produced 767·8 ± 160·8 seeds on dune crests and 1359·0 ± 234·4 seeds on the foredunes (overall mean across topographic locations = 1083·4 ± 144·4, n = 30). Considering that stem densities are only slightly greater on the dune crests than on the foredunes (10·4 vs. 8·0 stems m−2), a square metre of ground surface on the foredunes receives more seed than other topographic locations.
Seed predation
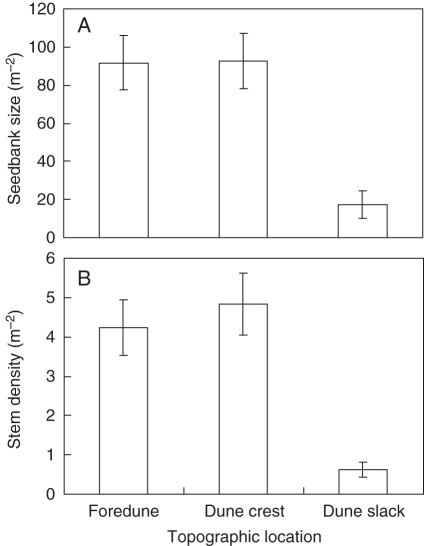
The main predator of I. woodii seeds that dispersed to the forest floor was an ant species, Pheidole spp. (Formicidae), with occasional sightings of Meranoplus spp. (Formicidae) at seed stations. The losses of post-dispersal seed to predation ranged from 32 % on dune crests to 54 % in dune slacks, but were statistically similar across these topographical locations (P > 0·1 in all cases, data not shown). Seed predation decreased with the size of the soil seed bank, stem density and height of I. woodii (Table 2). A decrease in seed predation with an increase in the mean number of inflorescences among a station and its two nearest neighbours was also found. This indicates that there were spatial density effects on predation at a broader scale (significant covariate inflorescence3, Table 2). However, in terms of the soil seed bank and stem density, there were no spatial density effects on predation (P > 0·05). There were significant interactions between topographic location and both seed bank size and stem density (Table 2), which reflects the lower stem density and soil seed bank size in the dune slacks (Fig. 3).
Table 2.
Analysis of covariance for predation of I. woodii seed at Cape Vidal
| Source | d.f. | MS | F | P |
|---|---|---|---|---|
| Seed bank | 1 | 1·799 | 8·66 | 0·004 |
| Seed bank × topography | 2 | 0·862 | 4·15 | 0·018 |
| Residual | 108 | 0·208 | ||
| Seed bank3 | 1 | 0·409 | 1·78 | 0·184 |
| Residual | 110 | 0·229 | ||
| Stem density | 1 | 1·163 | 5·63 | 0·019 |
| Stem density × topography | 2 | 1·398 | 6·77 | 0·002 |
| Residual | 108 | 0·206 | ||
| Stem density3 | 1 | 0·716 | 3·16 | 0·078 |
| Inflorescence | 1 | 0·803 | 3·56 | 0·062 |
| Residual | 110 | 0·226 | ||
| Inflorescence3 | 1 | 1·729 | 7·95 | 0·006 |
| Residual | 110 | 0·217 | ||
| Stem height | 1 | 2·415 | 11·44 | 0·001 |
| Residual | 110 | 0·211 |
Seed bank, stem density, stem height and number of inflorescences per stem are covariates. Density3, seed bank3 and inflorescence3 were derived by averaging their values at each station and the nearest two stations. The proportion of seed predated was arcsine square-root transformed before analysis.
Fig. 3.
Influence of topographic location on the soil seedbank and stem density of I. woodii at Cape Vidal.
