Abstract
Background and Aims
Grapevine (Vitis spp.) cold hardiness varies dynamically throughout the dormant season, primarily in response to changes in temperature. The development and possible uses of a discrete-dynamic model of bud cold hardiness for three Vitis genotypes are described.
Methods
Iterative methods were used to optimize and evaluate model parameters by minimizing the root mean square error between observed and predicted bud hardiness, using up to 22 years of low-temperature exotherm data. Three grape cultivars were studied: Cabernet Sauvignon, Chardonnay (both V. vinifera) and Concord (V. labruscana). The model uses time steps of 1 d along with the measured daily mean air temperature to calculate the change in bud hardiness, which is then added to the hardiness from the previous day. Cultivar-dependent thermal time thresholds determine whether buds acclimate (gain hardiness) or deacclimate (lose hardiness).
Key Results
The parameterized model predicted bud hardiness for Cabernet Sauvignon and Chardonnay with an r2 = 0·89 and for Concord with an r2 = 0·82. Thermal time thresholds and (de-)acclimation rates changed between the early and late dormant season and were cultivar dependent but independent of each other. The timing of these changes was also unique for each cultivar. Concord achieved the greatest mid-winter hardiness but had the highest deacclimation rate, which resulted in rapid loss of hardiness in spring. Cabernet Sauvignon was least hardy, yet maintained its hardiness latest as a result of late transition to eco-dormancy, a high threshold temperature required to induce deacclimation and a low deacclimation rate.
Conclusions
A robust model of grapevine bud cold hardiness was developed that will aid in the anticipation of and response to potential injury from fluctuations in winter temperature and from extreme cold events. The model parameters that produce the best fit also permit insight into dynamic differences in hardiness among genotypes.
Keywords: Cold hardiness, cold injury, differential thermal analysis, discrete model, grapevine, low-temperature exotherm, Vitis labruscana, Vitis vinifera
INTRODUCTION
Cold hardiness (Hc) in perennial plants during dormancy varies by species, cultivar and antecedent weather, among other causative factors (Schnabel and Wample, 1987; Wolf and Cook, 1992; Leinonen et al., 1995; Kanneganti et al., 1998; Ebel et al., 2005). Hardiness follows a general sequence of acclimation and deacclimation. During acclimation plants gain Hc as the temperature decreases, which coincides temporally with satisfying the chilling requirement often discussed in the dormancy literature (Richardson et al., 1974). During the subsequent deacclimation, plants are said to be eco-dormant (Lang et al., 1987), and changes in Hc respond to higher temperatures, finally leading up to budbreak in spring (Kalberer et al., 2006; Keller, 2010). Hardiness often remains quite stable in mid-winter as long as temperatures remain relatively low and stable; this has led some authors (Proebsting et al., 1980; Jiang and Howell, 2002) but not all (Wolf and Cook, 1992; Kwon et al., 2008) to treat this mid-winter period as physiologically unique.
Rates of acclimation and deacclimation vary dynamically and are reversible (Damborska, 1978; Wolf and Cook, 1992; Gu et al., 2002), but as budbreak approaches the loss of Hc ceases to be reversible (Fennell, 2004; Kalberer et al., 2006). In addition, Hc responds to fluctuations in ambient temperature (Proebsting et al., 1980; Wolf and Cook, 1992; Hubackova, 1996; Keller, 2010). In grapevines (Vitis spp.), the role of photoperiod is not as obvious as that of temperature. A short photoperiod induces bud dormancy, but low temperature seems to be required for full cold acclimation (Schnabel and Wample, 1987; Fennell and Hoover, 1991). Cultivars differ in rates of acclimation and deacclimation, maximum level of Hc and response to temperature fluctuations (Mills et al., 2006); thus, predictive models potentially can provide information on site suitability for grape production and for cultivar selection. Predictive models are also useful to growers who can respond with frost protection measures in advance of injury-inducing low temperatures or with adjustments to pruning in the aftermath of these low temperatures (Keller and Mills, 2007). Finally, such models may be used to estimate potential impacts of climate change scenarios.
Empirical estimates of Hc in woody perennials can be derived by several techniques, the most common of which is referred to as differential thermal analysis (DTA) or low temperature exotherm (LTE) analysis (Andrews et al., 1983; Tinus et al., 1985; Wolf and Pool, 1987; Burr et al., 1990; Wisniewski et al., 1990; Mills et al., 2006). This method measures the release of latent heat, termed LTE, when symplastic water freezes; LTE50 denotes the temperature at which 50 % of the organ or tissue samples are injured. Estimates of Hc are of particular interest for dormant buds that contain vegetative and reproductive primordia that represent the yield potential for the subsequent growing season. Empirical estimates of Hc can be used to develop dynamic models which, along with weather forecasts, may predict Hc in advance of critically low temperatures.
