Summary
Thrombosis, or complications from thrombosis, currently occupies the top three positions in the cardiovascular causes of morbidity and mortality in the developed world. There are a limited number of safe and effective drugs to prevent and treat thrombosis. Animal models of thrombosis are necessary to better understand the complex components and interactions involved in the formation of a clot. Tissue factor (TF) is required for the initiation of blood coagulation and likely plays a key role in both arterial and venous thrombosis. Understanding the role of TF in thrombosis may permit the development of new antithrombotic drugs. This review will focus on the role of TF in in vivo models of thrombosis.
Keywords: Tissue factor/factor VII, thrombosis, arterial thrombosis, venous thrombosis, animal models
Introduction
Tissue factor (TF) serves as the primary initiator of the coagulation cascade (1–2). It is the cellular receptor for the plasma factor VII/ VIIa (FVII/VIIa). The TF:FVIIa complex activates both FIX and FX. Activated FX (FXa), along with its cofactor FVa, is referred to as the prothombinase complex and cleaves prothrombin to thrombin, which then cleaves fibrinogen to fibrin. The transglutaminase FXIII then cross-links fibrin which acts to stabilise the platelet-rich thrombi. The TF:FVIIa complex is inhibited by a multivalent serine protease inhibitor, known as tissue factor pathway inhibitor (TFPI) (3).
Circulating blood is normally maintained in a fluidic state. The coagulation system is only activated at sites of vessel injury and this prevents excess blood loss. Following vessel injury, TF on adventitial cells, such as adventitial fibroblasts, pericytes and smooth muscle cells (SMCs), activate the clotting system (4–6). Haemostatic clots are localised to the vessel wall and do not greatly impair blood flow in the vessel. In contrast, thrombotic clots result in impairment of blood flow and even complete occlusion of the vessel. Thrombotic events commonly result from a pathologic response to vascular injury, such as atherosclerotic plaque rupture, the main cause of arterial thrombosis. Vessel wall-derived TF has been described to provide a “haemostatic envelope” around blood vessels in healthy individuals (5). In contrast, pathologic expression of TF within the vessel wall or in the blood may trigger thrombosis.
In addition to TF expression in the vessel wall, there are reports of TF within the blood, so-called “circulating TF”. Importantly, the level of circulating TF in the blood of healthy donors is extremely low and does not appear to contribute to haemostasis (3, 7). However, the level of circulating TF is elevated in various disease states. The relative contribution of vessel wall vs. circulating TF to thrombosis is currently a highly debated topic. Levels of TF in the vessel wall are reported to far exceed the amount in blood with an estimated ratio of 1,000:1 (~20 pM vessel wall TF vs. 20 fM circulating) (8–9). Other investigators have reported higher concentrations of TF in the blood of healthy individuals (10–12) with the highest levels (37 pM) found in a cohort of arthritis patients (13). This circulating TF is present at very low levels on monocytes and in the form of small membrane microparticles, also known as microvesicles (MVs) (14). MVs are derived from activated or apoptotic cells (15). TF expression can also be induced in blood monocytes and possibly endothelial cells during pathogenic states, such as sepsis (16–18). Levels of circulating TF are also increased in chronic pathologic conditions, such as cardiovascular disease, cancer, and sickle cell disease (19–24).
The role of TF in thrombosis was originally studied using inhibitory drugs in animal models of thrombosis. More recently, genetic mouse models have been used. However, deletion of the TF gene was found to be embryonic lethal. (25–27). In order to overcome this lethality, we generated a “low TF” mouse that express very low levels of human TF from a minigene (~1% normal levels) in the absence of mouse TF (28). These genetically modified mice, along with mice containing cell type-specific deletions of the TF gene, have allowed studies on the relative contribution of vessel wall vs. circulating TF to thrombosis. Additionally, TFPI heterozygous mice (TFPI+/−) or transgenic overexpression are also a useful tool to analyse the effects of increasing TF activity (29). The goal of this review is to summarise the literature on TF inhibition via small molecule inhibitors, blocking antibodies and recombinant TFPI (rTFPI) on thrombosis in animal models, as well as results from genetic mouse models.
Arterial thrombosis
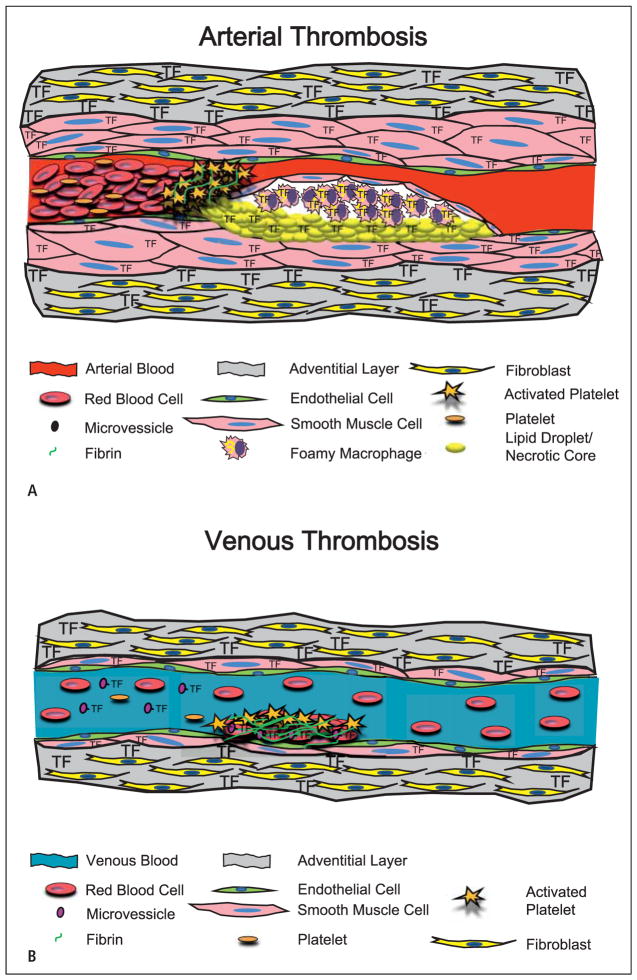
Arterial thrombosis is the most common cause of death in the developed world. The primary cause of arterial thrombosis is either instability or rupture of an atherosclerotic plaque resulting in localised clot formation and blockage of blood flow with subsequent myocardial infarction or stroke (Fig. 1A). Arterial thrombi are platelet-rich and are referred to as white clots. Thrombotic events are most devastating when they occur in the coronary and carotid arteries, which are prone to atherosclerotic disease. Various risk factors can increase the incidence of arterial thrombosis, such as smoking, hypertension, hyperlipidaemia, and diabetes mellitus. TF is present at high concentrations in atherosclerotic plaques in both cellular and acellular regions (30–32). Furthermore, levels of circulating TF are elevated in patients with cardiovascular disease, suggesting this source of TF may also contribute to thrombosis (33–34). This has led to the concept that targeting TF may reduce arterial thrombosis.
