Abstract
The lack of animal models of HIV-related pulmonary arterial hypertension (HIV-PAH) severely limits investigation of this serious disease. While histological evidence of HIV-PAH has been demonstrated in macaques infected with simian immunodeficiency virus (SIV) as well as with chimeric simian/human immunodeficiency virus (SHIV) containing HIV-1-derived Nef protein, other primate models have not been studied. The objective was to document and describe the development of pulmonary vascular changes in macaques infected with SIV or with SIV containing HIV-1-derived envelope protein (SHIV-env). Lung tissue was obtained at necropsy from 13 SHIV (89.6P)-env-infected macaques and 10 SIV (ΔB670)-infected macaques. Pulmonary arterial pathology, including arterial hyperplasia and the presence of plexiform lesions, was compared to normal monkey lung. Pulmonary artery hyperplasia was present in 8 of 13 (62%) SHIV-env-infected macaques and 4/10 (36%) SIV-infected macaques. The most common histopathological lesions were intimal and medial hyperplasia of medium and large pulmonary arteries. Hyperplastic lesions were predominantly due to smooth muscle cell hyperplasia. This is the first report of pulmonary vascular lesions in SHIV-env-infected macaques and confirms prior reports of pulmonary vasculopathy in SIV-infected macaques. The finding of pulmonary arteriopathy in monkeys infected with SHIV not containing HIV-nef suggests that other factors might also be important in the development of HIV-PAH. This SHIV-env model provides a new means to investigate HIV-PAH.
Introduction
Over the past two decades, medical breakthroughs in human immunodeficiency virus (HIV) management have greatly reduced the mortality of this disease. The decline in AIDS-related deaths has been accompanied by an increase in the percentage of deaths among HIV-infected individuals due to noninfectious diseases such as chronic cardiovascular and pulmonary disorders.1 The prevalence of 0.5% of HIV-associated pulmonary arterial hypertension (HIV-PAH) is much greater than that in the non-HIV-infected population and remains unchanged even after the development of combination antiretroviral therapy (CART).2,3 This disease may be even more common than previously thought as one study of echocardiography in HIV-infected outpatients found 35.2% had elevated pulmonary artery pressures.4 Associated with a 60% survival at 2 years after diagnosis, HIV-PAH has an even greater mortality in patients with more severe (New York Heart Association Class III–IV) disease.5 Although several reports have described clinical benefit with pulmonary hypertensive therapies in patients with HIV-PAH, we are far from a cure.6–9
A lack of adequate animal models has slowed understanding of this disease. Rodent models of PAH using monocrotaline or hypoxia have helped in understanding PAH pathogenesis, but these models do not replicate the potential effects of the HIV virus.10,11 Murine modeling of HIV and AIDS has greatly developed over the years to include transgenic and humanized mouse models.12–14 Although these models are useful, it would be difficult to determine whether end-organ effects such as PAH were truly from HIV infection or a phenomenon associated with the xenograft. The most studied animal models of HIV and HIV-PAH have been in simian immunodeficiency virus (SIV) and chimeric simian/human immunodeficiency virus (SHIV) models in nonhuman primates. In the SHIV models, HIV genes of interest (e.g., nef, env) are introduced into the SIV viral genome in order to accelerate the progression of immunosuppression.15 Previous studies have reported pulmonary arteriopathy in lung tissues from SIV-infected and SHIV-nef (chimeric virus with SIV backbone and HIV-1 Nef protein) macaques. In an early study, pulmonary artery lesions characterized by intimal thickening and luminal occlusion were seen in 19 of 85 SIV-infected rhesus macaques.16 More recently it was reported that 5 of 6 macaques infected with SHIV-nef developed histological lesions consistent with pulmonary hypertension, while none of 20 SIV-infected monkeys developed these lesions.17
The pathogenesis of HIV-PAH is not well understood. An early study of HIV-PAH pathogenesis demonstrated no evidence of direct HIV infection of pulmonary endothelial cells by electron microscopy, DNA in situ hybridization, or polymerase chain reaction (PCR).18 Although there is no direct infection of pulmonary endothelial cells with the virus, individual HIV viral proteins may actually have an important role in this disease.17,19 For example, in the SHIV-nef-infected macaques, HIV-1 Nef protein was detected in endothelial cells, and the authors postulated that HIV-1 Nef was important for the development of HIV-PAH.17,20 SHIV models containing other HIV proteins have not been studied, and it is possible that HIV proteins such as HIV envelope protein (Env) also play a role in the disease. Other mechanisms may be important as well. Infection with Pneumocystis in the setting of CD4 depletion followed by immune recovery in a murine model has been shown to produce pulmonary artery hyperplasia and right ventricular changes consistent with PAH.21 The relationship of HIV-PAH to severity of infection or immune function is debated, and while viral load and CD4 count have not been shown definitively to influence risk, individuals with HIV-PAH and overt AIDS seem to have more severe PAH.5,22,23
In this study, we determined the prevalence of PAH lesions in two nonhuman primate models without human HIV-nef in order to determine if PAH develops in animal models with other HIV proteins. We also examined the relationship of Pneumocystis to PAH as well as the relationship of PAH to various markers of immunosuppression.
Materials and Methods
Animal tissues
Lung tissue was obtained at necropsy from 13 SHIV-env (89.6P)-infected cynomolgus macaques (Macaca fasicularis), 10 SIV (ΔB670)-infected Rhesus macaques (Macaca mulatta), and one uninfected cynomolgus macaque.24–26 Tissues were fixed in 10% nonbuffered formalin and paraffin embedded, and five to six sections from each monkey were stained with hematoxylin and eosin (H&E). Investigators strictly adhered to animal use regulations as stated by the University of Pittsburgh Institutional Animal Care and Use Committee.