Modelling acclimation and deacclimation in perennial plants has been approached using varying degrees of detail [e.g. Kobayashi et al., 1983 (Cornus spp.); Rowland et al., 2005 (Vaccinium spp.); Ebel et al., 2005 (Citrus unshiu)]. To accommodate changes in acclimation, an empirical model of Hc in Lolium spp. fitted a logistic function that was derived from data obtained in a temperature gradient tunnel (Gay and Eagles, 1991). First-order discrete dynamic models were developed for overwintering cereals and alfalfa (Medicago sativa), where acclimation and deacclimation were defined as rate variables, and both time and photoperiod as driving variables (Kanneganti et al., 1998; Fowler et al., 1999). Cold hardiness was estimated by adding the change in Hc (ΔHc) on the current day to Hc from the previous day. The aforementioned work was conducted under controlled conditions and thus does not incorporate meteorological data or reflect natural fluctuations in ambient temperature. For Douglas fir (Pseudotsuga menziesii) there is a second-order dynamic model with a logistic component that includes a temperature-dependent maximum Hc (Leinonen et al., 1995). Andrews et al. (1987) published a dynamic conceptual model of cherry (Prunus avium) bud hardiness that calculated ΔHc on an hourly basis from hourly air temperatures. This model was specific to the mid-winter period defined as after autumn acclimation and before spring deacclimation.
Although there are several published models for determining grapevine chilling requirements and/or budbreak (McIntyre et al., 1987; Moncur et al., 1989; Dokoozlian, 1999; Kwon et al., 2008; Andreini et al., 2009; García de Cortázar-Atauri et al., 2009), there is no dynamic predictive model available for grapevine Hc. Current models are rudimentary and largely rely on post-hoc statistical analyses correlating Hc with ambient air temperature at or before the time of sampling. Proebsting et al. (1980) found a correlation between the average temperature of the preceding 2 d and grape bud hardiness. Wolf and Cook (1992) estimated correlations between current LTE50 and average air temperature for the previous 1, 3, 5 or 7 d. Jiang and Howell (2002) incorporated temperatures for the preceding 1–7 d in regression equations to predict Hc. The correlation approach does not apply a biological basis for model parameterization and, contrary to experience, it implies that Hc is independent of earlier weather conditions. Regression equations cannot provide insight into the Hc phenomenon beyond confirming the obvious importance of temperature.
To overcome these weaknesses, the present work took advantage of a 22-year database of LTE50, the most commonly used quantitative indicator of Hc, to develop and evaluate a discrete dynamic model to predict Hc of dormant grapevine buds. Instead of using a post-hoc correlation approach, the database, in combination with the integration of acknowledged concepts of bud dormancy, allowed us to apply a biological basis for model parameterization. This included identification of upper and lower temperature limits for Hc, temperature thresholds for acclimation and deacclimation, acclimation and deacclimation rates, and changes of these thresholds and rates as the buds transition from endo- to eco-dormancy. We assumed temperature, expressed as thermal time, to be the primary environmental determinant of Hc, as well as of its rate of change, while recognizing that these relationships are subject to seasonal variation. Model variants were parameterized for three distinct grape genotypes and permit biological insight beyond simple model equations.
MATERIALS AND METHODS
A database (1988–2010) of grapevine Hc maintained by Washington State University provided input data for model development and testing. The database comprises LTE50 values for dormant buds from genetically diverse cultivars of field-grown grapevines. The LTE50 values were determined by DTA (Andrews et al., 1983; Wample et al., 1990; Mills et al., 2006) conducted at varying intervals (2 d to 2 weeks), as time permitted, from leaf fall in autumn (normally at the time of the first frost) to budswell in spring. These two phenological events correspond to modified E-L stages 47 and 2, respectively (Coombe, 1995), and typically occurred in October and in April, respectively. Dormant buds were collected using the protocol described in Mills et al. (2006) from vines located at or within 1 km of the Irrigated Agriculture Research and Extension Center in Prosser, WA, USA (46·30 °N latitude, 119·75 °W longitude). Meteorological data for the on-site weather station were provided by the Washington State University Agricultural Weather Network (AgWeatherNet, http://weather.wsu.edu). We focused on two commercially important V. vinifera cultivars (Chardonnay and Cabernet Sauvignon) and one V. labruscana cultivar (Concord). The empirical data sets comprised 22 years for Cabernet Sauvignon, 14 years for Chardonnay and 15 years for Concord. Approximately half of the available dormant seasons (10 years for Cabernet Sauvignon, 6 years for Chardonnay and 7 years for Concord) were selected randomly to be used for model optimization; the remainder were reserved for model evaluation.
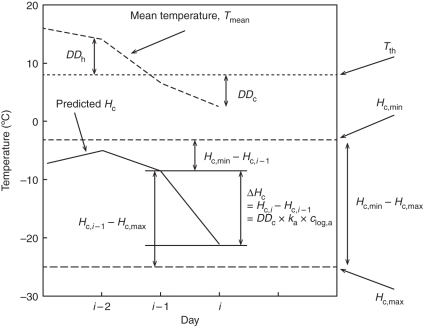
Abbreviations and units of measurement used in model parameterization and evaluation are listed in Table 1. Using a discrete dynamic approach (Fig. 1) with 1 d time steps from leaf fall to budswell, Hc, expressed as the predicted LTE50 of dormant buds, was estimated in finite difference form at the current time (i) from the previous day's value (Hc,i–1) and the change in hardiness (ΔHc):
| (1) |
Table 1.