Establishment of I. woodii seedlings in gaps during die-back
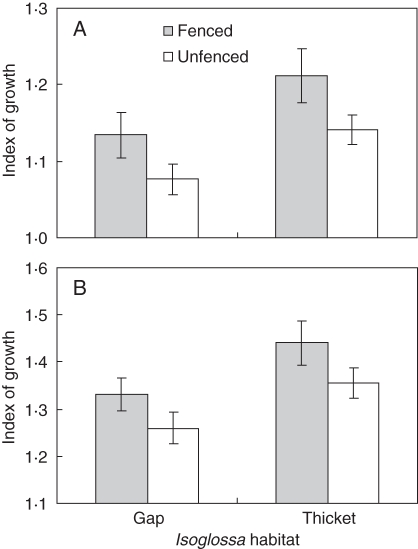
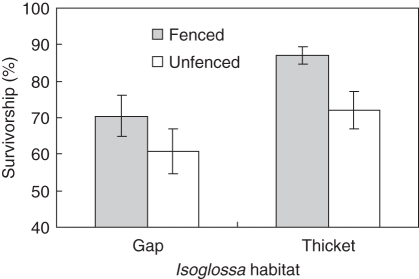
After 11 months, seedling growth was greater in the I. woodii thicket than in the gaps (F1,14 = 7·18, P = 0·02) and in the caged plots than in the open (F1,668 = 13·42 P < 0·001, Table 3a). These trends were evident only 8 months after the experiment was initiated (Fig. 4). Similarly, caging and the thicket habitat had higher seedling survivorship than uncaged and gap habitats (Table 3b, Fig. 5). These patterns show that accounting for herbivory, the I. woodii habitat appears to facilitate the establishment of I. woodii seedlings.
Table 3.
Growth (a) and survivorship (b) of I. woodii seedlings grown in caged and uncaged sub-plots in I. woodii die-back thicket and I. woodii gap habitats at Cape Vidal
| Source of variation | d.f. | MS | F | P |
|---|---|---|---|---|
| (a) Growth | ||||
| Site | 14 | 0·474 | 2·06 | 0·09 |
| Residual | 14 | 0·230 | ||
| Habitat | 1 | 1·566 | 7·18 | 0·02 |
| Residual | 14 | 0·218 | ||
| Site × habitat | 14 | 0·230 | 7·27 | <0·001 |
| Cage | 1 | 0·424 | 13·42 | <0·001 |
| Habitat × cage | 1 | 0·011 | 0·36 | 0·55 |
| Residual | 668 | 0·032 | ||
| Overall residual | 699 | 0·049 | ||
| (b) Survivorship | ||||
| Site | 14 | 0·050 | 0·69 | 0·75 |
| Residual | 14 | 0·072 | ||
| Habitat | 1 | 0·292 | 4·05 | 0·06 |
| Residual | 14 | 0·072 | ||
| Site × habitat | 14 | 0·072 | 4·25 | 0·001 |
| Cage | 1 | 0·227 | 13·35 | 0·001 |
| Habitat × cage | 1 | 0·011 | 0·65 | 0·43 |
| Residual | 28 | 0·017 | ||
| Overall residual | 59 | 0·046 | ||
Fig. 4.
Mean (± s.e.) growth of I. woodii seedlings transplanted into caged and uncaged plots in I. woodii gaps and thickets at Cape Vidal after (A) 8 months and (B) 11 months. Index of growth is based on the square-root transformation √(growth + 1).
Fig. 5.
Mean (± s.e.) survivorship of seedlings in caged and uncaged plots under habitats where Isoglossa woodii is present (thicket) and absent (gap) at Cape Vidal.
Establishment of I. woodii seedlings beneath adult conspecific plants
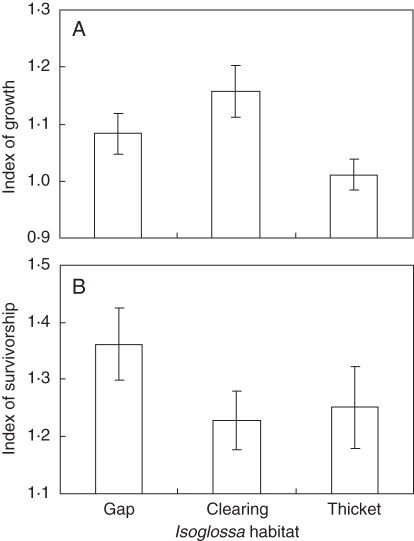
Eight months after the experiment was initiated at Mapelane, seedling growth was more suppressed in the thicket than in the gaps (F2,432 = 25·92, P < 0·001, Table 4), and was highest in the artificial gaps (artificial gap >natural gap >thicket, Fig. 6). Survival was greater in the natural gaps than in the thicket and artificial gaps (Fig. 6) but differences in survivorship among the gap habitats were not significant (Table 4b). Although gap size and stem density of cleared I. woodii plants influenced growth and survivorship of the I. woodii seedlings (Table 4), they were not important predictors of growth and survivorship (insignificant habitat × gap size and habitat × stem density interaction terms, Table 4).