Tissue and histological examination
Pulmonary blood vessels were examined for the presence of pulmonary vascular lesions by a pulmonologist and veterinary pathologist (M.P.G. and A.B.). Investigators were blinded to the identity of the monkeys and scored the slides according to the methods described by Pietra et al.27 Representative sections containing hyperplastic or hypertrophic vessels, if present, were selected from each monkey based on H&E analysis. If no such hyperplastic vessels were seen in any sections, then a representative slide with the most small, medium, and large pulmonary arteries was taken.
After identifying representative slides, we performed quantitative histological analyses of pulmonary artery thickening in SIV- and SHIV-infected macaques compared to an uninfected control. To more easily distinguish intimal, medial, and adventitial blood vessel layers, trichrome stains were performed on each representative section. Small, medium, and large-sized arteries were defined as follows: large pulmonary arteries were adjacent to a cartilaginous airway (lobar and segmental bronchi), medium arteries were adjacent to a noncartilaginous airway with airway epithelial cells (bronchioles), and small arteries were not adjacent to airways. On an uninfected control monkey, the numbers of cell layers were quantified within the intima and media from each caliber of blood vessel. Five separate histological sections were examined, and at least five small arteries, six medium arteries, and seven large arteries were enumerated from the uninfected control. Intimal thickness of small, medium, and large arteries in the control monkey were all two cell layers thick. Medial thickness of small, medium, and large arteries in the control monkey were two, three to five, and four to seven cell layers, respectively. We conservatively defined our normal range of cell layer thickness as the most number of cell layers seen in the intima and media of each similarly sized artery in the control monkey.
Cell layer thicknesses of intima and media of small, medium, and large arteries were quantified for the representative section from each SIV-infected and SHIV-env-infected monkey, and cell layers were considered to be hyperplastic if they were had a greater number of cell layers than the control. In small, medium, and large arteries, an intimal thickness greater than two cell layers was considered hyperplastic. Medial hyperplasia for small, medium, and large arteries was defined as greater than two cell layers, greater than five cell layers, and greater than seven cell layers, respectively.
Immunohistochemistry
Immunohistochemistry was performed on representative sections to distinguish endothelial cell hyperplasia from smooth muscle cell hyperplasia. Previously identified serial sections with representative hyperplastic lesions were deparaffinized, pretreated with 0.1 M sodium citrate (pH 6.0), and microwaved for 10 min. After cooling, they were stained with rabbit polyclonal antihuman von Willibrand factor (A0082, Dako, Carpinteria, CA) to identify endothelial cells and mouse monoclonal anti-alpha smooth muscle actin (A5228, Sigma-Aldrich, St. Louis, MO) to identify smooth muscle cells and corresponding isotype controls. Immunodetection was performed using the HRP polymer conjugate and DAB chromogen according to protocol in the ZymedSuperPicTure Kit (Invitrogen, Carlsbad, CA).
Bronchoalveolar lavage fluid and blood sample collection
Plasma, peripheral blood mononuclear cells (PBMCs), and bronchoalveolar lavage fluid (BAL) samples were collected on a monthly basis premortem from the SHIV-infected animals and from SIV-infected animals (although plasma and PBMC data were not available for all animals in the SIV-infected group), as described previously.28 Plasma SHIV viral levels and peripheral blood CD4 cell counts were determined as previously described.28,29 In addition, colonization with Pneumocystis (Pc) was determined by performing nested PCR on BAL cell lysate and determining anti-Pc endpoint antibody titers to Pc kexin (KEX1) by enzyme-linked immunosorbent assay (ELISA).28–30 Pc colonization was defined as a 3-fold increase in anti-KEX1 reciprocal endpoint titer over the baseline titer and detection of Pc DNA in BAL fluid.29 To diagnose Pneumocystis pneumonia (PCP), BAL fluid was stained using Gomori methenamine silver stain for detection of Pc, and tissue sections were examined for changes consistent with PCP.
Statistics
The prevalence and nature of PAH lesions in the SIV and SHIV groups were determined. Relationships between CD4 cell count, SHIV viral levels, and presence or absence of PAH histological lesions were determined using the Wilcoxon–Mann–Whitney rank sum test. The relationship between Pneumocystis colonization and the presence or absence of PAH lesions was tested using Fisher's exact test.
Results
Histology
Pulmonary artery hyperplasia and/or plexiform arteriopathy were present in 8 of 13 (62%) SHIV-infected macaques (Table 1) and 4 of 10 (40%) SIV-infected macaques. The most common histopathological lesions were intimal and medial hyperplasia of the medium and large pulmonary arteries, although complex arterial lesions (e.g., plexiform arteriopathy, recannalization) were occasionally seen (Figs. 1 and 2). We did not observe perivascular inflammation or evidence of vasculitis in SHIV-infected animals, although vacuolar changes were present in the tunica media in 6 of 13 (46%) animals. To further elucidate endothelial cell hyperplasia versus smooth muscle cell hyperplasia, we performed immunohistochemistry on selected sections using antibodies against Factor VIII, highlighting endothelial cells, and alpha smooth muscle actin, highlighting smooth muscle cells (Fig. 3). These stains demonstrated that the predominant cell type in the hyperplastic lesions consisted of smooth muscle cells.
Table 1.