Symbols, abbreviations and units of measurement used in text and model mathematics
| Abbreviation | Definition | Unit |
|---|---|---|
| B | Bias or mean error | °C |
| clog,a | Logistic component during acclimation | Dimensionless |
| clog,d | Logistic component during deacclimation | Dimensionless |
| DDc | Chilling degree-days | °C |
| DDh | Heating degree-days | °C |
| DTA | Differential thermal analysis | |
| EDB | Eco-dormancy boundary, DDc accumulation required to start eco-dormancy | °C |
| Hc | Cold hardiness | °C |
| Hc,i | Cold hardiness for day i | °C |
| Hc,i–1 | Cold hardiness for day i–1 | °C |
| Hc,initial | Initial cold hardiness, genotype-specific constant | °C |
| Hc,max | Maximum hardiness (most hardy condition), genotype-specific constant | °C |
| Hc,min | Minimum hardiness (least hardy condition), constant | °C |
| ΔHc | Change in cold hardiness | °C |
| ka,eco | Acclimation rate during eco-dormancy | °C °C−1 |
| ka,endo | Acclimation rate during endo-dormancy | °C °C−1 |
| kd,eco | Deacclimation rate during eco-dormancy | °C °C−1 |
| kd,endo | Deacclimation rate during endo-dormancy | °C °C−1 |
| LTE50 | Low-temperature exotherm, temperature lethal to 50 % of the population | °C |
| RMSE | Root mean square error | °C |
| Tmax | Maximum daily temperature | °C |
| Tmean | Mean daily temperature | °C |
| Tmin | Minimum daily temperature | °C |
| Tth,eco | Threshold temperature for calculating degree-days during eco-dormancy | °C |
| Tth,endo | Threshold temperature for calculating degree-days during endo-dormancy | °C |
Fig. 1.
Discrete dynamic approach to modelling cold hardiness (Hc), where Hc on the current day is equal to the change in Hc (ΔHc) added to the Hc from the previous day (Hc,i = ΔHc + Hc,i–1). Chilling degree-days (DDc) below a threshold temperature (Tth) lead to acclimation, whereas heating degree-days (DDh) above Tth lead to deacclimation.
The ΔHc is dependent on thermal time, which in turn is calculated from the mean daily air temperature, the driving force for changes in acclimation or deacclimation:
| (2) |
| (3) |
| (4) |
where DDc is the thermal time below a threshold temperature (Tth), expressed as chilling degree-days; DDh is the thermal time above a Tth, expressed as heating degree-days. These variables are conceptually familiar to scientists and growers who refer to growing degree-days to normalize rates of plant development across growing seasons. The Tth, as well as the acclimation rate constant (ka) and the deacclimation rate constant (kd), are unique by genotype and change during the dormant season (Table 2; cf. Kalberer et al., 2006). Using separate rates for acclimation and deacclimation accounts for hysteresis that is not captured if regression analysis is used to model Hc as a function of temperature (e.g. Hubackova, 1996; Jiang and Howell, 2002). The basis for the changes in Tth, ka and kd is illustrated in Fig. 2, which shows that the LTE50 curves of the three cultivars are nearly parallel during acclimation in autumn but diverge during deacclimation in spring. This phenomenon was modelled by dividing the dormant season into two periods, allowing Tth, ka and kd to be reset after a particular cultivar has accumulated a certain number of DDc. This division is conceptually similar to fulfilling a chilling requirement or to the boundary between endo-dormancy and eco-dormancy (EDB; Lang et al., 1987; Wolf and Cook, 1992; Kwon et al., 2008), and divides the winter into an endo-dormancy period dominated by acclimation and an eco-dormancy period dominated by deacclimation. Thus, Tth, ka and kd were optimized sequentially for these two periods, as indicated by subscripts endo and eco in the subsequent text.
Table 2.
Model parameters used to simulate cold hardiness (Hc) of three grape cultivars
| Parameter | Concord | Chardonnay | Cabernet Sauvignon |
|---|---|---|---|
| Hc,initial (°C) | –11·94 | –10·55 | –9·99 |
| Hc,max (°C) | –29·73 | –25·24 | –24·63 |
| Hc,min (°C) | –3·00 | –3·00 | –3·00 |
| Tth,endo (°C) | 12·50 | 11·75 | 11·75 |
| ka,endo (°C) | 0·13 | 0·14 | 0·14 |
| kd,endo (°C) | 0·01 | 0·01 | 0·01 |
| EDB (°C)* | –700 | –750 | –850 |
| Tth,eco (°C) | 4·25 | 3·75 | 5·75 |
| ka,eco (°C °C−1) | 0·01 | 0·07 | 0·01 |
| kd,eco (°C °C−1) | 0·16 | 0·13 | 0·12 |
| RMSE (°C) | 2·05 | 1·40 | 1·47 |
Using the optimization data set, parameters were estimated by iteration to minimize the root mean square error (RMSE) between observed and predicted Hc.
* DDc values are negative.
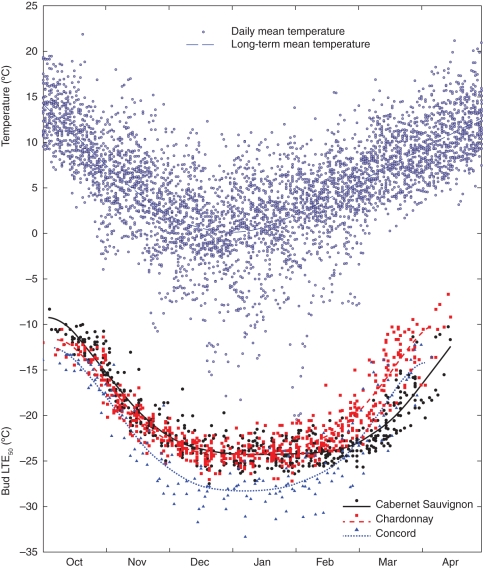
Fig. 2.