Figure 1. Models showing the cellular sources of TF that contributes to arterial and venous thrombosis.
A) Arterial thrombosis. The primary trigger of arterial thrombosis in a diseased vessel is the rupture of an atherosclerotic plaque. This involves disruption of the fibrous SMC cap exposing blood to TF in the plaque. The resultant clot is mainly composed of platelets with lower levels of cross-linking fibrin, and is referred to as a “white clot”. B) Venous thrombosis. Venous thrombosis occurs on a relatively undisturbed endothelial layer. Venous thrombosis may be triggered by circulating TF associated with MVs. The clot is mainly composed of fibrin with platelets and trapped red blood cells and is referred to as a “red clot”.
Four types of injury are primarily utilised to initiate arterial thrombosis in animal models. These consist of the Folts electrical-induction model, the balloon catheter injury model, the ferric chloride model (FeCl3), and the Rose Bengal laser-induced injury model. All of these models cause significant injury to the endothelium leading to exposure of blood to TF in the subendothelial media. The Folts electrical method can be used with or without arterial occlusion to 50% with a cuff or suture. The resulting positive electrical potential on the inner surface of the vessel leads to mechanical injury and endothelial cell disruption (35). As shown in Table 1, inhibition of TF utilising a monoclonal anti-rabbit TF antibody in a rabbit electrical model prevented thrombus formation in the abdominal aorta and the carotid artery (36–37). Similarly, an inhibitor of the TF:FVIIa complex (PHA-927) prevented electrical injury-induced femoral artery thrombosis (38). Furthermore, examination of fibrinolysis in electrical stimulation-induced coronary thrombosis demonstrated inhibitors of the TF:FVIIa complex or downstream inhibition of FXa prevented re-occlusion (39–40). Taken together, the results indicate that TF initiates thrombosis in the electrical stimulation model.
Table 1.
Role of tissue factor (TF) in arterial thrombosis models.
| Injury | Vascular location | Animal species | Inhibitor/gene | TF dependent | Study description | Reference |
|---|---|---|---|---|---|---|
| Electrical stimulation | Carotid artery | Rabbit | mab rabbit TF | Yes | Thrombosis was significantly attenuated in all nine rabbits tested | 37 |
| Electrical stimulation | Abdominal aorta | Rabbit | mab rabbit TF | Yes | Prevented procoagulant activity in circulating blood | 36 |
| Electrical stimulation | Femoral artery | N-H primate | PHA-927 | Yes | Prevented thrombus-induced vessel occlusion with little impact on bleeding | 38 |
| Balloon catheter injury | Thoracic aorta | Rabbit | rhTFPI | Yes | Bolus infusion of rTFPI inhibited fibrin formation and neointimal development | 42 |
| Balloon catheter injury | Coronary artery | Pig | rhTFPI | Yes | Reduced acute thrombosis and intimal hyperplasia after angioplasty | 43 |
| Balloon catheter injury | Femoral artery | Rabbit | FVIIai | Yes | Dose-dependently reduced thrombus formation | 44 |
| Ferric chloride | Carotid artery | Mouse | SMC Cre TFPI Flox deletion | Yes | Attenuation of arterial thrombosis without systemic side-effects | 29 |
| Ferric chloride | Carotid artery | Mouse | SMC Cre TF Flox deletion | Yes | Conditional deletion of SMC TF resulted in a marked reduction of thrombosis | 47 |
| Rose Bengal | Carotid artery | Mouse | TFPI+/− | Yes | TFPI+/− augmented thrombosis suggesting TFPI inhibition of TF attenuates coagulation | 50 |
| Rose Bengal | Carotid artery | Mouse | Low TF gene | Yes | Low TF mice were protected from carotid thrombosis | 9 |
Abbreviations: N-H: non-human; mab: monoclonal antibody; PHA-927: TF:FVII complex inhibitor; rh: recombinant human.
The balloon catheter injury model is used in larger animals to mimic atherosclerotic plaque disruption. Expansion of a intraluminal balloon results in disruption of the endothelium (41). Utilising this model in the coronary artery of pigs and the thoracic aorta of rabbits, two studies demonstrated that infusion of rTFPI reduced thrombosis (42–43). However, a TF:FVIIa inhibitor may not be as effective at reducing thrombosis in diseased vasculature, which contains increased levels of TF. This question was eloquently answered when rabbits were fed a hyperlipidaemic diet to induce atherosclerotic plaques in the femoral artery. These plaques were found to contain three times more TF than normal vessels. Mechanical rupture of the plaques using the balloon catheter injury-induced model of thrombosis was reduced with a TF:FVIIa inhibitor (44). These results indicate that the balloon catheter injury and subsequent thrombosis is dependent on TF activity.
The FeCl3 model induces a chemical injury when topically applied to the outer medial/adventitial artery wall, and is referred to as an outside-in model of injury. FeCl3 reaches the arterial lumen via an endocytic-exocytic pathway resulting in complete denudation of the endothelium, the presence of highly reactive oxygen species (ROS), and exposure of the blood to medial TF resulting in localised thrombosis (45). Thrombosis is monitored by a reduction in blood flow and time to occlusion of the vessel is used as a quantitative measure of thrombosis. One of the benefits of this model is that different doses of FeCl3 give different times of occlusion. This small animal model of thrombosis is thought to be comparable to arterial thrombosis in large animal models because there are cyclic flow variations induced by the presence of thrombi in arteries after FeCl3 injury (46). The role of TF has been examined in this model using mice with either a conditional deletion of the TF gene in SMC or with increased expression of TFPI in SMCs (29, 47). Both studies demonstrated that a reduction of TF activity was associated with an increase in the time to occlusion, indicating that “vessel wall” TF mediates FeCl3-induced thrombosis.
