Abstract
Despite significant advances in management of severe wounds such as burns and chronic ulcers, autologous split-thickness skin grafts are still the gold standard of care. The main problems with this approach include pain and discomfort associated with harvesting autologous tissue, limited availability of donor sites, and the need for multiple surgeries. Although tissue engineering has great potential to provide alternative approaches for tissue regeneration, several problems have hampered progress in translating technological advances to clinical reality. Specifically, engineering of skin substitutes requires long culture times and delayed vascularization after implantation compromises graft survival. To address these issues we developed a novel two-prong strategy for tissue regeneration in vivo: (1) vascularization of acellular dermal scaffolds by infiltration of angiogenic factors; and (2) generation of stratified epidermis by in situ delivery of epidermal keratinocytes onto the prevascularized dermal support. Using athymic mouse as a model system, we found that incorporation of angiogenic factors within acellular human dermis enhanced the density and diameter of infiltrating host blood vessels. Increased vascularization correlated with enhanced proliferation and stratification of the neoepidermis originating from the fibrin-keratinocyte cell suspension. This strategy promoted tissue regeneration in vivo with no need for engineering skin substitutes; therefore, it may be useful for treatment of major wounds when skin donor sites are scarce and rapid wound coverage is required.
Introduction
Patients with serious skin injuries such as burns or chronic wounds require immediate and specialized care to minimize morbidity and mortality. Moderate to severe burn injuries requiring hospitalization account for ∼100,000 of these cases, and about 5000 patients die each year from burn-related complications.1,2 In patients with severe burns over >40% of the total body surface area, 75% of all deaths are currently related to sepsis from burn wound infection or other infection complications and/or inhalation injury.3,4 On the other hand, chronic wounds, which commonly occur in diabetic patients, also require special medical intervention often resulting in drastic surgical procedures such as amputation of the infected limb. Despite intense efforts devoted into development of tissue-engineered skin,5–13 the autologous skin graft remains the gold standard for skin replacement.
Although autologous skin grafts are effective, they cause injury at the donor site and they are often limited by availability of donor sites. Tissue-engineered skin substitutes with epidermal and dermal components have been designed to provide the lost cellular functions of the epidermis and dermis, respectively.7,14,15 Although dermal substitutes may provide wound coverage and reduce pain, they still require an epidermal component, which is often provided through one or more applications of split-thickness autografts, necessitating multiple surgical procedures that injure the donor site and increase scar morbidity.16 On the other hand, cultured epithelial autografts without dermal component have shown unreliable take rate possibly owing to lack of adequate vascularization and produce an unstable epidermis that is prone to blistering or minor trauma.17 Finally, substitutes with autologous keratinocytes and fibroblasts require several weeks of culture for development before transplantation to the wound site. These constraints may be impractical in cases like burns that require immediate wound coverage to prevent patient death.
Several polymeric or natural biomaterials can be employed as dermal substrates, including fibroblast-containing nylon,7 fibroblast-containing biodegradable polyglactin matrix,15,18 collagen/chondroitin-6-sulfate composite (Integra),19–21 and decellularized dermis (Alloderm/Xenoderm).22,23 However, vascularization of the dermal support remains a major challenge limiting the survival and take rate of the multilayered epithelial component.16,17,24
Therefore, successful wound regeneration requires (1) fast wound coverage; (2) quick vascularization of the neodermis; and (3) full restoration of the epidermis with minimum culture time and no need for split-thickness autografts. In principle these requirements can be met by a two-step approach. First, employ a dermal substrate to provide immediate wound coverage and prevent infection and water loss. Then, keratinocytes can be seeded directly onto the prevascularized dermal support in situ to promote epidermal stratification and development of barrier function. This strategy saves time by circumventing the need for preparation of skin substitutes in vitro, but in situ cell delivery may be challenging especially in areas of the body with complex and uneven surface landscape. In this context, hydrogels such as fibrin that polymerize quickly after application and support cell growth may be used to prevent cell loss and facilitate uniform surface coverage. Fibrin hydrogels display these properties; in addition, they have been shown to maintain the epidermal stem cell phenotype1 and to promote epidermal wound healing in vitro and in vivo.25,26 In addition, fibrin has been used clinically to deliver keratinocytes directly to the wound site with promising results.17,24,27
Here we examined the hypothesis that a prevascularized dermal support such as acellular dermis may enhance wound re-epithelialization by in situ delivery of epidermal keratinocytes as cell suspension within a fibrin matrix. We showed that infiltration of acellular dermis with a mixture of angiogenic factors and fibrin promoted considerable vascularization as early as 1 week after transplantation onto full-thickness excisional wounds. In turn, prevascularized dermis enhanced proliferation and stratification of in situ-delivered keratinocytes to resurface the epidermis. Our strategy achieved immediate wound coverage and successful wound re-epithelialization with no need for split-thickness autografts or development of skin substitutes in vitro.
Materials and Methods
Cell isolation and culture
Keratinocytes were isolated from neonatal foreskin and were propagated as described previously.10 Briefly, keratinocytes were cocultivated on feeder layers of 3T3-J2 mouse fibroblasts (ATCC) that were pretreated with 15 μg/mL mitomycin-C (Sigma) for 3 h. The keratinocyte culture medium was a 3:1 mixture of Dulbecco's modified Eagle's medium (DMEM, high glucose; GIBCO) and Ham's F12 medium (GIBCO) supplemented with 10% (v/v) fetal bovine serum (FBS; GIBCO), adenine (24 μg/mL; Sigma), cholera toxin (Vibrio cholerae, type Inaba 569 B, 10-10M; Calbiochem); hydrocortisone (0.4 mg/mL; Calbiochem), insulin (5 mg/mL; Eli Lilly), transferrin (5 μg/mL; Sigma), triiodo-L-thyronine (2 × 10−9 M; Sigma), and penicillin-streptomycin (100 U/mL penicillin G and 100 μg/mL streptomycin sulfate; GIBCO). After 3 days, cells were replenished with a fresh keratinocyte culture medium supplemented with epidermal growth factor (EGF, 10 ng/mL; Collaborative Biomedical Products) until they reached 80%–90% confluence. Only the first two passages of keratinocytes were used for all experiments.
The human epidermal cell line HaCaT was cultured in DMEM supplemented with penicillin–streptomycin and 10% (v/v) FBS in a humidified atmosphere at 37°C and 10% CO2. For harvesting the conditioned medium, HaCaT cells (1 × 106 cells/well) were seeded in six-well tissue culture-treated plates (Greiner Bio-One), and 2 days later the medium was replaced with 1 mL of the culture medium containing 100 ng/mL EGF and 100 ng/mL keratinocyte growth factor (KGF; Amgen). The conditioned medium was collected after 12 h and stored at −80°C until use. The presence of vascular endothelial growth factor (VEGF) in this conditioned medium was verified by an ELISA kit for human VEGF according to the manufacturer's protocol (Invitrogen).