Daily mean air temperature and measured bud cold hardiness (Hc), expressed as low-temperature exotherms (LTE50), of three grape cultivars over the dormant season for the period 1988–2010. Note that Hc measurements usually started in early–mid October and ended in early–mid April. Symbols represent daily or measured values; lines represent long-term (n ≥14 years) averages.
We applied asymptotic bounds on the absolute lowest and highest possible values of Hc to ΔHc with logistic functions (Mooney and Swift, 1999):
| (5) |
| (6) |
where clog (dimensionless) varies from 0 to 1, and the subscripts a and d refer to acclimation and deacclimation, respectively. The physiological maximum bud hardiness (Hc,max) was computed as the mean lowest measured LTE50 by cultivar. The physiological minimum bud hardiness (Hc,min) corresponds to the highest LTE50 (i.e. least hardy) and was set to –3 °C, which is roughly the hardiness of green tissues (Fennell, 2004).
Initial conditions (Hc,initial) were computed by cultivar as the mean of the earliest measured LTE50 near leaf fall. Thus, model inputs were the cultivar-specific constants and Tmin and Tmax (2 m above ground) for the current day. Model output was a dynamic value of Hc (i.e. predicted LTE50) for dormant buds at the location represented by the input data. The model was coded and statistical analyses were conducted using SAS (version 9·1, SAS Institute, Cary, NC, USA). Model parameters were optimized by stepwise selection (Cesaraccio et al., 2004) and correlation (SAS, Proc. Reg.) of predicted against observed Hc, until the root mean square error (RMSE) from the 1:1 line was minimized (Willmott, 1982). In addition, the mean error or bias (B) of predicted against observed Hc was calculated to test model accuracy. Sensitivity analysis of the numerical model was performed by varying the parameters for each cultivar. At least 60 000 simulations were run, with each temperature parameter changing by 0·25 °C, each thermal time parameter changing by 50 DD and each rate parameter changing by 0·01 °C °C−1 while keeping other parameters constant. The model was evaluated using optimized parameters by correlation analysis (SAS, Proc. Reg.), using the data set aside for evaluation.
RESULTS
The optimized, cultivar-specific model parameters are presented in Table 2. Concord clearly was the hardiest of the three cultivars studied, with the lowest Hc,initial and Hc,max. The Hc,initial and Hc,max of the two V. vinifera cultivars (Chardonnay and Cabernet Sauvignon) both differ by <1 °C, as might have been expected from the measured LTE50 data (Fig. 2). However, bud Hc changes dynamically during the dormant season (Fig. 2), and the deacclimation rate during eco-dormancy (kd,eco) turned out to be very important in understanding cultivar-specific Hc patterns. Because of its high kd,eco, Concord lost hardiness quickly in the spring. Chardonnay ended the dormant season the least hardy due to its low Tth,eco, which corresponds to its vulnerability to late frosts. Cabernet Sauvignon, whose budbreak at this site occurs on average 10 d after that of Chardonnay and Concord, had the highest Hc,initial and Hc,max, required the most DDc to enter into eco-dormancy and had the highest threshold temperature during eco-dormancy (Tth,eco). In contrast, ka,endo and kd,endo were similar for all three cultivars, while Tth,endo and kd,eco were similar for Chardonnay and Cabernet Sauvignon but higher for Concord (Table 2).
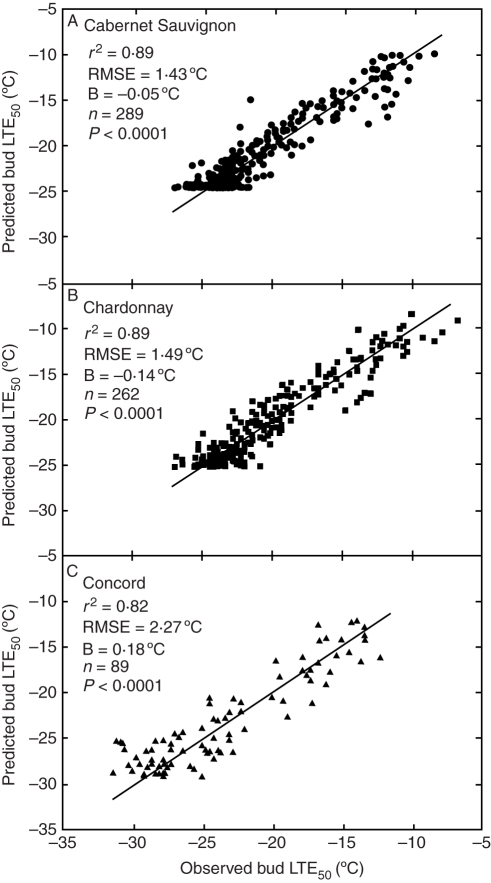
The numerical model derived from the optimization data set was fitted to the empirical data from the evaluation data set and generally showed good agreement. Correlation analysis demonstrated that the variation in observed bud Hc explained 89 % of the variation in predicted Hc for both Cabernet Sauvignon (Fig. 3A) and Chardonnay (Fig. 3B). The Concord version of the model is somewhat less accurate (Fig. 3C), most probably because of fewer years with less frequent measurements available (cf. Fig. 2). Nonetheless, even for Concord, the model showed both high precision (RMSE = 2·27 °C) and high accuracy (B = 0·18 °C). The lower bound or clipped feature on predicted Hc for all three cultivars (Fig. 3) resulted from using a fixed Hc,max (Table 2) across all years.