The Rose Bengal (tetrachlorotetraiodofluorescein) model of thrombosis is another model of human arterial thrombosis created by the generation of ROS (48). The Rose Bengal model utilises a photochemical reaction to cause localised induction of endothelial injury via ROS formation. Rose Bengal can be injected into the blood (inside-out injury) or is topically applied (outside-in injury). Exposure of Rose Bengal to a laser (543 nm) induces singlet oxygen radicals (49). Most studies inject Rose Bengal into the blood via the tail vein. Thrombosis is then monitored with a flow probe that determines the time to occlusion. Utilising the low TF mouse model and bone marrow transplantation, Day et al. demonstrated that TF in the carotid artery vessel wall, and not haematopoietic cell-derived TF, is responsible for arterial thrombosis (9). This study, together with the aforementioned FeCl3 SMC conditional deletion studies, strongly indicates that circulating TF does not contribute to arterial thrombosis in healthy mice. Another study with TFPI+/− mice, which have increased levels of TF activity, demonstrated a faster time to occlusion compared to TFPI+/+ mice when carotid atherosclerotic plaques were disrupted utilising Rose Bengal(50).
Venous thrombosis
Venous thromboembolism (VTE), which is a collective term for both deep-vein thrombosis (DVT) and pulmonary embolism (PE), is the third leading cause of cardiovascular death in the developed world. Commonly formed in the large veins of the leg, these clots are primarily composed of red cells and fibrin (known as red clots) (Fig. 1B). A common complication of venous thrombosis is PE, which occurs when part of the thrombus breaks away and travels to the lung resulting in partial or complete cessation of blood flow in the pulmonary artery. As opposed to arterial thrombosis, which occurs due to arterial injury and exposure of the sub-endothelium, venous thrombosis mainly occurs due to changes in the composition of the blood, changes in blood flow and/or activation of the endothelium (51–52). Many different factors can increase the incidence of VTE, such as the presence of cancer, obesity, and major surgery (53–54). Thrombophilia is the propensity to develop thrombosis as a result of changes in the blood itself. This can result from an increase in levels of circulating clotting factors, a resistance of clotting factors to inactivation (i.e. factor V Leiden), or a decrease in levels of anticoagulants.
While arterial thrombosis is caused by TF-derived from the vessel wall or within a rupture plaque, venous thrombosis occurs in the absence of gross vein wall disruption (55). It has been suggested that increased levels of circulating TF may trigger venous thrombus formation (56–57). Furthermore, mononuclear cell-associated TF is elevated 24 and 48 hours postoperatively and precedes clinical occurrence of VTE, strongly suggesting a positive association between surgery-induced TF expression and VTE (58). Interestingly, monocytes from patients with VTE had increased levels of TF antigen and activity compared with controls (59–61). In addition, TF antigen has been detected in human deep-vein thrombi (62). These data suggest that TF is present in venous thrombi and that it may play a key role in initiating venous thrombosis.
Two methods of inducing venous thrombosis are utilised in animal models (Table 2). These are the inferior vena cava (IVC) ligation/ stasis model and the collagen-coated thread technique. Although several studies have also utilised Rose Bengal and FeCl3 of veins, these are not widely accepted as good models due to their non-physiological ablation of the endothelium. The most commonly used model is ligation of the IVC, which results in stasis of the blood and formation of thrombi (63–64). A recent study by Zhou et al. using a rat IVC ligation model found TF staining in monocytes within the thrombi and endothelial cell adjacent to the thrombi (65). Szaloney et al. demonstrated that TF blockage with an inhibitor of the TF:FVIIa complex (PHA-796) reduced thrombosis in the IVC ligation model in non-human primates (66). In a translational study, Biro et al. demonstrated that MVs from cardiac surgery patients increased thrombosis in a rat model of IVC ligation (67). This effect was abolished when the MVs were preincubated with an anti-human TF antibody, demonstrating MVs promote venous thrombosis in a TF-dependent manner. Day and colleagues confirmed the role of TF in venous thrombosis by showing that low TF mice have smaller thrombi than controls in an IVC ligation model (9). However, they found that decreasing the level of TF expression in haematopoietic cells did not affect thrombosis in this model. Nevertheless, a reduction of TF activity either via use of an inhibitory drug, genetic manipulation, or inhibitory antibody reduced venous thrombosis in these animal models.
Table 2.
Role of tissue factor (TF) in venous and microvascular thrombosis models.
| Thrombotic method | Vascular location | Animal species | Inhibitor/gene | TF dependent | Study description | Reference |
|---|---|---|---|---|---|---|
| Ligation | IVC | N-H Primate | PHA-796 | Yes | Thrombosis was inhibited in a dose-dependent manner | 66 |
| Ligation | IVC | Rat | anti-TF Ab | Yes | Inhibition of TF abolished thrombosis from MVs collected from cardiac surgery patients | 67 |
| Ligation | IVC | Mouse | Low TF gene | Yes | Low TF mice were protected from venous thrombosis | 9 |
| Collagen-coated thread | Jugular Vein | Rabbit | anti-TF Ab | Yes | Inhibition of TF significantly downregulates fibrin accumulation | 68 |
| Laser injury | Cremaster arterioles | Mouse | Low TF gene | Yes | Inhibition of thrombus formation displaying small platelet thrombi lacking TF or fibrin | 74 |
| Laser injury | Cremaster arterioles | Mouse | Low TF gene | Yes | TF-bearing MVs derived from haematopoietic cells initiate thrombus formation | 75 |
Abbreviations: N-H: non-human; Ab: antibody; PHA-927: TF:FVII complex inhibitor
The collagen-coated thread technique has been utilised as a model of venous thrombosis in rabbits. The procedure consists of interrupting blood flow to the jugular vein, and either inserting a cotton thread pre-soaked in 1 mg/ml fibrillar collagen, and then restoring blood flow, or inserting a silicon catheter with the pre-soaked cotton thread attached to the lumen (68). Himber et al. demonstrated that a monoclonal antibody to rabbit TF inhibited fibrin accumulation and thrombus propagation in this model of venous thrombosis (68). Further, they demonstrated that leukocytes stained for TF within the thrombi. Further studies are needed to determine the cellular source(s) of TF responsible for initiating venous thrombosis.
While these models allow the analysis of pathways that contribute to venous thrombosis in animals, they both have limitations with regard to the formation of venous thrombi. The IVC ligation model has been criticised because this procedure may injure the vasculature during suture positioning, thus possibly releasing vessel wall TF into the blood. Further, the IVC ligation model stops all venous blood flow, which will reduce the delivery of MVs. In contrast, most venous thrombi occur in regions of disturbed or reduced flow. While the collagen-coated thread model has blood flow, the process of introducing the thread via clamping and insertion of a surgical graft, may also introduce vessel wall TF into the circulation. A new model of venous thrombosis is gaining popularity and is thought to be more physiologically relevant. Known as the St. Thomas’ Model of venous thrombosis, a stenotic reduction of blood flow (~80–90%) in the IVC is produced by a silk ligature, and then a clamp is used to damage the endothelium (69). This model of stenosis allows for delivery of leukocytes and MVs to the site of thrombosis to a greater extent than the ligation model. Kollnberger et al. demonstrated that mice lacking the TF gene in myeloid cells had a dramatic reduction in venous thrombus size when compared to controls when utilizing this stenosis model (70). This study demonstrates that IVC thrombosis is also dependent upon TF.