Histologic Findings in SIV-Infected and SHIV-Infected Macaquesa
| Animal | Species | Viral strain | Duration of infection (months) | Pulmonary vascular lesions | Lung pathologies |
|---|---|---|---|---|---|
| 2700 | Macaca mulatta | SIV ΔB670 | 24 | None | PCP, possible lung metastases of distant tumor |
| 2800 | Macaca mulatta | SIV ΔB670 | 21.5 | Vacuolar change | PCP |
| 3000 | Macaca mulatta | SIV ΔB670 | 45 | Vacuolar change | Persistently Pc colonized |
| 3200 | Macaca mulatta | SIV ΔB670 | 8.5 | None | PCP |
| 6697 | Macaca mulatta | SIV ΔB670 | 27.5 | Plexiform lesion, Small artery and Medium artery intimal hyperplasia, Vacuolar change | PCP |
| 10797 | Macaca mulatta | SIV ΔB670 | 24 | None | Mild PCP |
| 8299 | Macaca mulatta | SIV ΔB670 | 11.5 | Medium artery medial hyperplasia, vacuolar change | |
| 8799 | Macaca mulatta | SIV ΔB670 | 17 | Medium artery intimal hyperplasia | Acute PCP |
| 12099 | Macaca mulatta | SIV ΔB670 | 13.5 | Vacuolar change | PCP |
| 1700 | Macaca mulatta | SIV ΔB670 | 23 | Large artery intimal hyperplasia | Persistently Pc colonized |
| 11405 | Macaca fasicularis | SHIV-env (89.6P) | 15 | None | Pc colonized |
| 11505 | Macaca fasicularis | SHIV-env (89.6P) | 15 | Large artery intimal hyperplasia, vacuolar change | Pc colonized |
| 11605 | Macaca fasicularis | SHIV-env (89.6P) | 12.25 | Large artery intimal hyperplasia, vacuolar change | |
| 11705 | Macaca fasicularis | SHIV-env (89.6P) | 12.25 | Vacuolar change | |
| 11805 | Macaca fasicularis | SHIV-env (89.6P) | 15 | Large artery intimal and medial hyperplasia | Questionable Pc colonization status |
| 15405 | Macaca fasicularis | SHIV-env (89.6P) | 15 | Vacuolar change | Pc colonized |
| 15505 | Macaca fasicularis | SHIV-env (89.6P) | 15 | None | Pc colonized |
| 15605 | Macaca fasicularis | SHIV-env (89.6P) | 15 | Medium artery medial hyperplasia | Pc colonized |
| 15705 | Macaca fasicularis | SHIV-env (89.6P) | 15 | Medium artery medial hyperplasia, large artery intimal hyperplasia | Pc colonized |
| 15905 | Macaca fasicularis | SHIV-env (89.6P) | 12.25 | None | |
| 16005 | Macaca fasicularis | SHIV-env (89.6P) | 12.25 | Small artery hyperplasia, large artery intimal and medial hyperplasia, plexiform lesion | |
| 16105 | Macaca fasicularis | SHIV-env (89.6P) | 15 | Small artery hyperplasia, large artery intimal and medial hyperplasia, vacuolar change | Pc colonized |
| 16305 | Macaca fasicularis | SHIV-env (89.6P) | 15 | Plexiform lesion, vacuolar change | Pc colonized |
Pc, pneumocystis; PCP, pneumocystis pneumonia.
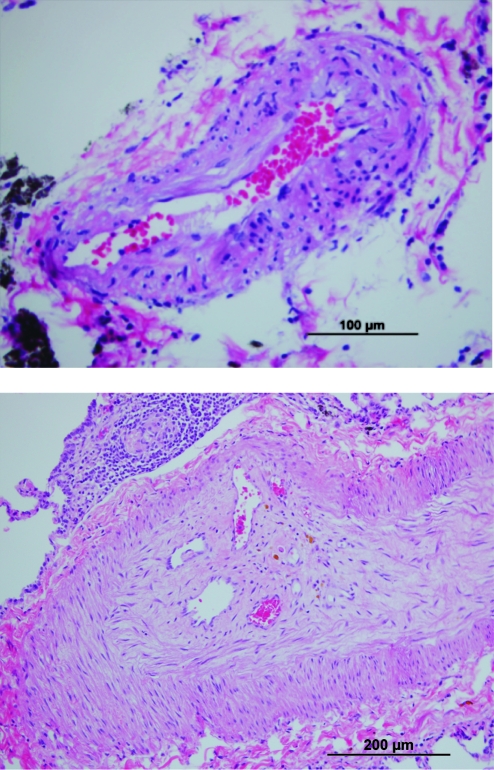
FIG. 1.
Examples of representative pulmonary hypertension lesions on H&E stain. Intimal hyperplasia in SHIV-env-infected macaque (above, magnification 40 ×) and complex vascular lesion with recannalization in SIV-infected macaque (below, magnification 20 ×). Color images available online at www.liebertonline.com/aid.
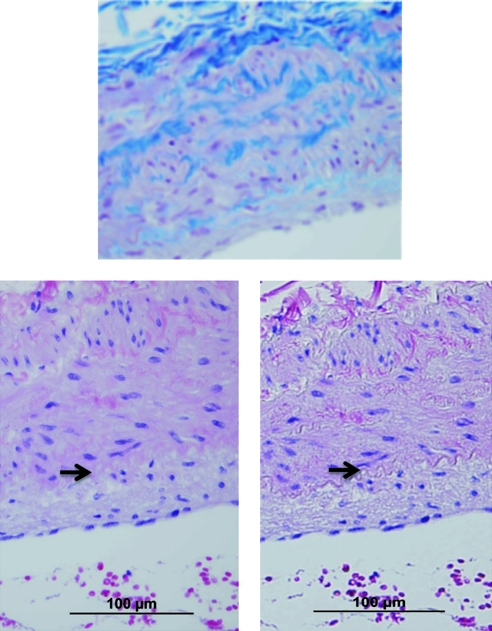
FIG. 2.