Fig. 3.
Comparison between model predictions and measurements of bud cold hardiness (expressed as low-temperature exotherms, LTE50) for Cabernet Sauvignon (A; 12 years), Chardonnay (B; 8 years) and Concord (C; 8 years) grapevines. Root mean square error (RMSE) calculated from 1:1 line, using the evaluation data set.
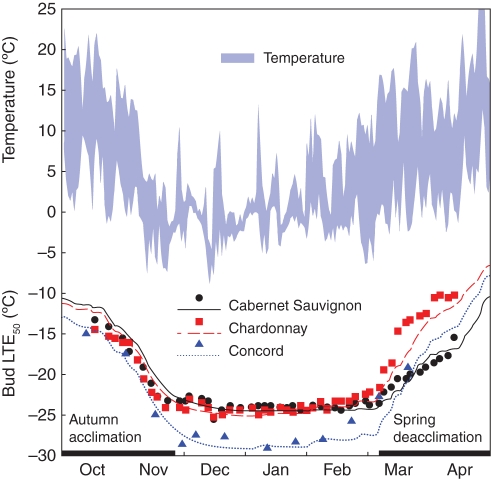
Model output for a single dormant season (Fig. 4) demonstrates the useful aspects of the discrete dynamic approach, as well as its limitations. By changing only the cultivar-specific parameters (Table 2), the model correctly predicted the genetically determined differences among cultivars in bud Hc (Fig. 4). The similarity in seasonal trends between the long-term LTE50 values shown in Fig. 2 and the single-year model output shown in Fig. 4 is evident. For instance, although Concord reached the lowest maximum Hc in mid-winter, its predicted hardiness curve crossed that of Cabernet Sauvignon, but not that of Chardonnay, in the period leading up to budbreak in spring. The predicted values were generally within 2 °C (90th percentile) of the observed LTE50 values, even when the model over-predicted Hc for Cabernet Sauvignon and Chardonnay during autumn acclimation and under-predicted Hc for Chardonnay and Concord during spring deacclimation (Fig. 4). However, while seasonal trends and cultivar differences were especially well predicted by the model, predictions of short-term changes driven by fluctuations in temperature were somewhat less accurate (Fig. 4).
Fig. 4.
Daily minimum and maximum temperatures, and measured bud cold hardiness (expressed as low-temperature exotherms, LTE50; symbols) and LTE50 predicted by a dynamic thermal time model (lines) for three grape cultivars during one dormant season (2000–2001).
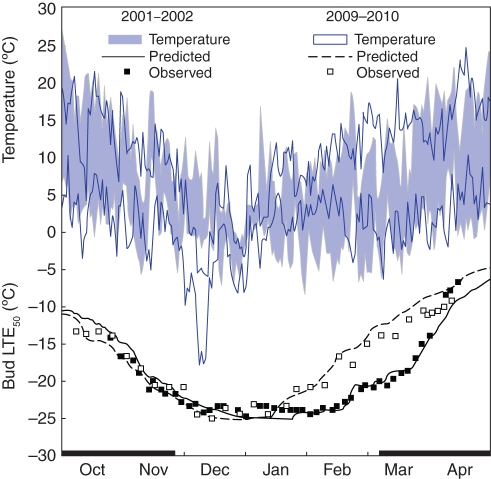
The model also simulated the response of grapevines to disparate temperature patterns (Fig. 5). The accuracy of prediction of Hc for Chardonnay buds was similar in 2001–2002 and in 2009–2010, although the former period was taken from the evaluation data set and the latter from the optimization data set. Both measured and predicted Hc followed the typical seasonal pattern of acclimation after leaf fall, prolonged Hc.max in mid-winter and subsequent deacclimation in spring. However, the cold event in early December 2009 that was associated with a 4·1 °C gain in measured LTE50 was absent in 2001. In addition, during the 2 months from mid January through mid March, 2010 had 46 d with Tmin >0 °C and 36 d with Tmax >10 °C. This contrasts with only 11 d with Tmin >0 °C and 27 d with Tmax >10 °C in 2002 (Fig. 5). Thus, the two dormant seasons also contrasted in the temperatures during the eco-dormancy period, during which 2010 was on average 2·8 °C warmer than 2002. The model accurately predicted the resulting early deacclimation and associated loss of Hc that occurred in 2010. However, during the period preceding budbreak the model underestimated Hc by 2·7 °C in 2001 and overestimated it by 2·1 °C in 2010.
Fig. 5.
Daily minimum and maximum temperatures, and predicted and observed bud cold hardiness (expressed as low-temperature exotherms, LTE50) of Chardonnay grapevines in two dormant seasons with disparate temperature patterns (2001–2002 drawn from the evaluation data set; 2009–2010 drawn from the optimization data set).