Microvascular thrombosis
Microvascular thrombosis examines clot formation in small cre-master or mesenteric arterioles of mice. Specifically, a laser is focused through an optical port of a microscope onto the target vessel. The laser beam is focused so that the resultant heat injury is only induced in a particular part of the vessel. Utilising this laser technology can physically injure a single or multiple endothelial cells with minimal damage to cells in the vessel wall. The thrombus is then observed by intravital microscopy to view the accumulation of TF, fibrin, and platelets. This model of thrombus formation has some advantages over the aforementioned arterial and venous models. The procedure is minimally invasive and requires minimal animal handling or perturbation. Furthermore, the injuries induced are small in size and multiple injuries can be produced in one mouse (71). However, the disadvantages of this model are the use of non-physiologic heat injury and the very small size of the vessels that may not mimic thrombosis in human coronary and carotid arteries.
Furie et al. have demonstrated that MVs from the blood rapidly accumulate into the thrombus. This is due to an interaction between P-selectin glycoprotein ligand 1 on the MV and P-selectin on the surface of the activated platelet. Accumulation of TF precedes fibrin formation within the thrombus (72–73). Recently, Chou et al. demonstrated that both vessel wall and haematopoietic cell-derived TF-positive MVs contributed to thrombosis in this model (Table 2) (74). Furthermore, additional studies demonstrated that circulating TF is specifically delivered via MVs (75). These data demonstrate that both vessel wall TF and haematopoietic cell-derived TF-positive MVs contribute to formation of a thrombus in the model of laser-injured microvasculature.
Disseminated intravascular coagulation
Disseminated intravascular coagulation (DIC) is characterised by microvascular thrombosis (76). Sepsis and endotoxaemia are the most common pathologic conditions leading to DIC (77–78). DIC can also be caused by severe trauma, such as surgery, resulting in the release of “tissue debris” into the circulation and subsequent activation of coagulation (79–81).
After introduction of endotoxin or live Gram-negative bacteria into experimental animals, thrombin generation is increased after a period of 3–5 hours (82). Originally it was thought that the contact system, comprised of FXI, FXII, and plasma kallikrein, mediated DIC-induced thrombosis. However, studies have demonstrated that these proteins were not activated in sepsis (83–84). Further experiments demonstrated that induction of TF expression and subsequent generation of thrombin was the mechanism of DIC-induced thrombosis. Most human patients diagnosed with DIC have detectable levels of TF antigen in their plasma (85). Moreover, sepsis induces the expression of TF by vascular cells, such as monocytes (86).
There are numerous studies that have examined the role of TF in DIC (Table 3). A monoclonal TF antibody (87), rTFPI (88), and FVIIa inhibitor (89) were all shown to reduce DIC and death in a lethal baboon model of sepsis. Furthermore, chimpanzees pre-injected with a monoclonal anti-TF antibody (90) or a monoclonal antibody to FVII/FVIIa (91) were also protected from endotoxin-induced coagulation. Similar results were found in a rabbit model of DIC, where a polyclonal rabbit TF inhibited activation of coagulation in animals injected with endotoxin (92). Recently, we found that low TF mice had reduced levels of thrombin-anti-thrombin (TAT) in a mouse model of endotoxaemia (93). Moreover, this study also demonstrated that haematopoietic-derived TF played a role in the activation of coagulation in endotoxaemic mice. Our recent studies indicate that TF expressed by both haematopoietic cells and non-haematopoietic cells contributes to activation of coagulation in endotoxaemic mice (94). Monocytes were the major haematopoietic cell that expressed TF. However, deletion of TF in endothelial cells did not reduce the activation of coagulation indicating that TF expression by other non-haematopoietic cells drives thrombosis in this model. In sum, these studies demonstrate TF plays a central role in DIC induced by endotoxaemia and sepsis.
Table 3.
Role of tissue factor (TF) in models of disseminated intravascular coagulation (DIC).
| Thrombotic method | Vascular location | Animal species | Inhibitor/gene | TF dependent | Study description | Reference |
|---|---|---|---|---|---|---|
| Endotoxin or TF Injection | Blood analysis | Rabbit | Polyclonal rabbit TF Ab | Yes | DIC was attenuated, however, endotoxic shock remained unchanged | 92 |
| Endotoxin injection | Blood analysis | Baboons | mAb TF | Yes | Protected against DIC activation of coagulation | 87 |
| Endotoxin injection | Blood analysis | Baboons | rTFPI | Yes | Activation of coagulation, measured by TAT, inhibited and decreased IL-6 | 88 |
| Endotoxin injection | Blood analysis | Baboons | FVIIai | Yes | Activation of coagulation inhibited, as well as IL-6 and IL-8, but not TNF-α | 89 |
| Endotoxin injection | Blood analysis | Chimps | mAb TF | Yes | Activation of coagulation inhibited, but no effect on IL-6 or TNF-α cytokines | 90 |
| Endotoxin injection | Blood analysis | Chimps | mAb FVII/FVIIa | Yes | DIC-induced coagulation inhibited | 91 |
| Endotoxin injection | Blood analysis | Mouse | Low TF Gene | Yes | Low TF mice are protected from endotoxaemia activation of coagulation | 93 |
| Endotoxin injection | Blood analysis | Mouse | LysM and Tie2 Cre TF Flox deletion | Yes | Conditional deletion of myeloid TF protects against endotoxaemia-induced coagulation | 94 |
Abbreviations: mab: monoclonal antibody; FVIIai: active site inhibitor of FVII; r: recombinant
Conclusion
In conclusion, all of the aforementioned models of arterial, venous, and microvascular thrombosis, as well as DIC, are all dependent on TF for initiating thrombosis. Models of arterial thrombosis induce vessel wall injury, similar to atherosclerotic plaque rupture, and are primarily dependent on vessel-wall derived TF for initiation of the coagulation cascade. Other studies indicate that circulating TF contributes to venous thrombosis and microvascular thrombosis. Finally, both haematopoietic and non-haematopoietic cell-derived TF activate coagulation in a mouse model of endotoxaemia. Further work is needed to elucidate the different cell types that express TF and their role in different models of thrombosis. Mice with cell type-specific deletion of the TF gene may be able to answer some of these questions.