Infiltration of acellular human dermis with conditioned medium
Human acellular dermis was prepared from human cadaveric skin as previously described.3,11,28 To infiltrate the dermis with HaCaT-conditioned medium, a piece of 1.5 cm2 acellular dermis was placed basement membrane side down in a vacuum device. Then, the dermis was infiltrated with a mixture of 200 μL of thrombin (2.5 mU/mL; Sigma) and 80 μL of HaCaT-conditioned medium for 1 min, followed by 200 μL of fibrinogen (8 mg/mL; Enzyme Research Laboratories) for 5 min or until a thin layer of fibrin was visible at the bottom of the dermis. Finally, the modified dermis was placed in a six-well tissue culture-treated plate and stored at 4°C for 24 h to preserve its biological activity.29
Preparation of epidermal equivalents within keratinocyte suspension in fibrin gels
A disk of polydimethylsiloxane with an opening of the same size as the dermis (area = 1 cm2) was placed in six-well tissue culture plate. Subsequently, a square piece of acellular dermis with the basement membrane side up was placed inside the opening of the insert. A freshly prepared suspension of keratinocytes (0.75 × 106 cells) was mixed with the indicated concentrations of fibrinogen (80 μL). Fibronectin (Sigma) (100 ng/mL) and KGF (80 ng/mL) were added to the fibrinogen fraction to promote cell attachment and proliferation. Finally, thrombin (2.5 mU/mL, 20 μL) was added to initiate polymerization on the surface of the dermis. As control, keratinocytes without fibrinogen were seeded on acellular dermis in the keratinocyte seeding medium (100 μL, see below) containing the same concentrations of fibronectin and KGF. After 1.5 h, the skin equivalents were submerged in the keratinocyte seeding medium. After 24 h, the keratinocyte seeding medium was replaced with the keratinocyte priming medium (see below) for 48 h. The skin equivalents were then placed on stainless steel screens, raised to the air–liquid interface, and cultured for 1 week with the air–liquid interface medium (see below) that was replaced every 2–3 days.
The keratinocyte seeding medium was a 3:1 mixture of DMEM (high glucose) (Gibco BRL) and Ham's F-12 medium (Gibco BRL) supplemented with 1% FBS (Gibco BRL), 10−10 M cholera toxin (V. cholerae, type Inaba 569 B; Calbiochem), 0.2 μg/mL hydrocortisone (Calbiochem), recombinant insulin (5 μg/mL; Sigma), ascorbic acid (50 μg/mL; Sigma), and penicillin–streptomycin. The keratinocyte priming medium was composed of the keratinocyte seeding medium supplemented with 24 μM bovine serum albumin (Sigma), 1.0 mM L-serine (Sigma), 10 μM L-carnitine (Sigma), and a cocktail of fatty acids: 25 μM oleic acid (Sigma), 15 μM linoleic acid (Sigma), 7 μM arachidonic acid (Sigma), and 25 μM palmitic acid (Sigma). The air–liquid interface medium composed of the serum-free keratinocyte priming medium supplemented with EGF (1 ng/mL).
Grafting modified acellular human dermis onto athymic mice
Male athymic nu/nu mice (Harlan Sprague Dawley) at 5–8 weeks of age were used to assess wound healing in vivo. To graft on the mouse, the animal was first anesthetized in a gas chamber filled with an inhaled anesthetic (Isoflourane; IsoFlo Abbott Laboratories) until it reached the desired level of anesthesia. After wiping the dorsum of the mouse with ethanol, a 1.5 cm2 full-thickness wound (including the panniculous carnosus) was created above the left shoulder of the mouse. Human acellular dermis infiltrated with the HaCaT-conditioned medium was trimmed to fit precisely into the defect and secured with 6-0 Prolene (nonabsorbable) suture (Ethicon; Johnson & Johnson), one stitch at each corner. Xeroform gauze (XEROFORM™ Petrolatum Gauze; Kendall) supplemented with triple antibiotic ointment (Neosporin; Johnson & Johnson) and the HaCaT-conditioned medium was applied to the graft to keep the graft moist. Subsequently, two stacked pieces of Telfa nonstick gauze (Kendall) supplemented with the HaCaT-conditioned medium were applied to the graft and secured with 6-0 Prolene sutures. A piece of polyurethane occlusive dressing (Tegaderm; 3M) was applied over the Telfa dressing. Finally, the graft was dressed with a trimmed 3M Sports Band-aid (3M) and waterproof adhesive tape (Johnson & Johnson). Bandages except for the Telfa were changed every 2 days for 1 week, at which time the Telfa was removed and a polydimethylsiloxane insert was sutured around the dermis (to create a surrounding wall) before cell seeding. Keratinocytes (1 × 106 cells) were added in a suspension of fibrin as discussed above. The final concentration of this cell-containing fibrin layer was 4 mg/mL fibrinogen, 2.5 mU/mL thrombin, 80 ng/mL KGF, and 100 ng/mL fibronectin. New Telfa containing the keratinocyte seeding medium was then placed on top of the fibrin gel and kept moist with the cell culture medium. The graft area was further covered with Tegaderm, Band-aid, and waterproof adhesive tape. The Telfa dressing was removed 3 days postkeratinocyte seeding to expose the cells to air and facilitate stratification. In this case, only a trimmed 3M Sports Band-aid and waterproof adhesive tape were used to protect the neoepidermis. Dressings were changed every 3 days for 2 weeks, at which time the grafts were harvested and a portion of the graft was embedded in paraffin, whereas the remaining portion was snap-frozen in Tissue Freezing Medium (TFM) (Triangle Biomedical Sciecnes). For control samples, keratinocytes in the keratinocyte seeding medium supplemented with fibronectin and KGF were directly seeded on the acellular dermis and covered with dressings and bandages as described above.
Histology and immunohistochemistry
Hematoxylin and Eosin staining was performed as described previously.11,25,26,28 The presence of keratin-10, laminin, KGF, and proliferating cell nuclear antigen (PCNA) in the tissue samples was detected in TFM-embedded tissues by immunohistochemistry. Briefly, the cryosections were washed twice in phosphate-buffered saline (PBS) followed by blocking in 10% goat serum/PBS for 1 h at room temperature. The samples were incubated with primary antibody: mouse anti human keratin-10 (1:100 dilution; Vector Laboratories); polyclonal rabbit anti-laminin (1:60 dilution; Sigma); monoclonal mouse anti KGF (1:100 dilution; R&D Systems); or mouse anti-PCNA (1:200 dilution; Biolegen) diluted in blocking buffer for 1 h at room temperature. Subsequently, the sections were washed three times with PBS (5 min/wash) and incubated with secondary antibody diluted in blocking buffer (Alexa 488–conjugated goat anti-mouse IgG, Alexa 594–conjugated goat anti-mouse IgG, or Alexa 594–conjugated goat anti-rabbit IgG, 5 μg/mL; Invitrogen), for half hour at room temperature. After washing with PBS for three times (5 min/wash), the samples were counterstained for nuclei with Hoechst 33258 (6.25 μg/mL; Invitrogen) for 5 min at room temperature. Finally, the sections were washed twice with PBS (5 min/wash). Fluorescent images were obtained using an inverted microscope (Diaphot-TMD; Nikon Corporation) that is connected with a Retiga 1300 digital camera (Quantitative Imaging Corporation). Fluorescent images taken at different channels were overlaid into one image using ImageJ 1.37 (National Institutes of Health).