Medial hyperplasia in SHIV-env-infected macaque demonstrated by two staining methods. Trichrome stain (upper) helps to distinguish intima and medial cell layers. H&E staining with (lower left) and without (lower right) condenser demonstrates that while the internal elastic lamina, separating the intima and media, can be visualized with all stains, it is most easily distinguished on trichrome and H&E without condenser. The arrow highlights the internal elastic lamina. Magnification 40 ×. Color images available online at www.liebertonline.com/aid
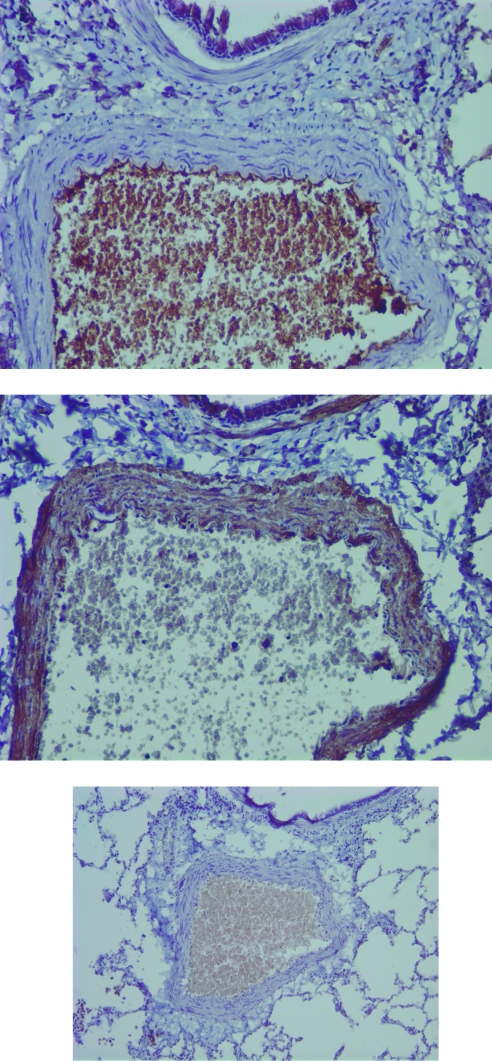
FIG. 3.
Immunohistochemistry demonstrates smooth muscle cell hyperplasia in a representative SHIV-env-infected macaque with histologic PAH. The Zymed DAB Chromogen SuperPicture Kit was used as a detection system after staining with primary antibodies. Primary antibodies include (above) rabbit polyclonal anti-human von Willibrand factor (enhances endothelium), (middle) mouse monoclonal anti-alpha smooth muscle actin (enhances smooth muscle cells), or (below) isotype control. Magnification 20 ×. Color images available online at www.liebertonline.com/aid
Duration of SIV/SHIV Infection and PAH
As a marker of SIV/SHIV viral exposure, we compared the duration of viral infection between the SIV- and SHIV-infected macaques. The median infection time among SIV-infected macaques was 22.25 months (range 8.5–45), and among SHIV-infected macaques was 15 months (range 12.25–15). There was no significant association between duration of infection and PAH in SIV-infected monkeys (p = 0.83) or SHIV-infected monkeys (p = 0.58), and survival times were not related to complications arising from PAH.
Markers of SHIV infection in SHIV-env-infected macaques
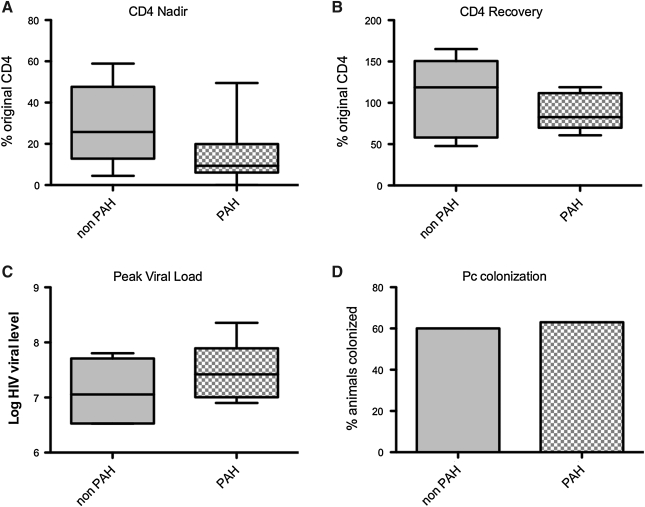
The median percent CD4 nadirs was 9.4% (range 0–49.5%) in animals with histological PAH and 25.8% (range 4.5–58.9%) in animals without histological PAH (p = 0.19) (Fig. 4A). While in 11 of 13 SHIV-infected animals the nadir CD4 count occurred within the first 8 weeks after infection, two animals did not have a notable CD4 decline until weeks 29 and 58. Neither of these animals with a late nadir developed histological PAH at the time of necropsy (15 months and 12.25 months, respectively).
FIG. 4.
Markers of SHIV infection and development of pulmonary hypertension. There were no significant relationships between (A) pulmonary hypertension and degree of CD4 decline (p = 0.19), (B) CD4 recovery (p = 0.77), (C) log HIV viral level (p = 0.31), or (D) pneumocystis (Pc) colonization (p = 0.58).
Many animals had a partial recovery of their CD4 percentages after the nadir CD4, but there was no relationship between percent recovery and PAH [median percent recovery 82.7% (range 60.7–119%) in animals with PAH, 118.8% (range 47.7–165.2) in animals without PAH, p = 0.56] (Fig. 4B). Interestingly, there was a trend toward later CD4 recovery in animals with PAH (median recovery at 42.5 weeks, range 16–58 weeks) compared to those without PAH lesions (16 weeks, range 12-37 weeks, p = 0.09). The mean peak log SHIV viral level in animals with histological PAH was 7.48 (SD ± 0.51) while the mean peak log viral level in animals without histological PAH was 7.12 (SD ± 0.59, p = 0.31) (Fig. 4C).