References
- 1.Edgington TS, Mackman N, Brand K, et al. The structural biology of expression and function of tissue factor. Thromb Haemost. 1991;66:67–79. [PubMed] [Google Scholar]
- 2.Bach RR. Initiation of coagulation by tissue factor. CRC Crit Rev Biochem. 1988;23:339–368. doi: 10.3109/10409238809082548. [DOI] [PubMed] [Google Scholar]
- 3.Broze GJ., Jr Tissue factor pathway inhibitor and the current concept of blood coagulation. Blood Coagul Fibrinolysis. 1995;6 (Suppl 1):S7–13. doi: 10.1097/00001721-199506001-00002. [DOI] [PubMed] [Google Scholar]
- 4.Drake TA, Morrissey JH, Edgington TS. Selective cellular expression of tissue factor in human tissues. Implications for disorders of hemostasis and thrombosis. Am J Pathol. 1989;134:1087–1097. [PMC free article] [PubMed] [Google Scholar]
- 5.Fleck RA, Rao LV, Rapaport SI, et al. Localization of human tissue factor antigen by immunostaining with monospecific, polyclonal anti-human tissue factor antibody. Thromb Res. 1990;59:421–437. doi: 10.1016/0049-3848(90)90148-6. [DOI] [PubMed] [Google Scholar]
- 6.Flossel C, Luther T, Muller M, Albrecht S, Kasper M. Immunohistochemical detection of tissue factor (TF) on paraffin sections of routinely fixed human tissue. Histochemistry. 1994;101:449–453. doi: 10.1007/BF00269495. [DOI] [PubMed] [Google Scholar]
- 7.Rauch U, Nemerson Y. Circulating tissue factor and thrombosis. Curr Opin Hematol. 2000;7:273–277. doi: 10.1097/00062752-200009000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Butenas S, Bouchard BA, Brummel-Ziedins KE, et al. Tissue factor activity in whole blood. Blood. 2005;105:2764–2770. doi: 10.1182/blood-2004-09-3567. [DOI] [PubMed] [Google Scholar]
- 9.Day SM, Reeve JL, Pedersen B, et al. Macrovascular thrombosis is driven by tissue factor derived primarily from the blood vessel wall. Blood. 2005;105:192–198. doi: 10.1182/blood-2004-06-2225. [DOI] [PubMed] [Google Scholar]
- 10.Diamant M, Nieuwland R, Pablo RF, et al. Elevated numbers of tissue-factor exposing microparticles correlate with components of the metabolic syndrome in uncomplicated type 2 diabetes mellitus. Circulation. 2002;106:2442–2447. doi: 10.1161/01.cir.0000036596.59665.c6. [DOI] [PubMed] [Google Scholar]
- 11.Giesen PL, Rauch U, Bohrmann B, et al. Blood-borne tissue factor: another view of thrombosis. Proc Natl Acad Sci USA. 1999;96:2311–2315. doi: 10.1073/pnas.96.5.2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siddiqui FA, Desai H, Amirkhosravi A, et al. The presence and release of tissue factor from human platelets. Platelets. 2002;13:247–253. doi: 10.1080/09537100220146398. [DOI] [PubMed] [Google Scholar]
- 13.So AK, Varisco PA, Kemkes-Matthes B, et al. Arthritis is linked to local and systemic activation of coagulation and fibrinolysis pathways. J Thromb Haemost. 2003;1:2510–2515. doi: 10.1111/j.1538-7836.2003.00462.x. [DOI] [PubMed] [Google Scholar]
- 14.Egorina EM, Sovershaev MA, Bjorkoy G, et al. Intracellular and surface distribution of monocyte tissue factor: application to intersubject variability. Arterioscler Thromb Vasc Biol. 2005;25:1493–1498. doi: 10.1161/01.ATV.0000168413.29874.d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morel O, Toti F, Hugel B, Bakouboula B, Camoin-Jau L, Dignat-George F, et al. Procoagulant microparticles: disrupting the vascular homeostasis equation? Arterioscler Thromb Vasc Biol. 2006;26:2594–2604. doi: 10.1161/01.ATV.0000246775.14471.26. [DOI] [PubMed] [Google Scholar]
- 16.Boden G, Rao AK. Effects of hyperglycemia and hyperinsulinemia on the tissue factor pathway of blood coagulation. Curr Diab Rep. 2007;7:223–227. doi: 10.1007/s11892-007-0035-1. [DOI] [PubMed] [Google Scholar]
- 17.Moosbauer C, Morgenstern E, Cuvelier SL, et al. Eosinophils are a major intravascular location for tissue factor storage and exposure. Blood. 2007;109:995–1002. doi: 10.1182/blood-2006-02-004945. [DOI] [PubMed] [Google Scholar]
- 18.Pawlinski R, Pedersen B, Erlich J, et al. Role of tissue factor in haemostasis, thrombosis, angiogenesis and inflammation: lessons from low tissue factor mice. Thromb Haemost. 2004;92:444–450. doi: 10.1160/TH04-05-0309. [DOI] [PubMed] [Google Scholar]
- 19.Soejima H, Ogawa H, Yasue H, et al. Heightened tissue factor associated with tissue factor pathway inhibitor and prognosis in patients with unstable angina. Circulation. 1999;99:2908–2913. doi: 10.1161/01.cir.99.22.2908. [DOI] [PubMed] [Google Scholar]
- 20.Kakkar AK, DeRuvo N, Chinswangwatanakul V, et al. Extrinsic-pathway activation in cancer with high factor VIIa and tissue factor. Lancet. 1995;346:1004–1005. doi: 10.1016/s0140-6736(95)91690-3. [DOI] [PubMed] [Google Scholar]
- 21.Sturk-Maquelin KN, Nieuwland R, Romijn FP, et al. Pro- and non-coagulant forms of non-cell-bound tissue factor in vivo. J Thromb Haemost. 2003;1:1920–1926. doi: 10.1046/j.1538-7836.2003.00361.x. [DOI] [PubMed] [Google Scholar]
- 22.Aras O, Shet A, Bach RR, et al. Induction of microparticle- and cell-associated intravascular tissue factor in human endotoxemia. Blood. 2004;103:4545–4553. doi: 10.1182/blood-2003-03-0713. [DOI] [PubMed] [Google Scholar]
- 23.Marsik C, Quehenberger P, Mackman N, et al. Validation of a novel tissue factor assay in experimental human endotoxemia. Thromb Res. 2003;111:311–315. doi: 10.1016/j.thromres.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 24.Key NS, Slungaard A, Dandelet L, et al. Whole blood tissue factor procoagulant activity is elevated in patients with sickle cell disease. Blood. 1998;91:4216–4223. [PubMed] [Google Scholar]