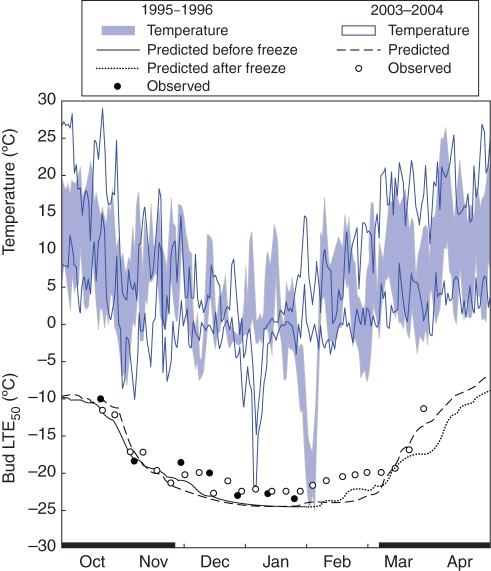
Taking Cabernet Sauvignon as an example, both DTA analysis and model simulation correctly predicted the lethal bud damage caused by the extreme freeze event (–25·3 °C) in late January 1996 (Fig. 6). Although LTE data were not collected for Chardonnay and Concord that winter, model simulations indicated that Chardonnay would sustain close to 50 % bud injury, whereas Concord would be hardy enough to escape serious injury (data not shown). These predictions were confirmed by sampling and dissection of compound buds (each with a primary, secondary and tertiary bud) following the freeze event: 63 % of primary buds, 15 % of secondary buds and 11 % of tertiary buds were killed in Cabernet Sauvignon (n = 289), Concord sustained 18 % lethal primary bud damage, 10 % secondary bud damage and 3 % tertiary bud damage (n = 442), but insufficient numbers of Chardonnay buds were sampled to permit any firm conclusions. Measurements of Hc for Cabernet Sauvignon were terminated after that event, because the condition of the available bud population was too erratic for DTA analysis. Nevertheless, the model was used to continue simulating the deacclimation phase of potentially surviving buds in late winter and spring (see dotted line in Fig. 6). A similar, but less severe, cold event (–21·7 °C) occurred in January 2004, and both the DTA analysis (LTE10 = –21·9 °C) and the model correctly indicated that Cabernet Sauvignon would sustain little if any bud damage at that temperature (Fig. 6). This was despite the model simulation not being as accurate in mid-winter of the 2003–2004 season, underestimating Hc by up to 3·2 °C. Chardonnay and Concord also did not sustain any bud injury that year, and both DTA analysis and model simulations showed these cultivars to have lower Hc at the time of the freeze event than did Cabernet Sauvignon (data not shown).
Fig. 6.
Daily minimum and maximum temperatures, and predicted and observed bud cold hardiness (expressed as low-temperature exotherms, LTE50) of Cabernet Sauvignon grapevines in two dormant seasons (1995–1996 and 2003–2004), with a lethal freeze event at the end of January 1996. Note that measurements ceased after the cold event due to unavailability of live buds.
DISCUSSION
We formulated and evaluated a numerical model, using a dynamic thermal time approach, which successfully simulates the changes in bud Hc of three distinct Vitis spp. genotypes throughout the dormant season and in multiple years. We are not aware of any published dynamic models of grapevine Hc, but the RMSEs in the present model compare favourably with those reported for Douglas fir by Leinonen et al. (1995). Fowler et al. (1999) reported r2 = 0·964 for their model of winter cereal hardiness. However, Leinonen et al. (1995) modelled only the acclimation period, relying on a single year of data, and Fowler et al. (1999) presented data for 2 years compared with our 6–10 years (depending on genotype) of grape bud LTE50 values used for model parameterization and optimization, and 8–12 years of LTE50 values used for model evaluation.
The optimized model parameters were especially effective at simulating seasonal trends of acclimation after leaf fall, prolonged mid-winter hardiness and deacclimation in spring. Another key strength was the model's ability to simulate Hc in response to disparate seasonal temperature patterns and unusual cold or warm spells. However, this seemed to be at the expense of capturing some of the short-term dynamics in Hc due to fluctuating temperatures. To improve the model's ability to extrapolate beyond measured Hc values, it could be re-optimized to enhance short-term accuracy on the temporal scale of weather forecasts, as opposed to season-long accuracy. However, some uncertainty is inherent in the use of ambient temperature in model parameterization and evaluation, because DTA analysis is conducted at bud temperature, and air and bud temperatures are often decoupled (Grace, 2006). Despite these limitations, the current model iteration accurately predicted not only the destructive freeze event of 1996, but also the low level of bud damage resulting from the less severe freeze event of 2004.
The model parameters computed here and the simulations run during the sensitivity analysis permit generalizations about how cold acclimation, mid-winter hardiness and deacclimation are influenced by grape genotype, dormancy status and temperature. The cultivars tested here had similar Hc,min, ka and kd during the autumn acclimation period, but differed in terms of Hc,initial, Hc,max, EDB, Tth, ka and kd during the deacclimation period following mid-winter. The present results confirm research comparing Vitis cultivars subjected to controlled temperature regimes (Damborska, 1978; Schnabel and Wample, 1987; Wolf and Cook, 1992; Gu et al., 2002). However, our results do not support the notion that, across plant species, acclimation occurs more slowly than deacclimation (Kalberer et al., 2006). Thus, the reversible nature of our model with independent acclimation and deacclimation may be of particular interest for improving dormancy and phenology models.