Data analysis
The surface area from the basal to the granular cell layers (Ae) and the length of the basement membrane (lBM) of each tissue image were measured using ImageJ software. Epidermal thickness was calculated by Ae/lBM. Keratinocyte density was determined by counting the total number of nuclei in the epithelium layer and divided by lBM. For vessel density, vessels were first identified by laminin staining. After counting the number of vessels on each image, vessel density was calculated as the number of vessels on each image divided by the cross-sectional area of the dermis. Vessel diameter was determined by measuring the cross-sectional area (Av) of the vessels using ImageJ. Then, the effective diameter was calculated from d = √ (4Av/π), where d is the effective diameter of the vessel. Statistical analysis was performed using a two-tailed paired t-test using Microsoft Excel (Microsoft) and statistical significance was defined as p < 0.05.
Results
Fibrin-mediated keratinocyte delivery maintains the normal differentiation and stratification potential of epidermal cells
Our goal is to deliver epidermal keratinocytes directly onto a prevascularized scaffold such as acellular dermis that has been used to cover the wound to prevent infection and/or water loss. Direct seeding of cells in vivo will avoid engineering of stratified epidermis in vitro and minimize the time of wound treatment. To this end, we hypothesized that fibrin may be an ideal cell delivery vehicle as it can be applied locally even on irregular surfaces and polymerized within seconds to secure the cells in place. However, it was not clear whether keratinocytes in a fibrin cell suspension would attach to the basement membrane of decellularized dermis to generate a stratified epidermis or whether they would remain as single cells within the fibrin hydrogel.
To examine this possibility, keratinocytes were mixed in fibrin hydrogels prepared with 4 mg/mL fibrinogen and seeded on the basement membrane side of acellular dermis, a substrate that was previously shown to promote epidermal stratification and facilitate grafting.3,10,30 Fibronectin (100 ng/mL) was added to support cell adhesion as keratinocytes lack the fibrin/fibrinogen receptor—integrin αvβ3—and consequently cannot bind and spread on fibrin matrix.31 In addition, KGF (80 ng/mL) was also added to support keratinocyte proliferation and stratification.10
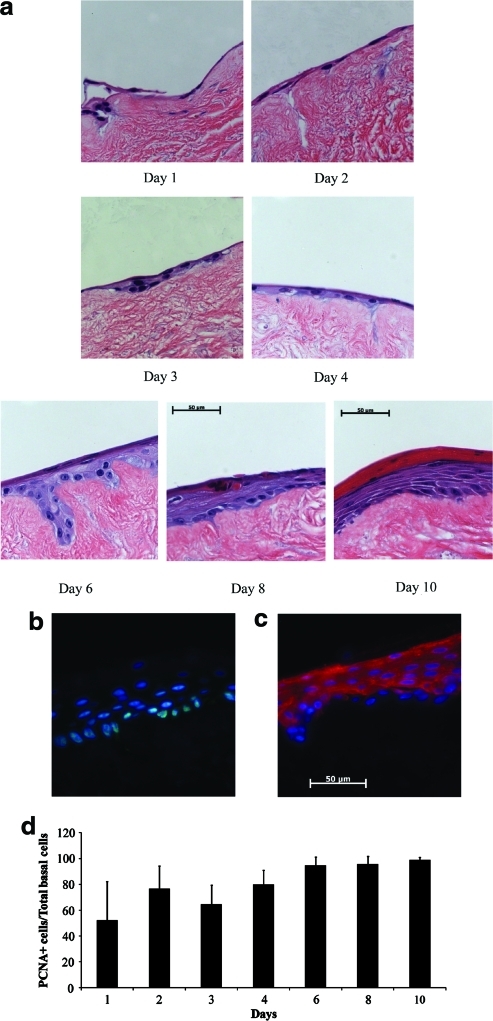
Within 24 h after seeding, keratinocytes degraded the hydrogel and attached to the dermis (Fig. 1a). A continuous monolayer of cells was observed in the first 3 days when the tissue constructs were kept submerged in the culture medium. Upon raising the tissue constructs to the air–liquid interface, keratinocytes stratified to form a multilayered epidermis, which by day 10 (day 7 at the air–liquid interface) contained basal, suprabasal, granular, and keratinized layers. PCNA staining showed that during the first 3 days when the tissues remained submerged, 50%–70% of basal cells were in proliferative state (Fig. 1b, d). The fraction of PCNA-positive basal cells increased to >90% at the air–liquid interface (Fig. 1d), in agreement with previously reported observations.32 In addition, cytokeratin 10 (K10) immunostaining showed that K10 was expressed in all cell layers except for the basal layer (Fig. 1d), indicating normal differentiation pattern.
FIG. 1.
Fibrin can be employed as a vehicle to deliver keratinocytes onto dermis. Tissue-engineered skin substitutes were constructed by seeding keratinocytes in fibrin hydrogels containing fibrinogen (4 mg/mL), fibronectin (100 ng/mL), and KGF (80 ng/mL) on the basement membrane side of decellularized dermis. At the indicated times after cell seeding, tissues were harvested. (a) Paraffin-embedded tissue sections were stained with hematoxylin and eosin. Frozen sections from tissues on day 10 postseeding were immunostained for (b) PCNA (green); or (c) K10 (red), and counterstained with the nuclear dye Hoechst (blue). bar = 50 μm. (d) The number of PCNA-positive cells along the basement membrane was counted and divided by the number of cells along the basement membrane at the indicated times postseeding. KGF, keratinocyte growth factor; PCNA, proliferating cell nuclear antigen. Color images available online at www.liebertonline.com/ten.
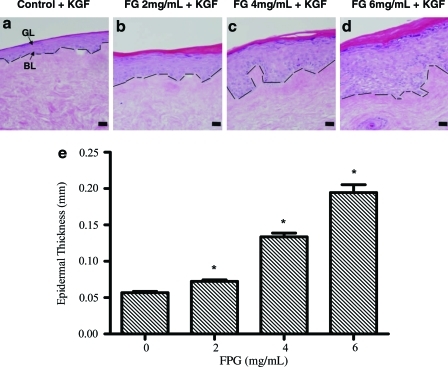
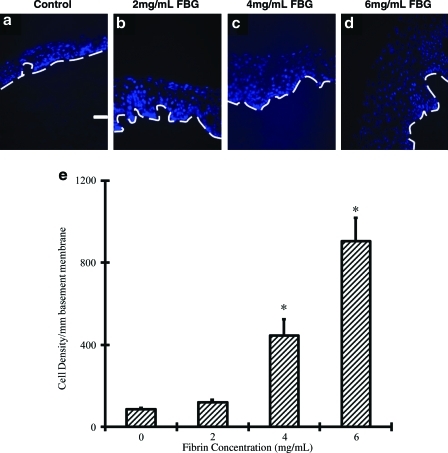
Next, we examined whether the fibrinogen concentration affected epidermal development on acellular dermis. To this end, we embedded keratinocytes in fibrin containing different concentrations of fibrinogen (0, 2, 4, and 6 mg/mL; n = 4 for each group). In 10 days postseeding, we found that cells from all groups attached to the basement membrane of acellular dermis, differentiated, and formed a well-stratified epidermis complete with basal, spinous, granular, and cornified layers (Fig. 2). Use of fibrin hydrogels enhanced epidermal thickness significantly and this effect depended strongly on the fibrinogen concentration (Fig. 2a–d). Specifically, keratinocyte delivery in fibrin containing 0, 2, 4, or 6 mg/mL fibrinogen yielded 57, 72, 134, or 195-μm-thick epidermis, respectively. Compared to control epidermal tissues (0 mg/mL fibrinogen), epidermal thickness increased approximately fourfold when cells were delivered in fibrin containing 6 mg/mL fibrinogen (Fig. 2e) (p < 0.05). Cell density (number of keratinocytes in all epidermal layers per unit lBM) increased by 10-fold from 86 to 904 cells per mm of basement membrane (p < 0.05) under the same conditions (Fig. 3). Collectively, our results suggested that epidermal keratinocytes maybe seeded directly onto the wound that is covered with acellular dermis.