- 25.Toomey JR, Kratzer KE, Lasky NM, et al. Targeted disruption of the murine tissue factor gene results in embryonic lethality. Blood. 1996;88:1583–1587. [PubMed] [Google Scholar]
- 26.Carmeliet P, Mackman N, Moons L, et al. Role of tissue factor in embryonic blood vessel development. Nature. 1996;383:73–75. doi: 10.1038/383073a0. [DOI] [PubMed] [Google Scholar]
- 27.Bugge TH, Xiao Q, Kombrinck KW, Flick MJ, Holmback K, Danton MJ, et al. Fatal embryonic bleeding events in mice lacking tissue factor, the cell-associated initiator of blood coagulation. Proc Natl Acad Sci USA. 1996;93:6258–6263. doi: 10.1073/pnas.93.13.6258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parry GC, Erlich JH, Carmeliet P, et al. Low levels of tissue factor are compatible with development and hemostasis in mice. J Clin Invest. 1998;101:560–569. doi: 10.1172/JCI814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pan S, Kleppe LS, Witt TA, et al. The effect of vascular smooth muscle cell-targeted expression of tissue factor pathway inhibitor in a murine model of arterial thrombosis. Thromb Haemost. 2004;92:495–502. doi: 10.1160/TH04-01-0006. [DOI] [PubMed] [Google Scholar]
- 30.Marmur JD, Thiruvikraman SV, Fyfe BS, et al. Identification of active tissue factor in human coronary atheroma. Circulation. 1996;94:1226–1232. doi: 10.1161/01.cir.94.6.1226. [DOI] [PubMed] [Google Scholar]
- 31.Tremoli E, Camera M, Toschi V, et al. Tissue factor in atherosclerosis. Atherosclerosis. 1999;144:273–283. doi: 10.1016/s0021-9150(99)00063-5. [DOI] [PubMed] [Google Scholar]
- 32.Wilcox JN, Smith KM, Schwartz SM, et al. Localization of tissue factor in the normal vessel wall and in the atherosclerotic plaque. Proc Natl Acad Sci USA. 1989;86:2839–2843. doi: 10.1073/pnas.86.8.2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Misumi K, Ogawa H, Yasue H, et al. Comparison of plasma tissue factor levels in unstable and stable angina pectoris. Am J Cardiol. 1998;81:22–26. doi: 10.1016/s0002-9149(97)00801-1. [DOI] [PubMed] [Google Scholar]
- 34.Mackman N. Role of tissue factor in hemostasis, thrombosis, and vascular development. Arterioscler Thromb Vasc Biol. 2004;24:1015–1022. doi: 10.1161/01.ATV.0000130465.23430.74. [DOI] [PubMed] [Google Scholar]
- 35.Sturgeon SA, Jones C, Angus JA, Wright CE. Adaptation of the Folts and electrolytic methods of arterial thrombosis for the study of anti-thrombotic molecules in small animals. J Pharmacol Toxicol Methods. 2006;53:20–29. doi: 10.1016/j.vascn.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 36.Speidel CM, Thornton JD, Meng YY, et al. Procoagulant activity on injured arteries and associated thrombi is mediated primarily by the complex of tissue factor and factor VIIa. Coron Artery Dis. 1996;7:57–62. [PubMed] [Google Scholar]
- 37.Pawashe AB, Golino P, Ambrosio G, et al. A monoclonal antibody against rabbit tissue factor inhibits thrombus formation in stenotic injured rabbit carotid arteries. Circ Res. 1994;74:56–63. doi: 10.1161/01.res.74.1.56. [DOI] [PubMed] [Google Scholar]
- 38.Suleymanov OD, Szalony JA, Salyers AK, et al. Pharmacological interruption of acute thrombus formation with minimal hemorrhagic complications by a small molecule tissue factor/factor VIIa inhibitor: comparison to factor Xa and thrombin inhibition in a nonhuman primate thrombosis model. J Pharmacol Exp Ther. 2003;306:1115–1121. doi: 10.1124/jpet.103.052779. [DOI] [PubMed] [Google Scholar]
- 39.Abendschein DR, Baum PK, Verhallen P, et al. A novel synthetic inhibitor of factor Xa decreases early reocclusion and improves 24-h patency after coronary fibrinolysis in dogs. J Pharmacol Exp Ther. 2001;296:567–572. [PubMed] [Google Scholar]
- 40.Lefkovits J, Malycky JL, Rao JS, et al. Selective inhibition of factor Xa is more efficient than factor VIIa-tissue factor complex blockade at facilitating coronary thrombolysis in the canine model. J Am Coll Cardiol. 1996;28:1858–1865. doi: 10.1016/S0735-1097(96)00401-9. [DOI] [PubMed] [Google Scholar]
- 41.Carmeliet P, Moons L, Collen D. Mouse models of angiogenesis, arterial stenosis, atherosclerosis and hemostasis. Cardiovasc Res. 1998;39:8–33. doi: 10.1016/s0008-6363(98)00108-4. [DOI] [PubMed] [Google Scholar]
- 42.Asada Y, Hara S, Tsuneyoshi A, et al. Fibrin-rich and platelet-rich thrombus formation on neointima: recombinant tissue factor pathway inhibitor prevents fibrin formation and neointimal development following repeated balloon injury of rabbit aorta. Thromb Haemost. 1998;80:506–511. [PubMed] [Google Scholar]
- 43.Roque M, Reis ED, Fuster V, et al. Inhibition of tissue factor reduces thrombus formation and intimal hyperplasia after porcine coronary angioplasty. J Am Coll Cardiol. 2000;36:2303–2310. doi: 10.1016/s0735-1097(00)01018-4. [DOI] [PubMed] [Google Scholar]
- 44.Chi L, Gibson G, Peng YW, et al. Characterization of a tissue factor/factor VIIa-dependent model of thrombosis in hypercholesterolemic rabbits. J Thromb Haemost. 2004;2:85–92. doi: 10.1111/j.1538-7836.2004.00547.x. [DOI] [PubMed] [Google Scholar]
- 45.Tseng MT, Dozier A, Haribabu B, et al. Transendothelial migration of ferric ion in FeCl3 injured murine common carotid artery. Thromb Res. 2006;118:275–280. doi: 10.1016/j.thromres.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 46.Farrehi PM, Ozaki CK, Carmeliet P, et al. Regulation of arterial thrombolysis by plasminogen activator inhibitor-1 in mice. Circulation. 1998;97:1002–1008. doi: 10.1161/01.cir.97.10.1002. [DOI] [PubMed] [Google Scholar]