The present model is based on a number of simplifications: LTE50 data were determined for only one location; thermal time was calculated from mean daily temperature; a fixed Hc,max was used; and an abrupt transition from endo- to eco-dormancy was applied. Extending the model to substantially different meso- or macroclimates may require full integration of thermal time over each day. Furthermore, one might argue that the EDB is only a convenient appropriation from the dormancy literature (Lang et al., 1987; Cesaraccio et al., 2004; Kwon et al., 2008). The chilling time required to reach this boundary found here places the EDB in mid-winter, in contrast to some authors (e.g. Pouget, 1972; Dokoozlian, 1999) who place dormancy release for grapes much earlier. However, Dokoozlian (1999) also reported that a chilling duration of 800 h at 0 °C, the longest and coldest treatment combination he tested, resulted in the most rapid and most uniform budbreak. These simplifications notwithstanding, the ability of our model to simulate Hc in distinct Vitis genotypes and over diverse dormant seasons suggests that it may be successfully applied to other climates and to questions regarding climate change effects.
Cold injury remains an environmental challenge in many fruit-producing regions. Implementing protective measures (e.g. wind machines) unnecessarily is expensive, whereas not implementing such measures when they would be needed and effective can lead to substantial economic loss across a region in a single cold event. Despite a general winter warming trend and a projected decrease in extreme cold events (Diffenbaugh et al., 2005), it is not clear whether the frequency and severity of cold injury will decrease in the future. Warmer autumn temperatures may be associated with slower cold acclimation (Keller et al., 2008), and the present data show that unseasonable warm spells are associated with a loss of Hc (cf. Kalberer et al., 2006). This can predispose plants for subsequent cold injury, especially if such episodes are followed by extreme cold. Therefore, future effects of overall warmer winters associated with global climate change are uncertain.
ACKNOWLEDGEMENTS
This work was supported by a grant from the US Department of Agriculture Viticulture Consortium (West) and by funds from the Washington State University Agricultural Research Center, WSU Extension and the Chateau Ste. Michelle Distinguished Professorship in Viticulture. We thank C. Longoria for technical support and G. Reisenauer for statistical advice. Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the US Department of Agriculture.
LITERATURE CITED
- Andreini L, Viti R, Scalabrelli G. Study on morphological evolution of bud break in Vitis vinifera L. Vitis. 2009;48:153–158. [Google Scholar]
- Andrews PK, Proebsting EL, Campbell GS. An exotherm sensor for measuring the cold hardiness of deep-supercooled flower buds by differential thermal analysis. HortScience. 1983;18:77–78. [Google Scholar]
- Andrews PK, Proebsting EL, Lee GS. A conceptual model of changes in deep supercooling of dormant sweet cherry flower buds. Journal of the American Society for Horticultural Science. 1987;112:320–324. [Google Scholar]
- Burr KE, Tinus RW, Wallner SJ, King RM. Comparison of three cold hardiness tests for conifer seedlings. Tree Physiology. 1990;6:351–369. doi: 10.1093/treephys/6.4.351. [DOI] [PubMed] [Google Scholar]
- Cesaraccio C, Spano D, Snyder RL, Duce P. Chilling and forcing model to predict bud-burst of crop and forest species. Agricultural and Forest Meteorology. 2004;126:1–13. [Google Scholar]
- Coombe BG. Adoption of a system for identifying grapevine growth stages. Australian Journal of Grape and Wine Research. 1995;1:100–110. [Google Scholar]
- Damborska M. The effect of higher winter temperatures on changes of the frost resistance of grapevine buds. Vitis. 1978;17:341–349. [Google Scholar]
- Diffenbaugh NS, Pal JS, Trapp RJ, Giorgi F. Fine-scale processes regulate the response of extreme events to global climate change. Proceedings of the National Academy of Sciences, USA. 2005;102:15774–15778. doi: 10.1073/pnas.0506042102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dokoozlian NK. Chilling temperature and duration interact on the budbreak of ‘Perlette’ grapevine cuttings. HortScience. 1999;34:1054–1056. [Google Scholar]
- Ebel RC, Campbell BL, Nesbitt ML, Dozer WA, Linsey JK, Wilkins BS. A temperature index model to estimate long-term freeze-risk of Satsuma mandarins grown on the northern coast of the Gulf of Mexico. Journal of the American Society for Horticultural Science. 2005;130:500–507. [Google Scholar]
- Fennell A. Freezing tolerance and injury in grapevines. Journal of Crop Improvement. 2004;10:201–235. [Google Scholar]
- Fennell A, Hoover E. Photoperiod influences growth, bud dormancy, and cold acclimation in Vitis labruscana and V. riparia. Journal of the American Society for Horticultural Science. 1991;116:270–273. [Google Scholar]
- Fowler DB, Limin AE, Ritchie JT. Low-temperature tolerance in cereals: model and genetic interpretation. Crop Science. 1999;39:626–633. [Google Scholar]
- García de Cortázar-Atauri I, Brisson N, Gaudillere JP. Performance of several models for predicting budburst date of grapevine (Vitis vinifera L.) International Journal of Biometeorology. 2009;53:317–326. doi: 10.1007/s00484-009-0217-4. [DOI] [PubMed] [Google Scholar]