FIG. 2.
Epidermal thickness increased with increasing concentration of fibrinogen. (a–d) Bioengineered epidermis was generated by seeding keratinocytes in fibrin hydrogels containing various concentrations of fibrinogen as indicated, fibronectin (100 ng/mL), and KGF (80 ng/mL) on the basement membrane side of decellularized dermis. After 1 week at the air–liquid interface paraffin-embedded tissue sections were stained with hematoxylin and eosin (bar = 50 μm; original magnification 20×). The dashed line denotes basement membrane. (e) Epidermal thickness was calculated as the ratio of the surface area from the basal layer (BL) to the granular cell layer (GL) over the length of the basement membrane. The symbol (*) denotes statistical significance (p < 0.05) compared to control tissues (n = 4 tissues per group). Color images available online at www.liebertonline.com/ten.
FIG. 3.
Epidermal cell number increased with increasing concentration of fibrin. (a–d) Bioengineered epidermis was generated by seeding keratinocytes in fibrin hydrogels containing various concentrations of fibrinogen as indicated, fibronectin (100 ng/mL) and KGF (80 ng/mL) on the basement membrane side of decellularized dermis. After 1 week at the air–liquid interface frozen sections were stained with the nuclear dye Hoechst. The dashed line denotes basement membrane (bar = 50 μm; original magnification 20×). (e) Cell density was measured as the number of cell nuclei per basement membrane length. The symbol (*) denotes statistical significance (p < 0.05) compared to control tissues (n = 4 tissues per group). Color images available online at www.liebertonline.com/ten.
Infiltration of acellular human dermis with angiogenic factors promotes angiogenesis in vivo
Next, we hypothesized that infiltration of acellular dermis with angiogenic factors might promote epidermal development by increasing neovascularization of the underlying wound bed. Since fibrin itself promotes angiogenesis,33 we reasoned that proangiogenic factors could be vacuum infiltrated in the dermis along with fibrinogen that will polymerize within the pores of the substrate shortly after infiltration.
First, we examined whether infiltration of angiogenic factor into acellular dermis in the presence of fibrin occurs evenly throughout the dermis. To this end, acellular human dermis was infiltrated with fibrin (4 mg/mL) and KGF (100 ng/mL), and the distribution of KGF was demonstrated by immunostaining of frozen tissue sections. As shown in Supplementary Figure S1 (Supplementary Data are available online at www.liebertonline.com/ten), KGF was uniformly distributed throughout the dermis, suggesting that this approach could be used with other factors as well.
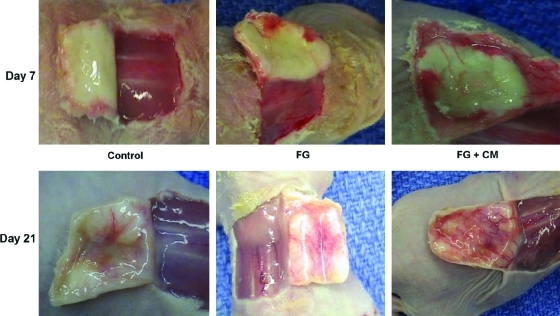
Next, we investigated whether dermis infiltrated with angiogenic factors could enhance angiogenesis. Since the human keratinocyte cell line HaCaT was shown to produce significant amount of VEGF when stimulated by serum as well as a combination of growth factors such as of EGF and KGF,34 we incubated HaCaT cells with EGF (100 ng/mL) and KGF (100 ng/mL) and collected the conditioned medium 12 h later. Indeed, using ELISA we found that the HaCaT-conditioned medium contained 1.5 ± 0.3 ng VEGF/mL. Subsequently, we infiltrated acellular dermis with fibrin alone (FG) or in combination with conditioned medium (FG + CM) and grafted onto the dorsum of athymic mice that were previously subjected to full-thickness wounding (including the panniculous carnosus). Noninfiltrated acellular human dermis served as control. Tissues were harvested on postoperative days 7 or 21 (n = 6/time point/condition) and the angiogenic response was evaluated macroscopically and histologically.
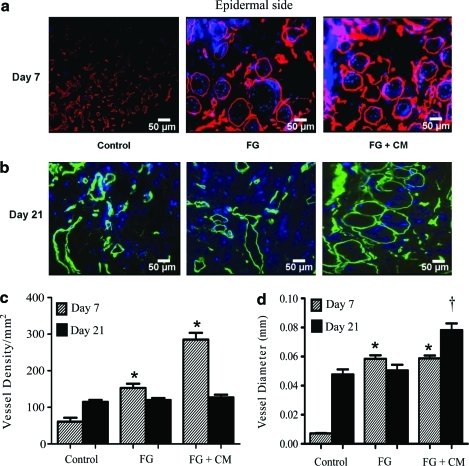
Macroscopic evaluation showed increased vascularization at the dermal–subcutaneous interface in the FG and the FG + CM groups at 7 days when compared to control (Fig. 4). At 21 days, all groups showed vasculature at the dermal–subcutaneous interface, but FG + CM was clearly superior to FG, whereas control dermis displayed the lowest blood vessel density. To obtain more quantitative data, we measured vessel density and diameter in tissue sections that were immunostained for laminin and counterstained with Hoechst (Fig. 5a, b). On day 7, vessel density increased by approximately twofold in the FG group and was further enhanced in the presence of FG + CM (Fig. 5c). Vessel diameter was significantly enhanced by FG and the presence of CM had no additional effect (Fig. 5d). As expected, vessel density decreased at 21 days and was similar for all three groups (Fig. 5c). On the other hand, vessel diameter remained higher in the FG + CM group (Fig. 5d). This suggests that even though fibrin provided an important stimulus for angiogenesis, addition of angiogenic factors further enhanced both vessel density (at 7 days) and diameter (at 21 days).
FIG. 4.
Increased angiogenic response by infiltration of acellular human dermis with angiogenic factors. Athymic mice (n = 6 animals per group) were subjected each to full thickness, excisional wound (including the panniculous carnosus) of ∼2.5 cm2 on their dorsum, and the skin was replaced with acellular human dermis of equal size. Acellular human dermis remained untreated (control) or was infiltrated with fibrin gel alone (FG) or in combination with conditioned medium (FG + CM). Pictures of taken at the time of tissue harvest on postoperative days 7 and 21. Color images available online at www.liebertonline.com/ten.
FIG. 5.