- 47.Wang L, Miller C, Swarthout RF, et al. Vascular smooth muscle-derived tissue factor is critical for arterial thrombosis after ferric chloride-induced injury. Blood. 2009;113:705–713. doi: 10.1182/blood-2007-05-090944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ambrosio G, Tritto I, Golino P. Reactive oxygen metabolites and arterial thrombosis. Cardiovasc Res. 1997;34:445–452. doi: 10.1016/s0008-6363(97)00101-6. [DOI] [PubMed] [Google Scholar]
- 49.Wilson CA, Hatchell DL. Photodynamic retinal vascular thrombosis. Rate and duration of vascular occlusion. Invest Ophthalmol Vis Sci. 1991;32:2357–2365. [PubMed] [Google Scholar]
- 50.Westrick RJ, Bodary PF, Xu Z, et al. Deficiency of tissue factor pathway inhibitor promotes atherosclerosis and thrombosis in mice. Circulation. 2001;103:3044–3046. doi: 10.1161/hc2501.092492. [DOI] [PubMed] [Google Scholar]
- 51.Mackman N. Triggers, targets and treatments for thrombosis. Nature. 2008;451:914–918. doi: 10.1038/nature06797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eppihimer MJ, Schaub RG. P-Selectin-dependent inhibition of thrombosis during venous stasis. Arterioscler Thromb Vasc Biol. 2000;20:2483–2488. doi: 10.1161/01.atv.20.11.2483. [DOI] [PubMed] [Google Scholar]
- 53.Cushman M. Epidemiology and risk factors for venous thrombosis. Semin Hematol. 2007;44:62–69. doi: 10.1053/j.seminhematol.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Urbach D, Matzen KA, Heitmann D, et al. Relation between peri-operative anti-thrombin activity and deep vein thrombosis after elective hip replacement surgery. Vasa. 2003;32:14–17. doi: 10.1024/0301-1526.32.1.14. [DOI] [PubMed] [Google Scholar]
- 55.Sevitt S. The structure and growth of valve-pocket thrombi in femoral veins. J Clin Pathol. 1974;27:517–528. doi: 10.1136/jcp.27.7.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hron G, Kollars M, Weber H, et al. Tissue factor-positive microparticles: cellular origin and association with coagulation activation in patients with colorectal cancer. Thromb Haemost. 2007;97:119–123. [PubMed] [Google Scholar]
- 57.Polgar J, Matuskova J, Wagner DD. The P-selectin, tissue factor, coagulation triad. J Thromb Haemost. 2005;3:1590–1596. doi: 10.1111/j.1538-7836.2005.01373.x. [DOI] [PubMed] [Google Scholar]
- 58.Johnson GJ, Leis LA, Bach RR. Tissue factor activity of blood mononuclear cells is increased after total knee arthroplasty. Thromb Haemost. 2009;102:728–734. doi: 10.1160/TH09-04-0261. [DOI] [PubMed] [Google Scholar]
- 59.Himber J, Kling D, Fallon JT, Nemerson Y, Riederer MA. In situ localization of tissue factor in human thrombi. Blood. 2002;99:4249–4250. doi: 10.1182/blood-2002-02-0557. [DOI] [PubMed] [Google Scholar]
- 60.Kamikura Y, Wada H, Nobori T, et al. Elevated levels of leukocyte tissue factor mRNA in patients with venous thromboembolism. Thromb Res. 2005;116:307–312. doi: 10.1016/j.thromres.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 61.Holschermann H, Haberbosch W, Terhalle HM, et al. Increased monocyte tissue factor activity in women following cerebral venous thrombosis. J Neurol. 2003;250:631–632. doi: 10.1007/s00415-003-1060-x. [DOI] [PubMed] [Google Scholar]
- 62.Lee A, Agnelli G, Buller H, et al. Dose-response study of recombinant factor VIIa/ tissue factor inhibitor recombinant nematode anticoagulant protein c2 in prevention of postoperative venous thromboembolism in patients undergoing total knee replacement. Circulation. 2001;104:74–78. doi: 10.1161/hc2601.091386. [DOI] [PubMed] [Google Scholar]
- 63.Reyers I, Mussoni L, Donati MB, et al. Failure of aspirin at different doses to modify experimental thrombosis in rats. Thromb Res. 1980;18:669–674. doi: 10.1016/0049-3848(80)90221-2. [DOI] [PubMed] [Google Scholar]
- 64.Wakefield TW, Strieter RM, Wilke CA, et al. Venous thrombosis-associated inflammation and attenuation with neutralizing antibodies to cytokines and adhesion molecules. Arterioscler Thromb Vasc Biol. 1995;15:258–268. doi: 10.1161/01.atv.15.2.258. [DOI] [PubMed] [Google Scholar]
- 65.Zhou J, May L, Liao P, et al. Inferior vena cava ligation rapidly induces tissue factor expression and venous thrombosis in rats. Arterioscler Thromb Vasc Biol. 2009;29:863–869. doi: 10.1161/ATVBAHA.109.185678. [DOI] [PubMed] [Google Scholar]
- 66.Szalony JA, Suleymanov OD, Salyers AK, et al. Administration of a small molecule tissue factor/factor VIIa inhibitor in a non-human primate thrombosis model of venous thrombosis: effects on thrombus formation and bleeding time. Thromb Res. 2003;112:167–174. doi: 10.1016/j.thromres.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 67.Biro E, Sturk-Maquelin KN, Vogel GM, et al. Human cell-derived microparticles promote thrombus formation in vivo in a tissue factor-dependent manner. J Thromb Haemost. 2003;1:2561–2568. doi: 10.1046/j.1538-7836.2003.00456.x. [DOI] [PubMed] [Google Scholar]
- 68.Himber J, Wohlgensinger C, Roux S, et al. Inhibition of tissue factor limits the growth of venous thrombus in the rabbit. J Thromb Haemost. 2003;1:889–895. doi: 10.1046/j.1538-7836.2003.00110.x. [DOI] [PubMed] [Google Scholar]
- 69.McGuinness CL, Humphries J, Waltham M, et al. Recruitment of labelled monocytes by experimental venous thrombi. Thromb Haemost. 2001;85:1018–1024. [PubMed] [Google Scholar]
- 70.Koellnberger M, von Bruehl M, Bergmeier W, et al. Platelets Contribute to Arterial and Venous Thrombosis in vivo [Abstract] Circulation. 2007;116(16 Supplement):II_75. Abstract: 448. [Google Scholar]