- Gay AP, Eagles CF. Quantitative analysis of cold hardening and dehardening in Lolium. Annals of Botany. 1991;67:339–345. [Google Scholar]
- Grace J. The temperature of buds may be higher than you thought. New Phytologist. 2006;170:1–3. doi: 10.1111/j.1469-8137.2006.01675.x. [DOI] [PubMed] [Google Scholar]
- Gu S, Ding P, Howard S. Effect of temperature and exposure time on cold hardiness of primary buds during the dormant season in ‘Concord’, ‘Norton’, ‘Vignoles’ and ‘St. Vincent’ grapevines. Journal of Horticultural Science and Biotechnology. 2002;77:635–639. [Google Scholar]
- Hubackova M. Dependence of grapevine bud cold hardiness on fluctuations in winter temperatures. American Journal of Enology and Viticulture. 1996;47:100–102. [Google Scholar]
- Jiang H, Howell GS. Correlation and regression analyses of cold hardiness, air temperatures, and water content of Concord grapevines. American Journal of Enology and Viticulture. 2002;53:227–230. [Google Scholar]
- Kalberer SR, Wisniewski M, Arora R. Deacclimation and reacclimation of cold-hardy plants: current understanding and emerging concepts. Plant Science. 2006;171:3–16. [Google Scholar]
- Kanneganti VR, Rotz CA, Walgenbach RP. Modeling freezing injury in alfalfa to calculate forage yield: I. Model development and sensitivity analysis. Agronomy Journal. 1998;90:687–697. [Google Scholar]
- Keller M. The science of grapevines – anatomy and physiology. Burlington, MA: Academic Press; 2010. [Google Scholar]
- Keller M, Mills LJ. Effect of pruning on recovery and productivity of cold-injured Merlot grapevines. American Journal of Enology and Viticulture. 2007;58:351–357. [Google Scholar]
- Keller M, Smithyman RP, Mills LJ. Interactive effects of deficit irrigation and crop load on Cabernet Sauvignon in an arid climate. American Journal of Enology and Viticulture. 2008;59:221–234. [Google Scholar]
- Kobayashi KD, Fuchigami LH, Weiser CJ. Modeling cold hardiness of red-osier dogwood. Journal of the American Society for Horticultural Science. 1983;108:376–381. [Google Scholar]
- Kwon E, Jung J, Chung U, Yun JI. Using thermal time to simulate dormancy depth and bud-burst of vineyards in Korea for the twentieth Century. Journal of Applied Meteorology and Climatology. 2008;47:1792–1801. [Google Scholar]
- Lang GA, Early JD, Martin GC, Darnell RL. Endo-, para-, and eco-dormancy: physiological terminology and classification for dormancy research. HortScience. 1987;22:371–377. [Google Scholar]
- Leinonen I, Repo T, Hänninen H, Burr KE. A second-order dynamic model for the frost hardiness of trees. Annals of Botany. 1995;76:89–95. [Google Scholar]
- McIntyre GN, Kliewer WM, Lider LA. Some limitations of the degree day system as used in viticulture in California. American Journal of Enology and Viticulture. 1987;38:1132. [Google Scholar]
- Mills LJ, Ferguson JC, Keller M. Cold-hardiness evaluation of grapevine buds and cane tissues. American Journal of Enology and Viticulture. 2006;57:194–200. [Google Scholar]
- Moncur MW, Rattigan K, Mackenzie DH, McIntyre GN. Base temperatures for budbreak and leaf appearance of grapevines. American Journal of Enology and Viticulture. 1989;40:21–26. [Google Scholar]
- Mooney DD, Swift R. A course in mathematical modeling. Washington, DC: Mathematical Association of America; 1999. [Google Scholar]
- Pouget R. Considérations générales sur le rythme végétatif et la dormance des bourgeons de la vigne. Vitis. 1972;11:198–217. [Google Scholar]
- Proebsting EL, Ahmedullah M, Brummund VP. Seasonal changes in low temperature resistance of grape buds. American Journal of Enology and Viticulture. 1980;31:329–336. [Google Scholar]
- Richardson EA, Seeley SD, Walker DR. A model for estimating the completion of rest for ‘Redhaven’ and ‘Elberta’ peach trees. HortScience. 1974;9:331–332. [Google Scholar]
- Rowland LJ, Ogden EL, Ehlenfeldt MK, Vinyard B. Cold hardiness, deacclimation kinetics, and bud development among 12 diverse blueberry genotypes under field conditions. Journal of the American Society for Horticultural Science. 2005;130:508–514. [Google Scholar]
- Schnabel BJ, Wample RL. Dormancy and cold hardiness in Vitis vinifera L. cv. White Riesling as influenced by photoperiod and temperature. American Journal of Enology and Viticulture. 1987;34:265–272. [Google Scholar]
- Tinus RW, Bourque JE, Wallner SJ. Estimation of cold hardiness of Douglas-fir and Engelmann spruce seedlings by differential thermal analysis of buds. Annals of Applied Biology. 1985;106:393–397. [Google Scholar]
- Wample RL, Reisenauer G, Bary A, Schuetze F. Microcomputer-controlled freezing, data acquisition and analysis system for cold hardiness evaluation. HortScience. 1990;25:973–976. [Google Scholar]
- Willmott CJ. Some comments on the evaluation of model performance. Bulletin of the American Meteorological Society. 1982;63:1309–1313. [Google Scholar]
- Wisniewski M, Lightner G, Davis G, Schiavone M. System configuration for microcomputer-controlled, low-temperature differential thermal analysis. Computers and Electronics in Agriculture. 1990;5:223–232. [Google Scholar]
- Wolf TK, Cook MK. Seasonal deacclimation patterns of three grape cultivars at constant, warm temperature. American Journal of Enology and Viticulture. 1992;43:171–179. [Google Scholar]
- Wolf TK, Pool RM. Factors affecting exotherm detection in the differential thermal analysis of grapevine dormant buds. Journal of the American Society for Horticultural Science. 1987;112:520–525. [Google Scholar]