Pneumocystis colonization
Of the SHIV-infected macaques, eight animals were Pc colonized, and four animals were Pc negative. The colonization status of one animal was indeterminate, as its antibody titers were not consistently significantly elevated above baseline and nested PCR results were inconsistent. Pulmonary infiltrates and overt PCP were not observed in any SHIV-infected animals. On histological review of distal airways, there was little overt inflammation, and the airways appeared grossly normal. Stains for Pc were negative. The prevalence of Pc colonization did not differ in PAH and non-PAH animals (63% among animals with PAH and 60% of animals without PAH, p = 0.58) (Fig. 4D). Of the SIV-infected macaques, three (30%) were Pc colonized and six (60%) had overt PCP as detected by histological review of lung tissue. No SHIV-infected animals had overt PCP.
Discussion
In this report, we describe PAH in macaques infected with SHIV (89.6P)-env (SHIV-env), a chimeric virus with SIV backbone and HIV-1 Env protein, thus introducing a novel nonhuman primate model of HIV-related pulmonary hypertension. We also detected changes associated with PAH in SIV-infected macaques, a finding that has been debated in the literature.16,17 The most common lesions were intimal and medial hyperplasia of the small and medium-sized pulmonary arteries with minimal parenchymal disease and inflammation. PAH lesions were not associated with severity of SHIV illness, as measured by peak SHIV viral level, severity of CD4 cell count decline, or degree of CD4 cell count recovery. There was a trend toward later CD4 recovery in animals that developed PAH lesions. PAH did not appear to be associated with Pc colonization or PCP.
The development of pulmonary arteriopathy in SIV-infected primates has been debated. Pulmonary arteriopathy, characterized by predominantly intimal and medial arterial thickening, was initially reported in 19 of 85 (22%) SIV-infected rhesus macaques.16 In contrast, Marecki and colleagues reported that none of 20 SIV-infected animals developed pulmonary vascular changes.17 In the current study, we found PAH in 36% of SIV-infected macaques. Several possibilities could explain the differences among these studies, including different SIV strains, different duration of SIV infection, and the presence of different concurrent pulmonary infections. The SIV viruses used in the three studies had slightly different tropisms and may have exerted different effects on the pulmonary vasculature. The duration of infection was also different among the different studies. In the Marecki study, the median infection time was shorter than either that reported here or in a previous report of PAH lesions in SIV-infected animals.16 It is also possible that the different pulmonary infections reported in each study influenced the development of PAH. The study by Chalifoux included several animals with avian tuberculosis, disseminated cytomegalovirus, septic endocarditis, and pulmonary thromboses, and most SIV-infected animals in the present study had overt PCP or were colonized by Pc.16 It is possible that the pulmonary hypertension lesions seen in the SIV-infected animals were related to hypoxemia or due to a primary pulmonary process other than SIV infection.
Pulmonary arterial changes have been documented in SHIV-infected primates as well.17 Chimeric viruses constructed using SIV genetic backbone and genetic substitutions of various HIV genes have been useful in modeling different aspects of HIV infection.31 The first report of pulmonary vascular lesions associated with SHIV infection involved macaques infected with SHIV that carried an HIV-1 nef gene in place of the SIV nef.17 Pulmonary arteriopathy, consisting of medial hyperplasia, intimal hypertrophy, vascular remodeling, and plexiform-like lesions, was reported in five out of six SHIV-nef-infected macaques.17 Nef protein was detected in pulmonary endothelial cells both in the monkeys and in humans with HIV and PAH, while monkeys infected with SIV did not have pathological evidence of PAH. These findings led the authors to hypothesize that Nef is important in the pathogenesis of HIV-related PAH and that the HIV-1 Nef protein differed from the SIV protein in the promotion of PAH.
The SHIV model used in this study differed from the SHIV-nef model because it contained an SIV-nef allele and the HIV-1 env gene. We found a fairly high frequency of pulmonary arteriopathy in these animals; however, the lesions differed somewhat from those described in the SHIV-nef model. For example, the pulmonary arterial lesions from the SHIV-nef-infected animals were reported to have more complex lesions, including plexiform arteriopathy. Other important differences in the models include a longer median duration of infection in the SHIV-nef cohort (median infection time 21 months). With a longer duration of infection, the SHIV-nef animals may have had more time to develop histological PAH, and if the SHIV-env animals had been studied for longer, more severe changes might have developed.