- 71.Rosen ED, Raymond S, Zollman A, et al. Laser-induced noninvasive vascular injury models in mice generate platelet- and coagulation-dependent thrombi. Am J Pathol. 2001;158:1613–1622. doi: 10.1016/S0002-9440(10)64117-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Falati S, Liu Q, Gross P, et al. Accumulation of tissue factor into developing thrombi in vivo is dependent upon microparticle P-selectin glycoprotein ligand 1 and platelet P-selectin. J Exp Med. 2003;197:1585–1598. doi: 10.1084/jem.20021868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Falati S, Gross P, Merrill-Skoloff G, et al. Real-time in vivo imaging of platelets, tissue factor and fibrin during arterial thrombus formation in the mouse. Nat Med. 2002;8:1175–1181. doi: 10.1038/nm782. [DOI] [PubMed] [Google Scholar]
- 74.Chou J, Mackman N, Merrill-Skoloff G, et al. Hematopoietic cell-derived micro-particle tissue factor contributes to fibrin formation during thrombus propagation. Blood. 2004;104:3190–3197. doi: 10.1182/blood-2004-03-0935. [DOI] [PubMed] [Google Scholar]
- 75.Gross PL, Furie BC, Merrill-Skoloff G, et al. Leukocyte-versus microparticle-mediated tissue factor transfer during arteriolar thrombus development. J Leukoc Biol. 2005;78:1318–1326. doi: 10.1189/jlb.0405193. [DOI] [PubMed] [Google Scholar]
- 76.Levi M, Ten Cate H. Disseminated intravascular coagulation. N Engl J Med. 1999;341:586–592. doi: 10.1056/NEJM199908193410807. [DOI] [PubMed] [Google Scholar]
- 77.Levi M, de Jonge E, van der Poll T, et al. Disseminated intravascular coagulation. Thromb Haemost. 1999;82:695–705. [PubMed] [Google Scholar]
- 78.Baglin T. Disseminated intravascular coagulation: diagnosis and treatment. Br Med J. 1996;312:683–687. doi: 10.1136/bmj.312.7032.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Levi M. Disseminated intravascular coagulation: What’s new? Crit Care Clin. 2005;21:449–467. doi: 10.1016/j.ccc.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 80.Levi M, van der Poll T, ten Cate H, et al. The cytokine-mediated imbalance between coagulant and anticoagulant mechanisms in sepsis and endotoxaemia. Eur J Clin Invest. 1997;27:3–9. doi: 10.1046/j.1365-2362.1997.570614.x. [DOI] [PubMed] [Google Scholar]
- 81.Gando S. Disseminated intravascular coagulation in trauma patients. Semin Thromb Hemost. 2001;27:585–592. doi: 10.1055/s-2001-18864. [DOI] [PubMed] [Google Scholar]
- 82.van Deventer SJ, Buller HR, ten Cate JW, et al. Experimental endotoxemia in humans: analysis of cytokine release and coagulation, fibrinolytic, and complement pathways. Blood. 1990;76:2520–2526. [PubMed] [Google Scholar]
- 83.van der Poll T, Buller HR, ten Cate H, et al. Activation of coagulation after administration of tumor necrosis factor to normal subjects. N Engl J Med. 1990;322:1622–1627. doi: 10.1056/NEJM199006073222302. [DOI] [PubMed] [Google Scholar]
- 84.Pixley RA, De La Cadena R, Page JD, et al. The contact system contributes to hypotension but not disseminated intravascular coagulation in lethal bacteremia. In vivo use of a monoclonal anti-factor XII antibody to block contact activation in baboons. J Clin Invest. 1993;91:61–68. doi: 10.1172/JCI116201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shimura M, Wada H, Wakita Y, et al. Plasma tissue factor and tissue factor pathway inhibitor levels in patients with disseminated intravascular coagulation. Am J Hematol. 1996;52:165–170. doi: 10.1002/(SICI)1096-8652(199607)52:3<165::AID-AJH5>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 86.Drake TA, Cheng J, Chang A, et al. Expression of tissue factor, thrombomodulin, and E-selectin in baboons with lethal Escherichia coli sepsis. Am J Pathol. 1993;142:1458–1470. [PMC free article] [PubMed] [Google Scholar]
- 87.Taylor FB, Jr, Chang A, Ruf W, et al. Lethal E. coli septic shock is prevented by blocking tissue factor with monoclonal antibody. Circ Shock. 1991;33:127–134. [PubMed] [Google Scholar]
- 88.Carr C, Bild GS, Chang AC, et al. Recombinant E. coli-derived tissue factor pathway inhibitor reduces coagulopathic and lethal effects in the baboon gram-negative model of septic shock. Circ Shock. 1994;44:126–137. [PubMed] [Google Scholar]
- 89.Taylor FB, Chang AC, Peer G, et al. Active site inhibited factor VIIa (DEGR VIIa) attenuates the coagulant and interleukin-6 and –8, but not tumor necrosis factor, responses of the baboon to LD100 Escherichia coli. Blood. 1998;91:1609–1615. [PubMed] [Google Scholar]
- 90.Levi M, ten Cate H, Bauer KA, et al. Inhibition of endotoxin-induced activation of coagulation and fibrinolysis by pentoxifylline or by a monoclonal anti-tissue factor antibody in chimpanzees. J Clin Invest. 1994;93:114–120. doi: 10.1172/JCI116934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Biemond BJ, Levi M, ten Cate H, et al. Complete inhibition of endotoxin-induced coagulation activation in chimpanzees with a monoclonal Fab fragment against factor VII/VIIa. Thromb Haemost. 1995;73:223–230. [PubMed] [Google Scholar]
- 92.Warr TA, Rao LV, Rapaport SI. Disseminated intravascular coagulation in rabbits induced by administration of endotoxin or tissue factor: effect of anti-tissue factor antibodies and measurement of plasma extrinsic pathway inhibitor activity. Blood. 1990;75:1481–1489. [PubMed] [Google Scholar]
- 93.Pawlinski R, Pedersen B, Schabbauer G, et al. Role of tissue factor and protease-activated receptors in a mouse model of endotoxemia. Blood. 2004;103:1342–1347. doi: 10.1182/blood-2003-09-3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pawlinski R, Mackman N. Cellular sources of tissue factor that contribute to activation of coagulation in endotoxemic mice. J Thromb Haemost. 2009;7(Suppl 2) doi: 10.1111/j.1538-7836.2009.03448.x. Abstract AS-MO-042. [DOI] [PMC free article] [PubMed] [Google Scholar]