Infiltration of acellular dermis with angiogenic factors enhanced the density and diameter of newly formed blood vessels. Athymic mice (n = 6 per group) were subjected each to full thickness, excisional wound (including the panniculous carnosus) of ∼2.5 cm2 on their dorsum, and the skin was replaced with acellular human dermis of equal size. Acellular human dermis remained untreated (control) or was infiltrated with fibrin gel alone (FG) or in combination with conditioned medium (FG + CM). (a, b) Frozen sections were stained for laminin [red in (a); green in (b)] and counterstained with Hoechst (blue). The epidermal side is at the top of each panel. Scale bar = 50 μm; original magnification 25×. Vessel density (c) and vessel diameter (d) were measured using NIH ImageJ software. The symbol (*) and (†) denote statistical significance (p < 0.05) compared to the respective control tissues. Color images available online at www.liebertonline.com/ten.
Formation of neoepidermis by in situ delivery of keratinocytes correlated with enhanced neovascularization of the underlying dermal support
Next, we examined whether increased vascularization of the implanted acellular dermis could lead to improved stratification of epidermal keratinocytes delivered in situ with the fibrin matrix. To this end, acellular dermis (2.25 cm2) was used directly or was infiltrated with FG or FG + CM and grafted onto athymic nude mice (Fig. 6). Seven days postimplantation cultured keratinocytes (4.4 × 105 cells/cm2) were mixed with fibrinogen (4 mg/mL), fibronectin (100 ng/mL), and KGF (100 ng/mL) and seeded directly onto the dermis (n = 6 per condition). Since epidermal thickness increased with increasing fibrinogen concentration in vitro (Fig. 2), we chose an intermediate concentration of 4 mg/mL for our in vivo studies to avoid epidermal hyperthickening, so that we can better evaluate the effect of dermal vascularization on epidermal development.
FIG. 6.
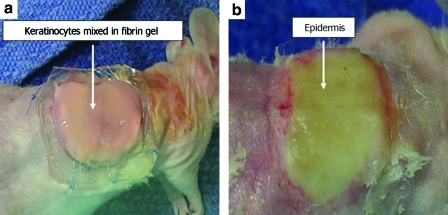
Fibrin as a vehicle for in situ delivery of cultured keratinocytes onto the prevascularized dermal substrate. Athymic mice (n = 6 per group) were subjected to full thickness, excisional wound (including the panniculous carnosus) of ∼2.5 cm2 on their dorsum, and the skin was replaced with acellular human dermis of equal size. Acellular human dermis remained untreated (control) or was infiltrated with fibrin gel alone (FG) or in combination with conditioned medium (FG + CM). One week postgrafting cultured keratinocytes were delivered directly onto the dermis in a fibrin hydrogel and covered with a moist Telfa dressing for 3 days before they were exposed to air (a). Neoepidermis at 2 weeks postseeding (b). Note the difference in the color of the graft on days 1 and 14. The yellow color on day 14 indicates development of the epidermis. Color images available online at www.liebertonline.com/ten.
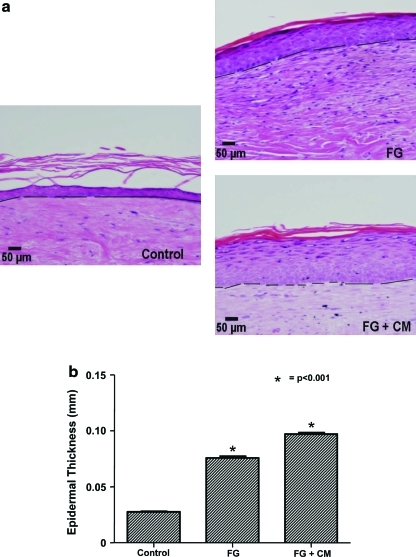
Two weeks later, the tissues were harvested and evaluated histologically. In all tissues, the epidermis was well-stratified with basal, suprabasal, granular, and cornified layers. However, epidermal thickness was significantly enhanced when the dermis infiltrated with FG or FG + CM as compared to control (Fig. 7a). Whereas control dermis supported formation of neoepidermis with average thickness of 28 μm, the presence of FG or FG + CM increased epidermal thickness to 75 or 97 μm, respectively (Fig. 7b). This emphasizes the importance of well-vascularized scaffolds to support proliferation and stratification of in situ delivered keratinocytes.
FIG. 7.
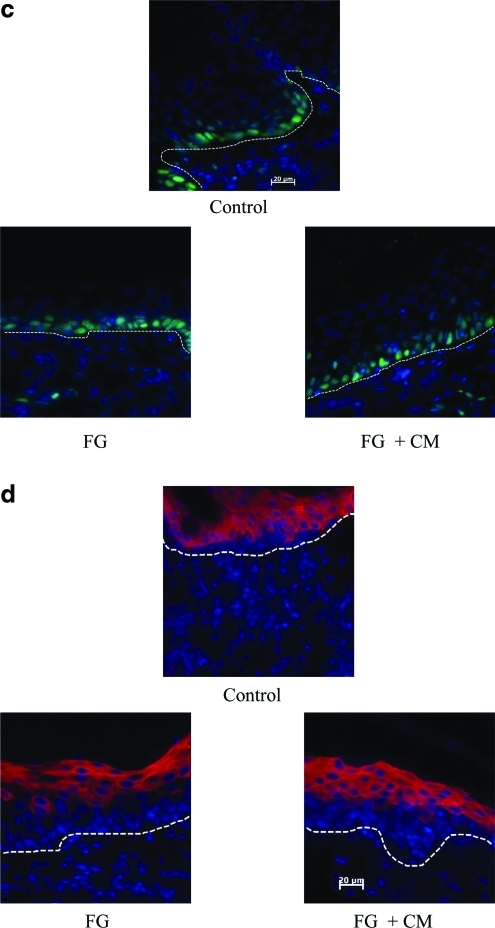
The thickness of the neoepidermis correlated with the extent of vascularization of the underlying dermal support. In vivo neoepidermis was generated by seeding cultured keratinocytes within fibrin hydrogels onto the prevascularized dermis at 1 week postgrafting. (a) Two weeks after keratinocyte delivery, tissues were excised and paraffin-embedded tissue sections were stained with hematoxylin and eosin (bar = 50 μm; original magnification 20×). The dashed line denotes basement membrane. (b) Epidermal thickness was calculated as the ratio of the surface area from the basal to the granular cell layers over the length of the basement membrane. The symbol (*) denotes statistical significance (p < 0.05) compared to control tissues (n = 6 animals per group). Paraffin-embedded tissue sections were immunostained for (c) PCNA (green) or (d) K10 (red) and counterstained for nuclei with Hoechst (blue). The dashed line denotes basement membrane; (c, d) Scale bar = 20 μm. Color images available online at www.liebertonline.com/ten.
Proliferation of transplanted cells was further evaluated by immunostaining for PCNA as shown in Figure 7c. Proliferation was similarly high in all tissues with the vast majority of cells (>90%) staining positive for PCNA (control: 92.6 ± 2.07; FG: 93.8 ± 5.5; FG + CM: 91.5 ± 8.6, p > 0.1 between FG or FG + CM and controls tissues), suggesting that at 2 weeks post-transplantation all tissues were in a proliferative state and had not reached homeostasis. In addition, the differentiation status of transplanted cells was evaluated by immunostaining for K10. As shown in Figure 7d, in control tissues K10 was present in all but the basal cell layer, indicating a normal pattern of differentiation. In FG and FG + CM tissues, two to three suprabasal cell layers were also negative for K10, indicative of a hyperproliferative epidermis.29 Collectively, our data indicated that the transplanted cells attached to the dermis, proliferated, and differentiated to form a well-stratified and keratinized epidermis.