Table 4.
Growth (a) and survivorship (b) of I. woodii seedlings grown in natural and artificial gaps of I. woodii, and thicket, at Mapelane
| Source | d.f. | MS | F | P |
|---|---|---|---|---|
| (a) Growth | ||||
| Habitat | 2 | 0·798 | 21·55 | <0·001 |
| Stem density | 1 | 0·009 | 0·23 | 0·63 |
| Stem height | 1 | 0·008 | 0·23 | 0·64 |
| Gap size | 1 | 0·398 | 10·76 | 0·001 |
| Habitat × gap size | 1 | 0·016 | 0·44 | 0·51 |
| Residual | 431 | 0·037 | ||
| Total | 438 | |||
| (b) Survivorship | ||||
| Habitat | 2 | 0·051 | 1·38 | 0·27 |
| Stem density | 1 | 0·183 | 4·96 | 0·037 |
| Stem height | 1 | 0·013 | 0·36 | 0·56 |
| Gap size | 1 | 0·015 | 0·41 | 0·53 |
| Habitat × stem density | 2 | 0·012 | 0·32 | 0·73 |
| Residual | 22 | 0·037 | ||
| Total | 30 | |||
Fig. 6.
Mean (± s.e.) growth (A) and survivorship (B) of I. woodii seedlings from Cape Vidal grown at three I. woodii habitats at Mapelane. The index of survivorship is based on arcsine square-root transformed values.
DISCUSSION
Seed predation
Little evidence was found for pre-dispersal flower and seed predation in a synchronously reproducing I. woodii population. Values of fruit set from this study (61 %) did not differ from the 60 % of Griffiths et al. (2010). As reproduction in the species is not pollen limited, fruit set did not differ between treatments and was similar to that found in natural conditions. At 39–47 %, seed set is similar to the 48 % reported in Griffiths et al. (2010).
Silvertown (1980) reported an increase in pre-dispersal seed survival with an increase in seed crop size for 24 of 59 data sets and concluded that these data sets supported the predator satiation hypothesis. Our high value (>97 %) of pre-dispersal seed survival during a synchronous reproductive event may be similarly associated with predator satiation (see below). Attempts to obtain seed from isolated plants flowering out of synchrony with the rest of the population in 2005 at St. Lucia, approx. 35 km south of Cape Vidal, and in 2006 at Cape Vidal, were largely unsuccessful because of high levels of pre-dispersal seed predation (Z. Tsvuura, pers. obs.). The latter strengthens the case for mast reproduction events satiating seed predators. At St Lucia, post-seed dispersal plants were visited several times until they senesced but no seedlings germinated beneath the dying canopies. However, during masting of the Cape Vidal and St Lucia populations in 2007 and 2008, respectively, seed could readily be obtained and a carpet of seedlings emerged within 2 months of seed fall. The low levels of pre-dispersal seed predation may therefore be an effect of masting, which satiates seed predators.
Silvertown (1980) identified three interdependent elements to the predator satiation hypothesis. The first is that there should be enough seed produced for predators to consume and spare some. The second is that the interval between masting events should be sufficiently long to result in marked declines of the predator population (or at least prevent sustaining a large predator population), and finally that reproduction should be synchronized within the population and between sympatric species sharing the same seed predators. In our study system the first condition is realized: the 3 month long seed fall period could result in up to 14 100 seeds m−2 depending on plant size, topographic location and/or stem density, and there was abundant recruitment of seedlings. The period between the last observed masting event for the I. woodii population at Cape Vidal was 7 years, which is sufficiently long to prevent the maintenance of large populations of invertebrate seed predators that specialize on I. woodii seed. The main post-dispersal predator of I. woodii seed, Pheidole spp., is a generalist seed-eating ant species, and as such is unlikely to manifest a numerical response to the stages of the reproductive cycle of I. woodii.