Our observations of pulmonary vascular lesions in SIV-infected and SHIV-env-infected macaques are significant in that they broaden the use of relevant primate models for PAH to include both SIV and SHIV-env-infected macaques. Our findings of pulmonary vascular lesions in both SIV-infected and SHIV-env-infected animals provide evidence that the HIV-1 nef allele is not required for the promotion of the pulmonary vascular changes observed. Although the mechanism underlying pathogenesis in these SIV and SHIV-env models is not yet fully elucidated, it is likely that multiple viral proteins play a role in the pathogenesis.17,19,32–34 Although the SIV and SHIV-env models differ from the SHIV-nef model in that they do not contain an HIV-1 nef allele, they do contain an SIV-nef allele, and SIV Nef is functionally similar to the HIV-1 Nef protein.35,36
Our findings of PAH lesions in SHIV-env infection suggest that the pathogenesis of HIV-related PAH is likely to be multifactorial. Although these results do not specifically implicate the Env protein in HIV-PAH pathogenesis, these results, together with findings from previously published work, suggest that it may play a role.33,34 HIV-1 Env is a 120-kDa HIV-1 surface glycoprotein that binds CD4 on target cells and is of potential interest in HIV-PAH since it may stimulate the secretion of endothelin-1 from monocytes and endothelial cells.33,34 Endothelin-1 is the most active isoform of the endothelin peptide family and is mainly produced by endothelial cells in the pulmonary vasculature. It induces vasoconstriction as well as smooth muscle proliferation among neighboring vascular cells.37,38 In vitro studies have also demonstrated that HIV-1 Env can stimulate the secretion of endothelin-1 from human lung microvascular endothelial cells.34 These findings along with the identification of soluble HIV-1 Env in the serum of AIDS patients and evidence that endothelin receptor antagonists seem to successfully treat HIV-PAH suggest a potential role for Env in HIV-PAH pathogenesis.8,9
We were not able to identify evidence of virus within host target tissues. In situ hybridization experiments in SHIV-env-infected macaques did not show evidence of virus in lymphoid tissue or lung tissue (data not shown). Furthermore, given the early peak viremia at 1–2 weeks after infection and the low (<10,000 copies/ml) or undetectable viral loads from approximately week 12 onward, we would not expect to see viral proteins in lung tissues in this model. The absence of tissue expression of viral proteins, such as Env or Nef, at the time of sacrifice does not exclude the possibility that they play an important role. It is possible that much of the vascular insult may occur early, at the time of peak viremia, and slowly progress even after clearance of the original inciting factor (SIV/SHIV virus).
Other pathogenic mechanisms may also be possible in the SIV/SHIV models. For example, the role of other infections in HIV-PAH has been debated. In a murine model of immune reconstitution and PCP, Swain et al. reported that animals which were CD4 depleted then infected with Pc developed pulmonary hypertension as measured by increased right ventricular thickness and pressure.21 These changes occurred after CD4 T-cell recovery and were sustained long after clearance of Pc. While Pc infection and chronic inflammation may be a potential mechanism contributing to pulmonary hypertension in the murine model, we did not find an association between pulmonary hypertension and Pc or degree of recovery after the CD4 nadir. We may not have detected an association with Pc colonization for several reasons. First, Pc may have been present in the lungs at very low levels without overt infection. Second, we did not initiate treatment with antiretroviral therapy to allow the animals to fully restore their immune function, which may play a role in PAH pathogenesis. Finally, our power might have been insufficient to detect an association.
We did not find any association between severity of SIV or SHIV infection and development of histological PAH, consistent with several previously published human studies describing a lack of association between HIV viral load, CD4 count, and development of PAH.5,22,23 It was interesting, however, that the two animals that developed late decline in CD4 in response to SHIV infection did not develop PAH lesions. The lack of histological PAH may have been due to the decreased overall severity of their SHIV illness. Conversely, it is possible that these animals would have developed histological PAH if they had lived for a longer period of time after their CD4 nadir. Therefore, although the severity of SHIV infection, as measured by viral load or median CD4 levels, may not be statistically associated with the development of PAH, it is plausible that there could be a threshold level and/or duration of infection required to develop histological PAH.
There were several weaknesses of the current study. Our study was limited by the fact that we did not have hemodynamic measurements to assess physiological abnormalities; however, histopathology has been considered the gold standard for diagnosis of PAH. Additionally, given that these studies were performed in historical cohorts, the SHIV-env-related histological findings were observed in cynomolgus macaques, whereas our SIV-related findings were seen in rhesus macaques. These species differences complicate direct comparisons of SIV-related lesions with SHIV-env-related lesions, as there could be species-specific variable susceptibilities to SIV or SHIV-env. Inclusion of a larger number of animals might have increased our power to detect differences in factors such as Pc colonization or changes in CD4 cell counts in the PAH and non-PAH animals, and inclusion of more uninfected controls would help in better defining the histology of normal animals. We also did not have adequately prepared heart specimens available to examine right ventricular hypertrophy or dilation. It is possible that the pathological changes observed, although mild, represent preclinical disease that could become symptomatic over the many years that HIV-infected patients now live. This study represents the initial description of pulmonary arteriopathy in SHIV-env-infected primates and forms a solid foundation for future investigations.
This article builds on previous work in several ways. We used a rigorous scoring system to classify pulmonary hypertension in our model. In addition to using the standard system by Pietra et al.,27 we developed a more quantitative scoring system, analyzing small, medium, and large arteries, and systematically compared each blood vessel caliber to a normal control. Because the pulmonary artery anatomy of macaques has not been previously described, the inclusion of a species-specific control improves on previous work using systems developed for humans. We are also the first to examine the SHIV-env model for pulmonary vascular changes. We examined potential relationships between histological PAH and SHIV disease duration and severity, as well as Pc colonization, avenues that had not been specifically explored in previous publications.
In the field of HIV-PAH research with few relevant models of disease, our observations are significant in that they broaden the use of primate models for PAH and suggest that the HIV-1 nef allele is not essential for pathogenesis. We have found that SHIV-env-infected primates develop pulmonary arterial lesions consistent with pulmonary hypertension representing a novel nonhuman primate model of HIV-PAH. We have also confirmed earlier reports documenting these lesions in SIV-infected animals. Factors such as Pc colonization or degree or duration of immunosuppression were not associated with the development of PAH lesions. This SHIV-env primate model will provide a new means to investigate the mechanisms of HIV-PAH, as well as a platform to test relevant therapeutic interventions, ultimately helping to improve outcomes in patients with HIV-PAH.