Discussion
We report that infiltration of acellular human dermis with angiogenic factors enhanced neovascularization as shown by higher density and larger diameter of the newly formed blood vessels as early as 1 week postimplantation. In turn, a prevascularized dermal substrate led to superior wound epithelialization upon keratinocyte delivery in fibrin hydrogels. Several studies used human acellular dermis as a matrix for culture of epidermal keratinocytes.3,30,35 Acellular dermis retains the biochemical components of the basement membrane (e.g., collagen IV, VII, and laminin), the microtopology of human dermis (rete-ridge pattern), and dermal porosity that promotes ingrowth of fibroblasts and blood vessels along the pathway of pre-existing vascular conduits. When keratinocytes are seeded on the basement membrane side of the dermis and raised to the air–liquid interface, they differentiate to form a fully stratified epidermis with basal, suprabasal, granular, and cornified cell layers exhibiting barrier function.10,11 In contrast to tissue constructs based on hydrogels such as collagen or fibrin, the dermis retains the mechanical strength and elasticity of human skin; therefore, it is easy to handle during transplantation. Thus, acellular dermis offers an effective off-the-shelf dressing for immediate coverage of wounds to avoid infection and reduce water loss.
It is well known that during wound healing in vivo many cytokines and growth factors are secreted in a well-orchestrated sequence and that delivery of a single growth factor in a plethora of studies has not had the desired effects in wound healing.36 For this reason, acellular dermis was infiltrated with the conditioned medium from a keratinocyte cell line that was previously shown to secrete angiogenic factors such as VEGF when stimulated with EGF and KGF.34,37 Although the conditioned medium is not well defined, we took this approach because it contained a cocktail of angiogenic factors to demonstrate the effectiveness of our novel strategy before proceeding to experiments with individual growth factors or well-defined cocktails. Our results clearly demonstrated that the presence of angiogenic factors in the dermis significantly enhanced vessel density and vessel diameter, especially at early times (day 7). By 3 weeks, vessel density decreased to the level of control samples, but vessel diameter remained large, possibly indicating immature vessels38 that might require longer times to reduce in size and reach homeostasis.
Notably, application of a keratinocyte-fibrin suspension onto acellular dermis resulted in cell attachment to the dermis, proliferation, and differentiation into well-stratified epidermis in vitro and in vivo, as evidenced by tissue morphology (hematoxylin and eosin), PCNA, and K10 staining. Epidermal thickness increased significantly with increasing concentrations of fibrinogen. Although the mechanism is unknown, we speculate that this might be caused by the increased density of the fibrin matrix resulting in slow diffusion of KGF out of the gel, thereby enhancing its effect on keratinocyte proliferation and stratification. Surprisingly, cells embedded in fibrin hydrogels with 4 mg/mL fibrinogen attached to the dermis and formed a continuous monolayer with no detectable fibrin hydrogels at 24 h postseeding. This result implies that keratinocytes may have degraded the fibrin and attached onto the dermis in <24 h, possibly facilitated by the presence of KGF and/or fibronectin. More studies are required to follow the kinetics of fibrin degradation under these conditions.
Although, previous studies have shown that application of keratinocytes in fibrin glue supported epidermal formation in vivo, the effect of vascularization of the dermal support has not been examined.24,27,39 This is a very important consideration as the variable take rate of cultured epithelial autografts has been attributed to poor vascularization of the dermis and reliance on nutrient diffusion to support epidermal proliferation for several days after transplantation.24,40 Indeed, we found that the thickness and cellular density of the neoepidermis increased as dermal vascularization improved by incorporation of angiogenic factors. Increased epithelialization may have been the result of increased blood supply or the presence of cytokines and growth factors secreted by the incoming endothelial cells, fibroblasts or other cells types. Continuous supply of growth factors enabled sustained keratinocyte proliferation even at 2 weeks after cell delivery. Indeed, immunostaining for K10 showed that in FG and FG + CM tissues besides the basal cells, two to three suprabasal cell layers were also devoid of K10, indicating a hyperproliferative epidermal phenotype.10 Long-term experiments are required to determine when the neoepidermis reaches homeostasis and whether epidermal homeostasis coincides with reduction in the size of the neovessels.
In the future we intend to apply well-established strategies for conjugation of proteins, to fibrin hydrogels11,41 to incorporate VEGF, platelet-derived growth factor-BB, and/or other growth factors individually or in combination to examine their effect on dermis vascularization in vivo. Plasmid DNA42–45 and recombinant viruses46,47 have also been incorporated into fibrin hydrogels by our group and others, and they may be also used in this setting to promote vascularization. For the most part, genetic modification in this setting is local to the cells that populate the dermis during the granulation tissue phase of wound healing, for example, macrophages, fibroblasts, and endothelial cells. The first cells infiltrating the wound are modified and secrete angiogenic factors, further promoting the angiogenic response. Temporary genetic modification through plasmid transfection may be more effective than growth factor delivery because of protein instability, especially at the protease-rich wound site.48 Finally, a combination of protein with plasmid DNA may be used, where the growth factor, for example, VEGF may be released quickly to accelerate migration into the wound site. Then, genetic modification of the infiltrating cells may provide other factor(s) that are needed at later phases of the healing response, for example, platelet-derived growth factor-BB, which has been shown to stabilize the nascent blood vessels.49
Overall, our data point to the importance of prevascularization of the dermal support for successful wound re-epithelialization and suggest a two-step method for treatment of wounds: (1) transplantation of a substrate such as acellular dermis infiltrated with angiogenic factors immediately after injury to close the wound and prevent infection and water loss; and (2) application of a keratinocyte suspension in fibrin hydrogels onto the prevascularized wound bed to generate a stratified epidermis and restore barrier function. This strategy circumvents the need for generation of skin substitutes, which takes ∼3 weeks and may also eliminate the need for split-thickness autografts, thereby reducing the number of surgical operations, pain, and injury at the donor sites. Since human keratinocytes did not mount an immune response upon transplantation of skin substitutes in vivo,50 it may be feasible to use allogeneic cells in the clinic, further reducing the time and cost of wound treatment.
Disclosure Statement
No competing financial interests exist.