Reproductive synchrony was evident in May 2007, when >90 % of the Cape Vidal I. woodii population flowered synchronously (Griffiths et al., 2010). We observed no other species of either monocarpic (e.g. Isoglossa cooperi C.B.Clarke) or iteroparous Acanthaceae in flower at the same time as I. woodii. A similar finding of non-synchronized flowering of sympatric monocarps was reported in India, where it was associated with predator satiation (Janzen, 1976), but a similar association was not detected at Kibale Forest in Uganda (Struhsaker, 1997). Synchronized mast fruiting among many species of the Dipterocarpaceae has also been explained in terms of predator satiation (Janzen, 1974; Ashton et al., 1988).
Post-dispersal predation of I. woodii seed decreased with an increase in the abundance of seed on the ground, the number of inflorescences on stems nearest to the seed station and with height and density of I. woodii stems at the seed station. Thus, levels of seed loss demonstrate fine-scale predation effects. However, we found similar levels of predation from site to site, which indicates the absence of coarse-scale (site) predation effects. At both scales of measurement of predation, seed availability surpassed seed loss, which is consistent with the expectations of the predator satiation hypothesis.
Post-reproductive senescence and seedling establishment
The facilitation of establishment of I. woodii seedlings by the death of adult I. woodii plants may occur through increased light levels (Read et al., 2006), through improved aeration and penetrability of the soil as a result of dying and decomposing roots, through improved water penetration and availability, or through the release of nutrients by the decomposing adult skeleton (see Vitousek and Denslow, 1986). Death of adult plants may also eliminate the transfer of allelopathic compounds and species-specific pests and diseases to establishing seedlings (Clark and Clark, 1984; Smith, 1984; Kitajima and Augspurger, 1989). Seedling establishment was greater under dying plants than in gaps, which suggests that the monocarpic strategy facilitates recruitment of seedlings. Gap sites may be unsuitable for recruitment of I. woodii in general (sensu Griffiths et al., 2007). However, at 6·3 ± 0·5 µmol m−2 s−1 the leaf-level light compensation point of the species is lower than background levels of light in understorey gaps (Tsvuura et al., 2010), which should not preclude I. woodii establishment. Nevertheless, our finding of greater I. woodii establishment beneath dying conspecific plants concurs with Foster (1977), who suggested that post-reproductive death of Tachigalia versicolor trees at Barro Colorado Island in Panama facilitates establishment of its seedlings. In fact, Kitajima and Augspurger (1989) found enhanced growth and survivorship of T. versicolor seedlings growing beneath dying conspecific adult trees compared with under the canopies of living conspecific and non-conspecific trees. Light gaps created by the death of a monocarpic plant may be open for colonization by seedlings of pioneer species, but for a species exhibiting prolific reproduction the vacant sites are rapidly taken over by emerging seedlings of the species. The absence of seed dormancy in these monocarps (Young, 1982; Taylor and Inouye, 1985) increases the probability that regeneration sites will be occupied by conspecific seedlings.
Struhsaker (1997) suggested that the death of parent plants of monocarpic Acanthaceae allows seedlings of the species to swamp seedlings of other species competitively, thereby maintaining the dominance of the monocarp. The death of mature I. woodii plants may also facilitate seedling establishment through competitive release. However, facilitative effects of understorey plant mortality may be accompanied by suppressive effects. For example, Paul et al. (2004) have shown that collapsing dead stems of a dominant undercanopy shrub, Acanthus pubescens, smother and suppress tree seedlings in logging gaps. This is in sharp contrast to the death and eventual collapse of an I. woodii plant. After seed dispersal, the I. woodii plant starts to die, beginning with the inflorescences and inflorescence stalks, followed by the small thin branches that support inflorescences, then proceeds gradually to the larger branches and main stem. In addition, over the flowering period, leaves undergo translocation of nutrients and drop off (Porizias and Balkwill, 2008). By the time the larger stem dies, the inflorescences and inflorescence-bearing stalks have fallen off so that at any one time very little biomass falls off an I. woodii plant. This mortality pattern is unlikely to smother I. woodii seedlings but could enhance their chances of establishment, as suggested for the mortality pattern of the monocarpic tree T. versicolor (Foster, 1977).