Acknowledgments
We would like to thank Siobhan Guyach and Kimberly Fuhrer at the University of Pittsburgh and Beth A. Gray at the Wisconsin Veterinary Diagnostic Laboratory for their assistance with tissue preparation and staining. The authors would like to also acknowledge Dr. Shulin Qin for assistance with the design of the immunohistochemistry protocol. Supported by NIH R01HL083462.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Crum NF. Riffenburgh RH. Wegner S, et al. Comparisons of causes of death and mortality rates among HIV-infected persons: Analysis of the pre-, early, and late HAART (highly active antiretroviral therapy) eras. J Acquir Immune Defic Syndr. 2006;41(2):194–200. doi: 10.1097/01.qai.0000179459.31562.16. [DOI] [PubMed] [Google Scholar]
- 2.Speich R. Jenni R. Opravil M. Pfab M. Russi EW. Primary pulmonary hypertension in HIV infection. Chest. 1991;100(5):1268–1271. doi: 10.1378/chest.100.5.1268. [DOI] [PubMed] [Google Scholar]
- 3.Sitbon O. Lascoux-Combe C. Delfraissy JF, et al. Prevalence of HIV-related pulmonary arterial hypertension in the current antiretroviral therapy era. Am J Respir Crit Care Med. 2008;177(1):108–113. doi: 10.1164/rccm.200704-541OC. [DOI] [PubMed] [Google Scholar]
- 4.Hsue PY. Deeks SG. Farah HH, et al. Role of HIV and human herpesvirus-8 infection in pulmonary arterial hypertension. AIDS. 2008;22(7):825–833. doi: 10.1097/QAD.0b013e3282f7cd42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nunes H. Humbert M. Sitbon O, et al. Prognostic factors for survival in human immunodeficiency virus-associated pulmonary arterial hypertension. Am J Respir Crit Care Med. 2003;167(10):1433–1439. doi: 10.1164/rccm.200204-330OC. [DOI] [PubMed] [Google Scholar]
- 6.Recusani F. Di Matteo A. Gambarin F. D'Armini A. Klersy C. Campana C. Clinical and therapeutical follow-up of HIV-associated pulmonary hypertension: Prospective study of 10 patients. AIDS. 2003;17(Suppl 1):S88–95. doi: 10.1097/00002030-200304001-00013. [DOI] [PubMed] [Google Scholar]
- 7.Ghofrani HA. Friese G. Discher T, et al. Inhaled iloprost is a potent acute pulmonary vasodilator in HIV-related severe pulmonary hypertension. Eur Respir J. 2004;23(2):321–326. doi: 10.1183/09031936.03.00057703. [DOI] [PubMed] [Google Scholar]
- 8.Sitbon O. Gressin V. Speich R, et al. Bosentan for the treatment of human immunodeficiency virus-associated pulmonary arterial hypertension. Am J Respir Crit Care Med. 2004;170(11):1212–1217. doi: 10.1164/rccm.200404-445OC. [DOI] [PubMed] [Google Scholar]
- 9.Barbaro G. Lucchini A. Pellicelli AM. Grisorio B. Giancaspro G. Barbarini G. Highly active antiretroviral therapy compared with HAART and bosentan in combination in patients with HIV-associated pulmonary hypertension. Heart. 2006;92(8):1164–1166. doi: 10.1136/hrt.2005.076794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rabinovitch M. Gamble W. Nadas AS. Miettinen OS. Reid L. Rat pulmonary circulation after chronic hypoxia: Hemodynamic and structural features. Am J Physiol. 1979;236(6):H818–827. doi: 10.1152/ajpheart.1979.236.6.H818. [DOI] [PubMed] [Google Scholar]
- 11.Okada K. Tanaka Y. Bernstein M. Zhang W. Patterson GA. Botney MD. Pulmonary hemodynamics modify the rat pulmonary artery response to injury. A neointimal model of pulmonary hypertension. Am J Pathol. 1997;151(4):1019–1025. [PMC free article] [PubMed] [Google Scholar]
- 12.Shacklett BL. Can the new humanized mouse model give HIV research a boost. PLoS Med. 2008;5(1):e13. doi: 10.1371/journal.pmed.0050013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rahim MM. Chrobak P. Hu C. Hanna Z. Jolicoeur P. Adult AIDS-like disease in a novel inducible human immunodeficiency virus type 1 Nef transgenic mouse model: CD4+ T-cell activation is Nef dependent and can occur in the absence of lymphophenia. J Virol. 2009;83(22):11830–11846. doi: 10.1128/JVI.01466-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Duyne R. Pedati C. Guendel I, et al. The utilization of humanized mouse models for the study of human retroviral infections. Retrovirology. 2009;6:76. doi: 10.1186/1742-4690-6-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu SL. Non-human primate models for AIDS vaccine research. Curr Drug Targets Infect Disord. 2005;5(2):193–201. doi: 10.2174/1568005054201508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chalifoux LV. Simon MA. Pauley DR. MacKey JJ. Wyand MS. Ringler DJ. Arteriopathy in macaques infected with simian immunodeficiency virus. Lab Invest. 1992;67(3):338–349. [PubMed] [Google Scholar]
- 17.Marecki JC. Cool CD. Parr JE, et al. HIV-1 Nef is associated with complex pulmonary vascular lesions in SHIV-nef-infected macaques. Am J Respir Crit Care Med. 2006;174(4):437–445. doi: 10.1164/rccm.200601-005OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mette SA. Palevsky HI. Pietra GG, et al. Primary pulmonary hypertension in association with human immunodeficiency virus infection. A possible viral etiology for some forms of hypertensive pulmonary arteriopathy. Am Rev Respir Dis. 1992;145(5):1196–1200. doi: 10.1164/ajrccm/145.5.1196. [DOI] [PubMed] [Google Scholar]