Supplementary Material
References
- 1.Marston W.A. Hanft J. Norwood P. Pollak R. The efficacy and safety of Dermagraft in improving the healing of chronic diabetic foot ulcers: results of a prospective randomized trial. Diabetes Care. 2003;26:1701. doi: 10.2337/diacare.26.6.1701. [DOI] [PubMed] [Google Scholar]
- 2.Facy V. Flouret V. Regnier M. Schmidt R. Reactivity of Langerhans cells in human reconstructed epidermis to known allergens and UV radiation. Toxicol In Vitro. 2005;19:787. doi: 10.1016/j.tiv.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 3.Medalie D.A. Eming S.A. Collins M.E. Tompkins R.G. Yarmush M.L. Morgan J.R. Differences in dermal analogs influence subsequent pigmentation, epidermal differentiation, basement membrane, and rete ridge formation of transplanted composite skin grafts. Transplantation. 1997;64:454. doi: 10.1097/00007890-199708150-00015. [DOI] [PubMed] [Google Scholar]
- 4.Ralston D.R. Layton C. Dalley A.J. Boyce S.G. Freedlander E. Mac Neil S. The requirement for basement membrane antigens in the production of human epidermal/dermal composites in vitro. Br J Dermatol. 1999;140:605. doi: 10.1046/j.1365-2133.1999.02758.x. [DOI] [PubMed] [Google Scholar]
- 5.Sheridan R.L. Tompkins R.G. Skin substitutes in burns. Burns. 1999;25:97. doi: 10.1016/s0305-4179(98)00176-4. [DOI] [PubMed] [Google Scholar]
- 6.Boyce S.T. Design principles for composition and performance of cultured skin substitutes. Burns. 2001;27:523. doi: 10.1016/s0305-4179(01)00019-5. [DOI] [PubMed] [Google Scholar]
- 7.Mansbridge J. Tissue-engineered skin substitutes. Expert Opin Biol Ther. 2002;2:25. doi: 10.1517/14712598.2.1.25. [DOI] [PubMed] [Google Scholar]
- 8.Bannasch H. Fohn M. Unterberg T. Bach A.D. Weyand B. Stark G.B. Skin tissue engineering. Clin Plast Surg. 2003;30:573. doi: 10.1016/s0094-1298(03)00075-0. [DOI] [PubMed] [Google Scholar]
- 9.Supp D.M. Boyce S.T. Engineered skin substitutes: practices and potentials. Clin Dermatol. 2005;23:403. doi: 10.1016/j.clindermatol.2004.07.023. [DOI] [PubMed] [Google Scholar]
- 10.Andreadis S.T. Hamoen K.E. Yarmush M.L. Morgan J.R. Keratinocyte growth factor induces hyperproliferation and delays differentiation in a skin equivalent model system. FASEB J. 2001;15:898. doi: 10.1096/fj.00-0324com. [DOI] [PubMed] [Google Scholar]
- 11.Geer D.J. Swartz D.D. Andreadis S.T. Biomimetic delivery of keratinocyte growth factor upon cellular demand for accelerated wound healing in vitro and in vivo. Am J Pathol. 2005;167:1575. doi: 10.1016/S0002-9440(10)61242-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rasmussen C.A. Gibson A.L. Schlosser S.J. Schurr M.J. Allen-Hoffmann B.L. Chimeric composite skin substitutes for delivery of autologous keratinocytes to promote tissue regeneration. Ann Surg. 2010;251:368. doi: 10.1097/SLA.0b013e3181c1ab5f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Butler C.E. Orgill D.P. Simultaneous in vivo regeneration of neodermis, epidermis, and basement membrane. Adv Biochem Eng Biotechnol. 2005;94:23. doi: 10.1007/b99998. [DOI] [PubMed] [Google Scholar]
- 14.Parenteau N. Sabolinski M. Prosky S. Nolte C. Kriwet K. Bilbo P. Biological and physical factors influencing the successful engraftment of a cultured skin substitute. Biotech Bioeng. 1996;52:3. doi: 10.1002/(SICI)1097-0290(19961005)52:1<3::AID-BIT1>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 15.Pinney E. Liu K. Sheeman B. Mansbridge J. Human three-dimensional fibroblast cultures express angiogenic activity. J Cell Physiol. 2000;183:74. doi: 10.1002/(SICI)1097-4652(200004)183:1<74::AID-JCP9>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 16.Wood F.M. Kolybaba M.L. Allen P. The use of cultured epithelial autograft in the treatment of major burn injuries: a critical review of the literature. Burns. 2006;32:395. doi: 10.1016/j.burns.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 17.Kopp J. Jeschke M.G. Bach A.D. Kneser U. Horch R.E. Applied tissue engineering in the closure of severe burns and chronic wounds using cultured human autologous keratinocytes in a natural fibrin matrix. Cell Tissue Bank. 2004;5:89. doi: 10.1023/B:CATB.0000034082.29214.3d. [DOI] [PubMed] [Google Scholar]
- 18.Liu K. Yang Y. Mansbridge J. Comparison of the stress response to cryopreservation in monolayer and three-dimensional human fibroblast cultures: stress proteins, MAP kinases, and growth factor gene expression. Tissue Eng. 2000;6:539. doi: 10.1089/107632700750022189. [DOI] [PubMed] [Google Scholar]
- 19.Yannas I.V. Burke J.F. Design of an artificial skin. I. Basic design principles. J Biomed Mater Res. 1980;14:65. doi: 10.1002/jbm.820140108. [DOI] [PubMed] [Google Scholar]
- 20.Yannas I.V. Burke J.F. Gordon P.L. Huang C. Rubenstein R.H. Design of an artificial skin. II. Control of chemical composition. J Biomed Mater Res. 1980;14:107. doi: 10.1002/jbm.820140203. [DOI] [PubMed] [Google Scholar]
- 21.Burke J.F. Yannas I.V. Quinby W.C., Jr. Bondoc C.C. Jung W.K. Successful use of a physiologically acceptable artificial skin in the treatment of extensive burn injury. Ann Surg. 1981;194:413. doi: 10.1097/00000658-198110000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Livesey S.A. Herndon D.N. Hollyoak M.A. Atkinson Y.H. Nag A. Transplanted acellular allograft dermal matrix. Potential as a template for the reconstruction of viable dermis. Transplantation. 1995;60:1. [PubMed] [Google Scholar]
- 23.Cuono C. Langdon R. McGuire J. Use of cultured epidermal autografts and dermal allografts as skin replacement after burn injury. Lancet. 1986;1:1123. doi: 10.1016/s0140-6736(86)91838-6. [DOI] [PubMed] [Google Scholar]
- 24.Horch R.E. Bannasch H. Stark G.B. Transplantation of cultured autologous keratinocytes in fibrin sealant biomatrix to resurface chronic wounds. Transplant Proc. 2001;33:642. doi: 10.1016/s0041-1345(00)02181-3. [DOI] [PubMed] [Google Scholar]
- 25.Geer D.J. Swartz D.D. Andreadis S.T. Fibrin promotes migration in a three-dimensional in vitro model of wound regeneration. Tissue Eng. 2002;8:787. doi: 10.1089/10763270260424141. [DOI] [PubMed] [Google Scholar]
- 26.Geer D.J. Andreadis S.T. A novel role of fibrin in epidermal healing: plasminogen-mediated migration and selective detachment of differentiated keratinocytes. J Invest Dermatol. 2003;121:1210. doi: 10.1046/j.1523-1747.2003.12512.x. [DOI] [PubMed] [Google Scholar]