Greater establishment of I. woodii seedlings was found in the artificial and natural gaps than beneath adult plants at Mapelane. This lends further support to the notion that the mortality of post-reproductive plants enhances establishment of their seedlings. Patterns of survivorship showed that the natural gaps were associated with higher seedling survivorship than the artificial gaps and I. woodii thicket, which had similar survivorship. This suggests mortality due to below-ground competition, perhaps for water as most mortality occurred in the dry season after winter rainfall.
As I. woodii reproduces synchronously within and among plants and produces large numbers of seed at supra-annual intervals, the selective advantage of masting in the species probably derives from its ability to satiate predators. Seedling establishment in the species is facilitated by synchronous mortality of post-seeding plants. We conclude that satiation of seed predators and the benefits of seedling establishment have driven the evolution of synchronous monocarpy in I. woodii.
ACKNOWLEDGEMENTS
We thank Hylton Adie and Thabane Moyeni for help in the field, and Ezemvelo KwaZulu-Natal Wildlife and the iSimangaliso Wetland Park Authority for permission to conduct fieldwork at Cape Vidal and at Mapelane Nature Reserve. This study was funded by the National Research Foundation (Focus area: Conservation and Management of Ecosystems and Biodiversity) of South Africa under grant number GUN: 2069339, and by financial support from the Andrew W. Mellon Foundation. The Mazda Wildlife Fund provided logistic support.
LITERATURE CITED
- Ashton PS, Givnish TJ, Appanah S. Staggered flowering in the Dipterocarpaceae: new insights into floral induction and the evolution of mast fruiting in the aseasonal tropics. American Naturalist. 1988;132:44–66. [Google Scholar]
- Augspurger CK. Mass flowering of a tropical shrub (Hybanthus prunifolius): influence on pollinator attraction and movement. Evolution. 1980;34:475–488. doi: 10.1111/j.1558-5646.1980.tb04837.x. [DOI] [PubMed] [Google Scholar]
- Augspurger CK. Experimental studies on effects of pollinators and seed predators on Hybanthus prunifolius (Violaceae) Ecology. 1981;62:775–789. [Google Scholar]
- Clark DA, Clark DB. Spacing dynamics of a tropical rain forest tree: evaluation of the Janzen–Connell model. American Naturalist. 1984;124:769–788. [Google Scholar]
- Foster RB. Tachigalia versicolor is a suicidal neotropical tree. Nature. 1977;268:624–626. [Google Scholar]
- Gadgil M, Prasad SN. Ecological determinants of life history evolution of two Indian bamboo species. Biotropica. 1984;16:161–172. [Google Scholar]
- Griffiths ME, Lawes MJ, Tsvuura Z. Understorey gaps influence regeneration dynamics in subtropical coastal dune forest. Plant Ecology. 2007;189:227–236. [Google Scholar]
- Griffiths ME, Tsvuura Z, Franklin DC, Lawes MJ. Pollination ecology of Isoglossa woodii, a long-lived, synchronously monocarpic herb from coastal forests in South Africa. Plant Biology. 2010;12:495–502. doi: 10.1111/j.1438-8677.2009.00222.x. [DOI] [PubMed] [Google Scholar]
- Ims RA. The ecology and evolution of reproductive synchrony. Trends in Ecology and Evolution. 1990;5:135–140. doi: 10.1016/0169-5347(90)90218-3. [DOI] [PubMed] [Google Scholar]
- Janzen DH. Why bamboos wait so long to flower. Annual Review of Ecology and Systematics. 1976;7:347–391. [Google Scholar]
- Janzen DH. Tropical blackwater rivers, animals and mast fruiting by the Dipterocarpaceae. Biotropica. 1974;6:69–103. [Google Scholar]
- Keeley JE, Bond WJ. Mast flowering and semelparity in bamboos: the bamboo fire cycle hypothesis. American Naturalist. 1999;154:383–391. doi: 10.1086/303243. [DOI] [PubMed] [Google Scholar]
- Kelly D. The evolutionary ecology of mast seeding. Trends in Ecology and Evolution. 1994;9:465–470. doi: 10.1016/0169-5347(94)90310-7. [DOI] [PubMed] [Google Scholar]