- 19.Caldwell RL. Gadipatti R. Lane KB. Shepherd VL. HIV-1 TAT represses transcription of the bone morphogenic protein receptor-2 in U937 monocytic cells. J Leukoc Biol. 2006;79(1):192–201. doi: 10.1189/jlb.0405194. [DOI] [PubMed] [Google Scholar]
- 20.Sehgal PB. Mukhopadhyay S. Patel K, et al. Golgi dysfunction is a common feature in idiopathic human pulmonary hypertension and vascular lesions in SHIV-nef-infected macaques. Am J Physiol Lung Cell Mol Physiol. 2009;297(4):L729–737. doi: 10.1152/ajplung.00087.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Swain SD. Han S. Harmsen A. Shampeny K. Harmsen AG. Pulmonary hypertension can be a sequela of prior Pneumocystis pneumonia. Am J Pathol. 2007;171:790–799. doi: 10.2353/ajpath.2007.070178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mehta NJ. Khan IA. Mehta RN. Sepkowitz DA. HIV-Related pulmonary hypertension: Analytic review of 131 cases. Chest. 2000;118(4):1133–1141. doi: 10.1378/chest.118.4.1133. [DOI] [PubMed] [Google Scholar]
- 23.Pellicelli AM. Barbaro G. Palmieri F, et al. Primary pulmonary hypertension in HIV patients: A systematic review. Angiology. 2001;52(1):31–41. doi: 10.1177/000331970105200105. [DOI] [PubMed] [Google Scholar]
- 24.Barouch DH. Fu TM. Montefiori DC. Lewis MG. Shiver JW. Letvin NL. Vaccine-elicited immune responses prevent clinical AIDS in SHIV(89.6P)-infected rhesus monkeys. Immunol Lett. 2001;79(1-2):57–61. doi: 10.1016/s0165-2478(01)00266-8. [DOI] [PubMed] [Google Scholar]
- 25.Reimann KA. Li JT. Voss G, et al. An env gene derived from a primary human immunodeficiency virus type 1 isolate confers high in vivo replicative capacity to a chimeric simian/human immunodeficiency virus in rhesus monkeys. J Virol. 1996;70(5):3198–3206. doi: 10.1128/jvi.70.5.3198-3206.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reimann KA. Li JT. Veazey R, et al. A chimeric simian/human immunodeficiency virus expressing a primary patient human immunodeficiency virus type 1 isolate env causes an AIDS-like disease after in vivo passage in rhesus monkeys. J Virol. 1996;70(10):6922–6928. doi: 10.1128/jvi.70.10.6922-6928.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pietra GG. Capron F. Stewart S, et al. Pathologic assessment of vasculopathies in pulmonary hypertension. J Am Coll Cardiol. 2004;43:25–32. doi: 10.1016/j.jacc.2004.02.033. [DOI] [PubMed] [Google Scholar]
- 28.Board KF. Patil S. Lebedeva I, et al. Experimental Pneumocystis carinii pneumonia in simian immunodeficiency virus-infected rhesus macaques. J Infect Dis. 2003;187(4):576–588. doi: 10.1086/373997. [DOI] [PubMed] [Google Scholar]
- 29.Kling HM. Shipley TW. Patil S. Morris A. Norris KA. Pneumocystis colonization in immunocompetent and simian immunodeficiency virus-infected cynomolgus macaques. J Infect Dis. 2009;199(1):89–96. doi: 10.1086/595297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wakefield AE. Pixley FJ. Banerji S, et al. Detection of Pneumocystis carinii with DNA amplification. Lancet. 1990;336(8713):451–452. doi: 10.1016/0140-6736(90)92008-6. [DOI] [PubMed] [Google Scholar]
- 31.Joag SV. Primate models of AIDS. Microbes Infect. 2000;2(2):223–229. doi: 10.1016/s1286-4579(00)00266-5. [DOI] [PubMed] [Google Scholar]
- 32.Rusnati M. Presta M. HIV-1 Tat protein and endothelium: From protein/cell interaction to AIDS-associated pathologies. Angiogenesis. 2002;5(3):141–151. doi: 10.1023/a:1023892223074. [DOI] [PubMed] [Google Scholar]
- 33.Ehrenreich H. Rieckmann P. Sinowatz F, et al. Potent stimulation of monocytic endothelin-1 production by HIV-1 glycoprotein 120. J Immunol. 1993;150(10):4601–4609. [PubMed] [Google Scholar]
- 34.Kanmogne GD. Primeaux C. Grammas P. Induction of apoptosis and endothelin-1 secretion in primary human lung endothelial cells by HIV-1 gp120 proteins. Biochem Biophys Res Commun. 2005;333(4):1107–1115. doi: 10.1016/j.bbrc.2005.05.198. [DOI] [PubMed] [Google Scholar]
- 35.Sinclair E. Barbosa P. Feinberg MB. The nef gene products of both simian and human immunodeficiency viruses enhance virus infectivity and are functionally interchangeable. J Virol. 1997;71(5):3641–3651. doi: 10.1128/jvi.71.5.3641-3651.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alexander L. Du Z. Howe AY. Czajak S. Desrosiers RC. Induction of AIDS in rhesus monkeys by a recombinant simian immunodeficiency virus expressing nef of human immunodeficiency virus type 1. J Virol. 1999;73(7):5814–5825. doi: 10.1128/jvi.73.7.5814-5825.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jacobs A. Preston IR. Gomberg-Maitland M. Endothelin receptor antagonism in pulmonary arterial hypertension––a role for selective ET(A) inhibition. Curr Med Res Opinions. 2006;22(12):2567–2574. doi: 10.1185/030079906X158020. [DOI] [PubMed] [Google Scholar]
- 38.Giaid A. Yanagisawa M. Langleben D, et al. Expression of endothelin-1 in the lungs of patients with pulmonary hypertension. N Engl J Med. 1993;328(24):1732–1739. doi: 10.1056/NEJM199306173282402. [DOI] [PubMed] [Google Scholar]