- 27.Bannasch H. Unterberg T. Fohn M. Weyand B. Horch R.E. Stark G.B. Cultured keratinocytes in fibrin with decellularised dermis close porcine full-thickness wounds in a single step. Burns. 2008;34:1015. doi: 10.1016/j.burns.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 28.Geer D.J. Swartz D.D. Andreadis S.T. In vivo model of wound healing based on transplanted tissue-engineered skin. Tissue Eng. 2004;10:1006. doi: 10.1089/ten.2004.10.1006. [DOI] [PubMed] [Google Scholar]
- 29.Chabbat J. Tellier M. Porte P. Steinbuch M. Properties of a new fibrin glue stable in liquid state. Thromb Res. 1994;76:525. doi: 10.1016/0049-3848(94)90281-x. [DOI] [PubMed] [Google Scholar]
- 30.Medalie D.A. Tompkins R.G. Morgan J.R. Evaluation of acellular human dermis as a dermal analog in a composite skin graft. ASAIO J. 1996;42:M455. doi: 10.1097/00002480-199609000-00030. [DOI] [PubMed] [Google Scholar]
- 31.Kubo M. Van de Water L. Plantefaber L.C. Mosesson M.W. Simon M. Tonnesen M.G. Taichman L. Clark R.A. Fibrinogen and fibrin are anti-adhesive for keratinocytes: a mechanism for fibrin eschar slough during wound repair. J Invest Dermatol. 2001;117:1369. doi: 10.1046/j.0022-202x.2001.01551.x. [DOI] [PubMed] [Google Scholar]
- 32.Koria P. Andreadis S.T. Epidermal morphogenesis: the transcriptional program of human keratinocytes during stratification. J Invest Dermatol. 2006;126:1834. doi: 10.1038/sj.jid.5700325. [DOI] [PubMed] [Google Scholar]
- 33.Dvorak H.F. Harvey V.S. Estrella P. Brown L.F. McDonagh J. Dvorak A.M. Fibrin containing gels induce angiogenesis. Implications for tumor stroma generation and wound healing. Lab Invest. 1987;57:673. [PubMed] [Google Scholar]
- 34.Frank S. Hubner G. Breier G. Longaker M.T. Greenhalgh D.G. Werner S. Regulation of vascular endothelial growth factor expression in cultured keratinocytes. Implications for normal and impaired wound healing. J Biol Chem. 1995;270:12607. doi: 10.1074/jbc.270.21.12607. [DOI] [PubMed] [Google Scholar]
- 35.Medalie D.A. Eming S.A. Tompkins R.G. Yarmush M.L. Krueger G.G. Morgan J.R. Evaluation of human skin reconstituted from composite grafts of cultured keratinocytes and human acellular dermis transplanted to athymic mice. J Invest Dermatol. 1996;107:121. doi: 10.1111/1523-1747.ep12298363. [DOI] [PubMed] [Google Scholar]
- 36.Andreadis S.T. Geer D.J. Biomimetic approaches to protein and gene delivery for tissue regeneration. Trends Biotechnol. 2006;24:331. doi: 10.1016/j.tibtech.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 37.Nakai K. Yoneda K. Moriue T. Igarashi J. Kosaka H. Kubota Y. HB-EGF-induced VEGF production and eNOS activation depend on both PI3 kinase and MAP kinase in HaCaT cells. J Dermatol Sci. 2009;55:170. doi: 10.1016/j.jdermsci.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 38.Pettersson A. Nagy J.A. Brown L.F. Sundberg C. Morgan E. Jungles S. Carter R. Krieger J.E. Manseau E.J. Harvey V.S. Eckelhoefer I.A. Feng D. Dvorak A.M. Mulligan R.C. Dvorak H.F. Heterogeneity of the angiogenic response induced in different normal adult tissues by vascular permeability factor/vascular endothelial growth factor. Lab Invest. 2000;80:99. doi: 10.1038/labinvest.3780013. [DOI] [PubMed] [Google Scholar]
- 39.Horch R.E. Bannasch H. Kopp J. Andree C. Stark G.B. Single-cell suspensions of cultured human keratinocytes in fibrin-glue reconstitute the epidermis. Cell Transplant. 1998;7:309. doi: 10.1177/096368979800700309. [DOI] [PubMed] [Google Scholar]
- 40.Wood F.M. Kolybaba M.L. Allen P. The use of cultured epithelial autograft in the treatment of major burn wounds: eleven years of clinical experience. Burns. 2006;32:538. doi: 10.1016/j.burns.2006.02.025. [DOI] [PubMed] [Google Scholar]
- 41.Zisch A.H. Lutolf M.P. Ehrbar M. Raeber G.P. Rizzi S.C. Davies N. Schmokel H. Bezuidenhout D. Djonov V. Zilla P. Hubbell J.A. Cell-demanded release of VEGF from synthetic, biointeractive cell ingrowth matrices for vascularized tissue growth. FASEB J. 2003;17:2260. doi: 10.1096/fj.02-1041fje. [DOI] [PubMed] [Google Scholar]
- 42.Andree C. Voigt M. Wenger A. Erichsen T. Bittner K. Schaefer D. Walgenbach K.J. Borges J. Horch R.E. Eriksson E. Stark G.B. Plasmid gene delivery to human keratinocytes through a fibrin-mediated transfection system. Tissue Eng. 2001;7:757. doi: 10.1089/107632701753337708. [DOI] [PubMed] [Google Scholar]
- 43.des Rieux A. Shikanov A. Shea L.D. Fibrin hydrogels for non-viral vector delivery in vitro. J Control Release. 2009;136:148. doi: 10.1016/j.jconrel.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kulkarni M. Breen A. Greiser U. O'Brien T. Pandit A. Fibrin-lipoplex system for controlled topical delivery of multiple genes. Biomacromolecules. 2009;10:1650. doi: 10.1021/bm900248n. [DOI] [PubMed] [Google Scholar]
- 45.Lei P. Padmashali R.M. Andreadis S.T. Cell-controlled and spatially arrayed gene delivery from fibrin hydrogels. Biomaterials. 2009;30:3790. doi: 10.1016/j.biomaterials.2009.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Breen A. Dockery P. O'Brien T. Pandit A. Fibrin scaffold promotes adenoviral gene transfer and controlled vector delivery. J Biomed Mater Res A. 2009;89:876. doi: 10.1002/jbm.a.32039. [DOI] [PubMed] [Google Scholar]
- 47.Raut S.D. Lei P. Padmashali R.M. Andreadis S.T. Fibrin-mediated lentivirus gene transfer: implications for lentivirus microarrays. J Control Release. 2010;144:213. doi: 10.1016/j.jconrel.2010.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liechty K.W. Sablich T.J. Adzick N.S. Crombleholme T.M. Recombinant adenoviral mediated gene transfer in ischemic impaired wound healing. Wound Repair Regen. 1999;7:148. doi: 10.1046/j.1524-475x.1999.00148.x. [DOI] [PubMed] [Google Scholar]
- 49.Richardson T.P. Peters M.C. Ennett A.B. Mooney D.J. Polymeric system for dual growth factor delivery. Nat Biotechnol. 2001;19:1029. doi: 10.1038/nbt1101-1029. [DOI] [PubMed] [Google Scholar]
- 50.Briscoe D.M. Dharnidharka V.R. Isaacs C. Downing G. Prosky S. Shaw P. Parenteau N.L. Hardin-Young J. The allogeneic response to cultured human skin equivalent in the hu-PBL-SCID mouse model of skin rejection. Transplantation. 1999;67:1590. doi: 10.1097/00007890-199906270-00014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.