- Kelly D, Sork VL. Mast seeding in perennial plants: why, how, where? Annual Review of Ecology and Systematics. 2002;33:427–447. [Google Scholar]
- Kéry M, Hatfield JS. Normality of raw data in general linear models: the most widespread myth in statistics. Bulletin of the Ecological Society of America. 2003;84:92–94. [Google Scholar]
- Kitajima K, Augspurger CK. Seed and seedling ecology of a monocarpic tropical tree, Tachigalia versicolor. Ecology. 1989;70:1102–1114. [Google Scholar]
- Louda SM, Potvin MA. Effect of inflorescence-feeding insects on the demography and lifetime fitness of a native plant. Ecology. 1995;76:229–245. [Google Scholar]
- Paul JR, Randle AM, Chapman CA, Chapman LJ. Arrested succession in logging gaps: is tree seedling growth and survival limiting? African Journal of Ecology. 2004;42:245–251. [Google Scholar]
- Poriazis DL, Balkwill K. Developmental variation in a species of Isoglossa (Acanthaceae: Ruellioideae) over a season. Bothalia. 2008;38:131–140. [Google Scholar]
- Read J, Sanson GD, Jaffré T, Burd M. Does tree size influence timing of flowering in Cerberiopsis candelabra (Apocynaceae), a long-lived monocarpic rain-forest tree? Journal of Tropical Ecology. 2006;22:621–630. [Google Scholar]
- Schauber EM, Kelly D, Turchin P, et al. Masting by eighteen New Zealand plant species: the role of temperature as a synchronizing cue. Ecology. 2002;83:1214–1225. [Google Scholar]
- Sharma MV, Kuriakose G, Shivanna KR. Reproductive strategies of Strobilanthes kunthianus, an endemic, semelparous species in southern Western Ghats, India. Botanical Journal of the Linnean Society. 2008;157:155–163. [Google Scholar]
- Silvertown JW. The evolutionary ecology of mast-seeding in trees. Biological Journal of the Linnean Society. 1980;14:235–250. [Google Scholar]
- Smith AP. Postdispersal parent–offspring conflict in plants: antecedent and hypothesis from the Andes. American Naturalist. 1984;123:354–370. [Google Scholar]
- Smith CS, Hamrick JL, Kramer CL. The advantage of mast years for wind pollination. American Naturalist. 1990;136:154–166. [Google Scholar]
- SPSS. SPSS for Windows, version 15. Chicago, IL: SPSS Inc; 2007. [Google Scholar]
- Stephenson AG. When does outcrossing occur in a mass-flowering plant? Evolution. 1982;36:762–767. doi: 10.1111/j.1558-5646.1982.tb05442.x. [DOI] [PubMed] [Google Scholar]
- Struhsaker TT. Ecology of an African rain forest: logging in Kibale and the conflict between conservation and exploitation. Gainesville: University of Florida Press; 1997. [Google Scholar]
- Taylor OR, Inouye DW. Synchrony and periodicity of flowering in Frasera speciosa (Gentianaceae) Ecology. 1985;66:521–527. [Google Scholar]
- Tsvuura Z, Griffiths ME, Lawes MJ. The effect of herbaceous understory cover on fruit removal and seedling survival in coastal dune forest trees in South Africa. Biotropica. 2007;39:428–432. [Google Scholar]
- Tsvuura Z, Griffiths ME, Gunton RM, Franks PJ, Lawes MJ. Ecological filtering by a dominant herb selects for shade tolerance in the tree seedling community of coastal dune forest. Oecologia. 2010;164:861–870. doi: 10.1007/s00442-010-1711-4. [DOI] [PubMed] [Google Scholar]
- Tweedie EM. Periodic flowering of some Acanthaceae on Mt. Elgon. Journal of the East African Natural History Society. 1965;15:92–94. [Google Scholar]
- Tweedie EM. Habitats and check-list of plants on the Kenya side of Mount Elgon. Kew Bulletin. 1976;31:227–257. [Google Scholar]
- Vitousek PM, Denslow JS. Nitrogen and phosphorus availability in treefall gaps. Journal of Ecology. 1986;74:1167–1178. [Google Scholar]
- Wurm PAS. A surplus of seeds: high rates of post-dispersal predation in flooded grassland in monsoonal Australia. Australian Journal of Ecology. 1998;23:385–392. [Google Scholar]
- Young TP. Bird visitation, seed set, and germination rates in two species of Lobelia on Mount Kenya. Ecology. 1982;63:1983–1986. [Google Scholar]